Abstract
NtcA is a transcriptional activator involved in global nitrogen control in cyanobacteria. In the absence of ammonium it regulates the transcription of a series of genes encoding proteins required for the uptake and assimilation of alternative nitrogen sources (I. Luque, E. Flores, and A. Herrero, EMBO J. 13:2862–2869, 1994). ntcA, present in a single copy in the marine Synechococcus sp. strain WH 7803, was cloned and sequenced. The putative amino acid sequence shows a high degree of identity to NtcA from freshwater cyanobacteria in two functional domains. The expression of ntcA was negatively regulated by ammonium from a putative transcription start point located downstream of an NtcA consensus recognition sequence. Addition of either rifampin or ammonium led to a rapid decline in ntcA transcript levels with half-lives of less than 2 min in both cases. Nitrate-grown cells showed high ntcA transcript levels, as well as the capacity for active nitrite uptake. However, ammonium-grown cells showed low levels of the ntcA transcript and did not utilize nitrite. The addition of ammonium to nitrite uptake-active cells resulted in a gradual decline in the rate of uptake over a 24-h period. Active nitrite uptake was not induced in cells transferred to medium lacking a nitrogen source despite evidence of elevated expression of ntcA, indicating that ntcA expression is not sufficient for uptake capacity to develop. Nitrate and nitrite addition led to the development of nitrite uptake, whereas the addition of leucine did not. Furthermore, nitrite addition triggered the de novo protein synthesis required for uptake capacity to develop. These data suggest that nitrite and nitrate act as specific inducers for the synthesis of proteins required for nitrite uptake.
The cyanobacteria are photosynthetic prokaryotes that generally fulfill their nitrogen requirements with inorganic nitrogenous compounds (14). Ammonium is the preferred source of nitrogen. This form of nitrogen does not require active uptake and is readily incorporated into amino acids. Other forms of inorganic nitrogen (nitrate, nitrite, and dinitrogen) must be reduced to ammonium internally, prior to incorporation into carbon skeletons, largely via the glutamine-synthetase/glutamate-synthase pathway (GS/GOGAT) (9, 14). The uptake and assimilation of such inorganic nitrogen sources are hampered by the presence of ammonium (9, 14). In the freshwater Synechococcus sp. strain PCC 7942, ammonium-promoted control is exerted at two levels. The addition of ammonium promotes an immediate cessation of nitrate and nitrite transport (24). Ammonium also prevents the transcription of the nirA operon, resulting in the repression of synthesis of proteins required for the assimilation of nitrate and nitrite (26, 44). These inhibitory effects are not direct, since ammonium must first be incorporated into the cell via the GS/GOGAT pathway (24, 44).
A similarly negative effect by ammonium is mediated by the ntr genes in the enterobacteria, in a two-component regulatory system (36). However, ntrB- and ntrC-like genes have not been found in cyanobacteria (26, 28). The isolation of a pleiotropic mutant of the freshwater cyanobacterium Synechococcus sp. strain PCC 7942, which is incapable of using inorganic nitrogen sources other than ammonium, led to the identification of NtcA, a transcriptional activator (46). This DNA binding protein belongs to the cyclic AMP receptor protein (CRP) family of transcriptional activators (47). In the absence of ammonium it binds upstream of its own gene as well as of a number of genes involved in nitrogen nutrition, positively regulating their expression. In Synechococcus sp. strain PCC 7942, NtcA positively regulates the expression of glnA and the nirA operon, encoding proteins essential for the uptake and reduction of nitrate and nitrite (26). A newly discovered operon, nirB, which contains genes necessary for normal growth on nitrate and nitrite, is also regulated by NtcA (45). In addition to some of the genes mentioned above, NtcA appears to be involved in the expression of the nif operon encoding the nitrogenase subunits, as well as genes involved in the differentiation of heterocysts in the filamentous Anabaena sp. strain PCC 7120 (12, 52). In both these freshwater cyanobacteria NtcA is essential for growth when ammonium is absent from the medium (12, 46). However, NtcA may not control expression of the nif operon in the marine unicellular nitrogen-fixing Cyanothece sp. strain BH68K (1).
The availability of nutrients often limits primary productivity in aquatic environments (31). Freshwater autotrophs are generally limited by phosphorus availability (16), whereas nitrogen is considered to be the nutrient limiting primary productivity in marine algae (3, 6, 8). Cyanobacteria of the genus Synechococcus are ubiquitous throughout the world’s oceans (21, 48). They are of particular importance in waters limited by nutrient availability, where these cyanobacteria contribute significantly to primary productivity (2, 20). It is thus of interest how this non-nitrogen-fixing cyanobacterial group meets its nitrogen requirements in the marine environment. Yet present knowledge about both the mechanisms and regulation of nitrogen acquisition and its assimilation in marine Synechococcus spp. is limited (4). However, it is known that ammonium interferes with nitrate utilization in marine cyanobacteria (13, 33).
Due to the integral differences in nutrient availability between marine and freshwater ecosystems, it is feasible that the regulation of nitrogen utilization differs for Synechococcus spp. from the two environments. We thus set out to investigate the regulation of nitrogen acquisition in a marine representative of this ecologically important group by addressing two questions. (i) Does NtcA play a role in the regulation of nitrogen acquisition in Synechococcus sp. strain WH 7803? (ii) How is the uptake of oxidized inorganic nitrogen regulated in this cyanobacterium? In this study we report on the identification of ntcA in the marine Synechococcus sp. strain WH 7803. We show that ntcA expression is controlled by ammonium in a manner similar to that found for freshwater Synechococcus sp. strain PCC 7942. However, in contrast to the freshwater Synechococcus strain, the marine Synechococcus strain did not have the capacity for nitrite uptake in nitrogen-depleted conditions, and the addition of ammonium did not cause an immediate cessation of nitrite uptake.
MATERIALS AND METHODS
Culturing conditions.
Cells were grown on ASW, a defined artificial sea water medium (53) with the addition of 5.9 mM NaHCO3 and the Va vitamin mix (49). A modified trace metal mix lacking inorganic nitrogen was used: cobalt nitrate was replaced with cobalt chloride, and ferric ammonium citrate was replaced with ferric chloride. The pH of the medium was adjusted to 8.0. This medium contains 9 mM NaNO3 as the sole nitrogen source and is referred to here as ASWNO3. For growth under ammonia conditions, nitrate was replaced by 2 mM NH4Cl (ASWNH4). Cultures grown on both ammonium and nitrate contained 2 mM NH4Cl and 9 mM NaNO3 (ASWNH4+NO3). For nitrogen-depleted conditions we omitted combined nitrogen sources from the medium (ASW0). In both ASWNH4 and ASW0, sodium ions were brought to the same concentration as in ASWNO3 by adding 9 mM NaCl. The purest quality chemicals available from Merck were used for medium preparation.
Cultures were grown at 25°C in continuous white light at an intensity of 25 to 30 μmol quanta · m−2 · s−1 with constant agitation on an orbital shaker at 125 rpm. Growth of cultures was monitored by determining the absorbance at 750 nm (A750). Doubling times under these conditions were in the range of 40 h when either NH4 or NO3 was used as the nitrogen source.
