Abstract
Background
Previous study confirmed that both TaohongSiwu decoction (THSWD) and Dubosiella newyorkensis improved hypertension-induced endothelial dysfunction. However, the mechanism of THSWD combined with Dubosiella newyorkensis remains unclear.
Purpos
e: We aimed to investigate the microecological mechanism underlying the THSWD combined with Dubosiella newyorkensis for the prevention of hypertensive vascular endothelial dysfunction.
Methods
Eight percent high-salt diet was applied to induce hypertension in a mouse model for 4 weeks. THSWD, Dubosiella newyorkensis and THSWD combined with Dubosiella newyorkensis were used to intervene in the model mice to observe the changes of systolic blood pressure (SBP), body weight, blood routine, endothelial function, gut contents microbiota and bile acid metabolites.
Results
Results revealed that THSWD combined with Dubosiella newyorkensis significantly restored blood pressure and regulated body weight, and markedly downregulating serum and vascular levels of endothelin-1 (ET-1), thrombin regulatory protein (TM), vascular hemophilia factor (vWF) and vascular endothelial growth factor (VEGF), and upregulating nitric oxide (NO) levels compared with the model group. Notably, It altered the diversity and community structure of gut contents microbiota in mice. Lactobacillus and Allobaculum was enormously up-regulated at the genus level. Serum bile acid differential metabolites cholic acid and chenodeoxycholic acid were markedly altered. Futhermore, there was a close relationship between Lactobacillus, Allobaculum and endothelial function indexes in mice.
Conclusion
Lactobacillus and Allobaculum play important roles in the prevention of vascular endothelial dysfunction in hypertension during the THSWD combined with Dubosiella newyorkensis.
Keywords: THSWD, Dubosiella newyorkensis, Hypertension, Vascular endothelial dysfunction, Microecological mechanism
1. Introduction
Hypertensive is clinical syndromes characterized by increased arterial blood pressure (systolic pressure and/or diastolic pressure) in the body circulation, which may be accompanied by functional or organic damage to the heart, brain, kidneys and other organs. Study confirmed that the prevalence of hypertension in Chinese adults aged ≥18 years was 23.2 %, with 245 million people affected; the prevalence of normal high blood pressure was 41.3 %, with 435 million people affected [1]. Various researches in recent years have shown, to varying degrees, an overall increasing trend in the prevalence of hypertension in the population [[2], [3], [4]]. Chronic high salt dietary intake is an important factor in triggering hypertension. In the global statistics of cardiovascular deaths, it was found that 10 % of the population deaths can be attributed to hypertension caused by a high salt diet [5]. Traditional Chinese medicine (TCM) believes that salt is salty and excessive consumption of salt can injure the blood vessels, resulting in poor blood flow. TaohongSiwu decoction (THSWD) is based on the Siwu decoction (Angelica sinensis (Oliv.) Diels (family Apiaceae), Ligusticum striatum DC. (family Apiaceae), Rehmannia glutinosa (Gaertn.) DC. (family Orobanchaceae), and Paeonia delavayi Franch. (family Paeoniaceae), with the addition of blood-stasis invigorating herbal pairs (Carthamus tinctorius L. (family Asteraceae) and Prunus davidiana (CarriŠre) Franch. (family Rosaceae). It is a classic representative of the formula of promoting blood circulation and removing blood stasis [6].
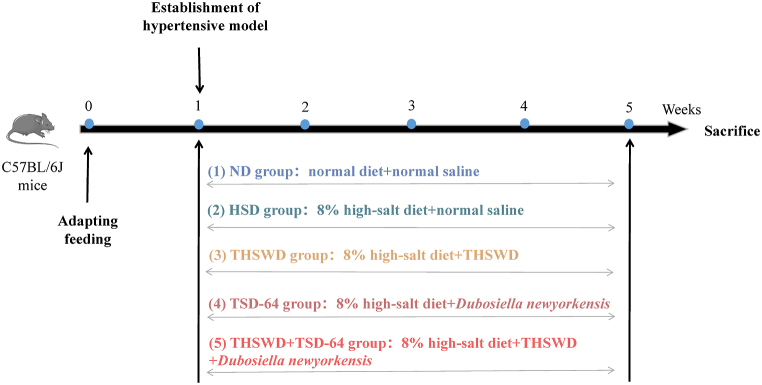
Endothelial dysfunction is one of the early risk markers of cardiovascular diseases (hypertension, diabetes, hypercholesterolemia, etc.) [7]. Recently, the research work of the applicant's group has focused on the relationship between vascular endothelial cell function and hypertension disease, including the inflammatory response of vascular endothelial cells, endoplasmic reticulum stress, apoptosis and miRNA mechanisms [[8], [9], [10]]. Of course, we have discovered that bacterium Dubosiella newyorkensis played a vital role in endothelial dysfunction in hypertension induced by high-salt diet [11]. High-salt diet lead to hypertensive downregulation of Dubosiella newyorkensis in mice, resulting in endothelial cell dysfunction; Dubosiella newyorkensis had protective effects on high salt-induced blood pressure and endothelial function in mice; protection of vascular endothelial function by THSWD could be achieved by upregulating Dubosiella newyorkensis; These above results suggested that THSWD might improve vascular endothelial dysfunction by modulating Dubosiella newyorkensis to prevent hypertension. Therefore, based on the previous experiments, this study intended to investigate in depth the microecological mechanism of THSWD combined with Dubosiella newyorkensis in the prevention and treatment of hypertension induced by by a high-salt diet, providing a scientific basis for the clinical application of classical TCM formulas, as well as providing a new strategy for the prevention and treatment of hypertension in TCM. The specific process was shown in Fig. 1.
Fig. 1.
Experimental flow chart.
2. Materials and methods
2.1. Animal treatment
Fifty pathogen free (SPF) grade C57BL/6 mice (male, 6–8 weeks) were purchased from Changzhou Cavens Laboratory Animals Co., Lot (Jiangsu, China) and were then acclimatized (12-h light/dark cycle) under standard conditions (temperature 22 ± 2 °C, humidity 50–60 %) with ad libitum access to food and water for 7 days. The ethics committee at the Animal Ethics Committee of Jiangnan University approved the animal experiment (Nos: IACUC-20190726-04), which was carried out in the university's animal facility. All animal studies were performed according to the institutional guidelines and complied with the ARRIVE guidelines.
The schematic illustration of the animal experimental design is shown as Fig. 1. A total of 50 mice were randomly assigned into five groups (n = 10 per group): the control group (ND), hypertension model group (HSD), THSWD treatment group (THSWD), Dubosiella newyorkensis treatment group (TSD-64) and THSWD + Dubosiella newyorkensis treatment group (THSWD + TSD-64). After the period of adaptation, a mouse model was given an 8 % high-salt diet for 4 weeks to develop hypertension in the HSD, THSWD, TSD-64 and THSWD + TSD-64 groups [11]. The ND group mice were given normal diet. Mice in the THSWD group, TSD-64 group and THSWD + TSD-64 group were gavaged 60 g/kg THSWD, 108–109 (cfus) Dubosiella newyorkensis and 60 g/kg THSWD+108–109 (cfus) Dubosiella newyorkensis respectively once daily for 4 weeks. Meanwhile, the other two groups mice were given the same volume of normal saline once a day for 4 weeks.
