Abstract
Currently, the soft-tissue adhesives used in clinical practice are glue-type organic adhesives. However, there is a demand for new types of adhesives, because the current organic adhesives present challenges in terms of their biocompatibility and adhesion strength. This review summarizes the discovery and development of inorganic and metallic adhesives designed for soft biological tissues while focusing on immobilization of medical divices on soft tissues. These new types of adhesives are in a solid state and adhere directly and immediately to soft tissues. Therefore, they are called “solid-state adhesives” to distinguish them from the currently used glue-type adhesives. In previous studies on inorganic solid-state adhesives, oxides and calcium phosphates were used as raw materials in the form of nanoparticles, nanoparticle-coated films, or nanoparticle-assembled porous plates. In previous studies on metallic solid-state adhesives, only Ti and its alloys were used as raw materials. This review also discusses the future perspectives in this active research area.
Keywords: Soft-tissue adhesive, Solid-state adhesion, Oxide, Calcium phosphate, Titanium
1. Introduction
In modern craniomaxillofacial surgery, medical devices that interface with non-keratinized soft tissues (e.g., facial contouring and prosthetic temporomandibular joint surgery, subperiosteal oral rehabilitation, and reconstructive cranial plating) are being increasingly utilized. Close attachment of non-keratinized soft tissues to the device surface is an optimal condition for preventing complications such as infections [1]. Moreover, the global increase in the aging population has led to increased demand for medical devices implanted in soft tissues, such as artificial heart pacemakers [2], deep brain stimulation devices [3], and spinal cord stimulation devices [4]. These devices should be tightly immobilized on target tissues for long durations, because common hardware-related complications, including the migration of electrode leads [2], [3], [4], [5] and pulse generators [6], [7], [8], can occur. Although suturing techniques are used to immobilize these devices, the suturing process is time consuming and highly dependent on the physician’s skill. Moreover, this process often results in secondary tissue damage, surgical site infection, and fluid leakage [9].
Soft tissue adhesives are promising alternatives to sutures for achieving a close attachment and tight immobilization of devices on target tissues within the body, and they have been used as alternatives to sutures for wound closure since 1960 [10]. Currently, three main glue-type adhesives are clinically used: cyanoacrylate [11], gelatin–resorcinol–formaldehyde/glutaraldehyde (GRFG) [12], and fibrin [13]. However, these tissue adhesives present challenges in terms of biocompatibility (for cyanoacrylate and GRFG adhesives) and adhesion strength (for fibrin adhesives) [10]. Notably, the lap shear adhesion strengths of commercially available fibrin glues are 2.2 kPa for Tissucol® on porcine skin and 18 kPa for Beriplast® on mouse dermis [14]. The adhesion strength of the GRFG glue (170 kPa), which involves the crosslinking of the tissue around the glue under dry conditions, is comparable to that of the cyanoacrylate glue. However, its adhesion strength decreases to 48 kPa under wet conditions [15]. Recently, unique adhesives with better adhesion strengths were developed using organic compounds, including hydrophobically modified gelatin [16], dissipative hydrogels coupled with bridging polymers [17], and mussel-inspired adhesives containing dihydroxyphenyl groups [15]. Readers interested in organic-based soft-tissue adhesives are advised to refer to recent reviews [18], [19]. However, organic adhesives have limitations related to mechanical weakness, time-intensive or heat-generating setting reactions, and toxicity owing to the presence of monomers, crosslinkers, or degradation products [18], [19]. Therefore, a new type of adhesive is required for soft biological tissues.
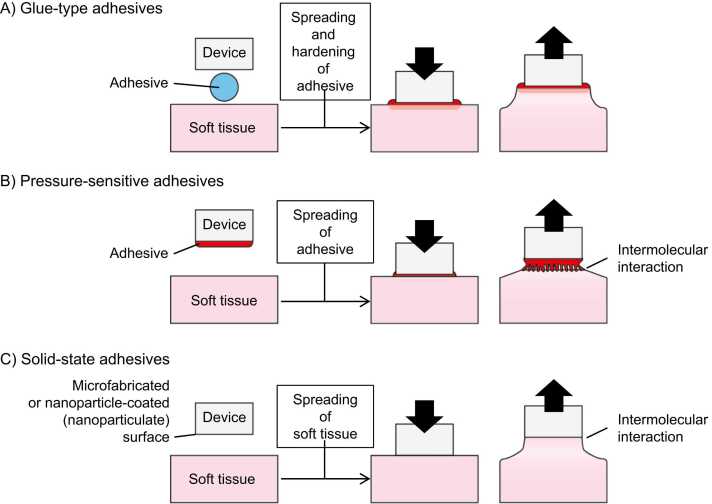
Soft-tissue adhesives can be classified into three types: (1) glue-type, (2) pressure-sensitive, and (3) solid-state adhesives, as shown in Fig. 1. Among them, glue-type and pressure-sensitive adhesives require setting/hardening abilities and viscoelastic properties, respectively; hence, their raw materials are limited to organic compounds. On the other hand, solid-state adhesives (such as gecko-inspired adhesives [20]) utilize intermolecular interactions at the interface between the adhesive and adherend; hence, various raw materials, including inorganic compounds and metals, can be used in solid-state adhesives. However, inorganic and metallic solid-state adhesives for soft tissues were not reported until 2014. These new adhesives have attracted attention of late, because they can overcome the drawbacks of the organic adhesives.
Fig. 1.
General concept and classification of soft-tissue adhesives for immobilizing devices.
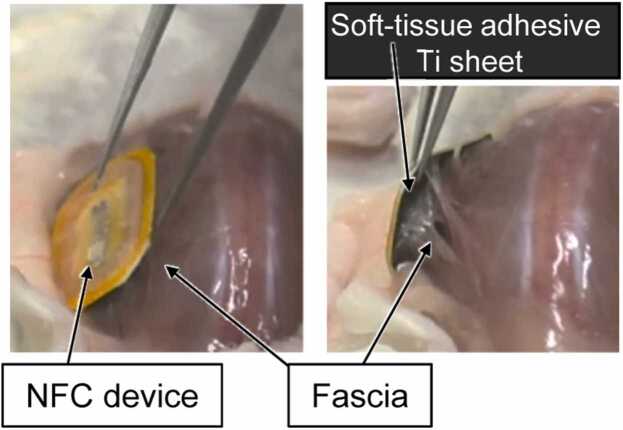
This review summarizes the recent progress in the use of inorganic and metallic adhesives for adhering medical devices to soft biological tissues. Medical devices immobilized on soft tissues are composed of biocompatible inorganic and metallic materials. Therefore, it is ideal to fabricate a solid-state adhesive using the biocompatible inorganic or metallic materials for developing a new type of self-adhesive device (Fig. 2) [21] that can be easily and securely fixed directly on the soft tissue.
