Abstract
Lamellar ichthyosis is a rare congenital disorder characterized by widespread epidermal hyperkeratinization. It is a rare clinical disorder throughout the entire planet, and newborns with this disease frequently have collodion membranes (adhering, supple, parchment‐like membrane). We present a 45‐day‐old infant who came to our facility complaining of a high‐grade persistent fever, high‐pitched crying, decreased feeding, odd body movements, rapid breathing, and grunting that lasted for 2 days. He was diagnosed with lamellar ichthyosis.
Keywords: autosomal recessive, infant, lamellar ichthyosis, skin hyperkeratinization
An image displaying bilateral cicatricial ectropion.
1. INTRODUCTION
The term “Ichthyosis” is derived from the Greek word “Ichthus,” which indicates “fish”. Ichthyosiform dermatoses are uncommon skin disorders characterized by abnormal skin desquamation. 1 Autosomal Recessive Congenital Ichthyosis (ARCI) is a term used to describe a group of heterogeneous keratinization disorders that are characterized by abnormal skin scaling on the entire body. The two classic forms of ARCI are lamellar ichthyosis (LI) and non‐bullous congenital ichthyosiform erythroderma (NBCE). 2 Lamellar ichthyosis is a rare (1:300,000 births) 3 autosomal recessively inherited congenital condition characterized by global hyperkeratinization of the epidermis, as first reported by Seelingman in 1841. Clinically, the infant is enclosed in a glossy membrane known as the ‘collodion baby,’ where the skin is often covered by tight, transparent collodion membranes at birth. 4 , 5 During the first few weeks of life, this membrane is typically shed, leaving behind scattered dry, scaly skin for the remaining life. 6 In between 45% and 80% of instances, bilateral ectropion is frequently seen with lamellar ichthyosis. 7 Ectropion can occur alone or in conjunction with exposure keratitis or lagophthalmos. If there is meibomian gland malfunction, the risk of exposure keratitis increases, and lamellar ichthyosis is likely to harm the meibomian glands due to their shared ectodermal origin. 8 We present a 45‐day‐old male infant who was diagnosed with lamellar ichthyosis and pyogenic meningitis.
2. CASE PRESENTATION
This is a 45‐day‐old male infant who visited our institution with complaints of high‐grade persistent fever, high‐pitched crying, decreased feeding, abnormal body movements, fast breathing, and grunting for 2 days. In association with these, his parents claimed that the baby had a history of scaling skin lesions all over the body since birth. The baby was born to parents with no personal or family history of similar complaints among them. He was born by spontaneous vaginal delivery at 38 weeks of gestation from a para two mother whose previous baby had no similar complaint. The delivery was at a nearby health center.
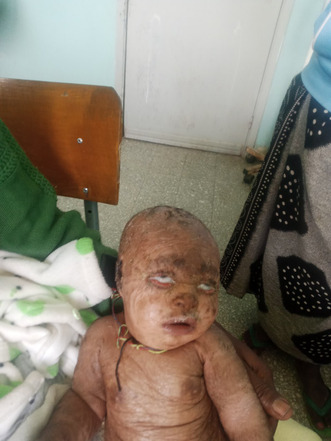
On physical examination, the baby appeared acutely sick with cardiorespiratory distress. Vital signs showed tachycardia (pulse rate = 190 bpm), tachypnea (respiratory rate = 72 breaths per minute), and fever (temperature = 40.1°C). Oxygen saturation levels ranged from 78%–82% on room air and 93%–98% on 4 L of INO2. Head, eye, ear, nose, and throat examination revealed pink conjunctiva, non‐icteric sclera, bilateral ectropion of the lower eyelid, and attached ear pinna that was easily separable from the scalp. The external ear canal appeared normal with no accumulation of scales (Figure 1). Figure 1: Picture showing bilateral cicatricial ectropion.
FIGURE 1.
