Abstract
Hypertension is a major public health issue worldwide. The imbalance of gut microbiota is thought to play an important role in the pathogenesis of hypertension. The authors conducted the systematic review and meta‐analysis to clarify the relationship between gut microbiota and hypertension through conducting an electronic search in six databases. Our meta‐analysis included 19 studies and the results showed that compared with healthy controls, Shannon significantly decreased in hypertension [SMD = −0.13, 95%CI (−0.22, −0.04), p = .007]; however, Simpson [SMD = −0.01, 95%CI (−0.14, 0.12), p = .87], ACE [SMD = 0.18, 95%CI (−0.06, 0.43), p = .14], and Chao1 [SMD = 0.11, 95%CI (−0.01, 0.23), p = .08] did not differ significantly between hypertension and healthy controls. The F/B ratio significantly increased in hypertension [SMD = 0.84, 95%CI (0.10, 1.58), p = .03]. In addition, Shannon index was negatively correlated with hypertension [r = −0.12, 95%CI (−0.19, −0.05)], but had no significant correlation with SBP [r = 0.10, 95%CI (−0.19, 0.37)] and DBP [r = −0.39, 95%CI (−0.73, 0.12)]. At the phylum level, the relative abundance of Firmicutes [SMD = −0.01, 95%CI (−0.37, 0.34), p = .94], Bacteroidetes [SMD = −0.15, 95%CI (−0.44, 0.14), p = .30], Proteobacteria [SMD = 0.25, 95%CI (−0.01, 0.51), p = .06], and Actinobacteria [SMD = 0.21, 95%CI (−0.11, 0.53), p = .21] did not differ significantly between hypertension and healthy controls. At the genus level, compared with healthy controls, the relative abundance of Faecalibacterium decreased significantly [SMD = −0.16, 95%CI (−0.28, −0.04), p = .01], while the Streptococcus [SMD = 0.20, 95%CI (0.08, 0.32), p = .001] and Enterococcus [SMD = 0.20, 95%CI (0.08, 0.33), p = .002] significantly increased in hypertension. Available evidence suggests that hypertensive patients may have an imbalance of gut microbiota. However, it still needs further validation by large sample size studies of high quality.
Keywords: gut microbiota, high throughput sequencing, hypertension, meta‐analysis, systematic review
1. INTRODUCTION
Hypertension (HTN) is one of the most prevalent public health problems in the world, which brings an increasingly serious economic burden to people and society, and it is reported that about one‐third of adults are affected by HTN every year. 1 In addition, the incidence of HTN varies widely and is increasing in different regions. 2 HTN has been proved to be a preventable risk factor for many chronic diseases, for example, ischemic heart disease, chronic kidney disease, dementia, etc. 3 , 4 Understanding the causes of HTN and taking targeted treatment are essential for disease prevention and control. Although the pathogenesis of HTN is complex, lifestyle and environmental factors are recognized as the most common influencing factors of HTN, and the gut microbiota (GM) is closely related to the lifestyle and environmental factors. 5 Therefore, some scholars have proposed that GM may also be risk factors for HTN's development. 6
Microbiota in the human intestinal tract consists of different bacteria classified by phylum, order, class, family, and genus; among them, Firmicutes and Bacteroidetes were the dominant phyla which accounted for 90% of the whole community, then followed by Actinobacteria and Proteobacteria; Firmicutes had 200 different genera, including Faecalibacterium, Enterococcus, Streptococcus, and Ruminococcus; Bacteroides and Prevotella were the main genera of Bacteroidetes. 7 The Firmicutes to Bacteroidetes ratio (F/B ratio) played an important role in maintaining normal intestinal homeostasis. 8 The increase of F/B ratio had been considered as an ecological imbalance, which was usually associated with the occurrence and development of diseases, such as obesity, 9 diabetes, 10 etc., and the increase of F/B ratio will increase the risk of cardiovascular disease. 11 Alpha diversity was the most common indicator for evaluating the health of GM and was also closely related to the disease status of the body. 12 Pinart and coworkers 13 found that the alpha diversity of obese adults was significantly lower than that of non‐obese adults. The latest meta‐analysis results by Choroszy and coworkers 14 showed that the alpha diversity was significantly reduced in patients with coronary heart disease. Reduced alpha diversity in the gut microbiome also appears to be a risk factor for cardiovascular disease.
In recent years, increasing studies have confirmed that GM participated in the occurrence and development of HTN. 15 , 16 A large number of animal studies have shown that GM was related to blood pressure (BP) regulation, and there also was evidence that HTN can be transferred through fecal microbiota transplantation. 17 Studies in human beings have also shown the correlation between BP and intestinal microbial community diversity, as well as composition. 18 However, in the TwinsUK cohort, self‐reported hypertensive patients were not associated with 68 microbiota markers. 19 It can be seen that the results of studies on the correlation between HTN and intestinal microorganisms are still inconsistent, which is worthy of further investigation.
At present, Guo and coworkers 20 had conducted a systematic review of some observational studies to explore if HTN and healthy controls (HC) have different gut microbiomes, but did not conduct a combined analysis of intestinal microbiome parameters. The recent meta‐analysis results of Qin and coworkers 21 indicated that changes in gut microbial parameters may be related to hypertensive patients in China. It is worth noting that although this study conducted meta‐analysis on gut microbial diversity indicators such as Shannon, Simpson, Chao1, ACE, the relative abundance of microbial groups was only semi‐quantitatively analyzed, and no combined analysis was conducted on them. In addition, due to the insufficient measurement depth and differences in measurement methods of the microbiome in the included studies, there was still no clear evidence to identify pathogenic bacteria closely related to HTN. 21
The gut microbiome's complexity can be quantified and unraveled using high‐throughput sequencing techniques, which also known as next‐generation sequencing. 13 With the rapid development of high‐throughput sequencing techniques and the smooth progress of deep sequencing, the importance of GM has been better recognized. 22 In order to reduce the heterogeneity among studies caused by differences in microbiome measurement techniques, this study will focus on the studies, those used high‐throughput sequencing techniques (such as 16S rDNA/rRNA sequencing, shotgun metagenomics) to explore the differences in intestinal microbiota species and quantity between HTN and HC, to provide new ideas and research directions for the prevention and treatment of HTN.
2. METHODS
We conducted this study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses checklist (Supplementary File 1). 23 This review protocol has been registered with PROSPERO (CRD42022355568).
2.1. Literature search
For this study, databases such as Embase, PubMed, Cochrane Library, Web of Science, Chinese Wanfang Database, and China National Knowledge Infrastructure (CNKI) were used for retrieval of the relevant literature from the inception of each database to March 2023. The information retrieval languages with access to the database were limited to Chinese and English. Determined relevant studies on the changes of intestinal microbiota between HTN and HC. To supplement and obtain relevant literature, we traced the references of the included literature as well as the articles citing it. Subject terms applied for the search were input together with free words, and the search words included: “Hypertension”, “High Blood Pressure”, “Gastrointestinal Microbiome”, “gut microflora”, “gastrointestinal flora”, “intestinal microflora”, “intestinal flora” and “enteric bacteria”. An complete strategy of the search can be found in Supplementary File 2.
2.2. Inclusion criteria
(1) The experimental group was adults with HTN (age ≥18 years); (2) The control group was HC; (3) A high‐throughput analysis of fecal samples was conducted to determine the intestinal microbiome (such as 16S rDNA/rRNA sequencing and shotgun metagenomics); (4) The primary outcome measures included gut microbiome alpha diversity (including indicators measuring community diversity such as Shannon index and Simpson index, and indicators estimating community richness such as ACE index and Chao1 index), 24 and F/B ratio. The secondary outcome measures were the abundance of microbial community at the level of phylum and genus, which are included in the meta‐analysis when the number of included studies was ≥3; (5) Design of the study: case‐control, cross‐sectional, or cohort; (6) Be able to obtain the mean and standard deviation of alpha diversity and F/B ratio in these two groups or have enough data to calculate these. In addition, the study that reported the correlation between GM and HTN, which with an effect indicator of Pearson's coefficient (r) or sufficient data to calculate it was also included in this study.
2.3. Exclusion criteria
(1) Pregnant women were selected as the study population; (2) The control group had a history of HTN; (3) Studies using duplicate samples; (4) Studies that focused on associations with diseases other than hypertension, such as kidney disease, liver disease, sleep apnea, or oral microbiota; (5) The research was published as a review, abstract, commentary, or editorial.
