Abstract
Response to antipsychotic medications (AP) is subjected to a wide and unpredictable variability and efforts were directed to discover predictive biomarkers to personalize treatment. Electroencephalography abnormalities in subjects with schizophrenia are well established, as well as a pattern of EEG changes induced by APs. The aim of this review is to provide a synthesis of the EEG features that are related to AP efficacy, including both pre-treatment signatures and changes induced by APs during treatment. A systematic review of English articles using PubMed, PsychINFO and the Cochrane database of systematic reviews was undertaken until july 2023. Additional studies were added by hand search. Studies having as an endpoint the relationship between AP-related clinical improvement and electroencephalographic features were included. Heterogeneity prevented a quantitative synthesis. Out of 1232 records screened, 22 studies were included in a final qualitative synthesis. Included studies evaluated resting-state and task-related power spectra, functional connectivity, microstates and epileptic abnormalities. At pre-treatment resting-state EEG, the most relevant predictors of a poor response were a change in theta power compared to healthy control, a high alpha power and connectivity, and diminished beta power. Considering EEG during treatment, an increased theta power, a reduced beta-band activity, an increased alpha activity, a decreased coherence in theta, alpha and beta-band were related to a favorable outcome. EEG is promising as a method to create a predictive biomarker for response to APs; further investigations are warranted to harmonize and generalize the contradictory results of reviewed studies.
Subject terms: Biomarkers, Neural circuits
Introduction
Antipsychotic medications (APs) are serotonin and/or dopamine receptor ligands exerting a widespread modulatory effect on the cerebral cortex and on the dopaminergic and serotoninergic subcortical projective systems. APs represent the cornerstone in the treatment of patients with schizophrenia (SZ) spectrum disorders and are among the main options for bipolar disorder and treatment-resistant depression1,2. Response rates to APs for SZ range from 47% for individuals who have received prior treatments to 66% for antipsychotic-naïve individuals3 while 1-year discontinuation rates reach 74% due to poor tolerability and/or efficacy4. For this reason, efforts based on several fields of experimental medicine (e.g., genomics, proteomics, neuroimaging) were directed to discover predictive biomarkers to anticipate therapeutic failures, but have so far been unsuccessful5,6.
Electroencephalography (EEG) is a neuroimaging technique, with very high temporal resolution and good spatial resolution of the whole brain activity caught in its essential nature of an ensemble of electrical oscillations7. In fact, quantitative EEG measures have been associated with many cognitive, motivational, sensorimotor, and emotional processes8–16
Typical EEG patterns were defined for many mental disorders; for SZ they include changes in power spectra in multiple frequency bands17–34, functional connectivity18,35–41, symmetry of the signal42–48, microstates (i.e. successive short time periods during which the configuration of the scalp potential field remains semi-stable)49 and evoked potentials (i.e. averaged electrical potentials aligned to repeated presentations of a stimulus)50–52. These EEG changes were also related to at-risk mental states and early stages of SZ53.
A general pattern of AP-induced EEG changes was described with the common features of an increase in delta and theta spectral-power20,54–60. Moreover, increased and decreased functional connectivity in AP-medicated patients were discovered in widespread brain areas across all frequency bands61. APs can be divided into first-generation APs, whose therapeutic effect is exerted through an antagonism to D2 dopamine receptor, second-generation APs, whose effect is also due the binding to serotonin receptors 5HT2A and 5HT1A and third-generation APs, characterized by a partial agonism to D2 and D3 dopamine receptors62. EEG changes specifically related to each category were described. Low-potency first-generation APs provoked a decrease of alpha and an increase of delta, theta and, less consistently, fast-beta activities. On the contrary, high-potency first-generation APs increased alpha and low-beta and decreased fast-beta frequencies58,63–68. However, these findings are contradictory, since other studies indicated an overall decrease in delta63,69,70 theta and alpha69 power and that chronic treatment induce a slowing of delta activity20. Concerning specific APs, Clozapine (CLZ) administration increased delta, theta and fast-beta and decreased alpha and slow-beta power in the resting-state71–76. An increased amplitude of the P300 component in evoked potentials was reported as well77,78. Olanzapine induced a similar array of changes, with an increase in theta and a decrease in alpha and beta power72. Concerning gamma frequencies, one report described that an atypical AP normalizes 30-50 Hz low-gamma power79, however, another study found no significant relationships in this respect80.
Overall, there is solid evidence for EEG alterations in patients with SZ and for AP-induced changes. However, the application of these findings to the clinic remains limited. As outlined above, one of the central challenges in the treatment of SZ and bipolar disorder is the prediction of AP response. Although several studies have addressed this research question, the literature remains limited and has to our knowledge not been systematically reviewed.
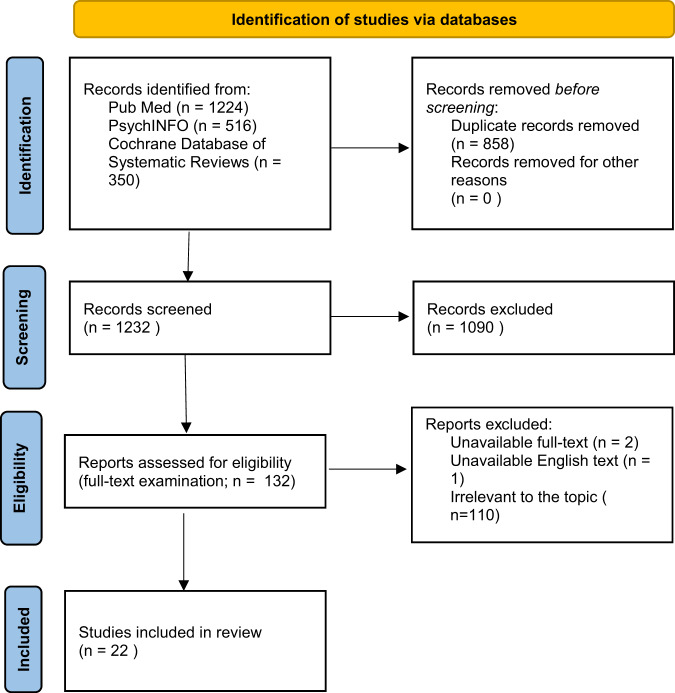
The aim of the present paper was to systematically review all the EEG features related to AP efficacy, including both pre-treatment and during-treatment recordings. The final target was to gain an insight over the possibility for EEG to become an instrument useful to personalize AP treatment (Fig. 1).
Fig. 1. PRISMA flowchart of included studies.
PRISMA 2020 flowchart diagram.
Methods
Study design
The Preferred Reporting Items for Systematic Reviews (PRISMA) statement has been followed to design and conduct the systematic review. We performed a comprehensive literature search on EEG signatures predicting the response to APs or associated to AP treatment outcome. A review protocol was enregistered in PROSPERO (CRD42023450068).
Article search strategy
A systematic literature search was conducted in three electronic databases: PubMed, PsycINFO and Cochrane Database of Systematic Reviews since inception to 31st July 2023 with no time limit and with English language as the only selected filter.
The following combination of search terms was used:
(“EEG” OR “electroencephalography” OR “Pharmaco-EEG” OR “EEG microstate” OR “dipole source localization” OR sLORETA OR LORETA OR eLORETA OR ERP OR “event-related potential” OR “spectral analysis” OR “frequency domain analysis” OR “spectral band” OR “neural oscillations” OR “spectral power” OR N100 OR N1 OR MMN OR “mismatch negativity” OR P300 OR P3a OR P3b OR “event-related” OR “evoked potential” OR “evoked-response”) AND (“antipsychotic*“ OR “clozapine” OR “risperidone” OR “olanzapine” OR “quetiapine” OR “paliperidone” OR “amisulpride” OR “aripiprazole” OR “brexpiprazole” OR “cariprazine” OR “lurasidone” OR “ziprasidone” OR “haloperidol” OR “zuchlopentixol” OR “chlorpromazine” OR “perphenazine”) AND (“response” OR “efficacy” OR “outcome” OR “effectiveness” OR “efficiency”).
Selection process and criteria
Firstly, any duplicate from the combination of the three databases was excluded. The remaining articles were included in the systematic review only if they met the following criteria:
Inclusion criteria
meta-analysis, reviews, clinical trials, case-control and cohort studies;
studies carried out in humans;
studies published in English;
studies conducted on patients affected by a disorder included in the DSM5 chapter “schizophrenia spectrum disorder” and/or “bipolar disorders”;
studies reporting on the relationship between EEG and AP efficacy, including both EEG features of pre-treatment recordings predicting response, and EEG features in a during-treatment recording, related to treatment outcome.
Exclusion criteria
books chapters, comments, editorials, case reports, theses, proceedings, letters, short surveys, notes;
studies irrelevant to the topic;
Two researchers (MDP, VR) independently screened for eligibility all the articles by titles and abstracts and then proceeded to read the full text. Any disagreements were resolved by consensus or by the decision of a third reviewer.
Data extraction
MDP and VR recorded the following variables from each included article: author/s, year of publication, socio-demographic and clinical features, assessment instruments for diagnosis, current and past pharmacological treatments, EEG methods and indexes and EEG data results (Tables 1–3).
Table 1.
