Abstract
The Latin American Brain Health Institute (BrainLat) has released a unique multimodal neuroimaging dataset of 780 participants from Latin American. The dataset includes 530 patients with neurodegenerative diseases such as Alzheimer’s disease (AD), behavioral variant frontotemporal dementia (bvFTD), multiple sclerosis (MS), Parkinson’s disease (PD), and 250 healthy controls (HCs). This dataset (62.7 ± 9.5 years, age range 21–89 years) was collected through a multicentric effort across five Latin American countries to address the need for affordable, scalable, and available biomarkers in regions with larger inequities. The BrainLat is the first regional collection of clinical and cognitive assessments, anatomical magnetic resonance imaging (MRI), resting-state functional MRI (fMRI), diffusion-weighted MRI (DWI), and high density resting-state electroencephalography (EEG) in dementia patients. In addition, it includes demographic information about harmonized recruitment and assessment protocols. The dataset is publicly available to encourage further research and development of tools and health applications for neurodegeneration based on multimodal neuroimaging, promoting the assessment of regional variability and inclusion of underrepresented participants in research.
Subject terms: Neurodegeneration, Biomarkers
Background & Summary
Dementia and neurodegenerative diseases significantly impact patients, families, the economy, and public health systems worldwide. However, such impact, coupled with prevalence, underdiagnosis, and assessment, is unequal. Latin America is one of the most unequal regions in the world, with a lack of adequate dementia diagnosis and care1–4. The current prevalence of dementia in LACs is estimated at 8.5% and is projected to be 19.33% by 2050, representing an increase of 220% approximately. Such prevalence is higher compared to other regions5,6 including Europe (current 6.9% and projected up to 7.7% by 2050) or North America (current 6.5% and projected up to 12.1% by 2050)4,5,7–10 Paradoxically, most global research on neurodegeneration is underrepresented in terms of Latino populations4,8,11–14 Most literature arises predominantly from the US, Europe, and other regions with high-income settings. Despite the pressing need to evaluate regional diversity and provide tailored evidence for underrepresented samples2,15–18, current scientific findings on neurodegeneration in Latin America do not meet this requirement. The situation seems more urgent given the recent evidence that the so-called non-stereotypic populations15 (participants from underrepresented populations in admixtures, genetics, cultural backgrounds, and demographics) defy the generalization of brain-phenotype models from stereotypical populations19–22. Thus, to evaluate diversity in dementia research is an immediate and significant gap that needs to be addressed.
Developing affordable, scalable, and widely available biomarkers is crucial for early diagnosis and intervention, specially Latin America4,8,11–14. While several multimodal neuroimaging databases and consortia for neurodegeneration exist (e.g., ADNI, LONI, HCP, UK Biobank, CAMCAN, ABCD, PPMI, ENIGMA), there is a lack of datasets from underrepresented, non-stereotypical samples, and few databases include EEG data. EEG is an advantageous technique for assessing neurodegeneration due to its cost-effectiveness, accessibility, scalability, and applicability to underserved populations. The opportunity to evaluate brain dynamics and networks with combined spatiotemporal methods represents a significant advance for clinical assessment23,24, as well as multimodal imaging and computational approaches to neuroscience25–27. However, to our knowledge, no other open datasets of multiple neurodegenerative diseases include resting-state recordings with high spatial (fMRI) and temporal (EEG) resolution.
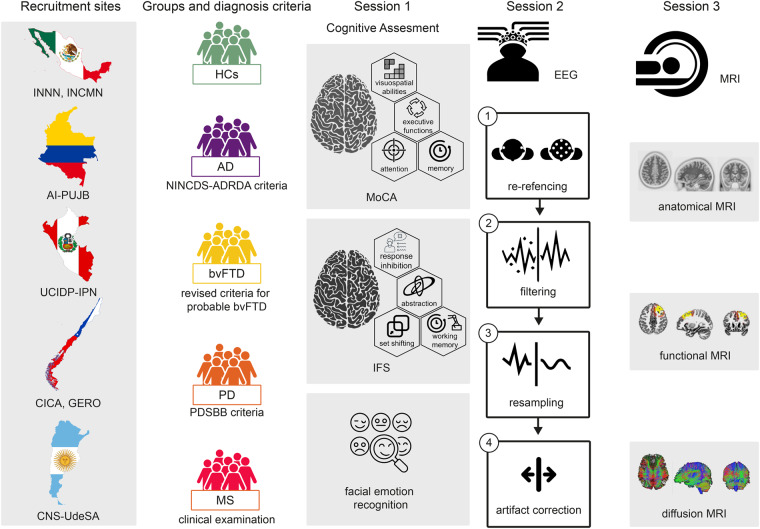
The BrainLat dataset28 (Fig. 1) is a pioneering dataset that addresses these gaps by providing data from a diverse group of Latin American patients with various neurodegenerative diseases, including Alzheimer’s disease (AD), behavioral variant frontotemporal dementia (bvFTD), Parkinson’s disease (PD), multiple sclerosis (MS), and healthy controls. It is a regional effort designed as a multicentric study with harmonized recruitment and neurocognitive assessment, led by the Latin American Brain Health Institute (BrainLat)29 and the Multi-partner consortium to explore dementia research in Latin America (ReDLat)10,30,31 with the support of various stakeholders. Details for harmonizing per the ReDLat procedures (recruitment and neurocognitive assessment) include a site manual, a checklist, and a tutorial, all available elsewhere30.
Fig. 1.
The BrainLat multimodal dataset of neurodegenerative diseases. The figure summarizes the entire protocol, encompassing various centers, participant groups, diagnostic criteria, cognitive assessments, and EEG and MRI recordings. The activities carried out by the participants during their three visits to the clinical center are also depicted. For the EEG session, the figure illustrates the key steps in the processing pipeline. Session three summarizes the different MRI recordings (anatomical, functional, and diffusion MRI). The recruitment sites included the INNN: Instituto Nacional de Neurología y neurocirugía, Ciudad de México, Mexico; INCMN: Geriatrics Department, Instituto Nacional de Ciencias médicas y nutrición Salvador Zubirán, Mexico City, Mexico; AI-PUJB: Aging Institute, Pontificia Universidad Javeriana, Bogotá, Colombia; UCIDP-IPN: Unit Cognitive Impairment and Dementia Prevention, Peruvian Institute of Neurosciences, Lima, Peru; CICA: Centro de Investigación Clínica Avanzada (CICA) Hospital Clínico Universidad de Chile, Chile: GERO: Neurology Department, Geroscience Center for Brain Health and Metabolism, Santiago, Chile; CNC-UdeSA Centro de Neurociencia Cognitiva, Universidad de San Andrés, Argentina. AD: Alzheimer’s disease, bvFTD: behavioral variant frontotemporal dementia, PD: Parkinson’s disease, MS: Multiple sclerosis, HCs: older healthy controls.
Along with cognitive and sociodemographic information, the BrainLat dataset28 includes anatomical MRI, resting-state fMRI, and resting-state EEG. Neuroimaging records have not been harmonized to allow dataset users to conduct custom analyses. Nevertheless, different post-recording harmonization (w- and z-scores, confusion matrices, data transformation/normalization, optimizers, and k-folds validation) have been successfully applied in this data32,33. Thus, the BrainLat dataset28 has been utilized for understanding neurodegeneration and developing multimodal markers32–59.
