Abstract
Approximately half of adolescents encounter a mismatch between their sleep patterns on school days and free days, also referred to as “social jetlag.” This condition has been linked to various adverse outcomes, such as poor sleep, cognitive deficits, and mental disorders. However, prior research was unsuccessful in accounting for other variables that are correlated with social jetlag, including sleep duration and quality. To address this limitation, we applied a propensity score matching method on a sample of 6335 11–12-year-olds from the 2-year follow-up (FL2) data of the Adolescent Brain Cognitive Development study. We identified 2424 pairs of participants with high sleep-corrected social jetlag (SJLsc, over 1 hour) and low SJLsc (<= 1 hour) at FL2 (1728 pairs have neuroimaging data), as well as 1626 pairs at 3-year follow-up (FL3), after matching based on 11 covariates including socioeconomic status, demographics, and sleep duration and quality. Our results showed that high SJLsc, as measured by the Munich Chronotype Questionnaire, was linked to reduced crystallized intelligence (CI), lower school performance—grades, and decreased functional connectivity between cortical networks and subcortical regions, specifically between cingulo-opercular network and right hippocampus. Further mediation and longitudinal mediation analyses revealed that this connection mediated the associations between SJLsc and CI at FL2, and between SJLsc and grades at both FL2 and FL3. We validated these findings by replicating these results using objective SJLsc measurements obtained via Fitbit watches. Overall, our study highlights the negative association between social jetlag and CI during early adolescence.
Keywords: sleep, brain imaging, adolescents, crystallized intelligence, hippocampus
Graphical Abstract
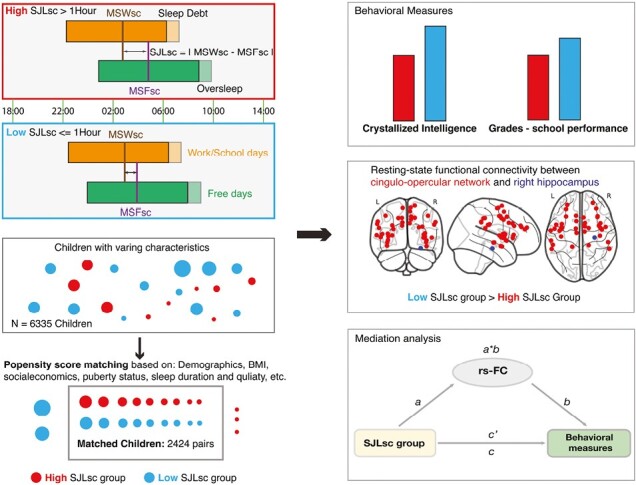
Statement of Significance.
The current study conducted on 6335 11–12-year-olds from the Adolescent Brain Cognitive Development study reveals a significant link between social jetlag and adverse behavioral and brain functional outcomes during early adolescence. Social jetlag, characterized by a mismatch between sleep patterns on school days and free days, was found to be associated with reduced crystallized intelligence, lower school performance, and reduced functional connectivity in certain regions, notably the right hippocampus. This comprehensive research, which factored in various variables such as socioeconomic status, sleep duration, and quality, employed propensity score matching to bolster result accuracy, shedding light on potential neural mechanisms behind these correlations. These findings underscore the significance of addressing social jetlag in adolescent sleep routines, given its potential impact on neurocognitive development.
Introduction
In today’s fast-paced and interconnected world, the prevalence of social jetlag (SJL) has become a significant health concern. SJL refers to the misalignment between an individual’s biological clock and their social schedules [1, 2]. While SJL presents throughout work life, adolescents often experience the most severe SJL [3], as their biological clock progressively shifts to the late phase during adolescence [4]. SJL has been associated with mood disorders, aggression and behavioral conduct problems, cognitive problems, and metabolic diseases in both adults [3, 5–7] and adolescents [8–11]. However, due to significant heterogeneity between study methodologies, the direct effects of SJL on behavior and brain are poorly understood. First, SJL significantly correlated with other sleep measurements, i.e. sleep duration and quality, as well as socioeconomic status. These factors typically have not been considered concurrently in previous studies. Given that our recent studies show that inadequate sleep is also related to mood disorders, crystallized intelligence (CI), and impulsivity [12–14], it was impossible to measure direct effects of SJL on above-mentioned behaviors unless other sleep measures are carefully controlled. Second, the calculation of SJL is inconsistent across studies. Classic calculation of SJL failed to consider oversleeping during weekends, which is common among adolescents [10, 11]. To account for the latter, a sleep-corrected SJL (SJLsc) takes the weekday–weekend sleep duration difference into account [15]. Third, most studies relied on self- or parent-reported sleep measures, which might overestimate child sleep as compared to objective sleep measures [16, 17], such as actigraphy. In addition, only a few studies investigated the functional connectivity associations of SJL in adults [18–20], and neural associations of SJL/SJLsc in adolescents are poorly understood.
