Abstract
One‐fourth of death in India is attributed to cardiovascular disease (CVD) and more than 80% is related to ischemic heart disease and stroke. The main risk factor for CVD is hypertension. Every third person in India suffers from hypertension and the prevalence increased drastically in the past 20 years, especially among the youngest age group of 20 and 44 years. Regardless of being under anti‐hypertension medication, the blood pressure (BP) control rate in the country is still low ranging between 6% and 28% only. Assessing the “true BP control rate” should be performed using both clinic BP measurement and out‐of‐office BP measurement as the latter shows better prognosis for patients’ hypertension and CVD outcomes. Home blood pressure monitoring (HBPM) shows superiority over ambulatory BP measurement as multiple measurements can be collected at the patient's convenience. Only limited evidence on HBPM in India is available and it's either lacking in hypertension participants or of a small sample size. This study will investigate the real BP control status among 2000 hypertensive patients from 18 centers in 12 states across Pan‐India. The outcome of this study will emphasize the value of establishing BP control management practice guidelines suitable for physicians and help policymakers in building proper strategies for hypertension management to reduce the CVD burden on the health situation in India.
Keywords: home blood pressure monitoring, hypertension, India, patient knowledge, real blood pressure control
1. BACKGROUND
In India, almost one‐fourth of all deaths are attributed to cardiovascular disease (CVD), and 52% of CVD death happens before the age of 70 years old. 1 , 2 , 3 More than 80% of CVD mortality is from ischemic heart disease and stroke, and the major risk factor is hypertension. 1 , 3 , 4 , 5 , 6 The prevalence of hypertension in India among adults‐general population aged 18 years and above is on average around 30%, meaning that every third person in the country suffers from hypertension, and this prevalence has increased drastically in the past 20 years in both urban and rural areas. 7 , 8 Despite the area of residency, the affected individuals are mostly among the youngest group between the age of 20 and 44 years as revealed in the national survey, showing that hypertension is not a disease for the older age exclusively. 7 , 9 Even though efforts are made in the country to diagnose and bring forth awareness of hypertension, the blood pressure (BP) control rate did not improve in the last two decades, ranging between 6% and 28% regardless of being under anti‐hypertension medications. 6 , 7 , 10 , 11 , 12 , 13 From the available national evidence, BP control rate is usually defined using clinic (office) BP (CBP) which is mainly measured on one occasion. There lies the shortcoming to using CBP as an index to BP control as it is impractical for patients to monitor their BP measurement frequently but during their visits to the clinic. Assessing the “true BP control rate” should be deducted not only from a single visit to the clinic but from multiple measurements out of the clinic setting. Using out‐of‐office BP measurements can relatively provide multiple numbers of BP measurements in the comfortable environment of each individual. 14 Existing global evidence supports the advantages of out‐of‐office BP measurement for the diagnosis of subtypes of hypertension, contributes to treatment efficacy evaluation, and assists in CVD prevention. 14 , 15 Among the out‐of‐office BP measurements, home blood pressure monitoring (HBPM) shows preference over ambulatory blood pressure monitoring (ABPM) from the perspective of the patients and clinical practicality. 16 , 17 , 18 , 19 , 20 A vast body of international evidence shows HBPM to have a superior prognostic value and as a better predictor over CBP for CVD outcomes and target organ damage. 21 A finding from the India Heart Study which investigated the agreement of two visits CBP and 7 days home blood pressure (HBP) showed poor agreement between the two readings, and stated the conclusion that when it comes to diagnosing hypertension, HBP should be the preferred method. 22 Summarizing the evidence thus far, HBPM appears to provide close to the true value of an individual's BP control better than CBP, while it shows benefit over ABPM as the patient can measure their BP multiple times for long period on several occasions at their own convenience. 19 , 20 Indian national evidence on HBPM is scarce either due to the limited number of studies conducted with HBPM or having a small sample size. 22 , 23 In the India Heart Study, they investigated HBPM in the country, however, the participants were of hypertension‐naive background and there is only limited evidence on HBPM in hypertensive patients in India. 22 In 2018, Asia BP@Home study determined HBP control status across 11 Asia countries/regions, among the countries involved in this study was India. 23 The limitations of the study were the low sample size (n = 97) and the data being collected from one center only. Therefore, there is still a need for nationwide HBPM evidence in India among hypertensive patients and with a larger sample size to show the real BP control rate in the country.
The GeogRaphic And socioecoNomic Distribution of real‐world Indian data of home blood pressure monitoring (GRAND Study) aims to create the first massive scale real‐world India data of HBPM among hypertensive patients which will answer various pertinent questions about hypertension and BP control, socioeconomic, demographic, and lifestyle factors affecting it, the distribution and variation of data of home and office measurement of BP, and the awareness and behavior of hypertensive patients regarding hypertension and HBPM in India.
2. METHODS AND STUDY DESIGN
2.1. Overview
This registry is a multi‐center, non‐interventional cross‐sectional study planned to recruit 2000 hypertensive patients in 18 medical centers across 7 regions and 12 states in India (Figure 1). We will investigate the BP control status and provide Indian real‐world data of BP monitoring by CBP and HBP. The study aims to determine the followings: (1) demographic comparison of CBP versus HBP, and BP control; (2)demographic, anthropometric, socioeconomic, medical, and lifestyle factors affecting hypertension subtypes which are white‐coat hypertension, masked hypertension, sustained uncontrolled hypertension, controlled hypertension, morning hypertension and evening hypertension in India; (3)BP and heart rate (HR) variability of visit‐to‐visit CBP and day‐by‐day HBP, morning HBP, and evening HBP and factors correlated to them; (4)regional differences in BP control; (5)hypertension patients’ behavioral association with BP control. The findings from this study will have an impact on filling the gap in the diagnostic dilemma of hypertension management and BP control in India, paving the path to developing guidance for physicians to be used in clinical practice, and building strategies by policymakers to improve the BP control and prevention of CVD outcomes.
