Abstract
Background
To improve research and care for patients with post-COVID-19 condition more insight into different subtypes of post-COVID-19 condition and their risk factors is urgently needed. We aimed to identify risk factors of post-COVID-19 condition in general and for specific symptom profiles.
Methods
This study is based on data collected within the Lifelines Coronavirus disease 2019 (COVID-19) cohort (N = 76 503). Mean pre- and post-SARS-CoV-2 infection symptom scores were compared to classify post-COVID-19 condition. Latent Profile Analysis was used to identify symptom profiles. Logistic and multinomial regression analyses were used to examine the association between demographic, lifestyle and health-related risk factors and post-COVID-19 condition, and symptom profiles, respectively.
Results
Of the 3465 participants having had COVID-19, 18.5% (n = 642) classified for post-COVID-19 condition. Four symptom profiles were identified: muscle pain, fatigue, cardiorespiratory and ageusia/anosmia. Female sex was a risk factor for the muscle pain and fatigue profiles. Being overweight or obese increased risk for all profiles, except the fatigue profile. Having a chronic disease increased the risk for all profiles except the ageusia/anosmia profile, with the cardiorespiratory profile being only significant in case of multimorbidity. Being unvaccinated increased risk of the ageusia/anosmia profile.
Conclusions
Findings from this study suggest that Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may trigger different pathophysiological mechanisms that may result in different subtypes of post-COVID-19 condition. These subtypes have shared and unique risk factors. Further characterization of symptom profiles and quantification of the individual and societal impact of specific symptom profiles are pressing challenges for future research.
Introduction
After the acute phase of COVID-19, a substantial proportion of individuals continues to experience symptoms of a physical, psychological and/or cognitive nature.1,2 The World Health Organization defined the ongoing experience of such symptoms 3–5 months from the onset of COVID-19 as post-COVID-19 condition.3 A recent study among the general Dutch population, using pre- and post-SARS-CoV-2 infection symptom measurements and a non-infected control group, estimated that COVID-19 causes persistent symptoms in 12.7% of individuals, including chest pain, painful muscles and difficulties breathing 3–5 months from the onset of COVID-19.4 To improve care for patients with post-COVID-19 condition, we urgently need more insight into subtypes of post-COVID-19 condition and their shared and unique risk factors.5 This may help understand heterogeneity in underlying mechanisms and facilitate the development of new treatments, which has great public health relevance given the medical and societal impact and costs.
Most previous studies have identified risk factors for post-COVID-19 condition with the assumption that post-COVID-19 condition is a homogeneous disorder. Two systematic reviews showed that female sex, increasing age, the presence of comorbidity, identifying as being part of a minority ethnicity, and acute disease severity increase the risk for post-COVID-19 condition.6,7 One of these reviews6 included hospitalized and non-hospitalized populations, while the other7 focused on hospitalized patients only. Yet, in practice, the majority of people with COVID-19 is not hospitalized.8 Furthermore, in both systematic reviews, the risk of selection and attrition bias was high for the majority of included studies. Since the publication of these reviews, the aforementioned risk factors were confirmed among large general population studies from the UK.9,10 In addition, these studies showed that smoking, being overweight and obese and specific comorbidities were associated with post-COVID-19 condition. However, research using pre- and post-SARS-CoV-2 infection symptom measurements is necessary to validate risk factors of post-COVID-19 condition by excluding symptoms that are not attributable to COVID-19.
Furthermore, it seems likely that post-COVID-19 condition encompasses multiple subtypes, that have shared and unique risk factors and mechanisms. The idea of post-COVID-19 condition subtypes is supported by studies on symptom profiles.9–13 Findings from these studies vary considerably as these have identified between 2 symptom profiles10 and 13 symptom11 profiles. Importantly, these studies could not establish whether symptoms were new or severely increased after the acute phase of COVID-19 or were a mere continuation of pre-existing symptoms. This may be problematic as misclassification of post-COVID-19 condition may not only obscure observed symptom profiles, but also the associations between risk factors and symptom profiles.14
We aim to study previously identified risk factors for post-COVID-19 condition and to examine whether such risk factors are associated with specific post-COVID-19 condition symptom profiles.
Methods
Study design and sample
This study used data from the Lifelines COVID-19 cohort (n = 76 503; mean age 53.6 (SD = 13.0) years; 60.8% female).15 This cohort examines the physical, psychological and societal impacts of the COVID-19 pandemic and is a digital add-on study to Lifelines.16 Lifelines is a multi-disciplinary prospective population-based cohort study examining the health and health-related behaviours of 167 729 persons living in the North of the Netherlands. It employs investigative procedures in assessing the biomedical, socio-demographic, behavioural, physical and psychological factors which contribute to the health and disease of the general population. Extensive information on the cohort, its design considerations and recruitment procedures are described elsewhere.15–17 In this study, we included data of 27 consecutive measurements from March 2020 until July 2022. Data were collected weekly in March, April and May 2020, biweekly from June until August 2020, monthly from August 2020 until June 2021 and every 1–3 months from July 2021. The response rates per wave varied between 28% and 49%. Lifelines and its add-on studies were conducted according to the guidelines of the Declaration of Helsinki, and all procedures were approved by the Medical Ethics Committee of the University Medical Center Groningen (2007/152). All participants provided written informed consent.
