QUESTIONS: What are the effects of empiric treatment with antibiotics in adults and children with suspected bacterial conjunctivitis? What are the effects of antibiotics in adults and children with culture-positive bacterial conjunctivitis?
INTERVENTIONS
Beneficial
Topical antibiotics (fluoroquinolones, polymyxin B sulfate combinations, aminoglycosides, and fusidic acid [fusidate sodium])
Trade-off
Topical chloramphenicol
DEFINITION
Conjunctivitis is any inflammation of the conjunctiva, generally characterized by irritation, itching, foreign body sensation, and tearing or discharge. Bacterial conjunctivitis may usually be distinguished from other types of conjunctivitis by the presence of a yellow-white mucopurulent discharge. Usually a papillary reaction—small bumps with fibrovascular cores on the palpebral conjunctiva, appearing grossly as a fine velvety surface—is also seen. Bacterial conjunctivitis is usually unilateral, as opposed to viral conjunctivitis that often starts in 1 eye and spreads to the other. This review covers only nongonococcal bacterial conjunctivitis.
Summary points
Bacterial conjunctivitis is usually self-limiting.
In people with suspected bacterial conjunctivitis, 1 systematic review found limited evidence from 1 randomized controlled trial (RCT) that topical norfloxacin use was associated with a significantly higher rate of clinical and microbiologic improvement than placebo. Comparative RCTs found no significant difference between different topical antibiotics in rates of clinical or microbiologic cure.
In people with bacterial conjunctivitis proved on culture, RCTs found faster clinical and microbiologic improvement with topical ciprofloxacin and ofloxacin than with placebo. Ofloxacin was associated with reduced relapse rate compared with placebo. Comparative RCTs found no significant difference between topical lomefloxacin and ciprofloxacin in clinical and microbiologic cure rates. One RCT found fusidate sodium to be superior to chloramphenicol.
Chloramphenicol is the only topical antibiotic possibly associated with serious systemic adverse effects (aplastic anemia).
We found no trials that examined the potential growth of resistant organisms from the use of antibiotics in bacterial conjunctivitis.
INCIDENCE/PREVALENCE
We found no good evidence on the incidence or prevalence of bacterial conjunctivitis.
ETIOLOGY/RISK FACTORS
Conjunctivitis may be infectious (caused by bacteria or viruses) or allergic. In adults, bacterial conjunctivitis is less common than viral conjunctivitis, although estimates vary widely (viral conjunctivitis has been reported to account for 8% to 75% of cases of acute conjunctivitis).1,2,3 Staphylococcus species are the most common bacterial pathogens, followed by Streptococcus pneumoniae and Haemophilus influenzae.4,5 In children, bacterial conjunctivitis is more common than viral and is mainly caused by H influenzae, S pneumoniae, and Moraxella catarrhalis.6,7
PROGNOSIS
Most bacterial conjunctivitis is self-limiting. In a systematic review of RCTs (primary sources Cochrane Controlled Trial Register [updated April 1999], Medline, bibliographies of identified trials, Science Citation Index, and personal contacts with investigators and pharmaceutical companies), 64% of people (99% confidence interval [CI], 54%-73%) had clinical cure or significant improvement on placebo within 2 to 5 days.8 Some organisms cause corneal or systemic complications, or both; otitis may develop in 25% of children with H influenzae conjunctivitis,9 and systemic meningitis may complicate primary meningococcal conjunctivitis in 18% of people.10 Conjunctivitis in children is more likely to be bacterial than viral, warranting heightened awareness of possible systemic complications.
AIMS
To achieve rapid resolution of inflammation and to prevent complications with minimal adverse effects of treatment.
OUTCOMES
Time to cure or improvement. Clinical signs or symptoms: hyperemia, discharge, papillae, follicles, chemosis, itching, pain, and photophobia. Most studies used a numbered scale to grade signs and symptoms. Some studies also included evaluation by investigators and patients regarding success of treatment. Culture results: usually number of colonies, sometimes with reference to a threshold level. Results were often classified into categories such as eradication, reduction, persistence, and proliferation.