2.4. Literature screening
Two researchers screened the literature as per inclusion and exclusion criteria independently. Firstly, literature management and duplication screening were performed with Endnote X9. Secondly, we removed those studies that weren't clearly relevant to the inclusion criteria after reading the abstract and title. Finally, we read the full text. Two researchers independently identified and cross‐checked to finally confirm the qualified study. In case of disagreement, a third reviewer helped resolve the issue.
2.5. Data extraction
Two researchers extracted and cross‐checked the date independently. In case of disagreement, a third reviewer helped resolve the issue. The data extracted included: (1) General information (author, publication year, location of the study, study design); (2) Patient characteristics (sex, age, body mass index, systolic blood pressure (SBP), diastolic blood pressure (DBP)); (3) Experimental methods (diagnostic criteria, sample size, microbiological evaluation techniques); (4) Outcome indicators: the index of Shannon, Simpson, ACE, and Chao1; F/B ratio; GM's relative abundance of phylum and genus levels. Contacted the corresponding author or used GetData Graph Digitizer 2.25 for digital processing to obtain sufficient data when necessary. 25
2.6. Quality assessment
Two researchers used the Newcastle Ottawa Quality Assessment Scale to assess the quality of the literature, and a third reviewer helped resolve the differences when necessary. The NOS included three criteria: selectivity, comparability, and exposure. 26 Each study can receive up to nine stars. The study with a score of ≥7 was considered of good quality, with a score of 5−6 of average quality, and a score of 0−4 was of poor quality.
2.7. Statistical analysis
We used RevMan 5.3 software for data analysis. For data that could be extracted to mean and standard deviation, we used standardized mean differences (SMD) with 95% confidence intervals (95% CI) as effect statistics. When the data reported was the Spearman's coefficient (rs), the conversion was performed by the formula r = 2sin(rs*π/6). 27 When the reported data were the beta coefficient, the conversion was performed according to the formula r = 0.998β + 0.05λ, where λ was 0 when β was negative and λ was 1 when β was positive. 28 For the data of correlation coefficient r value, we first performed Fisher's Z transformation and pooled to obtain summary fisher's Z value, and then calculated summary r value and 95%CI by inverse fisher's Z transformation. 29 If p < .05, it was considered statistically significant. Determined the existence of heterogeneity by I 2 tests. When there was good homogeneity among the included studies (I 2 < 50%, p > .1), we used the fixed effects model. Conversely, we used the random effects model if the included studies were significantly heterogeneous (I 2 ≥ 50%, p ≤ .1). To explore the source of heterogeneity, we conducted subgroup analysis through sample size, study population and sequencing method. We changed the effects model or excluded the study one by one to perform a sensitivity analysis. We also used funnel plots to investigate whether there was a publication bias.
3. RESULTS
3.1. The results of literature search
We retrieved 6681 articles from online databases. Of these, 1695 were duplicates and 4986 required preliminary screening. Upon reading the titles and abstracts, 4952 articles were eliminated. We read the remaining 34 studies fully and brought 19 eligible studies into this review finally. 18 , 19 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 Of the 19 studies included, 18 were from the PubMed database, 18 , 19 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 and one was from the CNKI database. 46 Figure 1 shows an illustration of the detailed selection process.
FIGURE 1.
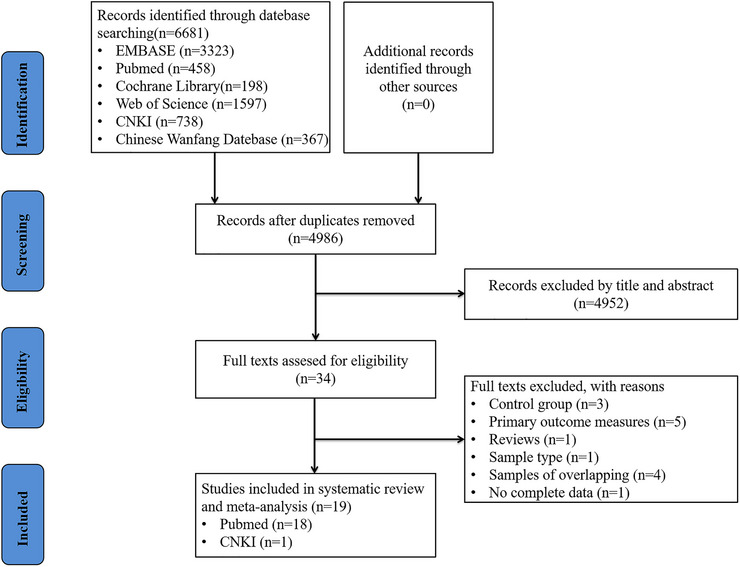
Literature screening process and results.
3.2. Study characteristics
Table 1 summarized and showed the study characteristics. The included studies were all published between 2017 and 2022. Study patients included 17944 individuals. The study location involved multiple countries, including 11 in China, 30 , 33 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 46 one in US, 18 one in Britain, 19 one in Spain, 31 one in Japan, 32 one in Australia, 34 one in Brazil, 35 one in Finland, 44 and one in Netherlands. 45 Eighteen were published in English, 18 , 19 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 and one in Chinese. 46 The 16S rRNA gene sequencing technology was used in 16 studies, 18 , 19 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 40 , 41 , 42 , 43 , 45 , 46 and the metagenomic shotgun sequencing technology was used in three studies. 38 , 39 , 44 In addition, 11 studies were case‐control studies, 30 , 32 , 33 , 34 , 35 , 36 , 37 , 39 , 41 , 42 , 46 seven were cohort studies, 18 , 19 , 38 , 40 , 43 , 44 , 45 and one was cross‐sectional study. 31
TABLE 1.
Included study characteristics for this meta‐analysis.
| Sex (Male/Female) | Age (Year, M ± SD) | BMI (Kg/m2, M ± SD) | SBP (mm Hg, M ± SD) | DBP (mm Hg, M ± SD) | Definition | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study/Location | HTN | HC | HTN | HC | p | HTN | HC | p | HTN | HC | p | HTN | HC | p | HTN | HC | BPLT | Measure‐ment method |
|
Mushtaq and coworkers (2019) China |
28/22 | 16/14 | 62.5 ± 10.4 | 60.5 ± 11.0 | .47 | NA | NA | NA | 180.34 ± 15.44 | 122.83 ± 7.6 | <.001 | 106.88 ± 10.1 | 79.63 ± 6.8 | <.001 | Patients with grade 3 hypertension | Healthy volunteers | NO | 1 |
|
Calderón‐Pérez and coworkers (2020) Spain |
10/19 | 16/16 | 53.7 ± 9.6 | 41.1 ± 9.1 | <.001 | 26.2 ± 2.5 | 23.8 ± 2.7 | <.001 | 153.1 ± 14.6 | 109.7 ± 7.1 | <.001 | 91.0 ± 8.8 | 65.7 ± 6.7 | <.001 | SBP between 140 and 159 mm Hg | SBP < 120 mm Hg | NO | 1 |
|
Takagi and coworkers (2020) Japan |
49/48 | 21/33 | 68.2 ± 10.0 | 63.2 ± 15.8 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | SBP≥140 mm Hg, DBP≥90 mm Hg, or current use of antihypertensive drugs | Healthy volunteers | NA | 1 |
|
Zhu and coworkers (2020) China |
67/54 | 54/50 | 54.6 ± 9.6 | 52.4 ± 7.9 | NA | 23.8 ± 0.9 | 23.7 ± 1.1 | NA | 146.95 ± 13.17 | 118.70 ± 3.72 | NA | 83.44 ± 11.00 | 78.15 ± 4.59 | NA | Patients with grade 3 hypertension | Healthy volunteers | YES | 1 |
|
Nakai and coworkers (2021) Australia |
15/8 | 16/31 | 60.3 ± 6.6 | 59.2 ± 7.7 | >.05 | 26.0 ± 2.6 | 24.9 ± 3.0 | >.05 | 139.7 ± 10.1 | 114.3 ± 8.5 | <.001 | 83.1 ± 6.3 | 69.6 ± 5.1 | <.001 | Hypertension was diagnosed using the European guidelines | Normotensive participants | NO | 1 |
|
Silveira‐Nunes and coworkers (2020) Brazil |
14/34 | 7/25 | 65.3 ± 15.5 | 63.3 ± 15.0 | .561 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
SBP>140 mm Hg, DBP>90 mm Hg |
Normotensive participants | YES | 1 |
|
Wan and coworkers (2021) China |
143/157 | 165/135 | 61.6 ± 11.9 | 62.0 ± 11.8 | >.05 | 20.47 ± 2.01 | 20.62 ± 1.85 | >.05 | NA | NA | NA | NA | NA | NA | BP>140/90 mm Hg | Healthy volunteers | NO | 1 |