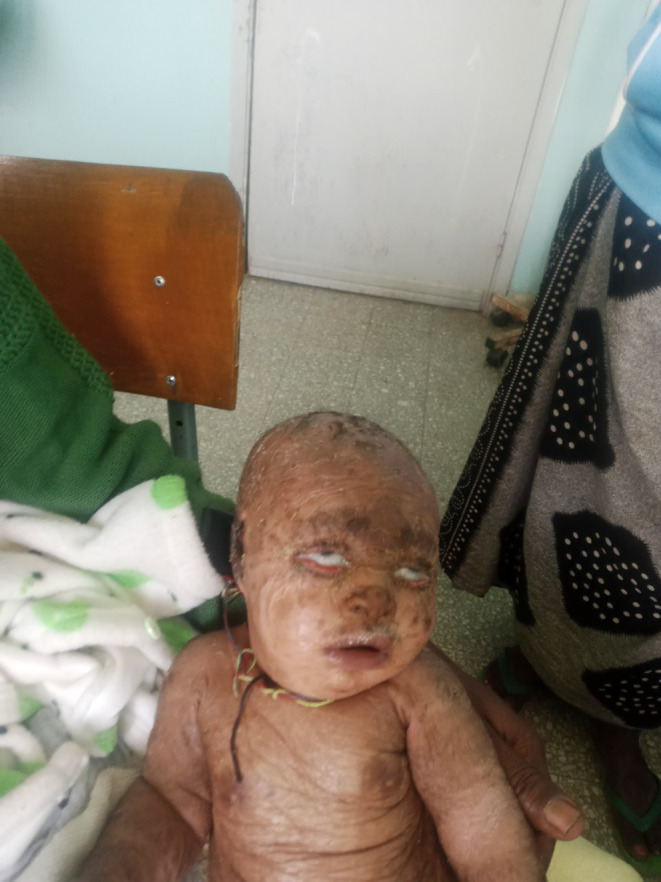
Picture showing bilateral cicatricial ectropion.
Respiratory examination showed significant intercostal and subcostal retractions, grunting, and flaring of the ala nasal, but the chest had clear and resonant sounds with good air entry. The integumentary system had dry, adherent skin lesions with scales all over the body, including the palms and soles (Figure 2). Figure 2: Picture showing dark, dry, scaly lesions all over the body.
FIGURE 2.
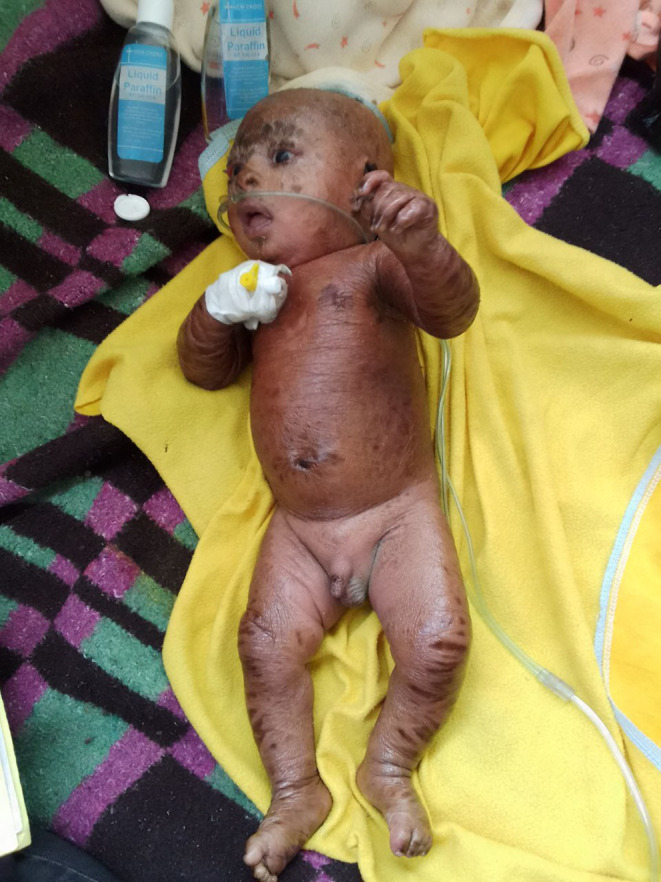
Picture showing dark, dry, scaly lesions all over the body.
