Abstract
Orobanchaceae root parasitic weeds cause significant damage to agriculture and become threats to global food security. Integrated pest management is a key concept in modern agriculture and requires chemicals with various modes of action. Planteose accumulates as a storage carbohydrate in the dry seeds of root parasitic weeds. In Orobanche minor seeds, planteose is hydrolyzed by an α-galactosidase, OmAGAL2, during germination. It was found that the OmAGAL2 inhibitor, PI-28, suppressed the radicle elongation of germinating O. minor seeds. This inhibitory activity against O. minor radicle elongation was evaluated for a series of aryloxyacetylthioureas synthesized based on the structure of PI-28. Compounds with a 3-Cl or 4-Cl substituent on the benzene ring in the phenoxy moiety in PI-28 exhibited more potent activity than the parent PI-28. This is the first report on the effect of aryloxyacetylthioureas on a root parasitic weed and will contribute to the development of control reagents for root parasitic weeds.
Keywords: aryloxyacetylthioureas, broomrape, Orobanche minor, parasitic weed, planteose, structure–activity relationship
Introduction
Agricultural damage caused by Orobanchaceae root parasitic weeds is one of the most challenging issues to overcome for global food security. Striga hermonthica greatly reduces the yields of cereal crops, bringing hunger and poverty to African countries.1) Moreover, Orobanche and Phelipanche spp. broomrapes parasitize various economically important crops worldwide and threaten sustainable agriculture and society.1) For example, significant damage to major oil crops, such as rapeseed and sunflower, is caused by the broomrapes.2) Numerous studies and practices have been conducted to solve root parasitic weed problems.
Integrated pest management (IPM), a sustainable strategy for pest management, is a key concept in modern agriculture.3) All measures for food production are effectively combined to minimize the agricultural impact on the environment in IPM. Therefore, chemicals with various modes of action exhibiting selective activities toward pests are required.
Germination stimulants specific to the root parasitic weeds are one promising approach in their IPM. Strigolactones are well-known germination stimulants of root parasitic weeds, as well as a class of plant hormones that is still intensively studied to reveal intrinsic active structures in planta.4,5) If root parasitic weeds are forced to germinate with the application of natural or synthetic strigolactones before crop cultivation, they will soon wither because they are completely dependent on the hosts. This approach is called suicidal germination and has been proven to be successful.6–8) However, the suicidal germination approach is not commonly adopted in agriculture mostly due to its costs, unfortunately.
Inhibitors of post-germination stimulation processes in root parasitic weeds can be utilized as chemicals with different modes of action from germination stimulants. Moreover, they have the potential to improve the efficacy of the suicidal germination approach, since a combination of germination stimulants and growth inhibitors may greatly reduce the probability of parasite attachment with hosts. Some plants attracted attention as intercrops owning to their allelopathic inhibition of radicle elongation of root parasitic weeds.9–12) It is expected to reveal the molecular mechanisms of allelochemicals to understand their mode of action and utilize the information in agriculture.13)
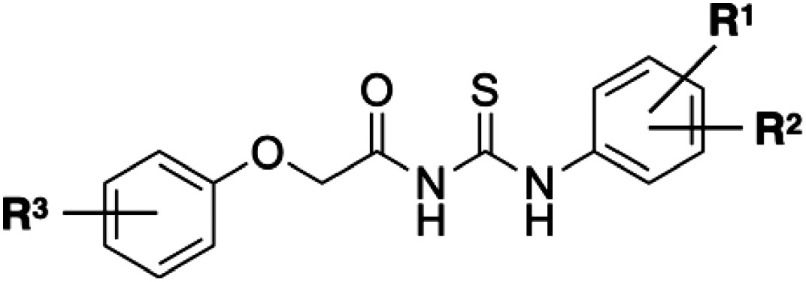
Metabolomics was conducted on the germination of O. minor, and planteose metabolism was found to be a possible target for growth inhibition.14) Planteose as a storage carbohydrate accumulates in the tissues surrounding the embryo in the dry seeds of O. minor and is hydrolyzed in the apoplast near the embryo via an α-galactosidase, OmAGAL2, after germination stimulation.15) Using recombinant ΔSP-OmAGAL2, which was expressed in Escherichia coli without a signal peptide at the N-terminus in OmAGAL2, inhibitors of OmAGAL2 were screened with a chemical library composed of ca. 15,000 compounds.16) Twenty-eight OmAGAL2 inhibitors were screened as a result, and some inhibitors suppressed the radicle elongation of O. minor.16) In this study, an aryloxyacetylthiourea, PI-28 (Fig. 1A), was selected as a lead compound among the screened inhibitors to design potent compounds inhibiting O. minor growth as bioactivity of this class of thioureas has not been reported so far.