|
Dan and coworkers (2019) China |
26/41 | 29/33 | 69.5 ± 9.6 | 69.3 ± 10.6 | .509 | 25.05 ± 4.44 | 26.09 ± 3.11 | .169 | 153.30 ± 14.92 | 122.94 ± 6.90 | <.05 | 84.31 ± 10.74 | 76.21 ± 6.90 | <.05 | SBP≥140 mm Hg or DBP≥90 mm Hg | Normal BP population | NO | 1 |
|
Li and coworkers (2017) China |
93/6 | 32/9 | 53.6 ± 5.5 | 53.7 ± 5.9 | .88 | 26 ± 3.5 | 25.2 ± 3.3 | .15 | 148.8 ± 14.2 | 115.3 ± 7.4 | <.001 | 94.7 ± 9.2 | 74.1 ± 6.5 | <.001 | SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg | SBP≤125 mm Hg, DBP≤80 mm Hg | NO | 2 |
|
Yan and coworkers (2017) China |
25/35 | 28/32 | 57.0 ± 9.6 | 56.0 ± 8.6 | .523 | 23.5 ± 2.9 | 23.4 ± 2.6 | .854 | 163.68 ± 6.86 | 161.78 ± 8.21 | <.001 | 23.5 ± 2.9 | 23.4 ± 2.6 | <.001 | Current BP ≥140/90 mm Hg | Current BP≤120/80 mm Hg | NO | 2 |
|
Qu and coworkers (2022) China |
32/31 | 18/16 | 59.8 ± 4.6 | 59.2 ± 6.2 | >.05 | 25.91 ± 2.93 | 24.82 ± 2.28 | >.05 | 159.06 ± 20.34 | 123.67 ± 5.83 | <.05 | 91.48 ± 12.89 | 77.31 ± 7.90 | <.05 |
(1) Without BPLT, SBP≥140 g and/or DBP≥90 mm Hg; (2) With a history of HTN, and have BPLT at present |
Spouses of Hypertension patients(Healthy people) | NA | 1 |
|
Wang JM and coworkers (2021) China |
29/64 | 3/12 | 61.4 ± 4.4 | 56.3 ± 7.7 | .299 | 24.87 ± 4.95 | 24.29 ± 0.71 | .169 | 144.69 ± 9.29 | 113.6 ± 9.0 | <.001 | 88.61 ± 8.15 | 75.14 ± 5.98 | <.001 | Be diagnosed as stage 1 hypertension | Healthy people | NO | 1 |
|
Liu and coworkers (2021) China |
16/10 | 16/10 | 56.9 ± 6.9 | 50.1 ± 6.0 | <.001 | 24.1 ± 5.1 | 23.5 ± 3.5 | .558 | 140.9 ± 14.3 | 122.7 ± 13.2 | <.001 | 83.2 ± 8.2 | 77.7 ± 9.6 | .031 | SBP≥140 mm Hg or DBP≥90 mm Hg | SBP≤139 mm Hg and DBP≤89 mm Hg | NA | 1 |
|
Chen and coworkers (2021) China |
23/15 | 10/10 | 46.6 ± 15.9 | 42.0 ± 14.1 | NA | 24.42 ± 1.98 | 23.37 ± 2.57 | NA | 151 ± 13.79 | 115.64 ± 9.72 | NA | 88.37 ± 12.76 | 73.45 ± 6.28 | NA | Essential hypertension was first diagnosed clinically | HC | NO | 1 |
|
Jackson and coworkers (2018) Britain |
301/2436 | 60 ± 12 | 26 ± 5 | NA | NA | A doctor or health professional had ever diagnosed. | NA | NA | 1 | |||||||||
|
Palmu and coworkers (2020) Finland |
3134/3819 | 49.2 ± 12.9 | 27 ± 4.66 | 135.6 ± 20.2 | 79.1 ± 11.2 | SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg, or use of antihypertensive medication | NA | NA | 2 | |||||||||
|
Verhaar and coworkers (2020) Netherlands |
2243/2429 | 49.8 ± 11.7 | 27.2 ± 4.9 | 129.9 ± 18.2 | 81.1 ± 10.6 | SBP > 140 mm Hg or DBP > 90 mm Hg or self‐reported use of BP‐lowering medication | NA | NA | 1 | |||||||||
|
Wang Y and coworkers (2021) China |
493/589 | 51.0 ± 9.1 | 23.8 ± 3.2 | 126.0 ± 17.4 | 80.7 ± 10.7 |
SBP≥140 mm Hg, DBP≥90 mm Hg, or self‐reported diagnosis |
NA | NO | 1 | |||||||||
|
Sun and coworkers (2020) USA |
244/285 | 55.3 ± 3.4 | 29.4 ± 6.3 | 119.4 ± 15.8 | 72.9 | current use of antihypertensive medication, SBP≥140 mm Hg, and/or a DBP≥90 mm Hg | NA | NA | 1 | |||||||||
Abbreviations: BMI, body mass index; BP, blood pressure; BPLT, blood‐pressure‐lowering treatment; DBP, diastolic blood pressure; HC, health control; HTN, Hypertension; M, Mean; NA, No Application; SBP, systolic blood pressure; SD, standard deviation; 1,16S rRNA gene sequencing; 2, shotgun metagenomic sequencing.
3.3. Quality assessment
Detailed results of the studies’ methodological quality were shown in Table 2. Fifteen of the 19 included studies were evaluated as “good quality”, 18 , 30 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 43 , 44 , 45 , 46 and four as “average quality”. 19 , 31 , 32 , 42
TABLE 2.
Methodological quality results of the included studies.
| Selection | Comparability | Exposure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | A | B | C | D | E | F | G | H | I | Score |
| Mushtaq and coworkers (2019) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
| Calderón‐Pérez and coworkers (2020) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | |||
| Takagi and coworkers (2020) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | |||
| Zhu and coworkers (2020) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | |
| Nakai and coworkers (2021) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
| Silveira‐Nunes and coworkers (2020) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | |
| Wan and coworkers (2021) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | |
| Dan and coworkers (2019) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | |
| Li and coworkers (2017) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 8 | |
| Yan and coworkers (2017) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
| Qu and coworkers (2022) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
| Wang JM and coworkers (2021) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
| Liu and coworkers (2021) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | |||
| Chen and coworkers (2021) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
| Jackson and coworkers (2018) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | |||
| Palmu and coworkers (2020) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
| Verhaar and coworkers (2020) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
| Wang Y and coworkers (2021) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
| Sun and coworkers (2020) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | ||
Note: A, adequate case definition or representativeness of the exposed cohort; B, representativeness of cases or selection of non‐exposed queues; C, selection of controls or determination of exposure; D, definition of controls or no outcome event occurred before the start of the research object; E, controlled for age; F, controlled for additional factors; G, ascertainment of exposure or evaluation of outcome events; H, same method for cases and controls or adequacy of follow‐up; I, non‐response rate or integrity of follow‐up.
3.4. The results of the meta‐analysis
3.4.1. Alpha diversity
Shannon index
Thirteen studies reported changes in the Shannon index in hypertensive patients’ intestinal flora. 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 46 The heterogeneity of studies was low (I 2 = 18%, p = .26), and we used a fixed effects model for analysis. We found that the Shannon index of HTN was significantly lower than HC [SMD = −0.13, 95%CI (−0.22, −0.04), p = .007] (Figure 2A). The number of studies reporting the correlation between Shannon index and HTN, 18 , 19 , 44 SBP, 18 , 43 , 44 , 45 and DBP 43 , 44 , 45 was 3,4,3, respectively. The heterogeneity among studies was significant (I2 = 92%, I2 = 99%, I2 = 100%), and we used a random effects model for analysis (Figure 3). After r was calculated by inverse Fisher's Z transformation, we found that Shannon index was negatively correlated with hypertension [r = −0.12, 95%CI (−0.19, −0.05)], but had no significant correlation with SBP [r = 0.10, 95%CI (−0.19, 0.37)] and DBP [r = −0.39, 95%CI (−0.73, 0.12)] (Table 3).