To address these shortcomings, we applied a data-driven, propensity score matching (PSM) method to investigate how SJLsc, measured by Munich Chronotype Questionnaire (MCTQ), is associated with functional connectivity and behavior in early adolescents after controlling for various covariates, such as other sleep measurements, i.e. sleep duration and quality, socioeconomic status, body mass index (BMI), puberty status, etc. We then verified the results using an objective measurement of SJLsc through wearable devices. Based on previous research, we hypothesized that SJLsc would have adverse effects on behavioral problems, cognitive performance, mental health, and brain functional connectivity. We predicted that identified functional connectivity would mediate the association between SJLsc and behavioral measures.
Methods
Study design and data source
In this longitudinal, observational cohort study, we utilized data from a population-based sample of 9–10-year-olds (11–13-year-olds onward in the current study) collected from 21 study sites across the United States as part of the ongoing Adolescent Brain Cognitive Development (ABCD) study. The data used in our study were obtained from the ABCD data release 4.0. This comprehensive dataset included behavioral and neural information gathered at baseline, 1-year, 2-year, and 3-year follow-ups. Only data from 2-year and 3-year follow-ups were used in the current study (collected between July 30, 2018, to January 15, 2021). The study employed rigorous protocols and stratified sampling techniques to ensure accurate data collection and representation of the diverse US population. Detailed protocols and designs were described previously [21]. Participants with missing data for relevant covariates used in PSM were excluded (see Supplementary Appendix 1.1 for details). This resulted in 6565 children out of 10 414 children. Informed consent was obtained from primary caregivers and assent from children. The study was approved by the Institutional Review Boards of all 21 study sites.
Subjective sleep measures
Starting from the 2-year follow-up (FL2), Munich Chronotype Questionnaire (MCTQ) was completed by children [1, 3, 22]. Sleep duration (SDweek) and SJLsc were derived from this MCTQ data. SDweek reflects average sleep duration across the whole week, i.e. school days and free days. Classic SJL was calculated as the absolute value of the mid-sleep (defined as midpoint between sleep onset and wake up) difference between work/school days (MSW) and weekends/free days (MSF) (Equation 1). However, classic SJL did not take the potential sleep deprivation on school days and/or oversleeping on free days into account [3, 15]. This effect can be minimized by SJLsc [15], which takes SDweek into account.
| (1) |
| (2) |
Here, in SJLsc calculation (Equation 2), instead of using MSW/MSF it added half of the average sleep duration across the week to the sleep onset on both work/school day (SOW) and free days (SOF). It should be noted that SJLsc actually reflects the difference in sleep onset between school days and free days. For quality control purposes, we excluded children whose reported sleep duration of school days/free days were longer than 15 hours or shorter than 3 hours per day (230 out of 6565 eligible children), because these abnormal sleep durations were more likely caused by mistakes that happened in data collection processes (Supplementary Appendix 1.3).
Total sleep disturbance score (TSD) is measured by the parent-reported Sleep Disturbance Scale for Children, which reflects the sum score of six sleep disorders, including disorders of initiating and maintaining sleep, sleep breathing disorders, disorder of arousal, sleep–wake transition disorders, disorders of excessive somnolence, and sleep hyperhidrosis.
Objective sleep measures
Data from a consumer sleep tracker, Fitbit Charge HR, were collected and processed at FL2 by the Data Analysis, Informatics, and Resource Center of the ABCD study. A subset of children (n = 5901 of 10 414) were instructed to wear Fitbit watches for 3 consecutive weeks after the onsite visit. We included data from 3821 children who had at least 7 nights [23, 24] of sleep recording within 3 weeks of the onsite visit and had at least 3 nights of sleep recording on school days/free days. Objective SJLsc (oSJLsc) was calculated based on data recorded from Fitbit watches. The reason for establishing the threshold of at least 7 nights is based on previous research findings, which suggest that to achieve a reliable measure of actigraphy-measured total sleep time, one would need to gather data from at least 7 nights [23]. For quality control purposes, we excluded sleep duration data that were longer than 15 hours or shorter than 3 hours.
Behavioral problems, cognition, mental health, and school performance measures
Behavioral problems.
We used the parent-reported Child Behavior Checklist at FL2 and 3-year follow-up (FL3) to measure a child’s behavioral problems in emotional, social, and behavioral domains; Cognition: we used scores from the US National Institutes of Health (NIH) Cognition Battery Toolbox at FL2 to assess a child’s cognitive functions. However, 4 out of 10 cognitive measures were not collected at FL2. Mental health: we estimated a child’s overall mental health at FL2 based on scores from the brief child version of the Prodromal Psychosis Scale (PPS); the youth version of the Urgency, Premeditation (lack of), Perseverance (lack of), Sensation Seeking, PU (UPPS-P) Impulsive Behavior Scale; and the Behavioral Inhibition Scale (a summary of these variable names used in the ABCD dataset is shown in Supplementary Appendix Table S1). School performance: grades from school during last year were used as an index for school performance at both FL2 and FL3. The school performance scale is inverted so that a higher score means higher performance in school. Note that cognitive performance and mental health measures were not collected at FL3. The details about the rationale and validity of behavioral measurements can be found elsewhere [25].