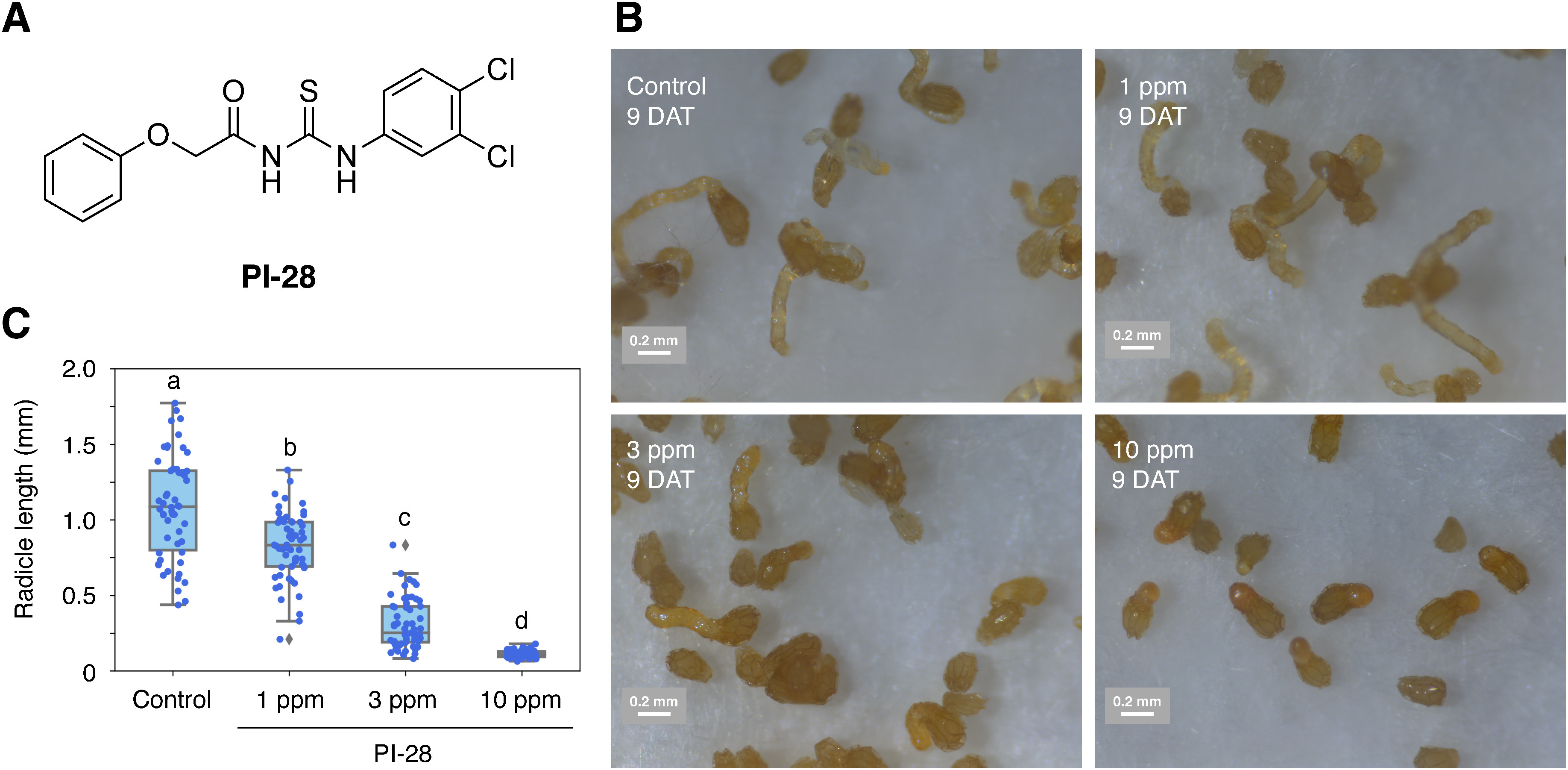
Fig. 1. The structure (A) and the effect of PI-28 (16) on the radicle elongation of O. minor (B, C). (B) The images were obtained at 9 DAT with 1, 3, and 10 ppm PI-28 or without PI-28 (control) together with 1 ppm rac-GR24 as a germination stimulant. (C) The radicle lengths of O. minor in each treatment at 9 DAT were measured using Image J software and shown as a boxplot. Different letters indicate significant differences (p<0.05) among the treatments evaluated via the Turkey–Kramer test, n=54–65.
Thioureas exhibit a wide range of biological activities toward viruses, bacteria, fungi, plants, insects, and mammals.17) Non-substituted thiourea, a plant growth regulator, improves abiotic stress tolerance in crops.18) Phenylureas and phenylthioureas are a class of widely used herbicides that inhibit photosynthesis and are grouped as C2 in the Herbicide Resistance Action Committee (HRAC) classification system.19) When thiourea and allylthiourea were added to the growth medium as organic nitrogen sources, the growth of S. hermonthica was suppressed.20) Moreover, when thiourea was applied with the synthetic germination stimulant, Nijmegen-1, simultaneously or 48 hr after germination treatment, germination of O. crenata was significantly inhibited.21) Accordingly, thioureas can potentially be utilized as reagents to control root parasitic weeds. However, studies on the mode of action or modification of the structure of thiourea against root parasitic weeds were not evaluated until now.
PI-28 and its derivatives can be synthesized in two steps or in a one-pot using commercially available phenols, 2-chloroacetamide, and N-aryl isothiocyanates.22) In this study, the inhibitory activity of PI-28 and its derivatives against O. minor radicle elongation was evaluated. Compounds exhibiting more potent activity than the parent PI-28 were obtained as a result. The structure–activity relationship (SAR) between aryloxyacetylthioureas and inhibitory activity toward O. minor radicle elongation is discussed.
Materials and methods
1. Chemicals
Derivatives of PI-28 were synthesized as reported previously (Table 1 and Fig. S1).22) Briefly, phenols with various substituents and 2-chloroacetamide reacted in acetone in the presence of potassium carbonate and potassium iodide, yielding 2-aryloxyacetamides. Next, 2-aryloxyacetamides reacted with aryl isothiocyanates with various substituents in dimethyl sulfoxide with sodium hydride as a base. The structures of the synthesized aryloxyacetylthioureas were confirmed via nuclear magnetic resonance and high-resolution mass spectrometry analyses. All the spectrometric data are available in Sonoda et al.22) clogP values were calculated using ChemDraw ver.20.1.0.112 (PerkinElmer, Waltham, MA) (Table 1).