Fig. 2.
Example of a self-adhesive device for soft biological tissues. A soft-tissue adhesive Ti sheet was attached to the bottom of a near-field communication (NFC) device, and the device was immobilized on the fascia at the back of a mouse. ©2021 Elsevier B.V. All rights reserved [21].
2. Inorganic solid-state adhesives
2.1. Oxides
In 2014 [22], Leibler and coworkers made the first demonstration of rapid adhesion between two synthetic poly(dimethylacrylamide) (PDMA) hydrogel specimens by spreading a silica (SiO2) nanoparticle suspension between them. They also demonstrated ex vivo adhesion of biological tissues by spreading a suspension of SiO2 nanoparticles between two pieces of a calf liver. In another study, Leibler and coworkers accomplished in vivo wound closure using SiO2 and Fe2O3 nanoparticulate adhesives [23]. The authors suggested that the adhesiveness of SiO2 nanoparticles depends on their ability to adsorb to polymer gels and thus act as connectors between the polymer chains and also on the ability of the polymer chains to reorganize and dissipate energy under stress when adsorbed to nanoparticles [22]. Notably, the ex vivo lap shear adhesion strength (300 mN/18 × 20 mm2 = 0.8 kPa) of SiO2 nanoparticles used to adhere two liver pieces was similar to that observed using fibrin glue, which is recognized to have insufficient adhesion strength, as described in the Introduction. In 2017 [24], Liu et al. re-evaluated these results and concluded that SiO2 nanoparticles were not essential for tissue adhesion; instead, the inorganic base (NaOH or KOH) added to stabilize the SiO2 nanoparticle dispersion played a crucial role in adhesion.
In 2017 [25], another research group compared the ex vivo lap shear adhesion strengths of SiO2, TiO2, and ZnO nanoparticle adhesives on liver tissues and reported that ZnO (adhesion strength of 1 kPa) provides a higher adhesion strength than SiO2 or TiO2 (adhesion strength of ∼0.2 kPa). Additionally, they realized in vivo wound closure using these nanoparticulate adhesives and claimed that ZnO nanoparticles facilitated successful wound closure and aesthetic wound healing, suggesting their potential as effective antimicrobial tissue adhesives. TaOx core/SiO2 shell nanoparticles [26] and silver- [27] and ceria-decorated [28] mesoporous SiO2 nanoparticles were also examined for in vivo wound closure, and the adhesion strength of these nanoparticulate adhesives was comparable to that of the fibrin glue.
In 2018 [29], Molinari and Angioletti-Uberti used coarse-grained modeling and molecular dynamics simulations to report that the properties of nanoparticles (such as the nanoparticle size and interaction force between the nanoparticles and hydrogel) and nanoparticle organization at the interface affect the adhesion strength. Based on their simulation results, Baik et al. claimed in 2022 [30] that when two hydrogels are adhered using a high-concentration nanoparticulate adhesive, resulting in the accumulation of a thick multilayer of nanoparticles between them, they can be easily separated via the propagation of a crack formed in the nanoparticle layer (i.e., owing to cohesive failure of the adhesive) because of weak interparticle forces. Consequently, the adhesion strength should be enhanced by increasing interparticle interactions in the coating material. Baik et al. achieved this by fabricating micron-sized spherical aggregates of nanoparticles, which led to an approximately 200 % increase in the adhesion energy compared with that of non-aggregated nanoparticles [30]. Another way to improve the adhesion strength (i.e., to avoid the cohesive failure of the adhesive layer) is to use monolayered nanoparticle coatings. Michel et al. reported hydrogel–nanoparticle–tissue adhesion by attaching a SiO2-nanoparticle-coated poly(ethylene glycol) hydrogel to porcine liver in 2020 [31]. Therefore, it is expected that a self-adhesive device can be fabricated by developing a monolayered nanoparticle coating on the targeted device.
2.2. Calcium phosphate
Hydroxyapatite (HAp) is a type of calcium orthophosphate. Synthetic HAp ceramics prepared in dense, porous, and granular forms have been used in the dental and medical fields for alveolar ridge reconstruction and augmentation, as fillers for bone defects, and in middle ear implants [32]. This is because HAp is bioactive (i.e., osteoconductive) in hard tissues and has the ability to encourage bone growth along its surface when placed in the vicinity of viable bone or differentiated bone-forming cells [33].
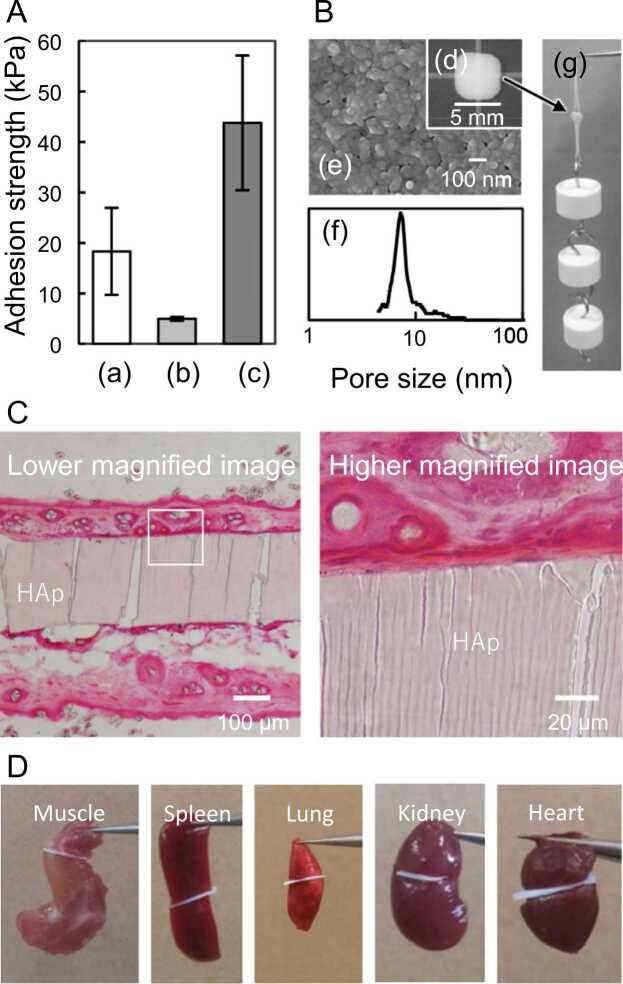
Inspired by a previous study [22] that used SiO2 nanoparticles, in 2017 [34], Okada et al. evaluated the use of HAp nanoparticles as soft-tissue adhesives and found that the adhesion strength of the HAp nanoparticulate adhesive was comparable to that of the SiO2 nanoparticulate adhesive but weaker than that of fibrin glue (Fig. 3A). The observation of the fractured interface of the HAp nanoparticle/hydrogel after lap shear adhesion tests revealed that the cohesive failure of the adhesive (i.e., the fracture of the deposited nanoparticle layer formed by water absorption from the aqueous nanoparticle suspension into the hydrogel adherends) was the primary cause of the weak adhesion strength. To prevent the cohesive failure of the adhesive, a plate-shaped specimen with interconnected pores was fabricated by assembling the HAp nanoparticles (Fig. 3B) and partially sintering the nanoparticle aggregates [35], [36]. The adhesion strength (approximately 44 kPa) of the HAp-nanoparticle-assembled plate was significantly higher than that of the nanoparticle suspension and more than twice that of the fibrin glue (Fig. 3A). The HAp nanoparticle-assembled plates, with continuous 8–30 nm pores and ∼50 vol% porosity, adhered immediately (i.e., within several seconds) to certain soft tissues when contacted (Fig. 3D). The instantaneous adhesion of solid-state adhesives is an excellent feature that is not observed with conventional glue-type adhesives, which are mainly cured via chemical reactions.
