最近,人们发现接种2019冠状病毒病(COVID-19)疫苗后出现了大量并发症,影响心脏、肾脏、胰腺、关节和皮肤。本文结合已报道的11例COVID-19疫苗(主要与信使RNA(mRNA)疫苗有关)接种后白癜风病例的数据和我们自己接种的1例非复制载体疫苗后的白癜风病例,分析探讨了其相关的病理机制。根据常见的原理,自身免疫性病变是通过病毒的抗原表位与某些人类蛋白质之间的分子模拟诱发的。我们认为这一过程的基础是胸腺负选择的破坏,导致自身反应性T细胞迁移到外周。在本文中,我们证实了疫苗和/或感染以及黑素细胞氧化应激是自身免疫反应的主要诱因。在这种情况下,白癜风的发病机制可被视为糖尿病或动脉粥样硬化等多种自身免疫性疾病发病机制的模型。此外,我们概述了一种病理机制,其中黑色素细胞氧化应激、自身反应性T细胞和胸腺功能障碍可作为干预策略的潜在目标。
Keywords: 白癜风, 2019冠状病毒病疫苗, 皮质醇, 去氢表雄酮, 自身反应性T细胞, 维生素D3
During the coronavirus disease 2019 (COVID-19) pandemic, vaccines help control the spread of infection. To date, 47 vaccines have been approved, with another 227 candidates in various stages of development. In the short period of time since the beginning of their use, evidence has begun to emerge of complications following vaccination in the form of the development or exacerbation of a number of pathological conditions (Block et al., 2022; Haseeb et al., 2022). For example, a population-based study in France identified 1612 cases of myocarditis and 1613 cases of pericarditis requiring hospital treatment within five months of vaccination (le Vu et al., 2022).
One type of complication is vitiligo. Vitiligo is an acquired chronic disease that affects between 0.06% and 2.28% of the general population (Krüger and Schallreuter, 2012). This article presents all known vitiligo cases published in scientific journals after COVID-19 vaccination, as well as our own case of autoimmune damage to melanocytes, and aims to discuss the possible causes of this process in more detail.
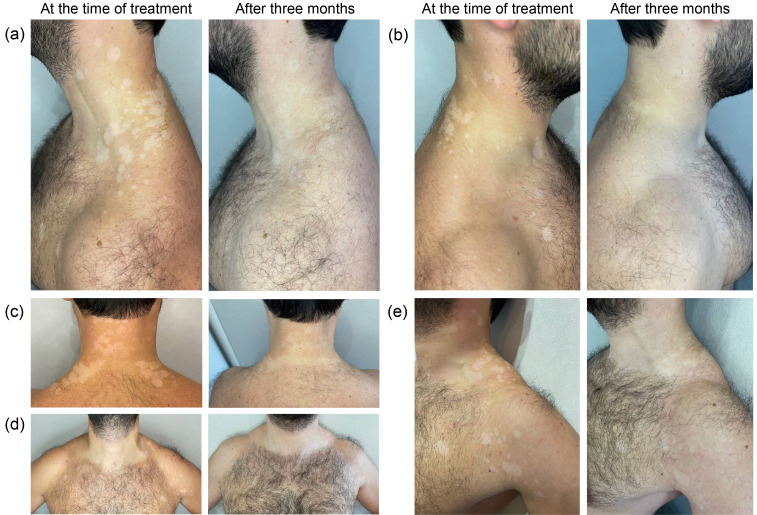
Here, we report a second case of vitiligo after using a nonreplicating viral vector vaccine. A 35-year-old man presented us with a 13-month history of well-defined, oval-to-linear, milky-white spots on his neck, chest, back, and shoulders (Fig. 1). The spots first appeared one month after the second dose of the nonreplicating vector vaccine Gam-COVID-Vac “Sputnik V” (Center Gamaleya) on the back of the neck in summer (as of Aug. 18, 2021). For six months afterwards, the spots did not increase in number or size. Then, after suffering mild COVID-19 and intense sun exposure, as of Feb. 24, 2022, the spots from the neck spread to the upper back, but the spots did not increase further, and no new spots appeared. In September of the same year, extensive spreading of spots on the sides of the neck, back, shoulders, and upper chest began. The spots began to grow and merge. The patient independently applied 1% (mass fraction) hydrocortisone ointment once a day for 7 d, but this did not stop the process from spreading. Then, the patient began using 0.1% (mass fraction) tacrolimus ointment once a day. This began to stop the development of large spots, but there were still small new lesions on the lower back, sides of the shoulders and forearms, after which the patient sought medical help. The patient was scheduled for tests for thyroid-stimulating hormone and thyroid hormones, thyroperoxidase antibodies, and cortisol. As of Oct. 19, 2022, the patient was found to have an elevated cortisol level of 723 nmol/L (the norm is 171‒536 nmol/L), the concentrations of the other hormones were normal, and there were no antibodies to thyroperoxidase. After 14 d of taking tacrolimus, the development of vitiligo stopped. There was no family history of vitiligo, and the patient had never suffered from it before. The white spots were clinically consistent with vitiligo and were examined under Wood’s lamp, confirming the diagnosis by the milky-white colouration of the spots. Three months later, the patient came in for an appointment. The patient showed repigmentation of the lesions (Fig. 1).