Cells were maintained for a minimum of five generations in exponential growth phase in the appropriate medium for experiments under steady growth conditions and prior to induction experiments. Nitrite uptake assays and RNA analyses were carried out with cells at mid-log phase (A750 = 0.10 to 0.12, which is the equivalent of 6 × 107 to 10 × 10−7 cells · ml−1). For induction experiments, cells were grown in ASWNH4, harvested by centrifugation at 10,000 × g for 10 min at 25°C, washed twice, and then resuspended in fresh growth medium. When stated, cells were allowed to acclimate to the conditions after transfer for 20 to 24 h. This period of acclimation was sufficient for cells to produce the uptake phenotype typical of cells in the new medium. Growth continued after transfer for at least 24 h in all media, showing that even the cells transferred into ASW0 were in decent physiological condition when cells were assayed or harvested. Wyman et al. (53) also reported that Synechococcus sp. strain WH 7803 grew for at least 24 h after transfer to an ASW medium devoid of combined nitrogen. Each experiment was repeated a minimum of two or three times.
Nucleic acid extraction.
Genomic DNA for Southern blot analyses was prepared by the method for RNA extraction described by Scanlan et al. (41), except that the RNA was degraded with pancreatic RNase A. Genomic DNA for PCRs was prepared according to Scanlan (40), with the omission of the dialysis step. High-quality plasmid DNA for sequencing and RNA probe preparation was made with Qiagen plasmid preparation kits. RNA was extracted from mid-log-phase cells as described in Scanlan et al. (41). Prior to their resuspension in ice-cold extraction buffer, the cells were harvested by gentle filtration onto 0.45-μm-pore-size polycarbonate membrane filters (Poretics). Total RNA was quantified spectrophotometrically and from ethidium bromide-stained RNA run on nondenaturing agarose gels. The latter method also served to ensure the integrity of the RNA before further analysis, as determined by discrete rRNA bands.
Plasmid construction.
DNA digestions were carried out with restriction enzymes from Promega. Fragment purification was carried out with the Geneclean II kit (Bio 101, Inc.). Ligations using T4 ligase (Promega) were carried out overnight at 15°C into either pGEM-5Zf+ (Promega) or Bluescript KS+ (Stratagene) or into pGEM-T (Promega) for PCR products. Plasmids were transformed into DH5α competent cells prepared by rubidium chloride treatment and subsequent heat shock (39).
PCR conditions.
Degenerate primers were designed to correspond to conserved regions of NtcA from freshwater cyanobacteria (11). Bases in parentheses denote degeneracy. Primer 1f, 5′ AT(CAT) TT(TC) TT(TC) CC(GATC) GG(GATC) GA(TC) CC(GATC) GC 3′, corresponds to bases 395 to 416 of the ntcA gene in Synechococcus sp. strain PCC 7942 as described previously (47); primer 2r, 5′ GG (GATC)GT (AG)AA (GATC)GC (GATC)AC (GATC)GC (AG)TG (AG)TA 3′, corresponds to bases 562 to 584 in PCC 7942; primer 3f, 5′ CA(AG) AC(GATC) GA(AG) ATG ATG AT(TCA) GA(GA) AC 3′, corresponds to bases 688 to 710 in PCC 7942; and primer 4r, 5′ AT (GATC)GC (TC)TC (GATC)GC (AGT)AT (GATC)GC (TC)TG (AG)T 3′, corresponds to bases 821 to 842 in PCC 7942.
Reactions were run in 50-μl volumes by using an MJ Research thermocycler with 2 mM MgCl2, 0.2 mM concentrations of each deoxynucleoside triphosphate (dNTP), 0.25 μM concentrations of each primer, and 1.25 U of Taq polymerase (Promega). The template consisted of ca. 1 ng of genomic DNA from Synechococcus sp. strain WH 7803. Thermocycling conditions for 40 cycles were as follows: denaturation at 94°C for 1.2 min, annealing at 55°C for 1 min, and elongation at 70°C for 1.5 min. Taq polymerase was added after the tubes had been heated to 95°C for 4 min.
Genomic library screening.
The 449-bp PCR product amplified from Synechococcus sp. strain WH 7803 with primers 1f and 4r was used as a probe for library screening and Southern blot analysis. DNA probes were synthesized with a random primer labeling kit (Biological Industries) with [α-32P]dATP (3,000 Ci/mmol). The probe was purified using a MicroSpin tube S-200 HR (Pharmacia). A λ Charon 35 genomic library of Synechococcus sp. strain WH 7803, carrying inserts of approximately 20 to 30 kb, was kindly provided by D. J. Scanlan. The Escherichia coli host strain for infections was K803. Plating and screening of the library were performed according to standard procedures (39). Plaques were immobilized on Schleicher & Schuell BA-85 nitrocellulose filters. Prehybridization and hybridization were carried out in the presence of 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5× Denhardt’s solution, 0.5% sodium dodecyl sulfate (SDS), and 100-μg · ml−1 samples of denatured, fragmented herring sperm DNA at 60°C. The final wash was carried out in 0.5× SSC–0.5% SDS at 60°C. Bacteriophage from positive plaques were isolated, and DNA was extracted according to standard procedures (39).
Southern blot analysis.
Genomic DNA from Synechococcus sp. strain WH 7803, digested with a number of restriction enzymes, was transferred to a positively charged nylon membrane (Sartorius) by capillary action in 13× SSC. Prehybridization and hybridization were carried out as described above for library screening but at 55°C. Hybridization was done in the absence of Denhardt’s solution. Two final 30-min washes in 0.1× SSC–0.1% SDS were carried out at the hybridization temperature.
DNA sequencing.
DNA sequencing was carried out with double-stranded plasmid templates using the T7 sequencing kit (Pharmacia) with α-35S-dATP (1,250 Ci/mmol). GC-rich regions were resolved on an automated cycle sequencer at 60°C in the presence of dimethyl sulfoxide. The sequence was verified by sequencing both strands.
Primer extension.
Primers 9r (5′ TGCGTTGGCGAGGCTCACGTTGCCT 3′) and 5r (5′ GGATCACCTCCAACAGGGTTCTG 3′) were end labeled with T4 polynucleotide kinase (MBI Fermentas) and [γ-32P]ATP. Labeled primer (300 ng) was annealed to 15 to 50 μg of total RNA in water by being heated to 68°C for 5 min and then transferred to ice for 10 min. Extension reactions were carried out at 50°C for 60 min with 0.15 U of Superscript reverse transcriptase (GIBCO BRL) per μl in 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, and 0.8 mM deoxynucleoside triphosphate. Ethanol-precipitated nucleic acids were resuspended in a formamide loading solution containing 300 mM NaOH. Sequencing was carried out with the same primer as that used in the extension experiments. Sequencing ladders were labeled with [α-32P]dATP.
RPAs.