Table 1. Structures and clogP of synthesized aryloxyacetylthioureas.
2. Plant material and germination treatment
Seeds of naturally grown O. minor were collected in Yokohama, Japan, in June 2013 and stored in darkness at 4°C. Seeds of Striga hermonthica were supplied by Prof. Abdel Gabar Babiker (National Center for Research, Sudan). Seed germination was induced as reported previously.15,23) The surface of the seeds (ca. 30 mg) was sterilized with 1% sodium hypochlorite containing 0.1% (w/v) Tween 20 for 2 min at 42°C with shaking at 750 rpm (Thermomixer comfort, Eppendorf). The seeds were rinsed several times with distilled water via vacuum-assisted suction filtration and dried on the filter paper in a vacuum. The seeds were then placed on two layers of glass microfiber filters (diameter 4.7 cm, Whatman GF/D, GE Healthcare) moistened with distilled water in a Petri dish (diameter 5.5 cm) for 7 days for O. minor and for 9 days for S. hermonthica. The upper layer of the glass microfiber filter with the conditioned seeds was transferred to a new Petri dish with a single glass microfiber filter, and strigolactone solution (rac-GR24 final concentration 1 ppm) was applied. The synthetic strigolactone rac-GR24 (a mixture of (+)-GR24 and (−)-GR24) is commonly used as a germination stimulant of Orobanchaceae parasitic weeds.23) The compounds were applied simultaneously with rac-GR24 to investigate the effect of aryloxyacetylthioureas on the radicle length of O. minor and S. hermonthica. Three Petri dishes were treated with each compound at each concentration, including the control (without aryloxyacetylthioureas).
3. Measurement of germination rate and radicle length
Images of germinating seeds were obtained with a digital microscope (DMS1000, Leica). The germination rate was measured as reported previously.24) The radicle lengths were measured using Image J software. The radicles of 10–30 seeds in two or three images obtained with a microscope as shown in Fig. 1B were measured as much as possible for samples in a Petri dish, with the average lengths of at least 30 radicles in three Petri dishes being calculated. The numbers of measured radicles were indicated in Table S1. Student’s t-test and Turkey–Kramer test were conducted with SciPy (Version 1.5.2) and Pandas (Version 1.1.3) modules, respectively, in Python (Version 3.8.5). Relative radicle lengths of O. minor treated with aryloxyacetylthioureas to those of the control were determined to compare the effects among different experimental batches.
Results
1. The effect of PI-28 on the radicle elongation of O. minor