Neurologically, the baby was lethargic with normotonic tone, absence of sucking reflex, and incomplete Moro reflex.
He was investigated with hematological tests. The complete blood counts showed white blood cell count of 15,490/μL, neutrophil = 61%, hemoglobin = 12.7%, platelet = 466,000, and hematocrit = 34.5%. Random blood sugar was 172 mg/dL. Liver function and renal function tests were all in the normal ranges. Likewise, serum electrolytes were also in the normal range. A lumbar puncture was not done since the patient was in cardiorespiratory distress. The patient was admitted to the pediatric ward with a diagnosis of pyogenic meningitis plus lamellar ichthyosis. The diagnoses were made based on characteristic signs and symptoms.
There is no definitive therapy to cure lamellar ichthyosis. The baby was supported with intranasal oxygen and treated with a broad‐spectrum meningeal dose of penicillin and cephalosporin (Ampicillin 200 mg/kg per day IV in four divided doses and Ceftriaxone 100 mg/kg per day in two divided doses) for 14 days, with significant improvement within the first 4 days with complete resolution within 2 weeks. He was also given emollients, which were applied twice daily. Topical corticosteroid (bethasone 0.1% cream) with a topical antibiotic (0.5% chloramphenicol eye ointment) and artificial tear twice daily for the ectropion, and the patient gradually improved within the first 2 days with complete and spontaneous resolution in 7 days of the admission (Figure 3).
FIGURE 3.
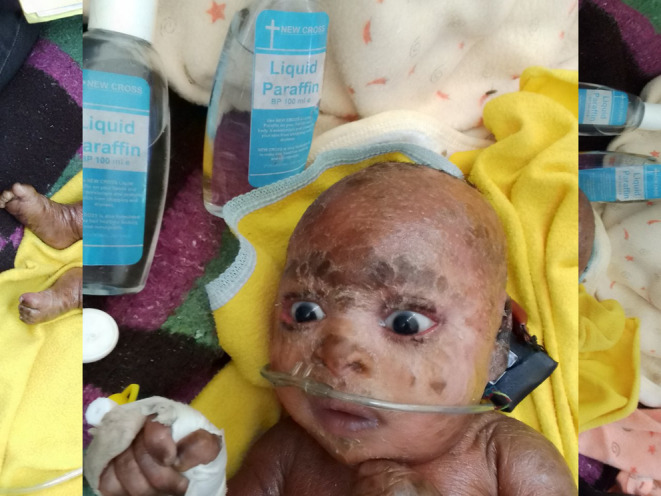
An image showing the resolution of ectropion after initiation of treatment.
The patient was monitored closely every day and found that the overall clinical condition of the baby improved significantly within the first 4 days of admission. He was discharged and completely improved within 2 weeks of admission and he has follow‐ups in our hospital every month.