By making the BrainLat dataset28 openly accessible, the project aims to encourage additional analyses and data exploitation. This dataset28 is the first to be released from a larger multicentric initiative, the Euro-LAD EEG consortium60, a Global EEG Platform for dementia research inclusive of diverse and underrepresented data. We hope this dataset28 will allow the future development of normative EEG datasets based on harmonized multicentric data, assessing sociodemographic variability, and promoting the development of tools and health applications for neurodegeneration based on multimodal neuroimaging.
Latin American populations display extensive heterogeneity triggered by the unique combination of genetic and environmental (i.e., socioeconomic) differences3,9. This open-access dataset28 fosters collaboration and facilitates the identification of new biomarkers, ultimately contributing to advancements in understanding and treating neurodegenerative diseases. While genetics and socioeconomic status information are not currently included in the BrainLat dataset28, we anticipate that these will be available upon completing the ReDLat protocol by 2026, when the dataset will be updated.
Methods
Participants
The BrainLat dataset28 contains neuroimaging and cognitive data from 780 subjects, including patients with AD (N = 278), bvFTD (N = 163), PD (N = 57) and MS (N = 32), and HCs (N = 250). Participants were enrolled in clinical sites from the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat), a regional effort to harmonize participant enrollment and neurocognitive assessment in multicentric studies10,30. Five ReDLat countries were included (Argentina, Chile, Colombia, Mexico, and Peru, see Table 1). The demographic information of the BrainLat dataset28 (global information) is presented in Table 2, while the information split for the recruitment sites is presented in Table 3 and stored in BrainLat_Demographic.csv. There was limited information available on the age of the participants at the onset of the disease. Consequently, the duration of the disease is not reported.
Table 1.
List of sites contributing to the BrainLat dataset.
| Short name | Country | Full name | Initials of the PI |
|---|---|---|---|
| INCMN | MX | Geriatrics Department, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City | AF |
| CICA | CL | Centro de Investigación Clínica Avanzada (CICA) Hospital Clínico Universidad de Chile | BE |
| UV | CO | Universidad del Valle, Cali | CA |
| UCIDP-IPN | PE | Unit Cognitive Impairment and Dementia Prevention, Peruvian Institute of Neurosciences, Lima | CU |
| CNC-UdeSA | AR | Centro de Neurociencia Cognitiva, Universidad de San Andrés, Buenos Aires | IB |
| AI-PUJB | CO | Aging Institute, Pontificia Universidad Javeriana, Bogotá | MA |
| GERO | CL | Neurology Department, Geroscience Center for Brain Health and Metabolism, Santiago | SL |
| INNN | MX | Instituto Nacional de Neurología y neurocirugía, Ciudad de México | SO |
Country codes meet the standards of the International Organization for Standardization (ISO). MX: Mexico, CL, Chile, CO: Colombia, PE: Perú, AR: Argentina. PI: principal investigator.
Table 2.
Demographic information of the BrainLat dataset.
| group | N | demography | |||
|---|---|---|---|---|---|
| sex (F: M) | age (years) | education (years) | HbP (right: left) | ||
| AD | 278 | 167:111 | 72.2 (7.9) | 11.9 (4.8) | 157:5* |
| bvFTD | 163 | 77:86 | 65.1 (10.5) | 13.1 (5.0) | 135:5* |
| PD | 57 | 19:38 | 69.9 (11.2) | 7.9 (5.5) | 55:1* |
| MS | 32 | 26:6 | 38.5 (8.9) | 16.5 (3.4) | — |
| HCs | 250 | 164:86 | 67.9 (8.9) | 14.7 (4.3) | 177:5* |
| total | 780 | 453:327 | 62.7 (9.5) | 12.8 (4.6) | 524:16* |
Age and years of formal education are presented as mean (standard deviation). Sex is the ratio between females (F) and males (M). HbP: Handedness by preference (self-referenced handedness). The symbol *indicates the field contains missing information. The symbol - indicates data is not available. AD: Alzheimer’s disease, bvFTD: behavioral variant frontotemporal dementia, PD: Parkinson’s disease, MS: Multiple sclerosis, HCs: healthy controls.
Table 3.
Demographic information of the BrainLat dataset split by recruitment site.
| group | total | recruitment site | demography | |||||
|---|---|---|---|---|---|---|---|---|
| N | sex (F: M) | age (years) | education (years) | HbP (right: left) | ||||
| AD | 279 | CICA | CL | 4 | 4:00 | 71.0 (10.1) | 5.5 (4.4) | 4:0 |
| UCIDP-IPN | PE | 73 | 30:43 | 72.5 (8.5) | 9.5 (3.6) | 71:1* | ||
| CNC-UdeSA | AR | 43 | 31:12 | 76.0 (6.1) | 9.3 (5.8) | 32:3* | ||
| AI-PUJB | CO | 23 | 14:90 | 67.8 (8.7) | 11.0 (4.5) | 23:0 | ||
| GERO | CL | 114 | 65:49 | 83.0 (7.2) | 16.5 (4.7) | 29:0* | ||
| INNN | MX | 21 | 10:11 | 76.8 (6.6) | 6.3 (6.9) | 18:1* | ||
| bvFTD | 165 | CICA | CL | 2 | 1:1 | 56.5 (0.7) | 14.5 (3.5) | 2:0 |
| UCIDP-IPN | PE | 1 | 0:1 | 61.0 - | 11.0 - | 1:0 | ||
| CNC-UdeSA | AR | 38 | 17:21 | 71.5 (17.2) | 13.0 (4.2) | 27:1* | ||
| AI-PUJB | CO | 81 | 46:35 | 61.0 (7.7) | 13.3 (5.1) | 78:3 | ||
| GERO | CL | 35 | 10:25 | 74.3 (10.5) | 13.8 (5.1) | 23:0* | ||
| INNN | MX | 6 | 4:2 | 68.8 (7.0) | 8.5 (6.2) | 4:1* | ||
| PD | 57 | CNC-UdeSA | AR | 11 | 5:6 | 74.0 (6.4) | 10.8 (5.6) | 11:0 |
| UV | CO | 25 | 3:22 | 64.5 (7.4) | 11.1 (5.8) | 28:0 | ||
| GERO | CL | 21 | 11:10 | 73.8 (6.9) | 10.9 (5.4) | 19:1* | ||
| MS | 32 | CNC-UdeSA | AR | 32 | 26:6 | 38.5 (8.9) | 16.5 (3.4) | — |
| HCs | 250 | INCMN | MX | 2 | 2:0 | 65.0 (1.4) | 15.0 - | * |
| CICA | CL | 28 | 18:00 | 63.8 (5.2) | 12.7 (4.7) | 28:0 | ||
| UCIDP-IPN | PE | 7 | 3:7 | 77.4 (9.0) | 12.0 (1.9) | 7:0 | ||
| CNC-UdeSA | AR | 80 | 58:22 | 66.4 (9.0) | 17.1 (3.3) | 65:4* | ||
| AI-PUJB | CO | 46 | 30:16 | 60.2 (8.6) | 14.4 (4.7) | 45:1 | ||
| GERO | CL | 87 | 60:17 | 73.3 (5.2) | 13.7 (4.0) | 30:0* | ||
Age and years of formal education are presented as mean (standard deviation). Country codes meet the standards of the International Organization for Standardization (ISO). Sex is the ratio between females (F) and males (M). HbP: Handedness by preference (self-referenced handedness). The symbol * indicates the field contains missing information. The symbol - indicates data is not available. AD: Alzheimer’s disease, bvFTD: behavioral variant frontotemporal dementia, PD: Parkinson’s disease, MS: Multiple sclerosis, HCs: healthy controls, AR: Argentina, CL, Chile, CO: Colombia, CNC- UniSA: Centro de Neurociencias Cognitivas, Universidad de San Andrés, Gero-CMYN: Clínica de Memoria y Neuropsiquistría, Centro de Gerociencia, Salud Mental y Metabolismo.