PI-28 is an aryloxyacetylthiourea screened as an inhibitor of recombinant ΔSP-OmAGAL2 expressed in E. coli (Fig. 1A).16) PI-28 was applied simultaneously with the germination stimulant, rac-GR24 (1 ppm), and the radicles of germinating O. minor seeds significantly shortened in a dose-dependent manner without affecting germination rates (Figs. 1B, 1C, and S2). The radicle lengths of O. minor germinated without PI-28 (control) at nine days after treatment (DAT) were 1.08±0.34 mm (mean±S.D.), while those with PI-28 at 1, 3, and 10 ppm were 0.82±0.22, 0.31±0.16, and 0.11±0.02 mm, respectively.
2. Effect of aryloxyacetylthioureas on O. minor radicle elongation
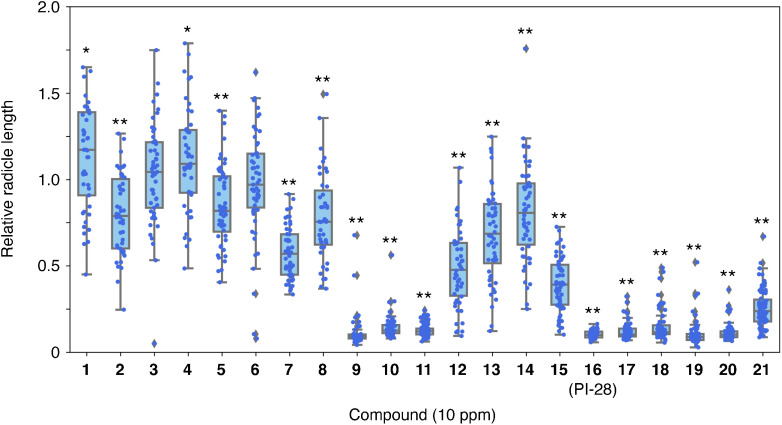
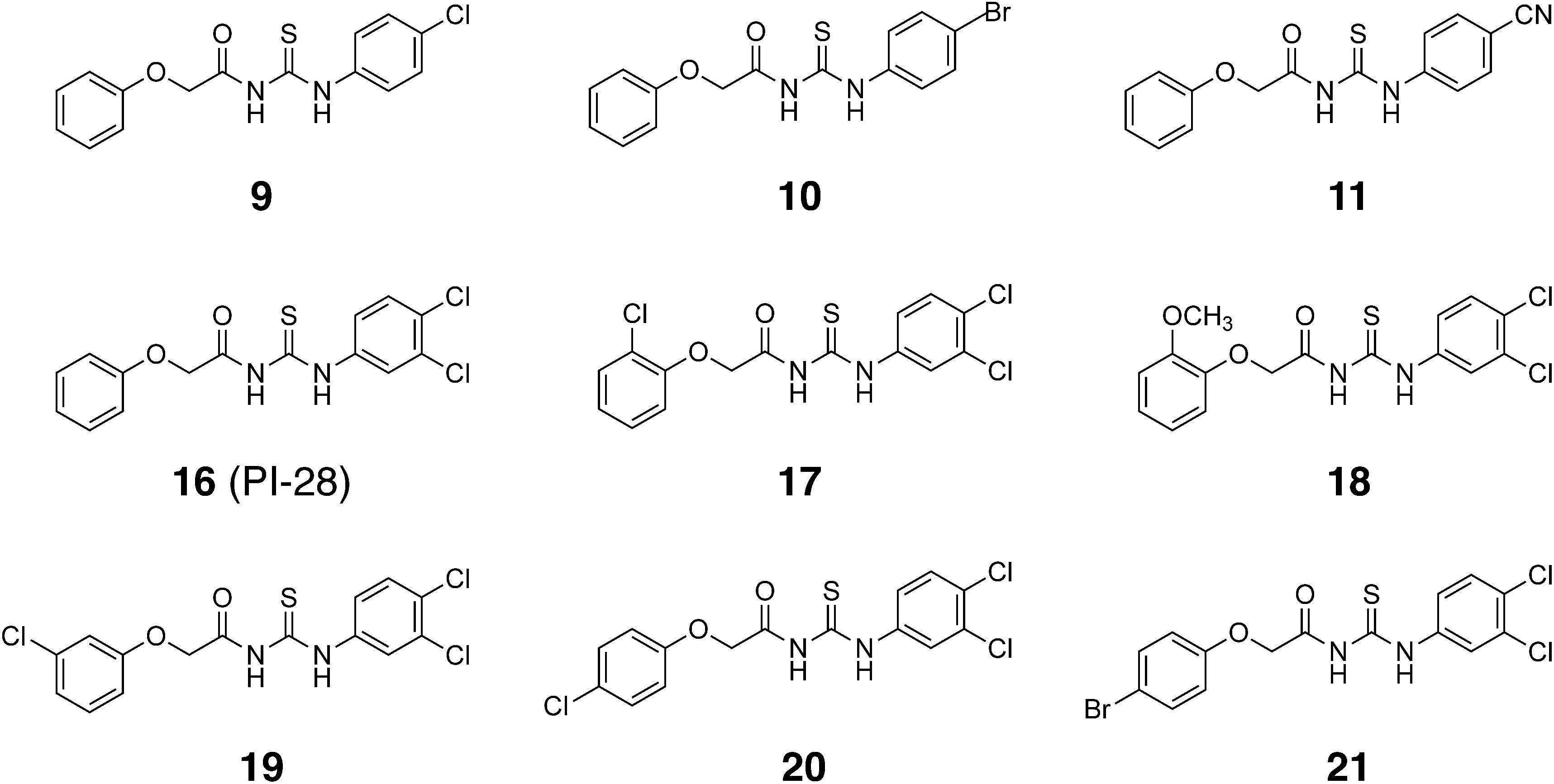
Since PI-28 exhibited a potent inhibitory effect on O. minor radicle elongation, the inhibitory effect of 20 PI-28 derivatives differing in substituents on two benzene rings22) was evaluated (Table 1 and Fig. S1). When applied simultaneously with 1-ppm rac-GR24, 17 among 21 derivatives, including PI-28, significantly reduced the O. minor radicle lengths at 10 ppm at 9 DAT (Fig. 2 and Table S1). Compound 1 without substituents on the benzene rings and compounds possessing 2-Cl (3), 2-OCH3 (4), and 3-CH3 (6) as R1 on the benzene ring in the phenylthiourea moiety did not exhibit an inhibitory effect on O. minor radicle elongation. The radicle lengths of O. minor treated with compounds 1 and 4 were slightly but significantly longer compared to the control. Monosubstituted compounds possessing 2-F (2), 3-F (5), 3-CF3 (7), 4-F (8), and 4-OCF3 (12) as R1, along with disubstituted compounds possessing 2,4-dichloro (14) and 3,4-difluoro (15) as R1 and R2 on the benzene ring in the phenylthiourea moiety moderately inhibited O. minor radicle elongation. Among the monosubstituted compounds, 4-Cl (9), 4-Br (10), and 4-CN (11) as R1 on the benzene ring in the phenylthiourea moiety derivatives exhibited potent inhibitory activity toward O. minor radicle elongation. All compounds possessing a substituent as R3 on the benzene ring in the phenoxy moiety (compounds 17–21) in PI-28 (16) also exhibited potent inhibitory activity toward radicle elongation. Figure 3 summarizes the structures of compounds inhibiting the radicle elongation to less than 30% of the control at 10 ppm.
Fig. 2. The effect of the synthesized aryloxyacetylthioureas at 10 ppm on the radicle elongation of O. minor at 9 DAT. The seeds were germinated with 1 ppm rac-GR24 with or without (control) the thioureas. Relative radicle lengths to the radicle lengths in the control in each treatment were shown as a boxplot. Significant differences were indicated an asterisk (p<0.05) or double asterisks (p<0.01) and were evaluated via the Student’s t-test, n=33–79.
Fig. 3. The structures of the aryloxyacetylthioureas exhibiting high inhibitory effects toward the radicle elongation of O. minor. These compounds reduced the O. minor radicle lengths to less than 30% of the control.