2.2. Sample collection
At the end of the experiment, mice were anesthetized under sodium pentobarbital for sample collection. Blood was drawn from the eyeball of the mice. Some of the blood samples were collected in the EDTA-K2 anticoagulation tubes for routine blood tests and the rest were collected in the sterile tubes for Enzyme-linked immunosorbent assay (ELISA) and Liquid chromotography with mass spectrometry (LC-MS). Mouse vascular tissues and colon contents was collected using sterile tubes and stored at −80 °C in the refrigerator for ELISA and high-throughput sequencing.
2.3. Weight body testing
The body weight of the mice was checked and recorded weekly.
2.4. Systolic blood pressure (SBP) measurements
We performed weekly blood pressure tests on mice using the tail artery manometry. The mouse platform temperature was pre-set to 37 °C according to the operating instructions. Each mouse was tested 15 times per round and its mean SBP was recorded. Measurements were taken weekly at 3 p.m. and each step of the operation was completed by the same operator using a blood pressure analysis program (BP2000, USA).
2.5. Blood routine assay
Blood samples for routine blood testing are tested using a fully automated haematology analyser by turning the blood collection tube over 2–3 times immediately after collection.
2.6. Detection of related indexes of vascular endothelial function
Blood samples for ELISA testing were left to stand for 2–4 h, then centrifuged at 3000 rpm for 5 min and the supernatant was taken in a 1.5 mL sterile EP tube. The collected mouse vascular tissues were ground, centrifuged at 3000 rpm for 5 min and the supernatants was taken in a 1.5 mL sterile EP tube for ELISA. According to the ELISA kit (ET-1, vWF, VEGF, TM, and NO) instructions provided by the method for setting plate layout, adding samples, adding enzymes, incubation, washing plate, color, termination reaction, machine detection.
2.7. Detection of gut contents microbiota
Microbial DNA was extracted by a E.Z.N.A.® soil DNA Kit (Omega Bio-Tek, Norcross, GA, U.S.) following the improved protocol based on the manufacturer's instructions. A NanoDrop 2000 UV–vis spectrophotometer (Thermo Scientific, Wilmington, USA) was used to assess the final DNA concentration and purification, and DNA quality was confirmed using 1 % agarose gel electrophoresis. The V3–V4 regions of the microbial 16S RNA were amplified with the paired primers (forward primer: 5′-ACTCCTACGGGAGGCAGCAG-3'; reverse primer: 5′-GGACTACHVGGGTWTCTAAT-3′). The PCR products were extracted from a 2 % agarose gel, purified with a AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA), and quantified with QuantiFluorTM-ST (Promega, USA) according to the manufacturer's instructions. All the quantified amplicons were pooled together at equalized concentrations for Illumina MiSeq sequencing (Illumina, Inc., CA, USA). Experiments including DNA extraction, quality assessment, library construction, and high-throughput sequencing were performed by Majorbio Bio-Pharm Technology Co. Ltd (Shanghai, China).
2.8. Targeted analysis of bile acid metabolism
The stock solution of individual bile acid was mixed and prepared in bile acid-free matrix to obtain a series of bile acid calibrators at a concentration of 25,000、15,000、5000、2500、500、250、50、25、15、5、2.5 or 1.5 ng/mL. Certain concentrations of GCA-d4, UDCA-d4, CA-d4, GCDCA-d4, LCA-d4 and CDCA-d4 were compounded and mixed as Internal Standard (IS). The stock solution of all of these and working solution were stored in refrigerator of −20 °C. Then, the samples (100 μL) were taken respectively and homogenized with 300 μL of acetonitrile/methanol (8 : 2) which contained mixed internal standards and centrifuged at 12,000 rpm for 10 min to remove the protein. Finally, the supernatant was injected into the LC-MS/MS system for analysis.
2.9. Statistical analysis
The results were expressed as the mean ± standard deviation and were analyzed by GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA). Multiple comparisons were performed using one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) post-hoc test, and statistical significance was set at P < 0.05.
3. Results
3.1. THSWD combined with Dubosiella newyorkensis treatment alleviated SBP in hypertensive mice
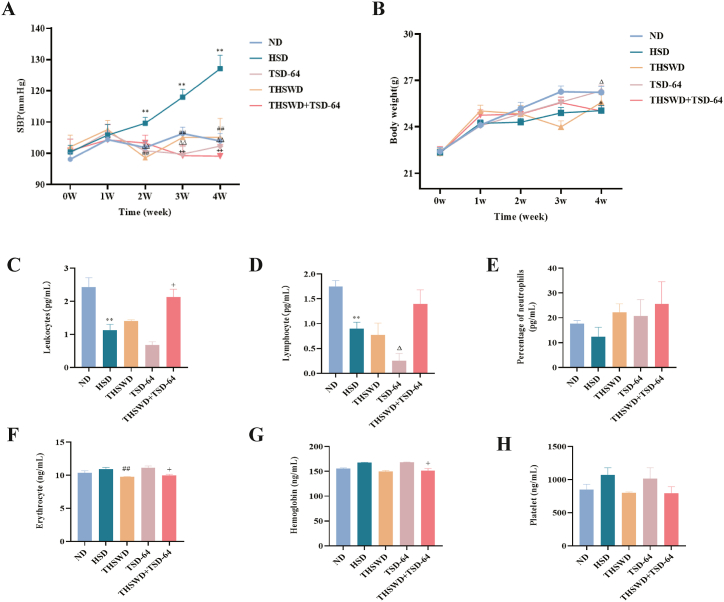
Compared with the ND group, the SBP of the HSD group increased with time, with a significant difference especially from the 2 nd week of the experiment. From the 2 nd week of the experiment, the SBP of the mice in the TSD-64, THSWD and THSWD + TSD-64 groups was obviously lower than that of the HSD group, with a greater reduction in the THSWD + TSD-64 group (Fig. 2A). Apparently, THSWD combined with Dubosiella newyorkensis treatment alleviated SBP in hypertensive mice.
Fig. 2.
Effect of THSWD combined with Dubosiella newyorkensis on SBP, body weight and blood routine of hypertensive mice. (A) SBP. (B) Body weight. (C) Leukocytes. (D) Lymphocyte. (E) Percentage of neutrophils. (F) Erythrocyte. (G) Hemoglobin. (H) Average haemoglobin concentration. (I) Platelet. ND, control group; HSD, hypertension model group; THSWD, THSWD treatment group; TSD-64, Dubosiella newyorkensis treatment group; THSWD + TSD-64, THSWD + Dubosiella newyorkensis treatment group. The values were expressed as mean ± standard deviation. **p < 0.01 (HSD vs ND), ##p < 0.01 (HSD vs THSWD), ΔΔp<0.01 (HSD vs TSD-64), ++p < 0.01 (HSD vs THSWD + TSD-64).
THSWD combined with Dubosiella newyorkensis treatment regulated body weight and blood routine in hypertensive mice.