FIGURE 1.
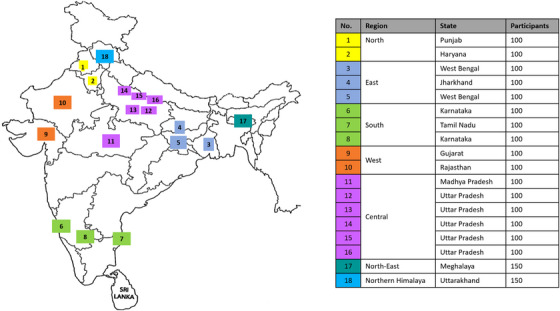
GRAND study centers’ distribution and the number of participants.
2.2. Study design and setting
The protocol and term of consent were approved by Independent Ethical Committee Narayana Diagnostics, Lucknow, Uttar Pradesh, India (Approve number: NIEC/INDT/APP/08/28/22‐02). The ethics committee is organized and operates according to the requirements of Good Clinical Practice (GCP), Indian Council of Medical Research (ICMR), and New Drug and Clinical Trial Rule 2019.
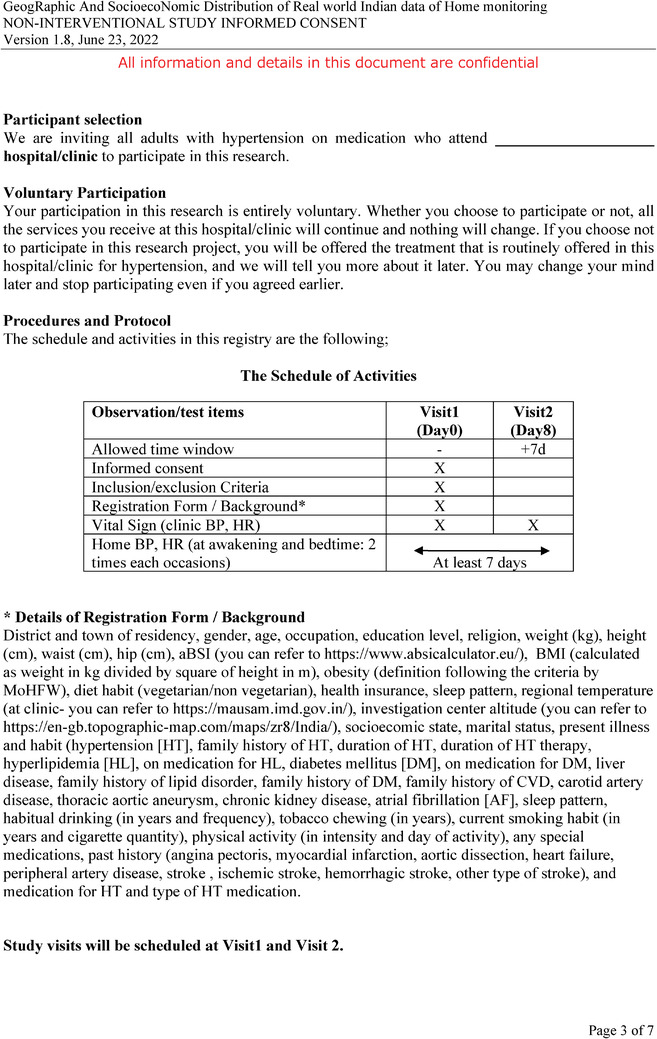
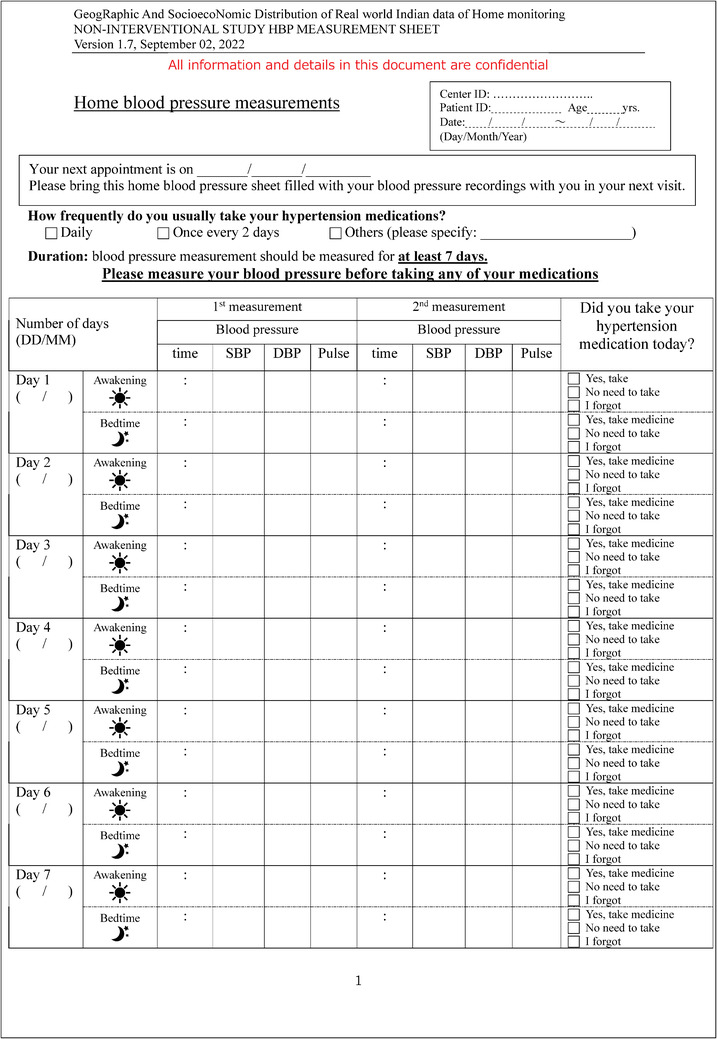
The study is designed to include two visits to the study center and at least 7 days of HBPM by the participants. Figure 2 shows the study schedule and data collected at each visit. The investigator or research nurse will recruit the patients and obtain the following list of items during the visit1: (1) written informed consent, (2) relevant background information filled in the registration form, and (3) patient Home BP survey. The patient Home BP survey is composed of 16 questions that will be used to understand the participants’ awareness and knowledge of hypertension, the practice of regular BP monitoring, their knowledge of HBP, and the measuring practice of HBP. Each study center will receive minimum 10 validated BP measurement devices (Omron HEM‐9210T) and these devices will be used in all the BP measurement data (CBP and HBP) throughout the study. The conditions for measuring CBP and HBP were based on the global instructions from guidelines 18 , 19 , 20 with minor adjustments to the resting time prior to the measurement from the standard 5 min to 2−5 min to accommodate practicality in measuring BP, limited time of consultation, and suitable alteration for the common cultural behavior by patients. The investigator or research nurse will measure CBP and (HR) following the instruction of the patient being in a sitting position in a quiet room without moving or talking while resting for 2−5 min and the cuff wrapped around the upper‐arm and positioned at the same level as the heart. The CBP measurement will be observed readings as the investigator or research nurse will operate measuring office BP. For a practical procedure, and to reflect the common practice and accommodate for limited consultation time by the investigators, CBP will be measured only two times within a gap of 1 min without removing the cuff. The two readings of each visit will be used in the analysis without discarding the first reading. The patients then will be provided with the same device used to measure their CBP along with the patient's Quick Guide on how to measure HBP and HBP measurement sheet. The day the patient will be recruited is considered day 0 in the study format and the next day will be considered day 1 of the HBPM and the participants will record their HBP for at least 7 days. HBP will be measured in the morning and evening; two times each occasion using provided BP device which will automatically store the measurement in the device memory digitally and the patients will also write down the readings manually on the HBP measurement sheet. The instructions for both measurements are described in Table 1.