The RNase protection assays (RPAs) were carried out with two antisense biotinylated RNA probes synthesized by using the Ambion BrightStar BiotinScript kit. An RNA probe internal to the ntcA gene was transcribed from a pGEM-T plasmid containing a PCR fragment amplified from Synechococcus sp. strain WH 7803 with primers 1f and 4r. The upstream RNA probe is complementary to 304 bases upstream and 96 bases downstream of the ntcA initiation codon. This probe was transcribed from a pGEM-T plasmid containing a PCR fragment amplified from Synechococcus sp. WH 7803 with primers 7f (5′ AGG CTG ACG TGG ACC TCA AC 3′) and 6r (5′ GTT GCG TTC GAT CAG CTC CGT G 3′). The Ambion RPAII kit was used for the RPAs as follows. The RNA probe was incubated for 16 to 20 h at 43°C with equal amounts of total RNA from the different treatments. The nonhybridized RNA and probe were digested with a solution of RNase A and T1 for 30 min at 37°C. After RNase inactivation and precipitation of the protected fragments (designated here as the transcript-probe duplexes), they were heat denatured and run on a 5% polyacrylamide–8 M urea gel. The denatured RNA and probe were then transferred to a positively charged nylon membrane (BrightStar Plus membrane; Ambion). Probe detection was carried out with the Ambion BrightStar BiotinDetect kit followed by exposure on X-ray film (Fuji). Single-strand RNA standards were synthesized from Century Marker template DNA (Ambion) with the Ambion BrightStar BiotinScript kit.
Nitrite uptake.
Nitrite uptake assays were initiated by the addition of 20 μM NaNO2 to mid-log-phase cells in growth medium under growth conditions. The concentration of nitrite remaining in the medium was followed after removal of the cells by centrifugation at 20,000 × g for 4 min. Nitrite concentrations were determined colorimetrically in duplicates as outlined by Parsons et al. (32). The chloramphenicol (20 μg · ml−1) and N,N′-dicyclohexylcarbodiimide (DCCD) (10 μM) used in uptake experiments were purchased from Sigma Chemical Co.
Nucleotide sequence accession number.
The sequence data presented here appear in the EMBL/GenBank/DDBJ nucleotide sequence data libraries under the accession number AF017020.
RESULTS
Identification of ntcA in Synechococcus sp. strain WH 7803.
PCR amplification with the degenerate primers resulted in products of expected sizes for all primer combinations when genomic DNA from the marine Synechococcus sp. strain WH 7803 was used as a template (data not shown). A 449-bp PCR product was used to probe a genomic library of Synechococcus sp. strain WH 7803 in λ Charon 35. The DNA extracted from positive plaques was subjected to restriction enzyme analysis. A 5-kb ApaI fragment, a 1.6-kb EcoRI fragment, and a 0.9-kb SacII fragment all produced 450-bp PCR products with primers 1f and 4r (data not shown). These fragments were subcloned, and sequencing of the SacII DNA fragment revealed an open reading frame of 672 nucleotides, with potential Shine-Dalgarno sequences found at 3 (AGGC) and 14 (AGCAGG) bases upstream from the GTG initiation codon. The DNA sequence of the coding region from Synechococcus sp. strain WH 7803 has 61.0, 56.8, 56.0, and 52.1% identity to that from Synechococcus sp. strain PCC 7942, Synechocystis sp. strain PCC 6803, Anabaena sp. strain PCC 7120, and Cyanothece sp. strain BH68K, respectively. The protein contains 224 amino acids and has 69 to 72% similarity and 60 to 65% identity to the NtcA proteins from the above-mentioned cyanobacterial strains. A comparison of amino acid sequences shows two regions of high identity (above 80%) among the five NtcA proteins between residues 30 and 95 and between residues 125 and 199 (Fig. 1). These regions correspond to two functional domains in the CRP family of transcriptional regulators: (i) the N-terminal region between residues 30 and 95 encodes a β-roll structure involved in binding the effector molecule and in dimer formation, and (ii) the C-terminal region between residues 125 and 199 contains the helix-turn-helix motif involved in DNA binding. The putative helix-turn-helix motif in NtcA from Synechococcus sp. strain WH 7803 is identical to that found for NtcA from other cyanobacteria except for one conserved substitution (isoleucine at position 191 instead of valine). Two regions of low identity were found in the 30 N-terminal residues and in a 20-amino-acid stretch beginning at residue 101 (Fig. 1).
FIG. 1.
Alignment of putative amino acid sequences of the NtcA proteins from Synechococcus sp. strain WH 7803, Synechococcus sp. strain PCC 7942, Synechocystis sp. strain PCC 6803, Anabaena sp. strain PCC 7120, and Cyanothece sp. strain BH68K (ATCC 51142). Asterisks mark the residues identical in all five proteins. Residues unique to one protein are in bold typeface. The putative helix-turn-helix motif is underlined.
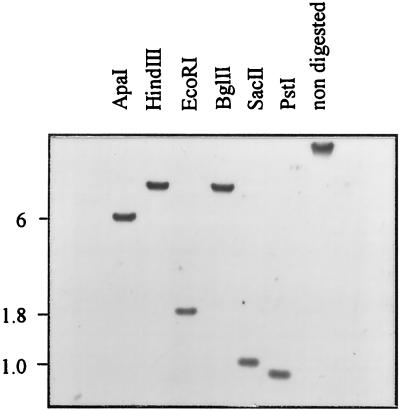
Once the presence of ntcA was established in Synechococcus sp. strain WH 7803, the number of ntcA gene copies found in the genome was determined. Genomic DNA was digested with a variety of restriction enzymes and subjected to Southern analysis with a homologous probe internal to the ntcA gene. The probe hybridized to single restriction fragments in all cases (Fig. 2), indicating that ntcA exists as a single copy in the genome of Synechococcus sp. strain WH 7803. The probe hybridized to fragments that were approximately 6, 1.8, and 1 kb when digested with ApaI, EcoRI, and SacII, respectively. These sizes correspond with those obtained from digests of the genomic DNA library used.
FIG. 2.
Southern blot of genomic DNA from Synechococcus sp. strain WH 7803 in which 5 μg of DNA was digested with ApaI, HindIII, EcoRI, BglII, SacII, or PstI, or left uncut (nondigested), as shown above the gel, and then run on a 0.7% agarose gel prior to capillary transfer. The Southern blot was probed with the 449-bp fragment internal to the ntcA gene from Synechococcus sp. strain WH 7803 amplified with primers 1f and 4r. The apparent sizes of the ApaI, EcoRI, and SacII fragments are indicated in kilobases.
ntcA expression in Synechococcus sp. strain WH 7803 grown under different nitrogen conditions.
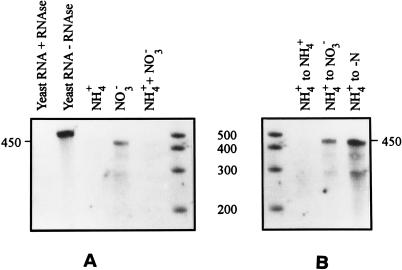
Use of an RNA probe encoded from within the gene (internal probe) enables relative quantification of the transcript levels in cells grown under different conditions. Subjection of total cellular RNA (8 μg) to the RPA with such a probe resulted in low levels of the transcript-probe duplex in cells grown on ASWNH4 and ASWNH4+NO3 (Fig. 3A). However, the levels of the ntcA transcript were much greater in ASWNO3-grown cells. When ASWNH4-grown cells were transferred and acclimated to ASWNO3 or ASW0 for 20 h, the levels of the ntcA transcript increased dramatically in comparison to cells transferred back to ASWNH4 (Fig. 3B). These results indicate that ntcA transcription in Synechococcus sp. strain WH 7803 was down-regulated by ammonium rather than induced by nitrate.