Fig. 1. Vitiliginous lesions at the time of treatment and after three months. (a) White spots on the left side of the neck and body; (b) White spots on the right side of the neck and body; (c) White spots on the back and neck; (d) White spots on chest and shoulders; (e) White spots on left neck, chest, and shoulder.
We obtained the patient’s informed consent for the publication of his case (images, medical history, and laboratory data). To the best of our knowledge, this is the 12th case of vitiligo associated with COVID-19 vaccination (Table 1).
Table 1.
Reported cases of vitiligo after vaccination and COVID-19
| Vaccine | Onset after vaccination | Localization | Age (years) | Sex | Related diseases | Reference |
|---|---|---|---|---|---|---|
| Pfier-BioNTech BNT162b2; vaccine type: mRNA | One week after the first dose | Face | 58 | M | Ulcerative colitis | Aktas and Ertuğrul, 2022 |
| Pfier-BioNTech BNT162b2; vaccine type: mRNA | Two weeks after the second dose, new spots | Multiple and widespread vitiligo of the upper and lower extremities, face, perioral and periocular areas. On the body, armpits, and genital area, there are large confluent foci of vitiligo. Approximately 70% of the total body surface area is affected. | 66 | М | Ten years ago, appearance of achromic, well-defined, and rounded spots, affecting the perioral and periocular regions, axillae, genital and sacral regions; Hashimoto's thyroiditis | Caroppo et al., 2022 |
| Pfier-BioNTech BNT162b2; vaccine type: mRNA | Three days after the second dose | Cervical-collar zone, face, abdomen, back, upper and lower extremities. Approximately 20% of the total surface area of his body is affected. | 69 | M | No history of autoimmunity | Nicolaidou et al., 2023 |
| mRNA-1273 (Moderna); vaccine type: mRNA | A few days after the first dose, with progression after the second dose | Face, neck, chest, and abdomen | 61 | F | No history of autoimmunity | Kaminetsky and Rudikoff, 2021 |
| Pfier-BioNTech BNT162b2; vaccine type: mRNA | One week after the first dose | Neck, upper back, and sternum area | 33 | F | Otherwise healthy, her father had vitiligo. | Ciccarese et al., 2022 |
| Biotech Sinovac; vaccine type: inactivated | Two weeks after the second dose | Face | 49 | M | Eight months before vitiligo, he was diagnosed with COVID-19. | Koç Yıldırım, 2022 |
| Pfier-BioNTech BNT162b2; vaccine type: mRNA | One week after the first dose. Between the first and second doses, the spots increased in size and new spots formed rapidly. After the second dose, the rate of progression slowed. | On both sides of the armpits and the flexor surfaces of the forearms | 47 | M | Ankylosing spondylitis for almost 40 years. He had previously taken sulfasalazine, but had not received any treatment for four years. | Uğurer et al., 2022 |
| mRNA-1273 (Moderna); vaccine type: mRNA | Two weeks after the first dose | Multiple spots on the limbs and body. Approximately 5% of the body surface area is affected. | 13 | F | She is otherwise healthy. Her father and a paternal uncle had vitiligo as an adult; however, the patient had never had vitiligo before. | Bukhari, 2022 |
| mRNA-1273 (Moderna); vaccine type: mRNA | Three weeks after the first dose | Left shoulder at injection site, several additional areas of depigmentation on face, head, and chest | 43 | F | Type II diabetes and psoriasis. One week after the first vaccine, she had a bilateral exacerbation of psoriasis on her hands and forearms. | Singh et al., 2022 |
| Astrazeneca ChAdOx1/AZD1222; vaccine type: non-replicating viral vector | Three days after the first dose | Face and both hands | 60 | F | Otherwise, healthy | López Riquelme et al., 2022 |
| Pfizer-BioNTech BNT162b2; vaccine type: mRNA | After receiving the second dose of the Comirnaty vaccine | Multiple depigmented patches on bilateral dorsal hands | 35 | F | Otherwise, healthy | Macca et al., 2022 |
COVID-19: coronavirus disease 2019; mRNA: messenger RNA; M: male; F: female.
Table 1 shows all described cases of vitiligo after COVID-19 vaccination. Interestingly, among all the above cases of vitiligo, including the one described by us, nine cases (75.0%) developed after immunization with messenger RNA (mRNA) vaccines. In 50.0% (six cases), there were lesions of the neck and upper back first, which later spread to the face and other parts of the body. In 16.7% (two cases) of observations, there were lesions only on the face, and in 33.3% (four cases), there were lesions on the back side of the hands. Lesions of the neck or upper back are not typical for vitiligo; in contrast, vitiligo most often affects the fingers, hands, and face (Speeckaert and van Geel, 2017). In 83.3% of cases, vitiligo was not present in family history. There are also a number of known cases of vitiligo after COVID-19 (Herzum et al., 2022; Schmidt et al., 2022).