As shown in Fig. 2B, it appeared that hypertension induced by the high-salt diet caused a reduction in body weight of mice. As the duration of the drug intervention increased, body weight of mice in the TSD-64, THSWD and THSWD + TSD-64 groups recovered, but the difference was not insignificant. Additionally, the percentage of leukocytes, lymphocytes and neutrophils of mice in the HSD group decreased compared to the ND group, and the contents of erythrocytes, haemoglobin and platelets increased. After THSWD combined with Dubosiella newyorkensis treatment, the above phenomena improved and gradually approached the ND group (Fig. 2C–H). Overall, THSWD combined with Dubosiella newyorkensis treatment had the regulating effect on body weight and blood routine in hypertensive mice.
3.2. THSWD combined with Dubosiella newyorkensis treatment attenuated vascular endothelial dysfunction in hypertensive mice
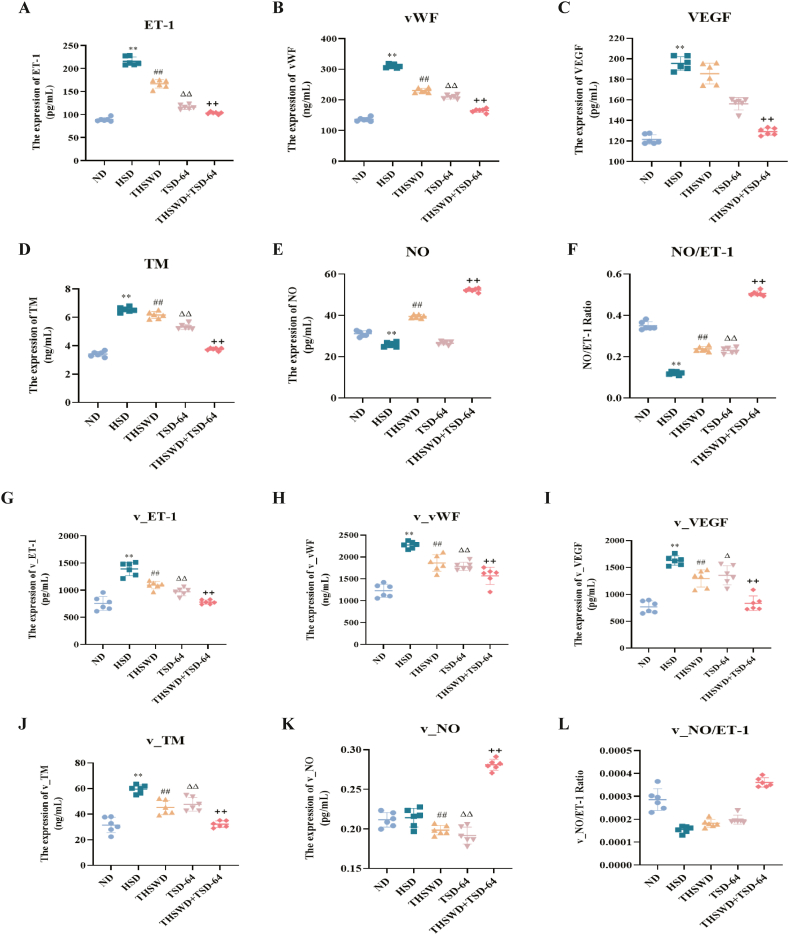
To investigate whether THSWD combined with Dubosiella newyorkensis intervention could improve endothelial dysfunction in hypertensive mice, we examined endothelial function by analysing serum and vascular levels of NO, VEGF, TM, vWF, ET-1 and NO/ET-1 (Fig. 3A-L). Compared to the HSD group, mice in the THSWD + TSD-64 group significantly lowered the serum and vascular levels of VEGF, TM, vWF and ET-1, whereas the serum and vascular levels of NO and NO/ET-1 exhibited dramatically improved. In addition, these results were also seen in the TSD-64 and THSWD groups of mice. In brief, THSWD combined with Dubosiella newyorkensis treatment attenuated vascular endothelial dysfunction in hypertensive mice.
Fig. 3.
Effect of THSWD combined with Dubosiella newyorkensis on vascular endothelial factors of hypertensive mice (n = 6). (A) ET-1 level in serum. (B) vWF level in serum. (C) VEGF level in serum. (D) TM level in serum. (E) NO level in serum. (F) NO/ET-1 in serum. (G) ET-1 level in blood vessels. (H) vWF level in blood vessels. (I) VEGF level in blood vessels. (J) TM level in blood vessels. (K) NO level in blood vessels. (L) NO/ET-1 in blood vessels. ND, control group; HSD, hypertension model group; THSWD, THSWD treatment group; TSD-64, Dubosiella newyorkensis treatment group; THSWD + TSD-64, THSWD + Dubosiella newyorkensis treatment group. The values were expressed as mean ± standard deviation. **p < 0.01 (HSD vs ND), ##p < 0.01 (HSD vs THSWD), ΔΔp<0.01 (HSD vs TSD-64), ++p < 0.01 (HSD vs THSWD + TSD-64).
3.3. THSWD combined with Dubosiella newyorkensis treatment reshaped gut contents microbiota in hypertensive mice
3.3.1. Sequencing data evaluation
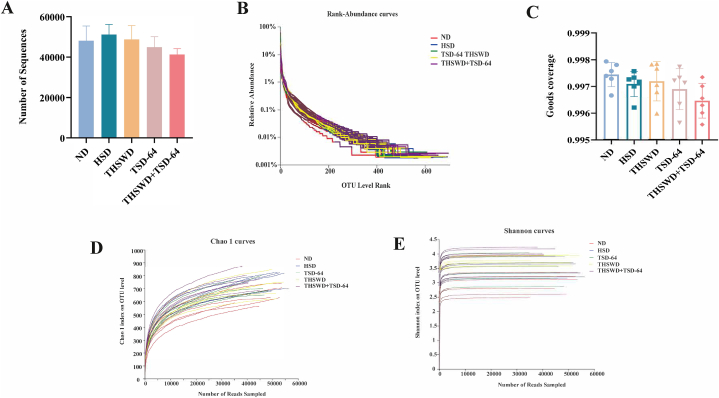
The raw sequencing data from the lower machine was quality controlled to obtain a total of 1406056 high quality sequences, and the amount of high quality sequences for each sample was shown in Fig. 4A. Then, we plotted the rank abundance distribution curve according to the abundance log2 value to analyse the community structure of gut microbiota in each group (Fig. 4B). The Good_coverage for each sample was greater than 99.5 % (Fig. 4C), demonstrating that the sequencing results were up to standard. The dilution curves presented that once the amount of sequence in each sample reaches 10,000, the curve plateaus and the amount of microorganisms detected in each sample approaches saturation (Fig. 4D and E), indicating that the current sequencing depth is sufficient to reflect the microbial diversity contained in the batch. It can be seen that the sequencing depth used in this study was reasonable and the amount of sequencing data collected is sufficient to represent the true nature of the microbial community in each sample, and can be used to analyse the microbial diversity composition of the samples.
Fig. 4.
Sequencing quality assessment of gut contents microbiota in mice (n = 6). (A) Number of Sequence. (B) Rank abundance distribution curve. (C) Good_coverage. (D) Dilution curve of Chao1. (E) Dilution curve of Shannon. ND, control group; HSD, hypertension model group; THSWD, THSWD treatment group; TSD-64, Dubosiella newyorkensis treatment group; THSWD + TSD-64, THSWD + Dubosiella newyorkensis treatment group.