FIGURE 2.
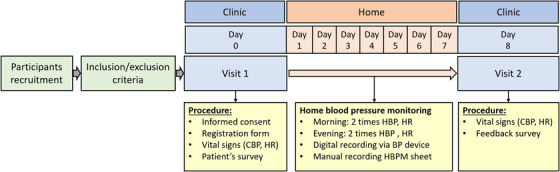
The study procedure and flow chart.
TABLE 1.
HBP morning and evening instructions to the participants.
| HBP morning (6:00–12:00) | HBP evening (18:00–24:00) |
|---|---|
|
|
Prior to CBP measurement, the investigator or research nurse will decide the arm BP will be measured by firstly measuring BP from both arms, starting from the non‐dominant upper arm (primarily the left arm). If the BP in the right and left arms differed considerably, the arm with the highest BP will be defined at the visit1 and used throughout the HBP measurements. After the 7 days of HBP measurement, and giving a buffer time, the patients will return within 15 days to the study center to visit2 with the BP device and the filled‐out HBP measurement sheet. Patients with less than 3 days of measurement recorded for both morning and evening will be excluded from the further data analysis.
During visit2, the investigator or research nurse upon obtaining the BP device and HBP measurement sheet will measure CBP (two times within a gap of 1 min) and HR following the same method performed in measuring CBP at visit1, and the patients will answer a feedback survey. The feedback survey is composed of 3 questions on the behavior change after experiencing 7 days of HBPM, and knowledge of HBPM benefits. The digitally stored BP recording in the device will be composed of 4 CBP measurements and at least 7 days HBP which will be transferred to a data management system prior to passing the BP device to the next patient.
Each patient's registration form, patient Home BP survey, and BP measurements (manually recorded and digitally) will be logged into a GRAND study data management system.
2.3. Participant recruitment
The total target sample size is a minimum of 2000 hypertension patients from 18 study centers across 12 states from 7 regions, and 100 patients per center with the exception of two centers in the North‐East and Northern Himalaya region which will collect 150 patients per center to balance out the number of patients per region (Figure 1).
In this study, the participants will be hypertensive patients under anti‐hypertensive medication treatment for more than 3 months. Eligible patients will be those who have understood, agreed, and signed the informed consent form for this study. The inclusion and exclusion criteria are shown in Table 2.
TABLE 2.
