Abstract
Background
Fetal assessment following preterm prelabour rupture of membranes (PPROM) may result in earlier delivery due to earlier detection of fetal compromise. However, early delivery may not always be in the fetal or maternal interest, and the effectiveness of different fetal assessment methods in improving neonatal and maternal outcomes is uncertain.
Objectives
To study the effectiveness of fetal assessment methods for improving neonatal and maternal outcomes in PPROM. Examples of fetal assessment methods that would be eligible for inclusion in this review include fetal cardiotocography, fetal movement counting and Doppler ultrasound.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 June 2014) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials comparing any fetal assessment methods, or comparing one fetal assessment method to no assessment.
Data collection and analysis
Two review authors independently assessed trials for inclusion into the review. The same two review authors independently assessed trial quality and independently extracted data. Data were checked for accuracy.
Main results
We included three studies involving 275 women (data reported for 271) with PPROM at up to 34 weeks' gestation. All three studies were conducted in the United States. Each study investigated different methods of fetal assessment. One study compared weekly endovaginal ultrasound scans with no assessment (n = 93), one compared amniocentesis with no assessment (n = 47), and one compared daily nonstress testing with daily modified biophysical profiling (n = 135). We were unable to perform a meta‐analysis, but were able to report data from individual studies.
There was no convincing evidence of increased risk of neonatal death in the group receiving endovaginal ultrasound scans compared with the group receiving no assessment (risk ratio (RR) 7.30, 95% confidence interval (CI) 0.39 to 137.54; one study, 92 women), or in the group receiving amniocentesis compared with the group receiving no amniocentesis (RR 1.00, 95% CI 0.07 to 15.00; one study, 44 women). For both these interventions, we inferred that there were no fetal deaths in the intervention or control groups. The study comparing daily nonstress testing with daily modified biophysical profiling did not report fetal or neonatal death. Primary outcomes of maternal death and serious maternal morbidity were not reported in any study. Overall, there were few statistically significant differences in outcomes between the comparisons.
The overall quality of evidence is poor, because participant blinding was not possible for any study.
Authors' conclusions
There is insufficient evidence on the benefits and harms of fetal assessment methods for improving neonatal and maternal outcomes in women with PPROM to draw firm conclusions. The overall quality of evidence that does exist is poor.
Further high‐quality randomised controlled trials are required to guide clinical practice.
Plain language summary
Fetal assessment methods after preterm prelabour rupture of membranes for improving outcomes for mothers and babies
In a small number of pregnancies, the sac (membranes) surrounding the baby ruptures preterm, before 37 weeks of gestation, and before onset of labour. Preterm prelabour rupture of membranes (PPROM) occurs in around a third of all preterm births but the cause is often unknown. PPROM can result in illness and death for both the mother and baby through complications such as compression of the umbilical cord and bacteria infecting the uterus. The biggest problem for the baby is an increased risk of respiratory distress, brain haemorrhage and infection because of being born early. Several methods are available for assessing the wellbeing of the unborn baby following PPROM, to help healthcare providers detect any problems with the baby and make decisions on whether to deliver the baby earlier that they otherwise would. Most women will go into spontaneous labour within several days of PPROM.
This review was carried out to evaluate whether these methods lead to improved health outcomes for the mother and her baby. The review included three randomised controlled studies that involved a total of 275 women (data reported for 271) with PPROM at up to 34 weeks' gestation. All three studies were from the USA. They each investigated different methods of fetal assessment, so no meta‐analysis could be conducted. Instead, the review reported the results of each individual study. One study compared weekly endovaginal ultrasound scans where the probe is placed inside the vagina versus no assessment, one compared an amniotic fluid test to measure levels of fetal lung surfactant with no assessment, and one compared a daily 'nonstress test' (recording the fetal heartbeat) with daily modified biophysical profiling (recording the fetal heartbeat as well as estimating the volume of amniotic fluid surrounding the baby). In each study, there were few statistically significant differences between groups in outcomes for the mother, fetus or neonate. The overall quality of the evidence was poor, because participants knew which group they were in. More studies are needed to assess the benefits and harms of fetal assessment methods for improving neonatal and maternal outcomes in women with PPROM before firm conclusions can be drawn.
Background
Description of the condition
Preterm prelabour rupture of membranes (PPROM) is defined as rupture of the chorioamniotic membranes before 37 weeks' gestation, where there is at least one hour between rupture of membranes and the onset of contractions. It occurs in around 1% to 2% of pregnancies and is associated with around 30% to 40% of preterm births (Douvas 1984; Maxwell 1993; Merenstein 1996).
PPROM is often idiopathic, but it has been associated with a number of factors including a history of previous preterm delivery or PPROM, vaginal bleeding during pregnancy, uterine overdistension (ACOG 1998), black race (Savitz 1991), smoking, cervical cerclage (Hadley 1990), amniocentesis, infection, and low socioeconomic status (Mercer 2003).
The mechanisms responsible for PPROM are largely unknown, but might include excessive stretching of the membranes, decreased collagen content, placental abruption or programmed amniotic cell death (Parry 1998). Between one‐quarter and one‐half of cases are associated with intrauterine infection and inflammation (Simhan 2005).
PPROM is associated with fetal and maternal morbidity and mortality. Cord prolapse, cord compression, placental abruption and maternal/neonatal infection are potential complications (Simhan 2005), and many women require interventions to expedite delivery, with induction of labour or caesarean section. The major cause of perinatal morbidity following PPROM arises from preterm birth, which is a significant problem because most women will go into spontaneous labour within several days of PPROM (Goldenberg 2008). The neonatal morbidity associated with preterm birth includes respiratory distress syndrome, intraventricular haemorrhage, and infection (Parry 1998).
Other Cochrane reviews have assessed interventions for improving outcomes following PPROM. Kenyon 2010 found some evidence that antibiotics may increase time to labour and decrease the risk of infection, but appear to have no effect on mortality. Mackeen 2011 found tocolytics increase time to labour but may also increase risk of chorioamnionitis, low Apgar scores and need for ventilation. Hofmeyr 2011 found transabdominal amnioinfusion decreased risk of neonatal sepsis, infection and death, but the evidence was insufficient to recommend routine use. Transcervical amnioinfusion improved fetal heart rate patterns and umbilical cord blood pH results but did not significantly improve substantive clinical outcomes. Abou El Senoun 2010 found home care is associated with a lower rate of caesarean section compared with hospital care, although there was only a small effect on outcomes such as perinatal mortality, neonatal and maternal infection, latency period and neonatal admission to intensive care.
Description of the intervention
This review focuses on fetal assessment methods following PPROM to improve pregnancy outcomes.
There are several methods for assessing fetal wellbeing, including fetal magnetic resonance imaging (MRI) for lung volume, fetal movement counting, fetal and umbilical artery or venous Doppler ultrasound, biophysical profile or modified biophysical profile, fetal cardiotocography and amniocentesis for fetal lung maturity. Although some of these methods may be useful in identifying intrauterine infection (Carroll 1995; Gauthier 1992; Goldstein 1988; Vintzileos 1986; Yücel 1997), their effectiveness in improving outcomes following PPROM is uncertain.
How the intervention might work
Fetal assessment may result in earlier delivery due to earlier detection of fetal compromise, which could improve neonatal and maternal outcomes. However, if the fetal assessment tool is inaccurate, it may cause inappropriate early delivery and worsen outcomes.
Why it is important to do this review
Following PPROM, accurate methods for assessing fetal wellbeing and prognosis are needed to aid obstetricians' decisions in planning the time and mode of delivery. However, the value of the various methods of fetal assessment in ultimately improving neonatal and maternal outcomes has yet to be established.
Objectives
This review aims to compare methods of fetal assessment in improving outcomes following preterm prelabour rupture of membranes (PPROM).
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant published and unpublished randomised controlled trials (RCTs). Quasi‐RCTs and cluster‐randomised trials were eligible for inclusion but none were found. We planned to exclude cross‐over trials because this is an unsuitable design to study fetal assessment during pregnancy. We planned to include abstracts if sufficient details were available and we planned to contact the authors of abstracts to obtain further information where necessary.
Types of participants
Women with preterm prelabour rupture of membranes (PPROM) before 37 + 0 weeks' gestation with no specific maternal or fetal contraindications to expectant management (defined by trialists).
Types of interventions
All methods of fetal assessment that could detect fetal compromise and provide indication for early delivery. These include the following.
Fetal movement counting ‐ mothers count fetal movements over a specified period or measure the time it takes for the fetus to make a specified number of movements. Reduced frequency of movements can be a sign of fetal compromise.
Fetal cardiotocography ‐ allows monitoring of the fetal heart rate over time. Changes in heart rate parameters outside of the accepted 'normal' limits can identify fetuses at risk of acute or chronic fetal hypoxia.