FIG. 3.
RPA showing ntcA transcript levels in Synechococcus sp. strain WH 7803 grown on different nitrogen sources. (A) The internal RNA probe was hybridized to 8 μg of total RNA extracted from cultures grown on ASWNH4 (NH4+), ASWNO3 (NO3−), and ASWNH4+NO3 (NH4+ + NO3−). Yeast RNA subjected to the RPA protocol (first lane) showed that the probe is degraded entirely when no homologous transcript is present, and yeast RNA subjected to the RPA protocol without RNase digestion (second lane) showed that the probe remained intact throughout the procedure. The last lane contains single-stranded-RNA standards. (B) The internal RNA probe was hybridized to 8 μg of total RNA extracted from cultures transferred from ASWNH4 and acclimated for 20 h in ASWNH4 (NH4+ to NH4+), ASWNO3 (NH4+ to NO3−), and ASW0 (NH4+ to −N). The first lane contains single-stranded-RNA standards. In both panels, the full length (in bases) of the transcript-probe duplex is indicated. This is shorter than the undigested probe which contains flanking regions of the cloning vector. The sizes of single-stranded-RNA standards are shown between the panels (in bases).
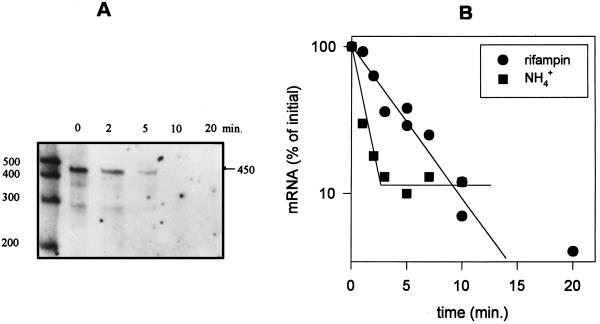
The stability of the ntcA transcript was monitored after the addition of rifampin or ammonium to nitrate-grown cells by RPA analysis with the internal probe. The levels of the ntcA transcript declined rapidly and could barely be detected by 10 min after rifampin addition (Fig. 4A). The addition of ammonium to nitrate-grown cells led to the reduction of ntcA transcript to basal levels along a similar time scale (data not shown). The levels of the transcript-probe duplex were quantified by densitometry and plotted as a function of time after rifampin or ammonium addition (Fig. 4B). The half-lives of the ntcA transcript were calculated from the linear region of the semilogarithmic plot and were found to be 1.83 ± 0.2 min (R2 = 0.95, n = 9) and 1.28 ± 0.2 min (R2 = 0.96, n = 4) after rifampin and ammonium addition, respectively.
FIG. 4.
ntcA transcript levels after the addition of rifampin or ammonium. (A) RPA analysis of 8 μg of total RNA extracted from cells grown on ASWNO3 at the indicated times after addition of rifampin (150 μg/ml). RPA was carried out with the internal RNA probe. Times (in minutes) after rifampin addition are shown above the lanes. Standards are the same as for Fig. 3. (B) The ntcA transcript levels from RPA analyses were quantified by densitometry and plotted relative to initial amounts (t = 0) at intervals after rifampin (150 μg/ml) and ammonium (2 mM) addition to ASWNO3-grown cells. The half-lives of the ntcA transcript were calculated by a linear regression of the data from the linear portion of the decline. The half-lives were 1.83 ± 0.2 (R2 = 0.95; n = 9) after rifampin addition and 1.28 ± 0.2 (R2 = 0.96; n = 4) after ammonium addition.
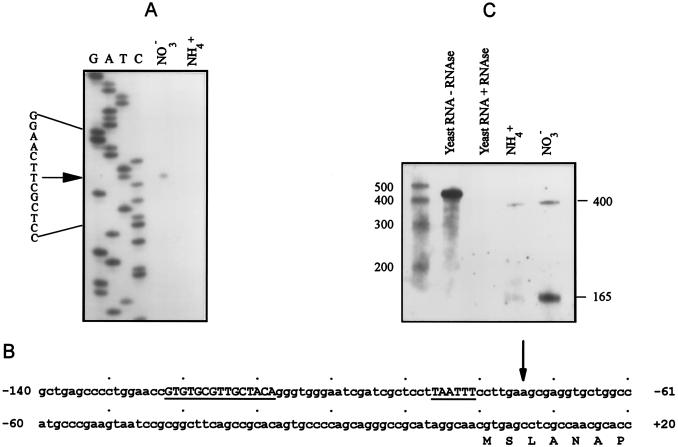
In order to determine whether ntcA in Synechococcus sp. strain WH 7803 is transcribed from variant transcription start points when grown on different nitrogen sources, we performed primer extension experiments. Total RNA (15 μg) from cells grown on ASWNO3 revealed a single extension product located 75 bases upstream of the first coding nucleotide (Fig. 5A and B). An extension product at this site was barely visible for ASWNH4-grown cells only upon overexposure of the autoradiogram when 50 μg of RNA was subjected to primer extension analysis (data not shown). These results were verified with both primers 9r and 5r. This putative transcription start point is located downstream of a −10 promoter-like box (TAATTT) and a palindromic sequence (GTGTGCGTTGCTACA) (Fig. 5B). The palindromic sequence bears a strong resemblence (identical in 11 of 15 bases) to the NtcA binding site found upstream of the ntcA gene in Synechococcus sp. strain PCC 7942.
FIG. 5.
Identification of transcription start points. (A) Primer extension analysis of 15 μg of total RNA from cells grown on ASWNH4 (NH4+) and ASWNO3 (NO3−). RNA was annealed to primer 9r and extended with reverse transcriptase. Sequencing was carried out on plasmid DNA containing the upstream region of the gene with the same primer and [α-32P]dATP. (B) Nucleotide sequence of the upstream region of ntcA from Synechococcus sp. strain WH 7803. The putative regulated transcription start point for cells grown on ASWNO3 is indicated by the arrow. The palindromic sequence and −10-promoter-like box are in uppercase and underlined. +1 corresponds to the first nucleotide of the GTG initiation codon. (C) RPA analysis of 30 μg of total RNA from cells grown on ASWNH4 (NH4+) and ASWNO3 (NO3−) with the upstream probe. The lengths of the transcript-probe duplexes are indicated. See Fig. 3 for the relevance of the yeast-containing lanes. The standards are the same as for Fig. 3.
