Abstract
Cognitive impairment and dementia are significant health burdens worldwide. Aging, hypertension, and diabetes are the primary risk factors for Alzheimer’s disease and Alzheimer’s disease and related dementias (AD/ADRD). There are no effective treatments for AD/ADRD to date. An emerging body of evidence indicates that cerebral vascular dysfunction and hypoperfusion precedes the development of other AD pathological phenotypes and cognitive impairment. However, vascular contribution to dementia is not currently well understood. This commentary highlights the emerging concepts and mechanisms underlying the microvascular contribution to AD/ADRD, including hypotheses targeting the anterograde and retrograde cerebral vascular pathways, as well as the cerebral capillaries and the venous system. We also briefly discuss vascular endothelial dysfunction, oxidative stress, inflammation, and cellular senescence that may contribute to impaired cerebral blood flow autoregulation, neurovascular uncoupling, and dysfunction of cerebral capillaries and the venous system.
Keywords: Aging, dementia, cerebral blood flow, neurovascular coupling, capillary, cerebral venous system
INTRODUCTION
Cognitive impairment and dementia are global healthcare crises. Alzheimer’s disease (AD) and vascular dementia are the most common forms of dementia, accounting for 50–75% and 20% of all cases, respectively. There are no cures for AD and AD-related dementias (AD/ADRD). Among six drugs (rivastigmine, galantamine, memantine, donepezil, memantine combined with donepezil, and aducanumab) approved by the U.S. Food and Drug Administration (FDA) for the treatment of AD to date, only aducanumab potentially slows the progression of AD (1). Many risk factors have been identified contributing to the onset and development of cognitive impairment and dementia, including age, genetics, cardiovascular and cerebral vascular diseases, traumatic brain injury, spinal cord injury, inadequate physical activity or sleep, excessive alcohol use, smoking, depression, and many more (2, 3). In the United States, eleven percent of people age 65 and older have dementia, and an estimated 6.2 million have AD as of 2021. The costs of treating and caring for dementia in 2021 were $355 billion. Moreover, AD is the fifth leading cause of death in people age 65 and older. In patients age 85 and older who died of COVID-19, eight and twenty percent had AD and vascular dementia, respectively (1).
AD/ADRD are progressive neurodegenerative disorders associated with memory deficits. Age is the greatest risk factor for AD/ADRD, especially in the late-onset cases correlated with genetic mutations in the apolipoprotein e4 gene (APOE-e4), the genes for amyloid precursor protein (APP) and the presenilin 1 and 2 (PS1 and PS2) proteins. However, only a small percentage of AD cases have been confirmed as a result of these genetic mutations, and cognitive impairment appears many years after pathological changes are detected in the brains of AD/ADRD patients and animals (4). Beta-amyloid (Aβ) plaques and intraneuronal tau-containing neurofibrillary tangles (NFTs) were thought to initiate the neurodegeneration and cognitive impairment seen in AD, but the failure of clinical trials targeting these pathways has led the community to reconsider other treatment options (5). Emerging studies have demonstrated that brain hypoperfusion is one of the causal factors of neurodegeneration that induces dementia in AD/ADRD (6). Cerebral microvascular mechanisms are now considered to be one of the major players in age-related cognitive impairment and dementia, similar to the original thoughts from Alois Alzheimer’s time (1900s) to the 20th century (7). Although underlying mechanisms remain elusive, the vascular hypothesis has been repeatedly validated in many community-based clinical-pathological studies and a recent human brain vascular atlas study demonstrating that 30 of the top 45 AD genome-wide association study (GWAS) genes are expressed in the cerebral vasculature (8). This editorial seeks to discuss the current understanding of the contribution of cerebral microvascular mechanisms to age-related cognitive impairment and dementia.
CEREBROVASCULAR MECHANISMS IN AGE-RELATED COGNITIVE IMPAIRMENT AND DEMENTIA
The brain is perfused by the internal carotid artery and the vertebral artery systems. These two arteries remit at the Willis circle, which acts as a base for the main cerebral arteries and provides blood flow to different brain regions. Pial large arterioles in the pia mater become smaller penetrating arterioles and parenchymal arterioles (PAs) that dive into the cortex to irrigate the gray and white matter. Vascular smooth muscle cells (VSMCs) or pericytes, endothelial cells (ECs), and extracellular matrix are major components of arteries and both large and small arterioles. The neurovascular unit (NVU) is located at the cerebral capillaries downstream of the PAs, which is composed of the ECs, pericytes, astrocyte end-feet, dendrites of neurons, and extracellular matrix (9). Several mechanisms have been suggested to contribute to cerebrovascular dysfunction, brain hypoperfusion and dementia. Here, we summarize as mechanisms targeting the anterograde and retrograde cerebral vascular pathways, as well as the cerebral capillaries and the venous system (Figure 1).