GRAND study participants’ inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
1. Patients living in India. 2. Patients diagnosed with hypertension and on stable hypertension medication ≥3 months. 3. Age between ≥20 and ≤70 years. 4. Patients who are thoroughly informed about the study and signed an informed consent form. |
1. Patients with Pregnancy‐Induced Hypertension. 2. Shift workers or patients working for odd hours (working between 5 pm and 9 am). 3. Patients with arm circumference of <22 cm or >42 cm. 4. Patients deemed unfit to participate in the study due to a health condition. |
2.4. Study withdrawal
Patients may withdraw or decide to discontinue the study at any time at their own request or at the discretion of the investigator for safety, behavioral, or administrative reasons. The investigator will act upon the withdrawal/discontinuation decision by requesting the participant or related family to fill in the proper form. When the patient withdraws from the study, this action will withdraw their consent for disclosure of future information, and no further evaluations nor additional data will be collected.
2.5. Outcomes
2.5.1. Primary outcomes/Clinical
Distribution of patients based on BP control status using hypertension diagnosis threshold by the difference in the clinic and home BP thresholds as shown in Figure 3. The BP control status will also be investigated by regions and expressed with graphical bars.
FIGURE 3.
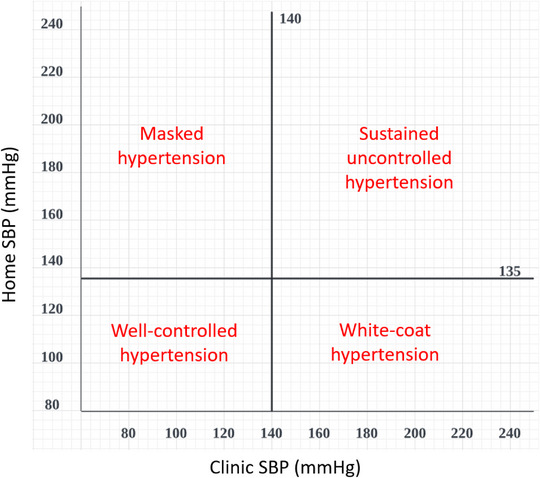
A plot template showing the distribution of uncontrolled, well‐controlled, masked, and white‐coat hypertension according to home and clinic systolic BP measurements. The cut‐off of average home SBP 135 and average clinic SBP 140 were assigned as proposed in the IGH‐IV 2019 and 2020 ISH Global Hypertension Practice Guidelines.
2.5.2. Secondary outcomes/Clinical
The distribution of BP control status using target threshold of CBP and HBP as <130/80 mmHg.
The distribution of BP control status for the population with diabetes mellitus using the target threshold of CBP <130/80 mmHg and HBP <125/75 mmHg. 24
The distribution of BP control status for the high‐risk population with diabetes mellitus, cardiovascular disease and chronic kidney disease using the target threshold of CBP <130/80 mmHg and HBP <125/75 mmHg.
To find out the correlation of demographic, anthropometric, socioeconomic, medical condition, medication use, and lifestyle factors to BP control status and HR, including data collected from unique demographics in India.
To examine BP and HR variability for visit‐to‐visit CBP, day‐by‐day HBP, morning HBP, and evening HBP.
Comparison between the digitally recorded BP measurement and manually recorded.
2.5.3. Secondary outcomes/Behavioral
To investigate the knowledge, attitude and practice (KAP) of HBPM by hypertensive patients in India.
Determine the association between KAP, physicians’ instructions of HBPM and prescription usage to BP control among hypertensive patients.
To investigate the change in patient's KAP regarding HBP from visit1 patient Home BP survey and visit two feedback surveys.
2.6. Data management
All the study‐related documents and database hardcopy will be maintained in a locked secured area under the study principal investigator (PI). All digital data will be stored securely under the responsibility of the study PI at King George's Medical University (KGMU), Lucknow, Uttar Pradesh, India. During the data collection, the project coordination, the tracking of study progress, follow‐up on the recruitment process, digital data management, and statistical analyses will be under the supervision and responsibility of the study PI and in coordination with TATA Consultancy Services.
2.7. Statistical method
The data will be collected centrally, and all data processing and statistical analyses will be done by using the software SAS 9.4 (SAS Institute; Cary, NC) and SPSS 21.0 (Release 21.0.0.0, IBM, USA). All the statistical analyses will be performed for the overall population and by the seven regions. Continuous and categorical variables will be presented as mean ± standard deviation (SD), and number (%), respectively. The average of all HBP readings versus the average of all CBP readings will be plotted to show the hypertension subtypes as in Figure 3. The chi‐square will be used to test the prevalence differences among the hypertension subtypes and regression analyses will be performed to describe the association between socio‐demographical, lifestyle, and clinical factors and each hypertension condition and hypertension subtype. From the aforementioned factors, determinants influencing each hypertension subtype will be identified. Additionally, the patient's Home BP awareness survey answers will be expressed as descriptive statistics of number (%). Both the demographic/clinical characteristics and the patient Home BP survey answers will be explored for association with hypertension subtypes. The change in patients’ behavior between visit 1 and visit 2 will be determined using paired proportions (McNemar's test). To analyze the BP variability of visit‐to‐visit CBP, day‐by‐day HBP, morning HBP, and evening HBP, the following parameters will be calculated which are SD, coefficient of variation (CV) calculated as SD divided by the average BP level and multiplying by 100, and average real variability (ARV) calculated as the average of absolute differences between successive measurements. We will also investigate the agreement for the diagnosis of hypertension among the aforementioned variables using the chi‐square test and kappa coefficient. Two‐sided p values will be presented for all analyses, and the significance of differences will be set at p < 0.05.
