Summary
Background
Duchenne muscular dystrophy (DMD) is a disabling and life-threatening, X-linked recessive disorder caused by mutations in dystrophin. Natural history studies can inform the disease characteristics of DMD, and data from these studies can be used to plan and design clinical trials and as external controls for long-term studies. We report 12-month results from the largest natural history study of individuals with DMD in China receiving standard of care treatment.
Methods
This ongoing, multicentre, prospective, single-cohort study (ClinicalTrials.gov: NCT03760029) was conducted in Chinese male participants with DMD (ambulatory aged <6 years [Group 1; n = 99]; ambulatory aged ≥6 years [Group 2; n = 177], and non-ambulatory of any age [Group 3; n = 36]. The follow-up period is ≥24 months, with some participants followed for 30 months. The primary endpoint was time to clinical milestones due to DMD disease progression, and motor, pulmonary, and cardiac function. Secondary endpoints were quality of life (QoL) assessments.
Findings
Mean (standard deviation [SD]) age at screening was 3.4 (1.2), 8.6 (2.0), 12.3 (2.7) and 7.4 (3.5) years in Groups 1, 2, 3 and total respectively. Mean (SD) North Star Ambulatory Assessment (NSAA) total score at baseline was 21.2 (5.8) in Group 1, 19.5 (8.3) in Group 2 and 20.0 (7.7) in ambulatory total. Overall, the time to clinical milestones due to DMD disease progression was consistent with previous findings, in which loss of ambulation occurred at 13 years. There was a trend towards a decline over 12 months in NSAA and timed motor function from age 6 years, with the greatest reductions observed thereafter. There were no consistent trends in measures of QoL, although participants of any age generally had poorer outcomes at Month 12 versus their domain scores at baseline.
Interpretation
This study improves the understanding of DMD progression according to the current standards of care in the Chinese DMD population and may inform selected endpoints and patient populations in clinical trials.
Funding
Pfizer Inc.
Keywords: Duchene muscular dystrophy, China, Natural history, Prospective study, Real-world, Disease characteristics
Research in context.
Evidence before this study
Duchenne muscular dystrophy (DMD) is a disabling and life-threatening, X-linked recessive disorder caused by mutations in dystrophin. Epidemiology data from large scale studies are limited in the Chinese DMD population; however, studies have shown that the incidence does not vary substantially by racial, ethnic, or geographic origins. The overall natural history of DMD has changed substantially over the past 20–30 years, although studies have primarily focused on the progression of lower extremity motor function of ambulation. This natural history prospective study was conducted in the largest population of individuals with DMD in China receiving standard of care treatment, providing a holistic evaluation of disease progression.
Added value of this study
This ongoing, multicentre, prospective, single cohort study in 312 participants with DMD demonstrates that the time to clinical milestones due to DMD disease progression was consistent with previous findings. There was a trend towards a decline over 12 months in function assessments, including North Star Ambulatory Assessment and timed motor function from the age of 6 years, followed by greater reductions. The findings were generally in line with disease progression in DMD. Although there were no consistent trends in measures of quality of life in this study, participants of any age generally tended to have poorer outcomes at Month 12 than their domain scores at baseline.
Implications of all the available evidence
All available evidence suggests an improved understanding of DMD progression according to the current standards of care is important in the Chinese DMD population and may inform selected endpoints and patient populations in clinical trials.