Figure 1. Mechanisms that target the anterograde and retrograde cerebral vascular pathways, as well as the cerebral capillaries and the venous system for age-related cognitive impairment and dementia.
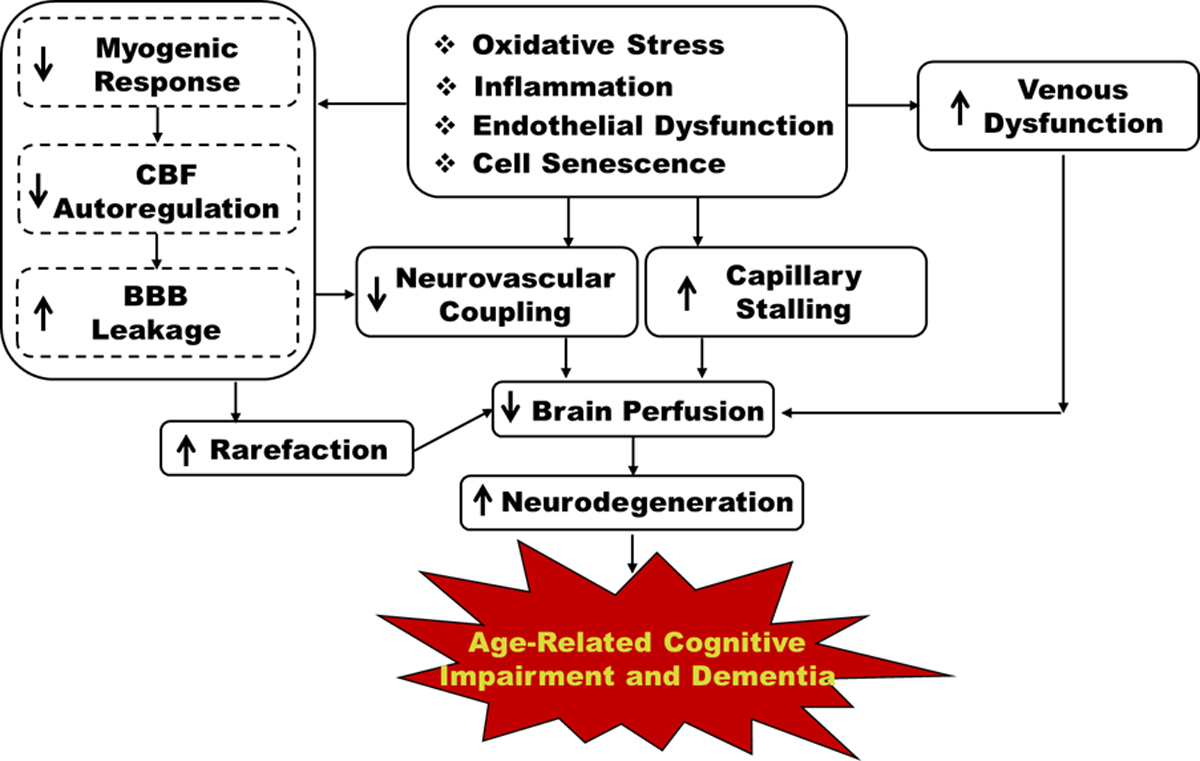
CBF: cerebral blood flow; BBB: blood-brain barrier.
Mechanisms targeting the anterograde cerebral vascular pathway
One of the most commonly affected vessels within the cerebral vasculature is the middle cerebral artery (MCA), which is the largest branch of the internal carotid artery (10). The PAs are high resistance vessels and key regulators of cerebral blood flow (CBF) that fine-tune capillary pressure and flow (11). Damage to the VSMCs and alpha-smooth muscle actin (α-SMA) positive pericytes have been reported to impair the myogenic response (MR) of cerebral arteries and arterioles, such as the MCAs and PAs, which diminishes CBF autoregulation in AD/ADRD (12–14). Impaired CBF autoregulation fails to prevent elevated pressure transmission to downstream capillaries resulting in blood-brain barrier (BBB) leakage, neurodegeneration, and cognitive impairment (15, 16). Diminished CBF autoregulation has been implicated in cerebral vascular disease, stroke, AD, hypertension-, and diabetes (DM)-related ADRD animal models (17–21). Thus, the MR and CBF autoregulation are mechanisms targeting the anterograde (artery-arteriole-capillary) cerebral vascular pathway to regulate brain perfusion and contribute to the development of age-related cognitive impairment and dementia.