Fig. 3.
(A) Lap shear adhesion strengths of mouse dermis attached with (a) commercially available fibrin glue, (b) HAp nanoparticle dispersion, and (c) HAp-nanoparticle-assembled plates. (B) Appearance and structure of HAp-nanoparticle-assembled plates: (d) digital photograph; (e) SEM image; (f) pore-size distribution; and (g) adhesion of two pieces of mouse dermis. (C) Histological sections of two pieces of mouse dermis attached with an HAp-nanoparticle-assembled plate. A condensed layer of tissue could be observed on the surface of plate. (D) HAp-nanoparticle-assembled plate could adhere to two pieces of certain mouse organs. ©2017 Elsevier B.V. All rights reserved [34].
Notably, complete sintering of the HAp nanoparticle aggregates to eliminate pores resulted in their inability to adhere to soft tissues. Therefore, capillary action (i.e., water migration from tissues into porous HAp) was suggested to be a key factor in the immediate adhesion of solid-state porous adhesives. First, capillary action enhanced the close macroscale contact between the tissue and solid-state adhesive owing to the suction of the tissues. Second, the number of molecular-scale interactions between the solid-state adhesive and the matrix organic molecules (such as collagen) of the tissue adherend increased with increasing concentration (i.e., condensation) of the matrix organics (Fig. 3C). As for molecular-scale interactions with HAp, the triple helix of collagen exposes the carbonyl groups on the surface, which can interact electrostatically with the calcium ions on the HAp surface [37]. Third, dehydration changes the viscosity, which is recognized as a critical factor in the tack (i.e., immediate adhesion) and shear/peel resistance of general pressure-sensitive adhesives [38], of the tissues. Of note, low viscosity is suitable for the tack, whereas high viscosity is necessary for large shear/peel resistance. Tissue dehydration can also cause salting-out-induced collagen aggregation [39] or the disruption of the triple helices (depending on the amino acid sequence [40]) of the collagen fibrils. Because the exposed hydrophobic moieties contribute to hydrophobic interactions with HAp adsorbed with lipids [41], the adhesion strength can be further enhanced by the surface modification of HAp with amphiphilic molecules [42] or low-energy electron irradiation [43].
In addition to HAp, a porous octacalcium phosphate (OCP) disk was developed by the dissolution–precipitation reaction of calcium hydrogen phosphate in a Na2HPO4 solution [44]. OCP is also biocompatible and can be used as a bone filler for replacing natural bone via metabolic processes [45]. The porous OCP disks also adhere immediately to certain soft tissues, and antibacterial agents can be incorporated in the pores of the OCP blocks.
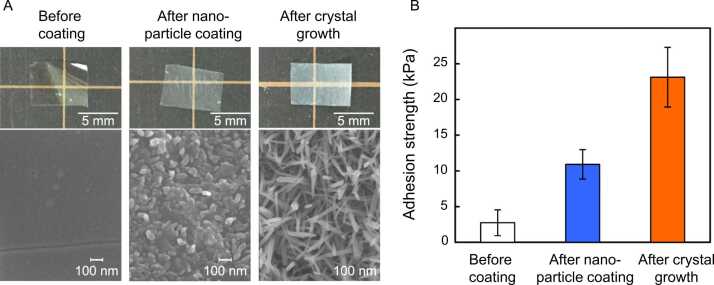
As mentioned previously, another way to improve the adhesion strength (i.e., to avoid the cohesive failure of the adhesive layer) of particulate adhesives is by forming a monolayered particle coating. In 2022 [46], Palierse et al. conducted a study in which nanostructured HAp–bioactive glass (BG) microparticles were coated onto a synthetic hydrogel crosslinked with poly(beta-thioester). Through ex vivo peeling experiments on porcine liver specimens, they demonstrated that HAp–BG coatings exhibit a two-fold increase in adhesion energy compared with that of the uncoated hydrogel. They also reported that HAp–BG coatings gradually degraded in physiological fluids, with nearly complete dissolution of the particles occurring within 21 d. Okada et al. fabricated an HAp nanoparticle-coated poly-L-lactide (PLLA) film (Fig. 4) [47]. The adhesion strength of the PLLA film on the synthetic hydrogels was improved by nanoparticle coating and further improved using the low-temperature crystal growth technique of HAp [48] owing to the enlarged surface area.Fig. 5.
Fig. 4.
(A) Digital photographs and SEM profiles of PLLA films before and after HAp nanoparticle coating followed by the crystal growth of HAp. (B) Lap shear adhesion strengths of synthetic hydrogels attached with PLLA films [47].
Fig. 5.