Sociodemographic and clinical features.
| Reference | N | Diagnosis | Age | Sex (F) | Education level | Duration of illness (months) | Age of Onset | Rating scale | Baseline severity |
|---|---|---|---|---|---|---|---|---|---|
| Itil 1981 | 13 | SZ | 49.4 | 0 | Na | 276 | Na | CGI, BPRS | Na |
| Ulrich1988 | 34 | SZ | 32.4 ± 12.1 | 14 | Na | Na | 25 ± 9.3 | BPRS, SZ-specific sub-score | 18.1 ± 7.78 |
| Czobor 1991 | 34 | SZ, SA | 33.8 ± 7.1 | Na | Na | 144 ± 72 | Na | BPRS | 51.1 ± 9.8 |
| Galderisi 1993 | 29 | SZ | 25.3 ± 4.7 | 12 | 8.6 ± 3.5 years | 48 ± 36 | 21.2 ± 3.8 | SANS, SAPS, CPRS | Na |
| Lacroix 1995 | 20 | TRS | 37.2 ± 7 | 10 | Na | 132 ± 60 | Na | BPRS | 71.15 ± 13.92 |
| Risby 1995 | 16 | SZ, SZ | Na | 5 | Na | Na | Na | BPRS | Na |
| Pillay 1996 | 86 | SZ, SA, BD, DM, others | 36.15 ± 10.98 | 48 | Na | Na | Na | CGI, GAF | Na |
| Merlo 1998 | 13 | FEP | 23.4 ± 6.2 | 7 | Na | Na | Na | AMDP | Na |
| Knott 2000 | 17 | TRS | 35.6 ± 8.9 | 1 | 11 ± 3 years | 180 ± 84 | 21 ± 4.5 | PANSS | Na |
| Kang 2001 | 10 | TRS | 31 ± 6.72 | 2 | Na | Na | Na | BPRS | 32 ± 9.1 |
| Gross 2004 | 16 | SZ | 32.6 | 9 | Na | 120 ± nd | Na | PANSSN | 30.3 ± 8.1 |
| PANSSP | 19.3 ± 5.8 | ||||||||
| PANSSG | 53.6 ± 11.4 | ||||||||
| Kikuchi 2005 | 16 | SZ, SZD | 27.3 ± 8.2 | 8 | 13.8 ± na years | 26.6 ± 42.4 | Na | BPRS | 52.6 ± 14.8 |
| Wichniak 2006 | 64 | SZ | 26.76 ± 7.84 | 27 | Na | 36 ± 60 | Na | Clinical judgement | / |
| Sumiyoshi 2006 | 5 | SZ | 39.2 ± 10.8 | 1 | 13.6 ± 2.2 years | 168 ± 84 | Na | BPRS | 14.6 ± 13.6 |
| AVLT | 19.8 ± 10.5 | ||||||||
| GAF | 55 ± 6.1 | ||||||||
| Kikuchi 2007 | 21 | SZ, SZD | 29.5 | 10 | Na | 23.6 | Na | BPRS | 56.2 |
| Khodayari-Rostamabad 2010 | 37 | TRS | 39.12 ± 9.3 | 17 | *3.2 ± 1.48 | Na | 21.2 ± 5 | PANSS, QCA | Na |
| Ravan 2014 | 47 | TRS | 37.3 ± 9.44 | 18 | Na | Na | Na | PANSS | Na |
| Mitra 2015 | 15 | SZ | 28.87 ± 6.81 | 3 |
*1 13.3% 2 46.7% 3 6.7% 6 33.3% |
55.13 | Na | PANSS | 83.87 ± 15.9 |
| PANSSP | 27.13 ± 7.46 | ||||||||
| PANNSN | 21.6 ± 10.3 | ||||||||
| PANSSG | 35.6 ± 8.26 | ||||||||
| Masychev 2020 | 62 | TRS | 37.3 ± 8.9 | 25 | *3 ± 0.78 | Na | 19.75 ± 2.75 | PANSSN | 30.72 ± 4.79 |
| PANSSP | 27.93 ± 5.8 | ||||||||
| PANSSG | 60.45 ± 8.4 | ||||||||
| Arikan 2021 | 24 | BD | 36.75 ± 13.09 | 13 | Na | Na | Na | YMRS | 6.26 ± 7.58 |
| Ciprian 2021 | 57 | TRS | 36.95 ± 922 | 20 | Na | Na | Na | PANSS | Na |
| Dominicus 2023 | 62 | FEP | 23.2 ± 4.7 | 29 |
*1 0% 2 9.7%, 3 61.3%, 4 19.4% |
11 ± 14.3 | Na | PANSS | 74.53 ± 16.97 |
| PANSSN | 27.5 ± 9.01 | ||||||||
| PANSSP | 18.47 ± 6.99 | ||||||||
| PANSSG | 18.57 ± 4.38 |
N number of patients, Na data not available, / not-applicable, AMDP Association of Methodology and Documentation in Psychiatry, PANSS positive and negative syndrome scale; PANSSG PANSS general psychopathology scale, PANSSN PANSS negative scale, PANSSP PANSS positive scale, BPRS brief psychiatric rating scale, YMRS Young mania rating scale, AVLT auditory verbal learning test, CGI clinical global impression; GAF global assessment of functioning, SANS scale for the assessment of negative symptoms, SAPS scale for the assessment of positive symptoms, CPRS comprehensive psychopathological rating scale, QCA quantitative clinical assessment questionnaire, TRS treatment-resistant schizophrenia, BD bipolar disorder; FEP first episode of psychosis, SZF schizophreniform disorder, SA schizo-affective disorder, MD major depression. Baseline severity of disease is expressed as the score of the corresponding rating scale, *education level expressed in categories: 1 grade 6 or less, 2 grade 7 to 12 without graduating, 3 graduated high-school, 4 admission to college, 5 graduate 2 years college, 6 graduate 4 years college, 7 part graduated/professional school, 8 completed graduated professional school.
Table 3.
EEG methods and statistics.
| Author year | Study design | EEG recording | Montage | Paradigm | EEG measures | Statistic test |
|---|---|---|---|---|---|---|
| Itil 1981 | Random order crossover placebo controlled, following washout | Pre-treatment and after 3 hours | Na | Resting-state and evoked potential (listening to 100 msec 1000 hz tones at 60 dB, every 2 sec) |
Absolute power spectra; Time spent in each frequency band |
Na |
| Ulrich 1988 | Cohort | Pre-treatment, 2 h after test dose, 4 h and 4 weeks of treatment | 8 ch | Resting-state (13 min) | Relative alpha power compared to total power spectra |
2-way ANOVA, Moore and Wallis trend test |
| Czobor 1991 | Double-blind crossover, placebo controlled | Pre-treatment, 3 and 6 weeks of treatment |
19 ch, 10-20 system |
Resting-state (10-15 min) | Relative power spectra | Multiple regression (with disease severity and prn drugs) |
| Galderisi 1993 | Cohort, following washout/drug naïve patients | Pre-treatment and 3, 6, 8 hours after test-dose | 21 ch | Resting-state | LAP and LRP power spectra |
Wilcoxon, Mann-Whitney U-test |
| Lacroix 1995 | Cohort | Pre-treatment and during treatment |
19 ch, 10–20 system |
Resting-state | Averaged cross power spectra squared root of power amplitude; coherence |
Wilcoxon, Logistic regression |
| Risby 1995 | Cohort | Pre-treatment and after 6 months of treatment |
21 ch, 10–20 system |
Resting-state | Epileptic abnormalities | Wilcoxon matched pairs signed rank-test, Mann-Whitney U test |
| Pillay 1996 | Cohort | During treatment | Na | Resting-state | Epileptic abnormalities | Mann-whitney U-test, Pearson correlation coefficient |
| Merlo 1998 | Case-control on drug naïve pz | Pre-treatment | 19 ch | Resting-state | Squared root of power spectra | ANOVAR |
| Knott 2000 | Open label case-control, following washout | Pre-treatment and 1.5 h after AP administration |
14 ch, 10–20 system |
Resting-state | Intrahemispheric and interhemispheric asymmetry | Pearson product moment correlation coefficient |
| Kang 2001 | Cohort |
8 ch, 10–20 system |
Resting-state | Non-linear (D2, PLE, MCP) | Wilcoxon signed-rank test | |
| Gross 2004 | Following washout | Pre-treatment and after 1, 3, 10 and 18 weeks of treatment | 21 ch | Resting-state | Theta absolute power spectra | Wilcoxon signed-rank, Spearman’s correlation |
| Kikuchi 2005 | Cohort, following washout or drug naive | Before treatment | 18 ch | Resting-state | CFFB and IAF power spectra | ANOVA, Spearman rank-order correlation |
| Wichniak 2006 | Cohort | During treatment | Na | Resting-state (20 min), after hyperventilation and photic stimulation | Epileptic abnormalities | Na |
| Sumiyoshi 2006 | Pilot case-control | Pre-treatment | 19 ch | Auditory oddball task | P300 CSD, laterality, amplitude | Wilcoxon signed-rank test |
| Kikuchi 2007 | Case-control | Pre-treatment and after 2-8 weeks of treatment |
16 ch, 10–20 system |
Resting-state | K-mean clustering algorithm for microsites, GFP, GFS | Pearson’s correlation coefficients |
| Khodayari-Rostamabad 2010 | Cohort | Pre-treatment |
16 ch, 10–20 system, midline discarded |
resting state (3 * 3.30 min) |
See candidate features, Table 4 |
Na |
| Ravan 2014 | Cohort | Pre-treatment | 20 ch, 10-20 system | Auditory odd-ball task |
See candidate features, Table 4 |
Welch t-test, paired student t-test |
| Mitra 2015 | Case control, on drug naïve or drug-free pz | Pre-treatment, after 4 and 8 weeks of treatment | 192 ch, 10-5 system |
Resting state 3 min |
Averaged power spectra | Mann-Whithney U-test, ANOVA, Friedman’s test, Spearman’s correlation, Bonferroni correction |
| Masychev 2020 | Cohort | Pre-treatment |
20 ch, 10-20 system |
Resting state |
See candidate features, Table 4 |
Na |
| Arikan, 2021 | Cohort | Pretreatment |
19 ch, 10-20 system |
Resting-state | Average absolute power spectra |
Mann-Whitney U- test |
| Ciprian 2021 | Cohort | Pre-treatment | 20 ch | Auditory oddball task |
See candidate features, Table 4 |
Na |
| Dominicus 2023 | Case-control | Pre-treatment | 64 ch | Resting-state |
See candidate features Table 4 |
Random forest regression, Mann-Whitney U-test, Bonferroni correction |
Na data not available, / not applicable or non-realized, ns not significant, Ch EEG channel, Cn-CV consensus nested cross validation, STE symbolic transfer entropy, mRMR minimum redundancy maximum relevance feature selection algorithm, PSD cross power spectrum density, LCMV linearly constrained minimum variance, ANOVA analysis of variance, D2 correlation dimension, PLE primary Lyapunov exponent, MCP mutual cross-prediction, IAF individual alpha frequency, CSD current source density, LORETA low-resolution electromagnetic tomography, GFP global field power, GFS global field synchronization. CFFB conventional fixed frequency band, IAF individual alpha frequency, LAP logarithmic absolute power, LRP logarithmic relative power.
Risk of bias was assessed using the Risk of Bias Assessment tool for Non-randomized Studies (RoBINS)81, including six domains: selection of participants, confounding variables, intervention measurements, blinding of outcome assessment, incomplete outcome data, selective outcome reporting. We classified studies as having low risk of bias if none of these domains was rated as high risk of bias and three or fewer were rated as unclear risk; moderate if four or more were rated as unclear risk; and all other cases were assumed to have high risk of bias.
The Assessing the methodological quality of systematic reviews (AMSTAR 2 checklist)82 was used to assess the quality and completeness of data.
Table 2.