3.3.2. Species diversity and overall structural analysis
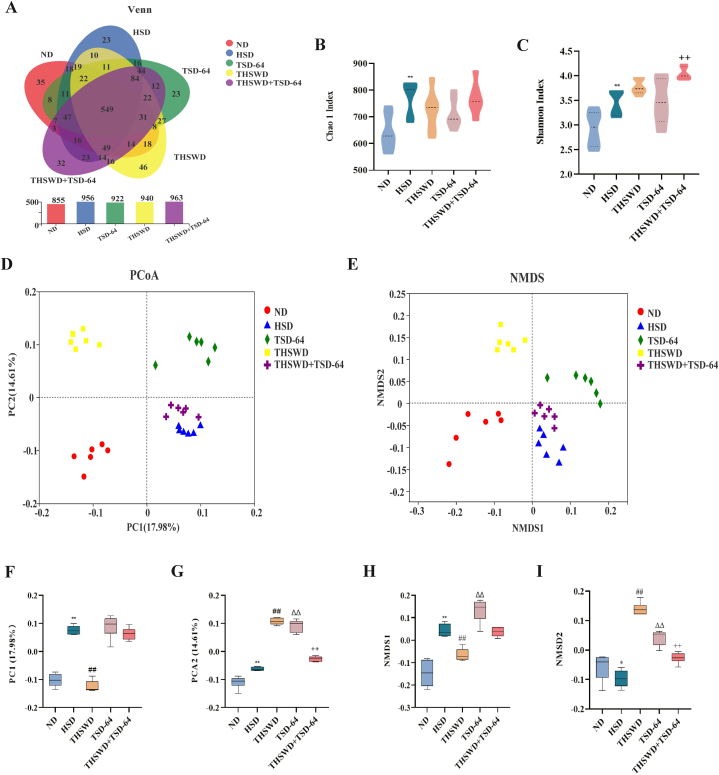
The Venn diagram can be used to calculate the number of sample shares or unique OTUs. Using the Qiime software platform, OTU clustering was performed according to 97 % similarity (Fig. 5A). There were 855 OTUs in the ND group, 956 in the HSD group, 940 in the THSWD group, 922 in the TSD-64 group, and 963 in the THSWD + TSD-64 group. There were 549 overlapping OTUs in the five groups. The results showed that the gut microbial community species of the mice were altered after THSWD combined with Dubosiella newyorkensis intervention, which formed some difference from those of the HSD group and there were relatively more of them than those of the HSD group.
Fig. 5.
Analysis of diversity and overall structure of gut contents microbiota in mice (n = 6). (A) Venn diagram. (B) Chao1 index. (C) Shannon index. (D) PcoA analysis. (E) NMDS analysis. (F) PCoA statistical analysis. (G) NMDS statistical analysis. ND, control group; HSD, hypertension model group; THSWD, THSWD treatment group; TSD-64, Dubosiella newyorkensis treatment group; THSWD + TSD-64, THSWD + Dubosiella newyorkensis treatment group. The values were expressed as mean ± standard deviation. **p < 0.01 (HSD vs ND), ##p < 0.01 (HSD vs THSWD), ΔΔp<0.01 (HSD vs TSD-64), ++p < 0.01 (HSD vs THSWD + TSD-64).
To determine whether the diversity of the microbial communities differed between samples, we calculated Alpha diversity indexes for five groups of gut contents samples. The results showed that indicators focusing on community richness, including Chao1 index, was significantly different between the ND and HSD groups (Fig. 5B, P < 0.05), indicating increased species and higher richness in the gut microbial community of hypertensive mice. The Shannan index, which reflected the homogeneity of the community, was also significantly higher in the HSD group than in the ND group (Fig. 5C, P < 0.05), suggesting that the number of species in the gut microbial community of hypertensive mice was more evenly distributed and more diverse. Compared with the HSD group, the Chao1 index of mice in the THSWD, TSD-64, THSWD + TSD-64 group pointed out the decreasing trends (P > 0.05; P > 0.05; P > 0.05) while the Shannon index increased, with a significant increase in the THSWD + TSD-64 group (P < 0.01). In summary, THSWD combined with Dubosiella newyorkensis has a beneficial effect on the diversity of the gut contents microbiota of mice.
To assess the similarities and differences in community structure between the different groups, we carried out Beta diversity analysis of the sequencing data from the five different groups. PCoA and NMDS analysis based on the Unweighted UniFrac distance matrix (Fig. 5D–G) presented significant dispersion between groups and significant clustering within groups. Among them, the THSWD + TSD-64 group and the HSD group had different structural distribution characteristics, so THSWD combined with Dubosiella newyorkensis had the regulatory effect on the gut contents bacterial community of mice with hypertension.
3.3.3. Analysis of dominant bacteria and characteristic bacteria
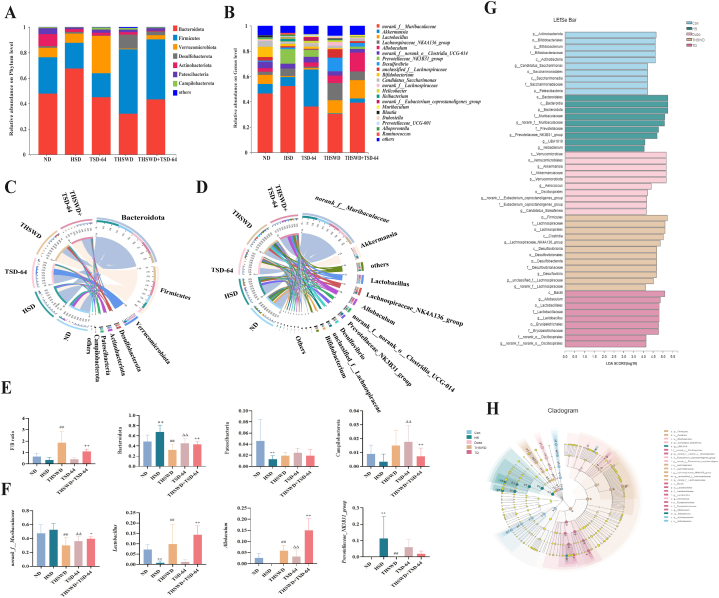
To obtain further information on the modulating effect of THSWD combined with Dubosiella newyorkensis on the gut contents microbiota of hypertensive mice, we analyzed the composition of the gut contents microbiota at different taxonomic levels. The results of the community composition at the phylum level (Fig. 6A, C) showed that Bacteroidota was the dominant phylum with the largest proportion in the five groups. Statistical analysis revealed (Fig. 6E) that the relative abundance of Bacteroidota was significantly higher in the gut contents microbiota of mice in the HSD group compared to the ND group (P < 0.01), while the relative abundance of Patescibacteria and Campilobacterota decreased (P < 0.01; P > 0.05). Compared with the HSD group, the relative abundance of Bacteroidota in the THSWD, TSD-64 and THSWD + TSD-64 groups was markedly lower (P < 0.01; P < 0.01) and Patescibacteria all presented an increasing trend (P > 0.05; P > 0.05; P > 0.05). The relative abundance of Campilobacterota was elevated in the TSD-64 and THSWD + TSD-64 groups, and the difference was significant. Besides, we evaluated the Firmicutes/Bacteroidota (F/B) ratio in each group of mice, and the results suggested that THSWD combinated with Dubosiella newyorkensis obviously promoted the F/B ratio in the gut contents microbiota of hypertensive mice.
