Abstract
Background
MALAT1 is one of the most abundant nuclear long non-coding RNAs, which has been found to be elevated in various types of cancers. However, conflicting reports on MALAT1 in breast cancer cell lines challenge understanding of MALAT1's involvement in breast cancer progression.
Aim
Measurement of normalized relative quantity (NRQ) of MALAT1 transcripts in cell lines representing triple-negative breast cancer (TNBC) and luminal breast cancer.
Materials and methods
The studies were performed using cell lines representing luminal breast cancer (T47D, MCF-7), TNBC (MDA-MB-468, CAL-51, MDA-MB-231), and MCF-10A cell line of normal breast epithelial cells. Total RNA was isolated from six independent cell cultures of each line, treated with DNase I, and used to synthesize complementary DNA, which was used in quantitative real-time PCR (qPCR) assays. Four MALAT1 fragments and reference genes CCSER2, ANKRD17, PUM1, GAPDH were amplified.
Results
Geometric means of the NRQ of MALAT1 in breast cancer cell lines had the shortest 95% confidence intervals when CCSER2 was used for normalization. MALAT1 major transcript levels thus estimated in TNBC cell lines were found to be statistically significantly reduced compared to levels in both MCF-10A cells and luminal breast cancer cell lines, while MALAT1 minority splice variants were found to be increased in almost all breast cancer cell lines.
Conclusion
CCSER2-normalized qPCR results indicate MALAT1 downregulation in cell lines representing the more aggressive breast cancer subtype compared to both the normal breast epithelial cell line and the estrogen receptor-positive breast cancer cell lines.
Keywords: Breast cancer cell lines, Non-coding RNA, lncRNA, Real-time PCR, Reference gene
Highlights
-
•
CCSER2-normalized relative quantities of MALAT1 in breast cancer cell lines.
-
•
Shortest confidence intervals of qPCR-estimated MALAT1 expression levels.
-
•
Reduction of MALAT1 major transcript in triple-negative breast cancer cell lines.
-
•
Increased levels of MALAT1 splice variants in breast cancer cell lines.
1. Introduction
Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) is essentially a single-exon gene whose transcript contains over eight thousand nucleotides of a long non-coding RNA that remains in the nucleus after minor processing [1], although minor splice variants also have been confirmed [2]. Elevated levels of MALAT1 have been found in metastatic cancer cells [3,4] and MALAT1 expression is thought to be upregulated in various types of cancer, which may also be related to a decrease in the sensitivity of cancer cells to cytotoxic drugs [5]. MALAT1 silencing in breast cancer MCF-7 cells resulted in an increase in apoptosis induction by Adriamycin and taxanes [6]. Also, exosome-delivered MALAT1 accelerated primary tumor growth in nude mice orthotopically injected with MCF-7 cells and treated with Adriamycin [7]. However, both the mechanistic complexity of MALAT1 function in cancer cells and the relevance of aberrant MALAT1 expression to breast cancer progression are still unclear [1,8]. On the one hand, the results of the meta-analysis showed that elevated MALAT1 expression in breast cancer was significantly associated with poorer overall survival [9]. On the other hand, the results of the mouse experiments seemed to support the hypothesis that murine MALAT1 inhibits breast cancer metastasis [10], and thus a downward pressure on MALAT1 expression in more aggressive breast cancer would rather be expected. In fact, the measurement of GAPDH-normalized MALAT1 in human breast cancer cell lines showed a downregulation of MALAT1 in cell lines representing the more aggressive basal-like/triple-negative breast cancer (TNBC) subtype [10]. However, neither previous nor subsequent measurements of MALAT1 in breast cancer cell lines published by other authors seemed to confirm this as, compared to the normal MCF-10A cell line, higher levels of MALAT1 were found in breast cancer cell lines of luminal and TNBC subtypes [[11], [12], [13], [14], [15]] or at least in a TNBC/basal cell line [16,17] or in luminal subtype breast cancer cell lines [18,19].