Antipsychotic treatment.
| Reference | Medication | Dose | Definition of response | Response rate | Duration of treatment | Co-medications | Previous antipsychotics |
|---|---|---|---|---|---|---|---|
| Itil 1981 | Molindole | 20 | Na | 42% | 1 week each test | No |
Long-acting fluphenazine Long-acting pipothiazine |
| Thiothixene | 20 | ||||||
| Fluphenazine | 10 | ||||||
| Ulrich 1988 | Perazine | 150 | 66% score decrease | 59% | 28 days | Na | Na |
| Czobor 1991 | Haloperidol | Na | 50% score decrease | Na | Na | Na | Na |
| Galderisi 1993 | Clopenthixol | 3 | 50% score decrease | Na | Single dose | No | Na |
| Haloperidol | 5 | ||||||
| Lacroix 1995 | Clozapine | 25 ± 75 | <30% or >35% score change | 50% | Na | Benzodiazepines procyclidine | Various APs, 850.4 ± 574.8 mg CPZ equivalent dose |
| Risby 1995 | Clozapine | 373.4 ± 99 | Any rating scale score improvement | / | 374 ± 177.4 days | Na | At least 2 AP attempts, minimum dosage 1000 CPZ equivalents/day |
| Pillay 1996 | Clozapine | Na | Any rating scale improvement | Na | Na | Na | Na |
| Merlo 1998 | Na | Na | 30% score decrease | 38% | Na | Na | no |
| Knott 2000 | Clozapine | 381.25 ± 124.33 | 20% score decrease | 84.6% | 6 weeks | Na | Various APs |
| Kang 2001 | Clozapine | 100–350 | 20% score decrease | 40% | 4 weeks | Na | Na |
| Gross 2004 | Clozapine | 50–800 | / | / | 18 weeks | Na | Various first-generation APs |
| Kikuchi 2005 | Haloperidol | 1-12 | Na | Na | 8 weeks |
Biperidene Diazepam Pimozide Estazolam Flunitrazepam perospirone |
Na |
| Risperidone | 2 | ||||||
| Levemepromazine | 25-80 | ||||||
| Nemonapride | 9 | ||||||
| Perphenazine | 8 | ||||||
| Wichniak 2006 | Olanzapine | 14.8 ± 6.4 | Clinical judgement + continuation of OLZ monotherapy + AP switch not due to side effects | Na | Na |
Risperidone Perazine Perphenazine Levomepromazine Flupenthixol Haloperidol Promethazine Sulpiride zuchlopenthixol |
Na |
| Olanzapine + BZD | 15.4 ± 4.3 | ||||||
| Olanzapine + another AP | 15.9 ± 5.8 | ||||||
| Sumiyoshi 2006 | Olanzapine | 3.6 | Na | Na | 6 months | bromazepam |
Haloperidol Risperidone Risperidone+perospirone |
| Kikuchi 2007 | Various | 175 CPZ equivalents/day | 20% score reduction | 50% | 2–8 weeks |
Other FGA or SGA Benzodiazepines anticholinergics antihystaminics |
Na |
| Khodayari-Rostamabad 2010 | Clozapine | 364.2 ± 139.27 | 50%, 30% or 25% score reduction | 50% or 30% | Na | Na | Na |
| Ravan 2014 | Clozapine | 347 | 35% score reduction | 42% | Na | Na | Na |
| Mitra 2015 | Olanzapine, risperidone, haloperidol |
At 4 weeks 516.62 ± 208.6 CPZ equivalents; At 8 weeks 496.26 ± 242.35 CPZ equivalents |
b/ | / | 8 weeks | Na | Na |
| Masychev 2020 | Clozapine | 375.8 ± 121.26 | 35% score reduction | 48.4% | 359.2 ± 171.75 days | Na | Na |
| Arikan 2021 | Aripiprazole | Na | 50% score reduction | 58 % | 6.26 ± 4.78 weeks | No | Na |
| Ciprian 2021 | Clozapine | 347 | 40% score reduction | 33.3% | 1.4 yrs | Na | Na |
| Dominicus 2023 | Aripiprazole | 10 ± 5.9 | / | / | 4-6 weeks |
Fluoxetine (20 mg n = 1) Zopiclone (7.5 mg n = 4) Codeine (2.5 mg n = 1) |
No |
| Amisulpride | 285 ± 170.9 |
Na data not available, / not applicable, AP antipsychotic, BZD benzodiazepines, CPZ chlorpromazine.
aa linear relationship between PANSS score and theta power is the measure considered;
bcorrelation between PANSS and frequency-band power is the measure considered.
Results
Characteristics of the included studies
The combined outcome of the three databases yielded a total of 1731 records, in addition 15 studies were added by hand search. Of the total studies, 858 were duplicates, leaving 1232 articles. After reading titles and abstracts, 1090 were excluded because they were not relevant to the topic or not respecting inclusion and exclusion criteria. The full-text of 132 articles were examined in details. Three studies were excluded due to full-text unavailability54,83 or unavailability of English full-text84. After the evaluation phase, a total number of 22 studies were finally identified as eligible for inclusion in the current review.
Resting state EEG
Resting-state power spectra
Power spectra are the power distribution of EEG series in the frequency domain: the local electrical power is estimated at each moment, and it is classically divided in the 5 bands delta (0–4 hz), theta (4–8 hz), alfa (8–12 hz), beta (13–30 hz) and gamma (30–100 hz)7.
Delta-band
In a pre-treatment EEG, Knott et al.85 examined indices of interhemispheric and intrahemispheric EEG asymmetry and their relationship to symptoms changes, using combinations of electrodes. Greater reduction in negative symptoms was associated with greater pretreatment intrahemispheric asymmetry between frontal-anterior-temporal and anterior-midtemporal delta ratios (i.e. the power ratio in delta band between the two ensembles of electrodes).
Theta-band
In a pre-treatment EEG, Galderisi et al.86 found that average theta2 power was reduced in responders compared to non-responders to AP; quite the opposite, Kikuchi et al.87 evidenced that response to AP was positively related to before-treatment theta power (both with the analysis of the average global signal and with the analysis of single electrode, where differences were widespread to almost all sites).
Galderisiet al.86 realized their study based on the single dose effect hypothesis (i.e. a single AP administration induces measurable quantitative changes of EEG and patients showing the same changes observed in healthy controls have a favorable clinical outcome): 6 hours after AP administration theta power was increased in responders and unchanged in non-responders.
In EEGs recorded during treatment, Kikuchi et al.87 showed that theta-band power was increased in responders, irrespective of the scalp position.
In a pre-treatment EEG Knott et al.85 found that a higher global intrahemispheric theta asymmetry was positively associated with overall symptoms reduction, and that a greater interhemispheric central-anterior-temporal theta ratio was associated with greater treatment-related reduction in positive symptoms.
Gross et al.88 focused on the fronto-central areas theta-band oscillations. After 3 weeks of treatment they detected a significant negative relationship between theta-power change as measured by midline electrodes and PANSS subscales for positive and negative symptoms – so positively correlated to the response to APs. After 10 weeks of treatment, the relationship was widespread to all of the electrodes, but significant only for PANSS-positive symptoms and PANSS general subscales. After 18 weeks this finding persisted for PANSS general and negative symptoms subscales, but not for the positive symptoms’ subscale.
Alpha-band
In a pretreatment EEG, Itil et al.66 found that nonresponders had an increased power in the alpha range, considering the signal from all over the brain; the study replicated the findings from the same group that was excluded from the review due to full-text unavailability.
Kikuchi et al.87 discovered that pre-treatment alpha2 power was positively related to response to APs on the average and at the single-electrode level in P4, T5, T6 and O2.
Czobor et al.89 discovered that higher alpha activity on a pre-treatment recording is associated with a poorer outcome at 3 and 6 weeks of treatment; however, the explained variance was low (13% after 3 weeks of treatment and 18% after 6 weeks). In term of localization, this relationship was significant for the anterior and the temporal areas at both 3 and 6 weeks of treatment, and for the posterior areas at 3 weeks only.
Merlo et al.90 evaluated the relationship of pre-treatment EEG features with speed of response to APs. Alpha2 band power were higher in early compared to late responders, widespread to almost all the electrode locations.
In a pretreatment recording, Galderisi et al.86 found differences concerning alpha band with responders having lower alpha1 and higher alpha2 power compared to non-responders. Six hours after AP administration, alpha1 was increased in responder and decreased in non-responders and alpha2 was unchanged in responders and increased in non-responders. Individual data showed a large overlap between responders and non-responders, except for alpha1 change after 6 hours AP administration, for which an opposite pattern was clearly observed between the two groups. For this reason, this frequency-band only was used for the sensitivity-specificity analysis, and its changes at the electrode C3 resulted as the best discriminant feature of responder and non-responder, including both baseline recording and the one realized after 6 h, with an accuracy of 89.3%.
Ulrich et al.91 focused on the individuation of alpha and non-alpha epochs (i.e. relative power spectrum density of alpha-band > or < 50%) in acutely ill, drug-free patients. Both in pretreatment and during treatment recordings, responders had more non-alpha epochs compared to non-responders. A further analysis focused on the dynamic of EEG vigilance (i.e. including time as a variable and thus observing the evolution of the signal along the recording) found a significantly increased density of non-alpha epochs in responders compared to non-responders, both in pre-treatment and during treatment EEG. Differences were mainly localized on bilateral anterior-occipital and anterior-frontal derivations. No other difference in power spectra emerged between responders and non-responders in this investigation.
Beta-band
Results concerning beta-band were only found regarding pre-treatment EEG. Two studies66,86 found that non-responders to APs had a diminished power in the beta range compared to responders. Moreover, beta band power in almost all regions was higher in patients having and early response to AP, compared to late responders90. Knott and colleagues found that a higher interhemispheric central-anterior-temporal asymmetry in the beta band is associated with a better improvement of negative symptoms in the course of the disorder85.
Gamma-band
Mitra et al.80 hypothesized that pre-treatment gamma oscillations would have been higher in SZ compared to HCs, and that there would have been a reduction in their activity over the course of AP treatment. Even if the primary outcome of the study was not to find EEG signatures of AP response, the analysis included correlations between AP efficacy and gamma frequencies, but no significant results emerged in this respect.
In a pre-treatment EEG, Arikan et al.92 discovered a lower high-gamma power in responders at multiple frontal, central, parietal, temporal and occipital regions (electrodes FP1, F3, F4, C3, P4, Pz, O1, F7, F8, T4 and T5). In contrast, Itil et al.66 found that nonresponders have a diminished gamma-band power in EEG recorded before starting an AP therapy.
Other results
Itil et al.66 found that non-responders had a higher EEG signal average amplitude and amplitude deviation compared to responders on a pre-treatment recording, considering all the frequencies together.
Lacroix et al.93 studied EEG recorded during treatment to assess changes concerning amplitude and coherence of the signal in the main frequency bands. Response to AP was not the primary outcome, but its relationship with resting-state amplitudes was studied finding no statistically meaningful associations. A significant result was found instead for connectivity measures, as described in the paragraph below.
Resting-state connectivity
EEG functional connectivity is the temporal coincidence of spatially distant neural activities that likely reflects the dynamic interregional communications in the brain, and it is calculated for all the power frequency bands described above7.