Brain measures
A subset of Children (n = 7675 of 10 414) had four standardized resting-state functional MRI scans and one structural MRI scan at FL2. The acquired images underwent processing and quality control at the Data Analysis, Informatics, and Resource Center of the ABCD study. Detailed protocols and processing steps can be found elsewhere [26]. To determine the resting-state functional connectivity (rs-FC) of cortical networks, the average Fisher-transformed correlation between the time courses of each pair of regions within or between 12 cortical networks defined by the Gordon atlas [27] was calculated. Additionally, rs-FC between the 12 cortical networks and 19 subcortical regions was also computed. In total, there were 306 unique functional connectivity measures. Gray matter volume, a structural measure, was extracted from 148 regions based on the Destrieux parcellation.
Covariates included in propensity score matching
We included the following covariates in PSM (Supplementary Appendix 1.1): (1) basic demographic characteristics of a participant (age in months, sex at birth, the interaction between age and sex, race, and study sites), (2) theoretically relevant factors, including parent education level and household income, pubertal status (assessed by ABCD Youth Pubertal Development Scale and Menstrual Cycle Survey History based on sex at birth: pds_p_ss_(fe)male_category_2) [28], and BMI, and (3) confounding sleep measures, including sleep duration, and total sleep disturbance score. It should be noted that there are 2973 out of 10 414 children who have missing values on BMI. When using baseline BMI as approximate, the main results stayed the same (Supplementary Appendix 2.2).
Statistical analysis
We conducted PSM to control for covariates in the observational data, allowing us to estimate the causal effects of SJLsc on the outcome measures. The MatchIt R package (version 4.3.0) was utilized for the PSM process. Participants were matched based on their probability of being in a comparison group, taking into account observed covariates using logistic regression. These above-mentioned covariates were included. The matching process involved pairing participants with low SJLsc (SJLsc ≤1 hour) with those with high SJL (SJLsc >1 hour) through one-to-one matching without replacement within a predefined propensity score radius (caliper = 0.1). To assess the quality of the matching, we examined the standardized mean difference of covariates between the sufficient sleep and insufficient sleep groups and found that all covariates were well-balanced between the groups after matching (Supplementary Appendix 1.1), indicating that any additional group differences could not be attributed to these covariates.
For assessing these group differences an independent-sample t-test was employed, as it tends to provide a more conservative estimate of effect size compared to a paired-sample t-test [29]. Subsequently, we examined how SJLsc influenced 39 behavioral domains that represented various aspects of adolescent behavior problems (e.g. aggression and rule-breaking; 20 items), cognitive functions (e.g. CI; 6 out of 10 items available at FL2), mental health (e.g. psychosis and impulsivity; 12 items), and school performance at FL2.
Regarding the analysis of fMRI data, our focus was on participant pairs that had passed quality control for each corresponding brain measure. Additionally, we regressed out mean frame-wise displacement and the number of fMRI time points remaining after preprocessing from network connectivity measurements, and we also regressed out the total intracranial volume from gray matter volume measures. Multiple comparisons were corrected based on the false discovery rate p < 0.05.
Based on the conceptual framework suggesting that brain activity patterns could act as mediators in the relationship between sleep patterns and developmental outcomes [12–14], we conducted additional tests to examine whether brain measures mediate the impact of SJLsc on behavioral measures at FL2, while controlling for the covariates used in the PSM process. The rationale behind conducting these mediation analyses is to unveil the neural and brain mechanisms through which SJLsc influences behavioral variables. These elucidated mechanisms could potentially be harnessed to mitigate the impact of SJLsc on behavior in the future. Our main focus was on brain and behavior assessments that exhibited a Cohen’s d greater than 0.15 between the high SJLsc and low SJLsc groups. This threshold represents a 50% increase in effect size compared to the typical range identified in the ABCD dataset (Cohen’s d 0.03–0.09) [30]. By using effect size but not P-value as a threshold, we aimed to enhance the replicability of neuroimaging findings [31].
To perform these analyses, we utilized a well-established neuroimaging mediation toolbox. Detailed processes can be found in Supplementary Appendix 1.2. The significance of the mediation analyses was determined through bootstrapping with 10 000 randomly generated samples, providing a robust assessment of the results. Additional longitudinal mediation analyses were performed to test whether brain measures at FL2 mediate the relationship between SJLsc at FL2 and behavior measures at FL3 while controlling for the corresponding behavior measures at FL2. These longitudinal time-lagged analyses aim to test whether the identified brain measures can serve as biomarkers of behavioral changes over time.
Next, we replicated the above-mentioned analyses using oSJLsc. Given that only a subset of children participated in the Fitbit data collection, we applied partial correlation to examine the association between oSJLsc and behavior and brain measurements.