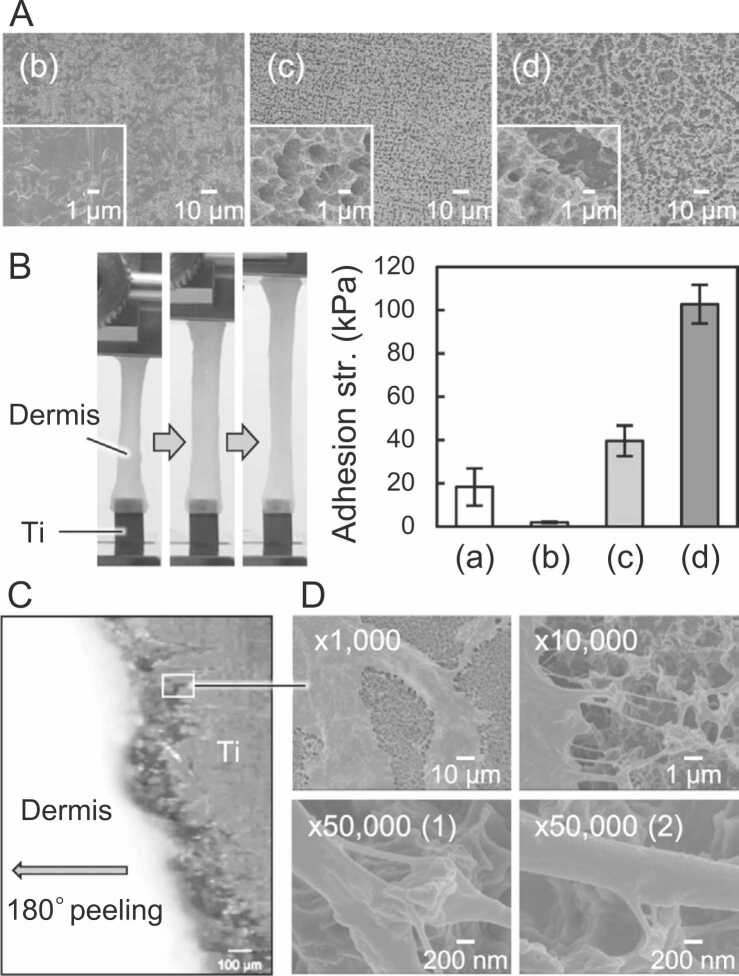
(A) SEM images and (B) lap shear adhesion test results obtained using mouse dermis [54]. (C) Optical micrograph during 180° peeling of mouse dermis attached to acid-treated CpTi, and (D) SEM images of CpTi after the adhesion test [21]. Collagen fibers retained on the submicron-sized irregularities formed on the CpTi surface after the acid treatment, indicating the adherend failure of dermal tissues. Lowercase alphabets indicate the adhesives: (a) commercially available fibrin glue, (b) non-treated CpTi, (c) acid-treated CpTi, and (d) acid-treated CpTi after sandblast pretreatment. ©2020 M. Okada et al. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim [55]. ©2021 Elsevier B.V. All rights reserved [21].
3. Metallic solid-state adhesives
Titanium (Ti), a metallic biomaterial, has better mechanical properties than polymer or ceramic biomaterials and greater biocompatibility than other metals [49]. Owing to these characteristics, Ti has been used as a substitute for hard tissues in orthopedics and dentistry. Furthermore, the outer casings of electrical stimulation therapy devices (e.g., artificial pacemakers, deep brain stimulation devices, and spinal cord stimulation devices) implanted in soft tissues are usually made of Ti. The common hardware-related complications of these devices include their migration, as mentioned in the Introduction.
Several Ti surface modification methods [50], [51] have been developed to promote osseointegration (i.e., bonding to hard tissues by increasing the interlocking capacity of the surface [52], [53]) of bone-anchored implants. Acid treatment is one such surface modification method for roughening the Ti implant surface, and it has been demonstrated to be an effective way to promote osseointegration [54]. Notably, osseointegration occurs over a long duration (i.e., a few months) because of complex processes, including protein adsorption, cell adhesion, cell differentiation, and mineral precipitation on the Ti implant surface. The application of Ti as a hard-tissue-anchored implant has been explored extensively; however, its application as a soft-tissue adhesive remains unexplored. In fact, unmodified Ti shows no immediate adhesion to biological soft tissues (Fig. 4).
In 2020 [55], Okada et al. reported that grade 1 commercially pure Ti (CpTi) films prepared by acid treatment with HCl/H2SO4 at 70 °C followed by air drying showed remarkable soft-tissue adhesiveness (i.e., 54 kPa on mouse dermis) and immediate attachment (i.e., within a few seconds) to some soft tissues. They also claimed that a simple autoclaving process at 121 °C, which usually denatures most organic polymer biomaterials, could be used to sterilize the CpTi films with no effect on the adhesion properties. This was demonstrated through ex vivo adhesion tests using mouse dermal tissues and in vivo implantation tests using mouse subcutaneous tissues. Acid treatment resulted in a submicron-scale roughness of the CpTi surface, and the dried surface exhibited hydrophobic characteristics. Further, sandblast pretreatment before acid treatment was effective in increasing the surface roughness, leading to immediate soft-tissue adhesion strength [21]. Notably, the maximum adhesion strength (102 kPa) of sandblast/acid-treated CpTi was significantly higher than that of the commercially available fibrin glue (e.g., 18 kPa [34]) or GRFG glue (e.g., 48 kPa under wet conditions [15]), as shown in Fig. 4A and 4B. After adhesion tests, fibrous tissues, such as collagen fiber bundles and split collagen fibers, remained attached to the CpTi film surface, indicating the failure of the adhered dermal tissues (Fig. 4C and 4D). The acid treatment of the CpTi film led to the formation of Ti hydrides, δ-TiHx, on the surface via a reaction between Ti and H2 generated during acid etching (Ti + x H+ → Tix+ + x/2 H2). Importantly, the amount of Ti hydride formed was directly proportional to the ex vivo adhesion strength of CpTi on mouse dermal tissues. Commercial Ti dental implants treated with a mixed H2SO4/HCl solution also contain Ti hydrides on their surfaces [56] and have been reported to exhibit excellent biocompatibility with hard tissue [57], [58]. For example, acid-treated CpTi (grade 2) implants were better encapsulated in thinner connective tissues than polished CpTi implants during both early (1–5 weeks) and late (6–11 weeks) stages of subcutaneous implantation in rats [59]. Therefore, after immediate adhesion to soft tissues, acid-treated Ti surfaces are expected to be encapsulated and fixed to connective tissues over the long term.