3. DISCUSSION
Results from other studies in India show that 42% of patients have a risk of misdiagnosis of hypertension because of the high percentage of masked hypertension and white‐coat hypertension. 22 Out‐of‐office BP measurement is an important tool for physicians to diagnose masked hypertension and white‐coat hypertension. Local evidence for ABPM is available in India, 25 , 26 , 27 and there is evidence of HBPM too, however, the available evidence is limited or the sample size is small. 23 National evidence from Longitudinal Ageing Study in India (LASI) explored many variables including hypertension situation. However, BP control was determined on only one occasion during the study, and no measurements at home were collected. 28 , 29 , 30 Data from the Indian Hypertension Control Initiative (IHCI) also lacked HBP measurement. 31 To find out the real BP control rate and medication efficacy, BP value measured at home is needed in India. Another aspect of India is, as a country, it is unique with multiple cultures and habits, and for that variation, it ranges widely in the non‐communicable disease (NCD) burden from 47.6% in the state of Bihar to 74.6% in Kerala. The life expectancy also has more than 10 years different with the lowest being in Uttar Pradesh for women (66.8 years old), and Assam for men (63.5 years old) to the highest in Kerala for both women and men, 78.7 years old and 73.8 years old, respectively. 32 Therefore, establishing an effective strategy to mitigate hypertension in the country must be tailored based on the area level of hypertension condition, keeping in mind each particular area's influencing factors to hypertension disease. Herein, the GRAND study will identify the real BP control rate, the factors associated with BP control, and the attitude of hypertensive patients in their hypertension management journey. From this study, addressing all the true factors that associate with hypertension may give a greater insight into how best to allocate resources to reduce the problem of hypertension and alleviate the burden of CVD on India's health situation. The study has notable strengths and limitations which were summarized in Table 3.
TABLE 3.
GRAND study strength and limitation.
| Strength | Limitation |
|---|---|
|
1. Investigate the real BP control rate from the agreement between CBP and HBP among hypertensive patients. 2. Assessing BP control by HBPM which might reflect the real reading of BP. 3. Determine the BP and HR variability (visit‐to‐visit of CBP, day‐by‐day, morning and evening of HBP). 4. Comparison between patients’ HBP recorded digital and manual readings. 5. Association of BP control with patients’ KAP of hypertension and HBPM. 6. Correlation of BP control with patients’ characteristics among hypertension in India. 7. Association between physicians’ Instructions with BP control and patients’ characteristics. 8. Patients’ behavior change after experiencing 7 days of HBPM. 9. Prescription usage for hypertension treatment. 10. Provide new evidence of hypertension from unique demographics in India. |
1. Selection Bias: participants are under hypertension treatment, and consulting with physicians. The participants are not randomized. 2. HBP measurement for 7 days only. 3. Moderate number of hypertensive patients (2,000 participant), however, the sample will be recruited from across India which might be representative of the country. 4. Recruitment centers are well‐distributed by 7 regions, however, in India, there are 29 states and 8 Union territories. This study covers less than half the number of states (12 states). |
3.1. Impact of study outcome
The study will help in conceptualizing the direction and strategies of policymaking for hypertension management and NCD burden mitigation projects in the country. Additionally, it will highlight the importance of establishing BP control management practice guidelines suitable for physicians and an educational opportunity for patients to practice HBPM in India.
AUTHOR CONTRIBUTIONS
Narsingh Verma conceived the study. Yutaka Imai and Takayoshi Ohkubo Advised on the design of the study. Noriko Matsushita and Ebtehal Salman drafted the manuscript. Narsingh Verma, Yutaka Imai, and Takayoshi Ohkubo reviewed the manuscript. Narsingh Verma and the GRAND Study Research Group are involved in the acquisition of data. All authors contributed to the critical revision of the manuscript for important intellectual content, and all authors read and approved the final manuscript.
CONFLICTS OF INTERESTS STATEMENT
The authors declare that they have no competing interests. Noriko Matsushita is an employee of Omron Healthcare Singapore Pte. Ltd. Ebtehal Salman is an employee of Omron Healthcare Co., Ltd. Takayoshi Ohkubo has received grants from Omron Healthcare Co., Ltd. Other authors declare that they have no conflict of interest.
TRIAL STATUS
This trial is at the recruitment stage.
CLINICAL TRIAL REGISTRATION STATUS
Registered at Clinical Trials Registry‐India (CTRI) under CTRI/2023/02/049486 on 06 February 2023.
CONSENT TO PARTICIPATE
All parties will ensure the protection of patient personal data and will not include patient names on reports, publications, or in any other disclosures, except where required by laws. The informed consent form will be in compliance with local regulatory requirements and legal requirements. The informed consent form used in this study, and any changes made during the course of the study, will be prospectively approved by the IEC. The investigator will ensure that each study patient, or his/her legally acceptable representative, is fully informed about the nature and objectives of the study and possible risks associated with participation. The investigator, or a person designated by the investigator, will obtain written informed consent from each patient or the patient's legally acceptable representative before any study‐specific activity is performed. The investigator will retain the original of each patient's signed consent form.
ADDITIONAL FILES
Additional file 1: Informed consent (PDF 297 kb).