Biophysical profile (BPP) ‐ an assessment of overall fetal wellbeing through the combined assessment of fetal cardiotocography along with ultrasonic measurement of fetal movements, fetal tone, fetal breathing and estimation of amniotic fluid volume.
Modified biophysical profile (MBPP) ‐ less time‐consuming than the BPP, the MBPP is an assessment of overall fetal wellbeing based only on fetal cardiotocography and estimation of amniotic fluid volume.
Fetal and umbilical artery or venous Doppler ultrasound ‐ ultrasonic measurement of blood flow through blood vessels of interest can indicate high vascular impedance and possible feto‐placental compromise.
Fetal MRI lung volumetrics ‐ fetal lung volume is measured by MRI and used as an indication of fetal lung development and pulmonary hypoplasia.
Amniocentesis for fetal lung maturity ‐ levels of fetal lung surfactant in the amniotic fluid are used to gauge fetal lung maturity.
Several Cochrane reviews have examined the use of some of these tests in improving maternal and fetal outcomes in normal and compromised pregnancies (Alfirevic 2010a; Alfirevic 2010b; Grivell 2010; Lalor 2008; Mangesi 2007). The scope of this review is to examine these assessment methods in improving outcomes in pregnancies affected by PPROM.
We made the following comparisons.
Any intervention versus no intervention.
One intervention versus another intervention.
Types of outcome measures
We have added additional secondary outcomes at the review stage ‐ these are noted below in italics.
Primary outcomes
Fetal death (antenatal). This was clarified from the original outcome of "any fetal death" in the review protocol.
Neonatal death (in the first 28 days of life). This was clarified from the original outcome of "any fetal death" in the review protocol. Neonatal death was included in the protocol as a secondary outcome.
Maternal death.
Serious maternal morbidity defined as: 1) septicaemia, 2) need for intensive care, 3) organ failure/need for ventilation, 4) need for hysterectomy.
Secondary outcomes
Maternal
Maternal chorioamnionitis (as defined by trialists).
Major postpartum haemorrhage.
Maternal endometritis (as defined by trialists).
Mode of delivery. This includes individual modes of delivery, e.g., caesarean delivery.
Induction of labour.
Postpartum maternal pyrexia (as defined by trialists).
Days of antenatal hospitalisation.
Days of postnatal hospitalisation.
Total days hospitalisation.
Breastfeeding initiated in hospital.
Breastfeeding at hospital discharge.
Maternal satisfaction.
Fetal
Gestational age at birth.
Days from randomisation/rupture of membrane to birth. This was clarified from the original "Days from randomisation to birth" in the protocol.
Birth within 48 hours after rupture of membranes.
Birth within seven days of rupture of membranes.
Birth before 37 + 0 weeks' gestation.
Birth before 34 + 0 weeks' gestation.
Birth before 28 + 0 weeks' gestation.
Neonatal
Postneonatal mortality ‐ death after 28 days of life but before one year.
Infant mortality ‐ death at or after 12 months of age.
Respiratory distress syndrome.
Use of surfactant.
Use of mechanical ventilation.
Days of mechanical ventilation.
Days of oxygen therapy.
Oxygen treatment greater than 28 days.
Oxygen therapy at 36 + 0 weeks' gestation.
Birthweight.
Birthweight less than 10th centile for gestational age.
Birthweight less than 2500 g.
Birthweight less than 1500 g.
Admission to neonatal intensive care unit.
Length of stay in neonatal intensive care unit.
Days from birth to discharge home from hospital.
Major cerebral abnormalities on ultrasound prior to discharge.
Necrotising enterocolitis.
Neonatal encephalopathy (as described by trialists).
Postural deformities (as defined by trialists).
Disability at time of childhood follow‐up (as defined by trialists).
Serious disability (as defined by trialists) after two years.
Diagnosis of fetal distress in labour (as defined by trialists).
Cardiotocographic abnormality in labour (as defined by trialists).
Cord pH less than 7.00.
Apgar scores less than seven at five minutes.
Other
Caregiver satisfaction.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group's Trials Register (30 June 2014).
The Cochrane Pregnancy and Childbirth Group's Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the 'Specialized Register' section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors (GC Sharp (GCS), S Stock (SS)) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, consultation with a third author (JE Norman (JEN)).
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (GCS, SS) extracted the data using the agreed form. Discrepancies were resolved through discussion or, if required, consultation with a third author (JEN). We entered data into The Cochrane Collaboration's statistical software, Review Manager (RevMan 2012), and checked for accuracy.
Assessment of risk of bias in included studies
Two review authors (GCS and SS) independently assessed risk of bias for each study using the criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third author (JEN).
1. Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
3.1. Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
3.2. Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as low, high or unclear risk of bias.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
5. Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
6. Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias; or
unclear whether there is risk of other bias.
7. Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analysis (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we used the mean difference (MD) if outcomes were measured in the same way between trials. If required, we planned to use the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials. However, in future updates of this review, we will include cluster‐randomised trials in the analyses if they are identified, along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Other unit of analysis issues
Studies with multiple pregnancies
Of the three included studies, one excluded multiple pregnancies. The other two studies did not exclude women with multiple pregnancies but did not report whether or not any women with multiple pregnancies took part. Therefore, we were unable to adjust our analyses for multiple pregnancies. In future updates of this review, if any studies that include multiple pregnancies are identified we will treat the infants as independent and note effects of estimates of confidence intervals in the review.
Studies with more than two treatment groups
We did not identify any studies using one or more treatment groups (multi‐arm studies). In future updates of this review, if any such studies are included we will combine groups to create a single pair‐wise comparison. We will use methods described in the Cochrane Handbook for Systematic Reviews of Interventions to ensure that we do not double count participants (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we planned to carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we planned to attempt to include all participants randomised to each group in the analyses, and all participants would have been analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial would have been the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We planned to measure heterogeneity of treatment effects between trials using the T2, I2 and Chi2 statistics. We planned to regard heterogeneity as substantial if an I2 was greater than 30% and either a T2 was greater than zero, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
Statistical analysis were carried out using the Review Manager software (RevMan 2012). Fixed‐effect meta‐analysis was used for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials' populations and methods were judged sufficiently similar. If there had been clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity had been detected, we planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. We planned to treat the random‐effects summary as the average range of possible treatment effects and we planned to discuss the clinical implications of treatment effects differing between trials. We planned not to combine trials if the average treatment effect was not clinically meaningful.
If, in future updates of this review, we use the random‐effects model, the results will be presented as the average treatment effect with 95% CIs, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
Multiple pregnancy versus singleton pregnancy.
Gestation at PPROM (before 20 + 0 weeks, 20 + 1 to 24 + 0 weeks, 24 + 1 to 28 + 0 weeks, 28 + 1 to 32 + 0 weeks, 32 + 1 to 37 + 0 weeks) versus each other category.
We planned to use the following outcomes in subgroup analysis.
Fetal death (antenatal).
Neonatal death (in the first 28 weeks of life).
Maternal death.
Serious maternal morbidity defined as: 1) septicaemia, 2) need for intensive care, 3) organ failure/need for ventilation, 4) need for hysterectomy.
We planned to assess subgroup differences by interaction tests available within the Review Manager software (RevMan 2012). We would have reported the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I2 value.
Sensitivity analysis
We planned to carry out sensitivity analysis to assess trial quality on completeness of data, adequacy of sequence generation and allocation of concealment. We also planned to use sensitivity analysis to explore the effects of fixed‐effect or random‐effects analysis for outcomes with statistical heterogeneity and the effects of any assumptions made.
We planned to restrict sensitivity analysis to the primary outcomes.
Results
Description of studies
Results of the search
See: Figure 1. The search of the Cochrane Pregnancy and Childbirth Group's Trials Register identified four studies in six reports. Three studies (five reports) reporting data on 271 women were included in this review (Carlan 1997; Cotton 1984; Lewis 1999), and one study (one report) was excluded (Li 2010).
1.
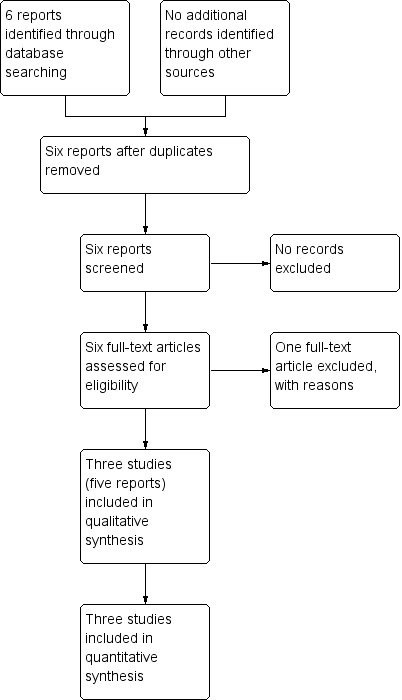
Study flow diagram.