In this study, we included participants with a COVID-19 diagnosis between March 2020 and April 2022 (n = 12 150). This allowed us to select participants with post-COVID-19 condition 90–150 days after SARS-CoV-2 infection.3 We only included participants for whom we could establish whether or not they had developed post-COVID-19 condition. We further excluded participants who did not join the Lifelines COVID-19 cohort during the first or second wave as information on several potential risk factors was only collected during these waves. Finally, we excluded participants with missing data on potential risk factors. Supplementary figure S1 gives a detailed overview of the sample selection. Characteristics of excluded (n = 8685) and included (n = 3465) participants were similar (Supplementary table S1).
Symptoms
Assessed symptoms included headache, dizziness, chest pain, lower back pain, nausea, painful muscles, difficulties breathing, feeling hot/cold alternately, tingling extremities, feeling a lump in the throat, general tiredness, heavy arms/legs, pain when breathing, runny nose, sore throat, dry cough, wet cough, fever, diarrhoea, stomach pain, ageusia/anosmia, sneezing and itchy eyes. The first 12 symptoms are derived from the Symptom CheckList-90 Somatization (SCL-90 SOM) subscale, which has been recommended for large-scale studies18 and has been shown to have sufficient measurement invariance, making it suitable to assess symptom experience repeatedly over time.19 The remainder of symptoms was added since these were considered to be COVID-19 related at the start of the Lifelines COVID-19 cohort. Experienced symptoms were assessed using an ordinal five-point Likert scale (1 ‘not at all’ and 5 ‘extremely’) in the past 7/14 days. The time frame changed when the follow-up time between measurements increased.
COVID-19
COVID-19 diagnosis was measured between March 2020 and April 2022 and in this study we only included participants’ first COVID-19 diagnosis. Between March 2020 and August 2020, a COVID-19 diagnosis was based on either a positive PCR test, antigen test or a physician’s diagnosis of COVID-19. The latter was included as the PCR testing regime in the Netherlands was strongly restricted until August 2020. From August 2020 onwards, we defined a COVID-19 diagnosis based on a positive PCR test at the Public Health Service, positive antigen tests at work or school, ‘testing for entry’ or another official institute. In line with a previous study in the Lifelines COVID-19 Cohort, self-tests were not included.4
Post-COVID-19 condition
Post-COVID-19 condition was classified by comparing mean post-SARS-CoV-2 infection symptom scores 90–150 days after infection with mean pre-infection symptom scores at least 7 days before infection.3,4 When the mean post-infection symptom score of at least one symptom was higher than three (i.e. moderate severity) and at least one point increased compared to the mean pre-infection score, participants were classified as having post-COVID-19 condition.4 We based this classification on the 10 symptoms that we previously identified as core symptoms using data from the Lifelines COVID-19 cohort.4 These core symptoms were chest pain, painful muscles, difficulties breathing, feeling hot/cold alternately, tingling extremities, feeling a lump in the throat, general tiredness, heavy arms/legs, pain when breathing and ageusia/anosmia.
Risk factors
Age was measured at the wave of SARS-CoV-2 infection and categorized into three age groups representing early adult life, mid-life and later life (i.e. 18–39, 40–59 and ≥60 years). Sex was categorized into male and female. Educational level was categorized into low, medium and high. Smoking was assessed with the question ‘Have you smoked in the last 7/14 days?’. Those answering ‘yes’ at one of the time points were categorized as smokers. BMI (in kg/m2) was calculated based on height previously collected in Lifelines, and self-reported weight from the questionnaire closest to SARS-CoV-2 infection and categorized into normal weight (BMI <25), overweight (BMI ≥25 to <30) and obesity (BMI ≥30). Chronic diseases were assessed in the first and second wave with single-item questions assessing the presence of 17 diseases. These were combined into cardiovascular disease, lung disease, diabetes, chronic muscle disease, autoimmune disease, psychiatric disorder and other disease (Supplement table S2). We also categorized participants as having no chronic disease, one chronic disease or multimorbidity. Vaccination status was measured from wave 18 onwards (start 25 February 2021) and categorized as fully vaccinated, partially vaccinated and unvaccinated at time of the SARS-CoV-2 infection. Participants with a SARS-CoV-2 infection until wave 18 were categorized as unvaccinated as the national vaccination campaign had not yet started.20 Hospitalization was assessed by the question ‘Have you been hospitalized for a COVID-19 infection?’ and was dichotomized into no/yes. We categorized participants as being infected by the Alpha (March 2020—July 2020), Delta (July 2020—January 2021) or Omicron (January 2021 to April 2022) variant based on the timing of their SARS-CoV-2 infection.21 Finally, we categorized the season of infection to correct for potential seasonal influences on symptom levels.
Statistical analyses
Characteristics of the study population were described for the total study sample and separately for participants without and with post-COVID-19 condition. Latent Profile Analysis (LPA) using the ‘tidyLPA’ package in R Studio 2022.02.0 was used to identify symptom profiles within participants categorized as having post-COVID-19 condition.22 LPA is a data-driven technique that identifies profiles of individuals based on scoring patterns across continuous input variables.23 Four possible model configurations pertaining variance and covariance within and between classes were examined to see which set of parameters fit the data best. One to 10 classes were analysed to determine the optimal number of groups. Decisions regarding model fit and class solution were based on the Akaike information criterion (AIC), Bayesian information criterion (BIC), entropy (≥0.8), minimal probability of belonging to a class (≥0.9), minimal group size (≥5% of the analytic sample) the bootstrapped likelihood test (BLRT) P values and conceptual meaningfulness.23 For the AIC and BIC, lower values represent better model fit. Characterization of profiles was based on a mean symptom score of ≥2.5. Univariable and multivariable logistic regression analyses were performed to examine associations between potential risk factors and post-COVID-19 condition. For these analyses, specific chronic diseases were used. To examine whether participant characteristics were associated with the identified profiles, we performed a multivariable multinomial logistic regression analysis using the potential risk factors as independent variables and profile membership as dependent variable. Participants without persistent symptoms were the reference category. For this analysis, we used the count variable regarding chronic diseases. We did not include hospitalization as risk factor due to the low number of hospitalizations.