As noted above, the BrainLat dataset28 included MS patients, where primary mechanisms are considered to have a larger inflammatory component compared to AD, bvFTD, and PD. Nonetheless, incorporating MS in the dataset holds significant relevance. Comparisons between MS and other neurodegenerative diseases are relevant and frequently reported47,61,62. Although the pathophysiological pathways differ, insightful comparisons between these conditions can be made. By leveraging multivariate data, comprehensive analyses can be performed to delineate shared and unique disease patterns63–66. Moreover, recent insights have emphasized shared mechanisms across different neurodegenerative diseases, including the role of inflammatory pathways65–68. Furthermore, the flexible nature of the dataset design allows for analyses to be conducted with patient groups combined or separated. This offers the opportunity to observe MS alone or in comparison with other conditions, providing a rich perspective in understanding complex neurodegenerative pathways.
Ethics
The institutional ethic boards of each recruitment site provided ethical approval for collecting and sharing data. The ethics approval reference codes for each participating institution (Table 1) are listed below.
Geriatrics Department, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMN), Mexico City, Mexico (reference code 09-CEI-011-2016-0627).
Centro de Investigación Clínica Avanzada (CICA) Hospital Clínico Universidad de Chile, Santiago, Chile (reference code FWA00029089).
Universidad del Valle, Cali, Colombia (reference code FWA00028864).
Unit Cognitive Impairment and Dementia Prevention, Peruvian Institute of Neurosciences, Lima, Peru (reference code 10360-19).
Centro de Neurociencia Cognitiva, Universidad de San Andrés, Buenos Aires, Argentina (reference code 0990-0279).
Aging Institute, Pontificia Universidad Javeriana, Bogotá, Colombia (reference code FM-CIE-0741-19).
Neurology Department, Geroscience Center for Brain Health and Metabolism, Santiago, Chile (reference code FWA00029089).
Instituto Nacional de Neurología y neurocirugía, Ciudad de México (reference code 12–20).
The ethics approvals were granted in accordance with the ethical regulations and guidelines of the countries where the centers are located, and in compliance with the Declaration of Helsinki.
On their first visit to the recruitment centers, participants were provided with both oral and written explanations about objectives, risks, and benefits of the study. Afterwards, participants proceed to sign a written consent form (Fig. 1). Patients were accompanied by a relative or legal representative, who signed the informed consent when necessary. The informed consent provided by the participants included for the open publication of the anonymized data. Consequently, participants were educated about processing information to protect the confidentiality of personally identifiable information. Information about sharing and publication of anonymized data was provided. For anonymization, the participants’ names were replaced by a code (section Usage Notes), and MRI images were defaced (section Data Records).
Recruitment, inclusion criteria, clinical and cognitive assessments
Information about the study was spread through networks of the recruitment centers and social media. The target audience was the HCs, patients with neurodegenerative diseases, and their families. The inclusion and exclusion criteria of the participants are outlined below. These criteria were reviewed and agreed upon by clinicians of the ReDLat consortium30.
The inclusion criteria for controls (HCs) were:
Possessing a Modified Clinical Dementia Rating (CDR) = 0 and a Mini-Mental State Examination (MMSE) score >25.
Meeting the criteria for fluency in Spanish (judged by the evaluator as sufficient to complete the assessment).
Having adequate visual and auditory acuity to complete cognitive testing.
Not having any proven history of substance abuse, or neurological or psychiatric disorders.
The inclusion criteria for participants with neurodegeneration were:
Having a clinical diagnosis of mild/moderate AD, bvFTD, PD, or MS. When needed, the diagnosis was supported by neuroimaging assessment (routine MRI or hypoperfusion/hypometabolism SPECT or PET).
Meeting criteria for fluency in Spanish (judged by the evaluator as sufficient to complete the assessment).
Must have adequate visual and auditory acuity for cognitive testing.
For patients with dementia (AD and bvFTD): having an informant who maintained frequent contact with the participant (e.g., family member, partner, friend, caregiver). The informant should be familiar with the participant’s daily activities and able to provide information on the participant’s cognitive and functional status. The duration of acquaintance with the patient should be at least six months.
Being able to sign the informed consent or be accompanied by an authorized representative who could do so.
The exclusion criteria for participants with neurodegenerative diseases were:
Mini-Mental State Examination (MMSE) score <14 (all groups), CDR = 3 (for AD), or FTLD-CDR (FTD) = 3.
Intoxication at the time of evaluation; multiple system atrophy, brain tumor, prion disease, Huntington’s disease, intracerebral hemorrhage, stroke.
Presence of ferromagnetic implants impacting MRI acquisition.
Clinically significant ischemic or hemorrhagic cerebrovascular disease, diffuse confluent white matter lesions (Fazekas Grade 3), intra or extra-axial masses revealed by MRI that compress brain parenchyma and that may affect cognition and/or behavior or may confound imaging analysis.
Deficiency of B12 (B12 < normal), hypothyroidism (TSH >150% of normal), HIV infection, renal insufficiency (creatinine >2), liver insufficiency (AST >2x normal), respiratory insufficiency (requiring oxygen), other significant systemic diseases (as judged by the attending neurologist).
Basic clinical criteria for other types of dementia or other neurological disorders.
Inability to communicate in Spanish.
Patients fulfilled either the current criteria of the National Institute of Neurological Disorders and Stroke–Alzheimer Disease and Related Disorders (NINCDS-ADRDA) working group for probable AD69, the revised criteria for probable bvFTD70, or the criteria of the United Kingdom Parkinson’s Disease Society Brain Bank (PDSBB) for PD71. Patients with MS were diagnosed by experts, considering standard clinical examination, magnetic resonance imaging, and lumbar puncture when necessary40.
Patients with AD and bvFTD were functionally impaired, as verified by caregivers. The AD patients were all sporadic, except for those recruited by one of the Colombian sites, who had PSEN1 mutations. The PD and AD groups had typical disease presentations, apart from the AD patients with PSEN1mutations that exhibited early-onset symptoms. The BraiLat dataset28 does not include records of late-onset AD or other atypical disease presentations. Additionally, participants with bvFTD exhibited noticeable changes in personality and social behavior. Participants with PD received levodopa treatment and were evaluated during the ‘on’ phase. Further details regarding this medication are unavailable.
ñA comprehensive assessment of the neurological, neuropsychiatric, and neuropsychological domains of the participants was conducted by ReDLat experts using semi-structured interviews and standardized cognitive and functional tests. The evaluation lasted up to three hours and comprised the test described below. The cognitive outcomes are stored in BrainLat_Cognition.csv.
Clinical assessments
Clinical dementia rating scale (CDR)
The CRD is an 8-item dementia rating scale that assesses cognitive and functional decline. Scores: 0 = Healthy, 0.5 = questionable dementia, 1 = mild dementia, 2 = moderate dementia, 3 = severe dementia72,73. Only AD patients were evaluated with this instrument.