To date, the etiopathogenesis of vitiligo is still debated, but there is evidence of an autoimmune process mediated by T cluster of differentiation 8-positive (CD8+) cells, where oxidative stress is the trigger. Immune activation by vaccination or during COVID-19 disease may trigger the development of vitiligo disease by shifting to adaptive type 1 (CD8+ and interferon-γ (IFN-γ)-producing T cells) immune responses (Post et al., 2021). However, it is not yet clear how autoreactivity of T cells develops and what is the relationship between age and the incidence of vitiligo, and we will try to answer these questions in this article.
For further discussion of this problem in the presented work, we provide the main links of the T-cell mechanism of pathogenesis. The disease occurs as a disruption of the immune system and melanocyte function, which leads to the induction of autoimmunity.
Link 1: Effects of trauma, stress, depression, acute and chronic infection, vaccination, cytostatics, and age-related involution of the thymus. All of these factors lead to inhibition of thymic function through negative feedback of corticosteroid levels (Ashwell et al., 2000; Huda et al., 2019) and sex hormones (Dumont-Lagacé et al., 2015; Paolino et al., 2021). Cortisol values and subsequent immune impairment are particularly high in patients with depression, which has a high prevalence among older adults (Duggal et al., 2015).
Link 2: Limitation of alternative mRNA splicing in the thymus leads to a “vulnerability” in the negative selection system. This occurs due to a decrease in the number of autologous peptides presented by thymic epithelial cells on major histocompatibility complex (MHC) molecules, which leads to the impossibility of subsequent apoptosis of thymocytes exhibiting a sufficiently high affinity to such autoantigens (Carter et al., 2022). As a result, autoreactive T cells specific to those isoform-specific epitopes that were not formed in the thymus or were presented there in insufficient quantities can survive and subsequently exit the thymus (Shilov et al., 2019).
Link 3: Disruption of negative selection of autoreactive cells during T-cell maturation in the thymus and their migration to the periphery under the influence of negative factors. In one lobe of the thymus, T cells mature into specialized disease-fighting cells with different functions (Daniels et al., 2006). However, only approximately 2% eventually undergo positive and negative selections and are further exposed to thymic hormones to complete maturation. The mature T cells then exit the thymus to perform their protective functions in organs and tissues (Thapa and Farber, 2019). Disruption of this process is due to thymus degradation occurring when cortisol levels increase through feedback. In turn, thymus degradation leads to a significant release of autoreactive T cells, which subsequently mature in various peripheral tissues (Baecher-Allan et al., 2001; Barthlott et al., 2005). The process of T-cell maturation is accompanied by the expression of MHC class I chain-related protein A/B (MICA/MICB) molecules in the outer layer of Hassall’s corpuscles of the medulla of normal thymus. In thymomas, MICA/MICB is overexpressed in the epithelial cells of the cortex and medulla of the thymus, which impairs their maturation. It is also known that in thymomas, there is an increase in the number of less mature T cells characterized by a reduced level of natural killer (NK) group 2D (NKG2D) expression (Hüe et al., 2003). Thus, it is evolutionarily established that immature autoreactive T cells have less ability to recognize MICA/MICB pathological cells, and in the absence of triggers, they remain inactive.
Link 4: Melanocyte oxidative stress triggers an immune attack. Exposure of melanocytes to ultraviolet light leads to the formation of reactive oxygen species (ROS), which creates oxidative stress. This process triggers a cascade of reactions leading to the release of damage-associated molecular pattern molecules (DAMPs) into the extracellular microenvironment, which are represented by melanocyte-specific neoantigens, inducible heat shock protein 70 (HSP70i), high-mobility group box-1 protein (HMGB1), S100 calcium-binding protein B (S100B), and mitochondrial DNA (Chen et al., 2021). These DAMPs bind to pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors, and activate the innate immune system (Richmond et al., 2013). In turn, this leads to melanocyte death and triggers adaptive immunity through cytokine/chemokine formation and antigen presentation (Tulic et al., 2019). When T cells have not undergone negative selection, these processes do not stop, creating a vicious cycle. The triggering of these systems leads to a greater recruitment of autoreactive cells to the inflammation focus, resulting in increased release of DAMPs, which interact with dendritic cells (DCs), NK cells, innate lymphoid cells (ILCs), keratinocytes, and melanocytes (Cui et al., 2019). In turn, enhanced delivery of melanocyte antigens to the MHC class II expressed on melanocytes enhances the immunogenicity of normal melanocytes to autoreactive T cells (Chen et al., 2021).
DAMPs bind to the PRRs on DCs, triggering their maturation and antigen-presentation process (Jacquemin et al., 2017; Cui et al., 2019). After DCs interact with DAMPs, they release a cytokine complex including IFN-α, interleukin-1β (IL-1β), and IL-18 (Jacquemin et al., 2017). DAMPs lead to keratinocyte-dependent recruitment of NK and melanocyte-specific CD8+ T cells through keratinocyte release of a wide range of cytokines and chemokines, including IL-1β, IL-8, C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, and CXCL16 (Cui et al., 2019). MICA/MICB expression is also increased by DCs. MICA/MICB as well as DAMPs activates the NKG2D receptor expressed by NK cells, ILCs, and effector memory T (Tem) cells, which consequently secretes IFN-γ to induce chemokines and cytokines and triggers melanocyte apoptosis with autoantigen release (Jacquemin et al., 2020). ILCs and NK cells express IFN-γ, and ILCs additionally produce the cytotoxic proteins perforin and granzyme. ILCs and NK cells function together as innate analogs of cytotoxic CD8+ T cells (Gordon et al., 2012). Nevertheless, the roles of ILCs and NK cells in autoimmune processes are still poorly understood.