Results
Of the 10 414 participants included in the FL2 data, 3849 were excluded due to having a least one missing value on the covariates used in the PSM, and an additional 230 were excluded for quality control for sleep duration data. After exclusion, 6335 eligible participants were included in this study, 2979 (47.02%) of whom were female, and 3356 (52.98%) were male. There were 3223 participants in the low SJLsc group and 3112 participants in the high SJLsc group. After PSM, we identified 2424 matching pairs at FL2, of which 1626 pairs had behavioral data at FL3.
We next tested how high SJLsc influenced the 43 behavioral measurements. Four cognitive measurements related to fluid intelligence were not tested in FL2. We found that high SJLsc had significant effects on 26 out of the remaining 39 available measures (FDR corrected p < 0.05, see Figure 1). Among these, six of them had an effect size larger than 0.15, including picture-vocabulary, oral reading recognition, CI (it is calculated as a sum score of picture-vocabulary and oral reading recognition, and it reflects knowledge that you have learned and stored), PPS total number of yes answers, positive urgency (PU), and grades. Surprisingly, contradictory to the previous research [9, 11], we found that high SJLsc was associated with low mood problems, i.e. depression and anxiety, with low effect size (FDR corrected p < 0.05, Cohen’s d < 0.1).
Figure 1.
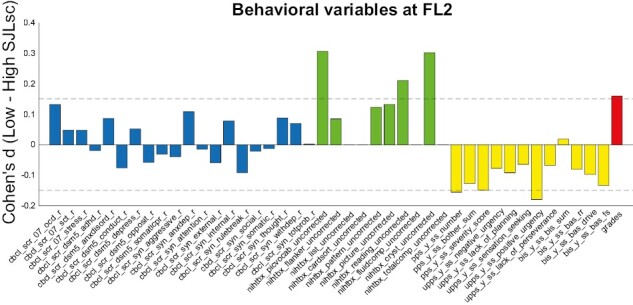
The effects of SJLsc on behavioral measures. Cohen’s d for behavior problems, cognition, mental health, and school performance in the comparison between high SJLsc and Low SJLsc groups at FL2 (Details for variable names can be found in Supplementary Appendix Table S1). Positive Cohen’s d value means the low SJLsc group has a higher value than that of the high SJLsc group. At FL2, four measures of cognition were not available: nihtbx_list_uncorrected, nihtbx_cardsort_uncorrected, nihtbx_fluidcomp_uncorrected, and nihtbx_totalcomp_uncorrected.
At FL3, only 21 out of 43 measures were obtained. Among these, four measures were significantly affected by SJLsc (FDR corrected p < 0.05). Of note, high SJLsc at FL2 is associated with low grades at FL3 (FDR corrected p < 0.05, Cohen’s d = 0.206 Supplementary Figure S2).
Out of 2424 pairs, 1728 pairs had fMRI data available. We next examined how SJLsc affects the intrinsic functional organization of brain networks in the developing brain at FL2. We found that 33 of the 306 unique network connectivity measurements showed significant differences between high SJLsc and low SJLsc (Figure 2). Among them, the top five connections (all have Cohen’s d value higher than 0.2) were cingulo-opercular—right hippocampus (cerc-hprh), cingulo-opercular—right amygdala (cerc-agrh), cingulo-parietal—left ventral DC (copa-vtdclh), sensorimotor hand—right caudate (smh-cderh), and sensorimotor hand—right putamen (smh-ptrh). Meanwhile, no gray matter volume measurements had Cohen’s d value higher than 0.15 (all false discovery rate correct p’s > 0.05). See Supplementary Appendix 3.1 for the belief discussion on lateralization of the hippocampus.
Figure 2.
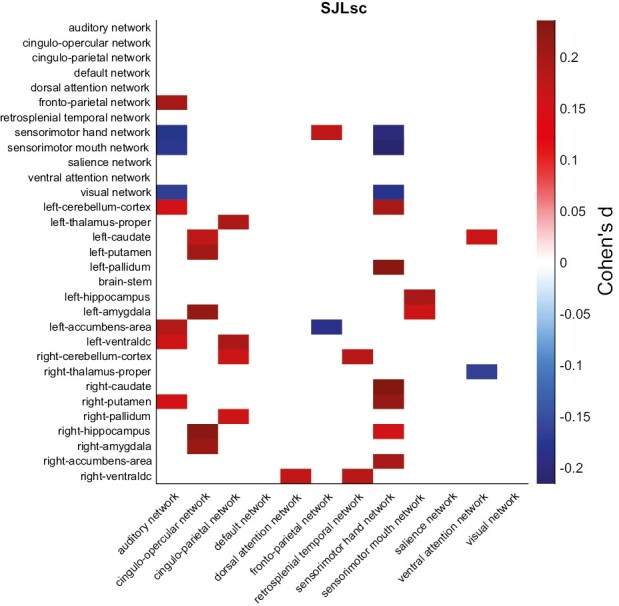
The effects of SJLsc on resting-state functional connectivity. Cohen’s d for resting-state functional connectivity measures in the comparisons between high SJLsc and low SJLsc group at FL2. Positive value denotes the Low SJLsc group has higher connectivity than the High SJLsc group, while negative value means the High SJLsc group has lower connectivity compared to the Low SJLsc group. Only connections that had Cohen’s d higher than 0.15 are shown in the figure. Within-network connectivity (i.e. visual network—visual network) is calculated by averaging unique functional connectivity pairs between all the regions in the network.