Frontotemporal lobar degeneration-modified clinical dementia rating (FTDL-CDR)
The FTDL-CDR is a 5-point scale characterizing six cognitive and functional domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care73. Additionally, it is used for assessing behavioral and motor domains in the case of the frontotemporal dementia spectrum. Only bvFTD patients were evaluated with this instrument.
Section 3 of the movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (MDS-UPDRS-III)
The MDS-UPDRS-III74 is a revised and expanded version of UPDRS75 consisting of twenty questions that needed to be answered by the patient or caregiver. The MDS-UPDRS has four parts, with part III dedicated to motor complications. The stage of the disease was rated with the Hoehn & Yahr (H&Y) scale76. Only PD patients were evaluated with these instruments.
The multiple sclerosis severity score (MSSS)
The MSSS77 relates scores of the Expanded Disability Status Scale (EDSS)78 to the distribution of disability in patients with comparable disease durations for detecting rates of disease progression. Only MS patients were evaluated with this instrument.
Cognitive tools
The montreal cognitive assessment (MoCA)
The MoCA79 is a cognitive screening for tracking mild cognitive impairment. The MoCa comprises 30 points evaluating short-term memory, visuospatial abilities, multiple aspects of executive functions, attention, memory, and working memory, language abilities, and orientation to time and place. Its maximum score is 30, with higher scores indicating better performance. All participants were evaluated with this tool.
The ineco frontal screening (IFS)
The IFS80 is a tool for screening executive function in patients with neurodegenerative diseases. The IFS evaluates response inhibition and set shifting, the capacity of abstraction, and working memory. The maximum score on the test is 30, with higher scores indicating better performance. All participants were evaluated with this tool.
Facial emotion recognition (FER)
In this task, participants identify emotional expressions depicted in a series of photos39 (thirty-five faces selected from the emotion face set81). Participants are instructed to associate faces with one of six possible emotions (happiness, surprise, sadness, fear, disgust, anger) or a neutral expression. A score (max. 15) is calculated from the percentage of correct responses. All HCs, AD, bvFTD, and PD participants were evaluated with this tool.
Functional ability assessments
Functional activities questionnaire (FAQ)
is a 10-item rating scale that measures instrumental activities of daily living (such as preparing meals and personal finance)82. A score above 9 suggests a possible impaired function and possible cognitive impairment. All HCs, AD, bvFTD, and PD participants were evaluated with this tool.
Frontotemporal dementia rating scale (FRS)
is a 30-item scale that evaluates severity in people with dementia83,84. Scores from 1.92 to −2.58 indicate a moderate/severe disease stage and from −2.58 to −6.66 very severe/profound disease stage. Only bvFTD participants were evaluated with this tool.
All clinical, cognitive, and functionality assessments are provided as raw data. However, these can be normalized and harmonized for comparisons as performed elsewhere with the current data31.
Neuroimaging data
EEG and MRI were acquired within 6 months after the neurological evaluation (second and third visits of the participants to each recruitment site), following the ReDLat protocol10,30. The duration of the assessment includes up to 2 hours for EEG, and up to 1 hour for MRI.
The duration of the assessment includes up to 2 hours for EEG, and up to 1 hour for MRI. Global information about the neuroimaging modalities and the data split by recruitment sites are presented in Tables 4, 5. Noteworthy, as in other available datasets, ours has some missing data. For most of the participants, one (MRI) or two (MRI + EEG) neuroimaging modalities were acquired (Tables 4, 5). Nevertheless, EEG was the only neuroimaging modality acquired in a reduced group of participants (Tables 4, 5). Reasons for missing data include the different objectives of the studies for which data was initially acquired, technological constraints, and the use of varied data storage formats. Detailed information about the neuroimage modalities acquired from each participant is provided in BrainLat_records.csv, which is deposited on Synapse.
Table 4.
Global neuroimaging information of the BrainLat dataset.
| Group | imagin | EEG | ||||
|---|---|---|---|---|---|---|
| scan | N | MRI | fMRI | DWI | ||
| AD | 1.5 | 156 | 155 | 14 | 3 | 35 |
| 3.0 | 91 | 90 | 72 | 51 | ||
| bvFTD | 1.5 | 28 | 28 | 15 | 0 | 19 |
| 3.0 | 97 | 96 | 82 | 91 | ||
| PD | 3.0 | 55 | 55 | 30 | 30 | 29 |
| MS | 3 | 31 | 31 | 30 | 0 | 32 |
| HC | 1.5 | 81 | 81 | 27 | 27 | 42 |
| 3 | 177 | 176 | 171 | 161 | ||
Imaging information is presented for anatomical (MRI), functional (fMRI), and diffusion-weighted (DWI) resonance magnetic imaging. The magnetic field of the scan (scan) is presented. N: number of subjects, AD: Alzheimer’s disease, bvFTD: behavioral variant frontotemporal dementia, PD: Parkinson’s disease, MS: Multiple sclerosis, HCs: healthy controls.
Table 5.
Neuroimaging information of the BrainLat dataset split by recruitment site.
| Group | recruitment site | N | imaging | EEG | ||||
|---|---|---|---|---|---|---|---|---|
| MRI | fMRI | DWI | +MRI | EEG only | ||||
| AD | CICA | CL | 4 | 4 | — | — | — | — |
| UCIDP-IPN | PE | 66 | 66 | — | — | — | — | |
| CNC-UdeSA | AR | 22 | 21 | 21 | 19 | — | — | |
| GERO | CL | 82 | 81 | 14 | 3 | — | — | |
| INNN | MX | 4 | 4 | — | — | — | — | |
| UCIDP-IPN | PE | 7 | — | — | — | — | — | |
| CNC-UdeSA | AR | 18 | 18 | 17 | 18 | 13 | 3 | |
| AI-PUJB | CO | 23 | 22 | 23 | 22 | — | — | |
| GERO | CL | 26 | 26 | 25 | 11 | 15 | 4 | |
| INNN | MX | 17 | 17 | 7 | — | — | — | |
| bvFTD | CICA | CL | 1 | 1 | — | — | — | — |
| GERO | CL | 23 | 23 | 15 | — | — | — | |
| INNN | MX | 4 | 4 | — | — | — | — | |
| CICA | CL | 1 | 1 | — | — | — | — | |
| UCIDP-IPN | PE | 1 | 1 | — | — | — | — | |
| CNC-UdeSA | AR | 39 | 39 | 37 | 35 | 12 | 1 | |
| AI-PUJB | CO | 81 | 80 | 69 | 79 | — | — | |
| GERO | CL | 13 | 13 | 12 | 11 | 3 | 3 | |
| PD | UV | CO | 25 | 25 | — | — | — | — |
| CNC-UdeSA | AR | 9 | 9 | 9 | 9 | 5 | 2 | |
| GERO | CL | 21 | 21 | 21 | 21 | 20 | 1 | |
| SM | CNC-UdeSA | AR | 31 | 31 | 30 | — | 31 | 1 |
| HCs | INCMN | MX | 2 | 2 | — | — | — | — |
| UCIDP-IPN | PE | 1 | 1 | — | — | — | — | |
| CNC-UdeSA | AR | 3 | 3 | 3 | — | — | — | |
| GERO | CL | 50 | 50 | 0 | 4 | — | — | |
| CICA | CL | 28 | 28 | 28 | 28 | — | — | |
| UCIDP-IPN | PE | 6 | 6 | 0 | 0 | — | — | |
| CNC-UdeSA | AR | 72 | 72 | 72 | 63 | 17 | 1 | |
| AI-PUJB | CO | 46 | 45 | 46 | 45 | — | — | |
| GERO | CL | 25 | 25 | 25 | 25 | 13 | 11 | |
Imaging information is presented for anatomical (MRI), functional (fMRI), and diffusion-weighted (DWI) resonance magnetic imaging. The magnetic field of the scan (scan) is presented. Codes for the recruitment sites are CICA: Centro de Investigación Clínica Avanzada (CICA) Hospital Clínico Universidad de Chile; Santiago, Chile; UCIDP-IPN: Unit Cognitive Impairment and Dementia Prevention, Peruvian Institute of Neurosciences, Lima, Peru; GERO: Neurology Department, Geroscience Center for Brain Health and Metabolism, Santiago, Chile; INNN: Instituto Nacional de Neurología y Neurocirugía, Ciudad de México, Mexico, CNC-UdeSA: Centro de Neurociencia Cognitiva, Universidad de San Andrés, Buenos Aires, Argentina; AI-PUJB: Aging Institute, Pontificia Universidad Javeriana, Bogotá, Colombia; INCMN: Geriatrics Department, Instituto Nacional de Ciencias médicas y nutrición Salvador Zubirán, Mexico City. The country codes meet the standards of the International Organization for Standardization (ISO). CL: Chile, PE: Peru, CO: Colombia, MX: Mexico, Argentina: AR. N: number of subjects, AD: Alzheimer’s disease, bvFTD: behavioral variant frontotemporal dementia, PD: Parkinson’s disease, MS: Multiple sclerosis, HCs: healthy controls.