Comparing pre- and post-treatment EEG, Lacroix et al.93 realized a connectivity analysis focused on EEG amplitude and coherence changes induced by CLZ. Changes in coherence in theta were negatively correlated with clinical improvement for couples of electrodes involving right anterior-medial temporal region against the frontal electrodes, and the left-parietal electrodes. A relationship between response and changes in coherence also in the alpha band, concerning left temporal electrodes paired with frontal electrodes. Moreover, in central electrodes, the amplitude of beta1-band was increased in less-responsive compared to more responsive patients, and the former showed a decrease in coherence in this band involving almost all electrodes.
By analyzing during-treatment EEG recordings, Kang et al.94 evaluated CLZ-induced EEG changes in functional connectivity, using non-linear methods. A result of their analysis is the subdivision of patients in two categories, one with frontal driving - occipital response (FDOR; i.e. a condition in which frontal regions are the inductors of the activity of the other regions) or one without this pattern. The study also reported that the clozapine induced the non-FDOR group to have a FDOR pattern after treatment and that this group showed a better clinical response, without a statistical measure in support.
Microstates
Microstates are global patterns of scalp potential topographies recorded using multichannel EEG arrays that dynamically vary over time in an organized manner. Broad-band spontaneous EEG activity at rest can be described by a limited number of scalp potential topographies, that remain stable for 60-120 milliseconds before transitioning to a different topography that remains stable again49.
One study reported microstates measurement linked to the response to AP, in a pre-treatment EEG. Kikuchi et al.95 found that responders had an overall shorter duration of microstates than controls or non-responders and a shorter duration of microstates A and D; occurrence of microstates B and C and of microstates in general was increased in responders compared to non-responders. Responders also had a lower total duration of microstate D compared to controls. Considering the percent change in microstate parameters induced by treatment (evaluated with a control EEG), responders had an increased duration of microstate A and D and overall microstate duration, a decreased duration of microstate C and reduced overall microstate occurrence compared to non-responders. The study moreover discovered a negative correlation between clinical improvement and duration of microstate D and total microstates duration, and a positive correlation with the occurrence of microstate A, microstate C and overall microstate occurrence. The analysis of global field synchronization (estimate of functional synchronization in the frequency domain) yielded no significant results.
Evoked potentials
An evoked potential or evoked response is an electrical potential recorded from the nervous system of a human or animal following the presentation of a stimulus. Resting-state analysis only reflects background brain processes, while evoked potentials explore the functioning related to task and active engagement in the environment7.
Sumiyoshi et al.96 aimed to assess changes in the configuration of the P300 wave generators in an auditory odd-ball task and following a treatment with olanzapine. The results showed an increase of a calculated laterality index of temporal lobe activation following 6 months of treatment; restoring the pattern observed in the control group. However, a significant relationship between this lateralization index and the response to treatment did not emerge, probably due to the small sample size.
Machine learning approach
Five studies used a machine learning approach to discover predictors of response to CLZ in treatment-resistant schizophrenia, using a pre-treatment EEG (Table 4).
Table 4.
Machine learning methods and results.
| First author year | Training set | Extraction process: candidate features | Features selection | Specification of classifier | Performance Evaluation | Top features |
|---|---|---|---|---|---|---|
| Khodayari-Rostamabad 2010 | EEG epochs and treatment outcome for each subject |
• Coherence between all electrode’s pairs at all frequencies • correlation and cross-correlation coefficients • mutual informations (Cover and Thomas 2006) • absolute and relative power levels • left-to-right hemisphere power ratio • anterior-posterior power gradient across frequencies and between electrodes |
Regularized features selection method followed by normalization (Peng 2006) |
Kernel partial least square regression procedure (Rospital & Kramer, 2006) Non-linear kernel principal component analysis (Muller 2001) |
L1O cross validation procedure |
• Mutual information: T3/P3; T3/O1; C3/P3 • Correlation F8/T4 • Coherence: 6hz T3/O1; 6hz T3/P3; 6hz C3/O1; 7 hz F3/P3; 8hz T6/P3; 9hz T3/O1; 10hz T3/T5; 10hz T3/P3; 11hz C3/P3; 11hz T3/P3; 12hz T3/T5; 13hz F7/F3 • Left-to-right PSD-ratio: 10 hz T5/T6; 11hz T5/T6; 12hz T5/T6; 16hz T5/T6 |
| Ravan 2014 | EEG responses for patients and HC and corresponding labels |
• auto-PSD values of 15 extracted source waveforms • c-PSD values between all pairs of source waveforms, at various frequencies (with transformation in a decibel scale and normalization) |
Regularized features selection (Peng) method followed by normalization |
Fuzzy c-mean method (Dunn 1973 & Bezdek 1981) | L1o cross validation procedure (Theodoridis, 2008) | CPSD: (FpM, TAR) at 19hz; (FpM, PR) at 19hz; (FpM, FM) at 16hz; (CM, PR) at 14hz; (OpM, PR) at 2hz |
| Masychev 2020 | EEG epochs and treatment outcome for each subject | STE connectivity values (all channels, all frequency bands) | Cn-CV; relief features method; KPCA | SVM, LNA, KNN, RF | Cn-CV algorithm | Effective connectivity: δ T3 to P4; θ F3 to T6; θ T6 to F3; α FP2 to Fz; δ T4 to O1; γ F1 to F2; γ F3 to F2; γ C3 to Cz |
| Ciprian 2021 | EEG responses to the oddball and corresponding labels, for patients and healthy controls | STE connectivity values (all channels, all frequency bands) | mRMR, CN-CV validation procedure; KPCA | SVM, LNA, KNN, RF | Cn-CV algorithm | Effective connectivity: θ right-IFG to left SPL; γ left LC to left LTL; θ right OFC to right ACC; θ left SFC to right PCC; θ left PCC to left AFC; γ left ACC to left IFG; β left FG to right SMA; θ left PCC to left AFC |
| Dominicus 2023 | EEG epochs and treatment outcome for each subject |
• Relative power spectra • mean phase lag index (PLI) • mean amplitude envelope correlation corrected (AEC-c) • Minimum spanning tree (MST) |
Random noise feature | RF | / | δ, Relative power; θ _MST – degree; θ, Relative power; θ _MST – Leaf fraction; α, Relative power; θ _MST – Diameter; β, Relative power; θ _MST – BCmax; δ, PLI; θ _MST – Ecc; θ, PLI; θ _MST – Tree hierarchy; α, PLI; α _MST – degree; β, PLI; α _MST – Leaf fraction; δ, AEC-c; α _MST – Diameter; θ, AEC-C; α _MST – BCmax; α, AEC-C; α _MST – Ecc; β, AEC-C; α _MST – Tree hierarchy; δ _MST_MST – degree; β _MST – degree; δ _MST_Leaf fraction; β _MST – Leaf fraction; δ _MST_Diameter; β _MST – Diameter; δ _MST – BCmax; β _MST – BCmax; δ _MST – Ecc; β _MST – Ecc; δ_MST _Tree hierarchy; β _MST – Tree hierarchy |
Cn-CV consensus nested cross validation, L1O leave one out, SVM support vector machine, LNA linear discrimination analysis, KNN K nearest neighbors, RF random forest, KPCA Kernelized principle component analysis with a polynomial kernel, STE symbolic transfer entropy, mRMR minimum redundancy maximum relevance feature selection algorithm, CPSD cross power spectrum density, MST minimum spanning tree, PLI phase lag index, AEC amplitude envelope correlation, BC betweenness centrality, IFG inferior frontal gyrus, SPL superior parietal lobule, LTL lower temporal lobule, OFC orbitofrontal cortex, SFC superior frontal cortex, PCC posterior cingulate cortex, AFC anterior frontal cortex, SMA supplementary motor area.
Khodayari-Rostamabad et al.97 evaluated resting-state EEG in two groups, used as discovery and replication cohorts, respectively. After the feature extraction and reduction processes, 8 features were applied for performance evaluation. A first step was realized on the discovery cohort, a second step used the discovery cohort as a training dataset, and tested the prediction performance in the replication cohort. A set of features was able to discriminate the responders form non-responders, including measures of mutual information (i.e. assessment of the amount of information about one signal contained in another signal), correlation (i.e. the quantification of the degree of association between apparently different signals), coherence (i.e. the normalized cross-power spectrum per frequency of two signals recorded simultaneously at different sites of the scalp) and left-to-right power spectra ratios involving both hemispheres on central, temporal and occipital areas, in theta, alpha and low-beta frequency-bands. Accuracy in classification of responders and non-responders was of 87.3% in the first step and of 85.7% in the second step.
Masychev et al.98, focused on EEG resting state functional connectivity between frequency bands, through measurements of symbolic transfer entropy. Using four different methods for classifier specification, patients were divided in most-responders and least-responders to treatment, and the ML process discovered 8 features effectively discriminating the two categories, consisting of measures of connectivity between frontal, prefrontal, central and temporal electrodes, at multiple frequency bands. The subsequent statistical-power analysis revealed high values of sensitivity and specificity with all the classifier methods. Accuracy in predicting responder status was of 89.9% ± 4.39 with support vectoring machine, of 87.98% ± 4.66 with linear discrimination analysis, of 87.98% ± 4.66 with K nearest neighbour and of 83.97% ± 6.32 with random forest.
The same research group realized another study99, with the same design but the EEG-paradigm, consisting in an auditory oddball task, and importantly the features were extracted from source reconstruction prior to ML. The discriminating features that were identified by mean of the ML process were then 8 measures of functional connectivity in the theta, gamma, and beta frequency ranges across multiple brain regions. In this study accuracy was of 95.83% with support vectoring machine, of 93.37% with random forest and of 91.48% with linear discrimination analysis.
Another ML study was realized by Dominicus et al.100, who tested a random forest regression model aimed to determine the response to an APs monotherapy after 4-6 weeks, in FEP patients. Sixty EEG features were included in the model, comprising measures of power spectra and functional connectivity in all frequency bands and widespread to multiple bilateral brain regions. The 5 more important features to predict response were the tree hierarchy in alpha band according to amplitude envelope correlation corrected (i.e. the absolute value of the Hilbert transform of a given cortical oscillation), phase lag index (i.e. a measure of the asymmetry of the distribution of phase differences between two signals) in beta band, betweenness centrality (i.e. the fraction of the second shortest path that pass through a node) in delta band, amplitude envelope corrected in theta-band and, tree hierarchy in beta-band according to phase lag index. A significant predictive power emerged only for PANSS positive subscale (P < 0.004), but with a low effect size (R2 = 0.23). No significant results emerged for PANSS total score and other PANSS subscales.