Included studies
We included three studies (Carlan 1997; Cotton 1984; Lewis 1999), involving 275 women in total (data reported for 271 women). Lewis 1999 was associated with three publications: one abstract (Lewis 1998), one answer from Lewis et al in response to a letter regarding the abstract (Harper 2000), and one full article describing the results of the trial (Lewis 1999).
Study design
All included studies were unmasked single‐centre randomised controlled trials.
Sample sizes
Carlan 1997 recruited 93 women (92 available for analysis), 46 were randomised to the intervention group and 47 to the control group. Cotton 1984 recruited 47 women (44 available for analysis), 25 were randomised to the intervention group and 22 to the control group. Lewis 1999 recruited 135 women (all 135 available for analysis), 66 were randomised to the group receiving daily modified biophysical profiling and 69 were randomised to the group receiving a daily nonstress test.
Study location
All included studies were conducted in the USA.
Participants
All included studies recruited women with a diagnosis of PPROM occurring ≤ 34 + 0 weeks. Lewis 1999 only recruited women with this diagnosis who were stable after 24 hours' monitoring.
Carlan 1997 excluded women with multiple pregnancies, cerclage in place, cervical dilatation on admission of more than 4 cm, active regular contractions, or any indication for delivery. Ninety‐three women were randomised to either the intervention group (n = 46) or the control group (n = 47). One woman was found to have a funic presentation and cervical dilation at the initial scan and was delivered by caesarean immediately. This left 92 participants available for analysis (45 in the intervention group and 47 in the control group).
Cotton 1984 excluded women with evidence of amnionitis, an indication for immediate delivery, sonographic evidence of gross fetal congenital anomalies, active labour with a cervical dilation of more than 4 cm, or an inadequate amount of amniotic fluid for amniocentesis (assessed by real time ultrasound). Forty‐seven women were randomised to either the intervention group (n = 25) or the control group (n = 22). In the intervention group, amniocentesis was unsuccessful in two women and one woman withdrew after the initial amniocentesis visit. This left 44 participants available for analysis (22 in each group).
Lewis 1999 excluded women with signs of obvious clinical infection or indications for immediate delivery. No women were excluded from analysis or lost to follow‐up, so 135 were available for analysis. Sixty‐six women were randomised to receive daily biophysical profile assessments, and 69 were randomised to receive daily nonstress tests.
All three studies reported no significant difference between groups in maternal age, but only Lewis 1999 reported the mean values (24.4 years in the nonstress test group and 25.7 years in the biophysical profile group). Similarly, all studies reported no significant difference in parity between groups, with Lewis 1999 reporting 30.4% primigravid in the nonstress test group and 19.7% primigravid in the biophysical profile group. Lewis 1999 also reported no significant difference in ethnicity (27.5% white, 72.5% African American in the nonstress test group; 21.2% white, 78.8% African American in the modified biophysical profile group). The ethnicity of the participants was not reported for Carlan 1997 or Cotton 1984.
Types of intervention
Carlan 1997 compared weekly endovaginal ultrasound scans versus no endovaginal ultrasound scans. Both groups received intravenous antibiotics until genital culture results were available. If the culture results were positive, women in both groups received intravenous antibiotics and were offered amniocentesis. Participants in both groups were offered steroids to accelerate pulmonary maturation until 31 + 0 weeks' gestation.
Cotton 1984 compared amniocentesis versus no amniocentesis. Amniocentesis was performed at admission, 48 hours later, and then at weekly intervals until pulmonary maturity or bacteria in the amniotic fluid was identified, at which point the baby was delivered. Both groups also received bi‐weekly electronic fetal heart rate monitoring. In the absence of fetal pulmonary maturity, infection or fetal compromise, both groups received intravenous tocolysis. Steroids were also administered to both groups at the discretion of the managing physician.
Lewis 1999 compared a daily nonstress test (a measurement of fetal cardiotocography) versus daily modified biophysical profile (as described by Chamberlain 1984: total score of 10, with points each for tone, amniotic fluid, movement, breathing, and a nonstress test). Both groups also received intravenous antibiotics until delivery or for a total of seven days.
Outcome measures
None of our primary outcomes (fetal death/neonatal death, maternal death, and serious maternal morbidity) were addressed by all trials. Neonatal deaths (in the first 28 days of life) were reported for Carlan 1997 and Cotton 1984. From this, we have inferred that there were no fetal deaths (before birth) in either study. Lewis 1999 did not report data for any of our primary outcomes.
Of our secondary outcomes, only maternal chorioamnionitis and respiratory distress syndrome were addressed by all three trials.
All addressed outcomes are listed in Characteristics of included studies.
Excluded studies
One study (Li 2010) was excluded because all the women recruited were diagnosed with PROM occurring after 37 + 0 weeks' gestation. Therefore, the study did not meet the inclusion criteria of this review. See Characteristics of excluded studies.
Risk of bias in included studies
See Characteristics of included studies, See Figure 2 for further details regarding 'Risk of bias' assessment. The overall risk of bias in the three studies was high.
2.
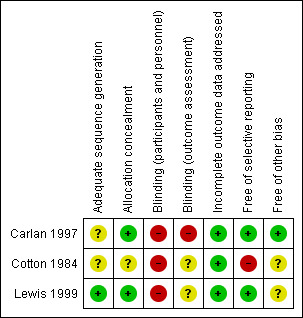
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation sequence generation and concealment was not described for Cotton 1984, so the risk of selection bias is unclear. For Carlan 1997, the authors state that sequences were "randomly generated" but do not describe the method. Therefore, we can only judge that the risk of bias is unclear. However, the allocations were concealed in sealed envelopes, which is associated with a low risk of bias. For Lewis 1999, there is an overall low risk of selection bias because random number tables were used to generate allocation sequences and allocations were concealed in opaque envelopes that were not opened until after informed consent was obtained.
Blinding
Participants and personnel
The nature of the interventions meant that blinding of participants and clinicians was not possible. This puts all three studies at a high risk of bias.
Outcome assessors
For Carlan 1997, it is assumed that outcome assessors were also not blinded because one of the outcomes (cervical dilation) was only recorded for the treatment group. Therefore, this study is associated with a high risk of bias. For Cotton 1984 and Lewis 1999, outcome assessor blinding is not discussed, so the risk of bias arising from this is unclear.
Incomplete outcome data
We have judged that there is a low risk of attrition bias because all three studies adequately described any loss of participants to follow‐up and any exclusion of participants after randomisation. Additionally, the level of attrition in all studies was low.
Selective reporting
For Carlan 1997 and Lewis 1999, the risk of reporting bias is low because there is no suggestion of selective outcome reporting in either study. For Cotton 1984, the risk of reporting bias is high because several outcomes listed in the methods section (gestation at delivery, birthweight and Apgar scores) are not described in the results section. Other outcomes (maternal antepartum and postpartum hospitalisation days, postpartum endometritis and fetal sepsis) are reported only as showing no difference between groups, without any quantitative data.
Other potential sources of bias
For Carlan 1997, we have no reason to suspect another potential source of bias. A potential source of bias in Cotton 1984 and Lewis 1999 arises from their treatment of women with multiple pregnancies. Neither study excludes women with multiple pregnancies but the reports do not mention how many (if any) women had multiple pregnancies. If women with multiple pregnancies are included in the analyses (without a subgroup analysis assessing their effect), then this is a potential source of bias.
Effects of interventions
Each of the included studies considered different interventions, so we considered these in separate comparisons.
Data on one outcome (days of postnatal hospitalisation) in one study (Cotton 1984) were not reported in a manner that could be included in the analysis (only the median and range were reported), these data were therefore omitted.
No study reported average gestation at delivery.
Comparison 1 ‐ Weekly endovaginal ultrasound scan versus no endovaginal ultrasound scan (one study, n available for analysis = 92)
All data for Comparison 1 were taken from Carlan 1997.
Primary outcomes
Fetal death
We inferred that there were no fetal deaths in either the intervention or control group (risk ratio (RR) not estimable; Analysis 1.1).
1.1. Analysis.
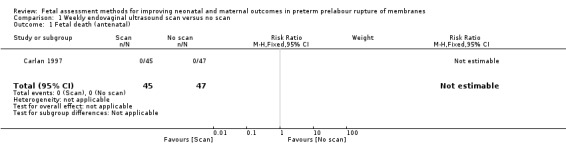
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 1 Fetal death (antenatal).
Neonatal death
Although there appeared to be an increased risk of neonatal death in the group assessed by endovaginal ultrasound compared to the control group (RR 7.30, 95% confidence interval (CI) 0.39 to 137.54; one study, 92 women; Analysis 1.2), there were very large confidence intervals, reflecting the small numbers, and findings were compatible with no effect.
1.2. Analysis.
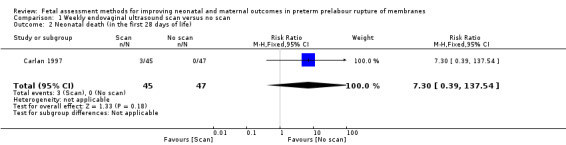
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 2 Neonatal death (in the first 28 days of life).