To determine whether ntcA is transcribed from a single transcription start point irrespective of nitrogen source or from additional transcription start points that are too far upstream to be detected by our primer extension experiments, we performed RPAs using a probe that overlaps both the coding (96 bases) and upstream (304 bases) regions of the gene. With such a probe two transcript-probe duplexes resulted from cells grown on ASWNO3 (Fig. 5C), indicating that there is indeed more than one transcription start point for ntcA in Synechococcus sp. strain WH 7803. The 165-bp transcript-probe duplex corresponds to the product received in primer extension analyses. The 400-bp duplex spans the length of the coding and upstream sections of the probe. (The full-length probe contains 55 bases from the cloning plasmid.) Thus, we assume that the putative transcription start point corresponding to this larger duplex is further upstream than the beginning of the probe, located 304 bases upstream from the first coding nucleotide.
The 400-bp transcript-probe duplex was found at similarly low levels in cells grown on both ASWNH4 and ASWNO3 (Fig. 5C). In comparison to the 400-bp duplex, the 165-bp transcript-probe duplex was found in highly elevated levels in cells grown on ASWNO3, yet it was absent from ASWNH4-grown cells (Fig. 5C). Note that the 165-bp transcript-probe duplex was more abundant than it appears relative to the 400-bp duplex, since 2.5 times fewer biotinylated cytosine bases remain in the shorter transcript-probe duplex. Cells transferred from ASWNH4 and acclimated for 20 h in ASW0 produced a profile of ntcA transcripts similar to that of the ASWNO3-grown cells (data not shown), and cells grown on ASWNH4+NO3 produced only the 400-bp duplex (data not shown). These data suggest that the 400-bp transcript-probe duplex corresponds to a transcript expressed constitutively at low levels, whereas the 165-bp fragment represents a regulated ntcA message transcribed in elevated levels in the absence of ammonium.
Uptake of nitrite in Synechococcus sp. strain WH 7803 grown under different nitrogen conditions.
To the best of our knowledge, the genes for the uptake and assimilation of nitrate and nitrite have not been identified or cloned for any marine cyanobacterium to date. As such, it is not possible to correlate ntcA expression with the transcription of the genes it is thought to activate in Synechococcus sp. strain WH 7803. We therefore undertook nitrite uptake assays to serve as an indication of whether elevated ntcA expression correlates with the activity of proteins required for uptake and assimilation of nitrogen sources other than ammonium. Nitrite rather than nitrate assays were undertaken since nitrite can be accurately and sensitively determined in the presence of both ammonium and nitrate. Nitrate and nitrite are transported by the same permease in Synechococcus sp. strain PCC 7942 (27) and probably in a variety of marine algae (7). Nitrate and nitrite uptake assays in intact cells measure the cells’ ability to both take up and reduce these compounds (19, 25, 38). Furthermore, nitrite reductase activity is necessary for the assimilation of nitrate. Therefore, the uptake of nitrite is likely to represent the cells’ ability to utilize nitrate as well.
In cyanobacteria nitrite uptake has both a passive and an active component depending on the pH (10, 29). Active uptake is inhibited by the ATPase inhibitor DCCD. Upon the addition of 10 μM DCCD (30 min prior to nitrite addition) to Synechococcus sp. strain WH 7803 grown in ASWNO3, the rate of uptake was less than 20% that of the nitrite uptake rate without DCCD (data not shown). This indicates that under the experimental conditions used here nitrite uptake was mainly an active process.
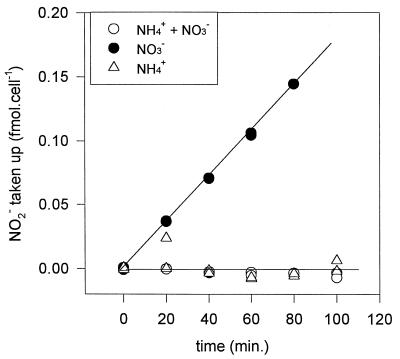
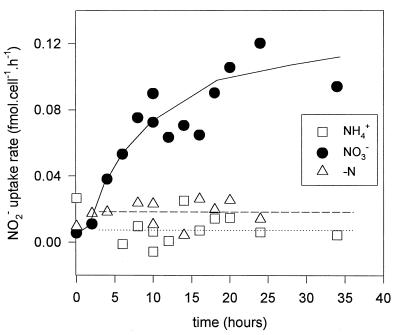
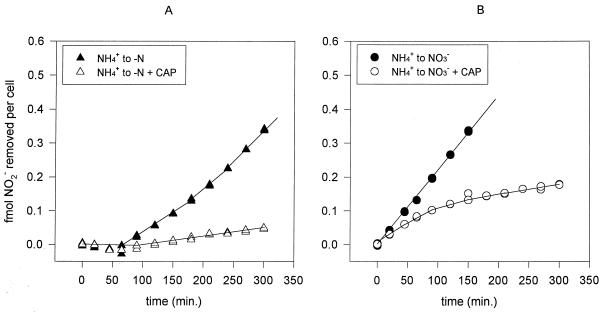
Cultures grown in ASWNH4 were not capable of taking up nitrite, whereas cells grown in ASWNO3 took up the added nitrite readily (Fig. 6). Cells grown on a combination of ammonium and nitrate did not take up nitrite upon its addition (Fig. 6). Within 4 to 6 h after the transfer of cells from ASWNH4 to ASWNO3, active nitrite uptake capacity had developed (Fig. 7). Maximal uptake rates were achieved by 20 to 24 h after transfer. However, cells transferred to ASW0 did not develop the capacity for nitrite uptake at any stage after transfer (Fig. 7). In further experiments we allowed a 20- to 24-h acclimation period when cells that were fully adapted to the new conditions after transfer were desired. The addition of nitrite to cells thus acclimated in ASW0 led to the induction of active nitrite uptake after a period of delay (Fig. 8A). The addition of chloramphenicol (a specific inhibitor of protein synthesis) 30 min prior to nitrite addition canceled the inducible effect of nitrite on uptake (Fig. 8A). This indicates that de novo protein synthesis was required for active nitrite uptake to develop after the addition of the nitrogen source. Nitrite uptake rates in ASWNO3-acclimated cells declined exponentially upon the addition of chloramphenicol (Fig. 8B), indicating that continual protein synthesis was required for sustained nitrite uptake.
FIG. 6.
Nitrite uptake by Synechococcus sp. strain WH 7803 grown on different nitrogen sources. Nitrite (20 μM) was added at time zero, and the amount remaining in the medium was monitored over time. Data were normalized as femtomoles of nitrite taken up per cell. No nitrite remained in the medium of ASWNO3-grown cells at 100 min after its addition; thus, this datum point is meaningless and has been omitted from the graph.
FIG. 7.
Nitrite uptake rates at intervals after the transfer of cells from ASWNH4 to ASWNO3 (NO3−), to ASW0 (−N), or back to ASWNH4 (NH4+). For each time point the amount of nitrite taken up from the medium was monitored for 20 min, and the uptake rate was then determined. Uptake rates were normalized as femtomoles of nitrite taken up per cell per hour. The dotted and dashed lines denote the average uptake rates for cells transferred to ASWNH4 and ASW0, respectively, and the solid line is a line of best fit through the data for cells transferred to ASWNO3.
FIG. 8.