3. DISCUSSION
Lamellar ichthyosis (LI) is an autosomal recessive condition that manifests at birth and lasts for the rest of one's life. 9 There is a defect in transglutaminase 1 (TGM1), a crucial epidermal enzyme that cross‐links epidermal proteins throughout the development and maturation of the stratum corneum. 9 , 10 Lamellar ichthyosis also has been associated with mutations in the CYP4F22, CERS3, PNPLA1, and ABCA12 genes. Most cases of LI are caused by TGM1 gene deficiencies, whereas CYP4F22 gene defects account for just 2%–6% of LI cases. The cornified envelope is absent in a patient with the TGM mutation because the TGM1 gene encodes one of the transglutaminase enzymes involved in the keratinized envelope's formation, which is located in the epidermis. 11
Ichthyosis patients frequently display skin signs and symptoms that can be challenging for the examining physician to succinctly describe or grade for severity in relation to a scientific study. The classic form of lamellar ichthyosis (LI) is characterized by drab‐colored plate‐like scales that form a mosaic or bark‐like pattern without significant erythroderma. As the condition progresses, the facial skin can become extremely taut, leading to clinical signs such as ectropion, eclabium, scarring alopecia affecting the scalp and eyebrows, and palmoplantar hyperkeratosis. Moreover, hearing problems and heat intolerance might result from the buildup of scales on the sweat ducts and the external ear. 12 Skin lesions often have scales that are lamellar, collodion‐like, cobblestone‐like, brownish, fine, and white. Scaling intensity can range from mild to severe, and lesions can be global or only occur focally. 13 Lamellar ichthyosis develops into huge, quadrilateral, dark scales that are free at the margins and attached at the center following the shedding of the collodion membrane, if present. Scaling is frequently noticeable and affects every surface of the body, including the flexural surfaces. The face frequently shows significant involvement, including ectropion and tiny, crinkled ears. Typically, the soles and palms are hyperkeratotic. The teeth and mucosal surfaces may be normal, but the hair may be thin and sparse. 14 , 15 Patients with ichthyosis may also experience skin infections, pruritus, thickening of the skin with cracking and fissuring, limited range of motion at joints, and diminished tactile sensitivity of the fingers. In certain cases, hypohidrosis and heat intolerance coexist. 16
Based on the clinical presentation (skin and related symptoms), a thorough medical and family history, skin biopsies, and laboratory results, an exact diagnosis of ichthyosis can be made. The classic NBCIE and LI phenotypes differ significantly and are clearly distinguishable from one another. Although both LI and NBCIE patients exhibit a wide range of clinical manifestations, the nature of the scales and the degree of the erythroderma serve as crucial clinical differentiating factors. 17 , 18 NBCIE typically has delicate, feathery scales that are white or light gray in color. On the lower legs, however, it is infrequently possible to see brownish, thick, lamellar scales that resemble LI scales and are shaped like plates. Generalized erythroderma is chronic in severe NBCIE patients. Nonetheless, erythroderma generally gets better in youth, especially in the lesser instances. 15 , 17 On the other hand, in the traditional LI phenotype, the entire body surface is affected by the scaling. However, in the milder types of LI, the huge, dark lamellar scales that are distinctive of the disease are only present at a few specific body areas, such as the forehead, upper arms, lower legs, and trunk. 17 , 19 , 20 Light microscopy reveals pronounced hyperkeratosis with only a few parakeratotic cells in skin lesions from patients with the characteristic phenotype of LI. The thickness of the granular layer is normal or slightly elevated. 15 To enable genetic counseling, which should be provided to the family or affected individual, the diagnosis must be confirmed by mutation analysis. 21 , 22 However, since we work in an elementary set up where there is no facility to conduct advanced genetic analysis, we were unable to undergo mutation analysis for our patient. We made the diagnosis based on typical physical characteristics of the condition.