FIGURE 2.
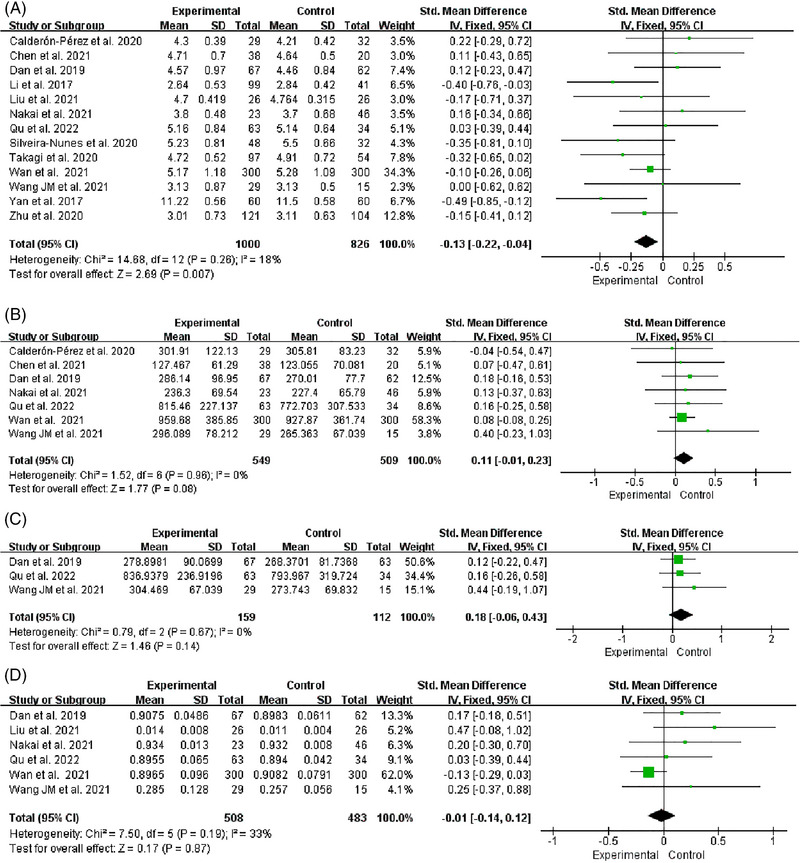
Forest plot of the Alpha diversity. (A) Shannon. (B) Chao1. (C) ACE. (D) Simpson.
FIGURE 3.
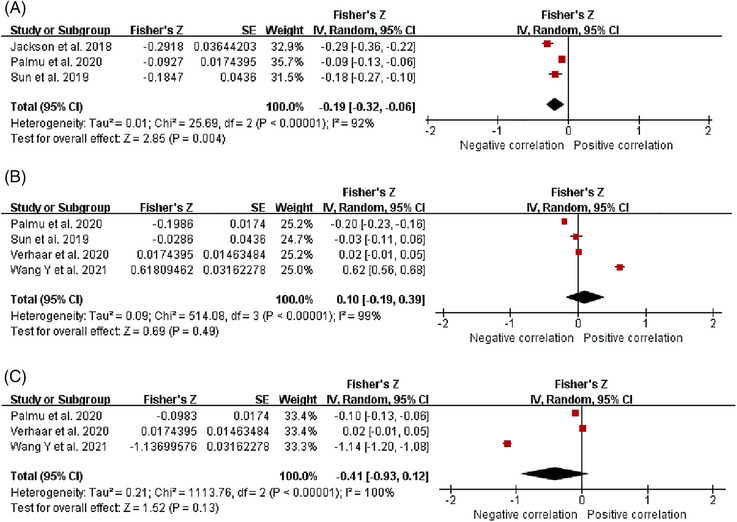
Fisher's Z comparison results of Shannon with HTN, SBP, and DBP. (A) HTN. (B) SBP. (C) DBP.
TABLE 3.
The correlation between Shannon index and HTN, SBP and DBP.
| Shannon | ||
|---|---|---|
| r | 95%CI | |
| HTN | ‐0.12 | ‐0.19, ‐0.05 |
| SBP | 0.10 | ‐0.19, 0.37 |
| DBP | ‐0.39 | ‐0.73, 0.12 |
Abbreviations: DBP, diastolic blood pressure; HTN, hypertension; SBP, systolic blood pressure.
Chao1 index
Seven studies reported changes in the Chao1 index in hypertensive patients’ intestinal flora. 31 , 34 , 36 , 37 , 40 , 41 , 46 The heterogeneity of studies was low (I2 = 0%, p = .96), and we used a fixed effects model for analysis. According to the results, the Chao1 index did not differ significantly between HTN and HC [SMD = 0.11, 95%CI (−0.01, 0.23), p = .08] (Figure 2B).
ACE index
Three studies reported changes in the ACE index in hypertensive patients’ intestinal flora. 37 , 40 , 41 The heterogeneity of studies was low (I2 = 0%, p = .67), and we used a fixed effects model for analysis. According to the results, the ACE index did not differ significantly between HTN and HC [SMD = 0.18, 95%CI (−0.06, 0.43), p = .14] (Figure 2C).
Simpson index
Six studies reported changes in the Simpson index in hypertensive patients’ intestinal flora. 34 , 36 , 37 , 40 , 41 , 42 The heterogeneity of studies was low (I2 = 33%, p = .19), and we used a fixed effects model for analysis. According to the results, the Simpson index did not differ significantly between HTN and HC [SMD = −0.01, 95%CI (−0.14, 0.12), p = .87] (Figure 2D).
Sensitivity analysis
We conducted sensitivity analysis by eliminating the study of Wan and coworkers, 36 which had the largest proportion of effect size, and the results showed no significant change in Shannon index [SMD = −0.15, 95%CI (−0.26, −0.03), p = .01], Chao1 index [SMD = 0.15, 95%CI (−0.04, 0.34), p = .13], and Simpson index [SMD = 0.19, 95%CI (−0.02, 0.39), p = .07]. In addition, we changed the effects model for sensitivity analysis, and the results did not significantly change in Shannon index [SMD = −0.13, 95%CI (−0.24, −0.02), p = .02], Chao1 index [SMD = 0.11, 95%CI (−0.01, 0.23), p = .08], ACE index [SMD = 0.18, 95%CI (−0.06, 0.43), p = .14], and Simpson index [SMD = 0.07, 95%CI (−0.12, 0.26), p = .46]. The sensitivity analysis showed that the results were relatively stable (Table 4). We conducted sensitivity analysis by eliminating literatures one by one and found that after removing the study of Sun and coworkers, 18 the 95%CI of Fisher's Z value of Shannon index and HTN was (−0.38, 0.01), with no statistical significance and indicating that the result was unstable.
TABLE 4.
The sensitivity analysis results of alpha diversity.
| Before sensitivity analysis | After sensitivity analysis | |||||
|---|---|---|---|---|---|---|
| Outcome | Effect estimate | p | I 2 (%) | Effect estimate | p | I 2 (%) |
| Shannon index | −0.13 (−0.22, −0.04) | .007 | 18 | −0.15 (−0.26, −0.03) a | .01 | 24 |
| −0.13 (−0.24, −0.02) b | .02 | 18 | ||||
| Chao1 index | 0.11 (−0.01, 0.23) | .08 | 0 | 0.15 (−0.04, 0.34) a | .13 | 0 |
| 0.11 (−0.01, 0.23) b | .08 | 0 | ||||
| ACE index | 0.18 (−0.06, 0.43) | .14 | 0 | 0.18 (−0.06, 0.43) b | .14 | 0 |
| Simpson index | −0.01 (−0.14, 0.12) | .87 | 33 | 0.19 (−0.02, 0.39) a | .07 | 0 |
| 0.07 (−0.12, 0.26) b | .46 | 33 | ||||
Is the effect estimate after eliminating the study of Wan and coworkers
Is the effect estimate after changing the effect model.
3.4.2. F/B ratio
Five studies reported the changes of the F/B ratio in hypertensive patients’ intestinal flora. 30 , 33 , 35 , 38 , 41 The heterogeneity among studies was significant (I2 = 92%, p < .001), and we used a random effects model for analysis. We found that the F/B ratio has a significant increase in HTN compared with HC [SMD = 0.84, 95%CI (0.10, 1.58), p = .03] (Figure 4).