Mechanisms targeting the retrograde cerebral vascular pathway
During intense local neuronal activation, independent of changes in blood pressure, healthy individuals display enhanced brain perfusion, which is achieved by functional hyperemia. This is due to the activation of an inward-rectifier K+ (Kir2.1) channel that hyperpolarizes capillary ECs at the NVU. The hyperpolarization signaling rapidly propagates, in a retrograde manner, along capillaries to upstream PAs via gap junctions, resulting in robust vasodilation and increased local CBF in the neuronal activated area. Neurovascular uncoupling has been found in AD/ADRD patients and animals associated with reduced cerebral capillary EC Kir2.1 activity (22–25).
Mechanisms targeting the cerebral capillaries
Another mechanism contributing to age-related cerebral microvascular dysfunction is capillary stalling and cessation of capillary blood flow. Many of the mechanisms underlying capillary stalling can be traced back to increased inflammation and reactive oxygen species (ROS) production, EC dysfunction, glial activation, capillary pericyte constriction, and altered leukocyte rolling and adhesion to the vascular wall (26, 27). The phenomenon of capillary stalling has been observed in older individuals and in AD/ADRD (27), therefore we depict it as a cerebral vascular mechanism targeting the capillaries to regulate brain perfusion.
Mechanisms targeting the cerebral venous system
There have been fewer studies focusing on understanding potential mechanisms targeting the cerebral venous system in age-related cognitive impairment and dementia. The blood pressure and flow velocity are low in the venous circulation, although it contains a large volume of fluid. In the elderly with AD/ADRD, cerebral venous system-related dysfunction potentially contributes to venous distension, BBB leakage, neuroinflammation, thrombosis and microhemorrhages, and neurodegeneration, which results in brain hypoperfusion and ischemic neuronal damage (28, 29).
CEREBRAL MICROVASCULAR ALTERATION IN AGING, DM, AND HYPERTENSION
Notably, despite the high prevalence, mortalities, and morbidities of AD/ADRD most commonly found in the elderly, aging alone is insufficient to cause AD/ADRD (1). Aging and associated pathophysiological processes alter the structural and functional integrity of cerebral vasculature that can significantly reduce brain perfusion (30, 31). Underlying mechanisms that contribute to these alterations include inflammation, oxidative stress, mitochondrial dysfunction, cellular senescence and apoptosis in all types of vascular cells concurrent with the activation of matrix metalloproteinases (MMPs) and enhanced vascular fibrosis that affect all cerebral vascular segments along the vascular beds (24, 32–38).
DM and hypertension are the most common diseases in the aging population that are associated with a higher risk of AD/ADRD. Recent studies suggest that aging, hypertension or DM-related ADRD and AD all exhibit cerebral microvascular dysfunction via various mechanisms that eventually lead to a final common outcome of localized cerebral hypoperfusion, neurodegeneration, brain atrophy, and cognitive impairment (6, 22, 39, 40). Our recent studies demonstrated that BBB leakage and the neurocognitive deficit in an old non-obese type 2 DM rat were reversed by a sodium-glucose co-transporter 2 inhibitor (SGLT2i) without altering blood pressure. These effects were possibly due to the reversal of hyperglycemia-induced mitochondrial dysfunction in cerebral VSMCs and pericytes, the restoration of impaired MR of cerebral arteries and arterioles, the reversal of neurovascular uncoupling, and enhanced reduced pericyte and tight junction coverage in the cortex and hippocampus (13, 14, 18, 23, 41). In young spontaneously hypertensive rats (SHRs) and angiotensin II (Ang II) rodent models of hypertension and young patients with essential hypertension, elevations in blood pressure promote inward remodeling of cerebral arteries and arterioles, resulting in an increase in the wall-to-lumen ratio and elevated cerebral vascular resistance which enhances the MR (34, 42, 43), similar to what is observed in young hypertensive individuals. Gradual loss of this crucial vascular protective mechanism in normal aging, especially when combined with other risk factors (such as DM and hypertension), shifts CBF autoregulation to lower pressures that fail to protect the cerebral microcirculation and the brain from damage (15, 33, 34). In addition, continued hypertrophy of vessels and endothelial damage in the elderly promotes arteriosclerosis which can further exacerbate cerebral hypoperfusion, neurodegeneration, and loss of cognitive function. Moreover, the elevated vascular resistance may have direct effects on the cerebral capillaries to reduce capillary perfusion. In contrast, Dahl salt-sensitive (Dahl SS) and Fawn-hooded hypertensive (FHH) rat models of hypertension display impaired MR of the MCAs and PAs and CBF autoregulation (44, 45). The Dahl SS and FHH rats contain genetic variants in the Cyp4a and Add3 vasoconstrictor genes, respectively, which are homologous mutations in CYP4A and ADD3, found in participants in the ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study) that have AD-like symptoms and pathological and cognitive features (46, 47). Therefore, these hypertension models with genetic defects in genes involved in CBF autoregulation seem to directly enter the pathological status of essential hypertension in aging that is associated with brain hypoperfusion due to BBB leakage-related ischemic damage (Figure 2). Additionally, loss of membrane expression of the ADD3 protein in the FHH rats dysregulates the actin cytoskeleton in VSMCs and podocytes, which also results in impaired renal hemodynamics and glomerular injury (48, 49).