Introduction
Duchenne muscular dystrophy (DMD) is a disabling and life-threatening, X-linked recessive disorder caused by mutations in the DMD gene encoding dystrophin, a protein that protects muscle from damage during contraction.1,2 DMD is characterised by muscle weakness and functional consequences affecting ambulation and upper limb use, and resulting in progressive musculoskeletal deformities, impaired airway clearance and ventilation, cardiomyopathy, and premature death.3
The disease course of DMD has changed substantially over the past 30 years, with glucocorticoid (GC) therapy delaying the loss of ambulation by approximately 3 years after diagnosis.4,5 Indeed, the course of DMD can be influenced by the age at which GC therapy is started, the GC regimen and mechanical ventilation.6, 7, 8, 9, 10
Natural history studies in DMD are typically conduced in patients receiving the current standard of care and/or emergent care and generate real-world evidence using data from disease registries.11 As DMD is a heterogenous disease, there is a need for improved, robust studies; as such, data from natural history studies can inform the disease characteristics in order to plan and design interventional clinical trials in DMD.4 Moreover, a report from a workshop on the meaningful outcome measures in DMD highlighted that patients and their caregivers supported the utilisation of data from natural history studies, which could be used as external controls for long-term studies, or to support accelerated approvals of biomarker drugs based on post-marketing trials that need to be carried out in other countries.12 In addition, as there are currently 4 agents under the accelerated (biomarker) approval pathway in the US, post-marketing studies required to support these approvals require post-marketing trials that need to be conducted in countries, other than the US.
Epidemiology data from large scale studies in the Chinese population are limited, and although data from some Chinese individuals with DMD have been published,13, 14, 15, 16 the natural history of DMD in the Chinese population remains poorly defined. Although several recent studies have provided insight into the natural history of DMD, these were primarily focused on the progression of lower extremity motor function, particularly on ambulation.17,18 Data showing the progression of upper extremity weakness or pulmonary disease in DMD are limited.19,20
Herein we report 12-month results from the largest prospective study to date of DMD in China and provide a holistic evaluation of disease progression, including motor function, pulmonary and cardiac function, and quality of life (QoL) for those receiving standard of care treatment.
Methods
Study design and participants
Study C3391004 is an ongoing, multicentre, prospective, single-cohort study (ClinicalTrials.gov: NCT03760029) designed to characterise the natural history of Chinese male participants with DMD. The follow-up period is at least 24 months, and some participants will be followed for 30 months. The study is being conducted at six sites (Chongqing, Fujian, Beijing, and Shanghai) in China, and this manuscript reports data from an unplanned interim analysis of the study. The Statistical Analysis Plan was finalized before this unplanned analysis; therefore, pre-specified analysis approaches were available.
All potentially eligible individuals were invited to participate. All questionnaires used were validated. Eligible participants were Chinese males of any age with a genetic diagnosis of DMD. Participants with any injury that would impact functional testing, the presence or history of other musculoskeletal or neurologic disease or somatic disorders not related to DMD, those aged ≥4 years who had not completed the varicella vaccination, and those involved in any clinical trial involving an investigational drug within 90 days of study entry were excluded.
Ethics
The study was conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects (Council for International Organisations of Medical Sciences 2002), International Council for Harmonisation Guidelines for Good Clinical Practice, and the Declaration of Helsinki. The protocol was approved by the relevant institutional review board/independent ethics committee at each study site. All participants (or parent/legal guardian) provided written informed consent.
Primary endpoints
The primary endpoints included time to clinical milestones due to DMD disease progression: age at failure to walk, age at failure to stand, and age at failure to self-feed. Data on loss of ability to walk, stand or self-feed were collected retrospectively in participants who were not able to perform these functions at baseline. Age at failure to walk, stand or self-feed was defined as the age at the date of failure to walk, stand or self-feed. Participants who did not have data for failure to walk, stand or self-feed were censored on the day of their last visit. Primary endpoints also consisted of change from baseline in function assessments, as listed below and outlined in Supplementary Table S1.
Function assessments
All functional assessments were conducted by trained physiotherapists (or exercise physiologists) (CEs). Training and confirmation that the functional assessments were performed reliably were provided by a vendor (master physiotherapist [MP]). Following the completion of training and reliability testing, a certificate was provided, which was in place at each site prior to conducting any functional assessments. In order to ensure ongoing quality of the CEs abilities to perform functional assessments, pre-specified visits were recorded, and videos were reviewed by the MPs to provide feedback to the CEs on the conduct of the method used to perform the functional assessments.
Motor function assessments
North Star Ambulatory Assessment and performance of the upper limb 2.0
Changes from baseline to Month 6 and Month 12 in the North Star Ambulatory Assessment (NSAA)21 total score (a 17-item test that grades functional skills) were reported in ambulatory participants aged ≥3 years.