EEG recordings
Both EEG acquisition and processing parameters are summarized in Table 6. Participants were seated in a comfortable chair inside a dimly lit, sound-attenuated, and electromagnetically shielded EEG room and instructed to remain still and awake. Ongoing (resting-state), eyes-closed EEG was recorded for ten minutes using the same amplifier across centers, a 128-channel Biosemi Active-two acquisition system (pin-type active, sintered Ag-AgCl electrodes). The reference electrodes were set to linked mastoids. Furthermore, external electrodes were placed in periocular locations to record blinks and eye movements. Analog filters were set at 0.03 and 100 Hz. The EEG was monitored online for detecting drowsiness, and myogenic and sweat artifacts.
Table 6.
Equipment and technical parameters for EEG acquisition and processing.
| Acquisition | Acquisition system | Biosemi Active II |
| Electrode layout | Biosemi 128 | |
| Reference choice | Linked mastoids | |
| Analog filters (frequency cutoff) | 0.03–100 Hz | |
| Processing | Digital filters (frequency cutoff) | 0.5 – 40 Hz |
| Sampling rate | 512 Hz | |
| Reference choice | Average reference |
The EEG was processed offline using an in-house pipeline built upon pre-existing EEGLab functions85. Only basic steps were implemented (i.e., re-referencing, filtering, and eliminating bad channels) to allow dataset users to conduct custom analyses. The row data (*.bdf extension) was imported into EEGLab using the BDFimport plugging and processed in the *.set extension (default EEGLab extension). Recordings were re-referenced to the average of all channels (average reference), and band-pass filtered between 0.5 and 40 Hz using a zero-phase shift Butterworth filter of order = 8. Data were down sampled to 512 Hz, and Independent Component Analysis (ICA) was used to correct EEG artifacts induced by blinking and eye movements. Malfunctioning channels were identified using a semiautomatic detection method and replaced using weighted spherical interpolation.
MRI acquisition
The MRI neuroimages were acquired with 1.5 or 3 Tesla scanners. The list of scanner models and institutions can be found in Table 7. T1-MPRAGE anatomical scans were acquired using a T1-weighted volumetric magnetization-prepared rapid gradient echo sequence. Diffusion and T2-FLAIR images were obtained through T2- and diffusion-weighted images, respectively. The number of slices depended on the acquisition protocol. Resting-state functional MRI completed eyes-open resting state multi-echo BOLD functional scans. Participants were instructed to remain still, keeping their eyes open, with normal breathing to reduce motion artifacts. Resting-state data were recorded using a multi-echo EPI sequence. While individual information has not been incorporated within the main body of the text due to its substantial volume, the details of the acquisition parameters for all subjects are available in the *.json files.
Table 7.
Equipment used for the MRI acquisition.
| MF (T) | Manufacturer | Model |
|---|---|---|
| 1.5 | GE | Signa HDxt |
| Siemens | Symphony | |
| Aera | ||
| Avanto | ||
| Philips | Achieva | |
| Prodiva CX | ||
| Intera | ||
| 3.0 | Siemens | Skyra |
| Verio | ||
| Philips | Achieva | |
| Ingenia |
Data Records
The neuroimaging data is hosted in the Synapse project “BrainLat-dataset”28. This is accompanied by the anonymized demographic information, and both cognitive and functional outcomes. Information is presented in *csv files (plain text, comma-separated values). Additionally, a dictionary containing all column headers from the demographic, cognitive, and neuroimaging csv files has been included in Synapse.
The neuroimaging data is organized according to the Brain Imaging Data Structure (BIDS) specifications86 to address the heterogeneity of data organization and follow the FAIR principles of findability, accessibility, and interoperability87 while protecting personal information. Initially developed to organize MRI data, the BIDS format has been extended to other neuroimaging modalities, including EEG. Accordingly, EEG data was converted into EEG-BIDS88. Conversion of the original files (i.e., e *.dcm for MRI and *.set for EEG) into the BIDS format was made using BIDScoin (for MRI)89 and the BIDS EEGLAB plugging88 (for EEG). For cases where MRI and EEG data were available from the same participant, the -MRI-BIDS and EEG-BIDS were combined in a single structure. The BIDS structures were validated using BIDS Validator v1.11.0 (https://bids-standard.github.io/bids-validator/). Personal information was removed from the EEG recordings during the EEG-BIDS conversion. The different MRI data were defaced using PyDeface 2.0.0 via Docker v4.12.0 (https://github.com/poldracklab/pydeface).
An example of the directory tree after structuring files according to the BIDS format is presented in Fig. 2. Participants’ data from the same group are stored in the same folder. For a given participant, the data of the different neuroimaging modalities are presented separately, being subfolders named “anat”, “func”, “dwi”, and “eeg”. The name of the files containing the data begins with the “sub-“ index, followed by the letter “P” and two letters referring to the PI responsible for the data acquisition (indicating the recruitment site). The name ends with the number of the subject (e.g., “00035”), followed by a string of characters indicating the neuroimaging modality. In individual folders, the files *.json contain information about the dataset and participants.
Fig. 2.