FIGURE 4.
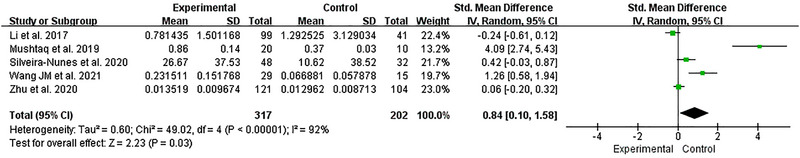
Forest plot of the F/B ratio.
When stratified according to sample size, study population, and sequencing method, the small sample group (n < 100) 30 , 35 , 41 [SMD = 1.78, 95%CI (0.22, 3.35), p = .03], the Chinese group 30 , 33 , 38 , 41 [SMD = 1.03, 95%CI (0.06, 2.00), p = .04], and the 16S rRNA sequencing group 30 , 33 , 35 , 41 [SMD = 1.22, 95%CI (0.23, 2.22), p = .02] both showed that the F/B ratio was significantly higher in patients with hypertension. However, the large sample group (n ≥ 100) 33 , 38 [SMD = −0.06, 95%CI (−0.35, 0.23), p = .67], the Brazilian group 35 [SMD = 0.42, 95%CI (−0.03, 0.87), p = .07], and the shotgun metagenomic sequencing group 38 [SMD = −0.24, 95%CI (−0.61, 0.12), p = .20] had no significant difference. There was significant heterogeneity between the subgroups of sample size (I2 = 80.6%, p = .02) and sequencing method (I2 = 86.5%, p = .007), indicating that sample size and sequencing method were not the sources of heterogeneity. The heterogeneity between the subgroups of study population was not significant (I2 = 19.8%, p = .26), indicating that study population may be the source of heterogeneity (Table 5).
TABLE 5.
Subgroup analysis for the F/B ratio.
| SMD (95% CI) | Heterogeneity | Subgroup differences | |||||
|---|---|---|---|---|---|---|---|
| Categories | Included study | Effect estimate | p−value | I 2 (%) | p−value | I 2 (%) | p−value |
| Sample size | 0.84 (0.10, 1.58) | .03 | 92 | <.001 | 80.6 | .02 | |
| Large sample group | Li and coworkers 2017 | −0.06 (−0.35, 0.23) | .67 | 42 | .19 | ||
| Zhu and coworkers 2020 | |||||||
| Small sample group | Mushtaq and coworkers 2019 | 1.78 (0.22, 3.35) | .03 | 93 | <.001 | ||
| Silveira‐Nunes and coworkers 2020 | |||||||
| Wang JM and coworkers 2021 | |||||||
| Study population | 0.84 (0.10, 1.58) | .03 | 92 | <.001 | 19.8 | .26 | |
| Chinese | Li and coworkers 2017 | 1.03 (0.06, 2.00) | .04 | 94 | <.001 | ||
| Zhu and coworkers 2020 | |||||||
| Mushtaq and coworkers 2019 | |||||||
| Wang JM and coworkers 2021 | |||||||
| Brazilians | Silveira‐Nunes and coworkers 2020 | 0.42 (−0.03, 0.87) | .07 | N/A | N/A | ||
| Sequencing methods | 0.84 (0.10, 1.58) | .03 | 92 | <.001 | 86.5 | .007 | |
| 16S rRNA sequencing | Zhu and coworkers 2020 | 1.22 (0.23, 2.22) | .02 | 93 | <.001 | ||
| Mushtaq and coworkers 2019 | |||||||
| Silveira‐Nunes and coworkers 2020 | |||||||
| Wang JM and coworkers 2021 | |||||||
| Shotgun metagenomic sequencing | Li and coworkers 2017 | −0.24 (−0.61, 0.12) | .20 | N/A | N/A | ||
Abbreviations: CI, confidence interval; N/A, not applicable; SMD, standardized mean difference.
3.4.3. The relative abundance of GM at phylum level
Firmicutes
Four studies reported changes in the relative abundance of Firmicutes in hypertensive patients. 32 , 33 , 38 , 41 The heterogeneity among studies was significant (I2 = 73%, p = .01), and we used a random effects model for analysis. We found that the relative abundance of Firmicutes did not differ significantly between HTN and HC [SMD = −0.01, 95%CI (−0.37, 0.34), p = .94] (Figure 5A). We used one‐by‐one elimination method for sensitivity analysis. When the study of Wang and coworkers 41 was excluded, no significant changes were observed in the combined results [SMD = −0.17, 95%CI (−0.38, 0.05), p = .13]; however, we observed a decrease in heterogeneity (I2 = 28%, p = .25), suggesting that heterogeneity might be caused by this study (Table 6).
FIGURE 5.
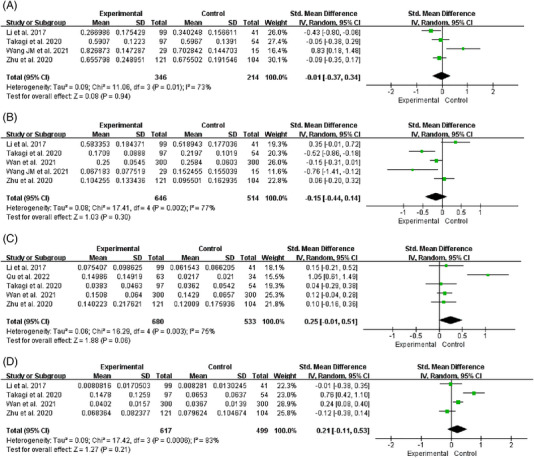
Forest plot of relative abundance of gut microbiota at phylum level. (A) Firmicutes. (B) Bacteroidetes. (C) Proteobacteria. (D) Actinobacteria.
TABLE 6.
The sensitivity analysis results of Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria.
| Before sensitivity analysis | After sensitivity analysis | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Effect estimate | p | I 2 (%) | Remove study | Effect estimate | p | I 2 (%) |
| Firmicutes | −0.01 (−0.37, 0.34) | .94 | 73 | Wang JM and coworkers 2021 | −0.17 (−0.38, 0.05) | .13 | 28 |
| Li and coworkers 2017 | 0.01 (−0.19, 0.21) | .91 | 71 | ||||
| Takagi and coworkers 2020 | −0.10 (−0.30, 0.10) | .33 | 82 | ||||
| Zhu and coworkers 2020 | −0.09 (−0.32, 0.14) | .47 | 82 | ||||
| Bacteroidetes | −0.15 (−0.44, 0.14) | .30 | 77 | Li and coworkers 2017 | −0.26 (−0.53, 0.02) | .07 | 71 |
| Takagi and coworkers 2020 | −0.05 (−0.35, 0.25) | .73 | 73 | ||||
| Wan and coworkers 2021 | −0.17 (−0.62, 0.27) | .44 | 83 | ||||
| Wang JM and coworkers 2021 | −0.07 (−0.35, 0.21) | 0.63 | 78 | ||||
| Zhu and coworkers 2020 | −0.22 (−0.60, 0.15) | .25 | 80 | ||||
| Proteobacteria | 0.25 (−0.01, 0.51) | .06 | 75 | Qu and coworkers 2022 | 0.11 (−0.01, 0.23) | .07 | 0 |
| Li and coworkers 2017 | 0.28 (−0.04, 0.61) | .09 | 82 | ||||
| Takagi and coworkers 2020 | 0.31 (−0.01, 0.64) | .06 | 81 | ||||
| Wan and coworkers 2021 | 0.25 (−0.01, 0.51) | .06 | 75 | ||||
| Zhu and coworkers 2020 | 0.31 (−0.05, 0.66) | .09 | 81 | ||||
| Actinobacteria | 0.21 (−0.11, 0.53) | .21 | 83 | Takagi and coworkers 2020 | 0.06 (−0.19, 0.31) | .64 | 65 |
| Li and coworkers 2017 | 0.28 (−0.13, 0.68) | .18 | 87 | ||||
| Wan and coworkers 2021 | 0.20 (−0.34, 0.74) | .46 | 88 | ||||
| Zhu and coworkers 2020 | 0.32 (−0.05, 0.70) | .09 | 81 | ||||
Bacteroidetes
Five studies reported changes in the relative abundance of Bacteroidetes in hypertensive patients. 32 , 33 , 36 , 38 , 41 The heterogeneity among studies was significant (I2 = 77%, p = .002), and we used a random effects model for analysis. We found that the relative abundance of Bacteroidetes did not differ significantly between HTN and HC [SMD = −0.15, 95%CI (−0.44, 0.14), p = .30] (Figure 5B). We used one‐by‐one elimination method for sensitivity analysis and no significant changes were observed in the combined results, suggesting that the results were relatively stable (Table 6).