To establish the relationship between Ti hydride formation and the immediate soft-tissue adhesiveness of Ti, Okada et al. subjected a Ti-6Al-4V alloy to the same conditions as CpTi. Pure Ti has a hexagonal close-packed (hcp) structure, referred to as α phase, at room temperature. The phase transformation from the hcp structure to a body-centered cubic (bcc) one, referred to as the β phase, occurs at 883 °C [57]. Previous investigations have revealed that the phase transformation temperature of Ti is strongly influenced by alloying elements [57]. CpTi is an α-type Ti, consisting of only α phase, with 0.20–0.50 wt% of Fe and 0.18–0.40 wt% of O [60], and Ti-6Al-4V is the most widely used (α + β)-type Ti alloy, accounting for 50 % of the total Ti production [61], and has higher strength than the α-type Ti. Because the (α + β)-type Ti alloy has a much higher hydrogen solubility [62] and diffusivity [62], [63] than α-type Ti, the effect of acid treatment on the immediate adhesiveness of the Ti-6Al-4V alloy differs from that of CpTi. It was revealed that the acid treatment of (α + β)-type Ti-6Al-4V alloys could also result in the formation of Ti hydrides and promote immediate soft-tissue adhesion [64]. However, the adhesion strength of acid-treated Ti-6Al-4V (i.e., ∼20 kPa after 20 min treatment) was significantly lower than that of CpTi (i.e., ∼54 kPa for 20 min treatment). The main cause of the decreased soft-tissue adhesiveness of the acid-treated Ti-6Al-4V is related to the decrease in both the amount of Ti hydrides formed and the hydrogen content of the Ti hydride. In general, the surface of Ti exposed to air is spontaneously oxidized to a oxide layer [65]. However, Ti hydride is impervious to oxygen [66], and the thickness of the oxidized layer decreases with increasing hydrogen content during air exposure [67]. Oxidized metal surfaces are generally hydrophilic, whereas hydride surfaces are hydrophobic. The water contact angles (73–85°) of acid-treated Ti-6Al-4V were lower than those of more hydrophobic acid-treated CpTi (91–100°). These results indicate that the immediate adhesion of acid-treated CpTi can be attributed to its greater hydrophobic interaction with the hydrophobic components of the extracellular matrix (ECM). Notably, compared with hydrophilic surfaces, hydrophobic surfaces generally adsorb greater amounts of proteins and have a faster protein adsorption rate [68]. Furthermore, proteins adhere more strongly to hydrophobic surfaces than to hydrophilic surfaces [69]. A previous in silico study on silicone (Si) revealed that the hydrophobic side chain groups of collagen XIV exhibited more stable interactions on H-terminated Si surfaces than on oxidized Si surfaces [70].
4. Summary and future perspectives
This article summarized the progress made in developing inorganic and metallic solid-state adhesives that adhere immediately to soft biological tissues upon contact.
Certain oxide and calcium phosphate nanoparticles were reported as raw materials for inorganic adhesives. However, the adhesion strengths of nanoparticulate adhesives were insufficient. Therefore, particle-coated films and plates assembled from nanoparticles were developed to prevent cohesive failure owing to weak interactions between the nanoparticles in nanoparticulate adhesives. The adhesion strength of these adhesives can be improved by optimizing the physicochemical characteristics of the inorganic materials, such as their surface functional groups and roughness. In the case of glue-type organic adhesives, high adhesion strength can be achieved by infiltrating the adherent tissues with organic precursor molecules, followed by hardening or crosslinking of the precursor molecules with the tissue matrix [19]. Similarly, the adhesive strength of inorganic solid-state adhesives can be improved further by releasing ions or molecules that strengthen the tissue matrix.
In the case of metallic solid-state adhesives, only titanium and its alloys have been used as raw materials. As the immediate adhesion of acid-treated Ti-based adhesives to soft biological tissues occurs owing to hydrophobic interactions attributable to Ti hydrates formed on their surfaces, self-adhering implantable devices made of other materials can be developed using Ti hydrate coatings [71]. With the recent advancement in the Internet of Things (IoT), nonmedical implantable devices such as biochips and biosensors have received significant attention [72], [73], [74]. For example, injectable radio frequency identification (RFID) tags [74] are widely used as biochips to manage livestock [75], domesticated or laboratory animals, and food supply chain products. Biosensors have been developed to monitor biorhythms [73] and as drug-release systems for treating cancers and endocrine diseases [74], including diabetes [76]. Implantable devices are expected to become prominent tools for electronic identification, internal body monitoring, and disease prevention and treatment in the near future. Nevertheless, after decades of market experience with animals, the intrabody migration of the current injectable RFID tags has been identified as a key issue [77], [78]. Intrabody migration of other implantable devices is also a major problem that hinders their further application. The development of self-adhering implantable devices incorporated with solid-state adhesives could solve intrabody migration problems.
To consider the possibility of the research-to-clinical translation of solid-state adhesives and self-adhering devices, it is necessary to estimate the actual force or stress at the interface between the target tissue and adhesives or devices. For example, when sandblasting and acid treatment were applied to the pulse generator of an artificial pacemaker, whose outer casing is typically made of Ti and has an approximate size of 5 cmϕ, the shear load capacity was calculated to be 41 kgf based on the ex vivo shear adhesion strength (102 kPa); the shear load capacity should be sufficiently larger than the actual force at the interface between the target tissue and adhesives or devices. Furthermore, because of the significant influence of the hardness and micro-nanostructure of materials on the fate of cells [79], long-term in vivo evaluation is necessary before clinical translation.
The next-generation tissue adhesives are expected to exhibit better adhesive properties in various tissue settings and incorporate a multitude of functionalities that enable them to serve as versatile therapeutic agents [19]. On-demand degradation or detachment from the tissues is another favorable function. Current strategies for degrading organic adhesives rely mostly on the incorporation of degradable polymers into adhesives [19], and the same strategy can be used for inorganic solid-state adhesives by incorporating inorganic components with high solubility. By changing the hydration structure, the hydrophobic interaction can be weakened (e.g., by lowering the salt concentration or adding chaotropic ions [80], [81]), enabling the development of on-demand detachable solid-state adhesives by controlling the hydration structures of the adhesive surfaces and matrix proteins.
Declaration of Competing Interest
None.
Acknowledgements
This work was partially supported by the Japan Society for the Promotion of Science, KAKENHI (Grant Nos. JP21K18828 and JP21H0312) and the Japan Science and Technology Agency CREST (Grant No. JPMJCR22L5).