In the search for a reference gene to normalize qPCR results measuring MALAT1 quantities in breast cancer cell lines relative to the normal MCF-10A cell line, we found that normalization to CCSER2 resulted in the shortest 95% confidence intervals of the estimated MALAT1 quantities. In this report, the CCSER2-normalized relative quantities of MALAT1 were determined for the T47D and MCF-7 luminal subtype breast cancer cell lines representing better prognosis estrogen receptor-positive breast cancers, and for the MDA-MB-231, MDA-MB-468 and CAL-51 cell lines (Table 1), representing TNBC/basal with a worse prognosis [20]. The MCF-7 cell line represents an early-stage disease model, while the MDA-MB-231 cell line is used to model late-stage breast cancer. The rarely used CAL-51 has been described as a nearly normal karyotype breast cancer cell line.
Table 1.
Basic information about the cell lines used.
| Cell line | Molecular subtypea | ER/PRb | TP53b | Source |
|---|---|---|---|---|
| MCF-10A | Basal B | – | wt | reduction mammoplasty |
| T47D | Luminal | + | mut | pleural effusion |
| MCF-7 | Luminal | + | wt | pleural effusion |
| MDA-MB-468 | Basal A | – | mut | pleural effusion |
| CAL-51 | Basal B | – | wt | pleural effusion |
| MDA-MB-231 | Basal B | – | mut | pleural effusion |
Based on information derived from Kao et al. [32].
ER/PR, the presence of estrogen and progesterone receptors; TP53 status: wild-type or mutated.
2. Materials and Methods
Cell lines and culture conditions One normal breast epithelial MCF-10A cell line and five breast cancer cell lines of luminal subtype (T47D, MCF-7) and TNBC/basal subtype (MDA-MB-231, MDA-MB-468, CAL-51) were used (Table 1). MCF-7 and T47D cell lines were obtained from European Collection of Authenticated Cell Cultures (ECACC, Porton Down, Salisbury, UK), MDA-MB-231 and MCF-10A cell lines were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). MDA-MB-468 and CAL-51 cell lines were obtained from DSMZ-German Collection of Microorganisms and Cell Cultures (Leibniz Institute, Germany). The cell lines have been maintained in the Hirszfeld Institute of Immunology and Experimental Therapy, Wroclaw, Poland.
MCF-10A cells were cultured in Ham's F12 nutrient mixture with glutamine supplemented with 5% horse serum (both from Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 0.02 μg/ml of Epidermal Growth Factor human, 10 μg/ml of insulin from bovine pancreas, 0.5 μg/ml of hydrocortisone, 0.05 μg/ml of cholera toxin from Vibrio cholerae (all from Sigma-Aldrich, Germany).
T47D cells were cultured in a medium composed of OptiMEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and RPMI 1640 with HEPES (IIET, PAS, Wroclaw, Poland) (1:1 ratio), supplemented with 5% fetal bovine serum (HyClone, Cytiva, Portsmouth, United Kingdom), 2 mM l-Glutamine and 8 μg/ml of insulin (both from Sigma-Aldrich, Germany).
MCF-7 cells were cultured in Eagle's medium (IIET, PAS, Wroclaw, Poland) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, Germany), 2 mM l-Glutamine, 1% MEM non-essential amino acid solution, 8 μg/ml of insulin (both from Sigma-Aldrich, Germany).
MDA-MB-231 and MDA-MB-468 cells were cultured in RPMI 1640 with Stable Glutamine (Biowest, Nuaillé, France) and 10% or 20% FBS, respectively.
CAL-51 cells were cultured in Dulbecco's modified Eagle medium (Biowest, Nuaillé, France) supplemented with 10% FBS and 2 mM l-Glutamine.
All cell lines were cultured with antibiotics: 100 U/ml penicillin (Sigma-Aldrich, Germany) and 0.1 mg/ml streptomycin (Polfa Tarchomin, Poland). Cells were cultured under normoxic conditions in a 37 °C humidified incubator with 95% air and 5% CO2.
Total RNA extraction and reverse transcription (RT) Total RNA was isolated from cultures of cells using TRI reagent (Molecular Research Center Inc., Cincinnati, OH, USA) and Direct-zol RNA MiniPrep (Zymo Research, Irvine, CA, USA) with RNase-free DNase I treatment in-column according to manufacturer's protocols. DNA-free extracted RNAs were quantified by NanoDrop (ND-2000, Thermo Fisher Scientific, Waltham, MA, USA) and the absorbance ratio at 260/280 nm and 260/230 nm were measured to assure RNA purity. RNA (1 μg) was reverse transcribed into complementary DNA (cDNA) using iScript (BioRad, Hercules, CA, USA) in 20 μl solution according to the manufacturer's protocol.