Proteobacteria
Five studies reported changes in the relative abundance of Proteobacteria in hypertensive patients. 32 , 33 , 36 , 38 , 40 The heterogeneity among studies was significant (I2 = 75%, p = .003), and we used a random effects model for analysis. We found that the relative abundance of Proteobacteria did not differ significantly between HTN and HC [SMD = 0.25, 95%CI (−0.01, 0.51), p = .06] (Figure 4C). We used one‐by‐one elimination method for sensitivity analysis. After excluding the study of Qu and coworkers, 40 no significant changes were observed in the combined results [SMD = 0.11, 95%CI (−0.01, 0.23), p = .07], however, we observed a decrease in heterogeneity (I2 = 0%, p = .97), suggesting that heterogeneity might be caused by this study (Table 6).
Actinobacteria
Four studies reported changes in the relative abundance of Actinobacteria in hypertensive patients. 32 , 33 , 36 , 38 The heterogeneity among studies was significant (I2 = 83%, p < .001), and we used a random effects model for analysis. We found that the relative abundance of Actinobacteria did not differ significantly between HTN and HC [SMD = 0.21, 95%CI (−0.11, 0.53), p = .21] (Figure 5D). We used one‐by‐one elimination method for sensitivity analysis. After excluding the study of Takagi and coworkers, 32 no significant changes were observed in the combined results [SMD = 0.06, 95%CI (−0.19, 0.31), p = .64], however, we observed a decrease in heterogeneity (I2 = 65%, p = .06), suggesting that heterogeneity might be caused by this study (Table 6).
3.4.4. The relative abundance of GM at genus level
Faecalibacterium
Four studies reported changes in the relative abundance of Faecalibacterium in hypertensive patients. 33 , 36 , 38 , 39 The homogeneity among studies was good (I2 = 0%, p = .46), and we used a fixed effects model for analysis. We found that the relative abundance of Faecalibacterium was significantly lower in HTN than HC [SMD = −0.16, 95%CI (−0.28, −0.04), p = .01] (Figure 6A). We changed the effects model for sensitivity analysis, and the results did not differ, suggesting a relatively stable result [SMD = −0.16, 95%CI (−0.28, −0.04), p = .01].
FIGURE 6.
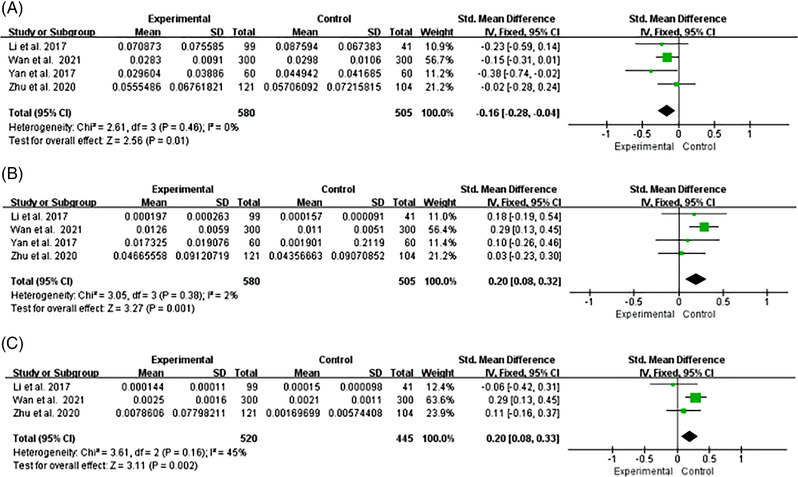
Forest plot of relative abundance of gut microbiota at genus level. (A) Faecalibacterium. (B) Streptococcus. (C) Enterococcus.
Streptococcus
Four studies reported changes in the relative abundance of Streptococcus in hypertensive patients. 33 , 36 , 38 , 39 The homogeneity among studies was good (I2 = 2%, p = .38), and we used a fixed effects model for analysis. We found that the relative abundance of Streptococcus in HTN was higher significantly as compared with HC [SMD = 0.20, 95%CI (0.08, 0.32), p = .001] (Figure 6B). We changed the effects model for sensitivity analysis, and the results did not differ, suggesting a relatively stable result [SMD = 0.20, 95%CI (0.08, 0.32), p = .001].
Enterococcus
Three studies reported changes in the relative abundance of Enterococcus in hypertensive patients. 33 , 36 , 38 The homogeneity among studies was good (I2 = 45%, p = .16), and we used a fixed effects model for analysis. We found that the relative abundance of Enterococcus in HTN was higher significantly as compared with HC [SMD = 0.20, 95%CI (0.08, 0.33), p = .002] (Figure 6C). We changed the effects model for sensitivity analysis, and the results changed, suggesting an unstable result [SMD = 0.16, 95%CI (−0.03, 0.36), p = .10].
3.5. Publication bias
The funnel plots were drawn for those outcome indicators which had more than five included studies. The funnel plots of Shannon index, Chao1 index, and Simpson index looked symmetrical, indicating no significant publication bias risk (Figure 7A–C).
FIGURE 7.
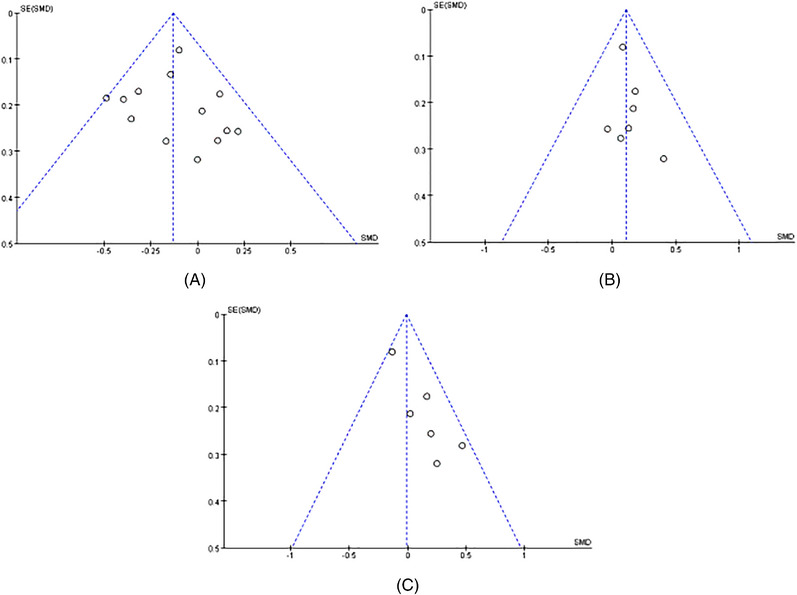
(A) Funnel plot of Shannon. (B) Funnel plot of Chao1. (C) Funnel plot of, Simpson.
4. DISCUSSION
As far as we know, this is the first meta‐analysis to explore if HTN and HC have different gut microbiomes, with emphasis on the application of high‐throughput sequencing techniques. This systematic review and meta‐analysis analyzed of 19 studies involving 17944 individuals. We observed that compared with healthy people, the microbiota diversity was significantly reduced and the F/B ratio was significantly increased in hypertensive patients. Shannon index was negatively correlated with HTN, but had no significant correlation with SBP and DBP. In addition, at the phylum level, the relative abundance of Faecalibacterium was significantly decreased, while at the genus level, the relative abundance of Streptococcus and Enterococcus was significantly increased. These results suggested that GM imbalance may exist in hypertensive patients.
In this study, the Shannon index of hypertensive patients decreased significantly, which was partially consistent with the results of Yang and coworkers 47 The difference was that there was no significant difference in Chao1 index between HTN and HC in this study. This result suggested that although the diversity of GM in hypertensive patients had been damaged to some extent, the richness had been retained as a whole. 21 In addition, we also found that there was a negative correlation between the Shannon index and HTN, which was consistent with the reported significant decrease in the Shannon index of GM in hypertensive patients in this study. However, after removing the study of Sun and coworkers, 18 the correlation between Shannon index and HTN was not statistically significant, and we speculated that it may be related to the difference in BMI of the study population. Because BMI may affect the composition of GM, 13 and it still needs to be demonstrated by rigorous design and high‐quality research in the future. Nevertheless, the findings of our study revealed that the diversity of GM may be closely related to the progression of HTN.