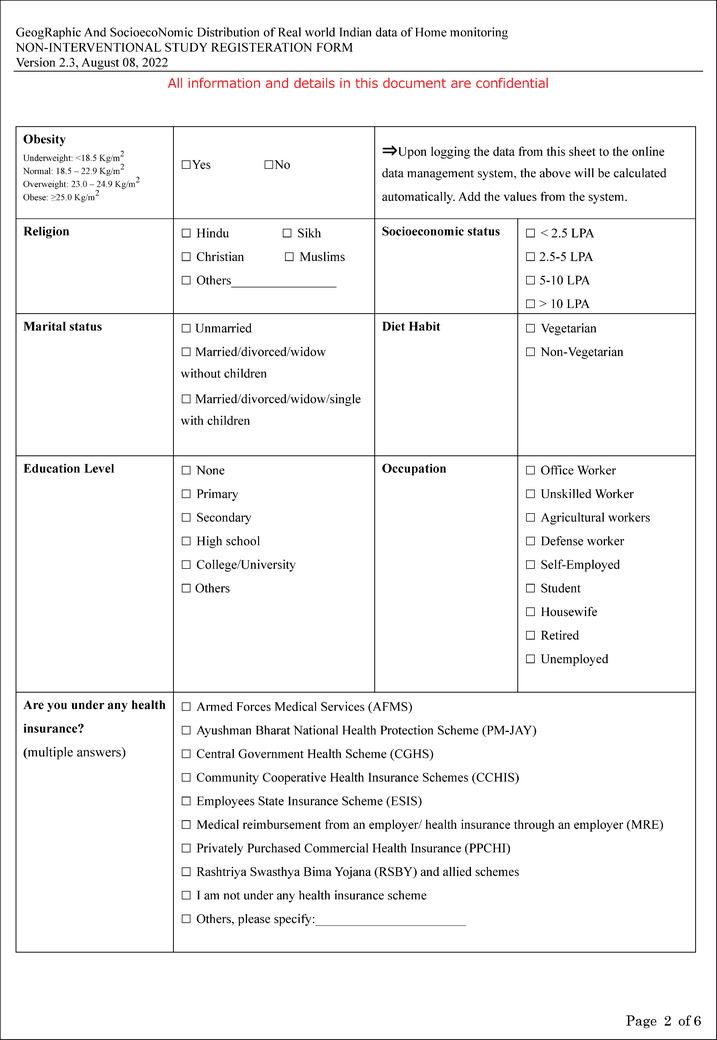
Additional file 2: Registration form and feedback survey (PDF 394 kb).

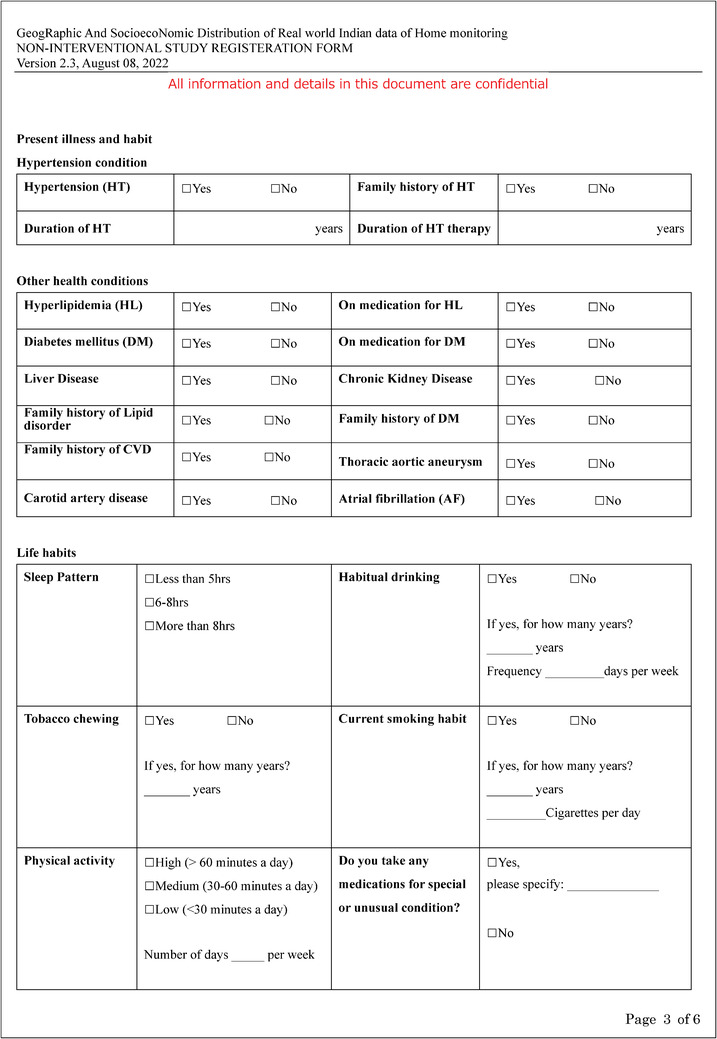
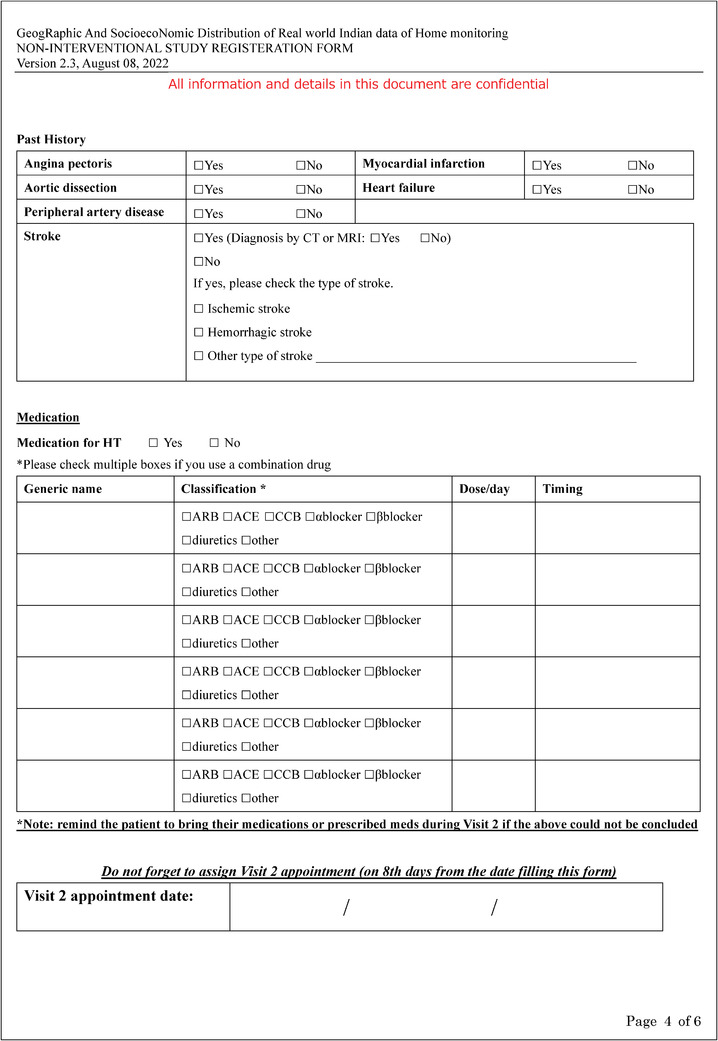


Additional file 3: Patient's Home BP survey (PDF 322 kb).





Additional file 4: HBP measurement sheet (PDF 320 kb).
Additional file 5: Withdrawal form (PDF 232 kb).


ACKNOWLEDGMENTS
This study is supported by research funding by Omron Healthcare Co., Ltd. Technical support for developing the data management system, IT support, and data analysis, by Tata Consultancy Services. The study protocol was developed by Principal Investigator (PI) and reviewed by 2 study advisors (Yutaka Imai, and Takayoshi Ohkubo). The members of the GRAND Study Research Group (not listed individually in the author list of the title page, alphabetically ordered) are as follows: Rituparna, Barooah; Smitha, Bhat; N, Bhavatharini; Arun, Goel; Aloke Kumar, Gupta; Amit, Gupta; Arvind, Gupta; Vitull Kumar, Gupta; Aravinda, Jagadeesha; Anuj, Maheshwari; Rachna, Parashar; Bijay, Patni; Banshi, Saboo; Divya, Saxena; N K, Singh; Ramji, Singh; Ruchi, Singh; Saurabh, Srivastava.
Verma N, Matsushita N, Salman E, Ohkubo T, Imai Y. GeogRaphic and socioecoNomic Distribution of real‐world Indian data of home blood pressure monitoring (GRAND Study): Study protocol for an observational study in 18 medical centers across India. J Clin Hypertens. 2023;25:1105–1134. 10.1111/jch.14713
REFERENCES
- 1. Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in India: current epidemiology and future directions. Circulation. 2016;133(16):1605‐1620. [DOI] [PubMed] [Google Scholar]
- 2. Harikrishnan S, Leeder S, Huffman M, Jeemon P, Prabhakaran D. A Race against Time: The Challenge of Cardiovascular Disease in Developing Economies. 2nd ed. New Delhi Centre for Chronic Disease Control; 2014. [Google Scholar]
- 3. Nag T, Ghosh A. Cardiovascular disease risk factors in Asian Indian population: a systematic review. J Cardiovasc Dis Res. 2013;4(4):222‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta R. Trends in hypertension epidemiology in India. J Hum Hypertens. 2004;18:73‐78. [DOI] [PubMed] [Google Scholar]
- 5. Gupta R, Xavier D. Hypertension: the most important non communicable disease risk factor in India. Indian Heart J. 2018;70(4):565‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anchala R, Kannuri NK, Pant H, et al. Hypertension in India: a systematic review and meta‐analysis of prevalence, awareness, and control of hypertension. J Hypertens. 2014;32(6):1170‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roy A, Praveen PA, Amarchand R, et al. Changes in hypertension prevalence, awareness, treatment and control rates over 20 years in National Capital Region of India: results from a repeat cross‐sectional study. BMJ Open. 2017;7(7):e015639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramakrishnan S, Zachariah G, Gupta K, et al. Prevalence of hypertension among Indian adults: results from the