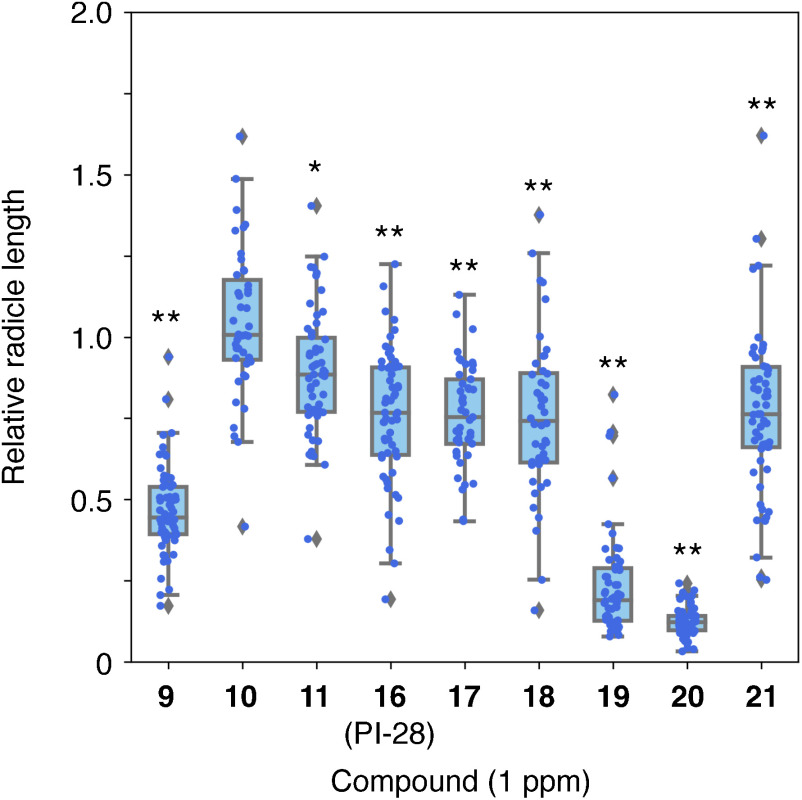
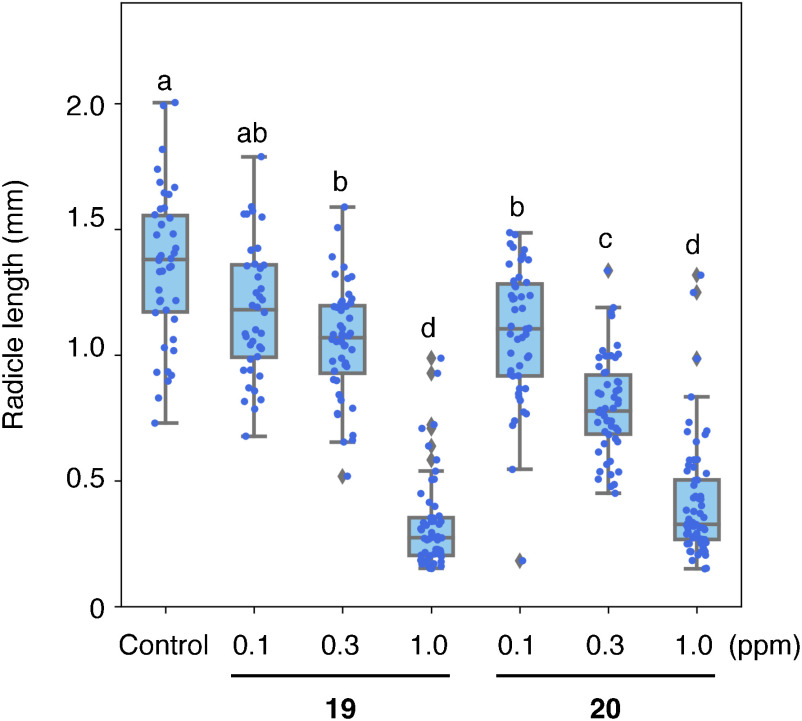
The effect of the compounds depicted in Fig. 3 at 1 ppm was evaluated to find the most potent inhibitors of O. minor radicle elongation. As a result, compounds 19 and 20 which possess chlorides as R3 on the benzene ring in the phenoxy moiety in PI-28 inhibited the radicle elongation to less than 30% of the control, even at 1 ppm (Fig. 4). Further analysis at lower concentrations revealed that compounds 19 and 20 inhibited the O. minor radicle elongation in a dose-dependent manner. The effects were even significant at 0.1 ppm (Fig. 5 and Table S1).
Fig. 4. The effect of the aryloxyacetylthioureas 9–11 and 16–21 at 1 ppm on the radicle elongation of O. minor at 9 DAT. The seeds were germinated with 1 ppm rac-GR24 with or without (control) the thioureas. Relative radicle lengths to the radicle lengths in the control in each treatment were shown as a boxplot. Significant differences were indicated an asterisk (p<0.05) or double asterisks (p<0.01) and were evaluated via the Student’s t-test, n=33–62.
Fig. 5. The effect of the aryloxyacetylthiourea 19 and 20 at 0.1, 0.3, and 1.0 ppm on the radicle elongation of O. minor at 9 DAT. The seeds were germinated with 1 ppm rac-GR24 with or without (control) the thioureas. The radicle lengths of O. minor in each treatment were measured using Image J software and shown as a boxplot. Different letters indicate significant differences (p<0.05) among the treatments evaluated via the Turkey–Kramer test, n=41–66.
3. Effect of compound 20 on S. hermonthica radicle elongation
Since compound 20 exhibited the highest inhibitory effect on O. minor radicle elongation, its effect on S. hermonthica was also evaluated. As a result, there was no effect on S. hermonthica radicles even at 10 ppm (Fig. S3).