Maternal death
Data for this outcome were not reported.
Serious maternal morbidity
Data for this outcome were not reported.
Secondary outcomes
Maternal
Maternal chorioamnionitis
The difference in risk of maternal chorioamnionitis between the intervention and control groups (RR 0.72, 95% CI 0.34 to 1.52; one study, 92 women; Analysis 1.3) did not reach statistical significance.
1.3. Analysis.
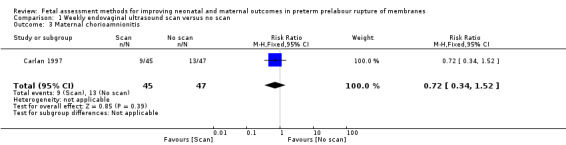
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 3 Maternal chorioamnionitis.
Major postpartum haemorrhage.
Data for this outcome were not reported.
Maternal endometritis
Compared with the group receiving no fetal assessment, Carlan 1997 found a statistically significant decreased risk of maternal endometritis in the group assessed by endovaginal ultrasound (RR 0.32, 95% confidence interval (CI) 0.11 to 0.91; one study, 92 women; Analysis 1.4). Any difference in the risk of infection may be clinically important.
1.4. Analysis.
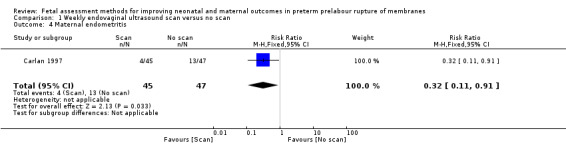
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 4 Maternal endometritis.
Mode of delivery
Women in the intervention group had a similar chance of caesarean delivery compared to women in the control group (RR 1.39, 95% CI 0.33 to 5.88; one study, 92 women; Analysis 1.5).
1.5. Analysis.
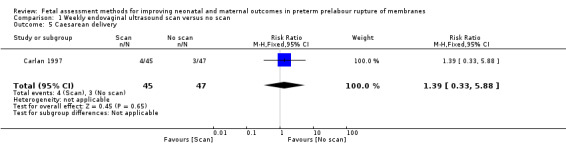
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 5 Caesarean delivery.
Induction of labour
Data for this outcome were not reported.
Postpartum maternal pyrexia
Data for this outcome were not reported.
Days of antenatal hospitalisation
Data for this outcome were not reported.
Days of postnatal hospitalisation
On average, women in the intervention group spent a similar length of time in hospital after delivery compared to women in the control group (mean difference (MD) ‐1.00 day, 95% CI ‐22.03 to 20.03; one study, 92 women; Analysis 1.6).
1.6. Analysis.
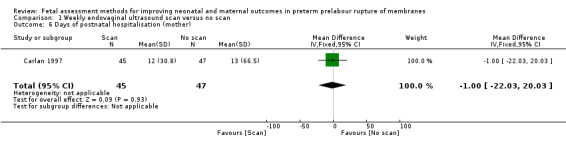
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 6 Days of postnatal hospitalisation (mother).
Total days hospitalisation
Data for this outcome were not reported.
Breastfeeding initiated in hospital
Data for this outcome were not reported.
Breastfeeding at hospital discharge
Data for this outcome were not reported.
Maternal satisfaction
Data for this outcome were not reported.
Fetal
Gestational age at birth
Data for this outcome were not reported.
Days from randomisation/rupture of membrane to birth
On average, the number of days from randomisation to birth was similar in the intervention group compared to the control group (MD 1.90 days, 95% CI ‐11.61 to 15.41; one study, 92 women; Analysis 1.7).
1.7. Analysis.
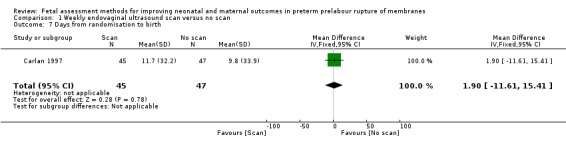
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 7 Days from randomisation to birth.
Birth within 48 hours after rupture of membranes
Data for this outcome were not reported.
Birth within seven days of rupture of membranes
Data for this outcome were not reported.
Birth before 37 + 0 weeks' gestation
Data for this outcome were not reported.
Birth before 34 + 0 weeks' gestation
Data for this outcome were not reported.
Birth before 28 + 0 weeks' gestation
Data for this outcome were not reported.
Neonatal
Postneonatal mortality
Data for this outcome were not reported.
Infant mortality
Data for this outcome were not reported.
Respiratory distress syndrome
Babies in the intervention group had a similar risk of respiratory distress syndrome compared to babies in the control group (RR 0.71, 95% CI 0.40 to 1.27; one study, 92 women; Analysis 1.8).
1.8. Analysis.
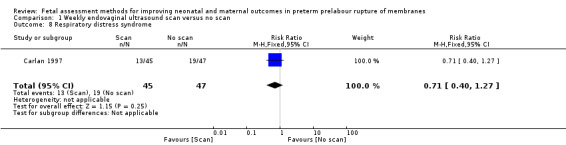
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 8 Respiratory distress syndrome.
Use of surfactant
Data for this outcome were not reported.
Use of mechanical ventilation
Data for this outcome were not reported.
Days of mechanical ventilation
Data for this outcome were not reported.
Days of oxygen therapy
Data for this outcome were not reported.
Oxygen treatment greater than 28 days
Data for this outcome were not reported.
Oxygen therapy at 36 + 0 weeks' gestation
Data for this outcome were not reported.
Birthweight
On average, babies in the intervention group were of similar birthweight to control group babies (MD 31.00 g, 95% CI ‐636.35 to 698.35; one study, 92 women; Analysis 1.9).
1.9. Analysis.
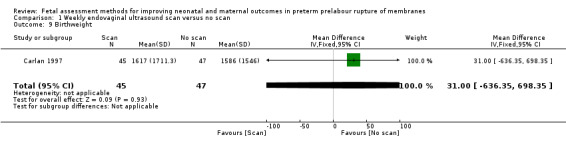
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 9 Birthweight.
Birthweight less than 10th centile for gestational age.
Data for this outcome were not reported.
Birthweight less than 2500 g
Data for this outcome were not reported.
Birthweight less than 1500 g
Data for this outcome were not reported.
Admission to neonatal intensive care unit
Data for this outcome were not reported.
Length of stay in neonatal intensive care unit
Data for this outcome were not reported.
Days from birth to discharge home from hospital
On average, babies in the intervention group spent a similar length of time in hospital after birth compared to control group babies (MD 4.00 days, 95% CI ‐45.82 to 53.82; one study, 92 women; Analysis 1.10).
1.10. Analysis.
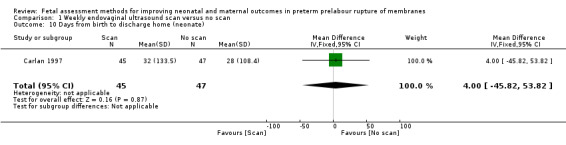
Comparison 1 Weekly endovaginal ultrasound scan versus no scan, Outcome 10 Days from birth to discharge home (neonate).
Major cerebral abnormalities on ultrasound prior to discharge
Data for this outcome were not reported.
Necrotising enterocolitis
Data for this outcome were not reported.
Neonatal encephalopathy (as described by trialists)
Data for this outcome were not reported.
Postural deformities (as defined by trialists)
Data for this outcome were not reported.
Disability at time of childhood follow‐up (as defined by trialists)
Data for this outcome were not reported.
Serious disability (as defined by trialists) after two years
Data for this outcome were not reported.
Diagnosis of fetal distress in labour (as defined by trialists)
Data for this outcome were not reported.
Cardiotocographic abnormality in labour (as defined by trialists)
Data for this outcome were not reported.
Cord pH less than 7.00
Data for this outcome were not reported.
Apgar scores less than seven at five minutes
Data for this outcome were not reported.
Other
Caregiver satisfaction
Data for this outcome were not reported.
Comparison 2 ‐ Amniocentesis versus no amniocentesis (one study, n available for analysis = 44)
All data for Comparison 2 were taken from Cotton 1984.
Primary outcomes
Fetal death
We inferred that there were no fetal deaths in either the control or the intervention group (RR not estimable; Analysis 2.1).
2.1. Analysis.
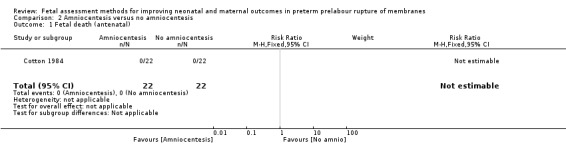
Comparison 2 Amniocentesis versus no amniocentesis, Outcome 1 Fetal death (antenatal).