Quantitative polymerase chain reaction (qPCR) qPCR in a 96-well plate was performed using Viia7 real-time PCR thermocycler (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). Each reaction was performed in duplicate and in a 10 μl volume containing 5 μl RT SYBR Green ROX qPCR Mastermix (Qiagen, Venlo, Netherlands), 0.4 μM of each primer and 25 ng of cDNA. The cycling conditions were as follows: 50°C-2 min, 95°C-10 min, 40 × (95°C-15 s, 60°C-1 min). The specificity of the PCR reaction was confirmed by a DNA melting curve analysis (95°C-15 s, 60°C-1 min, heating from 60 to 95°C-15 s with continuous fluorescence measurement) performed at the end of the PCRs to verify the formation of a single product. qPCR primer pairs that performed well in previous studies were used (Table 2). PCR primer efficiency was calculated from the slope of the linear regression line through the points of the log RNA quantity as the independent variable and the mean Ct values of triplicate qPCR as the dependent variable (r2 > 0.99 except 0.98 for CCSER2). qPCR was performed using cDNA serial dilutions corresponding to the following amounts of RNA isolated from MCF-7 cells: 10.00 ng, 1.00 ng, 0.10 ng, 0.01 ng (the latter amount only for MALAT1_1 and MALAT1_2). In Excel PCR-primer-efficiency (%)=(10^(−1/slope)-1)*100.
Table 2.
Sequence information for qPCR primers used.
| Gene/transcript | Primers/sequences (5’->3′) | Reference |
|---|---|---|
| MALAT1 | MALAT1_1 | [33] |
| NR_002819 | F: GAATTGCGTCATTTAAAGCCTAGTT | |
| R: GTTTCATCCTACCACTCCCAATTAAT | ||
| MALAT1_2 | [2] | |
| NR_002819 | F: CATCTTCTGAGTCATAACCAGCCT | |
| R: TGCCTTTACTTATCAATTCACCAA | ||
| MALAT1_3 | [2] | |
| Δ243-MALAT1a | F: TGGTAGCTTTTGTATTATCAAACTTT | |
| R: CTGCCAGGCTGGTTATGACT | ||
| MALAT1_4 | [2] | |
| Δ119-MALAT1a | F: GGCTCTTCCTTCTGTTCTAACTTCA | |
| R: GAACAGTACTGCATTTACTTGCCAA | ||
| CCSER2 | CCSER2 | [24] |
| NM_001284240 | F: GACAGGAGCATTACCACCTCAG | |
| R: CTTCTGAGCCTGGAAAAAGGGC | ||
| ANKRD17 | ANKRD17 | [24] |
| NM_032217 | F: CAAATGGTGGACACCTCGATGTG | |
| R: CTAAGTAGCGCACCACCTTCAC | ||
| PUM1 | PUM1 | [34] |
| NM_014676 | F: CTTTGGCAGAACGGATTCGAG | |
| R: TCTCATTCTGCTGGTCTGAAGG | ||
| GAPDH | GAPDH | |
| NM_002046 | F: ATGACATCAAGAAGGTGGTG | |
| R: CATACCAGGAAATGAGCTTG |
Splice variants with a deletion of 243 and 119 nucleotides of MALAT1, respectively. The qPCR forward primers span unique exon-exon junctions.
Data analysis and statistics The stability of expression of the endogenous reference genes in a series of independent cultures of normal and breast cancer cell lines was analyzed using the on-line tool RefFinder [21], which integrates the results of analyzes performed by four algorithms: the comparative ΔCt method, BestKeeper, NormFinder, and GeNorm.
Normalized relative quantities (NRQ) of MALAT1 in breast cancer cell lines, measured for two regions of full-length MALAT1 transcript and for two splice variants (Fig. 1), were calculated by the 2−ΔΔCt method and normalized to a single reference gene or calculated using the Multiple Reference Genes method of Vandesompele et al. [22], which allows normalization to the geometric mean of two or more reference genes. Briefly, for genej in samplek in the 2−ΔΔCt method:
Fig. 1.