Fig. 6.
Analysis of dominant bacteria and characteristic bacteria of gut contents in mice (n = 6). (A) Phylum level histogram. (B) Species level histogram. (C) Chord chart of phylum level. (D) Chord chart of species level. (E) Dominant phylum with significant variation. (F) Dominant species with significant variation. (G) LDA score chart. (H) Cladogram diagram. ND, control group; HSD, hypertension model group; THSWD, THSWD treatment group; TSD-64, Dubosiella newyorkensis treatment group; THSWD + TSD-64, THSWD + Dubosiella newyorkensis treatment group. The values were expressed as mean ± standard deviation. **p < 0.01 (HSD vs ND), ##p < 0.01 (HSD vs THSWD), ΔΔp<0.01 (HSD vs TSD-64), ++p < 0.01 (HSD vs THSWD + TSD-64).
The results of genus level community composition presented that norank_f_Muribaculaceae was the dominant genus with the largest proportion among the five groups (Fig. 6B, D). Statistical analysis revealed (Fig. 6E) that the relative abundance of norank_f_Muribaculaceae and Prevotellaceae_NK3B31_group in the HSD group was enriched compared to the ND group (P > 0.05; P < 0.01), while Lactobacillus and Allobaculum decreased (P < 0.01; P > 0.05). Compared to the HSD group, the relative abundance of norank_f_Muribaculaceae in the THSWD, TSD-64 and THSWD + TSD-64 groups was enormously downregulated (P < 0.01; P < 0.01; P < 0.01) and the Prevotellaceae_NK3B31_group in the THSWD group was remarkably lower (P < 0.01). The relative abundance of Lactobacillus and Allobaculum was elevated in the THSWD group and THSWD + TSD-64 group, and the differences were obvious. Hence, there were noteworthy changes in the composition of the dominant bacteria at the phylum and genus levels in the gut contents microbiota of mice after THSWD combined with Dubosiella newyorkensis.
To analyse changes in the composition of the microbiota of the gut contents microbiota of mice in the different treatment groups, we used linear discriminant effect size (LEfSe) analysis to identify characteristic taxa in each group. The results presented that 10, 9, 12, 10 and 9 characteristic taxa in the ND、HSD、THSWD、TSD-64 and THSWD + TSD-64 groups, respectively (Fig. 6G and H). Among them, the HSD group was markedly enhanced in genera norank_f_Muribaculaceae, Prevotellaceae_NK3B31_group, UBA1819 and Ileibacterium. The THSWD group was rich in genera Desulfovibrio and norank_f_Lachnospiraceae. The TSD-64 group was characterized by a greater increase in the abundance of genera Akkermansia, Aerococcus, norank_f_Eubacterium_coprostanoligenes_group and Candidatus_Soleaferrea. The THSWD + TSD-64 group was considerably enhanced in genera Allobaculum, Lactobacillus and norank_o_Oscillospirales. Collectively, there were significant differences in the characteristic bacteria among the five groups in the different classification systems.
THSWD combined with Dubosiella newyorkensis treatment affected serum bile acid metabolism in hypertensive mice.
3.3.4. Principal component analysis
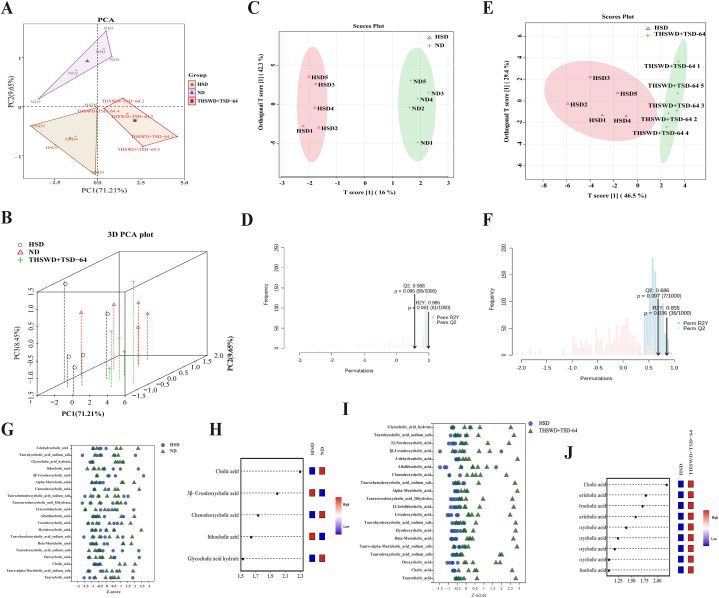
Principal component analysis (PCA) was used to reflect the overall metabolic differences between the HSD and THSWD + TSD-64 groups as a whole and the magnitude of variability between samples within the groups. In terms of differences between groups (Fig. 7A and B), the ND, HSD and THSWD + TSD-64 groups were clearly separated at PC1 (71.21 %) and PC2 (9.65 %), suggesting greater metabolic differences between groups.
Fig. 7.
Effect of THSWD combined with Dubosiella newyorkensis on serum bile acid metabolism in hypertensive mice (n = 5). (A) PCA score scatter plot. (B) PCA three-dimensional diagram. (C) OPLS-DA score plot (HSD vs ND). (D) Permutation test (HSD vs ND). (E) OPLS-DA score plot (HSD vs THSWD + TSD-64). (F) Permutation test (HSD vs THSWD + TSD-64). (G) Z-score analysis (HSD vs ND). (H) VIP value (HSD vs ND). (I) Z-score analysis (HSD vs THSWD + TSD-64). (J) VIP value (HSD vs THSWD + TSD-64). ND, control group; HSD, hypertension model group; THSWD + TSD-64, THSWD + Dubosiella newyorkensis treatment group.
3.3.5. Orthogonal partial least squares discriminant analysis
The metabolites that play an important role in the differences between groups were analyzed according to the Orthogonal partial least squares discriminant analysis (OPLS-DA) model and we plotted the scores for each subgroup. Q2, R2Y and R2X proformed the differences between the subgroups. In the comparison of the ND group and HSD group, the HSD and THSWD + TSD-64 group, the R2Y scores were all greater than 0.8 and close to 1, indicating that the model was relatively stable and reliable. Meanwhile, the Q2 of HSD and THSWD + TSD-64 groups was greater than 0.5 and p < 0.05, suggesting that THSWD combined with Dubosiella newyorkensis treatment caused differences in bile acid metabolism in hypertensive mice (Fig. 7C–F).
3.3.6. Differential metabolite screening
We performed a standard score (Z-score) conversion of the bile acid metabolite levels in the samples to measure the overall trend in serum bile acid metabolite levels at the same level in the ND and HSD groups, HSD and THSWD + TSD-64 groups of mice (Fig. 7G and H). Combining Variable Importance in the Projection (VIP) values ≥ 1, independent sample t-tests with P ≤ 0.05 and Fold change≥2 to find differentially expressed metabolites, five potentially characteristic metabolites that distinguished the ND group from the HSD group and nine potentially characteristic metabolites that distinguished the HSD from the THSWD + TSD-64 group (Fig. 7I and J). Cholic acid and chenodeoxycholic acid were positively regulated in the ND and HSD groups, while negatively regulated in the HSD and THSWD + TSD-64 groups. Here, we hypothesized that changes in cholic acid and chenodeoxycholic acid might be the main bile acid metabolites affecting THSWD combined with Dubosiella newyorkensis in the treatment of hypertensive mice.