Figure 2. Effects of Hypertension on age-related cognitive impairment and dementia.
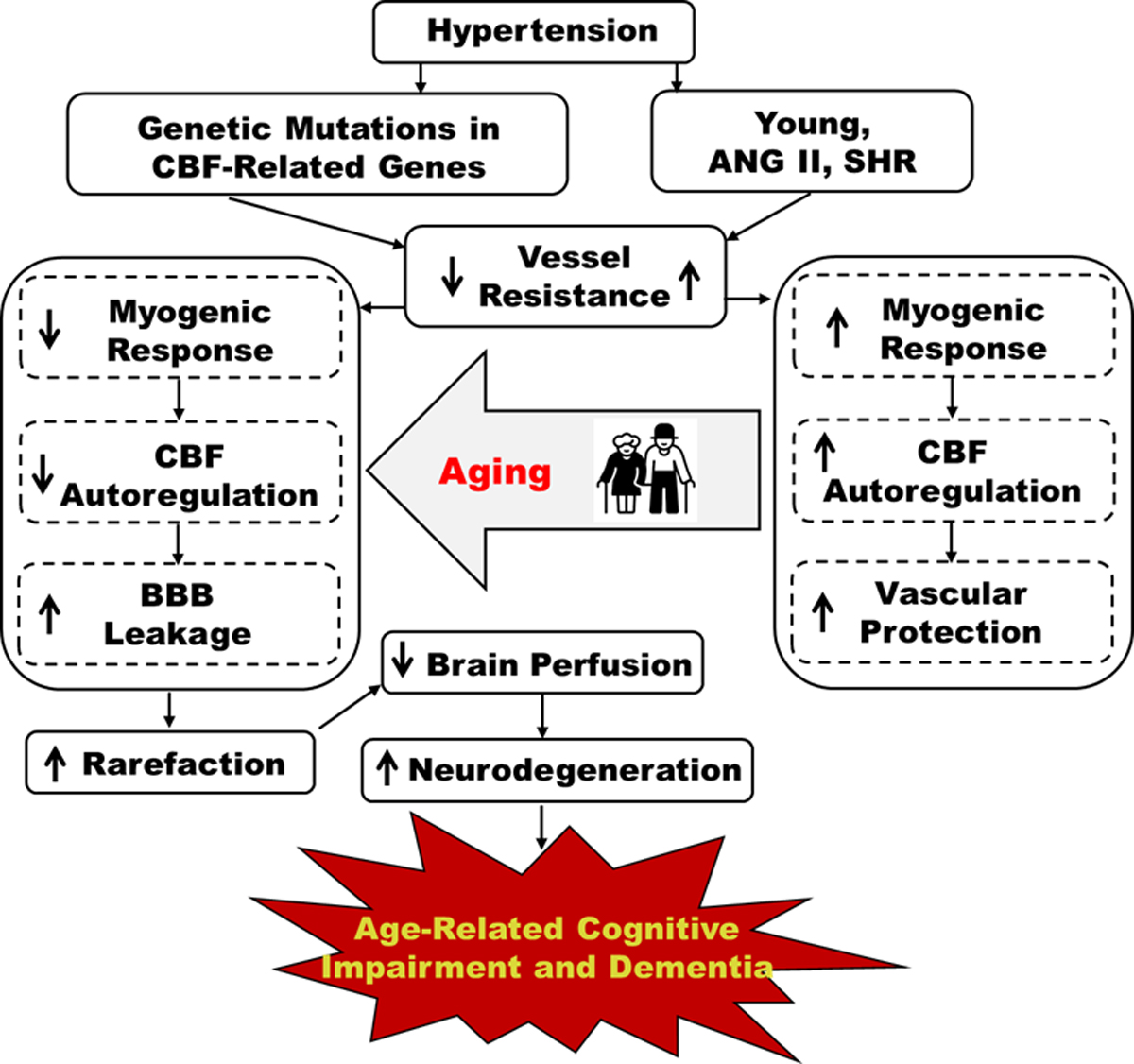
Vascular remodeling and elevated myogenic tone protect the cerebral microcirculation from damage in young patients and Ang II and SHR animal models of hypertension. This vascular protection is lost with aging, and genetic hypertension models with mutations in CBF-related genes, leading to impaired autoregulation, BBB leakage, capillary damage, and decreased CBF that contribute to age-related cognitive impairments and dementia. ANG II: angiotensin II; SHR: spontaneously hypertensive rat; CBF: cerebral blood flow; BBB cognitive dysfunction.