great India blood pressure survey. Indian Heart J. 2019;71(4):309‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramakrishanan S, Gupta K. Prevalence of hypertension among Indian adults: results from the great India blood pressure survey. Indian Heart J. 2020;72(3):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohanty SK, Pedgaonkar SP, Upadhyay AK, et al. Awareness, treatment, and control of hypertension in adults aged 45 years and over and their spouses in India: a nationally representative cross‐sectional study. PLoS Med. 2021;18(8):e1003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kothavale A, Puri P, Yadav S. The burden of hypertension and unmet need for hypertension care among men aged 15–54 years: a population‐based cross‐sectional study in India.J Biosoc Sci.. 2022;54(6):1078‐1099. [DOI] [PubMed] [Google Scholar]
- 12. Prenissl J, Manne‐Goehler J, Jaacks LM, et al. Hypertension screening, awareness, treatment, and control in India: a nationally representative cross‐sectional study among individuals aged 15 to 49 years. PLoS Med. 2019;16:e1002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amarchand R, Kulothungan V, Krishnan A, Mathur P. Hypertension treatment cascade in India: results from National Noncommunicable Disease Monitoring Survey [published online ahead of print, 2022 May 5] [published correction appears in J Hum Hypertens. 2022 Aug 2:]. J Hum Hypertens. 2022. 10.1038/s41371-022-00692-y [DOI] [Google Scholar]
- 14. Melville S, Byrd JB. Out‐of‐office blood pressure monitoring in 2018. JAMA. 2018;320(17):1805‐1806. [DOI] [PubMed] [Google Scholar]
- 15. Sharman JE, Howes FS, Head GA, et al. Home blood pressure monitoring: australian Expert Consensus Statement. J Hypertens. 2015;33(9):1721‐1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nasothimiou EG, Karpettas N, Dafni MG. Stergiou GS. Patients' preference for ambulatory versus home blood pressure monitoring. J Hum Hypertens. 2014;28(4):224‐229. [DOI] [PubMed] [Google Scholar]
- 17. Imai Y, Obara T, Asamaya K, Ohkubo T. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36(8):661‐672. [DOI] [PubMed] [Google Scholar]
- 18. Parati G, Stergiou GS, Bilo G, et al. Home blood pressure monitoring: methodology, clinical relevance and practical application: a 2021 position paper by the Working Group on Blood Pressure Monitoring and Cardiovascular Variability of the European Society of Hypertension. J Hypertens. 2021;39(9):1742‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stergiou GS, Palatini P, Parati G, et al. 2021 European Society of Hypertension practice guidelines for office and out‐of‐office blood pressure measurement. J Hypertens. 2021;39(7):1293‐1302. [DOI] [PubMed] [Google Scholar]
- 20. Imai Y, Kario K, Shimada K, et al. The Japanese Society of Hypertension Guidelines for self‐monitoring of blood pressure at home (Second Edition). Hypertens Res. 2012;35(8):777‐795. [DOI] [PubMed] [Google Scholar]