References
- 1.Zigterman B.G.R., Van den Borre C., Braem A., Mommaerts M.Y. Titanium surface modifications and their soft-tissue interface on nonkeratinized soft tissues—a systematic review (Review) Biointerphases. 2019;14 doi: 10.1116/1.5113607. [DOI] [PubMed] [Google Scholar]
- 2.Bennett M.T., Tung S.K.K. Long-term efficacy of cardiac pacemakers and implantable cardioverter/defibrillators. J Long Term Eff Med Implants. 2010;20:187–202. doi: 10.1615/jlongtermeffmedimplants.v20.i3.30. [DOI] [PubMed] [Google Scholar]
- 3.Morishita T., Hilliard J.D., Okun M.S., Neal D., Nestor K.A., Peace D., et al. Postoperative lead migration in deep brain stimulation surgery: incidence, risk factors, and clinical impact. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eldabe S., Buchser E., Duarte R.V. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med (United States) 2016;17:325–336. doi: 10.1093/pm/pnv025. [DOI] [PubMed] [Google Scholar]
- 5.Bowman R.G., Caraway D., Bentley I. Comparison of a novel fixation device with standard suturing methods for spinal cord stimulators. Neuromodulation. 2013;16:454–458. doi: 10.1111/j.1525-1403.2012.00480.x. [DOI] [PubMed] [Google Scholar]
- 6.Koulouris S., Pastromas S., Manolis A.S. An unusual distal abdominal migration of a pacemaker pulse generator with a complete epicardial lead fracture. Europace. 2008;10:1461. doi: 10.1093/europace/eun281. [DOI] [PubMed] [Google Scholar]
- 7.Ward J.L., Defrancesco T.C., Tou S.P., Atkins C.E., Griffith E.H., Keene B.W. Complication rates associated with transvenous pacemaker implantation in dogs with high-grade atrioventricular block performed during versus after normal business hours. J Vet Intern Med. 2015;29:157–163. doi: 10.1111/jvim.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russi I., Liechti R., Memeti E., Bertschy S., Weberndoerfer V., Kobza R. Intracolonic cardiac pacemaker: a case of device migration with colon perforation out of a subcutaneous epifascial pocket. Heart Case Rep. 2018;4:497–500. doi: 10.1016/j.hrcr.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauto A., Mawad D., Foster L.J.R. Adhesive biomaterials for tissue reconstruction. J Chem Technol Biotechnol. 2008;83:464–472. doi: 10.1002/jctb.1771. [DOI] [Google Scholar]
- 10.Matsuda M., Inoue M., Taguchi T. Adhesive properties and biocompatibility of tissue adhesives composed of various hydrophobically modified gelatins and disuccinimidyl tartrate. J Bioact Compat Polym. 2012;27:481–498. doi: 10.1177/0883911512455116. [DOI] [Google Scholar]
- 11.Tseng Y.-C., Suong-Hyu H., Ikada Y., Shimizu Y., Tamura K., Hitomi S. In vivo evaluation of 2-cyanoacrylates as surgical adhesives. J Appl Biomater. 1990;1:111–119. doi: 10.1002/jab.770010203. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga S., Karck M., Harringer W., Cremer J., Rhein C., Haverich A. The use of gelatin-resorcin-formalin glue in acute aortic dissection type A. Eur J Cardio-Thorac Surg. 1999;15:564–570. doi: 10.1016/S1010-7940(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 13.Dare E.V., Griffith M., Poitras P., Wang T., Dervin G.F., Giulivi A., et al. Fibrin sealants from fresh or fresh/frozen plasma as scaffolds for in vitro articular cartilage regeneration. Tissue Eng Part A. 2009;15:2285–2297. doi: 10.1089/ten.tea.2008.0228. [DOI] [PubMed] [Google Scholar]
- 14.Kull S., Martinelli I., Briganti E., Losi P., Spiller D., Tonlorenzi S., et al. Glubran2 surgical glue: in vitro evaluation of adhesive and mechanical properties. J Surg Res. 2009;157:e15–e21. doi: 10.1016/j.jss.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Bhagat V., Becker M.L. Degradable adhesives for surgery and tissue engineering. Biomacromolecules. 2017;18:3009–3039. doi: 10.1021/acs.biomac.7b00969. [DOI] [PubMed] [Google Scholar]
- 16.Nishiguchi A., Kurihara Y., Taguchi T. Underwater-adhesive microparticle dressing composed of hydrophobically-modified Alaska pollock gelatin for gastrointestinal tract wound healing. Acta Biomater. 2019 doi: 10.1016/J.ACTBIO.2019.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Celiz A.D., Yang J., Yang Q., Wamala I., Whyte W., et al. Tough adhesives for diverse wet surfaces. Science. 2017;357:378–381. doi: 10.1126/science.aah6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bal-Ozturk A., Cecen B., Avci-adali M., Topkaya S.N., Alarcin E., Yasayan G., et al. Tissue adhesives: from research to clinical translation. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101049.Tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam S., Mooney D. Polymeric tissue adhesives. Chem Rev. 2021;121:11336–11384. doi: 10.1021/acs.chemrev.0c00798. [DOI] [PubMed] [Google Scholar]
- 20.Mahdavi A., Ferreira L., Sundback C., Nichol J.W., Chan E.P., Carter D.J.D., et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc Natl Acad Sci. 2008;105:2307–2312. doi: 10.1073/pnas.0712117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yabe A., Okada M., Hara E.S., Torii Y., Matsumoto T. Self-adhering implantable device of titanium: enhanced soft-tissue adhesion by sandblast pretreatment. Colloids Surf B Biointerfaces. 2022;211 doi: 10.1016/j.colsurfb.2021.112283. [DOI] [PubMed] [Google Scholar]
- 22.Rose S., Prevoteau A., Elzière P., Hourdet D., Marcellan A., Leibler L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature. 2014;505:382–385. doi: 10.1038/nature12806. [DOI] [PubMed] [Google Scholar]
- 23.Meddahi-Pellé A., Legrand A., Marcellan A., Louedec L., Letourneur D., Leibler L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew Chem - Int Ed. 2014;53:6369–6373. doi: 10.1002/anie.201401043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H., Peng Y., Yang C., Wang M. Silica nanoparticles as adhesives for biological tissues? Re-examining the effect of particles size, particle shape, and the unexpected role of base. Part Part Syst Charact. 2017;34:1–7. doi: 10.1002/ppsc.201700286. [DOI] [Google Scholar]
- 25.Gao Y., Han Y., Cui M., Tey H.L., Wang L., Xu C. ZnO nanoparticles as an antimicrobial tissue adhesive for skin wound closure. J Mater Chem B. 2017;5:4535–4541. doi: 10.1039/c7tb00664k. [DOI] [PubMed] [Google Scholar]