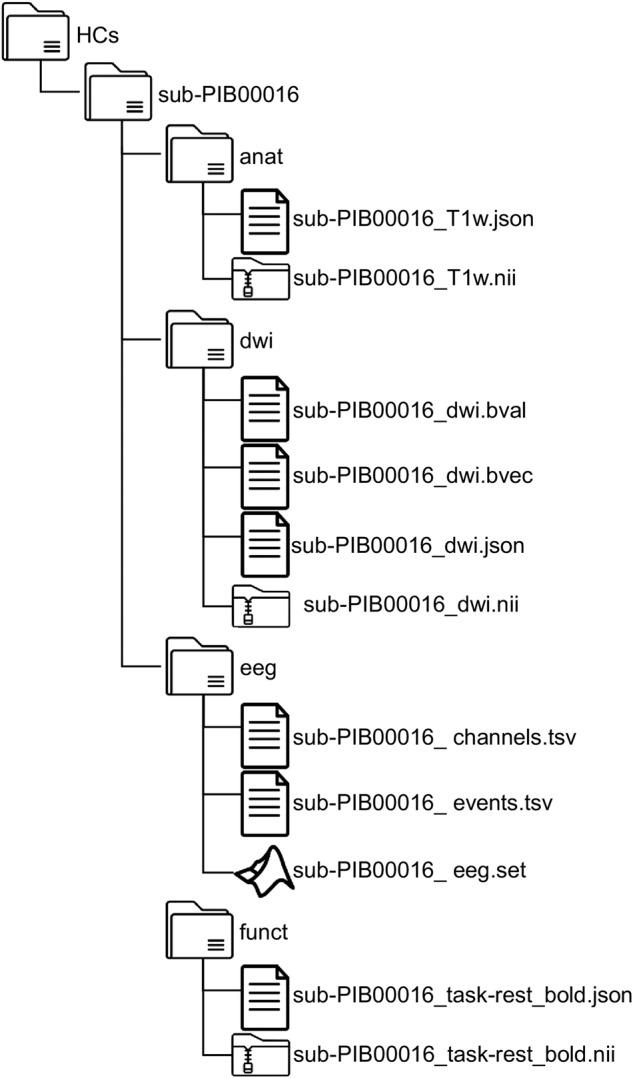
Illustrative diagram of the BrainLat dataset’s directory tree, organized according to the BIDS format. For MRI data, anatomical (anat), diffusion-weighted (dwi), and functional (funct) images are stored in specific files. The same applies to the EEG data.
Technical Validation
Quality checks included the implementation of standardized protocols for recruitment and psychophysiological assessment and quality control during the acquisition of neuroimaging data.
Recruitment
The recruitment comprised the following steps a) selection of HCs controls within the expected range; b) identification of the required control profiles to maintain SD < 2-3 for each match; c) searching for controls to meet the required parameters, such that HCs were matched for age, sex, and education with patients.
Diagnosis and psychological assessment
Multidisciplinary teams made the diagnosis as part of an ongoing multicentric protocol38,90. The cognitive and functional status were assessed following the standard protocols implemented by ReDLat30. Evaluators received a clinical certification from board-certified neurologists after completing training and a monitoring process to use standard procedures.
EEG
Incidences during the EEG acquisition were annotated for further visual inspection. Bad channels were detected using semiautomatic algorithms based on threshold amplitude. Automatic channel rejection and interpolation were implemented. On average, 3.2 ± 1.1 channels were interpolated per recording. Certified experts supervised the quality of the recording.
MRI
The quality control metrics for the T1w and functional BOLD MRI scans were computed by the MRIQC package91, which outputs several quality control metrics of different aspects of the data. These quality control metrics are stored in group_T1w.tsv and group_bold.tsv in the derivatives/mriqc folder.
Acknowledgements
The authors thank the ReDLat members as well as the patients and their relatives. The authors recognize the contributors of clinical investigators from Argentina (CNC-UdeSA), Chile (GERO, GERO), Colombia (AI-PUJB, UV), Mexico (INNN, INCMN), and Peru (UCIDP-IPN) that provided support for data collection and access. AI is partially supported by grants from ANID/FONDECYT Regular (1210195 and 1210176 and 1220995); ANID/FONDAP/15150012; ANID/PIA/ANILLOS ACT210096; FONDEF ID20I10152; ANID/FONDAP 15150012; Takeda CW2680521 and the MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA [ReDLat, supported by Fogarty International Center (FIC) and National Institutes of Health, National Institutes of Aging (R01 AG057234, R01 AG075775, R01 AG21051, CARDS-NIH), Alzheimer’s Association (SG-20-725707), Rainwater Charitable foundation – Tau Consortium, the Bluefield Project to Cure Frontotemporal Dementia, and Global Brain Health Institute)]. PP is partially supported by the USS grant USS-FIN-23-FAPE-09; AG is an Atlantic Fellow at the Global Brain Health Institute (GBHI) and is supported with funding from GBHI, Alzheimer’s Association, and Alzheimer’s Society (Alzheimer’s Association GBHI ALZ UK-22-865742); ANID (FONDECYT Regular 1210176); and Programa Interdisciplinario de Investigación Experimental en Comunicación y Cognición (PIIECC), Facultad de Humanidades, USACH. We also acknowledge the support from BrainLat via the Seed Grant BL-SRGP2020-02 awarded to MAP and AI in collaboration with FL and AG. The contents of this publication are solely the responsibility of the authors and do not represent the official views of these institutions. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
A.I. conceived the study and is the founder of the ReDLat consortium; A.I. and M.P. created the E.E.G. consortia; A.I., P.P., V.M. designed the study; S.M., J.M., R.G. provided data acquisition methods; H.S.M., F.L., D.A., A.B., A.S., A.L., S.F. and M.I.B. facilitated data collection, P.P., V.M., R.G., A.S., J.M., F.L., A.B. processed, analyzed, and organized the data; P.P., V.M. and A.I. wrote the paper. All authors contributed to the final version of the manuscript and approved the submission.
Code availability
This dataset28 only comprised raw data. No codes were generated for the creation of the repository. Data processing and generation of BIDS files were done with freely available software.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Pavel Prado, Vicente Medel.