- 21. Stergiou GS, Kario K, Kollias A, et al. Home blood pressure monitoring in the 21st century. J Clin Hypertens (Greenwich). 2018;20(7):1116‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaul U, Wander GS, Sinha N, et al. Self‐blood pressure measurement as compared to office blood pressure measurement in a large Indian population; the India Heart Study. J Hypertens. 2020;38(7):1262‐1270. [DOI] [PubMed] [Google Scholar]
- 23. Kario K, Tomitani N, Buranakitjaroen P, et al. Home blood pressure control status in 2017–2018 for hypertension specialist centers in Asia: results of the Asia BP@Home study. J Clin Hypertens (Greenwich). 2018;20(12):1686‐1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Umemura S, Arima H, Arima S, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235‐1481. [DOI] [PubMed] [Google Scholar]
- 25. Unnikrishnan S, Awadhiya O, Lahiri A, Pakhare AP, Joshi A, Joshi R. Accuracy of short‐term ambulatory blood pressure measurements for the diagnosis of hypertension. Cureus. 2021;13(9):e17871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ram CVS, Dani S, Oomman A, et al. Correlation between ambulatory blood pressure monitoring and office blood pressure measurement in patients with hypertension: a community study. Am J Med Sci. 2021;362(6):546‐552. [DOI] [PubMed] [Google Scholar]
- 27. Kaul U, Arambam P, Rao S, et al. Usefulness of ambulatory blood pressure measurement for hypertension management in India: the India ABPM study. J Hum Hypertens. 2020;34(6):457‐467. doi: 10.1038/s41371-019-0243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Institute for Population Sciences (IIPS), National Programme for Health Care of Elderly (NPHCE), MoHFW, Harvard T. H. Chan School of Public Health (HSPH) and the University of Southern California (USC) 2020 . Longitudinal Ageing Study in India (LASI) Wave 1, 2017–18, India Report. International Institute for Population Sciences, Mumbai.
- 29. Lee J, Wilkens J, Meijer E, Sekher TV, Bloom DE, Hu P. Hypertension awareness, treatment, and control and their association with healthcare access in the middle‐aged and older Indian population: a nationwide cohort study. PLoS Med. 2022;19(1):e1003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhatia M, Kumar M, Dixit P, Dwivedi LK. Diagnosis and treatment of hypertension among people aged 45 years and over in India: a sub‐national analysis of the variation in performance of Indian states. Front Public Health. 2021;9:766458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaur P, Kunwar A, Sharma M, et al. The India Hypertension Control Initiative‐early outcomes in 26 districts across five states of India, 2018–2020 [published online ahead of print, 2022 Aug 9]. J Hum Hypertens. 2022. doi: 10.1038/s41371-022-00742-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Indian Council of Medical Research, Public Health Foundation of India, and Institute for Health Metrics and Evaluation . India: Health of the Nation's States — The India State‐Level Disease Burden Initiative. ICMR, PHFI, and IHME; 2017. [Google Scholar]