In sensitivity analyses, we categorized participants as having post-COVID-19 condition based on the 23 measured symptoms and repeated all analyses to enable comparing our results to studies using a broader definition of post-COVID-19 condition. As more participants classify for post-COVID-19 condition with a broader definition, we used the specific chronic diseases instead of the count variable in the multinomial logistic regression analysis. Second, we re-ran the multinomial regression analysis with the least severe profile (i.e. least symptoms ≥2.5) as the reference category instead of participants without post-COVID-19 condition.
All analyses, except LPA, were performed in IBM SPSS Statistics version 28.
Results
Study population
A total of 80 338 questionnaires were completed by 3465 participants (mean age 55.8 years, SD 11.6, 64.3% female). Participants completed a median of 25 questionnaires (IQR: 21-27). Of the 3465 participants, 18.5% (n = 642) classified for post-COVID-19 condition (Table 1; Supplementary table S3). Hereof, 59.5% (n = 382) was aged 40–59 years and 72.6% (n = 466) was female.
Table 1.
Participant characteristics of the analytic study sample (n = 3465)
| Total study sample | COVID-19 | Post-COVID-19 condition | ||||
|---|---|---|---|---|---|---|
| (n = 3465) |
(n = 2823) |
(n = 642) |
||||
| Characteristic | n | % | n | % | N | % |
| Age | ||||||
| 18–39 | 343 | 9.9 | 282 | 10.0 | 61 | 9.5 |
| 40–59 | 1810 | 52.2 | 1428 | 50.6 | 382 | 59.5 |
| ≥60 | 1312 | 37.9 | 1113 | 39.4 | 199 | 31.0 |
| Sex | ||||||
| Male | 1238 | 35.7 | 1062 | 37.6 | 176 | 27.4 |
| Female | 2227 | 64.3 | 1761 | 62.4 | 466 | 72.6 |
| Educational level | ||||||
| High | 1185 | 34.2 | 964 | 34.1 | 221 | 34.4 |
| Medium | 1380 | 39.8 | 1102 | 39.0 | 278 | 43.3 |
| Low | 811 | 23.4 | 684 | 24.2 | 128 | 19.9 |
| Unknown | 89 | 2.6 | 74 | 2.6 | 15 | 2.3 |
| Smoking | ||||||
| No | 3132 | 90.4 | 2551 | 90.4 | 581 | 90.5 |
| Yes | 333 | 9.6 | 272 | 9.6 | 61 | 9.5 |
| Body mass index | ||||||
| Healthy | 1535 | 44.3 | 1304 | 46.2 | 231 | 36.0 |
| Overweight | 1386 | 40.0 | 1113 | 39.4 | 273 | 42.5 |
| Obese | 544 | 15.7 | 406 | 14.4 | 138 | 21.5 |
| Chronic diseases | ||||||
| Cardiovascular disease | 345 | 10.0 | 258 | 9.1 | 87 | 13.6 |
| Lung disease | 336 | 9.7 | 248 | 8.8 | 88 | 13.7 |
| Diabetes | 88 | 2.5 | 60 | 2.1 | 28 | 4.4 |
| Chronic muscle disease | 46 | 1.3 | 26 | 0.9 | 20 | 3.1 |
| Autoimmune disease | 145 | 4.2 | 110 | 3.9 | 35 | 5.5 |
| Psychiatric disorder | 76 | 2.2 | 49 | 1.7 | 27 | 4.2 |
| Other chronic disease | 523 | 15.1 | 385 | 13.6 | 138 | 21.5 |
| Chronic disease | ||||||
| No chronic disease | 2469 | 71.3 | 2080 | 73.7 | 389 | 60.6 |
| One chronic disease | 601 | 17.3 | 454 | 16.1 | 147 | 22.9 |
| Multimorbidity | 395 | 11.4 | 289 | 10.2 | 106 | 16.5 |
| Vaccination status | ||||||
| Fully vaccinated | 1584 | 45.7 | 1330 | 47.1 | 254 | 39.6 |
| Partially vaccinated | 98 | 2.8 | 80 | 2.8 | 18 | 2.8 |
| Not vaccinated | 1783 | 51.5 | 1413 | 50.1 | 370 | 57.6 |
| Virus variant | ||||||
| Omicron | 2656 | 76.7 | 2180 | 77.2 | 476 | 74.1 |
| Delta | 649 | 18.7 | 508 | 18.0 | 141 | 22.0 |
| Alpha | 160 | 4.6 | 135 | 4.8 | 25 | 3.9 |
| Hospitalization | ||||||
| No | 3340 | 96.4 | 2737 | 97.0 | 603 | 93.9 |
| Yes | 125 | 3.6 | 86 | 3.0 | 39 | 6.1 |
| Season of infection | ||||||
| Winter | 2555 | 73.7 | 2102 | 74.5 | 453 | 70.6 |
| Spring | 388 | 11.2 | 313 | 11.1 | 75 | 11.7 |
| Summer | 77 | 2.2 | 64 | 2.3 | 13 | 2.0 |
| Autumn | 445 | 12.8 | 344 | 12.2 | 101 | 15.7 |
Symptom profiles
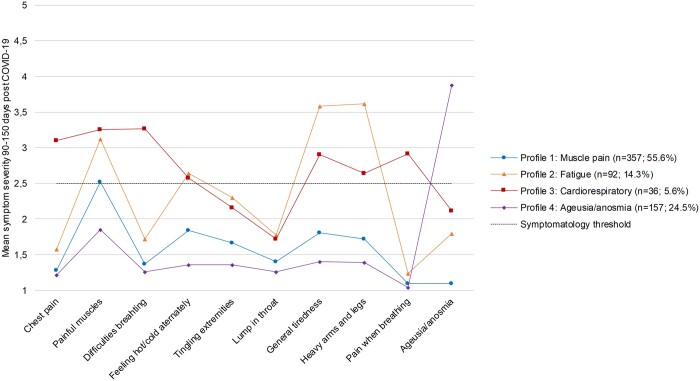