At the end of apoptosis, regulatory T cells (Tregs) should suppress autoreactive T cells. However, it is likely that signals from Tregs during the joint activation of resident memory T (Trm), T helper (Th), and autoreactive CD8+ cytotoxic cells are insufficient, leading to the recruitment of new autoreactive T cells (Chen et al., 2021), thus closing the “vicious circle.”
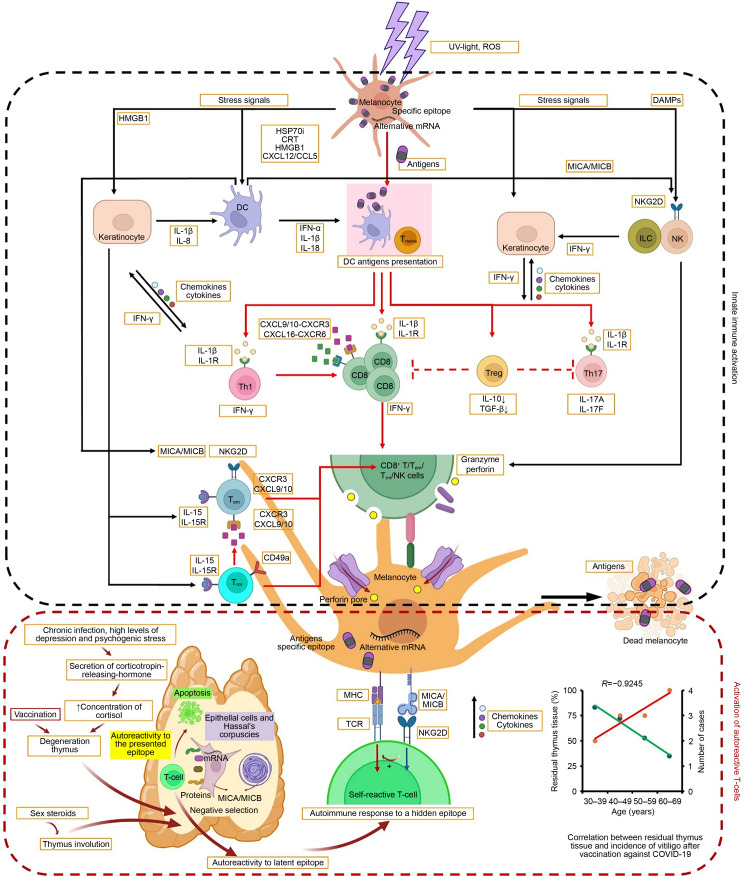
In support of the role of the thymus in the development of autoimmune melanocyte lesions, a computed tomography (CT) study of 597 patients found that increased fatty thymic replacement occurs with age (Drabkin et al., 2018). Overlaying these data with the incidence of vitiligo after vaccination (Table 1), we found a correlation of R=-0.9245 (Fig. 2). In addition, a number of publications have reported autoimmune diseases, including vitiligo in thymomas (Nakamagoe et al., 2009; Qiao et al., 2011; Warren et al., 2015), in contrast to the preservation of immune function observed in long livers (Strindhall et al., 2007). This emphasizes that thymus dysfunction plays a major role in the pathogenesis of vitiligo.
Fig. 2. Mechanism of pathogenesis of autoimmune melanocyte damage in vitiligo after COVID-19 vaccination. The interaction between innate immune cells is drawn by black long-tailed arrows, and the interaction between adaptive immune cells is drawn by red. COVID-19: coronavirus disease 2019; UV: ultraviolet; ROS: reactive oxygen species; HMGB1: high-mobility group box-1 protein; HSP70i: inducible heat shock protein 70; CRT: calreticulin; CXCL: C-X-C motif chemokine ligand; CXCR: C-X-C motif chemokine receptor; CCL: CC chemokine ligand; mRNA: messenger RNA; DAMPs: damage-associated molecular pattern molecules; MICA/MICB: major histocompatibility complex (MHC) class I chain-related protein A/B; IL: interleukin; IL-1R: IL-1 receptor; DC: dendritic cell; IFN: interferon; Tnaive: naive T cells; Treg: regulatory T cell; ILC: innate lymphoid cell; NK: nature killer; NKG2D: NK group 2D; Th: T-helper; CD8: cluster of differentiation 8; TGF-β: transforming growth factor-β; Tem: effector memory T cells; Trm: resident memory T cells; TCR: T cell receptor. This figure is partially reproduced from Chen et al. (2021) (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Common transcription factor IIi encoded by the general transcription factor II-I (GTF2I) gene is known to be a thymoma-specific oncogene, and some of its single nucleotide polymorphisms are associated with autoimmune disease (Kim et al., 2016; Liu et al., 2021). Mutation IIi of GTF2I leads to thymic epithelial cell transformation and overexpression of soluble growth factors, oncogenes, genes related to epithelial-mesenchymal transition (EMT), and antiapoptotic genes amidst decreased production of cell adhesion molecules, tumor suppressors, and cell death inducers (Manti et al., 2022). It is possible that this marker may have clinical significance not only for the verification/prognosis of thymoma but also for other diseases associated with thymus dysfunction and should be assessed as part of a diagnostic plan for autoimmune diseases. Thus, one of the main causes of vitiligo development after vaccination is the disruption of the thymus under the influence of cortisol and sex hormones, where the main trigger is the oxidative stress of melanocytes. On the one hand, this emphasizes the importance of developing reliable diagnostic methods for this organ, which should be used together with the determination of cortisol and dehydroepiandrosterone levels. On the other hand, the need to develop therapeutic approaches aimed at normalizing the hypothalamic‒pituitary‒adrenal axis responsible for cortisol and dehydroepiandrosterone levels, restoration of normal thymus functioning, and elimination of autoreactive T cells from the periphery.