- 26.Shin K., Choi J.W., Ko G., Baik S., Kim D., Park O.K., et al. Multifunctional nanoparticles as a tissue adhesive and an injectable marker for image-guided procedures. Nat Commun. 2017;8(1):12. doi: 10.1038/ncomms15807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu M.M., Bai J., Shao D., Qiu J., Li M., Zheng X., et al. Antibacterial and biodegradable tissue nano-adhesives for rapid wound closure. Int J Nanomed. 2018;13:5849–5863. doi: 10.2147/IJN.S177109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H., Li F., Wang S., Lu J., Li J., Du Y., et al. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials. 2018;151:66–77. doi: 10.1016/j.biomaterials.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Molinari N., Angioletti-Uberti S. Nanoparticle organization controls their potency as universal glues for gels. Nano Lett. 2018;18:3530–3537. doi: 10.1021/acs.nanolett.8b00586. [DOI] [PubMed] [Google Scholar]
- 30.Baik J.S., Kim S.A., Jung D.W., Chae W.S., Pang C., Bhang S.H., et al. Colloidal supraballs of mesoporous silica nanoparticles as bioresorbable adhesives for hydrogels. Chem Mater. 2022;34:584–593. doi: 10.1021/acs.chemmater.1c03072. [DOI] [Google Scholar]
- 31.Michel R., Roquart M., Llusar E., Gaslain F., Norvez S., Baik J.S., et al. Hydrogel-tissue adhesion using blood coagulation induced by silica nanoparticle coatings. ACS Appl Bio Mater. 2020;3:8808–8819. doi: 10.1021/acsabm.0c01158. [DOI] [PubMed] [Google Scholar]
- 32.Okada M., Matsumoto T. Synthesis and modification of apatite nanoparticles for use in dental and medical applications. Jpn Dent Sci Rev. 2015;51:85–95. doi: 10.1016/j.jdsr.2015.03.004. [DOI] [Google Scholar]
- 33.Hench L., Best S. In: An Introduction to Materials in Medicine. 2nd Ed. Ratner B., Hoffman A., Schoen F., Lemons J., editors. Elsevier Academic Press; Amsterdam: 2004. Ceramics, Glasses, and Glass-Ceramics; pp. 153–169. [Google Scholar]
- 34.Okada M., Nakai A., Hara E.S., Taguchi T., Nakano T., Matsumoto T. Biocompatible nanostructured solid adhesives for biological soft tissues. Acta Biomater. 2017;57:404–413. doi: 10.1016/j.actbio.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Okada M., Fujiwara K., Uehira M., Matsumoto N., Takeda S. Expansion of nanosized pores in low-crystallinity nanoparticle-assembled plates via a thermally induced increase in solid-state density. J Colloid Interface Sci. 2013;405:58–63. doi: 10.1016/j.jcis.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Okada M., Furuzono T. Low-temperature synthesis of nanoparticle-assembled, transparent, and low-crystallized hydroxyapatite blocks. J Colloid Interface Sci. 2011;360:457–462. doi: 10.1016/j.jcis.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 37.Cutini M., Corno M., Costa D., Ugliengo P. How does collagen adsorb on hydroxyapatite? Insights from Ab initio simulations on a polyproline type II model. J Phys Chem C. 2019;123:7540–7550. doi: 10.1021/ACS.JPCC.7B10013/SUPPL_FILE/JP7B10013_SI_001.PDF. [DOI] [Google Scholar]
- 38.Paul C.W. Pressure-Sensitive Adhesives (PSAs) Handb Adhes Technol. 2011:341–372. doi: 10.1007/978-3-642-01169-6_15. [DOI] [Google Scholar]
- 39.Komsa-Penkova R., Koynova R., Kostov G., Tenchov B.G. Thermal stability of calf skin collagen type I in salt solutions. Biochim Biophys Acta (BBA) - Protein Struct Mol Enzymol. 1996;1297:171–181. doi: 10.1016/S0167-4838(96)00092-1. [DOI] [PubMed] [Google Scholar]
- 40.Naito A., Tuzi S., Saitô H. A high-resolution 15N solid-state NMR study of collagen and related polypeptides. Eur J Biochem. 1994;224:729–734. doi: 10.1111/J.1432-1033.1994.00729.X. [DOI] [PubMed] [Google Scholar]
- 41.Warabino K., Ueno S., Hino T., Shimabayashi S. Preparation and adsorption properties of hydroxyapatite surface-modified by cetylphosphate. Phosphorus Res Bull. 2006;20:129–134. doi: 10.3363/prb.20.129. [DOI] [Google Scholar]
- 42.Li Y., Weng W. Surface modification of hydroxyapatite by stearic acid: characterization and in vitro behaviors. J Mater Sci: Mater Med. 2007;19(1):19–25. doi: 10.1007/S10856-007-3123-5. [DOI] [PubMed] [Google Scholar]
- 43.Aronov D., Rosen R., Ron E.Z., Rosenman G. Tunable hydroxyapatite wettability: effect on adhesion of biological molecules. Process Biochem. 2006;41:2367–2372. doi: 10.1016/j.procbio.2006.06.006. [DOI] [Google Scholar]
- 44.Sugiura Y., Okada M., Hirano K., Matsumoto T. Bone mineral analogue ceramic block as an instant adhesive to biological soft tissue. Adv Mater Interfaces. 2021;8 doi: 10.1002/admi.202002032. [DOI] [Google Scholar]
- 45.Kamakura S., Sasano Y., Homma H., Suzuki O., Kagayama M., Motegi K. Implantation of octacalcium phosphate (OCP) in rat skull defects enhances bone repair. J Dent Res. 1999;78:1682–1687. doi: 10.1177/00220345990780110401. [DOI] [PubMed] [Google Scholar]
- 46.Palierse E., Roquart M., Norvez S., Corté L. Coatings of hydroxyapatite-bioactive glass microparticles for adhesion to biological tissues. RSC Adv. 2022;12:21079–21091. doi: 10.1039/d2ra02781j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada M., Matsumoto T. Development of biocompatible soft-tissue adhesives with nanostructured inorganic biomaterials. Bull Ceram Soc Jpn. 2020;55:180–184. [Google Scholar]
- 48.Okada M., Hara E.S., Matsumoto T. In: Fabrication of Hydroxyapatite Nanofibers with High Aspect Ratio via Low-Temperature Wet Precipitation Methods Under Acidic Conditions. Endo K., Kogure T., Nagasawa H., editors. Biomineralization–From Molecular and Nano-structural Analyses to Environmental Science; New York: 2018. pp. 211–218. [DOI] [Google Scholar]
- 49.Saini M., Singh Y., Arora P., Arora V., Jain K. Implant biomaterials: a comprehensive review. World J Clin Cases. 2015;3:52–57. doi: 10.12998/wjcc.v3.i1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmquist A., Omar O.M., Esposito M., Lausmaa J., Thomsen P. Titanium oral implants: surface characteristics, interface biology and clinical outcome. J R Soc Interface. 2010;7:S515–S527. doi: 10.1098/rsif.2010.0118.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen P., Aso T., Sasaki R., Ashida M., Tsutsumi Y., Doi H., et al. Adhesion and differentiation behaviors of mesenchymal stem cells on titanium with micrometer and nanometer‐scale grid patterns produced by femtosecond laser irradiation. J Biomed Mater Res A. 2018;106:2735–2743. doi: 10.1002/jbm.a.36503. [DOI] [PubMed] [Google Scholar]
- 52.Butz F., Ogawa T., Nishimura I. Interfacial shear strength of endosseous implants. Int J Oral Maxillofac Implants n.d.;26:746–751. [PubMed]
- 53.Spriano S., Yamaguchi S., Baino F., Ferraris S. A critical review of multifunctional titanium surfaces: new frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018;79:1–22. doi: 10.1016/J.ACTBIO.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 54.Claes L., Steinemann S.G. Metallic implant anchorable to bone tissue for replacing a broken or diseased bone, 1995.