3.3.7. Correlation analysis of gut contents microbiota-bile acid metabolism-endothelial factor in mice
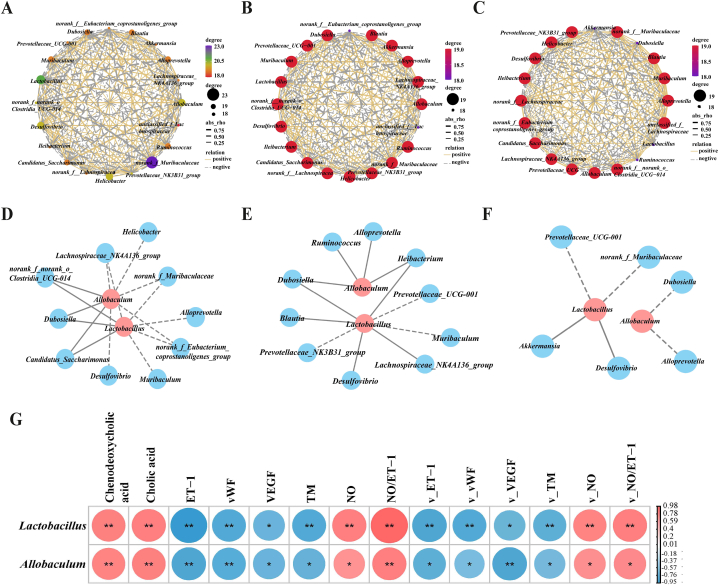
Screening spearman correlation analysis of interrelationships (Fig. 8A–C) was used to reveal the ecological relationships between microbiota in the overall gut contents microbial community of mice in the ND, HSD and THSWD + TSD-64 groups. The results presented that the overall regulatory relationship between gut contents microbiota was reduced in the THSWD + TSD-64 group of mice compared with the HSD group, and the positive regulatory effect between gut contents microbiota was increased and the negative regulatory effect was reduced. It was suggested that THSWD combined with Dubosiella newyorkensis changed the interactions between gut contents microbiota. Focusing on the association between Lactobacillus, Allobaculum and other characteristic bacteria (Fig. 8D–F), we found that THSWD combined with Dubosiella newyorkensis weakened the interaction between Lactobacillus, Allobaculum and other characteristic bacteria. Among them, the intercrossing networks specific to Lactobacillus and norank_f_Muribaculaceae, Lactobacillus and Akkermansia appeared in the THSWD + TSD-64 group. And the shared interaction network between Lactobacillus, Allobaculum and other characteristic bacteria was reduced compared to the HSD group. From the association as shown in Fig. 8G, Lactobacillus and Allobaculum were positively correlated with cholic acid and chenodeoxycholic acid, and the difference was significant. Besides, Lactobacillus and Allobaculum were negatively correlated with ET-1, vWF, VEGF, TM, v_ET-1, v_vWF, v_VEGF, v_TM, and negatively correlated with NO, NO/ET-1, v_NO, and v_NO/ET-1, and the differences were significant.
Fig. 8.
Correlation analysis of gut contents microbiota-bile acid metabolism-endothelial factor in mice. (A) Interaction network of “characteristic genera-characteristic genera” in the ND group. (B) Interaction network of “characteristic genera-characteristic genera” in the HSD group. (C) Interaction network of “characteristic genera-characteristic genera” in the THSWD + TSD-64 group. (D) Interaction network of “Lactobacillus, Allobaculum-others characteristic genera” in the ND group. (E) Interaction network of “Lactobacillus, Allobaculum-others characteristic genera” in the HSD group. (F) Interaction network of “Lactobacillus, Allobaculum-others characteristic genera” in the THSWD + TSD-64 group. (G) Associated Heatmap.
4. Discussion
Gut microbiota are known as the human "second genome" and play an important role in maintaining the health of the host [12]. Changes in gut microbiota can alter host physiology and increase the incidence and severity of many diseases [13]. Study confirmed [14] that transplantation of fecal bacteria from hypertensive patients to germ-free mice revealed a significant increase in systolic, diastolic and mean blood pressure in recipient mice compared to healthy controls. It indicated that the related alteration of gut microbiota might be a key cause of the elevated blood pressure and implied the importance of gut microbiota in blood pressure regulation. In our previous experiments, THSWD affected hypertension by regulating the composition of gut microbiota. Given the strong association between herbal medicine, gut microbiota, and hypertension, combining herbal medicine with targeted gut microbiota therapy may be a potential direction for the clinical treatment of hypertension. Combined with the results of this experiment, the microbiota diversity of mouse gut contents was significantly increased after the intervention of THSWD combined with Dubosiella newyorkensis. F/B, Lactobacillus and Allobaculum were significantly up-regulated in the gut contents, and Bacteroidota, Campilobacterota and norank_f_Muribaculaceae were markedly down-regulated. During the screening of characteristic genera, Lactobacillus and Allobaculum were significantly enriched in the THSWD + TSD-64 group. Combining the relative abundance and LEfSe analysis, we found that Lactobacillus and Allobaculum acted as both dominant and characteristic genera. Taken together, THSWD combined with Dubosiella newyorkensis reshaped the gut microbiota in hypertensive mice. Lactobacillus and Allobaculum might play an important role in the intervention of THSWD combined with Dubosiella newyorkensis in hypertension.
Besides, hypertension is a cardiovascular syndrome that is a major and common trigger and cause of the development of cardiovascular and cerebrovascular diseases; it is also a disease that is closely related to metabolism [15]. Metabolic disorders play an important role in the development of hypertension and in some cases have a causal relationship. Bile acids are the main components of bile, and their synthesis and metabolism are completed under the participation of gut microbiota [16]. Various pathological factors, on top of causing dysbiosis of the gut microbiota, affect bile acid homeostasis, which lead to the development and progression of diseases, including hypertension [17]. Among them, higher levels of Bacteroidota were found in the gut of hypertensive patients than in healthy controls, and chenodeoxycholic acid levels were elevated, analyzing the reason might be related to the bile salt hydrolysis of Bacteroidota [18]. Cholic acid also plays a bidirectional role in regulating blood pressure [19,20]. In summary, the interaction between bile acids and gut microbiota might affect the occurrence and development of hypertension. In our experiment, we also found that cholic acid and chenodeoxycholic acid might be involved as differential metabolites in the intervention of THSWD combined with Dubosiella newyorkensis in hypertension.The intestinal dialogue between gut microbiota and bile acids plays vital roles in host metabolism. Lactobacillus is the main probiotic in the gut and plays a crucial role in maintaining the microecological balance of the gastrointestinal tract [21]. The Study found that Lactobacillus was the main group of bacteria involved in the 7α-dehydroxylation reaction of cholic acid to DCA [22]. Lactobacillus showed positive regulation with chenodeoxycholic acid [23], which was consistent with our experimental results. Futhermore, Lactobacillus possessed bile salt hydrolase (BSH) activity, which promoted the hydrolysis of bile acids in the gut by regulating BSH activity [24,25]. Similarly, Ushiroda et al. confirmed an association between Allobaculum and cholic acid [26]. From this, we hypothesized that the interaction between Lactobacillus, Allobaculum and cholic acid, chenodeoxycholic acid might influence the course of THSWD combined with Dubosiella newyorkensis in the treatment of hypertension.