Ravan et al.101 used not only a pretreatment EEG, but also an after treatment recording, in both cases obtained during an odd-ball task. Comparing the two recordings, a decreased value of five measures of functional connectivity between frontal, parietal, temporal, central and occipital areas in beta and gamma bands was related to a better response to clozapine. Accuracy in predicting response to APs was 79.6%.
EEG epileptiform abnormalities and response to AP
Epileptiform abnormalities are abnormal synchronous electrical discharges generated by a group of neurons in the region of an epileptic focus102.
Two studies discovered that pre-treatment epileptiform abnormalities predicted a favorable response to APs. In one case the relationship was established only in patients with a psychotic disorder having a comorbid major depressive episode and in females103, in the other for the whole cohort; in this second study a relationship with response was defined also for an EEG repeated after 6 months104. A third study on the topic did not find a relationship between the presence of slow EEG activity (i.e., theta activity for more of 10% of a 1 second EEG epoch or delta occurrence) and/or EEG abnormalities, and treatment outcome and/or sleepiness105.
Discussion
Our systematic review illustrates how multiple EEG features are linked to response to APs. Given the heterogeneity of experimental paradigms and considered variables in the eligible studies, we did not carry out a meta-analysis. The EEG features we reviewed can be divided in two types: pre-treatment predictors of response to AP, and EEG changes occurring under treatment and mirroring APs efficacy. These findings support the notion that local and long-range brain network dysfunction underlying psychosis are mirrored by EEG recording, and that the effect of APs on disrupted neurocircuits produces measurable changes in EEG as well.
AP-induced EEG changes are known from previous studies not focused on response to APs or on the relationship with other clinical variables20,28,54,56,58–60,63–70; only some of them proved to be indeed related to response to treatment in the studies we systematically reviewed. The other changes could possibly be only an unspecific influence on brain rhythms or mirror CNS side effects of APs.
Prediction of response to AP with EEG features
In resting-state EEG, most of the findings concerned theta and alpha bands. At baseline theta power, either low86 or high87 was related to a favorable treatment outcome. A high alpha power predicted a poorer response in most of the studies66,86,87,89–91; moreover, alpha functional connectivity in frontal and temporal regions was inversely related to response93. A diminished beta-power on a pre-treatment RS-EEG was related to a worse treatment outcome66,85,86,90,93, such as a reduced functional connectivity after treatment93.
These findings could be read in light of psychosis pathophysiology and APs mechanism of action. Dopaminergic neurons fire either in a tonic way at 2-8 hz firing or in a phasic way, at 20 hz106,107, and the mesolimbic dopaminergic dysfunction is a mechanism at the origin of SZ108. Findings of a disrupted alpha, beta and theta in SZ could be a manifestation of the abnormalities of dopaminergic neuron firing; being the leverage for action of APs based on a modulation of dopaminergic receptor, it makes sense that these abnormalities can be changed by AP and that it corresponds to their future efficacy. Considering the different patterns of receptor bindings1, it could also be expected that the EEG signatures predicting response differ for different specific APs, but the studies under review don’t offer conclusions in this respect.
In gamma band, responders had a larger pre-treatment resting-state power in frontal, central, parietal, temporal and occipital areas80,92, while no differences emerged concerning functional connectivity. Gamma frequencies are considered the direct expression of the activity of the parvalbumin-containing GABAergic interneurons14,109,110, whose dysfunction is the other plausible pathophysiological process of SZ, based on the glutamatergic hypothesis2. Findings show disrupted power-spectra activities in SZ, and an action of APs on these rhythms in relation to the response, and this is in line with this theoretical background.
A smaller interhemispheric asymmetry at multiple frequency bands85 predicted a worse response to treatment.
In SZ a reduced asymmetry was defined both at structural111 and at the functional112 level, and linked to a reduced brain lateralization, especially in language areas. This reduced brain specialization has been proposed as one of the core pathogenetic processes in SZ113. Thus, we could speculate that a higher pre-treatment EEG asymmetry corresponds to a lesser brain impairment, facilitating a better response to therapeutics.
Machine learning studies used measures of functional connectivity involving all the frequency bands and across different brain regions as features in their predictive model, both at the resting state or during the execution of auditory tasks97–101. The relevance of functional connectivity measurements as an indicator of treatment response is in line with the conceptualization of SZ as a functional disconnection syndrome, whose core mechanism is not localized to a specific brain area or a function but involve large-scale neurocircuitries37,38,114.
On one side, the involvement of all frequency bands widespread to most brain regions is in line with a conceptualization of SZ as whole brain disease, linked to large-scale network disruptions115. On the other side, the link between frequency bands and the pathophysiology of psychosis is loose, except for gamma frequencies; the absence of a clear functional hypothesis for the other frequency bands concerning SZ contributes to the uncertainty surrounding the findings.
While most of the studies under review evaluated the relationship between general response to AP and EEG features, some80,88,98,100 explored the relationship with specific psychopathology domains, corresponding to the PANSS subscales for positive, and negative symptoms and general psychopathology. The fact that certain EEG features only relate to a peculiar category of symptoms indicates once more that SZ is a multidimensional disorder116,117. Each dimension possibly has a specific biological substrate in terms of type and location of neurophysiological activity.
Kikuchi et al.95 focused their investigation on microstates and observed that several characteristics of all 4 microstates (i.e. occurrence, duration and topography) were related to response to APs, considering both pre-treatment and during-treatment recordings. Of note, a meta-analysis118 summarized results on SZ and microstates, discovering that the key feature in SZ were a more frequent occurrence of microstate C and a shorter duration of microstates D compared to healthy control; the study of Kikuchi indicates that this C-D imbalance can be normalized by AP treatment
Changes in EEG features during treatment
An increased theta power86,88, a reduced beta band activity, a decreased coherence in theta, alpha and beta band88 were related to a favorable outcome.
Different frequency bands are altered in SZ, as detailed in the introduction; also, they are implicated in brain functions impaired in psychotic disorders. Delta frequency evoked responses play a role in motivation, emotions, and overall cognitive functioning8,9. Theta band orchestrates cognitive processes that are compromised in psychotic disorder, such as working memory, detection of new sensory stimuli and attentional control10. Neuronal oscillations in the alpha band play a pivotal role in cognition, consciousness, wakefulness, sensorimotor and emotional processing11. Beta oscillations were related to sensorimotor behavior, perceptual integration, working memory and top-down regulation of attention12,13,119. Gamma frequencies have been associated with sensation, perception, attention, cognitive processing, consciousness, memory and and more widely to synaptic plasticity14,15,115,120.
AP-inducing changes in these frequency bands possibly have their therapeutic effect mediated by a re-tuning of brain circuits underlying the sensorial, cognitive, and affective functions above-mentioned.
Only a pilot study evaluated an evoked potential as a marker of response, and suggested that olanzapine was able to restore the normal features of P300, which has a lower amplitude and a prolonged latency in SZ121.
Quality assessment
The AMSTAR checklist82 was used to improve the quality of this review. Included studies where described in details (in the text or in the tables) concerning population, intervention, comparators (i.e. control group, different diagnostic groups, same patient on different time points), outcomes (i.e. response to treatment) and research design. Methods were established prior to the conduct of the review, and all the types of studies suitable to investigate the variable of interest (i.e. EEG features related to response to AP) were included. Study selection and data extraction were performed in duplicate. The exclusion of initially retrieved studies was explained. Funding and authoring are clearly reported.
Risk of bias was assessed with RoBINS-I81, a tool that is normally used for interventional trial, but also useful to evaluate non-randomized observational trials as the studies under review. Concerning possible pre-intervention bias, we observed a wide variability in factors predicting response to treatment, including age, duration of illness, severity of disease, education level. In some studies, these parameters were not collected or they were not included as confounders in the statistical analysis. Selection of patients was globally correct, including a single diagnosis in each study. The intervention (i.e. the AP administration) was properly classified and reported. Controls subjects, when implicated, were well matched with patients for age, sex and education level. Significant attrition, detection and outcome reporting bias were not detected. Overall, a low to moderate risk of bias was assessed.
Limitations
Our systematic review was limited by the small number of studies available and by their methodological heterogeneity, which does not allow to draw univocal and generalizable conclusions.
Different rating scales were used to define response to treatment; even if the concordance between them is high122, a major limitation is represented by the use of different cut-offs for response, and of different time span for its determination.
EEG measures were heterogeneous between studies, in term of paradigm and analysis methods. Intra- and inter-subject variability of EEG recording was another major concern: EEG studies are generally characterized by a high variability within and even more between subjects, which might not always be indicative of any pathological status, making it difficult to generalize reports without applied normalization. Disparate EEG features were the focus in different studies; as discussed above, each frequency band correspond to distinct sensory, cognitive and emotional processes, both concerning power spectra and functional connectivity. However, the former mostly reflects local brain areas functioning, and the latter the long-range synchronization of distant regions. P300 correlate with memory, attention, auditory discrimination, processing of sequential information, and decision making123. Microstates reflects the fundamental functioning of consciousness49, of the visual system124, of the salience network125, of the default mode network124 and of the dorsal attention network49. Actually, it is possible to conclude that distinct EEG features reflect different aspect of brain functioning, each one having a peculiar relationship to psychopathology domains and being differently influenced by APs. As a consequence, the heterogeneity of finding precludes drawing generalizable conclusions.
Moreover, most of the data concern resting state activity, while only a minority is from task-related EEG, a difference that further prevent a generalization. Finally, even when the same EEG features were evaluated in different studies, different EEG analysis techniques account for heterogeneity in results.
Intersubjective variability in socio-demographic and clinical features such age, sex, educational level, duration and severity of disease was also considerable, and these parameters have been demonstrated to have a significant influence on EEG in patients with SZ18. Moreover, relevant prognostic factor/confounders for response to treatment such as education level, duration of illness, duration of untreated psychosis126,127 weren’t considered in the statistical analysis, exposing results to a risk of bias.
Overall, most of the studies did not report a significant amount of data about sociodemographic and clinical characteristics of patients.
Some of the features used for the connectivity analysis, such as coherence, correlation and mutual information are highly subject to volume conduction effect (i.e. at the level of each EEG channel, the effect of a mixture of active brain and nonbrain electrical sources whose activities are conducted to the scalp). This phenomenon could produce results wrongly indicating a functional connectivity or hemispheric asymmetries128.
Concerning the medication administered, most studies were conducted on clozapine, while the others on many different FGA, SGA and TGA. On one side this allowed to reproduce findings on CLZ, but on the other side deprives of information concerning other APs. Each AP has a peculiar binding profile, depending on intrinsic activity and constant of dissociation from serotonin and dopamine receptors; accordingly, the way they influence glutamatergic pyramidal neurons and dopaminergic neurons is substance specific, and it can be reflected by different EEG patterns. Moreover, the accessory effect of APs on histaminergic and cholinergic systems further differentiate AP and their influence on EEG activity2,129. In fact, the generic effect of APs (i.e. not related to response) on EEG is different when comparing different molecules20,54–80. This could explain why EEG markers of response are not overlapping from one AP to another.