Effect of nitrite and chloramphenicol addition on nitrite uptake by cells transferred from ASWNH4 and acclimated for 20 h to ASW0 (A) and ASWNO3 (B). Chloramphenicol (CAP) was added 30 min prior to the addition of 20 μM nitrite at 0 min. The amount of nitrite remaining in the medium was monitored with time. Data were normalized as femtomoles of nitrite taken up per cell.
To determine whether nitrate and nitrite act as specific inducers for the development of active nitrite uptake, it was necessary to find a nitrogen source that is utilized by the cell but that does not inhibit the capacity for nitrite uptake. The amino acid leucine can be utilized by a wide variety of cyanobacteria (30), including Synechococcus sp. strain WH 7803 (23). The addition of leucine to ASWNO3-grown cells did not lead to a reduction in nitrite uptake rates for up to 24 h after its addition. Cells acclimated for 20 h in ASW0 developed the capacity for nitrite uptake upon addition of nitrate in combination with leucine (data not shown). These data indicate that leucine did not interfere with nitrite uptake capacity or its induction. The addition of leucine alone to ASW0-acclimated cells did not lead to the induction of nitrite uptake capacity. Thus, despite the fact that leucine is a utilizable nitrogen source it did not lead to nitrite uptake, indicating that nitrogen starvation is not behind the lack of nitrite uptake in ASW0-acclimated cells. Our data also suggest that the presence of either nitrite or nitrate is required for the induction of nitrite uptake capacity.
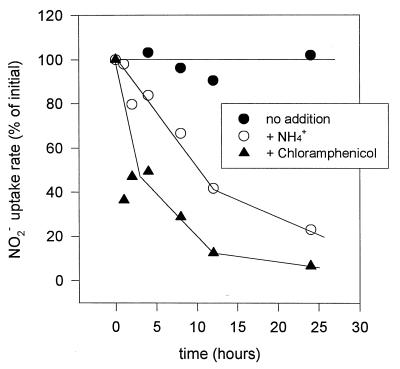
Addition of ammonium to cells capable of nitrite uptake brought about a gradual decline in uptake capacity (Fig. 9). At 4 and 10 h after ammonium addition, the cells retained 90 and 50% of their nitrite uptake capacities, respectively. Cells were still capable of active nitrite uptake 24 h after ammonium addition, as confirmed by the addition of DCCD (data not shown). The addition of chloramphenicol to nitrite uptake-active cells led to a faster decrease in uptake rates than did the addition of ammonium. These data show that no immediate shutdown of the nitrite uptake system occurred in Synechococcus sp. strain WH 7803 upon ammonium addition.
FIG. 9.
Nitrite uptake rates at intervals after the addition of ammonium (2 mM) or chloramphenicol (20 μg · ml−1) to cells grown steadily on ASWNO3. Nitrite taken up from the medium was monitored for 20 min, and the uptake rate per cell was determined for each datum point. Rates were calculated as a percentage of the initial uptake rate. The initial uptake rate was 0.13 fmol of NO2 per cell per h.
DISCUSSION
NtcA is a member of the CRP family of transcriptional activators (47). This family of proteins is characterized by an amino-terminal β-roll structure involved in effector molecule binding and dimer formation and a carboxy-terminal helix-turn-helix motif involved in DNA binding (42, 51). The helix-turn-helix motif in the available amino acid sequences of NtcA from other cyanobacteria is 100% conserved (1, 11). In Synechococcus sp. strain WH 7803, NtcA has one conserved substitution in this motif. The presence of isoleucine rather than valine at position 191 in the helix-turn-helix motif is unlikely to detract from its function in DNA binding. This position in the DNA binding motif, when filled with either isoleucine or valine, is one of three residues highly conserved across families of DNA binding proteins and is involved in determining the angle between the two helices (50).
NtcA-regulated genes possess a palindromic consensus sequence in their upstream regions (12, 26, 34, 35) to which the helix-turn-helix motif is thought to bind. The palindromic sequence in a number of these genes from both Synechococcus sp. strain PCC 7942 (ntcA, nirA, and glnA) and Anabaena sp. strain PCC 7120 (glnA and nirA) is positioned 21 to 22 bases upstream of the putative −10 promoter sequence and is centered 39.5 to 40.5 bases upstream of the regulated transcription start point (12, 26). In Synechococcus sp. strain WH 7803, the −10-like promoter box and the palindromic consensus sequence found upstream of ntcA conform to this precise positioning with respect to the putative transcription start point (Fig. 5B). This strongly suggests that elevated synthesis of NtcA in Synechococcus sp. strain WH 7803 is autoregulated in a way similar to those of the freshwater Synechococcus sp. strain PCC 7942 and Anabaena sp. strain PCC 7120 (26, 35). The positioning of the −10 promoter sequence and the transcription start point relative to the palindromic sequence is not retained in ntcA, rbcL, and genes encoding proteins involved in heterocyst formation and nitrogenase expression in Anabaena sp. strain PCC 7120 (34, 35).
A palindromic sequence identical in 12 of 15 bases to that found in this study was found upstream of the ureD gene (encoding an accessory protein required for urease activity) in the marine Synechococcus sp. strain WH 7805 (5). The presence of putative NtcA-binding sites upstream of ntcA in strain WH 7803 and ureD in strain WH 7805 suggests that NtcA plays a role in nitrogen acquisition in marine Synechococcus species.
The constitutively expressed ntcA message extended far upstream of the coding region of the gene, raising the possibility that it is expressed as part of a polycistronic message.
The two transcript populations detected by RPA analyses with the upstream probe were transcribed under different nitrogen conditions. The 400-bp transcript-probe duplex corresponded to an ntcA message that was constitutively expressed. Transcription of this constitutive mRNA was weak, and the level of this transcript did not change with changes in nitrogen source availability. The transcription of ntcA from the regulated transcription start point (corresponding to the 165-bp transcript-probe duplex) was down-regulated by ammonium. A nitrogen source was not required for the expression of the regulated transcript. This pattern of expression is identical to that reported for ntcA from Synechococcus sp. strain PCC 7942 (26).
The elevated level of ntcA expression from the regulated transcription start point in the absence of a nitrogen source suggests the presence of an active NtcA protein. Yet Synechococcus sp. strain WH 7803 was not capable of nitrite uptake. For uptake to occur, de novo protein synthesis was required. This contrasts with the situation in Synechococcus sp. strain PCC 7942, where the cell both expresses ntcA at elevated levels and has the capacity for nitrate and nitrite utilization in nitrogen-deficient conditions (compare references 26 and 14). Thus, although NtcA activity is likely to be necessary, its activity appears to be insufficient for the synthesis of proteins required for nitrite utilization in Synechococcus sp. strain WH 7803.