A four-profile solution with model one was identified as most optimal based on model fit statistics and conceptual meaningfulness (Supplementary table S4). The muscle pain profile (n = 357; 55.6%) is characterized by a high mean symptom level of muscle pain (figure 1; Supplementary table S5). The fatigue profile (n = 92; 14.3%) is characterized by heavy arms/legs, general tiredness, painful muscles and feeling hot/cold alternately. The cardiorespiratory profile (n = 36; 5.6%) is characterized by difficulties breathing, painful muscles, chest pain, pain when breathing, general tiredness, heavy arms/legs and feeling hot/cold alternately. The ageusia/anosmia profile (n = 157; 24.5%) is characterized by ageusia/anosmia. Supplementary table S6 shows participant characteristics stratified by symptom profile.
Figure 1.
Mean symptom severity 90–150 days post-COVID-19 by symptom profile
Risk factors
Multivariable logistic regression analysis showed that female sex, overweight and obesity, diabetes, chronic muscle disease, the category other chronic diseases, being unvaccinated and hospitalization for acute COVID-19 increased the risk for post-COVID-19 condition (table 2). A low educational level decreased the risk, just as participants most likely infected with the Alpha variant compared to Omicron. Age and smoking did not affect the risk for post-COVID-19 condition.
Table 2.
Associations between participant characteristics and post-COVID-19 condition
| Univariable | Multivariable | |||
|---|---|---|---|---|
|
| ||||
| n | % | OR (95% CI) | OR (95% CI) | |
| Age | ||||
| 18–39 | 343 | 9.9 | Ref | Ref |
| 40–59 | 1810 | 52.2 | 1.24 (0.92, 1.67) | 1.12 (0.82, 1.53) |
| ≥60 | 1312 | 37.9 | 0.83 (0.60, 1.13) | 0.78 (0.56, 1.10) |
| Sex | ||||
| Male | 1238 | 35.7 | Ref | Ref |
| Female | 2227 | 64.3 | 1.60 (1.32, 1.93) | 1.56 (1.28, 1.90) |
| Educational level | ||||
| High | 1185 | 34.2 | Ref | Ref |
| Medium | 1380 | 39.8 | 1.10 (0.90, 1.34) | 0.95 (0.77, 1.16) |
| Low | 811 | 23.4 | 0.82 (0.64, 1.04) | 0.71 (0.55, 0.91) |
| Unknown | 89 | 2.6 | 0.88 (0.50, 1.57) | 0.81 (0.44, 1.46) |
| Smoking | ||||
| No | 3132 | 90.4 | Ref | Ref |
| Yes | 333 | 9.6 | 0.98 (0.73, 1.32) | 0.96 (0.71, 1.30) |
| Body mass index | ||||
| Healthy | 1535 | 44.3 | Ref | Ref |
| Overweight | 1386 | 40.0 | 1.38 (1.14, 1.68) | 1.42 (1.17, 1.74) |
| Obese | 544 | 15.7 | 1.92 (1.51, 2.44) | 1.66 (1.29, 2.14) |
| Cardiovascular disease | ||||
| No | 3120 | 90.0 | Ref | Ref |
| Yes | 345 | 10.0 | 1.56 (1.20, 2.02) | 1.26 (0.95, 1.69) |
| Lung disease | ||||
| No | 3129 | 90.3 | Ref | Ref |
| Yes | 336 | 9.7 | 1.65 (1.27, 2.14) | 1.28 (0.96, 1.69) |
| Diabetes | ||||
| No | 3377 | 97.5 | Ref | Ref |
| Yes | 88 | 2.5 | 2.10 (1.33, 3.32) | 1.74 (1.06, 2.84) |
| Chronic muscle disease | ||||
| No | 3419 | 98.7 | Ref | Ref |
| Yes | 46 | 1.3 | 3.46 (1.92, 6.24) | 2.19 (1.16, 4.13) |
| Autoimmune disease | ||||
| No | 3320 | 95.8 | Ref | Ref |
| Yes | 145 | 4.2 | 1.42 (0.96, 2.10) | 0.99 (0.65, 1.51) |
| Psychiatric disorder | ||||
| No | 3389 | 97.8 | Ref | Ref |
| Yes | 76 | 2.2 | 2.48 (1.54, 4.01) | 1.66 (0.99, 2.75) |
| Other chronic disease | ||||
| No | 2942 | 84.9 | Ref | Ref |
| Yes | 523 | 15.1 | 1.73 (1.40, 2.15) | 1.42 (1.12, 1.80) |
| Vaccination status | ||||
| Fully vaccinated | 1584 | 45.7 | Ref | Ref |
| Partially vaccinated | 98 | 2.8 | 1.18 (0.69, 2.00) | 1.11 (0.64, 1.94) |
| Not vaccinated | 1783 | 51.5 | 1.37 (1.15, 1.64) | 1.29 (1.03, 1.62) |
| Virus variant | ||||
| Omicron | 2656 | 76.7 | Ref | Ref |
| Delta | 649 | 18.7 | 1.27 (1.03, 1.57) | 0.95 (0.68, 1.33) |