Neonatal death
There was a similar risk of neonatal death in the intervention group compared to the control group (RR 1.00, 95% CI 0.07 to 15.00; one study, 44 women; Analysis 2.2).
2.2. Analysis.
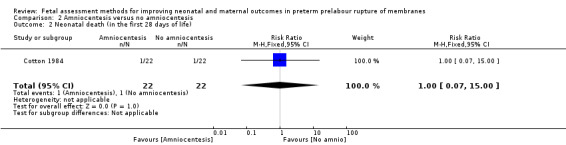
Comparison 2 Amniocentesis versus no amniocentesis, Outcome 2 Neonatal death (in the first 28 days of life).
Maternal death
Data for this outcome were not reported.
Serious maternal morbidity
Data for this outcome were not reported.
Secondary outcomes
Maternal
Maternal chorioamnionitis
Women in the intervention group had a similar risk of chorioamnionitis compared to women in the control group (RR 0.67, 95% CI 0.12 to 3.61; one study, 44 women; Analysis 2.3).
2.3. Analysis.
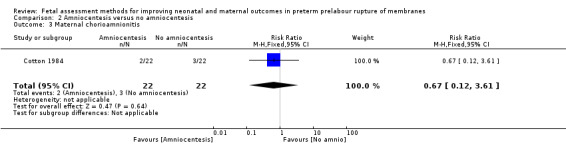
Comparison 2 Amniocentesis versus no amniocentesis, Outcome 3 Maternal chorioamnionitis.
Major postpartum haemorrhage.
Data for this outcome were not reported.
Maternal endometritis
Data for this outcome were not reported.
Mode of delivery
Data for this outcome were not reported.
Induction of labour
Data for this outcome were not reported.
Postpartum maternal pyrexia
Data for this outcome were not reported.
Days of antenatal hospitalisation
Data for this outcome were not reported.
Days of postnatal hospitalisation
Data for this outcome were not reported.
Total days hospitalisation
Data for this outcome were not reported.
Breastfeeding initiated in hospital
Data for this outcome were not reported.
Breastfeeding at hospital discharge
Data for this outcome were not reported.
Maternal satisfaction
Data for this outcome were not reported.
Fetal
Gestational age at birth
Data for this outcome were not reported.
Days from randomisation/rupture of membrane to birth
Data for this outcome were not reported.
Birth within 48 hours after rupture of membranes
There was a similar chance of birth within 48 hours after rupture of membranes in the intervention group compared to the control group (RR 1.33, 95% CI 0.71 to 2.51; one study, 44 women; Analysis 2.4).
2.4. Analysis.
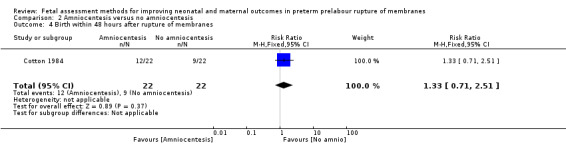
Comparison 2 Amniocentesis versus no amniocentesis, Outcome 4 Birth within 48 hours after rupture of membranes.
Birth within seven days of rupture of membranes
Data for this outcome were not reported.
Birth before 37 + 0 weeks' gestation
Data for this outcome were not reported.
Birth before 34 + 0 weeks' gestation
Data for this outcome were not reported.
Birth before 28 + 0 weeks' gestation
Data for this outcome were not reported.
Neonatal
Postneonatal mortality
Data for this outcome were not reported.
Infant mortality
Data for this outcome were not reported.
Respiratory distress syndrome
There was a similar risk of respiratory distress syndrome in the intervention group compared to the control group (RR 0.67, 95% CI 0.22 to 2.04; one study, 44 women; Analysis 2.5).
2.5. Analysis.
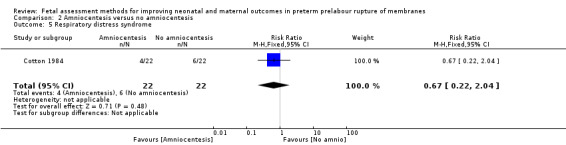
Comparison 2 Amniocentesis versus no amniocentesis, Outcome 5 Respiratory distress syndrome.
Use of surfactant
Data for this outcome were not reported.
Use of mechanical ventilation
Data for this outcome were not reported.
Days of mechanical ventilation
Data for this outcome were not reported.
Days of oxygen therapy
Data for this outcome were not reported.
Oxygen treatment greater than 28 days
Data for this outcome were not reported.
Oxygen therapy at 36 + 0 weeks' gestation
Data for this outcome were not reported.
Birthweight
Data for this outcome were not reported.
Birthweight less than 10th centile for gestational age.
Data for this outcome were not reported.
Birthweight less than 2500 g
Data for this outcome were not reported.
Birthweight less than 1500 g
Data for this outcome were not reported.
Admission to neonatal intensive care unit
Data for this outcome were not reported.
Length of stay in neonatal intensive care unit
Data for this outcome were not reported.
Days from birth to discharge home from hospital
Data for this outcome were not reported.
Major cerebral abnormalities on ultrasound prior to discharge
Data for this outcome were not reported.
Necrotising enterocolitis
Data for this outcome were not reported.
Neonatal encephalopathy (as described by trialists)
Data for this outcome were not reported.
Postural deformities (as defined by trialists)
Data for this outcome were not reported.
Disability at time of childhood follow‐up (as defined by trialists)
Data for this outcome were not reported.
Serious disability (as defined by trialists) after two years
Data for this outcome were not reported.
Diagnosis of fetal distress in labour (as defined by trialists)
Data for this outcome were not reported.
Cardiotocographic abnormality in labour (as defined by trialists)
There was a decreased risk of cardiotocographic abnormality in labour in the intervention group compared to the control group (RR 0.14, 95% CI 0.02 to 1.07; one study, 44 women; Analysis 2.6), although this difference did not reach statistical significance.
2.6. Analysis.
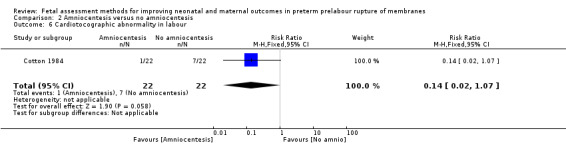
Comparison 2 Amniocentesis versus no amniocentesis, Outcome 6 Cardiotocographic abnormality in labour.
Cord pH less than 7.00
Data for this outcome were not reported.
Apgar scores less than seven at five minutes
Data for this outcome were not reported.
Other
Caregiver satisfaction
Data for this outcome were not reported.
Comparison 3 ‐ Daily nonstress test versus daily modified biophysical profile (one study, n available for analysis = 135)
All data for Comparison 3 were taken from Lewis 1999.
Primary outcomes
Fetal death
Data for this outcome were not reported.
Neonatal death
Data for this outcome were not reported.
Maternal death
Data for this outcome were not reported.
Serious maternal morbidity
Data for this outcome were not reported.
Secondary outcomes
Maternal
Maternal chorioamnionitis
The risk of maternal chorioamnionitis appeared lower in the group receiving daily nonstress tests than in the group receiving daily full biophysical profiles (RR 0.55, 95% CI 0.29 to 1.07; one study, 135 women; Analysis 3.1), although this difference did not reach statistical significance.
3.1. Analysis.
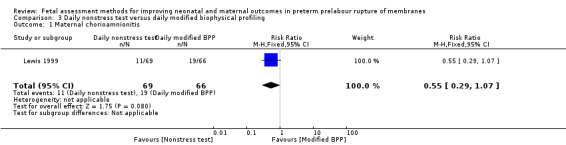
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 1 Maternal chorioamnionitis.
Major postpartum haemorrhage.
Data for this outcome were not reported.
Maternal endometritis
Compared with the group receiving daily full modified biophysical profiles, Lewis 1999 found a statistically significant decreased risk of maternal endometritis in the group receiving the daily nonstress test only (RR 0.25, 95% CI 0.10 to 0.64; one study, 135 women; Analysis 3.2), which could be clinically important.
3.2. Analysis.
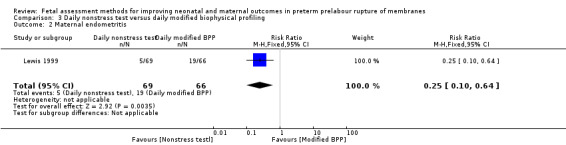
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 2 Maternal endometritis.
Mode of delivery
Women who received daily nonstress tests had a similar risk of caesarean delivery than women who received daily full biophysical profiles (RR 0.64, 95% CI 0.35 to 1.15; one study, 135 women; Analysis 3.3).
3.3. Analysis.
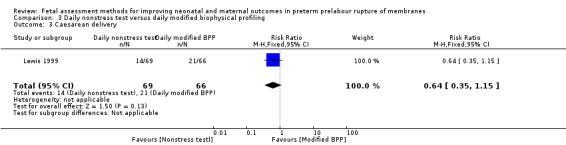
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 3 Caesarean delivery.