One promising complex to reduce autoimmune complications after vaccination and disease may be a combination of dehydroepiandrosterone (DHEA) and vitamin D3. A systematic review and meta-analysis of 11 clinical trials found that patients with severe COVID-19 had higher cortisol levels than patients with mild to moderate COVID-19 (Amiri-Dashatan et al., 2022). It was also found that dehydroepiandrosterone sulfate (DHEA-S) levels and the DHEA-S/cortisol ratio were significantly decreased in patients with increasing COVID-19 severity (Mahdavi et al., 2021). In addition, the risk of COVID-19 mortality correlated with a high DHEA-S/cortisol ratio. A high DHEA-S/cortisol ratio is called relative hypercortisolism (RHC). RHC predicts the severity and likelihood of death for community-acquired pneumonia and septic shock, which are the leading causes of death in COVID-19 (Cordell, 2020). DHEA, which affects cortisol concentration, maintains intracellular calcium homeostasis. The ratio of DHEA-S/cortisol determines the exon splicing of the calcium-sensitive big potassium (BKCa) channel. This channel exerts dominant control over cell polarization and the inner mitochondrial membrane. DHEA increases the large-conductance BKCa sensitivity to calcium and thereby reduces calcium influx into the cell and mitochondrial matrix. The effect of maintaining intracellular homeostasis together with vitamin D-enhanced calbindin-D9k/28k, magnesium, and andubiquinone provides protection against mitochondrial calcium overload. In the absence of these key factors, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), similar to other viruses, disrupts intracellular calcium homeostasis, causing mitochondrial calcium overload in susceptible hosts and leading to ROS accumulation (Amiri-Dashatan et al., 2022).
A one-year pilot study, the TRIIM (Thymus Regeneration, Immunorestoration, and Insulin Mitigation) trial, from 2015 to 2017, showed a pronounced thymic recovery effect when treated with human growth hormone in combination with metformin and DHEA (Fahy et al., 2019). After nine months, most participants had a greater thymic fat-free fraction, implying a restoration of thymic function. T-cell production and function improved, and it was demonstrated for the first time that this drug regimen reduced biological age by 2.5 years. The phase II TRIIM-X trial is currently underway.
However, there are concerns that uncontrolled supplementation with DHEA may potentiate SARS-CoV-2 antigen-induced vascular endotheliitis, contributing to multiorgan inflammatory responses, particularly acute responses in diabetic patients (Nyce, 2021). The poor glycemic control seen in many diabetic patients may further exacerbate the effects of DHEA. This underscores the importance of monitoring both cortisol and DHEA levels, especially in patients with atherosclerosis and diabetes.
Vitamin D has many important health benefits. However, they are little known among medical professionals and the general public. Vitamin D deficiency is known to be associated with decreased thymus volume during fetal development. This observation is consistent with the fact that inadequate maternal vitamin D levels during pregnancy can increase the risk of multiple sclerosis in offspring (Handel et al., 2018). Immune cells express the vitamin D receptor and are therefore targets of vitamin D. High expression of the vitamin D receptor has been shown in bone marrow and thymus immune cell precursors. In the periphery, monocytes, neutrophils, and macrophages have very high expression of this receptor (88%‒98%). A lower level of expression is found in lymphocytes (60%‒70%) and in ILCs. Moreover, the level of vitamin D receptor expression in T cells corresponds to slower proliferation and lower IFN-γ production (Arora et al., 2022). The interaction of vitamin D with its receptor triggers a cascade of reactions through the regulation of many genes, leading to modulation of the immune system. It has been shown that vitamin D can increase Treg activity, which in turn helps to suppress autoreactive cells and reduce the action of proinflammatory cytokines (Aygun, 2022). In a study involving 4599 COVID-19 patients, it was found that the greatest risk of hospitalization and death was observed with lower vitamin D concentrations (Seal et al., 2022). In a study with 2148 patients, vitamin D intake prior to COVID-19 infection was found to reduce disease severity and hospitalization rates (Nimer et al., 2022). Vitamin D has been shown to reduce COVID-19 complications by modulating proinflammatory cytokines, antiviral proteins, and autophagy. Grant et al. (2022) provide evidence that optimal vitamin D concentrations for health and wellbeing exceed 30 ng/mL (75 nmol/L) for cardiovascular disease and all-cause mortality, while thresholds for several other outcomes can be as high as 40 or 50 ng/mL in serum.