| Alpha | 160 | 4.6 | 0.85 (0.55, 1.31) | 0.46 (0.26, 0.81) |
| Hospitalization | ||||
| No | 3340 | 96.4 | Ref | Ref |
| Yes | 125 | 3.6 | 2.06 (1.40, 3.04) | 2.34 (1.49, 3.65) |
| Season of infection | ||||
| Winter | 2555 | 73.7 | Ref | Ref |
| Spring | 388 | 11.2 | 1.11 (0.85, 1.46) | 1.01 (0.71, 1.45) |
| Summer | 77 | 2.2 | 0.94 (0.51, 1.73) | 0.67 (0.34, 1.30) |
| Autumn | 445 | 12.8 | 1.36 (1.07, 1.74) | 1.21 (0.87, 1.70) |
Female sex was a risk factor for the muscle pain and fatigue profiles while age was not a risk factor for any of the profiles (table 3). A low educational level was protective for the cardiorespiratory and ageusia/anosmia profiles. For lifestyle-related factors, being overweight or obese increased the risk for all profiles except the fatigue profile, while smoking was not a risk factor for any of the profiles. Regarding health status, having a chronic disease increased the risk for all profiles except the ageusia/anosmia profile, with the cardiorespiratory profile being only significant in case of multimorbidity. Finally, being unvaccinated increased the risk for the ageusia/anosmia profile, whereas the Alpha variant was associated with a decreased risk in this profile compared to Omicron.
Table 3.
Multivariable multinomial logistic regression analysis of determinants for profile status with participants without post-COVID-19 condition (n = 2749) as the reference groupa
| Muscle pain (n = 349) | Fatigue (n = 92) | Cardiorespiratory (n = 35) | Ageusia/anosmia (n = 151) | |
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age | ||||
| 18–39 | Ref | Ref | Ref | Ref |
| 40–59 | 1.03 (0.68, 1.55) | 0.98 (0.47, 2.04) | 3.94 (0.52, 29.7) | 1.44 (0.75, 2.76) |
| ≥60 | 0.77 (0.48, 1.16) | 0.72 (0.33, 1.61) | 1.46 (0.17, 12.4) | 1.10 (0.55, 2.20) |
| Sex | ||||
| Male | Ref | Ref | Ref | Ref |
| Female | 1.63 (1.26, 2.10) | 2.09 (1.24, 3.51) | 1.67 (0.76, 3.64) | 1.10 (0.78, 1.57) |
| Educational level | ||||
| High | Ref | Ref | Ref | Ref |
| Medium | 1.11 (0.85, 1.44) | 0.99 (0.61, 1.62) | 0.46 (0.22, 0.98) | 0.78 (0.54, 1.13) |
| Low | 0.85 (0.62, 1.18) | 0.95 (0.53, 1.72) | 0.30 (0.10, 0.84) | 0.53 (0.33, 0.87) |
| Smoking | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.72 (0.47, 1.11) | 1.53 (0.82, 2.82) | 1.19 (0.40, 3.53) | 1.07 (0.61, 1.88) |
| Body mass index | ||||
| Healthy | Ref | Ref | Ref | Ref |
| Overweight | 1.53 (1.18, 1.98) | 0.76 (0.46, 1.24) | 3.08 (1.18, 8.08) | 1.62 (1.12, 2.36) |
| Obese | 1.77 (1.28, 2.44) | 1.24 (0.71, 2.17) | 6.28 (2.29, 17.2) | 1.82 (1.12, 2.98) |
| Chronic disease | ||||
| No | Ref | Ref | Ref | Ref |
| One | 1.78 (1.35, 2.35) | 2.31 (1.39, 3.86) | 2.16 (0.90, 5.22) | 1.21 (0.78, 1.88) |
| Multimorbidity | 1.82 (1.31, 2.54) | 3.13 (1.80, 5.45) | 4.77 (2.09, 10.9) | 1.05 (0.60, 1.84) |
| Vaccination status | ||||
| Fully vaccinated | Ref | Ref | Ref | Ref |
| Not vaccinated | 1.01 (0.76, 1.33) | 1.38 (0.81, 2.35) | 2.23 (0.95, 5.27) | 2.45 (1.65, 3.79) |
| Virus variant | ||||
| Omicron | Ref | Ref | Ref | Ref |
| Delta | 0.79 (0.51, 1.22) | 1.41 (0.71, 2.78) | 0.70 (0.21, 2.39) | 1.00 (0.59, 1.69) |
| Alpha | 0.87 (0.45, 1.67) | 0.42 (0.12, 1.55) | 2.81 (0.49, 16.2) | 0.22 (0.05, 0.99) |
| Season | ||||
| Winter | Ref | Ref | Ref | Ref |
| Spring/summer | 1.02 (0.68, 1.54) | 1.44 (0.75, 2.75) | 0.48 (0.11, 2.15) | 0.81 (0.45, 1.47) |
| Autumn | 1.19 (0.76, 1.87) | 1.02 (0.48, 2.14) | 1.58 (0.45, 5.61) | 1.37 (0.79, 2.40) |
Due to a low number of participants in some cells: unknown education excluded; partly vaccinated combined with fully vaccinated; spring and summer combined.