Discussion
In this study, derivatives of an aryloxyacetylthiourea, PI-28, (Table 1 and Fig. S1) were evaluated for their inhibitory activity against O. minor radicle elongation. PI-28 was screened as a recombinant ∆SP-OmAGAL2 inhibitor, which might target the first step of planteose metabolism.16) Planteose is hydrolyzed to sucrose and galactose, and sucrose is further hydrolyzed to glucose and fructose in the early germination process after germination stimulation in the root parasitic weeds.14) When the hydrolysis of sucrose was inhibited via nojirimycin treatment, the germination rate of O. minor was significantly reduced, indicating that carbohydrate metabolism is essential for germination.14,25,26) In this study, PI-28 significantly reduced the O. minor radicle lengths, suggesting that planteose metabolism promotes radicle elongation. Since a certain amount of sucrose is contained in dry seeds of O. minor, germination might be initiated via sucrose hydrolysis, even without planteose metabolism. This demonstrates the possibility of using PI-28 or related aryloxyacetylthiourea derivatives as a control reagent for root parasitic weeds because the probability of reaching host roots is greatly reduced with the shortened radicles. This is the first finding that this class of thioureas affects the growth of root parasitic weeds.
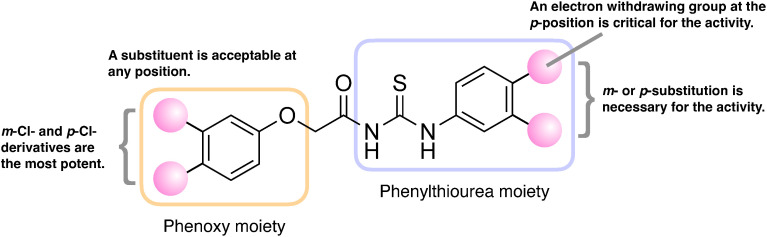
PI-28 possesses 3-Cl and 4-Cl substituents as R1 and R2 on the benzene ring in the phenylthiourea moiety. Compound 1, possessing no substituent on two benzene rings, completely lost the activity, indicating a substitution on the benzene ring in the phenylthiourea moiety is necessary for the activity (Fig. 6). Compounds 2–4, possessing substituents at the o-position on the benzene ring in the phenylthiourea moiety, exhibit no or weak activity, indicating that substitution at the o-position does not improve the activity (Fig. 2 and Table S1). Only the 2-F derivative (compound 2) showed a weak inhibitory effect, suggesting the electrostatic properties of the benzene ring in the phenylthiourea moiety affected the activity. The statistical analysis resulted in significant radicle elongation by the treatments with compounds 1 and 4 compared to the control. However, further studies are required to reveal whether these compounds activate the radicle elongation or if the results were derived merely from unknown experimental artifacts, such as minor environmental differences during the culture. Among m-substituted compounds 5–7, the 3-CH3 derivative (compound 6) did not exhibit activity, while the 3-CF3 derivative (compound 7) showed moderate activity that was more potent than that of the 3-F derivative (compound 5) (Fig. 2 and Table S1). Accordingly, electron-withdrawing groups at the m-position on the benzene ring in the phenylthiourea moiety were favored for the activity. Compounds 9–11, possessing 4-Cl, 4-Br, and 4-CN, respectively, exhibited potent inhibitory activity against O. minor radicle elongation among p-substituted compounds 8–13. Contrastingly, compounds 8, 12, and 13, possessing 4-F, 4-OCF3, and 4-N(CH3)2, respectively, showed moderate activity (Fig. 2 and Table S1). This revealed that an electron-withdrawing group at the p-position on the benzene ring in the phenylthiourea moiety is critical for the activity (Fig. 6). When 2-Cl was also introduced to the 4-Cl derivative (compound 14), the activity was greatly reduced, indicating its introduction at the o-position is not favorable for the activity. The 3,4-difluoro derivative (compound 15) showed less activity than PI-28 (3,4-dichloro), indicating electrostatic properties or bulkiness of the substituents deter activity (Fig. 2 and Table S1). All PI-28 derivatives possessing substituents on the benzene ring in the phenoxy moiety as R3 at o-, m-, and p- positions (compounds 17–21) exhibited potent activities, indicating that any substitution at any position is acceptable for the activity (Figs. 2, 6, and Table S1). Compounds 19 and 20 possessing 3-Cl and 4-Cl, respectively, exhibited the highest activity among the synthesized PI-28 derivatives in this study (Figs. 4, 6, and Table S1). These compounds reduced the radicle lengths of O. minor to 24% and 12% of the control at 1 ppm (2.6 µM) and 87% and 80% at 0.1 ppm (0.26 µM), respectively (Fig. 5 and Table S1). The half maximal inhibitory concentrations (IC50) of these compounds were calculated as 1.3 µM for both, while that of PI-28 was 6.3 µM. The 4-Cl-substituent was a suitable size for the activity since the 4-Br substituent (compound 21) weakened the activity. Figure 6 summarizes the SAR of synthesized aryloxyacetylthioureas. On the benzene ring in the phenylthiourea moiety, m- or p- substitution is necessary, and an electron-withdrawing group at the p-position is critical for the inhibitory effect on O. minor radicle elongation. Substitutions are acceptable at any position on the benzene ring in the phenoxy moiety, and 3-Cl and 4-Cl derivatives exhibited the most potent activities. Based on this information, we are synthesizing more PI-28 derivatives that might exhibit higher activities than compounds 19 and 20. No correlation between lipophilicity and activity was observed since the most hydrophilic compound 11 with clogP of 1.97 exhibited potent activity as PI-28 with clogP of 3.84 (Table 1). It should be noted that the SAR observed in the study is against in vivo activity, which is influenced by absorption, distribution, metabolism, and excretion (ADME).27) SAR study of the in vitro inhibitory activity of aryloxyacetylthioureas against OmAGAL2 is our future work.