Induction of labour
Data for this outcome were not reported.
Postpartum maternal pyrexia
Data for this outcome were not reported.
Days of antenatal hospitalisation
Data for this outcome were not reported.
Days of postnatal hospitalisation
Data for this outcome were not reported.
Total days hospitalisation
Data for this outcome were not reported.
Breastfeeding initiated in hospital
Data for this outcome were not reported.
Breastfeeding at hospital discharge
Data for this outcome were not reported.
Maternal satisfaction
Data for this outcome were not reported.
Fetal
Gestational age at birth
Data for this outcome were not reported.
Days from randomisation/rupture of membrane to birth
On average, the number of days from randomisation to birth was similar in the group receiving the full biophysical profile compared to the daily nonstress test (MD 1.00 day, 95% CI ‐2.83 to 4.83; one study, 135 women; Analysis 3.4).
3.4. Analysis.
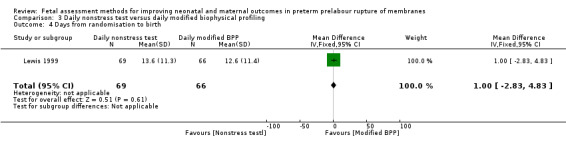
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 4 Days from randomisation to birth.
Birth within 48 hours after rupture of membranes
Data for this outcome were not reported.
Birth within seven days of rupture of membranes
Data for this outcome were not reported.
Birth before 37 + 0 weeks' gestation
Data for this outcome were not reported.
Birth before 34 + 0 weeks' gestation
Data for this outcome were not reported.
Birth before 28 + 0 weeks' gestation
Data for this outcome were not reported.
Neonatal
Postneonatal mortality
Data for this outcome were not reported.
Infant mortality
Data for this outcome were not reported.
Respiratory distress syndrome
There was a lower risk of respiratory distress syndrome in the group receiving daily nonstress tests compared to the group receiving daily full biophysical profiles (RR 0.58, 95% CI 0.33 to 1.03; one study, 135 women; Analysis 3.5), although this difference did not reach statistical significance.
3.5. Analysis.
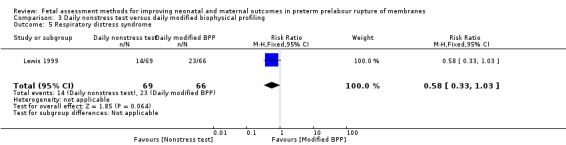
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 5 Respiratory distress syndrome.
Use of surfactant
Data for this outcome were not reported.
Use of mechanical ventilation
Data for this outcome were not reported.
Days of mechanical ventilation
Data for this outcome were not reported.
Days of oxygen therapy
Data for this outcome were not reported.
Oxygen treatment greater than 28 days
Data for this outcome were not reported.
Oxygen therapy at 36 + 0 weeks' gestation
There was a similar chance of oxygen therapy after 36 + 0 weeks in the group receiving the daily nonstress test compared to the group receiving the daily full biophysical profile (RR 0.77, 95% CI 0.21 to 2.73; one study, 135 women; Analysis 3.6).
3.6. Analysis.
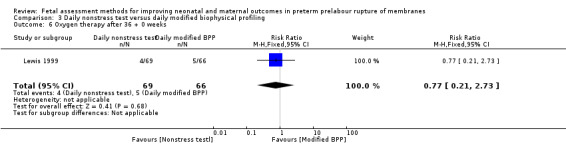
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 6 Oxygen therapy after 36 + 0 weeks.
Birthweight
On average, babies in the daily full biophysical profile group had similar birthweights to babies in the nonstress test group (MD 126.20g, 95% CI ‐56.32 to 308.72; one study, 135 women; Analysis 3.7).
3.7. Analysis.
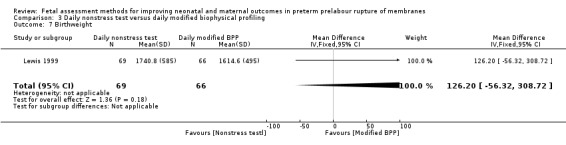
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 7 Birthweight.
Birthweight less than 10th centile for gestational age.
Data for this outcome were not reported.
Birthweight less than 2500 g
Data for this outcome were not reported.
Birthweight less than 1500 g
Data for this outcome were not reported.
Admission to neonatal intensive care unit
Data for this outcome were not reported.
Length of stay in neonatal intensive care unit
Data for this outcome were not reported.
Days from birth to discharge home from hospital
On average, the number of days from birth to discharge was similar in the group receiving the daily nonstress test compared to the group receiving the daily biophysical profile (MD ‐5.60, 95% CI ‐16.88 to 5.68; one study, 135 women; Analysis 3.8).
3.8. Analysis.
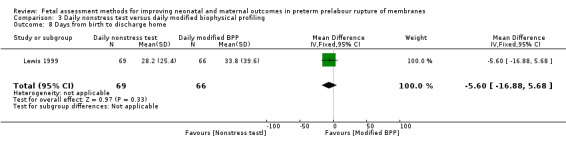
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 8 Days from birth to discharge home.
Major cerebral abnormalities on ultrasound prior to discharge
Data for this outcome were not reported.
Necrotising enterocolitis
There was a similar risk of necrotising enterocolitis (RR 0.64, 95% CI 0.11 to 3.70; one study, 135 women; Analysis 3.9) in the group receiving the nonstress test compared to the group receiving the full biophysical profile.
3.9. Analysis.
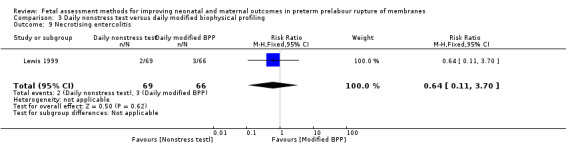
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 9 Necrotising entercolitis.
Neonatal encephalopathy (as described by trialists)
Data for this outcome were not reported.
Postural deformities (as defined by trialists)
Data for this outcome were not reported.
Disability at time of childhood follow‐up (as defined by trialists)
Data for this outcome were not reported.
Serious disability (as defined by trialists) after two years
Data for this outcome were not reported.
Diagnosis of fetal distress in labour (as defined by trialists)
Data for this outcome were not reported.
Cardiotocographic abnormality in labour (as defined by trialists)
Data for this outcome were not reported.
Cord pH less than 7.00
Data for this outcome were not reported.
Apgar scores less than seven at five minutes
There was a similar chance of Apgar scores less than seven at five minutes in the group receiving a daily nonstress test compared to the group receiving a daily biophysical profile (RR 0.78, 95% CI 0.35 to 1.77; one study, 135 women; Analysis 3.10).
3.10. Analysis.
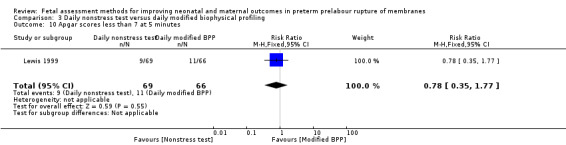
Comparison 3 Daily nonstress test versus daily modified biophysical profiling, Outcome 10 Apgar scores less than 7 at 5 minutes.
Other
Caregiver satisfaction
Data for this outcome were not reported.
Discussion
Summary of main results
This review looked at fetal assessment methods for improving neonatal and maternal outcomes in preterm prelabour rupture of membranes (PPROM). The search returned six reports describing four studies. Of the three studies that met the inclusion criteria of this review, all were unmasked single‐centred randomised controlled trials and all investigated different fetal assessment methods. Carlan 1997 investigated endovaginal ultrasound versus no assessment (number of women randomised = 93, number available for analysis = 92, number of women analysed in the intervention group = 45, number of women analysed in the control group = 47). Cotton 1984 investigated amniocentesis versus no assessment (number of women randomised = 47, number available for analysis = 44, number of women analysed in the treatment group = 22, number of women analysed in the control group = 22). Lewis 1999 investigated daily nonstress tests versus daily modified biophysical profiling (number of women randomised = 135, number available for analysis = 135, number of women analysed in the nonstress test group = 69, number of women analysed in the biophysical profile group = 66). Overall, there were few statistically significant differences in maternal or fetal outcomes between the two groups in any of the studies, but several findings might be clinically important. For our primary outcomes, Carlan 1997 and Cotton 1984 found an increased risk of neonatal death in their intervention groups compared to their control groups, but these differences did not reach statistical significance. Carlan 1997 and Cotton 1984 did not report on fetal death, maternal death or serious maternal morbidity. Lewis 1999 did not report on any of our primary outcomes.
Overall completeness and applicability of evidence
There are too few trials in this area to provide enough data to draw any firm conclusions. Additionally, none of the identified studies reported all of our primary outcomes, and few of our secondary outcomes were reported. None of the outcomes that were reported were statistically significantly affected by any of the assessment methods, so the evidence cannot be applied in a clinical setting.