Many important information about the treatment were often missing, such as dose, duration of treatment, co-medications, previous medications, and when present displayed a large variability among studies and subjects under study.
Some of the studies were specifically focused on treatment-resistant SZ, which represents a specific category of SZ patients, differing from the nontreatment-resistant one130.
The statistical analysis in most of the studies was minimal, excluding a sensitivity-specificity analysis and the effect-size determination, precluding a formal evaluation of EEG feature as predictive biomarkers.
Only a part of the studies had a case-control design, the other being cohort studies.
Conclusions
EEG still is a promising method for brain study and to create a predictive biomarker for response to APs, since it is non-invasive and allows a direct in-vivo observation of brain oscillations. Further investigations are warranted to address the limits of the studies under review; novel studies should have a larger sample size, display a better clinical and demographic characterization of the sample, extensively evaluate all of the APs, use HD-EEG and EEG paradigms including auditory, cognitive and emotional tasks. While the studies reviewed here were mostly of exploratory nature, we believe that the most promising approach for novel studies would be to select frequencies, regions of interest and connectivity measures mirroring a sound theoretical hypothesis on the pathogenesis of SZ and the mechanism of action of APs. If large data sets are available, an alternative to this hypothesis-driven approach could be data-driven approaches based on machine learning.
Supplementary information
Acknowledgements
Internal funding only.
Author contributions
Conception or design of the work, acquisition analysis and interpretation of data: M.D.P., V.R., S.K. Drafting the work and revising it critically: M.D.P., V.R., M.S., C.M., S.K. Final approval of the completed manuscript: M.D.P., V.R., M.S., C.M., S.K. All the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41537-023-00419-z.
References
- 1.Stahl, S. M. Stahl’s Essential Psychopharmacology Neuroscientific Basis and Practical Applications. 4th ed. (Cambridge, ed.); (2013).
- 2.Stahl, S. M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 23. 10.1017/S1092852918001013 (2018). [DOI] [PubMed]
- 3.Haddad, P. M., & Correll, C. U. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 8. 10.1177/2045125318781475 (2018). [DOI] [PMC free article] [PubMed]
- 4.Lieberman, J. A. et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. New Engl. J. Med. 353. 10.1056/NEJMoa051688 (2005). [DOI] [PubMed]
- 5.Perkovic, M. et al Theranostic Biomarkers for Schizophrenia. Int. J. Mol. Sci. 18. 10.3390/ijms18040733 (2017). [DOI] [PMC free article] [PubMed]
- 6.Lozupone, M. et al. The Role of Biomarkers in Psychiatry. In: 135–162. 10.1007/978-3-030-05542-4_7 (2019). [DOI] [PubMed]
- 7.Michel, C. M., Koenig, T., Brandeis, D., Gianotti, L. R. R., & Wackermann, J., eds. Electrical Neuroimaging. Cambridge University Press; 10.1017/CBO9780511596889 (2009).
- 8.Güntekin B, Başar E. Review of evoked and event-related delta responses in the human brain. Int. J. Psychophysiol. 2016;103:43–52. doi: 10.1016/j.ijpsycho.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Harmony, T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 7. 10.3389/fnint.2013.00083 (2013). [DOI] [PMC free article] [PubMed]
- 10.Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazanova OM, Vernon D. Interpreting EEG alpha activity. Neurosci. Biobehav. Rev. 2014;44:94–110. doi: 10.1016/j.neubiorev.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Liddle EB, et al. Abnormal salience signaling in schizophrenia: The role of integrative beta oscillations. Hum. Brain Mapp. 2016;37:1361–1374. doi: 10.1002/hbm.23107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spitzer B, Haegens S. Beyond the Status Quo: A Role for Beta Oscillations in Endogenous Content (Re)Activation. eNeuro. 2017;4:ENEURO.0170–17.2017. doi: 10.1523/ENEURO.0170-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNally JM, McCarley RW. Gamma band oscillations. Curr. Opin. Psychiatry. 2016;29:202–210. doi: 10.1097/YCO.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reilly TJ, et al. Gamma band oscillations in the early phase of psychosis: A systematic review. Neurosci. Biobehav. Rev. 2018;90:381–399. doi: 10.1016/j.neubiorev.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Perrottelli A, et al. Unveiling the Associations between EEG Indices and Cognitive Deficits in Schizophrenia-Spectrum Disorders: A Systematic Review. Diagnostics. 2022;12:2193. doi: 10.3390/diagnostics12092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbert T, Lutzenberger W, Rockstroh B, Berg P, Cohen R. Physical aspects of the EEG in schizophrenics. Biol. Psychiatry. 1992;32:595–606. doi: 10.1016/0006-3223(92)90072-8. [DOI] [PubMed] [Google Scholar]
- 18.John ER, et al. Quantitative electrophysiological characteristics and subtyping of schizophrenia. Biol. Psychiatry. 1994;36:801–826. doi: 10.1016/0006-3223(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 19.Miyauchi T, et al. Computerized EEG in Schizophrenic patients. Biol. Psychiatry. 1990;28:488–494. doi: 10.1016/0006-3223(90)90482-H. [DOI] [PubMed] [Google Scholar]
- 20.Itil TM. Qualitative and Quantitative EEG Findings in Schizophrenia. Schizophr. Bull. 1977;3:61–79. doi: 10.1093/schbul/3.1.61. [DOI] [PubMed] [Google Scholar]
- 21.Narayanan B, et al. Resting State Electroencephalogram Oscillatory Abnormalities in Schizophrenia and Psychotic Bipolar Patients and Their Relatives from the Bipolar and Schizophrenia Network on Intermediate Phenotypes Study. Biol. Psychiatry. 2014;76:456–465. doi: 10.1016/j.biopsych.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venables NC, Bernat EM, Sponheim SR. Genetic and Disorder-Specific Aspects of Resting State EEG Abnormalities in Schizophrenia. Schizophr. Bull. 2009;35:826–839. doi: 10.1093/schbul/sbn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clementz BA, Sponheim SR, Iacono WG, Beiser M, Resting EEG. in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology. 1994;31:486–494. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 24.Harris A, Melkonian D, Williams L, Gordon E. Dynamic Spectral Analysis Findings In First Episode And Chronic Schizophrenia. Int. J. Neurosci. 2006;116:223–246. doi: 10.1080/00207450500402977. [DOI] [PubMed] [Google Scholar]
- 25.Pascual-Marqui RD, et al. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. Neuroimag. 1999;90:169–179. doi: 10.1016/S0925-4927(99)00013-X. [DOI] [PubMed] [Google Scholar]
- 26.van Tricht MJ, et al. Can quantitative EEG measures predict clinical outcome in subjects at Clinical High Risk for psychosis? A prospective multicenter study. Schizophr. Res. 2014;153:42–47. doi: 10.1016/j.schres.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Renaldi R, et al. Predicting Symptomatic and Functional Improvements over 1 Year in Patients with First-Episode Psychosis Using Resting-State Electroencephalography. Psychiatry Investig. 2019;16:695–703. doi: 10.30773/pi.2019.06.20.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saletu B, Küfferle B, Grünberger J, Anderer P. Quantitative EEG, SPEM, and Psychometric Studies in Schizophrenics before and during Differential Neuroleptic Therapy. Pharmacopsychiatry. 1986;19:434–437. doi: 10.1055/s-2007-1017283. [DOI] [PubMed] [Google Scholar]
- 29.Begré S, et al. Reduced hippocampal anisotropy related to anteriorization of alpha EEG in schizophrenia. Neuroreport. 2003;14:739–742. doi: 10.1097/00001756-200304150-00016. [DOI] [PubMed] [Google Scholar]
- 30.Koenig T, et al. Decreased functional connectivity of EEG theta-frequency activity in first-episode, neuroleptic-naïve patients with schizophrenia: preliminary results. Schizophr. Res. 2001;50:55–60. doi: 10.1016/S0920-9964(00)00154-7. [DOI] [PubMed] [Google Scholar]
- 31.Guenther W, Breitling D, Banquet JP, Marcie P, Rondot P. EEG Mapping of left hemisphere dysfunction during motor performance in schizophrenia. Biol. Psychiatry. 1986;21:249–262. doi: 10.1016/0006-3223(86)90046-6. [DOI] [PubMed] [Google Scholar]
- 32.Morihisa JM. Brain Electrical Activity Mapping (BEAM) in Schizophrenic Patients. Arch. Gen. Psychiatry. 1983;40:719. doi: 10.1001/archpsyc.1983.01790060017002. [DOI] [PubMed] [Google Scholar]
- 33.Morstyn R, Duffy FH, McCarley RW. Altered topography of EEG spectral content in schizophrenia. Electroencephalogr. Clin. Neurophysiol. 1983;56:263–271. doi: 10.1016/0013-4694(83)90251-1. [DOI] [PubMed] [Google Scholar]
- 34.Uhlhaas PJ. High-Frequency Oscillations in Schizophrenia. Clin. EEG Neurosci. 2011;42:77–82. doi: 10.1177/155005941104200208. [DOI] [PubMed] [Google Scholar]
- 35.Krukow P, Jonak K, Grochowski C, Plechawska-Wójcik M, Karakuła-Juchnowicz H. Resting-state hyperconnectivity within the default mode network impedes the ability to initiate cognitive performance in first-episode schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;102:109959. doi: 10.1016/j.pnpbp.2020.109959. [DOI] [PubMed] [Google Scholar]
- 36.Newson JJ, Thiagarajan TC. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum Neurosci. 2019;12:521. doi: 10.3389/fnhum.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Lorenzo, G. et al. Altered resting-state EEG source functional connectivity in schizophrenia: the effect of illness duration. Front. Hum. Neurosci. 9. 10.3389/fnhum.2015.00234 (2015). [DOI] [PMC free article] [PubMed]
- 38.Lehmann D, et al. Functionally aberrant electrophysiological cortical connectivities in first episode medication-naive schizophrenics from three psychiatry centers. Front. Hum. Neurosci. 2014;8:635. doi: 10.3389/fnhum.2014.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tauscher J, Fischer P, Neumeister A, Rappelsberger P, Kasper S. Low frontal electroencephalographic coherence in neuroleptic-free schizophrenic patients. Biol. Psychiatry. 1998;44:438–447. doi: 10.1016/S0006-3223(97)00428-9. [DOI] [PubMed] [Google Scholar]