Fig. 6. Structure–activity relationship of aryloxyacetylthioureas for the inhibitory effect on radicle elongation of O. minor germinating seeds. On the benzene ring in the phenoxy moiety, any substitution at any position was acceptable for the activity. Cl-substitution at the m- or p- position had the most potent inhibitory effect. On the benzene ring in the phenylthiourea moiety, a substitution at the m- or p- position was essential for the activity. Specifically, an electron-withdrawing group at the p-position was critical for the activity.
One of the control methods of root parasitic weeds is utilization of intercrops which inhibit their growth through their allelopathic effects.28) Desmodium uncinatum is used in East Africa to control Striga hermonthica in maize production.11,12) Isoshaftoside, a C-glycosylflavonoid, was isolated as allelochemical inhibiting the growth of S. hermonthica and S. asiatica even at 100 pM.11) Other allelochemicals, quercetin analogues and ryecyanatine A had inhibitory activities against root parasitic weeds at 50 µM and 160 µM, respectively.9,10) The most potent aryloxyacetylthiourea compound 20 exhibited inhibitory activity against the radicle elongation of O. minor with IC50 of 1.6 µM which is more active than reported allelochemicals by an order except for isoshaftoside. Accordingly, their activity might be high enough for controlling root parasitic weeds in practical use after a thorough examination of their safety toward organisms other than their targets and environmental impacts as in the case of other agrochemicals.
Unfortunately, the most potent compound 20 failed to inhibit the radicle elongation of S. hermonthica (Fig. S3). It is assumed that planteose metabolism can be a control target also in S. hermonthica as planteose is accumulated in the dry seeds.14) Additionally, an orthologous gene of OmAGAL2 should exist in S. hermonthica because it was confirmed in S. asiatica.15) Two possibilities to explain the results are; the germination of S. hermonthica is less dependent on planteose metabolism compared with O. minor, or the synthesized aryloxyacetylthiourea are highly specific to OmAGAL2. Further studies focusing on planteose metabolism using other Orobanchaceae species such as Phelipanche spp. will contribute to generalizing the results in Orobanchaceae root parasitic weeds.
Having been screened as an OmAGAL2 inhibitor that hydrolyzes a storage carbohydrate planteose in the early process of O. minor germination, a series of derivatives of PI-28 were evaluated for their effects on germinating O. minor seeds. Several compounds exhibited higher inhibitory activity against O. minor radicle elongation than the lead compound, PI-28. This is the first report of the effect of aryloxyacetylthioureas on a root parasitic weed. Further structural optimization of aryloxyacetylthioureas will contribute to the development of control reagents for root parasitic weeds targeting planteose metabolism as a novel mode of action.
Acknowledgements
This research is supported in part by JST/JICA SATREPS (JPMJSA1607 to A.O. and Y.S.), the JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (JP20H02924 to A.O., D.O., and M.S.), the Fund for the Promotion of Joint International Research (Fostering Joint International Research (B) (JP20KK0131 to A.O. and Y.S.), and the Kobayashi Foundation (to M.S.). The authors would like to thank Prof. Abdel Gabar Babiker for providing the seeds of S. hermonthica and Tomoko Sakai for technical assistance.
Electronic supplementary materials
The online version of this article contains supplementary materials (Table S1, Figs S1, S2, and S3) which is available at https://www.jstage.jst.go.jp/browse/jpestics/.
Supplementary Data
References
- 1) C. Parker: The parasitic weeds of the Orobanchaceae. In “Parasitic Orobanchaceae,” ed. by D. M. Joel, J. Gressel and L. J. Musselman, Springer-Verlag, Berlin, pp. 313–344, 2013.
- 2) S. S. Rathore, K. Shekhawat, O. P. Premi, B. K. Kandpal and J. S. Chauhan: Biology and management of the fast-emerging threat of broomrape in rapeseed-mustard. Weed Biol. Manage. 14, 145–158 (2014). [Google Scholar]
- 3) S. K. Dara: The new integrated pest management paradigm for the modern age. J. Integr. Pest Manag. 10, 12 (2019). [Google Scholar]
- 4) H. Bouwmeester, C. Li, B. Thiombiano, M. Rahimi and L. Dong: Adaptation of the parasitic plant lifecycle: Germination is controlled by essential host signaling molecules. Plant Physiol. 185, 1292–1308 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5) K. Yoneyama: Recent progress in the chemistry and biochemistry of strigolactones. J. Pestic. Sci. 45, 45–53 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6) R. E. Eplee and R. S. Norris: Chemical control of Striga. In “Parasitic Weeds in Agriculture,” ed. by L. J. Musselman, CRC Press, Boca Raton, pp. 173–182, 1987.