Data available to date from freshwater cyanobacteria suggest that the capacity for nitrate and nitrite utilization in the absence of a combined nitrogen source is dependent on the ability of the organism to fix molecular nitrogen. Non-nitrogen fixers are capable of immediate utilization of nitrate and nitrite in the absence of a nitrogen source, whereas nitrogen-fixing cyanobacteria can utilize nitrate and nitrite only when these compounds are available (14, 17, 18, 33, 43). Our data show that the marine non-nitrogen-fixing Synechococcus sp. strain WH 7803 was incapable of nitrite uptake upon transfer to a medium lacking a nitrogen source. The lack of induction of nitrite uptake in the presence of leucine further suggests that nitrite and nitrate may act as specific inducers for the de novo synthesis of proteins required for uptake in Synechococcus sp. strain WH 7803. Marine Synechococcus spp. often inhabit nitrogen-depleted environments for extended periods. Thus the lack of synthesis of proteins required for utilization of inorganic nitrogen in the absence of a specific substrate may be metabolically advantageous.
In two marine Synechococcus strains (WH 7803 and WH 8018), the presence of ammonium interfered with the uptake of nitrate from the medium (13). We have shown here that the presence of ammonium also interfered with nitrite uptake.
The short half-life of the ntcA transcript in Synechococcus sp. strain WH 7803 upon ammonium addition corresponds to reports for other genes involved in nitrogen nutrition in cyanobacteria (22, 37, 44). This suggests a common theme of rapid regulation by ammonium at the level of transcription of genes required for the utilization of nitrogen sources other than ammonium. Such a fast turnover in transcript levels would enable the cell to respond immediately to changes in ammonium availability. However, the addition of ammonium to cells capable of nitrite uptake led to a gradual decline in nitrite uptake capacity in Synechococcus sp. strain WH 7803. The gradual decline may be due to nonspecific protein degradation coupled with a specific lack of new synthesis of proteins required for nitrite utilization due to the presence of ammonium. Thus even though the cell responds within minutes to ammonium addition by stopping transcription, the physiological response takes hours to have much of an effect in Synechococcus sp. strain WH 7803.
ACKNOWLEDGMENTS
This work was supported by German Ministry for Science and Technology grant GR1307 and Ecological Foundation of the Keren Kayemet Le’Israel grant 190/1/702/6. This study was further supported by the “Moshe Shilo” Minerva Center for Marine Biogeochemistry, Minerva Stiftung-Gesellschaft fuer die Forschung, Munich, Germany.
We thank D. J. Scanlan and J. Newman for providing us with the λ Charon 35 genomic library and for helpful discussions. We also thank E. Flores, D. Scanlan, B. Brahamsha, and two anonymous reviewers for helpful comments on the manuscript.
REFERENCES
- 1.Bradley R L, Reddy K J. Cloning, sequencing, and regulation of the global nitrogen regulator gene ntcA in the unicellular diazotrophic cyanobacterium Cyanothece sp. strain BH68K. J Bacteriol. 1997;179:4407–4410. doi: 10.1128/jb.179.13.4407-4410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkill P H, Leakey R J G, Owens N J P, Mantoura R F C. Synechococcus and its importance to the microbial food web of the northwestern Indian Ocean. Deep-Sea Res. 1993;40:773–782. [Google Scholar]
- 3.Carpenter E J, Capone D G. Nitrogen in the marine environment. New York, N.Y: Academic Press, Inc.; 1983. [DOI] [PubMed] [Google Scholar]
- 4.Carr N G, Mann N H. The oceanic cyanobacterial picoplankton. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 27–48. [Google Scholar]
- 5.Collier, J. L., and B. Palenik. Unpublished data.
- 6.Dugdale R C. Nutrient limitation in the sea: dynamics, identification and significance. Limnol Oceanogr. 1967;12:685–695. [Google Scholar]
- 7.Falkowski P G. Enzymology of nitrogen assimilation. In: Carpenter E J, Capone D G, editors. Nitrogen in the marine environment. New York, N.Y: Academic Press, Inc.; 1983. pp. 839–868. [Google Scholar]
- 8.Falkowski P G. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387:272–275. [Google Scholar]
- 9.Flores E, Herrero A. Assimilatory nitrogen metabolism and its regulation. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 487–517. [Google Scholar]
- 10.Flores E, Herrero A, Guerrero M G. Nitrite uptake and its regulation in the cyanobacterium Anacystis nidulans. Biochim Biophys Acta. 1987;896:103–108. [Google Scholar]
- 11.Frias J E, Merida A, Herrero A, Martin-Nieto J, Flores E. General distribution of the nitrogen control gene ntcA in cyanobacteria. J Bacteriol. 1993;175:5710–5713. doi: 10.1128/jb.175.17.5710-5713.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frias J E, Flores E, Herrero A. Requirement of the regulatory protein NtcA for the expression of nitrogen assimilation and heterocyst development genes in the cyanobacterium Anabaena sp. PCC 7120. Mol Microbiol. 1994;14:823–832. doi: 10.1111/j.1365-2958.1994.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 13.Glibert P M, Ray R T. Different patterns of growth and nitrogen uptake in two clones of marine Synechococcus spp. Mar Biol (Berlin) 1990;107:273–280. [Google Scholar]
- 14.Guerrero M G, Lara C. Assimilation of inorganic nitrogen. In: Fay P, Van Baalen C, editors. The cyanobacteria. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1987. pp. 163–186. [Google Scholar]
- 15.Harrison W G. Nitrogen utilization in chlorophyll and primary productivity maximum layers: an analysis based on the f-ratio. Mar Ecol Prog Ser. 1990;60:85–90. [Google Scholar]
- 16.Hecky R E, Kilham P. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol Oceanogr. 1988;33:796–822. [Google Scholar]
- 17.Herrero A, Flores E, Guerrero M G. Regulation of nitrate reductase levels in the cyanobacteria Anacystis nidulans, Anabaena sp. strain 7119, and Nostoc sp. strain 6719. J Bacteriol. 1981;145:175–180. doi: 10.1128/jb.145.1.175-180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrero A, Flores E, Guerrero M G. Regulation of nitrate reductase cellular levels in the cyanobacteria Anabaena variabilis and Synechocystis sp. FEMS Microbiol Lett. 1985;26:21–25. [Google Scholar]
- 19.Herrero A, Guerrero M G. Regulation of nitrite reductase in the cyanobacterium Anacystis nidulans. J Gen Microbiol. 1986;132:2463–2468. [Google Scholar]
- 20.Iturriaga R, Mitchell B G. Chrococcoid cyanobacteria: a significant component in the food web dynamics of the open ocean. Mar Ecol Prog Ser. 1986;28:291–297. [Google Scholar]
- 21.Johnson P W, Sieburth J M. Chrococcoid cyanobacteria in the sea: a ubiquitous and diverse phototrophic biomass. Limnol Oceanogr. 1979;24:928–935. [Google Scholar]