Thus, one of the rational and affordable ways to prevent complications after COVID-19 vaccination and disease can be to control the concentration of vitamin D, the DHEA/cortisol ratio, and the timely administration of vitamin D and DHEA supplements to achieve the required concentrations in the body.
In summary, we report a case of vitiligo after COVID-19 vaccination and review the literature and pathogenesis. We provided evidence that tissue damage and thymus dysfunction are the “fertile soil” on which the “seeds” of autoimmune diseases sprout by disrupting negative selection in the thymus. This leads to the release of autoreactive T cells from it and their migration to the periphery. Nonsignificant external environmental factors, such as ultraviolet light against the background of vaccination, can lead to oxidative stress in melanocytes and trigger a cascade of uncontrolled autoimmune reactions. This understanding of the pathogenesis of autoimmune lesions may be important in the context of developing new diagnostic and therapeutic methods and standards. The mechanism of autoimmune lesions is presented in Fig. 2 for better understanding. In general, the role of the thymus as well as the identification of the main molecular mechanisms in autoimmunity has become known only in the last few years, and further research and collection of clinical cases of autoimmune lesions are needed.
Author contributions
Denis KUZNETSOV: conceptualization, methodology, data curation, formal analysis, project administration, writing – original draft, and visualization. Oleg KALYUZHIN and Andrey MIRONOV: conceptualization, data curation, methodology, formal analysis, and writing – review & editing. Valery NESCHISLIAEV: writing – review & editing and formal analysis. Anastasiia KUZNETSOVA: visualization and writing – review & editing. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Denis KUZNETSOV, Oleg KALYUZHIN, Andrey MIRONOV, Valery NESCHISLIAEV, and Anastasiia KUZNETSOVA declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Biomedical Ethics Committee of the G.N. Gabrichevsky Scientific and Research Institute of Epidemiology and Microbiology, extract from Minutes No. 60A) and with the Helsinki Declaration of 1975, as revised in 2013. Informed consent was obtained from the patient for being included in the study. Additional informed consent was obtained from the patient for whom identifying information is included in this article.
References
- Aktas H, Ertuğrul G, 2022. Vitiligo in a COVID-19-vaccinated patient with ulcerative colitis: coincidence? Clin Exp Dermatol, 47(1): 143-144. 10.1111/ced.14842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri-Dashatan N, Koushki M, Parsamanesh N, et al. , 2022. Serum cortisol concentration and COVID-19 severity: a systematic review and meta-analysis. J Invest Med, 70(3): 766-772. 10.1136/jim-2021-001989 [DOI] [PubMed] [Google Scholar]
- Arora J, Wang JP, Weaver V, et al. , 2022. Novel insight into the role of the vitamin D receptor in the development and function of the immune system. J Steroid Biochem Mol Biol, 219: 106084. 10.1016/j.jsbmb.2022.106084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell JD, Lu FWM, Vacchio MS, 2000. Glucocorticoids in T cell development and function. Annu Rev Immunol, 18: 309-345. 10.1146/annurev.immunol.18.1.309 [DOI] [PubMed] [Google Scholar]
- Aygun H, 2022. Vitamin D can reduce severity in COVID-19 through regulation of PD-L1. Naunyn-Schmiedeberg’s Arch Pharmacol, 395(4): 487-494. 10.1007/s00210-022-02210-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown JA, Freeman GJ, et al. , 2001. CD4+CD25high regulatory cells in human peripheral blood. J Immunol, 167(3): 1245-1253. 10.4049/jimmunol.167.3.1245 [DOI] [PubMed] [Google Scholar]
- Barthlott T, Moncrieffe H, Veldhoen M, et al. , 2005. CD25+CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int Immunol, 17(3): 279-288. 10.1093/intimm/dxh207 [DOI] [PubMed] [Google Scholar]
- Block JP, Boehmer TK, Forrest CB, et al. , 2022. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep, 71(14): 517-523. 10.15585/mmwr.mm7114e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari AE, 2022. New-onset of vitiligo in a child following COVID-19 vaccination. JAAD Case Rep, 22: 68-69. 10.1016/j.jdcr.2022.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo F, Deotto ML, Tartaglia J, et al. , 2022. Vitiligo worsened following the second dose of mRNA SARS-CoV-2 vaccine. Dermatol Ther, 35(6): e15434. 10.1111/dth.15434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JA, Strömich L, Peacey M, et al. , 2022. Transcriptomic diversity in human medullary thymic epithelial cells. Nat Commun, 13: 4296. 10.1038/S41467-022-31750-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Li SL, Li CY, 2021. Mechanisms of melanocyte death in vitiligo. Med Res Rev, 41(2): 1138-1166. 10.1002/med.21754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarese G, Drago F, Boldrin S, et al. , 2022. Sudden onset of vitiligo after COVID-19 vaccine. Dermatol Ther, 35(1): e15196. 10.1111/dth.15196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell W, 2020. The mechanisms linking relative hypercortisolism—the common feature across COVID-19 risks—to ARDS, septic shock, and cytokine dysregulation. SSRN, preprint. 10.2139/ssrn.3721693 [DOI] [Google Scholar]