- 7) T. Miyakawa, Y. Xu and M. Tanokura: Molecular basis of strigolactone perception in root-parasitic plants: Aiming to control its germination with strigolactone agonist/antagonists. Cell. Mol. Life Sci. 77, 1103–1113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8) H. Samejima, A. G. Babiker, H. Takikawa, N. Sasaki and Y. Sugimoto: Practicality of the suicidal germination approach for controlling Striga hermonthica. Pest Manag. Sci. 72, 2035–2042 (2016). [DOI] [PubMed] [Google Scholar]
- 9) A. Cimmino, M. Fernández-Aparcio, F. Avolio, K. Yoneyama, D. Rubiales and A. Evidente: Ryecyanatines A and B and ryecarbonitrilines A and B, substituted cyanatophenol, cyanatooenzo[1,3]dioxole, and benzo[1,3]dioxolecarbonitriles from rye (Secale cereale L.) root exdates: Novel metabolites with allelopathic activity on Orobanche seed germination and radicle growth. Phytochemistry 109, 57–65 (2015). [DOI] [PubMed] [Google Scholar]
- 10) M. Fernández-Aparicio, M. Masi, A. Cimmino, S. Vilariño and A. Evidente: Allelopathiic effect of quercetin, a flavonoid from Fagopyrum esculentum roots in the radicle growth of Phelipanche ramose: Quercetin natural and semisynthetic analogues were used for a structure–activity relationship investigation. Plants 10, 543 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11) A. M. Hooper, M. K. Tsanuo, K. Chamberlain, K. Tittcomb, J. Scholes, A. Hassanali, Z. R. Khan and J. A. Picket: Isoschaftoside, a C-glycosylflavonoid from Desmodium unicinatum root exudate, is an allelochemical against the development of Striga. Phytochemistry 71, 904–908 (2010). [DOI] [PubMed] [Google Scholar]
- 12) Z. R. Khan, A. Hassanali, W. Overholt, T. M. Khamis, A. M. Hooper, J. A. Picket, L. J. Wadhams and C. M. Woodcock: Control of witchweed Strigaa hermonthica by intercropping with Desmodium spp., and the mechanism defined as allelopathic. J. Chem. Ecol. 28, 1871–1885 (2002). [DOI] [PubMed] [Google Scholar]
- 13) S. O. Duke, Z. Pan and J. Bajsa-Hirschel: Proving the mode of action of phytotoxic phytochemicals. Plants 9, 1756 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14) T. Wakabayashi, B. Joseph, S. Yasumoto, T. Akashi, T. Aoki, K. Harada, S. Muranaka, T. Bamba, E. Fukusaki, Y. Takeuchi, K. Yoneyama, T. Muranaka, Y. Sugimoto and A. Okazawa: Planteose as a storage carbohydrate required for early stage of germination of Orobanche minor and its metabolism as a possible target for selective control. J. Exp. Bot. 66, 3085–3097 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15) A. Okazawa, A. Baba, H. Okano, T. Tokunaga, T. Nakaue, T. Ogawa, S. Shimma, Y. Sugimoto and D. Ohta: Involvement of α-galactosidase OmAGAL2 in planteose hydrolysis during seed germination of Orobanche minor. J. Exp. Bot. 73, 1992–2004 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16) A. Okazawa and A. Baba: (Osaka Prefecture University): Jp. Pat. JP2019-147749A (2018).
- 17) U. Zahra, A. Saeed, T. A. Fattah, U. Flörke and M. F. Erben: Recent trends in chemistry, structure, and various applications of 1-acyl-3-substituted thiourea: A detailed review. RCS Adv 12, 12710–12745 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18) M. A. Waqas, C. Kaya, A. Riaz, M. Farooq, I. Nawaz, A. Wilkes and Y. Li: Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front. Plant Sci 10, 1336 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19) A. Forouzesh, E. Zand, S. Soufizadeh and S. S. Foroushani: Classification of herbicides according to chemical family for weed resistance management strategies: An update. Weed Res. 55, 334–358 (2015). [Google Scholar]
- 20) I. Igbinnosa, K. F. Cardwell and S. N. C. Okonkwo: The effect of nitrogen on the growth and development of giant witchweed Striga hermonthica Benth.: Effect on cultured germinated seedlings in host absence. Eur. J. Plant Pathol. 102, 77–86 (1996). [Google Scholar]
- 21) C. Kannan, P. Aditi and B. Zwanenburg: Quenching the action of germination stimulants using borax and thiourea, a new method for controlling parasitic weeds: A proof of concept. Crop Prot. 70, 92–98 (2015). [Google Scholar]
- 22) M. Sonoda, Y. Mimura, S. Noda and A. Okazawa: Synthesis of aryloxyacetylthiourea derivatives for the development of radicle elongation inhibitor of parasitic weeds. Tetrahedron 135, 133333 (2023). [Google Scholar]
- 23) D. C. Nelson: The mechanism of host-induced germination in root parasitic plants. Plant Physiol. 185, 1353–1373 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24) A. Okazawa, H. Samejima, S. Kitani, Y. Sugimoto and D. Ohta: Germination stimulatory activity of bacterial butenolide hormones from Streptomyces albus J1074 on seeds of the root parasitic weed Orobanche minor. J. Pestic. Sci. 46, 242–247 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25) K. Harada, Y. Kurono, S. Nagasawa, T. Oda, Y. Nasu, T. Wakabayashi, Y. Sugimoto, H. Matsuura, S. Muranaka, K. Hirata and A. Okazawa: Enhanced production of nojirimycin via Streptomyces ficellus cultivation using marine broth and inhibitory activity of the culture for seeds of parasitic weeds. J. Pestic. Sci. 42, 166–171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26) A. Okazawa, T. Wakabayashi, T. Muranaka, Y. Sugimoto and D. Ohta: The effect of nojirimycin on the transcriptome of germinating Orobanche minor seeds. J. Pestic. Sci. 45, 230–237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27) D. Chen, G. Hao and B. Song: Finding the missing property concepts in pesticide-likeness. J. Agric. Food Chem. 70, 10090–10099 (2022). [DOI] [PubMed] [Google Scholar]
- 28) Y. Goldwasser and J. Rodenburg: Integrated agronomic management of parasitic weed seed banks. In “Parasitic Orobanchaceae,” ed. by D. M. Joel, J. Gressel and L. J. Musselman, Springer-Verlag, Berlin, pp. 393–413, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.