Next, to assess how network connections mediate the relationship between SJLsc and behavioral outcomes, we tested whether the five selected network connections mediated the effects of SJLsc (group: high vs. low SJLsc) on the seven behavioral measures identified with a Cohen’s d greater than 0.15. In this analysis, we regressed out covariances used in the PSM plus two rs-FC quality control indexes (mean motion and the number of fMRI time points remaining after preprocessing). We found that all five network connections significantly mediated the association between SJLsc (group) and Picture-vocabulary, reading, CI, PU, and grades (p’s < 0.05 using bootstrap sampling with 10 000 random-generated samples, see Figure 3). In addition, it was found that two out of five network connections mediated the association between SJLsc and PPS number. In terms of longitudinal mediation analyses, we found that cerc-hprh, cerc-agrh, and smh-ptrh at FL2 mediated the effect of SJLsc at FL2 on grades at FL3 (p’s < 0.05 using bootstrap sampling with 10 000 random-generated samples, see Supplementary Figure S3), controlling for grades at FL2.
Figure 3.
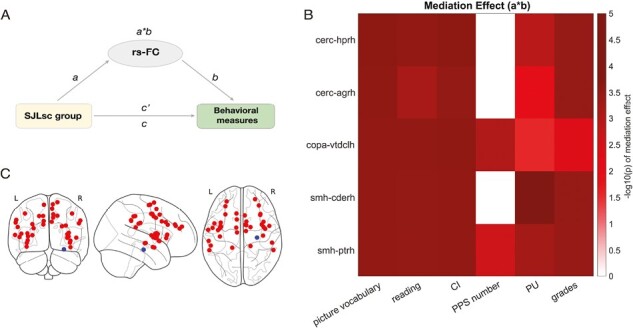
Brain measures mediated the effect of SJLsc group on cognition, positive urgency, and grades. (A) Diagram of the mediation models. (B) Based on matched group comparisons, we have examined how brain measures identified with larger effect sizes (i.e. 5 rs-FC Y-axis) would mediate the effects of SJLsc on the six behavioral measures identified with a Cohen’s d greater 0.15 (i.e. picture vocabulary, picture memory, reading, CI, PPS number, PU, and grades; X-axis). Color bars are coded based on log-transferred bootstrapped P-values of the mediation effects. Only the effects reached statistical significance (p < 0.05) using bootstrap sampling with 10 000 random-generated samples shown in the figure. (C) Visualization of nodes in the cerc (red) and hprh (blue). rs-FC, resting-state functional connectivity; cerc, cingulo-opercular network; copa, cingulo-parietal network; smh, sensorimotor hand network; hprh, right hippocampus; agrh, right amygdala; vtdlh, left ventral diencephalon; cderh, right caudate; ptrh, right putamen.
Last, out of 4848 matched children, 2310 had oSJLsc data available and passed quality control. In this subsample, we found that oSJLsc was weakly correlated with SJLsc (r = 0.23, p < 0.0001, Supplementary Figure S4). However, by using partial-correlation analyses, the correlation patterns between oSJLsc and behavioral measures (n = 2310, see Supplementary Figure S5 and Appendix 2.1) and between oSJLsc and rs-FC (n = 1655, see Supplementary Figure S6 and Appendix 2.1) were quantitatively similar to the effects of SJLsc on behavior and rs-FC (Figures 1 and 2, respectively).
Discussion
By controlling various important covariates linked to social jetlag, we assessed the impact of SJLsc on both behavior and brain. Unlike previous studies, we did not discover a meaningful correlation between SJLsc and affective functions such as depression. We found a connection between SJLsc and CI and school performance, which was most significantly mediated by the functional connectivity between the cingulo-opercular network and the right hippocampus. The association between SJLsc and school performance lasted for at least 1 year. Our results suggest that, in addition to sufficient sleep duration and quality, SJLsc may play a crucial and unique role in the neurocognitive development of early adolescents.
In contrast with previous research demonstrating a positive connection between SJL or SJLsc and mood disorders among adults [18] and adolescents [10, 11], we found that after matching for other sleep measures and related covariates, the associations between SJLsc and depression or anxiety were negative instead of positive and with low effect size (effect size lower than 0.1). This aligns well with recent discoveries indicating that the associations between SJLsc and poor mood diminish when puberty status and other sleep issues are taken into consideration [8]. Consequently, future investigations into social jetlag should include the control of other sleep metrics, such as sleep duration and quality, as well as additional sleep-related covariates.