METHODS
Clinical Evidence search and appraisal August 1999. All identified systemic reviews and RCTs were reviewed.
QUESTION: What are the effects of empiric treatment with topical antibiotics in adults and children with suspected bacterial conjunctivitis?
One systematic review found limited evidence from 1 RCT that topical norfloxacin was associated with a significantly higher rate of clinical and microbiologic improvement than placebo. Comparative RCTs found no significant difference between different topical antibiotics in rates of clinical or microbiologic cure.
Benefits
Versus placebo: We found 1 systematic review (updated in 1999) that identified 1 RCT comparing the effects of topical norfloxacin use (143 adults) versus placebo (141 adults); 50.4% of participants were culture-positive.8 Significantly higher rates of clinical and microbiologic cure or improvement at 5 days were obtained with norfloxacin (88.1% [95% CI, 81%-93%] vs 71.6% [95% CI, 63%-79%], P < 0.01). Versus each other: We found no systematic review. We found 18 comparative RCTs involving adults and children, and these found no significant difference between different topical antibiotics in rates of clinical or microbiologic cure (see table on the Web site www.clinicalevidence.org). Five of the RCTs (evaluating lomefloxacin, fusidic acid, rifamycin, chloramphenicol, and tobramycin sulfate) included grading by patients of effectiveness and tolerability. No significant differences were found.11,12,13,14,15
Harms
The placebo-controlled RCT reported minor adverse events in 4.2% of people using norfloxacin and 7.1% using placebo (no statistical analysis available).5 Placebo contained a higher proportion of benzalkonium chloride (0.01% vs 0.0025% in the norfloxacin solution). One nonsystematic review described complications of topical antibiotic use.16 These included 4 reported cases of idiosyncratic aplastic anemia associated with topical chloramphenicol and 3 cases of Stevens-Johnson syndrome associated with topical sulfonamides, but no figures were given on the number of people using these drugs.
Comment
The placebo-controlled RCT did not address the effect of using topical antibiotics on antibiotic resistance, which would be of interest given the self-limiting nature of the disease.5 None of the trials specified their method of selecting participants. The findings may not be generalizable to primary care patients. Most trials included children and adults, and the ratio of children to adults was usually not specified. Single-blind comparisons were used for lomefloxacin versus chloramphenicol and fusidate and norfloxacin versus fusidate. Lomefloxacin and fusidate are not available in the United States, and chloramphenicol is rarely used in the United States because of reports of idiosyncratic aplastic anemia.
QUESTION: What are the effects of topical antibiotics in adults and children with culture-positive conjunctivitis?
Randomized controlled trials in people with bacterial conjunctivitis proved on culture found faster clinical and microbiologic improvement with the use of ciprofloxacin, ofloxacin, or the combination of polymyxin B sulfate and bacitracin zinc than with placebo. The use of ofloxacin was associated with reduced relapse rates. Comparative RCTs found no significant difference between tobramycin and ciprofloxacin or between the combination of trimethoprim and polymyxin B sulfate and sulfacetamide sodium in clinical and microbiologic cure rates. One RCT found greater clinical efficacy and less microbiologic resistance with fusidate than with chloramphenicol.