The treatment of lamellar ichthyosis primarily focuses on managing symptoms, as there are currently no curative treatments available. The main approach is to provide supportive care aimed at enhancing quality of life, optimizing function, and minimizing complications. This typically involves a multidisciplinary approach, with specialists from relevant fields working together to provide comprehensive care. 23 The child's unsightly appearance and the unpleasant smell caused by bacterial colonization of macerated scales could lead to major psychological issues for the patients. 2 Uncomfortable conditions are minimized by high humidity in the winter and air conditioning in the summer. Emollients and keratolytic substances such as lactic or glycolic acid (5%–12%), urea (10%–40%), tazarotene (0.1% gel), and retinoic acid (0.1% cream) should be applied liberally and frequently to prevent scaling. Nevertheless, these substances irritate when applied to skin that has fissures. Although oral retinoids are effective in treating these disorders, they do not treat the underlying problem, necessitating continuous administration. These substances have notable side effects (teratogenicity and bone toxicity), making them less beneficial in the long term. 14 The treatment of eyes might be conservative or surgical. Hyaluronic acid injections, 5% hypertonic saline application, chloramphenicol eye ointment lubrication, and eye patching are all nonsurgical treatments used to treat eye problems. While temporal tarsorrhaphy, subconjunctival hyaluronic acid injection, fornix sutures, and full‐thickness skin transplant of the upper lid are surgical treatments. Since they are very susceptible to infection, it is crucial to use antibiotics to either prevent or treat infection. 24
4. CONCLUSION
Lamellar ichthyosis (LI) is a lifelong autosomal recessive disorder that first appears at birth. The traditional type of LI is distinguished by drab‐colored, plate‐like scales that form a mosaic or bark‐like pattern without a lot of erythroderma. Patients with ichthyosis frequently exhibit skin signs and symptoms that can be difficult for the examining doctor to briefly describe or assign a severity level to in the context of a scientific investigation. Major psychological problems could result from the child's unattractive appearance and the foul smell brought on by bacterial colonization of macerated scales.
AUTHOR CONTRIBUTIONS
Telila Mesfin: Data curation; formal analysis; writing – original draft; writing – review and editing. Mesfin Tsegaye: Data curation; investigation. Kenbon Seyoum: Conceptualization; writing – review and editing. Girma Geta: Conceptualization; writing – review and editing. Neway Ejigu: Conceptualization; writing – review and editing. Tesfaye Elala: Conceptualization; writing – review and editing. Degefa Gomora: Conceptualization; supervision. Biniyam Sahiledengle: Conceptualization; supervision. Eshetu Mesfin Tadesse: Conceptualization; supervision. Getu Kusa: Software; writing – review and editing. Teketel Tilahun: Software; writing – review and editing.
FUNDING INFORMATION
We received no funding for this case report.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
CONSENT
Written informed consent was obtained from the patient's parents to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
None.
Mesfin T, Tsegaye M, Seyoum K, et al. An infant with lamellar ichthyosis presenting with meningitis. Clin Case Rep. 2023;11:e8329. doi: 10.1002/ccr3.8329
DATA AVAILABILITY STATEMENT
Data on the case clinical information, informed consent form, and images are available for review from the corresponding author upon reasonable request.
REFERENCES
- 1. Huang J‐J, Huang M‐Y, Huang T‐Y. Lamellar ichthyosis with severe bilateral ectropion and self‐healing collodion membrane. Biomark Genom Med. 2013;5(3):110‐112. [Google Scholar]
- 2. Cortés H, Rojas‐Márquez M, Reyes‐Hernández OD, et al. Increased risk of depression and impairment in quality of life in patients with lamellar ichthyosis. Dermatol Ther. 2021;34(1):e14628. [DOI] [PubMed] [Google Scholar]