- 40.Winterer G, et al. An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophr. Res. 2001;49:129–143. doi: 10.1016/S0920-9964(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 41.Kam JWY, Bolbecker AR, O’Donnell BF, Hetrick WP, Brenner CA. Resting state EEG power and coherence abnormalities in bipolar disorder and schizophrenia. J. Psychiatr. Res. 2013;47:1893–1901. doi: 10.1016/j.jpsychires.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shagass C. Relationships Between Psychiatric Diagnosis and Some Quantitative EEG Variables. Arch. Gen. Psychiatry. 1982;39:1423. doi: 10.1001/archpsyc.1982.04290120053011. [DOI] [PubMed] [Google Scholar]
- 43.Roemer RA, Shagass C, Dubin W, Jaffe R, Siegal L. Quantitative EEG in elderly depressives. Brain Topogr. 1992;4:285–290. doi: 10.1007/BF01135566. [DOI] [PubMed] [Google Scholar]
- 44.d’Elia G, et al. Changes in psychopathology in relation to EEG variables and visual averaged evoked responses (V. AER) in schizophrenic patients treated with penfluridol or thiothixene. Acta Psychiatr. Scand. 1977;55:309–318. doi: 10.1111/j.1600-0447.1977.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 45.Etevenon P, et al. Intra- and interhemispheric EEG differences quantified by spectral analysis. Acta. Psychiatr. Scand. 1979;60:57–68. doi: 10.1111/j.1600-0447.1979.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 46.Coger RW. Electroencephalographic Similarities Between Chronic Alcoholics and Chronic, Nonparanoid Schizophrenics. Arch. Gen. Psychiatry. 1979;36:91. doi: 10.1001/archpsyc.1979.01780010097011. [DOI] [PubMed] [Google Scholar]
- 47.Serafetinides EA, Coger RW, Martin J, Dymond AM. Schizophrenic symptomatology and cerebral dominance patterns: A comparison of EEG, AER, and BPRS measures. Compr. Psychiatry. 1981;22:218–225. doi: 10.1016/0010-440X(81)90072-9. [DOI] [PubMed] [Google Scholar]
- 48.Flor-Henry P. Lateralized Temporal-Limbic Dysfunction And Psychopathology. Ann. N Y Acad. Sci. 1976;280:777–795. doi: 10.1111/j.1749-6632.1976.tb25541.x. [DOI] [PubMed] [Google Scholar]
- 49.Michel CM, Koenig T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. Neuroimage. 2018;180:577–593. doi: 10.1016/j.neuroimage.2017.11.062. [DOI] [PubMed] [Google Scholar]
- 50.Kwon JS, et al. Gamma Frequency–Range Abnormalities to Auditory Stimulation in Schizophrenia. Arch. Gen. Psychiatry. 1999;56:1001. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishnan GP, et al. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim, H. K., Blumberger, D. M., & Daskalakis, Z. J. Neurophysiological Biomarkers in Schizophrenia—P50, Mismatch Negativity, and TMS-EMG and TMS-EEG. Front. Psychiatry. 11. 10.3389/fpsyt.2020.00795 (2020). [DOI] [PMC free article] [PubMed]
- 53.Perrottelli, A., Giordano, G. M., Brando, F., & Giuliani, L., Mucci A. EEG-Based Measures in At-Risk Mental State and Early Stages of Schizophrenia: A Systematic Review. Front. Psychiatry. 12. 10.3389/fpsyt.2021.653642 (2021). [DOI] [PMC free article] [PubMed]
- 54.Small JG, et al. Computerized EEG profiles of haloperidol, chlorpromazine, clozapine and placebo in treatment resistant schizophrenia. Clin. Electroencephalogr. 1987;18:124–135. [PubMed] [Google Scholar]
- 55.Saletu B, Küfferle B, Anderer P, Grünberger J, Steinberger K. EEG-brain mapping in schizophrenics with predominantly positive and negative symptoms. Euro. Neuropsychopharmacol. 1990;1:27–36. doi: 10.1016/0924-977X(90)90007-W. [DOI] [PubMed] [Google Scholar]
- 56.Kemali D, et al. Computerized EEG topography findings in schizophrenic patients before and after haloperidol treatment. Int. J. Psychophysiol. 1992;13:283–290. doi: 10.1016/0167-8760(92)90078-P. [DOI] [PubMed] [Google Scholar]
- 57.Galderisi S, et al. C-EEG brain mapping in DSM-III schizophrenics after acute and chronic haloperidol treatment. Euro. Neuropsychopharmacol. 1990;1:51–54. doi: 10.1016/0924-977X(90)90011-X. [DOI] [PubMed] [Google Scholar]
- 58.Coppola R, Herrmann WM. Psychotropic Drug Profiles: Comparisons by Topographic Maps of Absolute Power. Neuropsychobiology. 1987;18:97–104. doi: 10.1159/000118400. [DOI] [PubMed] [Google Scholar]
- 59.Fink M. EEG and Human Psychopharmacology. Annu. Rev. Pharmacol. 1969;9:241–258. doi: 10.1146/annurev.pa.09.040169.001325. [DOI] [PubMed] [Google Scholar]
- 60.Pfeiffer CC, Goldstein L, Murphree HB. Effects of Parenteral Administration of Haloperidol and Chlorpromazine in Man. I. Normal Subjects: Quantitative EEG and Subjective Response. J. Clin. Pharmacol. J. New Drugs. 1968;8:79–88. doi: 10.1002/j.1552-4604.1968.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 61.Mackintosh AJ, et al. Psychotic disorders, dopaminergic agents and EEG/MEG resting-state functional connectivity: A systematic review. Neurosci. Biobehav. Rev. 2021;120:354–371. doi: 10.1016/j.neubiorev.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 62.Mailman R, Murthy V. Third Generation Antipsychotic Drugs: Partial Agonism or Receptor Functional Selectivity. Curr. Pharm. Des. 2010;16:488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saletu B, Grünberger J. Drug profiling by computed electroencephalography and brain maps, with special consideration of sertraline and its psychometric effects. J. Clin. Psychiatry. 1988;49:59–71. [PubMed] [Google Scholar]
- 64.Galderisi S, Mucci A, Mignone ML, Maj M, Kemali D. CEEG mapping in drug-free schizophrenics. Schizophr. Res. 1991;6:15–23. doi: 10.1016/0920-9964(91)90016-K. [DOI] [PubMed] [Google Scholar]
- 65.Herrmann W, Schärer E, Delini-Stula A. Predictive Value of Pharmaco-Electroencephalography in Early Human-Pharmacological Evaluations of Psychoactive Drugs. Pharmacopsychiatry. 1991;24:196–205. doi: 10.1055/s-2007-1014469. [DOI] [PubMed] [Google Scholar]
- 66.Itil TM, Shapiro D, Schneider SJ, Francis IB. Computerized EEG as a Predictor of Drug Response in Treatment Resistant Schizophrenics. J. Nerv. Ment. Dis. 1981;169:629–637. doi: 10.1097/00005053-198110000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Ozaki T, Toyomaki A, Hashimoto N, Kusumi I. Quantitative Resting State Electroencephalography in Patients with Schizophrenia Spectrum Disorders Treated with Strict Monotherapy Using Atypical Antipsychotics. Clin. Psychopharmacol. Neurosci. 2021;19:313–322. doi: 10.9758/cpn.2021.19.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshimura M, et al. A pharmaco-EEG study on antipsychotic drugs in healthy volunteers. Psychopharmacology (Berl) 2007;191:995–1004. doi: 10.1007/s00213-007-0737-8. [DOI] [PubMed] [Google Scholar]
- 69.Nagase Y, Okubo Y, Toru M. Electroencephalography in schizophrenic patients: Comparison between neuroleptic-naive state and after treatment. Biol. Psychiatry. 1996;40:452–456. doi: 10.1016/0006-3223(96)00304-6. [DOI] [PubMed] [Google Scholar]
- 70.Begić D, Hotujac L, Jokić-Begić N. Quantitative EEG in Schizophrenic Patients before and during Pharmacotherapy. Neuropsychobiology. 2000;41:166–170. doi: 10.1159/000026650. [DOI] [PubMed] [Google Scholar]
- 71.Mucci A, Volpe U, Merlotti E, Bucci P, Galderisi S. Pharmaco-EEG in Psychiatry. Clin. EEG Neurosci. 2006;37:81–98. doi: 10.1177/155005940603700206. [DOI] [PubMed] [Google Scholar]
- 72.Tislerova B, et al. LORETA Functional Imaging in Antipsychotic-Naive and Olanzapine-, Clozapine- and Risperidone-Treated Patients with Schizophrenia. Neuropsychobiology. 2008;58:1–10. doi: 10.1159/000154474. [DOI] [PubMed] [Google Scholar]
- 73.Hyun J, Baik MJ, Kang UG. Effects of Psychotropic Drugs on Quantitative EEG among Patients with Schizophrenia-spectrum Disorders. Clin. Psychopharmacol. Neurosci. 2011;9:78–85. doi: 10.9758/cpn.2011.9.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knott V, Labelle A, Jones B, Mahoney C. Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophr. Res. 2001;50:41–53. doi: 10.1016/S0920-9964(00)00165-1. [DOI] [PubMed] [Google Scholar]
- 75.Roubicek J, Major I. EEG profile and behavioral changes after a single dose of clozapine in normals and schizophrenics. Biol Psychiatry. 1977;12:613–633. [PubMed] [Google Scholar]
- 76.Saletu B, Grünberger J, Linzmayer L, Anderer P. Comparative Placebo-controlled Pharmacodynamic Studies with Zotepine and Clozapine Utilizing Pharmaco-EEG and Psychometry. Pharmacopsychiatry. 1987;20:12–27. doi: 10.1055/s-2007-1017125. [DOI] [PubMed] [Google Scholar]
- 77.Umbricht D, et al. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol. Psychiatry. 1998;44:716–725. doi: 10.1016/S0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 78.Niznikiewicz MA, et al. Clozapine action on auditory P3 response in schizophrenia. Schizophr. Res. 2005;76:119–121. doi: 10.1016/j.schres.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 79.Alegre M, et al. Atypical antipsychotics normalize low-gamma evoked oscillations in patients with schizophrenia. Psychiatry Res. 2017;247:214–221. doi: 10.1016/j.psychres.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 80.Mitra S, Nizamie SH, Goyal N, Tikka SK. Evaluation of resting state gamma power as a response marker in schizophrenia. Psychiatry Clin Neurosci. 2015;69:630–639. doi: 10.1111/pcn.12301. [DOI] [PubMed] [Google Scholar]
- 81.Sterne JA, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. Published online October. 2016;12:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shea BJ, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. Published Online September. 2017;21:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Itil TM, Marasa J, Saletu B, Davis S, Mucciardi AN. Computerized EEG: predictor of outcome in schizophrenia. J Nerv Ment Dis. 1975;160:118–203. doi: 10.1097/00005053-197503000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Koukkou M, Angst J, Zimmer D. Paroxysmal EEG Activity and Psychopathology During the Treatment with Clozapine. Pharmacopsychiatry. 1979;12:173–183. doi: 10.1055/s-0028-1094608. [DOI] [PubMed] [Google Scholar]