MOLECULAR AND CELLULAR MECHANISMS ASSOCIATED WITH CEREBRAL VASCULAR DYSFUNCTION IN AGE-RELATED COGNITIVE IMPAIRMENT AND DEMENTIA
Many potential molecular and cellular mechanisms have been proposed to contribute to cerebral vascular dysfunction in age-related cognitive impairment and dementia. Here, we briefly discuss several aspects that have been actively studied.
Oxidative stress
A large number of studies have implicated that increased oxidative stress contributes to cerebral microvascular dysfunction in aging. Excessive oxidative stress has been associated with aging, age-related cardiovascular, cerebral vascular, and neurodegenerative diseases in humans and animal models (30, 50–52). Elevated ROS may attribute to the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, dysfunction of mitochondria in the vascular cells, and increased numbers of perivascular macrophages. Increases in ROS influence many facets of vascular function, including reduced production or inactivation of endothelium-derived NO, induction of redox-sensitive transcription factors, and oxidation of critical proteins involved in vascular contractile mechanisms. Reduced NO diminishes vascular protective effects, inhibits platelet aggregation and endothelial apoptosis, and reduces anti-inflammatory properties. Additionally, increased ROS promotes cerebral microhemorrhages by activating ROS-MMP axis in old hypertensive mice (53). Mitochondria-derived H2O2 induces inflammation in the ECs by activating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB), resulting in overexpression of cytokines in aged rats (30, 50). Loss of VSMC and pericyte contractile capabilities that contribute to the impaired MR and CBF autoregulation have been found in old DM rats with cognitive deficits, which was thought to be due to increased mitochondrial fission protein expression associated with reduced adenosine triphosphate (ATP) production (13, 14).
Inflammation
Continuous low-grade inflammation has been reported in laboratory animals and humans with age-related cognitive impairment and dementia, which is closely linked to increased activity of NADPH oxidates and ROS production in the cerebral vasculature. On the other hand, inflammatory mediators are potent inducers of cellular oxidative stress. Moreover, growing evidence also suggests a significant interaction between vascular inflammation and endothelial cell senescence (30). Potential mechanisms of cerebral vascular inflammation in aging individuals with dementia may be attributed to BBB leakage due to impaired CBF autoregulation, EC dysfunction, and capillary stalling, which leads to infiltrate circulated inflammatory factors, such as tumor necrosis factor (TNF)-α, interleukin 6 (IL-6), IL-1β to the microenvironment in the brain. The activation of perivascular macrophages and gut microbiome also contribute to neurovascular and cognitive deficits (54).
Endothelial dysfunction
Age-dependent endothelial dysfunction is a multifactorial process associated with the deterioration of cerebral vascular ECs. It is closely associated with increased production of ROS, inflammation, cellular senescence, altered leukocyte rolling and adhesion to the vascular wall (26, 27, 30). The common end-points of EC dysfunction are the reduction and release of vasodilatory endothelial factors, such as nitric oxide (NO), prostacyclin, and the other endothelium-derived hyperpolarizing factors. Loss of critical roles of ECs in cardiovascular homeostasis also results in dysregulation of blood fluidity and fibrinolysis, angiogenesis, and platelet aggregation (30).
Cell senescence
Cell replicative senescence is a fundamental biological process in aging that can be evoked by age-associated pathophysiological or environmental stress events (55–57). It is a phenomenon of the cessation of cell proliferation, leading to cell cycle arrest due to the shortening of telomeres-induced DNA damage, oxidative stress, inflammation, mitochondrial dysfunction, autophagy, and many other stressors. Accelerated cell senescence has been found in cerebral VSMCs, ECs, and other vascular cells in age-related cognitive impairment and dementia, which may play a role in the development of vascular dysfunction and brain hypoperfusion in these individuals (30, 50).