Sensitivity analyses
Using 23 symptoms, 1024 (29.5%) participants classified for post-COVID-19 condition (Supplementary tables S7 and S8). Profile solutions were similar, although the muscle pain profile became an unspecific symptom profile (Supplementary tables S9–S11). Logistic regression analyses showed similar results as well (Supplementary table S12). Multinomial regression analyses, most importantly, showed that chronic muscle disease was a risk factor for the fatigue and cardiorespiratory profiles, lung- and auto-immune diseases were risk factors for the cardiorespiratory profile and diabetes for the fatigue profile (Supplementary table S13). Hospitalization increased the risk for the fatigue and cardiorespiratory profiles. Multinomial regression analysis with the muscle pain profile as the reference category showed that smoking was positively associated with the fatigue profile, obesity and multimorbidity with the cardiorespiratory profile and not being vaccinated with the ageusia/anosmia profile (Supplementary table S14). Overweight and a lower educational level were negatively associated with the fatigue and cardiorespiratory profile, respectively.
Discussion
This study identified four symptom profiles for post-COVID-19 condition with shared and unique risk factors, suggesting that SARS-CoV-2 may trigger different pathophysiological mechanisms that may lead to different subtypes of post-COVID-19 condition. Patients in the seemingly most severe profile, i.e. the cardiorespiratory profile, had the highest likelihood of being overweight, obese and having multimorbidity.
The following limitations should be acknowledged before interpreting the results of this study. First, consistent longitudinal data on cognitive symptoms and post-exertional malaise were not available while these symptoms are potentially relevant for post-COVID-19 condition.24 Second, there may be some selection bias as participants in the Lifelines COVID-19 cohort were older and more often female compared to the general Lifelines population. Third, misclassification of post-COVID-19 condition may have occurred as an increase in symptoms may be related to other causes.4 As reasons for symptom increases in those potentially misclassified are likely heterogeneous, we do not expect important differences in results if misclassification could completely be avoided. Fourth, the small sample size of the cardiorespiratory profile and within some of the variable categories may lead to considerable uncertainty around some estimates and potentially false non-significant findings. Fifth, the virus variant was assigned based on the dominant variant at wave of infection.21 Some misclassification is therefore not ruled out. Sixth, while we adjusted analyses for vaccination status we did not take type and timing of vaccination into account, while this may be important for the development of post-COVID-19 condition.25,26
A major strength of this study is the use of longitudinal data from 27 consecutive waves since the start of the pandemic. This allows for the calculation of pre-COVID-19 symptom severity, enabling us to classify participants as developing post-COVID-19 condition based on increased or new symptoms, reducing the risk of misclassification.14 In addition, the study sample completed a median of 25 questionnaires, which is relatively high. Finally, the SCL-90 SOM subscale is a validated instrument, suitable for assessing symptoms in large-scale cohort studies over time.
In line with previous studies, we found that female sex,6,7,9,10 weight status,9,10 comorbidity,6,7,9 being unvaccinated27 and hospitalization10 were determinants of post-COVID-19 condition. In contrast to previous studies, older age,9,10 smoking,9,10 cardiovascular disease, lung disease and psychiatric disorder9 were not significantly associated with post-COVID-19 condition, although point estimates for cardiovascular disease, lung disease and psychiatric disorder were increased. The relatively low percentage of COVID-19 cases may be explained by the low prevalence in the Northern Netherlands during the initial stage of the pandemic,15 the drop-out during the first phase of the pandemic and the fact that we did not include positive self-tests. While we most likely underestimate the prevalence of COVID-19, we expect that this did not influence the associations and profiles. While drop-out may have been selective in the entire Lifelines COVID-19 cohort, characteristics of those excluded (n = 8450) from our initial sample of 12 150 participants with COVID-19 were similar to the characteristics of those included in the analytic study sample.
We further found that participants most likely infected with the Alpha variant were at lower risk to develop post-COVID-19 condition compared to Omicron. Recently, a British study showed that people with Omicron had a lower risk to develop post-COVID-19 condition than people with the Delta variant.25 While we found that Omicron reduced the risk in a univariable analysis, this result was not maintained in our multivariable analysis. Differences in classification of post-COVID-19 condition, covariate adjustment and selection of participants may explain this difference. The proportion of fully vaccinated participants was lower in the analytic study sample than in those excluded. This can be explained by the increased time between measurement waves later in the pandemic in combination with the increase in vaccinations. Since this is thus directly related to the study design, we do not expect it to influence the validity of the findings.