Change history
1/2/2024
A Correction to this paper has been published: 10.1038/s41597-023-02870-0
References
- 1.Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. The Lancet Global Health. 2019;7:e596–e603. doi: 10.1016/S2214-109X(19)30074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston G, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santamaria-Garcia H, et al. Factors associated with healthy aging in Latin American populations. Nat Med. 2023;29:2248–2258. doi: 10.1038/s41591-023-02495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibáñez A, et al. Dementia caregiving across Latin America and the Caribbean and brain health diplomacy. The Lancet Healthy Longevity. 2021;2:e222–e231. doi: 10.1016/S2666-7568(21)00031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribeiro FS, Teixeira-Santos AC, Leist AK. The prevalence of mild cognitive impairment in Latin America and the Caribbean: a systematic review and meta-analysis. Aging Ment Health. 2022;26:1710–1720. doi: 10.1080/13607863.2021.2003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Custodio N, Wheelock A, Thumala D, Slachevsky A. Dementia in Latin America: Epidemiological Evidence and Implications for Public Policy. Front Aging Neurosci. 2017;9:221. doi: 10.3389/fnagi.2017.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibanez, A. et al. The impact of SARS‐CoV‐2 in dementia across Latin America: A call for an urgent regional plan and coordinated response. A&D Transl Res & Clin Interv6, (2020). [DOI] [PMC free article] [PubMed]
- 9.Duran-Aniotz C, et al. Systematic Review: Genetic, Neuroimaging, and Fluids Biomarkers for Frontotemporal Dementia Across Latin America Countries. Front Neurol. 2021;12:663407. doi: 10.3389/fneur.2021.663407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.for The Latin America and the Caribbean Consortium on Dementia (LAC-CD) Ibanez A, Parra MA, Butler C. The Latin America and the Caribbean Consortium on Dementia (LAC-CD): From Networking to Research to Implementation Science. JAD. 2021;82:S379–S394. doi: 10.3233/JAD-201384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parra MA, et al. Biomarkers for dementia in Latin American countries: Gaps and opportunities. Alzheimer’s & Dementia. 2023;19:721–735. doi: 10.1002/alz.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parra MA, et al. Dementia in Latin America: Paving the way toward a regional action plan. Alzheimer’s & Dementia. 2021;17:295–313. doi: 10.1002/alz.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parra MA, et al. Dementia in Latin America: Assessing the present and envisioning the future. Neurology. 2018;90:222–231. doi: 10.1212/WNL.0000000000004897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibanez A, Kosik KS. COVID-19 in older people with cognitive impairment in Latin America. The Lancet Neurology. 2020;19:719–721. doi: 10.1016/S1474-4422(20)30270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene AS, et al. Brain–phenotype models fail for individuals who defy sample stereotypes. Nature. 2022;609:109–118. doi: 10.1038/s41586-022-05118-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fatumo S, et al. A roadmap to increase diversity in genomic studies. Nat Med. 2022;28:243–250. doi: 10.1038/s41591-021-01672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Precision medicine needs an equity agenda. Nat Med27, 737–737 (2021). [DOI] [PubMed]
- 18.Wojcik GL, et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature. 2019;570:514–518. doi: 10.1038/s41586-019-1310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes AJ, Patrick LM. The Myth of Optimality in Clinical Neuroscience. Trends in Cognitive Sciences. 2018;22:241–257. doi: 10.1016/j.tics.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SM, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18:1565–1567. doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravindranath V, et al. Regional research priorities in brain and nervous system disorders. Nature. 2015;527:S198–S206. doi: 10.1038/nature16036. [DOI] [PubMed] [Google Scholar]
- 22.Duncan L, et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun. 2019;10:3328. doi: 10.1038/s41467-019-11112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pievani M, Filippini N, Van Den Heuvel MP, Cappa SF, Frisoni GB. Brain connectivity in neurodegenerative diseases—from phenotype to proteinopathy. Nat Rev Neurol. 2014;10:620–633. doi: 10.1038/nrneurol.2014.178. [DOI] [PubMed] [Google Scholar]
- 24.Pievani M, Pini L, Cappa SF, Frisoni GB. Brain networks stimulation in dementia: insights from functional imaging. Current Opinion in Neurology. 2016;29:756–762. doi: 10.1097/WCO.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 25.Ibanez A. The mind’s golden cage and cognition in the wild. Trends in Cognitive Sciences. 2022;26:1031–1034. doi: 10.1016/j.tics.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lord L-D, Stevner AB, Deco G, Kringelbach ML. Understanding principles of integration and segregation using whole-brain computational connectomics: implications for neuropsychiatric disorders. Phil. Trans. R. Soc. A. 2017;375:20160283. doi: 10.1098/rsta.2016.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luppi AI, et al. Dynamical models to evaluate structure–function relationships in network neuroscience. Nat Rev Neurosci. 2022;23:767–768. doi: 10.1038/s41583-022-00646-w. [DOI] [PubMed] [Google Scholar]
- 28.Prado P, 2023. BrainLat-dataset. Synapse. [DOI]
- 29.Duran‐Aniotz C, et al. The Latin American Brain Health Institute, a regional initiative to reduce the scale and impact of dementia. Alzheimer’s & Dementia. 2022;18:1696–1698. doi: 10.1002/alz.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibanez A, et al. The Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat): Driving Multicentric Research and Implementation Science. Front. Neurol. 2021;12:631722. doi: 10.3389/fneur.2021.631722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maito MA, et al. Classification of Alzheimer’s disease and frontotemporal dementia using routine clinical and cognitive measures across multicentric underrepresented samples: a cross sectional observational study. The Lancet Regional Health - Americas. 2023;17:100387. doi: 10.1016/j.lana.2022.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birba A, et al. Allostatic-Interoceptive Overload in Frontotemporal Dementia. Biological Psychiatry. 2022;92:54–67. doi: 10.1016/j.biopsych.2022.02.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birba A, et al. Multimodal neurocognitive markers of naturalistic discourse typify diverse neurodegenerative diseases. Cerebral Cortex. 2022;32:3377–3391. doi: 10.1093/cercor/bhab421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prado P, et al. Source space connectomics of neurodegeneration: One-metric approach does not fit all. Neurobiology of Disease. 2023;179:106047. doi: 10.1016/j.nbd.2023.106047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cruzat J, et al. Temporal Irreversibility of Large-Scale Brain Dynamics in Alzheimer’s Disease. J. Neurosci. 2023;43:1643–1656. doi: 10.1523/JNEUROSCI.1312-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legaz A, et al. Multimodal mechanisms of human socially reinforced learning across neurodegenerative diseases. Brain. 2022;145:1052–1068. doi: 10.1093/brain/awab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herzog R, et al. Genuine high-order interactions in brain networks and neurodegeneration. Neurobiology of Disease. 2022;175:105918. doi: 10.1016/j.nbd.2022.105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salamone PC, et al. Interoception Primes Emotional Processing: Multimodal Evidence from Neurodegeneration. J. Neurosci. 2021;41:4276–4292. doi: 10.1523/JNEUROSCI.2578-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fittipaldi S, et al. A multidimensional and multi-feature framework for cardiac interoception. NeuroImage. 2020;212:116677. doi: 10.1016/j.neuroimage.2020.116677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salamone PC, et al. Altered neural signatures of interoception in multiple sclerosis. Hum Brain Mapp. 2018;39:4743–4754. doi: 10.1002/hbm.24319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dottori M, et al. Towards affordable biomarkers of frontotemporal dementia: A classification study via network’s information sharing. Sci Rep. 2017;7:3822. doi: 10.1038/s41598-017-04204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibáñez A, et al. Motor-language coupling: Direct evidence from early Parkinson’s disease and intracranial cortical recordings. Cortex. 2013;49:968–984. doi: 10.1016/j.cortex.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Hazelton JL, et al. Thinking versus feeling: How interoception and cognition influence emotion recognition in behavioural-variant frontotemporal dementia, Alzheimer’s disease, and Parkinson’s disease. Cortex. 2023;163:66–79. doi: 10.1016/j.cortex.2023.