Quality of the evidence
Overall, the quality of the three studies included in this review is poor. In two (Carlan 1997; Cotton 1984), out of three studies, the method used to generate allocation sequences was not described and in one study (Cotton 1984) the concealment of these allocations was also not described. Although the nature of the interventions prevented blinding of participants and clinicians, blinding of outcome assessors was also either not attempted or not reported in any study. Two studies were free of selective reporting, but one study (Cotton 1984) did not report data for several outcomes mentioned in the methods section. For Cotton 1984 and Lewis 1999 there was an unclear risk of bias associated with the failure to perform a subgroup analysis of women with multiple pregnancies. Attrition was not a problem in any of the studies and reasons for any women lost to follow‐up were also described adequately.
The small number of studies and limited applicability of the evidence mean that findings of these studies and this review may not be generalisable to other populations.
Potential biases in the review process
The applicability of this review is limited by the small number of studies identified and the poor quality of evidence. In an effort to reduce biases from other potential sources, study eligibility for inclusion, data extraction and 'Risk of bias' assessment were independently conducted by two review authors.
Agreements and disagreements with other studies or reviews
We are not aware of any other studies or reviews assessing fetal assessment methods for improving neonatal and maternal outcomes in PPROM.
Authors' conclusions
Implications for practice.
Currently, there is insufficient evidence from randomised controlled trials on the benefits and harms of fetal assessment methods for improving neonatal and maternal outcomes in women with preterm prelabour rupture of membranes (PPROM) to draw firm conclusions that might be applied in clinical practice.
Implications for research.
This is an important clinical question and further randomised controlled trials are required. These should collect data on a wide range of neonatal and maternal outcomes and should include subgroup analyses to assess the effect of multiple pregnancies.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Weekly endovaginal ultrasound scan versus no scan.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fetal death (antenatal) | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Neonatal death (in the first 28 days of life) | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.30 [0.39, 137.54] |
| 3 Maternal chorioamnionitis | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.34, 1.52] |
| 4 Maternal endometritis | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.11, 0.91] |
| 5 Caesarean delivery | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.33, 5.88] |
| 6 Days of postnatal hospitalisation (mother) | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐22.03, 20.03] |
| 7 Days from randomisation to birth | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐11.61, 15.41] |
| 8 Respiratory distress syndrome | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.40, 1.27] |
| 9 Birthweight | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 31.0 [‐636.35, 698.35] |
| 10 Days from birth to discharge home (neonate) | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐45.82, 53.82] |
Comparison 2. Amniocentesis versus no amniocentesis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fetal death (antenatal) | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Neonatal death (in the first 28 days of life) | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.00] |
| 3 Maternal chorioamnionitis | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.61] |
| 4 Birth within 48 hours after rupture of membranes | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.71, 2.51] |
| 5 Respiratory distress syndrome | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.22, 2.04] |
| 6 Cardiotocographic abnormality in labour | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.07] |
Comparison 3. Daily nonstress test versus daily modified biophysical profiling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal chorioamnionitis | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.29, 1.07] |
| 2 Maternal endometritis | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.10, 0.64] |
| 3 Caesarean delivery | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.15] |
| 4 Days from randomisation to birth | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.83, 4.83] |
| 5 Respiratory distress syndrome | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.33, 1.03] |
| 6 Oxygen therapy after 36 + 0 weeks | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.21, 2.73] |
| 7 Birthweight | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | 126.20 [‐56.32, 308.72] |
| 8 Days from birth to discharge home | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐5.60 [‐16.88, 5.68] |
| 9 Necrotising entercolitis | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.70] |
| 10 Apgar scores less than 7 at 5 minutes | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.35, 1.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carlan 1997.
| Methods | A single‐centre unmasked randomised controlled trial conducted from May 1993 until June 1996 (the study was interrupted from March 1994 until December 1994 due to technical difficulties with the sonography machine). | |
| Participants | 93 women with a diagnosis of PPROM between 24 + 0 and 34 + 0 weeks' gestation at Arnold Palmer Hospital for Children and Women (Orlando, Florida, USA). 46 women (45 available for analysis) were randomised to the intervention group and 47 (47 available for analysis) were randomised to the control group. | |
| Interventions | Weekly endovaginal ultrasound scans versus no endovaginal ultrasound scans. | |
| Outcomes |
|
|
| Notes | The trial addressed a further outcome (the relation of cervical length to the time of labour in women who went into spontaneous labour), but this was not included in our review because it did not compare the effect of the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Sequences were "randomly generated", but the method is not described. |
| Allocation concealment | Low risk | Allocations were concealed in sealed envelopes. |
| Blinding (participants and personnel) | High risk | The nature of the intervention prohibited participant and clinician blinding. |
| Blinding (outcome assessment) | High risk | It is presumed that the outcome assessor was also not blinded, because the outcomes include cervical length measurement, which was only available for the treatment group. |
| Incomplete outcome data addressed All outcomes | Low risk | Loss of participants to follow‐up and exclusion of participants after randomisation are described adequately. Only 1 participant was excluded after randomisation, representing a low risk of bias. |
| Free of selective reporting | Low risk | There is no suggestion of selective reporting of outcomes. All main outcomes described in the methods are reported in the results. |
| Free of other bias | Low risk | No reason to suspect other bias. |
Cotton 1984.
| Methods | A single‐centre unmasked randomised controlled trial. | |
| Participants | 47 women with a diagnosis of PPROM between 26 + 0 and 34 + 0 weeks' gestation at Hermann Hospital (Houston, Texas, USA). 25 (22 available for analysis) women were randomised to the intervention group and 22 (22 available for analysis) women were randomised to the control group. | |
| Interventions | Amniocentesis versus no amniocentesis. | |
| Outcomes |
|
|
| Notes | Days of postnatal hospitalisation could not be included in our review because data were presented as the median and range, which was insufficient to calculate the mean difference between the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not described. |
| Allocation concealment | Unclear risk | Not described. |
| Blinding (participants and personnel) | High risk | The nature of the intervention prohibited participant and clinician blinding. |
| Blinding (outcome assessment) | Unclear risk | Outcome assessor blinding is not described, therefore the risk of bias arising from this is unclear. |
| Incomplete outcome data addressed All outcomes | Low risk | Loss of participants to follow‐up and exclusion of participants after randomisation are described adequately. |
| Free of selective reporting | High risk | Several outcomes listed in the methods section are not described in the results section. Some outcomes are reported only as showing no difference between the 2 groups, without any quantitative data. |
| Free of other bias | Unclear risk | The number of women with multiple gestations is not reported. These women were not excluded from the study, but a subgroup analysis of their effect has not been described. Therefore, the risk of bias arising from the inclusion of women with multiple gestations in the analyses is unclear. |
Lewis 1999.
| Methods | A single‐centre unmasked randomised controlled trial conducted during a 36‐month period. | |
| Participants | 135 women with a diagnosis of PPROM ≤ 34 + 0 weeks' gestation at Louisiana State University School of Medicine, Shreveport, Louisiana, USA, and who were stable after 24 hours' monitoring. 66 (66 available for analysis) women were randomised to the biophysical profile group and 69 (69 available for analysis) women were randomised to the nonstress test group. | |
| Interventions | Daily nonstress test versus daily modified biophysical profile. | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Random number tables with a 1:1 match were used to generate allocation sequences. |
| Allocation concealment | Low risk | Allocation cards were kept in opaque envelopes and not opened until after informed consent was obtained. |
| Blinding (participants and personnel) | High risk | The nature of the intervention prohibited participant or clinician blinding. |
| Blinding (outcome assessment) | Unclear risk | Outcome assessor blinding is not described, therefore the risk of bias arising from this is unclear. |
| Incomplete outcome data addressed All outcomes | Low risk | No loss of participants to follow‐up at each data collection point. No exclusion of participants after randomisation. |
| Free of selective reporting | Low risk | There is no suggestion of selective reporting of outcomes. All main outcomes described in the methods are reported in the results. |
| Free of other bias | Unclear risk | The number of women with multiple gestations is not reported. These women were not excluded from the study, but a subgroup analysis of their effect has not been described. Therefore the risk of bias arising from the inclusion of women with multiple gestations in the analyses is unclear. |
PPROM: preterm prelabour rupture of membranes
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Li 2010 | The participants in this study are all over 37 + 0 weeks' gestation. This review addresses PPROM, defined as occurring before 37 + 0 weeks' gestation. |
PPROM: preterm prelabour rupture of membranes
Differences between protocol and review
Primary outcomes
We clarified our first primary outcome (any fetal death) by splitting it into two separate outcomes "fetal death (antenatal)" and "neonatal death (in the first 28 days of life)".
Secondary outcomes
Clarification of outcomes
We further clarified two of our secondary outcomes.