Scheme of qPCR primer location for amplification of MALAT1 transcript fragments: NR_002819 and splice variants Δ243-MALAT1 and Δ119-MALAT1. qPCR product lengths: 85 bp MALAT1_1 (1811–1895), 85 bp MALAT1_2 (4915–4999), 65 bp MALAT1_3 (4636–4943 Δ243), 91 bp MALAT1_4 (6451–6660 Δ119), where the numbers in parentheses refer to the position in the sequence NR_002819. The triangles indicate qPCR primer positions complementary to the genomic and cDNA sequences (open triangles) or only to the cDNA sequences of unique splice variants (closed triangles).
NRQ [of genej in samplek] = 2^ - (Ct [genej in samplek] – Ct [geneR in samplek] – AVERAGE (Ct [genej in calibrator samples] – Ct [geneR in calibrator samples])), where Ct is the qPCR quantification cycle, MCF-10A cells are the calibrator, and geneR is the reference gene. AVERAGE is the arithmetic mean of ΔCt = Ct [genej]-Ct [geneR] of all calibrator samples.
In turn, calculations according to the Multiple Reference Genes method for two reference geneR1 and geneR2 were performed as follows:
NRQ [of genej in samplek] = RQ [genej in samplek]/GEOMEAN (RQ [geneR1 in samplek]; RQ [geneR2 in samplek]), where the relative quantity (RQ) for genex (where x is j or R1 or R2) in samplek is calculated as follows:
RQ [genex in samplek] = POWER(Ex; AVERAGE (Ct [genex in calibrator samples]) –Ct [genex in samplek]), where the base number Ex = PCR-primer-efficiency (%)/100 + 1, and AVERAGE is the arithmetic mean of Ct [genex] in all calibrator samples.
Estimated NRQ of the genej in six independent cultures of a given cell line is presented as a geometric mean with a 95% confidence interval (CI). The upper and lower bounds of the 95% CI were calculated in Excel as follows:
Upper/Lower CI = EXP (LN (GEOMEAN(X)) ± CONFIDENCE.T (0.05; LN (GSD); N)), where X = NRQ of genej in all samples of a given cell line, N is the number of samples, and the geometric standard deviation (GSD) is calculated as:
| GSD = EXP(SQRT(SUM(LN(X/GEOMEAN(X))^2)/(N-1))) |
The calculated NRQ of MALAT1 in breast cancer cell lines were subjected to statistical analysis after prior logarithmic transformation. The Shapiro-Wilk test was performed to test the null hypothesis that the original qPCR Ct values and the calculated LOG (NRQ) values are normally distributed. Differences in NRQ of MALAT1 in different breast cancer cell lines were analyzed by one-way analysis of variance (ANOVA) with post-hoc Tukey's HSD test using online calculators [23]. A value of p < 0.05 was regarded statistically significant.
3. Results
Endogenous reference gene In the search for a reference gene with stable expression in breast cancer cell lines, suitable for normalization of qPCR results, which would allow for a reliable comparison of MALAT1 levels, the commonly used GAPDH has already proven to be too variable in previous studies [24]. Similarly, for the breast cancer cell lines we tested, the coefficient of variation (CV%) of the obtained Ct values for GAPDH was 10.6%, which seemed too high to normalize the MALAT1 measurements whose CV% of the obtained Ct values ranged from 6.6 to 9.1% depending on the primer pair. On the other hand, CV% was 5.9% for the CCSER2, 6.0% for PUM1 and 6.7% for ANKRD17. Then, the RefFinder tool was used to analyze the obtained Ct values and the analyzes indicated CCSER2 as the recommended reference gene for normalization of qPCR results in the studied breast cancer cell lines, followed by ANKRD17 and PUM1. Moreover, the sum of the 95% CI of the geometric means of the NRQ of MALAT1 estimated for the tested cell lines was lowest when CCSER2 was used to normalize the qPCR results (Fig. 2).
Fig. 2.