- 55.Okada M., Hara E.S., Yabe A., Okada K., Shibata Y., Torii Y., et al. Titanium as an instant adhesive for biological soft tissue. Adv Mater Interfaces. 2020;7 doi: 10.1002/admi.201902089. [DOI] [Google Scholar]
- 56.Szmukler-Moncler S., Bischof M., Nedir R., Ermrich M. Titanium hydride and hydrogen concentration in acid-etched commercially pure titanium and titanium alloy implants: a comparative analysis of five implant systems. Clin Oral Implants Res. 2010;21:944–950. doi: 10.1111/j.1600-0501.2010.01938.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L., Chen L. A review on biomedical titanium alloys: recent progress and prospect. Adv Eng Mater. 2019;21 doi: 10.1002/adem.201801215. [DOI] [Google Scholar]
- 58.Wong M., Eulenberger J., Schenk R., Hunziker E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J Biomed Mater Res. 1995;29:1567–1575. doi: 10.1002/jbm.820291213. [DOI] [PubMed] [Google Scholar]
- 59.Kim H., Murakami H., Chehroudi B., Textor M., Brunette D.M. Effects of surface topography on the connective tissue attachment to subcutaneous implants. Int J Oral Maxillofac Implants. 2006;21:354–365. [PubMed] [Google Scholar]
- 60.Elias C.N., Fernandes D.J., Resende C.R.S., Roestel J. Mechanical properties, surface morphology and stability of a modified commercially pure high strength titanium alloy for dental implants. Dent Mater. 2015;31:e1–e13. doi: 10.1016/j.dental.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Sidambe A.T. Biocompatibility of advanced manufactured titanium implants-a review. Materials. 2014;7:8168–8188. doi: 10.3390/ma7128168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prando D., Brenna A., Diamanti M.V., Beretta S., Bolzoni F., Ormellese M., et al. Corrosion of titanium: Part 1: aggressive environments and main forms of degradation. J Appl Biomater Funct Mater. 2017;15:e291–e302. doi: 10.5301/jabfm.5000387. [DOI] [PubMed] [Google Scholar]
- 63.Nelson H.G. Environmental hydrogen embrittlement of an α-β titanium alloy: effect of hydrogen pressure. Metall Trans. 1973;4:364–367. doi: 10.1007/BF02649639. [DOI] [Google Scholar]
- 64.Wang Y., Okada M., Xie S.C., Jiao Y.Y., Hara E.S., Yanagimoto H., et al. Immediate soft-tissue adhesion and mechanical properties of Ti-6Al-4V alloy after long-term acid treatment. J Mater Chem B. 2021 doi: 10.1039/D1TB00919B. [DOI] [PubMed] [Google Scholar]
- 65.Hristova E., Arsov Lj, Popov B.N., White R.E. Ellipsometric and Raman spectroscopic study of thermally formed films on titanium. J Electrochem Soc. 1997;144:2318–2323. doi: 10.1149/1.1837811. [DOI] [Google Scholar]
- 66.Fang Z.Z., Paramore J.D., Sun P., Chandran K.S.R., Zhang Y., Xia Y., et al. Powder metallurgy of titanium-past, present, and future. Int Mater Rev. 2018;63:407–459. doi: 10.1080/09506608.2017.1366003. [DOI] [Google Scholar]
- 67.Zhang Y., Fang Z.Z., Xu L., Sun P., Van Devener B., Zheng S., et al. Mitigation of the surface oxidation of titanium by hydrogen. J Phys Chem C. 2018;122:20691–20700. doi: 10.1021/acs.jpcc.8b04684. [DOI] [Google Scholar]
- 68.Sigal G.B., Mrksich M., Whitesides G.M. Effect of surface wettability on the adhesion of proteins and detergents. J Am Chem Soc. 1998;120:3464–3473. [Google Scholar]
- 69.Sethuraman A., Han M., Kane R.S., Belfort G. Effect of surface wettability on the adhesion of proteins. Langmuir. 2004;20:7779–7788. doi: 10.1021/la049454q. [DOI] [PubMed] [Google Scholar]
- 70.Cole D.J., Payne M.C., Ciacchi L.C. Water structuring and collagen adsorption at hydrophilic and hydrophobic silicon surfaces. Phys Chem Chem Phys. 2009;11:11395–11399. doi: 10.1039/b816125a. [DOI] [PubMed] [Google Scholar]
- 71.Shimizu R., Sasahara Y., Oguchi H., Yamamoto K., Sugiyama I., Shiraki S., et al. Fabrication of atomically abrupt interfaces of single-phase TiH2 and Al2O3. APL Mater. 2017;5 doi: 10.1063/1.4996984. [DOI] [Google Scholar]
- 72.Smith C. Human microchip implantation introduction. J Technol Manag Innov. 2008;3:151–160. [Google Scholar]
- 73.Koydemir H.C., Ozcan A. Wearable and implantable sensors for biomedical applications. Annu Rev Anal Chem. 2018;11:127–146. doi: 10.1146/annurev-anchem-061417-125956. [DOI] [PubMed] [Google Scholar]
- 74.Gray M., Meehan J., Ward C., Langdon S.P., Kunkler I.H., Murray A., et al. Implantable biosensors and their contribution to the future of precision medicine. Vet J. 2018;239:21–29. doi: 10.1016/j.tvjl.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 75.Troyk P.R. Injectable electronic identification, monitoring, and stimulation systems. Annu Rev Biomed Eng. 1999;1:177–209. doi: 10.1146/annurev.bioeng.1.1.177. [DOI] [PubMed] [Google Scholar]
- 76.Scholten K., Meng E. A review of implantable biosensors for closed-loop glucose control and other drug delivery applications. Int J Pharm. 2018;544:319–334. doi: 10.1016/j.ijpharm.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 77.ISO 15639-1:2015, Radio frequency identification of animals -- Standardization of injection sites for different animal species -- Part 1: Companion animals (cats and dogs). n.d.
- 78.Mader D.R., Divers S.J., Norton T.M., Andrews K.M., Smith L.L. Techniques for working with wild reptiles. Curr Ther Reptile Med Surg. 2014:310–340. doi: 10.1016/B978-1-4557-0893-2.00029-6. [DOI] [Google Scholar]
- 79.Elosegui-Artola A., Andreu I., Beedle A.E.M., Lezamiz A., Uroz M., Kosmalska A.J., et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397–1410.e14. doi: 10.1016/J.CELL.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Parmar A.S., Muschol M. Hydration and hydrodynamic interactions of lysozyme: effects of chaotropic versus kosmotropic ions. Biophys J. 2009;97:590–598. doi: 10.1016/j.bpj.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salvi G., De Los Rios P., Vendruscolo M. Effective interactions between chaotropic agents and proteins. Protein: Struct, Funct Genet. 2005;61:492–499. doi: 10.1002/prot.20626. [DOI] [PubMed] [Google Scholar]