Benefits
Versus placebo: We found 1 systematic review (updated 1999) that identified 3 placebo-controlled RCTs evaluating polymyxin-bacitracin, ciprofloxacin, and ofloxacin in bacterial conjunctivitis proved by culture. The review identified no trials of gentamicin sulfate that included only culture-proven conjunctivitis.8 One RCT of children (n = 84)17 found that in culture-proven H influenzae and S pneumoniae bacterial conjunctivitis, a higher clinical cure rate at days 3 to 5 was seen with polymyxin-bacitracin than with placebo, although by days 8 to 10, the difference was not significant (62% vs 28% at days 3-5 [P < 0.02], and 91% vs 72% at days 8-10 [P > 0.05]). The microbiologic cure rate was significantly higher with antibiotics at days 3 to 5 and 8 to 10. When antibiotics were given parenterally for concurrent problems, no significant difference was found between groups, but the numbers were too small to rule out a clinically important difference. Two other trials identified in the review did not specify the age of participants. One did not evaluate clinical outcome but found significantly greater microbiologic improvement at day 3 in people treated with ciprofloxacin (n = 177).18 One RCT published as an abstract only found significantly greater clinical and microbiologic improvement at day 2 in people treated with ofloxacin (n = 132): 64.1% versus 22.1% improved at day 2 (P < 0.001). It also found a lower relapse rate in people treated with ofloxacin 2 days after treatment stopped.19 A 4th trial was published in the Japanese language and was not reviewed.20
Versus each other: We found no systematic review. We found 1 RCT that did not specify the age of participants and 3 RCTs in children only. The former found no significant difference in microbiologic eradication or improvement between ciprofloxacin (n = 140) and tobramycin (n = 111); eradication or improvement occurred in 94.3% versus 91.9% (P > 0.5 [no CI available]).18 Clinical cure was not evaluated. Of the 3 RCTs in children, 2 found no significant difference in clinical or overall microbiologic efficacy between ciprofloxacin and tobramycin (n = 70 vs 71 [eradication, 90.1% vs 84.3%, P = 0.29; cure, 87% vs 89.9%, P = 0.6]) or between trimethoprimpolymyxin B, gentamicin, and sulfacetamide (n = 53 vs 57 vs 46 [eradication, 83% vs 68% vs 72%, P > 0.1; cure, 84% vs 88% vs 89%, P > 0.1]).21,22 The third (nonblinded) RCT found more clinical efficacy and less microbiologic resistance with fusidate than with chloramphenicol (n = 114 vs 25 [cure, 85% vs 48%, P < 0.001; resistance, 16% vs 55%, statistical analysis not provided]).23
Harms
Of the 116 children initially enrolled in the first RCT described above, 1 was excluded because of possible allergic reaction to the ointment; the other exclusions were unrelated to adverse effects.17 In RCTs that included people with both culture-proven and suspected bacterial conjunctivitis, minor adverse events were reported with antibiotics: burning, bitter taste, pruritus, or punctate epithelial erosions (35% with tobramycin vs 20% with ciprofloxacin; no statistical detail available from abstract)24; bad taste (20% with norfloxacin vs 6% with fusidate)25; and stinging (50% with norfloxacin vs 37% with fusidate) and burning (gentamicin more than lomefloxacin).13
Comment
None of the RCTs addressed the effect on antibiotic resistance of using topical antibiotics in bacterial conjunctivitis, which would be of interest given the self-limiting nature of the disease. Furthermore, they did not report on patient-oriented outcomes or look at rates of reinfection.
Figure 1.