Upper and lower bounds of the 95% confidence interval (Upper/Lower CI) of the geometric mean of six estimates of normalized relative quantity (NRQ) of a given transcript, depending on normalization to a single reference (REF) gene or to a geometric mean (GEOMEAN) of ANKRD17 and PUM1. The Upper/Lower CI calculation formula is provided in the Materials and Methods section. The ‘Sum of CI’ was calculated in Excel as SUM(Upper CI – Lower CI) for a total of six cell lines. The smallest numerical value of the ‘Sum of CI’ for estimating the NRQ of a given transcript in the tested cell lines is marked in bold. The confidence interval [Upper CI, Lower CI] determined for a given cell line that does not at least partially overlap with the confidence interval for MCF-10A is marked in red. The yellow background indicates a statistically significant difference (ANOVA with Tukey's HSD post-hoc test, p < 0.05) in MALAT1 levels in cells of this line relative to T47D.
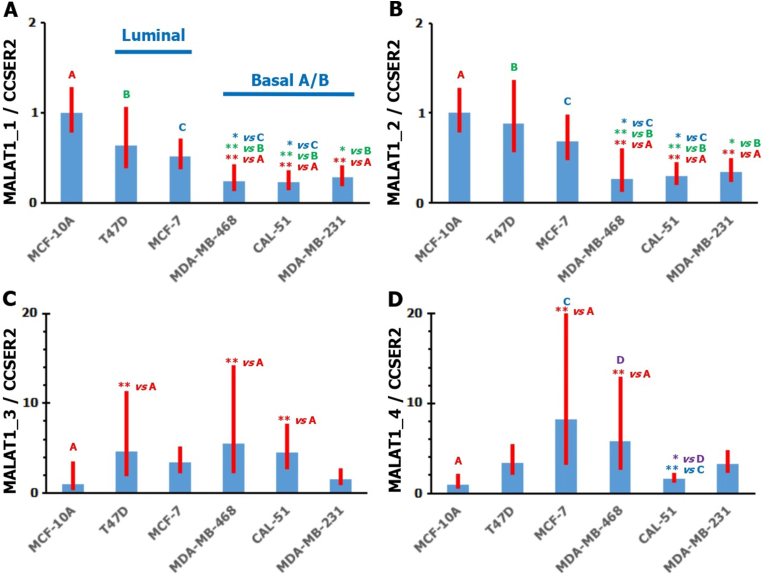
Normalized relative quantity of MALAT1 Correlations between the obtained qPCR Ct values suggest that the PCR primers MALAT1_1 and MALAT1_2 (Fig. 1) amplify different regions of the same MALAT1 molecules (Pearson's coefficient 0.97). The measured NRQ of MALAT1 was found to be statistically significantly lower in TNBC/basal cell lines compared to both the normal MCF-10A cell line and the luminal subtype breast cancer cell lines (Fig. 3A and B). In contrast, the lower correlation of the obtained Ct values in qPCR with the primer pairs MALAT1_3 and MALAT1_4 (Pearson's coefficient 0.78) may suggest that these are at least partly amplifications of different MALAT1 splice variants. Compared to the MCF-10A cell line, elevated levels of MALAT1 splice variants were found in four out of five breast cancer cell lines (Fig. 3C and D).
Fig. 3.
CCSER2-normalized relative quantity (NRQ) of MALAT1 in luminal (T47D, MCF-7) and TNBC/basal (MDA-MB-468, CAL-51, MDA-MB-231) breast cancer cell lines relative to the MCF-10A cell line. Shown are results for the transcript NR_002819 of MALAT1 with two primer sets (A, B) and for the transcripts Δ243-MALAT1 (C) and Δ119-MALAT1 (D), which are minor splice variants. Bars with whiskers represent geometric means and 95% confidence intervals for NRQs measured in six independent cultures of a given cell line. Statistical significance of differences in MALAT1 levels between cell lines was analyzed by one-way ANOVA with Tukey's HSD post-hoc test and marked *(p < 0.05), **(p < 0.01) vs A (MCF-10A), B (T47D), C (MCF-7).