CONCLUSION
In summary, age-related cognitive impairment and dementia are often seen in DM and hypertension, which are all associated with cerebral vascular dysfunction that leads to cerebral hypoperfusion. Potential underlying mechanisms include EC dysfunction, oxidative stress, inflammation, and cellular senescence, resulting in impaired CBF autoregulation, neurovascular uncoupling, and dysfunction of cerebral capillaries and the venous system. Reversing cerebral vascular dysfunction to enhance brain perfusion could be a potential treatment for AD/ADRD.
SOURCES OF FUNDING
This study was supported by grants AG057842, HL138685, P20GM104357 from the National Institutes of Health; Medical Student Research Program (MSRP) from the University of Mississippi Medical center.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17: 327–406, 2021. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Roman RJ, and Fan F. Duration and magnitude of bidirectional fluctuation in blood pressure: the link between cerebrovascular dysfunction and cognitive impairment following spinal cord injury. J Neurobiol Physiol 2: 15–18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csipo T, Lipecz A, Owens C, Mukli P, Perry JW, Tarantini S, Balasubramanian P, Nyúl-Tóth Á, Yabluchanska V, Sorond FA, Kellawan JM, Purebl G, Sonntag WE, Csiszar A, Ungvari Z, and Yabluchanskiy A. Sleep deprivation impairs cognitive performance, alters task-associated cerebral blood flow and decreases cortical neurovascular coupling-related hemodynamic responses. Sci Rep 11: 20994, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang X, Zhang H, Wang S, Liu Y, Gao W, Roman RJ, and Fan F. Abstract P076: Cerebral Vascular Dysfunction Precedes Cognitive Impairment In Alzheimer’S Disease. Hypertension 76: AP076–AP076, 2020. [Google Scholar]
- 5.Huang LK, Chao SP, and Hu CJ. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci 27: 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan F, and Roman RJ. Reversal of cerebral hypoperfusion: a novel therapeutic target for the treatment of AD/ADRD? Geroscience 43: 1065–1067, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iadecola C The pathobiology of vascular dementia. Neuron 80: 844–866, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang AC, Vest RT, Kern F, Lee DP, Maat CA, Losada PM, Chen MB, Agam M, Schaum N, Khoury N, Calcuttawala K, Pálovics R, Shin A, Wang EY, Luo J, Gate D, Siegenthaler JA, McNerney MW, Keller A, and Wyss-Coray T. A human brain vascular atlas reveals diverse cell mediators of Alzheimer’s disease risk. bioRxiv 2021.2004.2026.441262, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipolla MJ. The Cerebral Circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2009. [PubMed] [Google Scholar]
- 10.Wang S, Zhang H, Liu Y, Li L, Guo Y, Jiao F, Fang X, Jefferson JR, Li M, Gao W, Gonzalez-Fernandez E, Maranon RO, Pabbidi MR, Liu R, Alexander BT, Roman RJ, and Fan F. Sex differences in the structure and function of rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 318: H1219–H1232, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Zhang C, Liu Y, Gao W, Wang S, Fang X, Guo Y, Li M, Liu R, Roman RJ, Sun P, and Fan F. Influence of dual-specificity protein phosphatase 5 on mechanical properties of rat cerebral and renal arterioles. Physiol Rep 8: e14345, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Zhang H, Wu CY, Yu T, Fang X, Ryu JJ, Zheng B, Chen Z, Roman RJ, and Fan F. 20-HETE-promoted cerebral blood flow autoregulation is associated with enhanced pericyte contractility. Prostaglandins Other Lipid Mediat 154: 106548, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Wang S, Liu Y, Fan L, Booz GW, Roman RJ, Chen Z, and Fan F. Accelerated cerebral vascular injury in diabetes is associated with vascular smooth muscle cell dysfunction. Geroscience 42: 547–561, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Zhang H, Wang S, Guo Y, Fang X, Zheng B, Gao W, Yu H, Chen Z, Roman RJ, and Fan F. Reduced pericyte and tight junction coverage in old diabetic rats are associated with hyperglycemia-induced cerebrovascular pericyte dysfunction. Am J Physiol Heart Circ Physiol 320: H549–H562, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, Booz GW, and Roman RJ. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci (Landmark Ed) 21: 1427–1463, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, and Li Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J Alzheimers Dis 63: 1223–1234, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Claassen J, Thijssen DHJ, Panerai RB, and Faraci FM. Regulation of Cerebral Blood Flow in Humans: Physiology and Clinical Implications of Autoregulation. Physiol Rev 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Jiao F, Border JJ, Fang X, Crumpler RF, Liu Y, Zhang H, Jefferson J, Guo Y, Elliott PS, Thomas KN, Strong LB, Urvina AH, Zheng B, Rijal A, Smith SV, Yu H, Roman RJ, and Fan F. Luseogliflozin, a sodium-glucose cotransporter-2 inhibitor, reverses cerebrovascular dysfunction and cognitive impairments in 18-mo-old diabetic animals. Am J Physiol Heart Circ Physiol 322: H246–h259, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan F, Pabbidi M, Lin RCS, Ge Y, Gomez-Sanchez EP, Rajkowska GK, Moulana M, Gonzalez-fernandez E, Sims J, Elliott MR, Paul IA, Alexander AP, Mosley TH, Harder DR, and Roman RJ. Impaired myogenic response of MCA elevates transmission of pressure to penetrating arterioles and contributes to cerebral vascular disease in aging hypertensive FHH rats. The FASEB Journal 30: 953.957–953.957, 2016. [Google Scholar]
- 20.Fan F, Geurts AM, Pabbidi MR, Smith SV, Harder DR, Jacob H, and Roman RJ. Zinc-finger nuclease knockout of dual-specificity protein phosphatase-5 enhances the myogenic response and autoregulation of cerebral blood flow in FHH.1BN rats. PLoS One 9: e112878, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toth P, Tarantini S, Csiszar A, and Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 312: H1–H20, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mughal A, Harraz OF, Gonzales AL, Hill-Eubanks D, and Nelson MT. PIP2 Improves Cerebral Blood Flow in a Mouse Model of Alzheimer’s Disease. Function (Oxf) 2: zqab010, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Roman R, and Fan F. Hippocampus is more susceptible to hypoxic injury: has the Rosetta Stone of regional variation in neurovascular coupling been deciphered? GeroScience 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarantini S, Tran CHT, Gordon GR, Ungvari Z, and Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol 94: 52–58, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, and Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol 306: H299–308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crumpler R, Roman RJ, and Fan F. Capillary Stalling: A Mechanism of Decreased Cerebral Blood Flow in AD/ADRD. J Exp Neurol 2: 149–153, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz Hernández JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M, Park L, Vinarcsik LK, Ivasyk I, Rivera DA, Kang Y, Cortes-Canteli M, Peyrounette M, Doyeux V, Smith A, Zhou J, Otte G, Beverly JD, Davenport E, Davit Y, Lin CP, Strickland S, Iadecola C, Lorthois S, Nishimura N, and Schaffer CB. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci 22: 413–420, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulop GA, Tarantini S, Yabluchanskiy A, Molnar A, Prodan CI, Kiss T, Csipo T, Lipecz A, Balasubramanian P, Farkas E, Toth P, Sorond F, Csiszar A, and Ungvari Z. Role of age-related alterations of the cerebral venous circulation in the pathogenesis of vascular cognitive impairment. Am J Physiol Heart Circ Physiol 316: H1124–h1140, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molnár A, Nádasy GL, Dörnyei G, Patai BB, Delfavero J, Fülöp G, Kirkpatrick AC, Ungvári Z, and Merkely B. The aging venous system: from varicosities to vascular cognitive impairment. Geroscience 43: 2761–2784, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungvari Z, Tarantini S, Donato AJ, Galvan V, and Csiszar A. Mechanisms of Vascular Aging. Circ Res 123: 849–867, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yabluchanskiy A, Nyul-Toth A, Csiszar A, Gulej R, Saunders D, Towner R, Turner M, Zhao Y, Abdelkari D, Rypma B, and Tarantini S. Age-related alterations in the cerebrovasculature affect neurovascular coupling and BOLD fMRI responses: Insights from animal models of aging. Psychophysiology 58: e13718, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipecz A, Csipo T, Tarantini S, Hand RA, Ngo BN, Conley S, Nemeth G, Tsorbatzoglou A, Courtney DL, Yabluchanska V, Csiszar A, Ungvari ZI, and Yabluchanskiy A. Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience 41: 341–349, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, and Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab 35: 527–530, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, and Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, and Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci 69: 1339–1352, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, and Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 40: 513–521, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan F, Wang SX, Mims PN, Maeda KJ, Li LY, Geurts AM, and Roman RJ. Knockout of matrix metalloproteinase-9 rescues the development of cognitive impairments in hypertensive Dahl salt sensitive rats. FASEB J 31: 842.846–842.846, 2017. [Google Scholar]