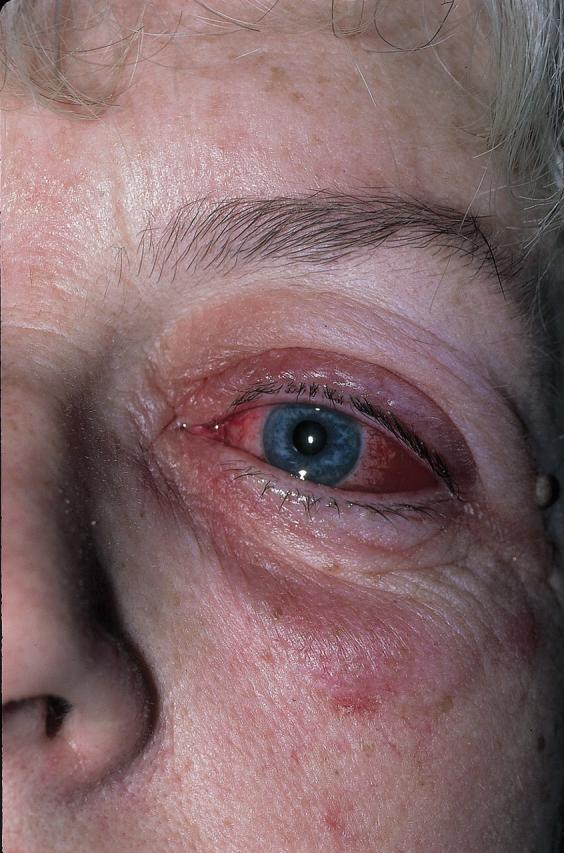
The left eye of a woman with acute conjunctivitis and periorbital edema
© Science Photo Library
Competing interests: None declared
This article comes from Clinical Evidence (2000;3:305-310), a new resource for clinicians produced jointly by the BMJ Publishing Group and the American College of Physicians—American Society of Internal Medicine. Clinical Evidence is an extensively peer-reviewed publication that summarizes the best available evidence on the effects of common clinical interventions gleaned from thorough searches and appraisal of the world literature. It became available in the United States late last year. Please see advertisement for more information or, alternatively, visit the web site at www.evidence.org.
References
- 1.Wishart PK, James C, Wishart MS, Darougar S. Prevalence of acute conjunctivitis caused by chlamydia, adenovirus, and herpes simplex virus in an ophthalmic casualty department. Br J Ophthalmol 1984;68: 653-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitch CP, Rapoza PA, Owens S, et al. Epidemiology and diagnosis of acute conjunctivitis at an inner-city hospital. Ophthalmology 1989;96: 1215-1220. [DOI] [PubMed] [Google Scholar]
- 3.Woodland RM, Darougar S, Thaker U, et al. Causes of conjunctivitis and keratoconjunctivitis in Karachi, Pakistan. Trans R Soc Trop Med Hyg 1992;86: 317-320. [DOI] [PubMed] [Google Scholar]
- 4.Seal DV, Barrett SP, McGill JI. Aetiology and treatment of acute bacterial infection of the external eye. Br J Ophthalmol 1982;66: 357-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller IM, Wittreich J, Vogel R, Cook TJ, for the Norfloxacin-Placebo Ocular Study Group. The safety and efficacy of topical norfloxacin compared with placebo in the treatment of acute bacterial conjunctivitis. Eur J Ophthalmol 1992;2: 58-66. [DOI] [PubMed] [Google Scholar]
- 6.Gigliotti F, Williams WT, Hayden FG, et al. Etiology of acute conjunctivitis in children. J Pediatr 1981;98: 531-536. [DOI] [PubMed] [Google Scholar]
- 7.Weiss A, Brinser JH, Nazar-Stewart V. Acute conjunctivitis in childhood. J Pediatr 1993;122: 10-14. [DOI] [PubMed] [Google Scholar]
- 8.Sheikh A, Hurwitz B, Cave J. Antibiotics for acute bacterial conjunctivitis. In: The Cochrane Library, Issue 4, 1999. Oxford, England: Update Software; updated April 1999.