We replicated the associations between SJLsc and cognition and school performance in a population-based, carefully controlled study. SJLsc had a large effect on CI with an effect size of around 0.3, which is three times larger than a typical effect size found on the ABCD dataset (Cohen’s d 0.03–0.09) [30]. While these associations have been demonstrated before [3, 5, 11], we further revealed that the neural basis of such effect involved the functional connection between the cingulo-opercular network and hippocampus (cerc-hprh). The cingulo-opercular network is known as a cognitive control network, e.g. controlling cognitive resources during episodic memory search tasks [32, 33]. Hence, higher resting-state connectivity between cingulo-opercular network and hippocampus in low SJLsc group might reflect better cognitive resource relocation, especially for memory-related tasks. A recent review paper [34] highlighted that hippocampal function in memory encoding is not fully developed during middle childhood (i.e. 6–12 years old). It is possible that Low SJLsc (no more than 1 hour) promotes hippocampal development and hence children with low SJLsc perform better in school and memory-related tasks, i.e. tasks measuring CI.
Despite the fact that there was only a weak correlation between subjective and objective SJLsc, quantitatively similar effects were found. It is common to have notable disparities between subjective and objective assessments of sleep measures [23]. In addition, Aili et al. [23], found objective sleep duration during weekdays was more reliable than that during weekends. It is possible that more data from actigraphy are needed, e.g. longer than 3 consecutive weeks in the current study, to reach a high agreement between subjective and objective SJLsc.
The current study offers several significant contributions. Firstly, through the utilization of PSM, the group comparison between individuals with low and high SJLsc was conducted while accounting for variables such as sleep duration, sleep quality, puberty status, BMI, socioeconomic status, and other relevant covariates. This approach effectively addressed the conflicting findings regarding the association between SJLsc and mental health, providing compelling evidence that SJLsc is indeed linked to CI and school performance. Secondly, we demonstrated SJLsc at FL2 predicted school performance one year later, and the connection between cingulo-opercular network and hippocampus mediated this time-lagged association. These results highlight early sleep intervention might have positive effects on school performance.
Several limitations should be acknowledged. First, due to a large amount of missing data (due to coronavirus disease 2019) on BMI, we excluded about 26% of children. However, almost all the children with missing BMI data do not have neuroimaging data and/or Fitbit data. In addition, when using baseline measures of BMI as proximate values to include more children, the results stayed the same (see Appendix 2.3). Second, our sample only had limited longitudinal data on school performance. While we demonstrated a consistent association between SJLsc and grades at both FL2 and FL3, it would be valuable for future studies to explore the longitudinal association between SJLsc and other variables as more data becomes available in the ABCD study. Third, with data mostly from one-time points, the current study is not able to distinguish within-person and between-person effects. A random-intercept cross-lagged panel model can mitigate this issue when more visits are available in future ABCD data releases. Fourth, although the objective sleep data measured with Fitbit Charge HR show strong agreement with polysomnography (PSG), it exhibits low specificity in detecting wakefulness in healthy adolescents [35]. This challenge could be amplified when applied to adolescents with sleep disturbances. However, we controlled sleep disturbance in the current study to mitigate this issue. Fifth, the sleep disturbance score was generated by parent reports, while MCTQ was generated by children’s reports. There might be bias between parent-reported and children-reported sleep data. Future studies might take this discrepancy into consideration.
The current study estimates the effects of high SJLsc (>1 hour vs. ≤1 hour) on neurocognitive development in early adolescence while carefully controlling for key covariates, including other sleep measures, socioeconomic status, puberty status, and physical health indicators. Our results indicate that the connectivity between the cingulo-opercular network and the right hippocampus plays a significant role in mediating the effects of SJLsc on cognitive ability and school performance. These effects are likely to last at least 1 year. Considering the prevalence of SJLsc and its negative consequences, early interventions such as extending sleep duration on school days [36] are necessary to enhance neurocognitive development outcomes in early adolescents.
Supplementary Material
Acknowledgment
This research was supported (in part) by the Intramural Research Program of the NIH, NINDS. We thank the ABCD consortium and NIH for providing the data for performing the research in this work. Data used in the preparation of this article were obtained from the ABCD Study (https://abcdstudy.org/) and are held in the NIMH Data Archive. This is a multisite, longitudinal study designed to recruit more than 10 000 children aged 9–10 and follow them over 10 years into early adulthood.
Contributor Information
Fan Nils Yang, Advanced MRI Section, Laboratory of Functional and Molecular Imaging, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA.
Dante Picchioni, Advanced MRI Section, Laboratory of Functional and Molecular Imaging, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA.
Jeff H Duyn, Advanced MRI Section, Laboratory of Functional and Molecular Imaging, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA.