- 22.Kikuchi H, Aichi M, Suzuki I, Omata T. Positive regulation by nitrite of the nitrate assimilation operon in the cyanobacteria Synechococcus sp. strain PCC 7942 and Plectonema boryanum. J Bacteriol. 1996;178:5822–5825. doi: 10.1128/jb.178.19.5822-5825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer J G. The effect of irradiance and specific inhibitors on protein and nucleic acid synthesis in the marine cyanobacterium Synechococcus sp. WH 7803. Arch Microbiol. 1990;154:280–285. [Google Scholar]
- 24.Lara C, Rodriguez R, Guerrero M G. Nitrate transport in the cyanobacterium Anacystis nidulans. Physiol Plant. 1993;89:582–587. [Google Scholar]
- 25.Lara C, Rodriguez R, Guerrero M G. Sodium-dependent nitrate transport and energetics of cyanobacteria. J Phycol. 1993;29:389–395. [Google Scholar]
- 26.Luque I, Flores E, Herrero A. Molecular mechanism for the operation of nitrogen control in cyanobacteria. EMBO J. 1994;13:2862–2869. doi: 10.1002/j.1460-2075.1994.tb06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luque I, Flores E, Herrero A. Nitrate and nitrite transport in the cyanobacterium Synechococcus sp. PCC 7942 are mediated by the same permease. Biochim Biophys Acta. 1994;1184:296–298. [Google Scholar]
- 28.Mann N H. How do cells express nutrient limitation at the molecular level? NATO ASI Ser G. 1995;38:171–190. [Google Scholar]
- 29.Martin-Nieto J, Herrero A, Flores E. Regulation of nitrate and nitrite reductases in dinitrogen-fixing cyanobacteria and Nif− mutants. Arch Microbiol. 1989;151:475–478. [Google Scholar]
- 30.Montesinos M L, Herrero A, Flores E. Amino acid transport in taxonomically diverse cyanobacteria and identification of two genes encoding elements of a neutral amino acid permease putatively involved in recapture of leaked hydrophobic amino acids. J Bacteriol. 1997;179:853–862. doi: 10.1128/jb.179.3.853-862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons T R, Takahashi M, Hargrave B. Biological oceanographic processes. 3rd ed. Elmsford, N.Y: Pergamon Press, Inc.; 1984. [Google Scholar]
- 32.Parsons T R, Maita Y, Lalli C M. A manual of chemical and biological methods of sea water analysis. Elmsford, N.Y: Pergamon Press, Inc.; 1984. [Google Scholar]
- 33.Rai A N, Borthakur M, Bergman B. Nitrogenase derepression, its regulation and metabolic changes associated with diazotrophy in the nonheterocystous cyanobacterium Plectonema boryanum PCC 73110. J Gen Microbiol. 1992;138:481–491. [Google Scholar]
- 34.Ramasubramanian T S, Wei T F, Golden J W. Two Anabaena sp. strain PCC 7120 DNA-binding factors interact with vegetative cell- and heterocyst-specific genes. J Bacteriol. 1994;176:1214–1223. doi: 10.1128/jb.176.5.1214-1223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramasubramanian T S, Wei T F, Oldham A K, Golden J W. Transcription of the Anabaena sp. strain PCC 7120 ntcA gene: multiple transcripts and NtcA binding. J Bacteriol. 1996;178:922–926. doi: 10.1128/jb.178.3.922-926.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reitzer L J, Magasanik B. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium. Washington, D.C: American Society for Microbiology; 1987. pp. 302–320. [Google Scholar]
- 37.Reyes J C, Muro-Pastor M I, Florencio F J. Transcription of glutamine synthetase (glnA and glnN) from the cyanobacterium Synechocystis sp. strain PCC 6803 is differently regulated in response to nitrogen availability. J Bacteriol. 1997;179:2678–2689. doi: 10.1128/jb.179.8.2678-2689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez R, Lara C, Guerrero M G. Nitrate transport in the cyanobacterium Anacystis nidulans R2. Kinetic and energetic aspects. Biochem J. 1992;282:639–643. doi: 10.1042/bj2820639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Scanlan D J, Bloye S A, Mann N H, Hodgson D A, Carr N G. Construction of lacZ promoter probe vectors for use in Synechococcus: application to the identification of CO2-regulated promoters. Gene. 1990;90:43–49. doi: 10.1016/0378-1119(90)90437-v. [DOI] [PubMed] [Google Scholar]
- 41.Scanlan D J, Mann N H, Carr N G. The response of the picoplanktonic marine cyanobacterium Synechococcus species WH 7803 to phosphate starvation involves a protein homologous to the periplasmic phosphate-binding protein of Escherichia coli. Mol Microbiol. 1993;10:181–191. doi: 10.1111/j.1365-2958.1993.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 42.Shaw D J, Rice D W, Guest J R. Homology between CAP and Fnr, a regulator of anaerobic respiration in Escherichia coli. J Mol Biol. 1983;166:241–247. doi: 10.1016/s0022-2836(83)80011-4. [DOI] [PubMed] [Google Scholar]
- 43.Sivak M N, Lara C, Romero J M, Rodriguez R, Guerrero M G. Relationship between a 47-kDa cytoplasmic membrane polypeptide and nitrate transport in Anacystis nidulans. Biochem Biophys Res Commun. 1989;158:257–262. doi: 10.1016/s0006-291x(89)80206-2. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki I, Sugiyama T, Omata T. Primary structure and transcriptional regulation of the gene for nitrite reductase from the cyanobacterium Synechococcus PCC 7942. Plant Cell Physiol. 1993;34:1311–1320. [Google Scholar]
- 45.Suzuki I, Horie N, Sugiyama T, Omata T. Identification and characterization of two nitrogen-regulated genes of the cyanobacterium Synechococcus sp. strain PCC 7942 required for maximum efficiency of nitrogen assimilation. J Bacteriol. 1995;177:290–296. doi: 10.1128/jb.177.2.290-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vega-Palas M A, Madueno F, Herrero A, Flores E. Identification and cloning of a regulatory gene for nitrogen assimilation in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1990;172:643–647. doi: 10.1128/jb.172.2.643-647.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vega-Palas M A, Flores E, Herrero A. NtcA, a global nitrogen regulator from the cyanobacterium Synechococcus that belongs to the CRP family of bacterial regulators. Mol Microbiol. 1992;6:1853–1859. doi: 10.1111/j.1365-2958.1992.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 48.Waterbury J B, Watson S W, Guillard R R L, Brand L E. Widespread occurrence of a unicellular, marine, planktonic cyanobacterium. Nature. 1979;277:293–294. [Google Scholar]
- 49.Waterbury J B, Willey J M. Isolation and growth of marine planktonic cyanobacteria. Methods Enzymol. 1988;167:100–105. [Google Scholar]
- 50.Weber I T, McKay D B, Steitz T A. Two helix DNA binding motif of CAP found in lac repressor and gal repressor. Nucleic Acids Res. 1982;10:5085–5102. doi: 10.1093/nar/10.16.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber I T, Steitz T A. Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 Å resolution. J Mol Biol. 1987;198:311–326. doi: 10.1016/0022-2836(87)90315-9. [DOI] [PubMed] [Google Scholar]
- 52.Wei T-F, Ramasubramanian T S, Golden J W. Anabaena sp. strain PCC 7120 ntcA gene required for growth on nitrate and heterocyst development. J Bacteriol. 1994;176:4473–4482. doi: 10.1128/jb.176.15.4473-4482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyman M, Gregory R P F, Carr N G. Novel role for phycoerythrin in a marine cyanobacterium, Synechococcus strain DC2. Science. 1985;230:818–820. doi: 10.1126/science.230.4727.818. [DOI] [PubMed] [Google Scholar]