Among 642 participants with post-COVID-19 condition, we identified four symptom profiles that may represent subtypes of post-COVID-19 condition. In line with previous studies,9–13 we found two symptom profiles that were characterized by general tiredness, heavy limbs and painful muscles. One of these profiles was characterized by the additional cardiorespiratory symptoms chest pain, difficulties breathing and pain while breathing. While this concurs with a meta-regression analysis,13 separate profiles for respiratory symptoms have been identified as well.10,11,13 Differences in sample size and study design compared to previous studies may explain why we did not find a separate respiratory profile. In contrast to previous studies, neither in the main analyses including previously defined core symptoms4 nor in sensitivity analyses did we identify profiles characterized by upper respiratory symptoms like cough or sore throat.9,11 This may indicate that previous studies could not distinguish between symptoms attributable to COVID-19, and those being a mere continuation of pre-existing symptoms. Finally, in line with studies from the UK12 and Germany11 we found a clear profile of patients experiencing solely ageusia/anosmia with few additional symptoms. Predictors for post-COVID-19 condition and for belonging to a more severe symptom profile mainly include excessive weight status and pre-existing health conditions. Excessive weight status and existing health conditions seem biological plausible risk factors for post-COVID-19 condition as they affect e.g. the immune, endocrine, circulatory and respiratory systems. Findings highlight the public health relevance of a healthy lifestyle and the prevention of overweight and chronic diseases.
In the current study, the cardiorespiratory profile seems to represent a small group of patients with a multitude of debilitating symptoms. The finding that the risk increase by overweight, obesity and multimorbidity is more pronounced for this particular profile than for the other profiles, indicates that existing pathology might contribute to a more severe subtype of post-COVID-19 condition. Similar results regarding weight status were observed in the UK, where overweight and obese COVID-19 patients were also at risk to develop persistent respiratory symptoms after COVID-19.9 Although the cardiorespiratory profile represents only 1% of our total study sample, the consequences on a population level could be substantial if indeed one in every hundred COVID-19 patients develops such a severe combination of persistent symptoms. Our findings regarding the size of symptom profiles are supported by a meta-regression analysis on three pre-defined symptom clusters, showing that 3.2% and 3.7% of individuals with symptomatic SARS-CoV-2 infection experienced persistent fatigue with bodily pain and ongoing respiratory problems, respectively.13
Our results suggest that it is important to distinguish between subtypes of post-COVID-19 condition when examining underlying mechanisms, as risk factors vary across subtypes. Future research should aim to disentangle how different profiles can be further characterized based on pathophysiological markers, preferably using pre- and post-SARS-CoV-2 measurements and a control group. It may be hypothesized that the cardiorespiratory profile is characterized by underlying organ damage in e.g. the lungs and heart,1 whereas the muscle pain and fatigue profiles may resemble known post-infectious somatic syndromes. Long term consequences regarding well-being and social participation may vary as well.11,28 One of the most pressing challenges is to further quantify the impact of specific symptom profiles on well-being and social participation, including work, in non-biased studies that are representative for the general population.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centers delivering data to Lifelines, and all the study participants. Lifelines Corona Research Initiative: H. Marike Boezen†1, Jochen O. Mierau2,3,9, H. Lude Franke4, Jackie Dekens4,6, Patrick Deelen4, Pauline Lanting4, Judith M. Vonk1, Ilja Nolte1, Anil P.S. Ori4,5, Annique Claringbould4, Floranne Boulogne4, Marjolein X.L. Dijkema4, Henry H. Wiersma4, Robert Warmerdam4, Soesma A. Jankipersadsing4, Irene van Blokland4,7, Geertruida H. de Bock1, Judith G.M. Rosmalen5,8, Cisca Wijmenga4. †Professor H. Marike Boezen passed away in October 2021. 1Department of Epidemiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands. 2Department of Economics, Econometrics & Finance, Faculty of Economics and Business, University of Groningen, Groningen, The Netherlands. 3Lifelines Cohort Study and Biobank, Groningen, The Netherlands. 4Department of Genetics, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands. 5Department of Psychiatry, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands. 6Center of Development and Innovation, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands. 7Department of Cardiology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands. 8Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands. 9Team Strategy &External Relations, University of Groningen, University Medical Center Groningen, the Netherlands.
Contributor Information
Sander K R van Zon, Department of Health Sciences, Community and Occupational Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Aranka V Ballering, Department of Psychiatry, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Sandra Brouwer, Department of Health Sciences, Community and Occupational Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Judith G M Rosmalen, Department of Psychiatry, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; Department of Internal Medicine, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
for the Lifelines Corona Research Initiative:
H Marike Boezen, Jochen O Mierau, H Lude Franke, Jackie Dekens, Patrick Deelen, Pauline Lanting, Judith M Vonk, Ilja Nolte, Anil P S Ori, Annique Claringbould, Floranne Boulogne, Marjolein X L Dijkema, Henry H Wiersma, Robert Warmerdam, Soesma A Jankipersadsing, Irene van Blokland, Geertruida H de Bock, Judith G M Rosmalen, and Cisca Wijmenga
Supplementary data
Supplementary data are available at EURPUB online.
Funding
This work was supported by the ZonMw COVID-19 programme (10430302110002), the European Union’s Horizon Europe research and innovation programme (101057553) and the Swiss State Secretariat for Education, Research and Innovation (SERI) (22.00094). The Lifelines initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG), Groningen University and the Provinces in the North of the Netherlands (Drenthe, Friesland, Groningen).
Conflicts of interest: None declared.