- 38.Fan F, Booz GW, and Roman RJ. Aging diabetes, deconstructing the cerebrovascular wall. Aging (Albany NY) 13: 9158–9159, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, and Csiszar A. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nature reviews Nephrology 17: 639–654, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyúl-Tóth Á, Tarantini S, Kiss T, Toth P, Galvan V, Tarantini A, Yabluchanskiy A, Csiszar A, and Ungvari Z. Increases in hypertension-induced cerebral microhemorrhages exacerbate gait dysfunction in a mouse model of Alzheimer’s disease. Geroscience 42: 1685–1698, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Lv W, Zhang H, Liu Y, Li L, Jefferson JR, Guo Y, Li M, Gao W, Fang X, Paul IA, Rajkowska G, Shaffery JP, Mosley TH, Hu X, Liu R, Wang Y, Yu H, Roman RJ, and Fan F. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience 42: 1387–1410, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roman RJ, and Fan F. 20-HETE: Hypertension and Beyond. Hypertension 72: 12–18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, and Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol 305: H1698–1708, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan F, Geurts AM, Murphy SR, Pabbidi MR, Jacob HJ, and Roman RJ. Impaired myogenic response and autoregulation of cerebral blood flow is rescued in CYP4A1 transgenic Dahl salt-sensitive rat. Am J Physiol Regul Integr Comp Physiol 308: R379–390, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan F, Pabbidi MR, Ge Y, Li L, Wang S, Mims PN, and Roman RJ. Knockdown of Add3 impairs the myogenic response of renal afferent arterioles and middle cerebral arteries. Am J Physiol Renal Physiol 312: F971–F981, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan F, Simino J, Auchus AP, Knopman DS, Boerwinkle E, Fornage M, Mosley TH, and Roman RJ. Functional variants in CYP4A11 and CYP4F2 are associated with cognitive impairment and related dementia endophenotypes in the elderly. In: The 16th International Winter Eicosanoid Conference. Baltimore: 2016, p. CV5. [Google Scholar]
- 47.Thomas K, Wang S, Zhang H, Crumpler R, Elliott P, Ryu J, Fang X, Strong L, Liu Y, Zheng B, Fan F, and Roman R. Abstract 35: Gamma Adducin Dysfunction Leads To Cerebrovascular Distention, Blood Brain Barrier Leakage, And Cognitive Deficits In The Fawn-hooded Hypertensive Rats. Hypertension 78: 2021. [Google Scholar]
- 48.Fan F, Geurts AM, Pabbidi MR, Ge Y, Zhang C, Wang S, Liu Y, Gao W, Guo Y, Li L, He X, Lv W, Muroya Y, Hirata T, Prokop J, Booz GW, Jacob HJ, and Roman RJ. A Mutation in gamma-Adducin Impairs Autoregulation of Renal Blood Flow and Promotes the Development of Kidney Disease. J Am Soc Nephrol 31: 687–700, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao W, Liu Y, Fan L, Zheng B, Jefferson JR, Wang S, Zhang H, Fang X, Nguyen BV, Zhu T, Roman RJ, and Fan F. Role of γ-adducin in actin cytoskeleton rearrangements in podocyte pathophysiology. Am J Physiol Renal Physiol 320: F97–f113, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, and Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci 13: 5056–5070, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Berkowitz BA, Podolsky RH, Childers KL, Gow A, Schneider BL, Lloyd SC, Bosse KE, Conti AC, Roberts R, Berri AM, Graffice E, Sinan K, Eliwat W, and Shen Y. Age-related murine hippocampal CA1 laminae oxidative stress measured in vivo by QUEnch-assiSTed (QUEST) MRI: impact of isoflurane anesthesia. Geroscience 42: 563–574, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Logan S, Royce GH, Owen D, Farley J, Ranjo-Bishop M, Sonntag WE, and Deepa SS. Accelerated decline in cognition in a mouse model of increased oxidative stress. Geroscience 41: 591–607, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, and Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell 14: 400–408, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Yang Y, Su J, Zheng X, Wang C, Chen S, Liu J, Lv Y, Fan S, Zhao A, Chen T, Jia W, and Wang X. Age-related compositional changes and correlations of gut microbiome, serum metabolome, and immune factor in rats. Geroscience 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anerillas C, Abdelmohsen K, and Gorospe M. Regulation of senescence traits by MAPKs. Geroscience 42: 397–408, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arancio W Progerin expression induces a significant downregulation of transcription from human repetitive sequences in iPSC-derived dopaminergic neurons. Geroscience 41: 39–49, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Attaallah A, Lenzi M, Marchionni S, Bincoletto G, Cocchi V, Croco E, Hrelia P, Hrelia S, Sell C, and Lorenzini A. A pro longevity role for cellular senescence. Geroscience 42: 867–879, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