4. Discussion
Rather unexpectedly, the CCSER2-normalized relative quantities of the MALAT1 major transcript found in breast cancer cell lines, reported in this study, are consistent with the results of Kim et al. [10] and thus stand in opposition to the published results of almost all MALAT1 measurements performed by the authors who used various reference genes to normalize qPCR results, such as GAPDH [11,[13], [14], [15],17,18,25,26], ACTB [12,16,27], PUM1 [28], RPLP0 [19]. The results of qPCR measurements, repeated by us for five independent cell cultures of the tested breast cancer lines, indicate a rather high GAPDH variability in the breast cancer cell lines. Also, the use of ANKRD17 or PUM1 or geometric mean of ANKRD17 and PUM1 for normalization led to the estimation of the geometric mean of the NRQ of MALAT1 with a wider 95% CI than normalization to CCSER2 (Fig. 2).
Differences in the preparation of RNA samples for analysis may also contribute to the misestimation of MALAT1 levels. Unfortunately, there is no information in some publications whether the RNA samples were treated with DNase I to remove potential genomic DNA contamination that could contribute to the overestimation of MALAT1 levels, as previously shown [29]. Although in studies by Arshi et al. [28] RNA samples were treated with DNase I and the less variable PUM1 was used as a normalizer for qPCR data, and despite this, the NRQ of MALAT1 in the MCF-7 and MDA-MB-231 cell lines were estimated to be higher than in MCF-10A. The finding that hypoxia upregulates MALAT1 in breast cancer cell lines but not in MCF-10A cells [30] suggests that differences in cell line culture conditions may also contribute to the discrepancy in MALAT1 level measurements. Finally, it cannot be excluded that MCF-10A sublines were used in various measurements [31]. Therefore, it seems more important that both this report and the two previous publications [10,18] showed measurement results indicating a lower level of MALAT1 expression in cell lines representing more aggressive TNBC compared to cell lines representing the luminal subtype of breast cancer.
5. Conclusion
First, the use of the CCSER2 transcript to normalize the qPCR data resulted in the shortest 95% confidence intervals of the geometric means of the normalized relative quantities of MALAT1 in breast cancer cell lines. Second, analysis of CCSER2-normalized relative quantities of the MALAT1 major transcript indicates its statistically significant reduction in TNBC cell lines compared to both the normal breast epithelial cell line and the luminal subtype breast cancer cell lines. Third, increased expression levels of MALAT1 minor splice variants were found in the breast cancer cell lines tested, with the exception of MDA-MB-231.
Authors’ contributions
D.K.: data curation; funding acquisition; investigation; methodology; resources; validation; J.M.: conceptualization; formal analysis; methodology; project administration; visualization; writing – original draft, review & editing.
All authors who contributed to the article approved the submitted version.
Funding
This work was supported by the statutory funds of the Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Contributor Information
Dagmara Klopotowska, Email: dagmara.klopotowska@hirszfeld.pl.
Janusz Matuszyk, Email: janusz.matuszyk@hirszfeld.pl.
Data availability
Data will be made available on request.
References
- 1.Sun Y., Ma L. New insights into long non-coding RNA MALAT1 in cancer and metastasis. Cancers. 2019;11:216. doi: 10.3390/cancers11020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meseure D., Vacher S., Lallemand F., Alsibai K.D., Hatem R., Chemlali W., Nicolas A., De Koning L., Pasmant E., Callens C., Lidereau R., Morillon A., Bieche I. Prognostic value of a newly identified MALAT1 alternatively spliced transcript in breast cancer. Br. J. Cancer. 2016;114:1395–1404. doi: 10.1038/bjc.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J., Ling C. Construction of a predictive model for breast cancer metastasis based on lncRNAs. Transl. Cancer Res. 2023;12:387–397. doi: 10.21037/tcr-23-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen L., Chen L., Wang Y., Jiang X., Xia H., Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J. Neuro Oncol. 2015;121:101–108. doi: 10.1007/s11060-014-1613-0. [DOI] [PubMed] [Google Scholar]