In our study, the F/B ratio of hypertensive patients increased significantly. The increase of F/B ratio has been widely considered as a sign of GM imbalance and has been related to obesity, diabetes, and cardiovascular disease. 47 This finding was consistent with the findings of our study, we observed a significant increase in the F/B ratio in hypertensive patients, indicating that hypertensive patients may have intestinal flora disorders. The mechanism of GM disorder leading to hypertension may be related to the following aspects. Firstly, the imbalance of GM impaired the host's gut barrier function, making microbial products penetrate into mesentery fat, trigger immune response, and stimulate the production of inflammatory cytokines. 48 Inflammation promoted oxidative stress, which can activate immune cells, and the immune cells in turn released reactive oxygen species (ROS) and effector inflammatory molecules. 49 When endothelial cells stimulated by ROS and inflammatory molecules, them can produce the endothelin‐1 (ET‐1). 50 ET‐1 can stimulate vasoconstriction by activating ETA and ETB2 receptors on smooth muscle cells, 51 thus promoting the development of HTN. Secondly, the bioactive metabolites which produced by GM, including short‐chain fatty acids (SCFAs), trimethylamine N‐oxides (TMAO), bile acids, etc., promoted the formation of HTN through various pathways. SCFAs could affect BP by activating the G protein‐coupled receptor pathway when they were absorbed by the intestinal epithelium into the host circulatory system. 52 TMAO could induce oxidative stress and inflammatory reaction in vascular endothelium, damage the production and bioavailability of endothelial nitric oxide, resulting in endothelial dysfunction, vasoconstriction and elevated BP. 53 In addition, the GM may also stimulate sympathetic drives through enteric nervous system‐brain interactions or by promoting neuroinflammation, while increased sympathetic activity may promote the development of hypertension by stimulating low‐grade systemic inflammation. 54 We conducted subgroup analysis based on sample size, study population, and sequencing methods, and found that the study population may be the source of heterogeneity. However, there was still significant heterogeneity within Chinese subgroups, and we speculated that it may be related to significant differences in sex and age between populations studied. 45 In addition, the results of different studies may also be influenced by factors such as diet, season, and latitude of the study site. 55
At the phylum level, we found that there was no significant difference in the relative abundance of Bacteroidetes, Proteobacteria, Actinobacteria, and Firmicutes between HTN and HC, and there was significant heterogeneity among the studies. We speculate that it may be related to the differences in population, grade of hypertension and dietary habits. 30 However, there were only a limited number of studies included, and original gene sequencing data were difficult to obtain, we were unable to explore the sources of heterogeneity by subgroup analyses. At the genus level, we observed a lower relative abundance of Faecalibacterium in the hypertensive population. It is known that Faecalibacterium produces SCFAs, 56 which can help maintain intestinal health and play an important role in producing anti‐inflammatory metabolites. 57 A recent study showed that Faecalibacterium transplantation reduced neurological deficits and inflammation in elderly stroke mice and increased SCFAs concentration in the intestine, brain, and plasma. 58 SCFAs, especially butyrate, can mediate anti‐inflammatory effects by inhibiting histone deacetylase (HDAC). 59 , 60 In spontaneously hypertensive rats, the activation of HDAC was associated with HTN. 61 We speculated that the decrease of Faecalibacterium may reduce the production of SCFAs, leading to the enhancement of HDAC activity, and thus promoting the occurrence and development of HTN. Our results confirmed previous evidence of a decrease in the relative abundance of some SCFAs (mainly butyrate) producing genera in hypertensive patients, which were generally considered as beneficial components of human GM, such as Faecalibacterium. 38 , 39 In the hypertensive population, apart from the significant reduction of Faecalibacterium, we also found a significant increase in some common opportunistic pathogens, such as Streptococcus and Enterococcus. 39 The large cohort study of Verhaar and coworkers 45 showed that Streptococcus was positively correlated with SBP and DBP. Kang and coworkers 24 found that Streptococcus was negatively correlated with BP control compliance in hypertensive populations. Liu and coworkers 42 also reported that compared with the healthy group, there were fewer bacteria producing SCFAs in the GM of patients with primary Hyperaldosteronism, and more Streptococcus related to inflammation. The results of all these studies support the results of our study to some extent. However, the increase of Streptococcus and Entercoccus may be the result or cause for the progression of HTN, and further research is needed to assess the causal relationship. In conclusions, the relative abundance of Faecalibacterium in hypertensive patients decreased significantly, suggesting that Faecalibacterium may be a potential “beneficial” microbiota against HTN. On the contrary, the relative abundance of Streptococcus and Enterococcus were increased significantly in HTN, suggesting that they may be a potential “harmful” microbiota related to the pathogenesis of HTN.
Our study had the following limitations. First, the database was only accessible in Chinese and English as information retrieval languages, and only six databases were retrieved, so the retrieval may be incomplete. Second, as it was difficult to obtain the original data from all included studies, we were unable to conduct a consolidated analysis of more indicators, such as the Βeta Diversity indicators. Third, we had only discussed the structure and composition of the GM and failed to gain insight into the transcriptomics and proteomics of GM function.
5. CONCLUSIONS
In this study, we observed that Shannon index was negatively correlated with HTN, Shannon index and the relative abundance of Faecalibacterium were significantly decreased in the HTN, while the relative abundance of Streptococcus and Enterococcus and the F/B ratio were significantly increased. The ecological imbalance of GM may promote the development of HTN. In future work, a large sample size and high‐quality research will be necessary to further verify the role of GM in HTN development with the help of metagenomics and metabolomics techniques.
AUTHOR CONTRIBUTIONS
Research design: Lin YJ and Cai ML; Later stages of the design: Cai ML and Lin LY; Identifying and screening of the included studies: Lin LY and Jiang F; Data analysis and evaluation: Peng YC and Li SL; Writing of this paper: Cai ML; Manuscript revision: Lin YJ and Chen LW. Suggestions to the data analysis, helping in interpretation of results and final manuscript reading and approval: All authors.
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This work was supported by the Medical Innovation Project of Fujian Provincial Health Commission (grant number 2021CXA017); Guiding Project of Fujian Science and Technology (grant number 2021Y0023); Fujian Provincial Special Reserve Talents Laboratory (Fujian Medical University Union Hospital); and Key Laboratory of Cardio‐Thoracic Surgery (Fujian Medical University), Fujian Province University.
Cai M, Lin L, Jiang F, et al. Gut microbiota changes in patients with hypertension: A systematic review and meta‐analysis. J Clin Hypertens. 2023;25:1053–1068. 10.1111/jch.14722
Meiling Cai and Lingyu Lin are co‐first authors.
Contributor Information
Liangwan Chen, Email: fjxhlwc@163.com.