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibañez A, et al. Predicting and Characterizing Neurodegenerative Subtypes with Multimodal Neurocognitive Signatures of Social and Cognitive Processes. JAD. 2021;83:227–248. doi: 10.3233/JAD-210163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrevaya S, et al. The Road Less Traveled: Alternative Pathways for Action-Verb Processing in Parkinson’s Disease. JAD. 2016;55:1429–1435. doi: 10.3233/JAD-160737. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez Campo C, et al. Fatigue in multiple sclerosis is associated with multimodal interoceptive abnormalities. Mult Scler. 2020;26:1845–1853. doi: 10.1177/1352458519888881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abrevaya S, et al. At the Heart of Neurological Dimensionality: Cross-Nosological and Multimodal Cardiac Interoceptive Deficits. Psychosom Med. 2020;82:850–861. doi: 10.1097/PSY.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moguilner S, et al. Visual deep learning of unprocessed neuroimaging characterises dementia subtypes and generalises across non-stereotypic samples. eBioMedicine. 2023;90:104540. doi: 10.1016/j.ebiom.2023.104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Cordero I, et al. Metacognition of emotion recognition across neurodegenerative diseases. Cortex. 2021;137:93–107. doi: 10.1016/j.cortex.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanz Perl Y, et al. Model-based whole-brain perturbational landscape of neurodegenerative diseases. eLife. 2023;12:e83970. doi: 10.7554/eLife.83970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moguilner S, et al. Multi-feature computational framework for combined signatures of dementia in underrepresented settings. J. Neural Eng. 2022;19:046048. doi: 10.1088/1741-2552/ac87d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perl, Y. S. et al. The impact of regional heterogeneity in whole-brain dynamics in the presence of oscillations. Network Neuroscience 1–29, 10.1162/netn_a_00299 (2023). [DOI] [PMC free article] [PubMed]
- 53.Díaz-Rivera MN, et al. Multidimensional inhibitory signatures of sentential negation in behavioral variant frontotemporal dementia. Cerebral Cortex. 2022;33:403–420. doi: 10.1093/cercor/bhac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moguilner S, et al. Dynamic brain fluctuations outperform connectivity measures and mirror pathophysiological profiles across dementia subtypes: A multicenter study. NeuroImage. 2021;225:117522. doi: 10.1016/j.neuroimage.2020.117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachli MB, et al. Evaluating the reliability of neurocognitive biomarkers of neurodegenerative diseases across countries: A machine learning approach. NeuroImage. 2020;208:116456. doi: 10.1016/j.neuroimage.2019.116456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moguilner S, et al. Weighted Symbolic Dependence Metric (wSDM) for fMRI resting-state connectivity: A multicentric validation for frontotemporal dementia. Sci Rep. 2018;8:11181. doi: 10.1038/s41598-018-29538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sedeño L, et al. Tackling variability: A multicenter study to provide a gold-standard network approach for frontotemporal dementia: Multicenter Assessment of FC in bvFTD. Hum. Brain Mapp. 2017;38:3804–3822. doi: 10.1002/hbm.23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santamaría-García H, et al. A lesion model of envy and Schadenfreude: legal, deservingness and moral dimensions as revealed by neurodegeneration. Brain. 2017;140:3357–3377. doi: 10.1093/brain/awx269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melloni M, et al. Your perspective and my benefit: multiple lesion models of self-other integration strategies during social bargaining. Brain. 2016;139:3022–3040. doi: 10.1093/brain/aww231. [DOI] [PubMed] [Google Scholar]
- 60.Parra‐Rodriguez, M. A. et al. The EuroLaD‐EEG consortium: towards a global EEG platform for dementia, for seeking to reduce the regional impact of dementia. Alzheimer’s & Dementia18, (2022).
- 61.Varma-Doyle AV, Lukiw WJ, Zhao Y, Lovera J, Devier D. A hypothesis-generating scoping review of miRs identified in both multiple sclerosis and dementia, their protein targets, and miR signaling pathways. J Neurol Sci. 2021;420:117202. doi: 10.1016/j.jns.2020.117202. [DOI] [PubMed] [Google Scholar]
- 62.Giovannoni G. Should we rebrand multiple sclerosis a dementia? Mult Scler Relat Disord. 2017;12:79–81. doi: 10.1016/j.msard.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Li H, et al. The role of plasma cortisol in dementia, epilepsy, and multiple sclerosis: A Mendelian randomization study. Front Endocrinol (Lausanne) 2023;14:1107780. doi: 10.3389/fendo.2023.1107780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luczynski P, Laule C, Hsiung G-YR, Moore GRW, Tremlett H. Coexistence of Multiple Sclerosis and Alzheimer’s disease: A review. Mult Scler Relat Disord. 2019;27:232–238. doi: 10.1016/j.msard.2018.10.109. [DOI] [PubMed] [Google Scholar]
- 65.Ibanez A, Zimmer ER. Time to synergize mental health with brain health. Nat. Mental Health. 2023;1:441–443. doi: 10.1038/s44220-023-00086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ibáñez A, Legaz A, Ruiz-Adame M. Addressing the gaps between socioeconomic disparities and biological models of dementia. Brain. 2023;146:3561–3564. doi: 10.1093/brain/awad236. [DOI] [Google Scholar]
- 67.Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. 2023;8:267. doi: 10.1038/s41392-023-01486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao C, Jiang J, Tan Y, Chen S. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct Target Ther. 2023;8:359. doi: 10.1038/s41392-023-01588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rascovsky K, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery & Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes CP, Berg L, Danziger W, Coben LA, Martin RL. A New Clinical Scale for the Staging of Dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 73.Knopman DS, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results: MDS-UPDRS: Clinimetric Assessment. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 75.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 76.Goetz CG, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations The Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 77.Roxburgh RHSR, et al. Multiple Sclerosis Severity Score: Using disability and disease duration to rate disease severity. Neurology. 2005;64:1144–1151. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- 78.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1444. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 79.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment: MOCA: A BRIEF SCREENING TOOL FOR MCI. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 80.Torralva T, Roca M, Gleichgerrcht E, López P, Manes F. INECO Frontal Screening (IFS): A brief, sensitive, and specific tool to assess executive functions in dementia–CORRECTED VERSION. J Int Neuropsychol Soc. 2009;15:777–786. doi: 10.1017/S1355617709990415. [DOI] [PubMed] [Google Scholar]
- 81.Ekman, P. & Friesen, W. V. Unmasking the face: a guide to recognizing emotions from facial clues. (Prentice-Hall, 1975).
- 82.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of Functional Activities in Older Adults in the Community. Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 83.Mioshi E, et al. The Impact of Dementia Severity on Caregiver Burden in Frontotemporal Dementia and Alzheimer Disease. Alzheimer Disease & Associated Disorders. 2013;27:68–73. doi: 10.1097/WAD.0b013e318247a0bc. [DOI] [PubMed] [Google Scholar]
- 84.Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74:1591–1597. doi: 10.1212/WNL.0b013e3181e04070. [DOI] [PubMed] [Google Scholar]
- 85.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 86.Gorgolewski KJ, et al. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data. 2016;3:160044. doi: 10.1038/sdata.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilkinson MD, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3:160018. doi: 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pernet CR, et al. EEG-BIDS, an extension to the brain imaging data structure for electroencephalography. Sci Data. 2019;6:103. doi: 10.1038/s41597-019-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zwiers MP, Moia S, Oostenveld R. BIDScoin: A User-Friendly Application to Convert Source Data to Brain Imaging Data Structure. Front. Neuroinform. 2022;15:770608. doi: 10.3389/fninf.2021.770608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Donnelly‐Kehoe PA, et al. Robust automated computational approach for classifying frontotemporal neurodegeneration: Multimodal/multicenter neuroimaging. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2019;11:588–598. doi: 10.1016/j.dadm.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Esteban O, et al. MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One. 2017;12:e0184661. doi: 10.1371/journal.pone.0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Prado P, 2023. BrainLat-dataset. Synapse. [DOI]
Data Availability Statement
This dataset28 only comprised raw data. No codes were generated for the creation of the repository. Data processing and generation of BIDS files were done with freely available software.