- 85.Knott V, Labelle A, Jones B, Mahoney C. EEG Hemispheric Asymmetry as a Predictor and Correlate of Short-term Response to Clozapine Treatment in Schizophrenia. Clin. Electroencephalogr. 2000;31:145–152. doi: 10.1177/155005940003100308. [DOI] [PubMed] [Google Scholar]
- 86.Galderisi S, Maj M, Mucci A, Bucci P, Kemali D. QEEG alpha1 changes after a single dose of high-potency neuroleptics as a predictor of short-term response to treatment in schizophrenic patients. Biol Psychiatry. 1994;35:367–374. doi: 10.1016/0006-3223(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 87.Kikuchi M, et al. Individual Analysis of EEG Band Power and Clinical Drug Response in Schizophrenia. Neuropsychobiology. 2005;51:183–190. doi: 10.1159/000085593. [DOI] [PubMed] [Google Scholar]
- 88.Gross A, Joutsiniemi SL, Rimon R, Appelberg B. Clozapine-Induced QEEG Changes Correlate with Clinical Response in Schizophrenic Patients: a Prospective, Longitudinal Study. Pharmacopsychiatry. 2004;37:119–122. doi: 10.1055/s-2004-818989. [DOI] [PubMed] [Google Scholar]
- 89.Czobor P, Volavka J. Pretreatment EEG predicts short-term response to haloperidol treatment. Biol Psychiatry. 1991;30:927–942. doi: 10.1016/0006-3223(91)90006-8. [DOI] [PubMed] [Google Scholar]
- 90.Merlo MCG, Kleinlogel H, Koukkou M. Differences in the EEG profiles of early and late responders to antipsychotic treatment in first-episode, drug-naive psychotic patients. Schizophr Res. 1998;30:221–228. doi: 10.1016/S0920-9964(97)00156-4. [DOI] [PubMed] [Google Scholar]
- 91.Ulrich G, Gaebel W, Pietzcker A, Müller-Oerlinghausen B, Stieglitz RD. Prediction of neuroleptic on-drug response in schizophrenic in-patients by EEG. Eur Arch Psychiatry Neurol Sci. 1988;237:144–155. doi: 10.1007/BF00451282. [DOI] [PubMed] [Google Scholar]
- 92.Arıkan MK, Günver G, İlhan R, Öksüz Ö, Metin B. Gamma oscillations predict treatment response to aripiprazole in bipolar disorder. J. Affect Disord. 2021;294:159–162. doi: 10.1016/j.jad.2021.07.044. [DOI] [PubMed] [Google Scholar]
- 93.Lacroix D, et al. Quantified EEG changes associated with a positive clinical response to clozapine in schizophrenia. Prog Neuropsychopharmacol Biol. Psychiatry. 1995;19:861–876. doi: 10.1016/0278-5846(95)00116-D. [DOI] [PubMed] [Google Scholar]
- 94.Kang UG, et al. Non-linear dynamic analysis of clozapine-induced electroencephalographic changes in schizophrenic patients - a preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:1229–1239. doi: 10.1016/S0278-5846(01)00183-X. [DOI] [PubMed] [Google Scholar]
- 95.Kikuchi M, et al. Native EEG and treatment effects in neuroleptic-naïve schizophrenic patients: Time and frequency domain approaches. Schizophr. Res. 2007;97:163–172. doi: 10.1016/j.schres.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 96.Sumiyoshi T, et al. Electrical brain activity and response to olanzapine in schizophrenia: A study with LORETA images of P300. Prog. Neuropsychopharmacol Biol. Psychiatry. 2006;30:1299–1303. doi: 10.1016/j.pnpbp.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 97.Khodayari-Rostamabad A, Hasey GM, MacCrimmon DJ, Reilly JP, Bruin Hde. A pilot study to determine whether machine learning methodologies using pre-treatment electroencephalography can predict the symptomatic response to clozapine therapy. Clin. Neurophysiol. 2010;121:1998–2006. doi: 10.1016/j.clinph.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 98.Masychev K, Ciprian C, Ravan M, Manimaran A, Deshmukh A. Quantitative biomarkers to predict response to clozapine treatment using resting EEG data. Schizophr. Res. 2020;223:289–296. doi: 10.1016/j.schres.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 99.Ciprian C, Masychev K, Ravan M, Reilly JP, Maccrimmon D. A Machine Learning Approach Using Effective Connectivity to Predict Response to Clozapine Treatment. IEEE Trans. Neural Syst. Rehabilit. Engineer. 2020;28:2598–2607. doi: 10.1109/TNSRE.2020.3019685. [DOI] [PubMed] [Google Scholar]
- 100.Dominicus LS, et al. Macroscale EEG characteristics in antipsychotic-naïve patients with first-episode psychosis and healthy controls. Schizophrenia. 2023;9:5. doi: 10.1038/s41537-022-00329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ravan M, Hasey G, Reilly JP, MacCrimmon D, Khodayari-Rostamabad A. A machine learning approach using auditory odd-ball responses to investigate the effect of Clozapine therapy. Clin. Neurophysiol. 2015;126:721–730. doi: 10.1016/j.clinph.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 102.Kural MA, et al. Criteria for defining interictal epileptiform discharges in EEG. Neurology. 2020;94:e2139–e2147. doi: 10.1212/WNL.0000000000009439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pillay S, et al. EEG Abnormalities Before Clozapine Therapy Predict a Good Clinical Response to Clozapine. Ann. Clin. Psychiat. 1996;8:1–5. doi: 10.3109/10401239609149083. [DOI] [PubMed] [Google Scholar]
- 104.Risby ED, et al. Clozapine-induced EEG abnormalities and clinical response to clozapine. J. Neuropsychiat. Clin. Neurosci. 1995;7:466–470. doi: 10.1176/jnp.7.4.466. [DOI] [PubMed] [Google Scholar]
- 105.Wichniak A, Szafrański T, Wierzbicka A, Waliniowska E, Jernajczyk W. Electroencephalogram slowing, sleepiness and treatment response in patients with schizophrenia during olanzapine treatment. J. Psychopharmacol. 2006;20:80–85. doi: 10.1177/0269881105056657. [DOI] [PubMed] [Google Scholar]
- 106.Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J. Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aggarwal M, Wickens JR. A Role for Phasic Dopamine Neuron Firing in Habit Learning. Neuron. 2011;72:892–894. doi: 10.1016/j.neuron.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 108.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-U. [DOI] [PubMed] [Google Scholar]
- 109.Williams S, Boksa P. Gamma oscillations and schizophrenia. J. Psychiat. Neurosci. 2010;35:75–77. doi: 10.1503/jpn.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schijven, D. et al. Large-scale analysis of structural brain asymmetries in schizophrenia via the ENIGMA consortium. Proc. Natl. Acad. Sci.120. 10.1073/pnas.2213880120 (2023). [DOI] [PMC free article] [PubMed]
- 112.Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G. Abnormal Asymmetry of Brain Connectivity in Schizophrenia. Front. Hum. Neurosci. 2014;8:1010. doi: 10.3389/fnhum.2014.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Crow T. Schizophrenia as the price that Homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res. Rev. 2000;31:118–129. doi: 10.1016/S0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 114.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta. Psychiatr. Scand. 1999;99:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 115.Uhlhaas PJ. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr. Opin. Neurobiol. 2013;23:283–290. doi: 10.1016/j.conb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 116.Demjaha, A., & Murray, R. M. Overview of Schizophrenia. In;5-15. 10.1016/B978-0-12-800981-9.00001-8 (2016).
- 117.Salokangas RKR. Symptom dimensions and outcome in schizophrenia. World Psychiat. 2003;2:172–178. [PMC free article] [PubMed] [Google Scholar]
- 118.Rieger, K., Diaz Hernandez, L., Baenninger, A., & Koenig, T. 15 Years of Microstate Research in Schizophrenia – Where Are We? A Meta-Analysis. Front Psychiat.7. 10.3389/fpsyt.2016.00022 (2016). [DOI] [PMC free article] [PubMed]
- 119.Brinkman L, Stolk A, Dijkerman HC, de Lange FP, Toni I. Distinct Roles for Alpha- and Beta-Band Oscillations during Mental Simulation of Goal-Directed Actions. J. Neurosci. 2014;34:14783–14792. doi: 10.1523/JNEUROSCI.2039-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bosman CA, Lansink CS, Pennartz CMA. Functions of gamma-band synchronization in cognition: from single circuits to functional diversity across cortical and subcortical systems. Eur. J. Neurosci. 2014;39:1982–1999. doi: 10.1111/ejn.12606. [DOI] [PubMed] [Google Scholar]
- 121.Hassan WA, et al. P300 cognitive assessment in patients with first-episode psychosis: a prospective case-control study. Middle East Curr. Psychiat. 2020;27:23. doi: 10.1186/s43045-020-00031-2. [DOI] [Google Scholar]
- 122.Leucht S, et al. Linking the PANSS, BPRS, and CGI: Clinical Implications. Neuropsychopharmacology. 2006;31:2318–2325. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
- 123.Casali RL, et al. Comparison of auditory event-related potentials between children with benign childhood epilepsy with centrotemporal spikes and children with temporal lobe epilepsy. Epilepsy Behavior. 2016;59:111–116. doi: 10.1016/j.yebeh.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 124.Seitzman BA, et al. Cognitive manipulation of brain electric microstates. Neuroimage. 2017;146:533–543. doi: 10.1016/j.neuroimage.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Britz J, Van De Ville D, Michel CM. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage. 2010;52:1162–1170. doi: 10.1016/j.neuroimage.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 126.Carbon, M., & Correll, C. U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 16. 10.31887/DCNS.2014.16.4/mcarbon (2014). [DOI] [PMC free article] [PubMed]
- 127.Bozzatello, P., Bellino, S., & Rocca, P. Predictive Factors of Treatment Resistance in First Episode of Psychosis: A Systematic Review. Front. Psychiatry. 10. 10.3389/fpsyt.2019.00067 (2019). [DOI] [PMC free article] [PubMed]
- 128.Brunner, C., Billinger, M., Seeber, M., & Mullen, T. R., Makeig S. Volume Conduction Influences Scalp-Based Connectivity Estimates. Front. Comput. Neurosci. 10. 10.3389/fncom.2016.00121 (2016). [DOI] [PMC free article] [PubMed]
- 129.Horacek J, et al. Mechanism of Action of Atypical Antipsychotic Drugs and the Neurobiology of Schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- 130.Potkin SG, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6:1. doi: 10.1038/s41537-019-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.