- 3. Odom RB, James WD, Berger TG. Andrews' Diseases of the Skin: Clinical Dermatology (Vol. 1135). WB Saunders Company Philadelphia; 2000. [Google Scholar]
- 4. Singh M, Kaur M, Kaur R, Singh S. Severe ectropion in lamellar ichthyosis managed medically with oral acitretin. Pediatr Dermatol. 2018;35(2):e117‐e120. [DOI] [PubMed] [Google Scholar]
- 5. Gupta A, Patel K, Nathwani Y, et al. Congenital bilateral ectropion in collodion baby: a rare case report. Delhi J Ophthalmol. 2019;29(4):90‐92. [Google Scholar]
- 6. Subramanian N, Nivean PD, Alam MS. Combined medical and surgical management for cicatricial ectropion in lamellar ichthyosis: a report of three cases. Indian J Ophthalmol. 2020;68(11):2615‐2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shindle RD, Leone CR. Cicatricial ectropion associated with lamellar ichthyosis. Arch Ophthalmol. 1973;89(1):62‐64. [DOI] [PubMed] [Google Scholar]
- 8. Palamar M, Karaca I, Onay H, Ertam I, Yagci A. Dry eye and meibomian gland dysfunction with meibography in patients with lamellar ichthyosis. Contact Lens Anterior Eye. 2018;41(2):154‐156. [DOI] [PubMed] [Google Scholar]
- 9. Cao X, Lin Z, Yang H, et al. New mutations in the transglutaminase 1 gene in three families with lamellar ichthyosis. Clin Exp Dermatol. 2009;34(8):904‐909. [DOI] [PubMed] [Google Scholar]
- 10. Judge M, McLean W, Munro C. Disorders of keratinization. Rook's Textbook of Dermatology. 2010;1:1‐122. [Google Scholar]
- 11. Xiao X, Wu Z‐C, Chou K‐C. A multi‐label classifier for predicting the subcellular localization of gram‐negative bacterial proteins with both single and multiple sites. PloS One. 2011;6(6):e20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gulasi S. Congenital ichthyosis: a case treated successfully with acitretin. Iran J Pediatr. 2016;26(5):e2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fischer J, Bourrat E. Genetics of inherited ichthyoses and related diseases. Acta Derm Venereol. 2020;100(7):186‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kliegman RM, Toth H, Bordini BJ, et al. Nelson Pediatric Symptom‐Based Diagnosis E‐Book. Elsevier Health Sciences; 2022. [Google Scholar]
- 15. Akiyama M, Sawamura D, Shimizu H. The clinical spectrum of nonbullous congenital ichthyosiform erythroderma and lamellar ichthyosis. Clin Exp Dermatol. 2003;28(3):235‐240. [DOI] [PubMed] [Google Scholar]
- 16. DiGiovanna JJ, Robinson‐Bostom L. Ichthyosis: etiology, diagnosis, and management. Am J Clin Dermatol. 2003;4:81‐95. [DOI] [PubMed] [Google Scholar]
- 17. Griffiths W, Judge M, Leigh I. Disorders of Keratinization,“In” Rook, Textbook of Dermatology. Blackwell; 1998. [Google Scholar]
- 18. Williams ML, Elias PM. Heterogeneity in autosomal recessive ichthyosis: clinical and biochemical differentiation of lamellar ichthyosis and nonbullous congenital ichthyosiform erythroderma. Arch Dermatol. 1985;121(4):477‐488. [DOI] [PubMed] [Google Scholar]
- 19. Akiyama M, Matsuo I, Takizawa Y, Suzuki Y, Ishiko A, Shimizu H. Compound heterozygous TGM1 mutations including a novel missense mutation L204Q in a mild form of lamellar ichthyosis. J Invest Dermatol. 2001;116(6):992‐995. [DOI] [PubMed] [Google Scholar]
- 20. Laiho E, Niemi KM, Ignatius J, Kere J, Palotie A, Saarialho‐Kere U. Clinical and morphological correlations for transglutaminase 1 gene mutations in autosomal recessive congenital ichthyosis. Eur J Hum Genet. 1999;7(6):625‐632. [DOI] [PubMed] [Google Scholar]
- 21. Patel N, Spencer LA, English JC III, Zirwas MJ. Acquired ichthyosis. J Am Acad Dermatol. 2006;55(4):647‐656. [DOI] [PubMed] [Google Scholar]
- 22. Oji V, Traupe H. Ichthyosis: clinical manifestations and practical treatment options. Am J Clin Dermatol. 2009;10:351‐364. [DOI] [PubMed] [Google Scholar]
- 23. Paller AS, Butala S. Inherited ichthyosis: overview of management. p. 12–1 2022.
- 24. Sayomi B, Eyekpegha OT, Joel‐Medewase VI, et al. Collodion baby, lamellar ichthyosis; a report of two cases and literature review. J African Neonatol. 2023;1(1):31‐34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data on the case clinical information, informed consent form, and images are available for review from the corresponding author upon reasonable request.