- 9.Bodor FF. Conjunctivitis-otitis media syndrome: more than meets the eye. Contemp Pediatr 1989;6: 55-60. [Google Scholar]
- 10.Barquet N, Gasser I, Domingo P, Moraga FA, Macaya A, Elcuaz R. Primary meningococcal conjunctivitis: report of 21 patients and review. Rev Infect Dis 1990;12: 838-847. [DOI] [PubMed] [Google Scholar]
- 11.Kettenmeyer A, Jauch A, Boscher M, et al. A double-blind double-dummy multicenter equivalence study comparing topical lomefloxacin 0.3% twice daily with norfloxacin 0.3% four times daily in the treatment of acute bacterial conjunctivitis. J Clin Res 1998;1: 75-86. [Google Scholar]
- 12.Agius-Fernandez A, Patterson A, Fsadni M, et al. Topical lomefloxacin versus topical chloramphenicol in the treatment of acute bacterial conjunctivitis. Clin Drug Invest 1998;15: 263-269. [DOI] [PubMed] [Google Scholar]
- 13.Montero J, Casado A, Perea E, et al. A double-blind double-dummy comparison of topical lomefloxacin 0.3% twice daily with topical gentamicin 0.3% four times daily in the treatment of acute bacterial conjunctivitis. J Clin Res 1998;1: 29-39. [Google Scholar]
- 14.Adenis JP, Arrata M, Gastaud P, Limon S, Massin M. A multicenter randomized study of fusidic acid ophthalmic gel and rifamycin eyedrops in acute conjunctivitis [in French]. J Fr Ophtalmol 1989;12: 317-322. [PubMed] [Google Scholar]
- 15.Huerva V, Ascaso FJ, Latre B, et al. Tolerancia y eficacia de la tobramicina topica vs cloranfenicol en el tratamiento de las conjunctivitis bacterianas. Ciencia Pharmaceutica 1991;1: 221-224. [Google Scholar]
- 16.Stern GA, Killingsworth DW. Complications of topical antimicrobial agents. Int Ophthalmol Clin 1989;29: 137-142. [DOI] [PubMed] [Google Scholar]
- 17.Gigliotti G, Hendley JO, Morgan J, Michaels R, Dickens M, Lohr J. Efficacy of topical antibiotic therapy in acute conjunctivitis in children. J Pediatr 1984;104: 623-626. [DOI] [PubMed] [Google Scholar]
- 18.Leibowitz HM. Antibacterial effectiveness of ciprofloxacin 0.3% ophthalmic solution in the treatment of bacterial conjunctivitis. Am J Ophthalmol 1991;112(suppl 1): 29S-33S. [PubMed] [Google Scholar]
- 19.Ofloxacin Study Group III. A placebo-controlled clinical study of the fluoroquinolone ofloxacin in patients with external infection [abstract]. Invest Ophthalmol Vis Sci 1990;31: 572. [Google Scholar]
- 20.Mitsui Y, Matsuda H, Miyajima T, et al. Therapeutic effects of ofloxacin eye drops (DE-055) on external infection of the eye: multicentral double blind test [in Japanese]. J Rev Clin Ophthalmol 1986;80: 1813-1828. [Google Scholar]
- 21.Gross RD, Hoffman RO, Lindsay RN. A comparison of ciprofloxacin and tobramycin in bacterial conjunctivitis in children. Clin Pediatr 1997;36: 435-444. [DOI] [PubMed] [Google Scholar]
- 22.Lohr JA, Austin RD, Grossman M, Hayden GF, Knowlton GM, Dudley SM. Comparison of three topical antimicrobials for acute bacterial conjunctivitis. Pediatr Infect Dis J 1988;7: 626-629. [DOI] [PubMed] [Google Scholar]
- 23.van Bijsterveld OP, el Batawi Y, Sobhi FS, Massar MW. Fusidic acid in infections of the external eye. Infection 1987;15: 16-19. [DOI] [PubMed] [Google Scholar]
- 24.Alves MR, Kara JN. Evaluation of the clinical and microbiological efficacy of 0.3% ciprofloxacin drops and 0.3% tobramycin drops in the treatment of acute bacterial conjunctivitis. Rev Bras Oftalmol 1993;52: 371-377. [Google Scholar]
- 25.Wall AR, Sinclair N, Adenis JP. Comparison of Fucithalmic (fusidic acid viscous eye drops 1%) and Noroxin (norfloxacin ophthalmic solution 0.3%) in the treatment of acute bacterial conjunctivitis. J Clin Res 1998;1: 316-325. [Google Scholar]