- Cui TT, Zhang WG, Li SL, et al. , 2019. Oxidative stress–induced HMGB1 release from melanocytes: a paracrine mechanism underlying the cutaneous inflammation in vitiligo. J Invest Dermatol, 139(10): 2174-2184.e4. 10.1016/j.jid.2019.03.1148 [DOI] [PubMed] [Google Scholar]
- Daniels MA, Teixeiro E, Gill J, et al. , 2006. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature, 444(7120): 724-729. 10.1038/nature05269 [DOI] [PubMed] [Google Scholar]
- Drabkin MJ, Meyer JI, Kanth N, et al. , 2018. Age-stratified patterns of thymic involution on multidetector CT. J Thorac Imaging, 33(6): 409-416. 10.1097/RTI.0000000000000349 [DOI] [PubMed] [Google Scholar]
- Duggal NA, Upton J, Phillips AC, et al. , 2015. NK cell immunesenescence is increased by psychological but not physical stress in older adults associated with raised cortisol and reduced perforin expression. AGE, 37: 11. 10.1007/s11357-015-9748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont-Lagacé M, St-Pierre C, Perreault C, 2015. Sex hormones have pervasive effects on thymic epithelial cells. Sci Rep, 5: 12895. 10.1038/srep12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy GM, Brooke RT, Watson JP, et al. , 2019. Reversal of epigenetic aging and immunosenescent trends in humans. Aging cell, 18(6): e13028. 10.1111/acel.13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SM, Chaix J, Rupp LJ, et al. , 2012. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity, 36(1): 55-67. 10.1016/j.immuni.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WB, al Anouti F, Boucher BJ, et al. , 2022. A narrative review of the evidence for variations in serum 25-hydroxyvitamin D concentration thresholds for optimal health. Nutrients, 14(3): 639. 10.3390/nu14030639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel AE, Irani SR, Holländer GA, 2018. The role of thymic tolerance in CNS autoimmune disease. Nat Rev Neurol, 14(12): 723-734. 10.1038/s41582-018-0095-7 [DOI] [PubMed] [Google Scholar]
- Haseeb AA, Solyman O, Abushanab MM, et al. , 2022. Ocular complications following vaccination for COVID-19: a one-year retrospective. Vaccines, 10(2): 342. 10.3390/vaccines10020342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzum A, Micalizzi C, Molle MF, et al. , 2022. New-onset vitiligo following COVID-19 disease. Skin Health Dis, 2(1): e86. 10.1002/ski2.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda MN, Ahmad SM, Alam J, et al. , 2019. Infant cortisol stress–response is associated with thymic function and vaccine response. Stress, 22(1): 36-43. 10.1080/10253890.2018.1484445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüe S, Monteiro RC, Berrih-Aknin S, et al. , 2003. Potential role of NKG2D/MHC class I-related chain A interaction in intrathymic maturation of single-positive CD8 T cells. J Immunol, 171(4): 1909-1917. 10.4049/jimmunol.171.4.1909 [DOI] [PubMed] [Google Scholar]
- Jacquemin C, Rambert J, Guillet S, et al. , 2017. Heat shock protein 70 potentiates interferon alpha production by plasmacytoid dendritic cells: relevance for cutaneous lupus and vitiligo pathogenesis. Br J Dermatol, 177(5): 1367-1375. 10.1111/bjd.15550 [DOI] [PubMed] [Google Scholar]
- Jacquemin C, Martins C, Lucchese F, et al. , 2020. NKG2D defines a subset of skin effector memory CD8 T cells with proinflammatory functions in vitiligo. J Invest Dermatol, 140(6): 1143-1153.e5. 10.1016/j.jid.2019.11.013 [DOI] [PubMed] [Google Scholar]
- Kaminetsky J, Rudikoff D, 2021. New-onset vitiligo following mRNA-1273 (Moderna) COVID-19 vaccination. Clin Case Rep, 9(9): e04865. 10.1002/ccr3.4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Bang SY, Ikari K, et al. , 2016. Association-heterogeneity mapping identifies an Asian-specific association of the GTF2I locus with rheumatoid arthritis. Sci Rep, 6: 27563. 10.1038/srep27563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koç Yıldırım S, 2022. A new-onset vitiligo following the inactivated COVID-19 vaccine. J Cosmet Dermatol, 21(2): 429-430. 10.1111/jocd.14677 [DOI] [PubMed] [Google Scholar]
- Krüger C, Schallreuter KU, 2012. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol, 51(10): 1206-1212. 10.1111/j.1365-4632.2011.05377.x [DOI] [PubMed] [Google Scholar]