Funding
The ABCD Study is supported by the National Institutes of Health (NIH) and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners/. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. We are not paid to write this article by a pharmaceutical company or other agency.
Author Contributions
F.N.Y. conceptualized the study, analyzed the data, generated figures, and wrote the original draft. D.P. edited the manuscript. J.H.D. supervised the study and edited the manuscript.
Disclosure Statement
Financial disclosure: The authors declare no competing financial interests. Nonfinancial disclosure: The authors declare no competing nonfinancial interests.
Ethical Approval
ABCD study received ethical approval in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Data Availability Statement
The ABCD data used in this study are available in the National Institutes of Mental Health Data Archive (https://nda.nih.gov/abcd/). Information about the ABCD consortium is available on their website (https://abcdstudy.org/principal-investigators/).
References
- 1. Roenneberg T, Wirz-Justice A, Merrow M.. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18(1):80–90. doi: 10.1177/0748730402239679 [DOI] [PubMed] [Google Scholar]
- 2. Wittmann M, Dinich J, Merrow M, Roenneberg T.. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1-2):497–509. doi: 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- 3. Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC.. Chronotype and social jetlag: a (self-) critical review. Biology. 2019;8(3):54. doi: 10.3390/biology8030054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T.. Chronotypes in the US – influence of age and sex. PLoS One. 2017;12(6):e0178782. doi: 10.1371/journal.pone.0178782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beauvalet JC, Quiles CL, Oliveira MAB de, Ilgenfritz CAV, Hidalgo MPL, Tonon AC.. Social jetlag in health and behavioral research: a systematic review. ChronoPhysiology Ther. 2017;7:19–31. doi: 10.2147/CPT.S108750 [DOI] [Google Scholar]
- 6. Caliandro R, Streng AA, van Kerkhof LWM, van der Horst GTJ, Chaves I.. Social jetlag and related risks for human health: a timely review. Nutrients. 2021;13(12):4543. doi: 10.3390/nu13124543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taillard J, Sagaspe P, Philip P, Bioulac S.. Sleep timing, chronotype and social jetlag: impact on cognitive abilities and psychiatric disorders. Biochem Pharmacol. 2021;191:114438. doi: 10.1016/j.bcp.2021.114438 [DOI] [PubMed] [Google Scholar]
- 8. Chen CX, Li TMH, Zhang J, et al. The impact of sleep-corrected social jetlag on mental health, behavioral problems, and daytime sleepiness in adolescents. Sleep Med. 2022;100:494–500. doi: 10.1016/j.sleep.2022.09.027 [DOI] [PubMed] [Google Scholar]
- 9. Díaz-Morales JF, Escribano C.. Social jetlag, academic achievement and cognitive performance: understanding gender/sex differences. Chronobiol Int. 2015;32(6):822–831. doi: 10.3109/07420528.2015.1041599 [DOI] [PubMed] [Google Scholar]
- 10. Henderson SEM, Brady EM, Robertson N.. Associations between social jetlag and mental health in young people: a systematic review. Chronobiol Int. 2019;36(10):1316–1333. doi: 10.1080/07420528.2019.1636813 [DOI] [PubMed] [Google Scholar]
- 11. Tamura N, Komada Y, Inoue Y, Tanaka H.. Social jetlag among Japanese adolescents: association with irritable mood, daytime sleepiness, fatigue, and poor academic performance. Chronobiol Int. 2022;39(3):311–322. doi: 10.1080/07420528.2021.1996388 [DOI] [PubMed] [Google Scholar]
- 12. Yang FN, Liu TT, Wang Z.. Corticostriatal connectivity mediates the reciprocal relationship between parent-reported sleep duration and impulsivity in early adolescents. J Child Psychol Psychiatry. 2023;n/a(n/a). doi: 10.1111/jcpp.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang FN, Liu TT, Wang Z.. Functional connectome mediates the association between sleep disturbance and mental health in preadolescence: a longitudinal mediation study. Hum Brain Mapp. 2022;43(6):2041–2050. doi: 10.1002/hbm.25772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang FN, Xie W, Wang Z.. Effects of sleep duration on neurocognitive development in early adolescents in the USA: a propensity score matched, longitudinal, observational study. Lancet Child Adolesc Health. 2022;10(6):705–712. doi: 10.1016/S2352-4642(22)00188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jankowski KS. Social jet lag: sleep-corrected formula. Chronobiol Int. 2017;34(4):531–535. doi: 10.1080/07420528.2017.1299162 [DOI] [PubMed] [Google Scholar]
- 16. Dayyat EA, Spruyt K, Molfese DL, Gozal D.. Sleep estimates in children: parental versus actigraphic assessments. Nat Sci Sleep. 2011;3:115–123. doi: 10.2147/NSS.S25676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perpétuo C, Fernandes M, Veríssimo M.. Comparison between actigraphy records and parental reports of child’s sleep. Front Pediatr. 2020;8:567390. doi: 10.3389/fped.2020.567390. https://www.frontiersin.org/articles/10.3389/fped.2020.567390. Accessed May 23, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jia Y, Tian Y, Wang H, Lei X.. Functional connectivity from dorsolateral prefrontal cortex mediates the impact of social jetlag on depressive tendency in young adults. Chronobiol Int. 2023;40(0):824–833. doi: 10.1080/07420528.2023.2212755 [DOI] [PubMed] [Google Scholar]