Maternal
"Mode of delivery" includes individual modes of delivery (such as caesarean section)
Fetal
"Days from randomisation to birth" was edited to "Days from randomisation/rupture of membrane to birth"
Contributions of authors
Gemma Sharp (GCS), Sarah Stock (SS) and Jane Norman (JEN) conceived and designed the protocol. SS and JEN provided a clinical perspective. GCS wrote the protocol with input from SS and JEN.
GCS and SS independently assessed studies for inclusion and extracted the data. JEN settled any disputes. GCS wrote the review with input from SS and JEN.
Sources of support
Internal sources
-
University of Edinburgh Principal's Career Development Scholarship, UK.
We are grateful to the University of Edinburgh's Principal Career Development fund for providing a scholarship to GCS.
External sources
Tommy's the baby charity, UK.
The Albert McKern Bequest, UK.
Declarations of interest
Gemma Sharp and Sarah Stock: none known.
Jane Norman has research grants from (non commercial) funding agencies for pregnancy related conditions (full list available on request), consultancy funds from UK government agencies for providing reports on maternal deaths (less than £5000), honoraria for book chapters and books in obstetrics and gynaecology. She has provided paid consultancy to a small drug company (Preglem) with an interest in obstetric/gynaecological drugs (less than £5000; 2010 to 2012) and unpaid consultancy to Hologic (who manufacture fibronectin amongst others).
New
References
References to studies included in this review
Carlan 1997 {published data only}
- Carlan SJ, Richmond LB, O'Brien WF. Randomized trial of endovaginal ultrasound in preterm premature rupture of membranes. Obstetrics & Gynecology 1997;89:458‐61. [DOI] [PubMed] [Google Scholar]
Cotton 1984 {published data only}
- Cotton DB, Gonik B, Bottoms SF. Conservative vs aggressive management of preterm rupture of membranes. A randomized trial of amniocentesis. American Journal of Perinatology 1984;1:322‐4. [DOI] [PubMed] [Google Scholar]
Lewis 1999 {published data only}
- Harper M. Nonstress tests versus biophysical profiles [letter]. American Journal of Obstetrics and Gynecology 2000;183(3):777‐8. [DOI] [PubMed] [Google Scholar]
- Lewis DF, Adair CD, Weeks JW, Barrilleaux PS, Edwards MS, Garite TJ. A randomized clinical trial of daily nonstress testing versus biophysical profile in the management of preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 1999;181:1495‐9. [DOI] [PubMed] [Google Scholar]
- Lewis DF, Adair CD, Weeks JW, Barrilleaux PS, Edwards MS, Garite TJ. A randomized clinical trial of nonstress test versus biophysical profile in preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S197. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Li 2010 {published data only}
- Li J, Ma QL, Chen RX, Li HN, Zhang HY. Effect of different pre‐labor positions for premature rupture of membranes with vertex and engaged presentation on the maternal and neonatal outcomes. Chinese Journal of Evidence‐Based Medicine 2010;10(12):1415‐8. [Google Scholar]
Additional references
Abou El Senoun 2010
- Abou El Senoun G, Dowswell T, Mousa HA. Planned home versus hospital care for preterm prelabour rupture of the membranes (PPROM) prior to 37 weeks' gestation. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD008053.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACOG 1998
- American College of Obtetricians and Gynecologists. ACOG practice bulletin. Premature rupture of membranes. Clinical management guidelines for obstetrician‐gynecologists. International Journal of Gynaecology and Obstetrics 1998;63(1):75‐84. [PubMed] [Google Scholar]
Alfirevic 2010a
- Alfirevic Z, Stampalija T, Gyte GML. Fetal and umbilical Doppler ultrasound in high‐risk pregnancies. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007529.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alfirevic 2010b
- Alfirevic Z, Stampalija T, Gyte GML. Fetal and umbilical Doppler ultrasound in normal pregnancy. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD001450.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 1995
- Carroll S, Papaioannou S, Nicolaides K. Assessment of fetal activity and amniotic fluid volume in the prediction of intrauterine infection in preterm prelabor amniorrhexis. American Journal of Obstetrics and Gynecology 1995;172(5):1427‐35. [DOI] [PubMed] [Google Scholar]
Chamberlain 1984
- Chamberlain PF, Manning FA, Morrison I, Harman CR, Lange IR. Ultrasound evaluation of amniotic fluid volume. I. The relationship of marginal and decreased amniotic fluid volumes to perinatal outcome. American Journal of Obstetrics and Gynecology 1984;150(3):245‐9. [PUBMED: 6385713 ] [DOI] [PubMed] [Google Scholar]
Douvas 1984
- Douvas SG, Brewer MJ, McKay, ML, Rhodes PG, Kahlstorf JH, Morrison JC. Treatment of premature rupture of the membranes. Journal of Reproductive Medicine 1984;29(100):741‐4. [PubMed] [Google Scholar]
Gauthier 1992
- Gauthier D, Meyer W, Bieniarz A. Biophysical profile as a predictor of amniotic fluid culture results. Obstetrics and Gynecology 1992;80(1):102‐5. [PubMed] [Google Scholar]
Goldenberg 2008
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371(9606):75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldstein 1988
- Goldstein I, Romero R, Merrill S, Wan M, O'Connor T, Mazor M, et al. Fetal body and breathing movements as predictors of intraamniotic infection in preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 1988;159(2):363‐8. [DOI] [PubMed] [Google Scholar]
Grivell 2010
- Grivell RM, Alfirevic Z, Gyte GML, Devane D. Antenatal cardiotocography for fetal assessment. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007863.pub2] [DOI] [PubMed] [Google Scholar]
Hadley 1990
- Hadley CB, Main DM, Gabbe SG. Risk factors for preterm premature rupture of the fetal membranes. American Journal of Perinatology 1990;7(4):374‐9. [DOI] [PubMed] [Google Scholar]
Harper 2000
- Harper M. Nonstress tests versus biophysical profiles [letter]. American Journal of Obstetrics and Gynecology 2000;183(3):777‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2011
- Hofmeyr GJ, Essilfie‐Appiah G, Lawrie TA. Amnioinfusion for preterm premature rupture of membranes. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000942.pub2] [DOI] [PubMed] [Google Scholar]
Kenyon 2010
- Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD001058.pub2] [DOI] [PubMed] [Google Scholar]
Lalor 2008
- Lalor JG, Fawole B, Alfirevic Z, Devane D. Biophysical profile for fetal assessment in high risk pregnancies. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000038.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 1998
- Lewis DF, Adair CD, Weeks JW, Barrilleaux PS, Edwards MS, Garite TJ. A randomized clinical trial of nonstress test versus biophysical profile in preterm premature rupture of membranes [SPO abstract]. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S197. [DOI] [PubMed] [Google Scholar]
Mackeen 2011
- Mackeen AD, Seibel‐Seamon J, Grimes‐Dennis J, Baxter JK, Berghella V. Tocolytics for preterm premature rupture of membranes. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007062.pub2] [DOI] [PubMed] [Google Scholar]
Mangesi 2007
- Mangesi L, Hofmeyr GJ, Smith V. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004909.pub2] [DOI] [PubMed] [Google Scholar]
Maxwell 1993
- Maxwell GL. Preterm premature rupture of membranes. Obstetrics and Gynecology Survey 1993;48(8):576‐83. [DOI] [PubMed] [Google Scholar]
Mercer 2003
- Mercer BM. Preterm premature rupture of the membranes. Obstetrics and Gynecology 2003;101(1):178‐93. [DOI] [PubMed] [Google Scholar]
Merenstein 1996
- Merenstein GB, Weisman LE. Premature rupture of the membranes: neonatal consequences. Seminars in Perinatology 1996;20(5):375‐80. [DOI] [PubMed] [Google Scholar]
Parry 1998
- Parry S, Strauss JF. Premature rupture of the fetal membranes. New England Journal of Medicine 1998;338(10):663‐70. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Savitz 1991
- Savitz DA, Blackmore CA, Thorp JM. Epidemiologic characteristics of preterm delivery: etiologic heterogeneity. American Journal of Obstetrics and Gynecology 1991;164:467‐71. [DOI] [PubMed] [Google Scholar]
Simhan 2005
- Simhan HN, Canavan TP. Preterm premature rupture of membranes: diagnosis, evaluation and management strategies. British Journal of Obstetrics and Gynaecology 2005;112(1):32‐7. [DOI] [PubMed] [Google Scholar]
Vintzileos 1986
- Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ, Mirochnick MH, Escoto DT. Fetal biophysical profile versus amniocentesis in predicting infection in preterm premature rupture of the membranes. Obstetrics and Gynecology 1986;68(4):488‐94. [PubMed] [Google Scholar]
Yücel 1997
- Yücel N, Yücel O, Yekeler H. The relationship between umbilical artery Doppler findings, fetal biophysical score and placental inflammation in cases of premature rupture of membranes. Acta Obstetricia et Gynecologica Scandinavica 1997;76(6):532‐5. [DOI] [PubMed] [Google Scholar]