- 5.Goyal B., Yadav S.R.M., Awasthee N., Gupta S., Kunnumakkara A.B., Gupta S.C. Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim. Biophys. Acta Rev. Cancer. 2021;1875 doi: 10.1016/j.bbcan.2021.188502. [DOI] [PubMed] [Google Scholar]
- 6.Yu J., Jin T., Zhang T. Suppression of long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) potentiates cell apoptosis and drug sensitivity to taxanes and Adriamycin in breast cancer. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.922672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao S., Bai Z., Liu Y., Gao Y., Zhou J., Zhang Y., Li J. Exosomes derived from tumor cells initiate breast cancer cell metastasis and chemoresistance through a MALAT1-dependent mechanism. J Oncol. 2022;2022 doi: 10.1155/2022/5483523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arun G., Spector D.L. MALAT1 long non-coding RNA and breast cancer. RNA Biol. 2019;16:860–863. doi: 10.1080/15476286.2019.1592072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Zhang Y., Hu K., Qiu J., Hu Y., Zhou M., Zhang S. Elevated long noncoding RNA MALAT-1 expression is predictive of poor prognosis in patients with breast cancer: a meta-analysis. Biosci. Rep. 2020;40 doi: 10.1042/BSR20200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J., Piao H.L., Kim B.J., Yao F., Han Z., Wang Y., Xiao Z., Siverly A.N., Lawhon S.E., Ton B.N., Lee H., Zhou Z., Gan B., Nakagawa S., Ellis M.J., Liang H., Hung M.C., You M.J., Sun Y., Ma L. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat. Genet. 2018;50:1705–1715. doi: 10.1038/s41588-018-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajibabaei S., Nafissi N., Azimi Y., Mahdian R., Rahimi-Jamnani F., Valizadeh V., Rafiee M.H., Azizi M. Targeting long non-coding RNA MALAT1 reverses cancerous phenotypes of breast cancer cells through microRNA-561-3p/TOP2A axis. Sci. Rep. 2023;13:8652. doi: 10.1038/s41598-023-35639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue X., Wu W.Y., Dong M., Guo M. LncRNA MALAT1 promotes breast cancer progression and doxorubicin resistance via regulating miR-570-3p. Biomed. J. 2021;44:S296–S304. doi: 10.1016/j.bj.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao J., Zhang Q., Wang P., Wang Z. LncRNA MALAT1 promotes breast cancer progression by sponging miR101-3p to mediate mTOR/PKM2 signal transmission. Am. J. Transl. Res. 2021;13:10262–10275. PMID: 34650695; PMCID: PMC8507063. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao C., Ling X., Xia Y., Yan B., Guan Q. The m6A methyltransferase METTL3 controls epithelial-mesenchymal transition, migration and invasion of breast cancer through the MALAT1/miR-26b/HMGA2 axis. Cancer Cell Int. 2021;21:441. doi: 10.1186/s12935-021-02113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang N., Cao S., Wang X., Zhang L., Yuan H., Ma X. lncRNA MALAT1/miR-26a/26b/ST8SIA4 axis mediates cell invasion and migration in breast cancer cell lines. Oncol. Rep. 2021;46:181. doi: 10.3892/or.2021.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes C.P., Nóbrega-Pereira S., Domingues-Silva B., Rebelo K., Alves-Vale C., Marinho S.P., Carvalho T., Dias S., Bernardes de Jesus B. An antisense transcript mediates MALAT1 response in human breast cancer. BMC Cancer. 2019;19:771. doi: 10.1186/s12885-019-5962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin C., Yan B., Lu Q., Lin Y., Ma L. Reciprocal regulation of Hsa-miR-1 and long noncoding RNA MALAT1 promotes triple-negative breast cancer development. Tumour Biol. 2016;37:7383–7394. doi: 10.1007/s13277-015-4605-6. [DOI] [PubMed] [Google Scholar]