Studies have shown that the development of hypertension is closely related to blood rheological abnormalities and microcirculatory disorders caused by vascular endothelial dysfunction [27]. The vascular endothelium is the largest organ with endocrine function in the body, and damage to the vascular endothelium causes dependent diastolic dysfunction and promotes the progression of hypertension [28]. ET-1 and NO are important vasoactive substances secreted by vascular endothelial cells (VEC). Under physiological conditions, ET is always in balance with NO, thus maintaining normal vasodilation and contraction, and is a representative indicator of vascular endothelial function [29]. VEGF, a more specific vascular endothelial cytokine, leads to increased vascular permeability, vascular endothelial dysfunction, and increased sensitivity to vascular pressor substances, resulting in varying degrees of pathological changes [30]. As a multimeric glycoprotein synthesized and released by vascular endothelial cells, when vascular endothelial cells are damaged, vWF is rapidly released into the blood to cause platelet coagulation and further thrombus formation [31]. TM is a thrombin-regulating protein, which is synthesized and secreted by vascular endothelial cells. Endothelial cells in a physiological state do not secrete and release TM, and when vascular endothelial cells are damaged, serum TM can be an important indicator of the degree of vascular endothelial cell damage [32]. It was found that THSWD promoted endothelial cell proliferation, regulated VEC synthesis and released of active substances, inhibitd endothelial cell apoptosis, and promoted angiogenesis [33]. In the previous experiments, we also confirmed the protective effect of Dubosiella newyorkensis on high salt-induced endothelial function in mice. Therefore, we investigated the effects of THSWD combined with Dubosiella newyorkensis on vascular endothelial function in hypertensive mice. The results presented that compared with the HSD group, the levels of ET-1, TM, vWF and VEGF in the serum and blood vessels of mice in the THSWD + TSD-64 group were decreased, while NO and NO/ET-1 were increased, and the above indexes were significantly different. To sum up, THSWD combined with Dubosiella newyorkensis markedly improved hypertension-induced vascular endothelial dysfunction. Moreover, gut microbiota plays an active role in improving the disease process by regulating vascular endothelial function. Wang et al. demonstrated that Lactobacillus acidophilus could increase NO bioavailability and protect endothelial function thus exerting an anti-atherosclerotic effect [34]. Malik et al. pointed out that Lactobacillus plantarum 299v supplementation had the effect of improving vascular endothelial dysfunction in patients with stable coronary artery disease [35]. In the correlation analysis of this experiment, we confirmed the close association between the Lactobacillus, Allobaculum and indicators related to vascular endothelial function. So, we speculated that Lactobacillus and Allobaculum might be the modulators in THSWD combined with Dubosiella newyorkensis to improve hypertension-induced vascular endothelial dysfunction. However, the exact mechanism still needs to be further explored.
Additionally, the main active ingredients of THSWD, such as saffron yellow pigment, gallic acid, and ligustrolactone, are known to lower blood pressure, protect cardiovascular and cerebrovascular, and regulate human physiological functions [[36], [37], [38]]. In previous experiments, we confirmed the function of Dubosiella newyorkensis to protect against high salt-induced blood pressure in mice. So, we investigated the effects of THSWD combined with Dubosiella newyorkensis on SBP, body weight and blood routine in hypertensive mice. The results pointed out that the mice in the THSWD + TSD-64 group had higher body weight and significantly lower SBP than those in the HSD group, suggesting that the intervention of THSWD combined with Dubosiella newyorkensis was able to reduce SBP and promote body weight in hypertensive mice.
5. Conclusions
To sum up, the current study emphasized that treatment with THSWD combined with Dubosiella newyorkensis attenuated endothelial dysfunction and effectively controlled blood pressure in hypertensive mice. The mechanism of action was related to gut microbiota and bile acid metabolism. These data suggested that gut microbiota played an important role in THSWD combined with Dubosiella newyorkensis in the regulation of hypertensive vascular endothelial dysfunction induced by high-salt diet. But, the tissue of vascular endothelial dysfunction in mice was not pathologically analyzed, only some indicators reflecting endothelial dysfunction were analyzed, which is also the limitation of this study. Of course, further studies are needed to further investigate the detailed mechanisms of the complete metabolic profile and then to relate it to health and disease conditions.
Data availability statement
The data underlying this study was available within the manuscript. The gut content microbiota sequencing data has been uploaded to the NCBI database (https://www.ncbi.nlm.nih.gov/), no. PRJNA919161.
CRediT authorship contribution statement
Tianhao Liu: Conceptualization, Methodology, Writing – original draft. Xiaoya Li: Conceptualization, Methodology, Visualization. Chenyang Zhang: Visualization, Writing – review & editing. Lin Zhao: Conceptualization, Methodology, Visualization. Yahong Zhou: Conceptualization, Methodology, Writing – original draft. Xue Li: Conceptualization, Methodology, Writing – original draft. Yusheng Yu: Conceptualization, Methodology, Visualization.Yuzheng Xue: Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was supported by the National Natural Sciences Foundation of China (82305072, 82305064, 82174148), Natural Science Foundation of Jiangsu Province (BK20230184), Science and technology development project of Jiangsu Provincial Administration of Traditional Chinese Medicine (YB2020043), Wuxi Municipal Health Commission Scientific Research Fund Youth Project (Q202106, Q202225), The Taihu Lake Talent Project of Wuxi Science and Technology Bureau (K20221027), and Doctoral talent startup fund Affiliated of Hospital of Jiangnan University.
Contributor Information
Yahong Zhou, Email: yhzhoutwo@163.com.
Yuzheng Xue, Email: 9862018034@jiangnan.edu.cn.