- le Vu S, Bertrand M, Jabagi MJ, et al. , 2022. Age and sex-specific risks of myocarditis and pericarditis following Covid-19 messenger RNA vaccines. Nat Commun, 13: 3633. 10.1038/s41467-022-31401-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CX, Yan SX, Chen HZ, et al. , 2021. Association of GTF2I, NFKB1, and TYK2 regional polymorphisms with systemic sclerosis in a Chinese Han population. Front Immunol, 12: 640083. 10.3389/fimmu.2021.640083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Riquelme I, Fernández Ballesteros MD, Serrano Ordoñez A, et al. , 2022. COVID-19 and autoimmune phenomena: vitiligo after Astrazeneca vaccine. Dermatol Ther, 35(7): e15502. 10.1111/dth.15502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macca L, Peterle L, Ceccarelli M, et al. , 2022. Vitiligo-like lesions and COVID-19: case report and review of vaccination- and infection-associated vitiligo. Vaccines, 10(10): 1647. 10.3390/vaccines10101647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi MRV, Ardestani SK, Rezaei A, et al. , 2021. COVID-19 patients suffer from DHEA-S sufficiency. Immunoregulation, 3(2): 135-144. 10.32598/Immunoregulation.3.2.5 [DOI] [Google Scholar]
- Manti PG, Trattaro S, Castaldi D, et al. , 2022. Thymic stroma and TFII-I: towards new targeted therapies. Trends Mol Med, 28(1): 67-78. 10.1016/j.molmed.2021.10.008 [DOI] [PubMed] [Google Scholar]
- Nakamagoe K, Furuta JI, Shioya A, et al. , 2009. A case of vitiligo vulgaris showing a pronounced improvement after treatment for myasthenia gravis. BMJ Case Rep, 2009: bcr07. 2009.2091. 10.1136/bcr.07.2009.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaidou E, Vavouli C, Koumprentziotis IA, et al. , 2023. New-onset vitiligo after COVID-19 mRNA vaccination: a causal association? J Eur Acad Dermatol Venereol, 37(1): e11-e12. 10.1111/jdv.18513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer RM, Khabour OF, Swedan SF, et al. , 2022. The impact of vitamin and mineral supplements usage prior to COVID-19 infection on disease severity and hospitalization. Bosn J Basic Med Sci, 22(5): 826-832. 10.17305/bjbms.2021.7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyce J, 2021. Alert to US physicians: DHEA, widely used as an OTC androgen supplement, may exacerbate COVID-19. Endocr-Relat Cancer, 28(2): R47-R53. 10.1530/ERC-20-0439 [DOI] [PubMed] [Google Scholar]
- Paolino M, Koglgruber R, Cronin SJF, et al. , 2021. RANK links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy. Nature, 589(7842): 442-447. 10.1038/s41586-020-03071-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post NF, Luiten RM, Wolkerstorfer A, et al. , 2021. Does autoimmune vitiligo protect against COVID-19 disease? Exp Dermatol, 30(9): 1254-1257. 10.1111/exd.14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao JJ, Zhou GG, Ding YG, et al. , 2011. Multiple paraneoplastic syndromes: myasthenia gravis, vitiligo, alopecia areata, and oral lichen planus associated with thymoma. J Neurol Sci, 308(1-2): 177-179. 10.1016/j.jns.2011.05.038 [DOI] [PubMed] [Google Scholar]
- Richmond JM, Frisoli ML, Harris JE, 2013. Innate immune mechanisms in vitiligo: danger from within. Curr Opin Immunol, 25(6): 676-682. 10.1016/j.coi.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AF, Rubin A, Milgraum D, et al. , 2022. Vitiligo following COVID-19: a case report and review of pathophysiology. JAAD Case Rep, 22: 47-49. 10.1016/j.jdcr.2022.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Bertenthal D, Carey E, et al. , 2022. Association of vitamin D status and COVID-19-related hospitalization and mortality. J Gen Intern Med, 37(4): 853-861. 10.1007/s11606-021-07170-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilov ES, Gorshkova EA, Minnegalieva AR, et al. , 2019. Splicing pattern of mRNA in thymus epithelial cells limits the transcriptome available for negative selection of autoreactive T cells. Mol Biol, 53(1): 87-96. 10.1134/S0026893319010151 [DOI] [PubMed] [Google Scholar]
- Singh R, Cohen JL, Astudillo M, et al. , 2022. Vitiligo of the arm after COVID-19 vaccination. JAAD Case Rep, 28: 142-144. 10.1016/j.jdcr.2022.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeckaert R, van Geel N, 2017. Vitiligo: an update on pathophysiology and treatment options. Am J Clin Dermatol, 18(6): 733-744. 10.1007/s40257-017-0298-5 [DOI] [PubMed] [Google Scholar]
- Strindhall J, Nilsson BO, Löfgren S, et al. , 2007. No immune risk profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study. Exp Gerontol, 42(8): 753-761. 10.1016/j.exger.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Thapa P, Farber DL, 2019. The role of the thymus in the immune response. Thorac Surg Clin, 29(2): 123-131. 10.1016/j.thorsurg.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulic MK, Cavazza E, Cheli Y, et al. , 2019. Innate lymphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat Commun, 10: 2178. 10.1038/s41467-019-09963-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uğurer E, Sivaz O, Altunay İK, 2022. Newly-developed vitiligo following COVID-19 mRNA vaccine. J Cosmet Dermatol, 21(4): 1350-1351. 10.1111/jocd.14843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S, Nehal K, Querfeld C, et al. , 2015. Graft-versus-host disease-like erythroderma: a manifestation of thymoma-associated multiorgan autoimmunity. J Cutan Pathol, 42(10): 663-668. 10.1111/cup.12642 [DOI] [PMC free article] [PubMed] [Google Scholar]