- 18.Jadaliha M., Zong X., Malakar P., Ray T., Singh D.K., Freier S.M., Jensen T., Prasanth S.G., Karni R., Ray P.S., Prasanth K.V. Functional and prognostic significance of long non-coding RNA MALAT1 as a metastasis driver in ER negative lymph node negative breast cancer. Oncotarget. 2016;7:40418–40436. doi: 10.18632/oncotarget.9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng T., Shao F., Wu Q., Zhang X., Xu D., Qian K., Xie Y., Wang S., Xu N., Wang Y., Qi C. miR-124 downregulation leads to breast cancer progression via LncRNA-MALAT1 regulation and CDK4/E2F1 signal activation. Oncotarget. 2016;7:16205–16216. doi: 10.18632/oncotarget.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai X., Cheng H., Bai Z., Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J. Cancer. 2017;8:3131–3141. doi: 10.7150/jca.18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie F., Wang J., Zhang B. RefFinder: a web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genomics. 2023;23:125. doi: 10.1007/s10142-023-01055-7. [DOI] [PubMed] [Google Scholar]
- 22.Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Statistics Kingdom. 2017. https://www.statskingdom.com/180Anova1way.html Available at: [Google Scholar]
- 24.Tilli T.M., Castro Cda S., Tuszynski J.A., Carels N. A strategy to identify housekeeping genes suitable for analysis in breast cancer diseases. BMC Genom. 2016;17:639. doi: 10.1186/s12864-016-2946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang N.S., Chi Y.Y., Xue J.Y., Liu M.Y., Huang S., Mo M., Zhou S.L., Wu J. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 (MALAT1) interacts with estrogen receptor and predicted poor survival in breast cancer. Oncotarget. 2016;7:37957–37965. doi: 10.18632/oncotarget.9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu S., Sui S., Zhang J., Bai N., Shi Q., Zhang G., Gao S., You Z., Zhan C., Liu F., Pang D. Downregulation of long noncoding RNA MALAT1 induces epithelial-to-mesenchymal transition via the PI3K-AKT pathway in breast cancer. Int. J. Clin. Exp. Pathol. 2015;8:4881–4891. PMID: 26191181; PMCID: PMC4503053. [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Chen N., Zhou L., Wang C., Wen X., Jia L., Cui J., Hoffman A.R., Hu J.F., Li W. Genome-wide target interactome profiling reveals a novel EEF1A1 epigenetic pathway for oncogenic lncRNA MALAT1 in breast cancer. Am. J. Cancer Res. 2019;9:714–729. PMID: 31105998; PMCID: PMC6511647. [PMC free article] [PubMed] [Google Scholar]
- 28.Arshi A., Sharifi F.S., Khorramian Ghahfarokhi M., Faghih Z., Doosti A., Ostovari S., Mahmoudi Maymand E., Ghahramani Seno M.M. Expression analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in breast cancer tissues from young women and women over 45 Years of age. Mol. Ther. Nucleic Acids. 2018;12:751–757. doi: 10.1016/j.omtn.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markou A.N., Smilkou S., Tsaroucha E., Lianidou E. The effect of genomic DNA contamination on the detection of circulating long non-coding RNAs: the paradigm of MALAT1. Diagnostics. 2021;11:1160. doi: 10.3390/diagnostics11071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone J.K., Kim J.H., Vukadin L., Richard A., Giannini H.K., Lim S.S., Tan M., Ahn E.E. Hypoxia induces cancer cell-specific chromatin interactions and increases MALAT1 expression in breast cancer cells. J. Biol. Chem. 2019;294:11213–11224. doi: 10.1074/jbc.RA118.006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall A.M., Pai V.P., Sartor M.A., Horseman N.D. In vitro multipotent differentiation and barrier function of a human mammary epithelium. Cell Tissue Res. 2009;335:383–395. doi: 10.1007/s00441-008-0719-0. [DOI] [PubMed] [Google Scholar]
- 32.Kao J., Salari K., Bocanegra M., Choi Y.L., Girard L., Gandhi J., Kwei K.A., Hernandez-Boussard T., Wang P., Gazdar A.F., Minna J.D., Pollack J.R. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng X., Ren J., Peng B., Ye J., Wu X., Zhao W., Li Y., Chen R., Gong X., Bai C., Wang Y., Zhao H., Zhang Y. MALAT1 overexpression promotes the growth of colon cancer by repressing β-catenin degradation. Cell. Signal. 2020;73 doi: 10.1016/j.cellsig.2020.109676. [DOI] [PubMed] [Google Scholar]
- 34.Hauck T., Kadam S., Heinz K., Garcia Peraza M., Schmid R., Kremer A.E., Wolf K., Bauer A., Horch R.E., Arkudas A., Kengelbach-Weigand A. Influence of the autotaxin-lysophosphatidic acid axis on cellular function and cytokine expression in different breast cancer cell lines. Sci. Rep. 2022;12:5565. doi: 10.1038/s41598-022-09565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.