References
- 1.Wang Z.W., Chen Z., Zhang L.F., Wang X., Hao G., Zhang Z.G. Status of hypertension in China: results from the China hypertension survey, 2012-2015. Circulation. 2018;137:2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 2.Hunter P.G., Chapman F.A., Dhaun N. Hypertension: current trends and future perspectives. Br. J. Clin. Pharmacol. 2021;87:3721–3736. doi: 10.1111/bcp.14825. [DOI] [PubMed] [Google Scholar]
- 3.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touyz R.M., Schiffrin E.L. A compendium on hypertension: new advances and future impact. Circ. Res. 2021;128:803–807. doi: 10.1161/CIRCRESAHA.121.319181. [DOI] [PubMed] [Google Scholar]
- 5.Paczula A., Wiecek A., Piecha G. Cardiotonic steroids-a possible link between High-Salt diet and organ damage. Int. J. Mol. Sci. 2019;20:590. doi: 10.3390/ijms20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Q. Curative effect observation of hypertension nephropathy with modified Shengqidihuang Decoction and Taohongsiwu Decoction. J. Henan. Univer. Chin. Med. 2006;6:1–3. [Google Scholar]
- 7.Konukoglu D., Uzun H. Endothelial dysfunction and hypertension. Adv. Exp. Med. Biol. 2017;956:511–540. doi: 10.1007/5584_2016_90. [DOI] [PubMed] [Google Scholar]
- 8.Chen W.H., Liu T.H., Liang Q.E., Chen X.D., Tao W.C., Fang M.X., Xiao Y., Chen L.G. miR-1283 contributes to endoplasmic reticulum stress in the development of hypertension through the activating transcription factor-4 (ATF4)/C/EBP-Homologous protein (CHOP) signaling pathway. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2021;27 doi: 10.12659/MSM.930552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Q.E., Liu T.H., Guo T.T., Tao W.C., Chen X.D., Chen W.H., Chen L.G., Xiao Y. ATF4 promotes renal tubulointerstitial fibrosis by suppressing autophagy in diabetic nephropathy. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118686. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y., Chen W.H., Chen R.X., Luo A.L., Chen D.Y., Liang Q.E., Liu T.H., Chen X.D., Tan W. Exosomal MicroRNA expression profiling analysis of the effects of lycium barbarum polysaccharide on gestational diabetes mellitus mice. Evid. Based. Complement. Alternat. Med. 2020;2020 doi: 10.1155/2020/2953502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T.H., Tao W.C., Liang Q.E., Tu W.Q., Xiao Y., Chen L.G. Gut microbiota-related evidence provides new insights into the association between activating transcription factor 4 and development of salt-induced hypertension in mice. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.585995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y.T., Huang W.G., Zhao R. Diagnostic value of intestinal microeclogic balance monitoringin pregnancy-induced-hypertension. Internet J. Lab. Med. 2022;43:1389–1394. [Google Scholar]
- 13.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases: a review. Antonie. Van. Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 14.Li J., Zhao F.Q., Wang Y.D., Chen J.R. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu D., Fang N.Y., Wang H.Y. The relationship between bile acid and hypertension. Chin. J. Geriatrics. Res. 2021;8:41–44. [Google Scholar]
- 16.Zhao Y.C., Cui N.Q. Research progress on the correlation between bile acids and intestinal flora. Chin. J. Sur. Inte. Trad. Wes. Med. 2018;24:666–671. [Google Scholar]
- 17.Jin H., Zhang L.L. Bile acid regulation is a potential new intervention approach for hypertension. Chin. J. Hypertension. 2022;30:318–323. [Google Scholar]
- 18.Li X. Shanghai Jiaotong University; 2020. Study on Gastrointestianal Microbiota and its Relationship with Bile Acid Metabolism in Hypertension Patients with or without Nephropathy. [Google Scholar]
- 19.Morris D.J., Souness G.W. Endogenous 11 beta-hydroxysteroid dehydrogenase inhibitors and their role in glucocorticoid Na+ retention and hypertension. Endocr. Res. Commun. 2012;22:793–801. doi: 10.1080/07435809609043778. [DOI] [PubMed] [Google Scholar]
- 20.Al-Salami H., Mamo J.C., Mooranian A., Negrulj R., Lam V., Elahy M., Takechi R. Long-term supplementation of microencapsulated ursodeoxycholic acid prevents hypertension in a mouse model of insulin resistance. Exp. Clin. Endocrinol. Diabetes. 2016;125:28–32. doi: 10.1055/s-0042-106084. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z.X., Wang T., Jiang Z.Z., Ye Y.F., Xie C. Research progress of FXR regulating bile acid synthesis and transport. Central. South. Phar. 2010;8:374–377. [Google Scholar]
- 22.Jia W., Xie G., Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018;15:111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Zhao H., Sun G., Duan Y., Guo Y., Xie L., Ding X.F. Alterations in the gut microbiome and metabolome profiles of septic rats treated with aminophylline. J. Transl. Med. 2022;20:69. doi: 10.1186/s12967-022-03280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Valdez G.F., Martos G., Taranto M.P. Influence of bile on beta-galactosidase activity and cell viability of Lactobacillus reuteri when subjected to freeze-drying. J. Dairy Sci. 1997;80:1955–1958. doi: 10.3168/jds.S0022-0302(97)76137-X. [DOI] [PubMed] [Google Scholar]
- 25.Saikia D., Manhar A.K., Deka B. Hypocholesterolemic activity of indigenous probiotic isolate Saccharomyces cerevisiae ARDMC1 in a rat model. J. Food Drug Anal. 2018;26:154–162. doi: 10.1016/j.jfda.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ushiroda C., Naito Y., Takagi T. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019;65:34–46. doi: 10.3164/jcbn.18-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C., Wu Y., Ma P.D. Effect of aerobic exercise on microvascular rarefaction in skeletal muscle of spontaneously hypertensive rats. Chin. J. Arteriosclerosis. 2018;26:691–697. [Google Scholar]
- 28.Wang Y.P., Zheng W.W., Xia M. Lactobacillus acidophilus regulates NO and its oxidative mediator in atherosclerosis model rats. Tianjin Med. J. 2019;47:32–37. [Google Scholar]
- 29.Sonja S. The clinical significance of endocardial endothelial dysfunction. Medicina. 2017;53:295–302. doi: 10.1016/j.medici.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Breslow M.J., Jordan D.A., Christopherson R. Epidural morphine decreases postoperative hyperactivity. Jama. J. American. Med. Associat. 2016;261:3577–3581. [PubMed] [Google Scholar]
- 31.Springer T.A. von Willebrand factor, Jedi knight of the bloodstream. Blood. 2014;124:1412–1425. doi: 10.1182/blood-2014-05-378638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Q.S., Bai C.M., Bian X. Investigation of thrombomodulin and plasminogen activator inhibitor type-I in pregnancy induced hypertension and its clinical significance. Chin. Med. Sci. J. 2001;16:169–171. [PubMed] [Google Scholar]
- 33.Liu Q.Z., Peng D.Y., Yin D.K. Research progress on the effects of Taohong Siwu Decoction and its active components on vascular endothelial cells. J. Anhui. Univ. Chin. Med. 2021;31:78–80. [Google Scholar]
- 34.Wang Y.P., Zheng W.W., Xia M. Lactobacillus acidophilus regulates NO and its oxidative mediator in atherosclerosis model rats. Tianjin Med. J. 2019;47:32–37. [Google Scholar]
- 35.Malik M., Suboc T.M., Tyagi S. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ. Res. 2018;123:1091–1102. doi: 10.1161/CIRCRESAHA.118.313565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li F., Deng Y., Duan C.H. The research progress on the mechanism of safflow er anti cerebral ischemia injury. Chin. Med. Mod. Dis. Edu. China. 2018;16:151–154. [Google Scholar]
- 37.Zhang Q.S. Dalian Medical University; 2021. Role and Molecular Mechanism of Gallic Acid in Angiotensin Ⅱ-induced Hypertension. [Google Scholar]
- 38.Yi L., Wang L.H., Ji L.F. Effects of ligustilide on AT1R gene expression in spontaneously hypertensive rats. J. Tradit. Chin. Vet. Med. 2018;37:9–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study was available within the manuscript. The gut content microbiota sequencing data has been uploaded to the NCBI database (https://www.ncbi.nlm.nih.gov/), no. PRJNA919161.