Yanjuan Lin, Email: fjxhyjl@163.com.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Valenzuela PL, Carrera‐Bastos P, Gálvez BG, et al. Lifestyle interventions for the prevention and treatment of hypertension. Nat Rev Cardiol. 2021;18(4):251‐275. [DOI] [PubMed] [Google Scholar]
- 2. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134(6):441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang T, Richards EM, Pepine CJ, et al. The gut microbiota and the brain‐gut‐kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14(7):442‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou B, Perel P, Mensah GA, et al. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18(11):785‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol. 2018;15(1):20‐32. [DOI] [PubMed] [Google Scholar]
- 6. Muralitharan RR, Jama HA, Xie L, et al. Microbial peer pressure: the role of the gut microbiota in hypertension and its complications. Hypertension. 2020;76(6):1674‐1687. [DOI] [PubMed] [Google Scholar]
- 7. Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stojanov S, Berlec A, Štrukelj B. the influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8(11):1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chávez‐Carbajal A, Nirmalkar K, Pérez‐Lizaur A, et al. Gut microbiota and predicted metabolic pathways in a sample of mexican women affected by obesity and obesity plus metabolic syndrome. Int J Mol Sci. 2019;20(2):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qi B, Ren D, Li T, et al. Fu Brick tea manages HFD/STZ‐induced Type 2 diabetes by regulating the gut microbiota and activating the IRS1/PI3K/Akt signaling pathway. J Agric Food Chem. 2022;70(27):8274‐8287. [DOI] [PubMed] [Google Scholar]
- 11. Zhou W, Cheng Y, Zhu P, et al. Implication of gut microbiota in cardiovascular diseases. Oxid Med Cell Longev. 2020;2020:5394096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WillisAD. Rarefaction, alpha diversity, and statistics. Front Microbiol. 2019;10:2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pinart M, Dötsch A, Schlicht K, et al. Gut microbiome composition in obese and non‐obese persons: a systematic review and meta‐analysis. Nutrients. 2021;14(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choroszy M, Litwinowicz K, Bednarz R, et al. Human gut microbiota in coronary artery disease: a systematic review and meta‐analysis. Metabolites. 2022;12(12):1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richards EM, Pepine CJ, Raizada MK, et al. The gut, its microbiome, and hypertension. Curr Hypertens Rep. 2017;19(4):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qi Y, Kim S, Richards EM, et al. Gut microbiota: potential for a unifying hypothesis for prevention and treatment of hypertension. Circ Res. 2017;120(11):1724‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adnan S, Nelson JW, Ajami NJ, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun S, Lulla A, Sioda M, et al. Gut microbiota composition and blood pressure. Hypertension. 2019;73(5):998‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jackson MA, Verdi S, Maxan ME, et al. Gut microbiota associations with common diseases and prescription medications in a population‐based cohort. Nat Commun. 2018;9(1):2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo Y, Li X, Wang Z, et al. Gut microbiota dysbiosis in human hypertension: a systematic review of observational studies. Front Cardiovasc Med. 2021;8:650227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin Y, Zhao J, Wang Y, et al. Specific alterations of gut microbiota in chinese patients with hypertension: a systematic review and meta‐analysis. Kidney Blood Press Res. 2022;47(7):433‐447. [DOI] [PubMed] [Google Scholar]
- 22. Virgin HW. The virome in mammalian physiology and disease. Cell. 2014;157(1):142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777‐784. [DOI] [PubMed] [Google Scholar]
- 24. Kang G, He H, Miao H, et al. Predictive value of gut microbiota in long‐term blood pressure control: a cross‐sectional study. Eur J Med Res. 2023;28(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen T, Yue Y, He T, et al. The association between the gut microbiota and Parkinson's disease, a meta‐analysis. Front Aging Neurosci. 2021;13:636545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernández‐de‐Las‐Peñas C, Navarro‐Santana MJ, Cleland JA, et al. Evidence of bilateral localized, but not widespread, pressure pain hypersensitivity in patients with upper extremity tendinopathy/overuse injury: a systematic review and meta‐analysis. Phys Ther. 2021;101(8):pzab131. [DOI] [PubMed] [Google Scholar]
- 27. Kou C, Wang Q, Li C, et al. A meta‐analysis of the correlation coefficient between blood pressure variability and left ventricular mass index in patients with essential hypertension. J Lanzhou Univ (Med Sci). 2020;46(6):1‐8. [Google Scholar]
- 28. Peterson RA, Brown SP. On the use of beta coefficients in meta‐analysis. J Appl Psychol. 2005;90(1):175‐181. [DOI] [PubMed] [Google Scholar]
- 29. Kollias A, Ntineri A, Stergiou GS. Association of night‐time home blood pressure with night‐time ambulatory blood pressure and target‐organ damage: a systematic review and meta‐analysis. J Hypertens. 2017;35(3):442‐452. [DOI] [PubMed] [Google Scholar]
- 30. Mushtaq N, Hussain S, Zhang S, et al. Molecular characterization of alterations in the intestinal microbiota of patients with grade 3 hypertension. Int J Mol Med. 2019;44(2):513‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calderón‐Pérez L, Gosalbes MJ, Yuste S, et al. Gut metagenomic and short chain fatty acids signature in hypertension: a cross‐sectional study. Sci Rep. 2020;10(1):6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takagi T, Naito Y, Kashiwagi S, et al. Changes in the gut microbiota are associated with hypertension, hyperlipidemia, and type 2 diabetes mellitus in Japanese subjects. Nutrients. 2020;12(10):2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu LL, Ma ZJ, Ren M, et al. Distinct features of gut microbiota in high‐altitude Tibetan and middle‐altitude Han hypertensive patients. Cardiol Res Pract. 2020;2020:1957843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakai M, Ribeiro RV, Stevens BR, et al. Essential hypertension is associated with changes in gut microbial metabolic pathways: a multisite analysis of ambulatory blood pressure. Hypertension. 2021;78(3):804‐815. [DOI] [PubMed] [Google Scholar]
- 35. Silveira‐Nunes G, Durso DF, Jr LRAO, et al. Hypertension is associated with intestinal microbiota dysbiosis and inflammation in a Brazilian population. Front Pharmacol. 2020;11:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wan C, Zhu C, Jin G, et al. Analysis of gut microbiota in patients with coronary artery disease and hypertension. Evid Based Complement Alternat Med. 2021;2021:7195082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dan X, Mushi Z, Baili W, et al. Differential analysis of hypertension‐associated intestinal microbiota. Int J Med Sci. 2019;16(6):872‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan Q, Gu Y, Li X, et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017;7:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qu L, Dong Z, Ma S, et al. Gut microbiome signatures are predictive of cognitive impairment in hypertension patients‐a cohort study. Front Microbiol. 2022;13:841614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang JM, Yang MX, Wu QF, et al. Improvement of intestinal flora: accompany with the antihypertensive effect of electroacupuncture on stage 1 hypertension. Chin Med. 2021;16(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Y, Jiang Q, Liu Z, et al. Alteration of gut microbiota relates to metabolic disorders in primary aldosteronism patients. Front Endocrinol (Lausanne). 2021;12:667951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Y, Wang H, Howard AG, et al. Gut microbiota and host plasma metabolites in association with blood pressure in Chinese adults. Hypertension. 2021;77(2):706‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palmu J, Salosensaari A, Havulinna AS, et al. Association between the gut microbiota and blood pressure in a population cohort of 6953 individuals. J Am Heart Assoc. 2020;9(15):e016641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verhaar BJH, Collard D, Prodan A, et al. Associations between gut microbiota, faecal short‐chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur Heart J. 2020;41(44):4259‐4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen J, Chen X, Sun L, et al. Analysis of intestinal microflora characteristics based on 16S rRNA detection in salt‐sensitive hypertension patients in Ningbo. Modern Pract Med. 2021;33(11):1458‐1459+1463+1542. [Google Scholar]
- 47. Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferguson JF, Aden LA, Barbaro NR, et al. High dietary salt‐induced dendritic cell activation underlies microbial dysbiosis‐associated hypertension. JCI Insight. 2019;5(13):e126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Small HY, Migliarino S, Czesnikiewicz‐Guzik M, et al. Hypertension: focus on autoimmunity and oxidative stress. Free Radic Biol Med. 2018;125:104‐115. [DOI] [PubMed] [Google Scholar]
- 50. Ambrosino P, Bachetti T, D'Anna SE, et al. Mechanisms and clinical implications of endothelial dysfunction in arterial hypertension. J Cardiovasc Dev Dis. 2022;9(5):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kostov K. The causal relationship between endothelin‐1 and hypertension: focusing on endothelial dysfunction, arterial stiffness, vascular remodeling, and blood pressure regulation. Life (Basel). 2021;11(9):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mutengo KH, Masenga SK, Mweemba A, et al. Gut microbiota dependant trimethylamine N‐oxide and hypertension. Front Physiol. 2023;14:1075641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verhaar BJH, Prodan A, Nieuwdorp M, et al. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients. 2020;12(10):2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74(9):1251‐1262. [DOI] [PubMed] [Google Scholar]
- 56. Nishiwaki H, Hamaguchi T, Ito M, et al. Short‐chain fatty acid‐producing gut microbiota is decreased in Parkinson's Disease but not in rapid‐eye‐movement sleep behavior disorder. mSystems. 2020;5(6):e00797‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferreira‐Halder CV, Faria AVS, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol. 2017;31(6):643‐648. [DOI] [PubMed] [Google Scholar]
- 58. Lee J, d'Aigle J, Atadja L, et al. Gut microbiota‐derived short‐chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127(4):453‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chang PV, Hao L, Offermanns S, et al. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111(6):2247‐2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606‐613. [DOI] [PubMed] [Google Scholar]
- 61. Cardinale JP, Sriramula S, Pariaut R, et al. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56(3):437‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.