Key points.
Studies incorporating pre-existing symptoms in the classification of post-COVID-19 condition are lacking, which may not only obscure observed symptom profiles, it may also obscure associations between risk factors and post-COVID-19 condition and specific symptom profiles.
We report a study that includes pre- and post-SARS-CoV-2 infection symptom scores to classify post-COVID-19 condition, enabling us to adjust for symptom presence unrelated to COVID-19.
Our unique approach identified four novel symptom profiles characterized by (i) muscle pain, (ii) fatigue, (iii) cardiorespiratory symptoms and (iv) ageusia/anosmia.
Shared and unique demographic, lifestyle and health-related risk factors were identified for the four symptom profiles.
Results from our study may guide future research regarding underlying pathophysiological mechanisms and quantification of the individual and societal impact of specific symptom profiles, which will further inform tailored care for patients with varying persistent symptoms.
Data availability
The data underlying this article were provided by Lifelines. Lifelines is a facility that is open for all researchers. Information on application and data access procedure is summarized on www.lifelines.nl.
References
- 1. Crook H, Raza S, Nowell J, et al. Long COVID-mechanisms, risk factors, and management. BMJ 2021;374:n1648. [DOI] [PubMed] [Google Scholar]
- 2. Nasserie T, Hittle M, Goodman SN.. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open 2021;4:e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soriano JB, Murthy S, Marshall JC, et al. ; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102-07–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM; Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022;400:452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alwan NA. The road to addressing long COVID. Science 2021;373:491–3. [DOI] [PubMed] [Google Scholar]
- 6. Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health 2021;6:e005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maglietta G, Diodati F, Puntoni M, et al. Prognostic factors for post-COVID-19 syndrome: a systematic review and meta-analysis. JCM 2022;11:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ballering AV, Oertelt-Prigione S, Hartman TCO, Rosmalen JGM.. Response to Rossato et al. J Womens Health (Larchmt) 2022;31:896–8. [DOI] [PubMed] [Google Scholar]
- 9. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med 2022;28:1706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitaker M, Elliott J, Chadeau-Hyam M, et al. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat Commun 2022;13:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peter RS, Nieters A, Krausslich HG, et al. ; EPILOC Phase 1 Study Group. Post-acute sequelae of COVID-19 six to 12 months after infection: population based study. BMJ 2022;379:e071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canas LS, Molteni E, Deng J, et al. Profiling post-COVID syndrome across different variants of SARS-CoV-2: a prospective longitudinal study in unvaccinated wild-type, unvaccinated alpha-variant, and vaccinated delta-variant populations. Lancet Digit Health 2023;5:e421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wulf Hanson S, Abbafati C, Aerts JG, et al. ; Global Burden of Disease Long COVID Collaborators. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022;328:1604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wisk LE, Nichol G, Elmore JG.. Toward unbiased evaluation of postacute sequelae of SARS-CoV-2 infection: challenges and solutions for the long haul ahead. Ann Intern Med 2022;175:740–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mc Intyre K, Lanting P, Deelen P, et al. Lifelines COVID-19 cohort: investigating COVID-19 infection and its health and societal impacts in a Dutch population-based cohort. BMJ Open 2021;11:e044474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scholtens S, Smidt N, Swertz MA, et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol 2015;44:1172–80. [DOI] [PubMed] [Google Scholar]
- 17. Klijs B, Scholtens S, Mandemakers JJ, et al. Representativeness of the LifeLines Cohort Study. PLoS One 2015;10:e0137203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zijlema WL, Stolk RP, Lowe B, et al. ; BioSHaRE. How to assess common somatic symptoms in large-scale studies: a systematic review of questionnaires. J Psychosom Res 2013;74:459–68. [DOI] [PubMed] [Google Scholar]
- 19. Rytila-Manninen M, Frojd S, Haravuori H, et al. Psychometric properties of the Symptom Checklist-90 in adolescent psychiatric inpatients and age- and gender-matched community youth. Child Adolesc Psychiatry Ment Health 2016;10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Institute for Public Health and the Environment. Cijfers COVID-19 vaccinatie programma (Figures COVID-19 vaccination program). Available at: https://www.rivm.nl/covid-19-vaccinatie/cijfers-vaccinatieprogramma (10 October 2022. date last accessed).
- 21. National Institute for Public Health and the Environment. Variants of the Coronavirus SARS-CoV-2. Available at: https://www.rivm.nl/coronavirus-covid-19/virus/varianten (9 September 2022 date last accessed).
- 22. Rosenberg JM, Beymer PN, Anderson DJ, et al. tidyLPA: an R package to easily carry out latent profile analysis (LPA) using open-source or commercial software. JOSS 2018;3:978. [Google Scholar]
- 23. Wardenaar K. Latent profile analysis in R: a tutorial and comparison to Mplus. PsyArXiv, 2021.
- 24. Al-Aly Z, Xie Y, Bowe B.. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021;594:259–64. [DOI] [PubMed] [Google Scholar]
- 25. Antonelli M, Pujol JC, Spector TD, et al. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022;399:2263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022;399:924–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al-Aly Z, Bowe B, Xie Y.. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022;28:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lunt J, Hemming S, Burton K, et al. What workers can tell us about post-COVID workability. Occup Med (Lond) 2022;kqac086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by Lifelines. Lifelines is a facility that is open for all researchers. Information on application and data access procedure is summarized on www.lifelines.nl.