- 19. Nechifor RE, Ciobanu D, Vonica CL, et al. Social jetlag and sleep deprivation are associated with altered activity in the reward-related brain areas: an exploratory resting-state fMRI study. Sleep Med. 2020;72:12–19. doi: 10.1016/j.sleep.2020.03.018 [DOI] [PubMed] [Google Scholar]
- 20. Nechifor RE, Popita C, Bala C, et al. Regional homogeneity and degree of centrality in social jetlag and sleep deprivation and their correlations with appetite: a resting-state fMRI study. Biol Rhythm Res. 2022;53(6):966–986. doi: 10.1080/09291016.2020.1854991 [DOI] [Google Scholar]
- 21. Casey BJ, Cannonier T, Conley MI, et al.; ABCD Imaging Acquisition Workgroup. The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang S, Wang H, Deng X, Lei X.. Validation of the Munich Chronotype Questionnaire (MCTQ) in Chinese college freshmen based on questionnaires and actigraphy. Chronobiol Int. 2023;40(5):661–672. doi: 10.1080/07420528.2023.2202246 [DOI] [PubMed] [Google Scholar]
- 23. Aili K, Åström-Paulsson S, Stoetzer U, Svartengren M, Hillert L.. Reliability of actigraphy and subjective sleep measurements in adults: the design of sleep assessments. J Clin Sleep Med. 2017;13(1):39–47. doi: 10.5664/jcsm.6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giddens NT, Juneau P, Manza P, Wiers CE, Volkow ND.. Disparities in sleep duration among American children: effects of race and ethnicity, income, age, and sex. Proc Natl Acad Sci. 2022;119(30):e2120009119. doi: 10.1073/pnas.2120009119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barch DM, Albaugh MD, Avenevoli S, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Dev Cogn Neurosci. 2018;32:55–66. doi: 10.1016/j.dcn.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hagler DJ, Hatton SN, Cornejo MD, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202:116091. doi: 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE.. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26(1):288–303. doi: 10.1093/cercor/bhu239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herting MM, Uban KA, Gonzalez MR, et al. Correspondence between perceived pubertal development and hormone levels in 9-10 year-olds from the adolescent brain cognitive development study. Front Endocrinol. 2020;11:549928. doi: 10.3389/fendo.2020.549928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan RCK, Xie W, Geng F, et al. Clinical utility and lifespan profiling of neurological soft signs in schizophrenia spectrum disorders. Schizophr Bull. 2016;42(3):560–570. doi: 10.1093/schbul/sbv196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Owens MM, Potter A, Hyatt CS, et al. Recalibrating expectations about effect size: a multi-method survey of effect sizes in the ABCD study. PLoS One. 2021;16(9):e0257535. doi: 10.1371/journal.pone.0257535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vandekar SN, Stephens J.. Improving the replicability of neuroimaging findings by thresholding effect sizes instead of p-values. Hum Brain Mapp. 2021;42(8):2393–2398. doi: 10.1002/hbm.25374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gratton C, Sun H, Petersen SE.. Control networks and hubs. Psychophysiology. 2018;55(3):1. doi: 10.1111/psyp.13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sestieri C, Corbetta M, Spadone S, Romani GL, Shulman GL.. Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. J Cogn Neurosci. 2014;26(3):551–568. doi: 10.1162/jocn_a_00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee JK, Johnson EG, Ghetti S.. Hippocampal development: structure, function and implications. In: Hannula DE, Duff MC, eds. The Hippocampus from Cells to Systems: Structure, Connectivity, and Functional Contributions to Memory and Flexible Cognition. Switzerland: Springer International Publishing; 2017:141–166. doi: 10.1007/978-3-319-50406-3_6 [DOI] [Google Scholar]
- 35. de Zambotti M, Baker FC, Willoughby AR, et al. Measures of sleep and cardiac functioning during sleep using a multi-sensory commercially–available wristband in adolescents. Physiol Behav. 2016;158:143–149. doi: 10.1016/j.physbeh.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dewald-Kaufmann JF, Oort FJ, Meijer AM.. The effects of sleep extension on sleep and cognitive performance in adolescents with chronic sleep reduction: an experimental study. Sleep Med. 2013;14(6):510–517. doi: 10.1016/j.sleep.2013.01.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ABCD data used in this study are available in the National Institutes of Mental Health Data Archive (https://nda.nih.gov/abcd/). Information about the ABCD consortium is available on their website (https://abcdstudy.org/principal-investigators/).