Changes from baseline to Month 6 and Month 12 in the performance of the upper limb [PUL] 2.0 total score (a scale with assessment of shoulder, elbow, wrist, and hand function) were reported in participants aged ≥10 years.
Strength assessments
Muscle strength was quantified by means of a handheld myometry. Changes from baseline to Month 6 and Month 12 in muscle strength included knee extension, elbow flexion, elbow extension, and shoulder abduction and were reported in participants aged ≥5 years.
Range of motion
Change from baseline to Month 6 and Month 12 in range of motion (ROM) included bilateral ankles and elbows were reported in participants of all ages.
Timed motor function
Changes from baseline to Month 6 and Month 12 timed function tests from the NSAA; rise from floor velocity (in ambulatory participants aged ≥3 years) and 10-m walk/run (10 m w/r) velocity (in ambulatory participants aged ≥3 years). The rise from floor velocity was defined as the reciprocal of the time to rise from the floor. For participants with an NSAA Equivalent Activity score of 0 on the NSAA item of rise from floor, the velocity was set to 0. For participants with a missing score on the NSAA item of rise from floor, the velocity was set to missing. The 10-m w/r velocity was defined as 10 divided by the time to complete the 10 m w/r test. For participants with an NSAA Equivalent Activity score of 0 on the NSAA item of run (10 m), the velocity was set to 0. For participants with a missing score on the NSAA item of run (10 m), the velocity was set to missing.
Pulmonary function assessments (aged ≥6 years)
Pulmonary function testing was performed in participants aged ≥6 years to evaluate the maximal lung function using spirometry. Assessments included change from baseline to Month 12 in pulmonary function tests, including percent predicted forced vital capacity (%pFVC), percent predicted forced expiratory volume in 1 s (%pFEV1), maximum inspiratory pressure (MIP), maximum expiratory pressure (MEP), and peak cough flow (PCF).
Echocardiogram (aged ≥6 years)
Echocardiograms were performed using a 2-D imaging collection method. Change from baseline to Month 12 in left-ventricular ejection fraction (LVEF) in participants aged ≥6 years was reported.
Secondary assessments
Quality of life
The self-administered EuroQoL 5 Dimension three-level version (EQ-5D-3L) questionnaire was completed to assess the current overall health status of participants aged ≥16 years. The EQ-5D-Y (adapted from the EQ-5D) questionnaire was used for participants aged <16 years.
The descriptive system of questionnaires comprised five dimensions, including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension had three potential responses (no problems, some problems, and extreme problems). These responses were subsequently converted into a single index value ranging from 0 (death) to 1 (full health) using the specific Chinese value set.22,23 An index value <0 was classified as a bad health status worse than death.
The visual analogue scale (VAS) of the questionnaire recorded self-rated health ranging from 0 (the worst health) to 100 (the best health). Both the index values and VAS were used to describe the QoL of participants, with greater values indicating better QoL.
Data analysis
No formal statistical hypothesis testing was performed for this study. The study included three groups of participants: ambulatory participants aged <6 years (Group 1); ambulatory participants aged ≥6 years (Group 2), and non-ambulatory participants of any age (Group 3).
Data for continuous endpoints were summarised using descriptive statistics, including sample size, mean, standard deviation (SD), median, minimum, and maximum. Data for categorical endpoints were summarised using descriptive statistics using count and percentage. Time-to-event endpoints (age at failure to walk, age at failure to stand, and age at failure to self-feed) for each participant were defined as the time (in age) from the date of birth to the date of the event. Estimates of median time to event and survival probabilities by Kaplan–Meier method were presented.
Role of funding source
This study (NCT03760029; C3391004) was sponsored by Pfizer Inc. YG, YZ, CJ and JF are employees of Pfizer, and may hold stock and/or stock options. TP was an employee at the time of initiation of the manuscript and may hold stock and/or stock options.
Results
Participants
In total, 318 participants were screened between 24 July 2019 and 31 March 2021. Six participants did not meet eligibility criteria, and 312 participants comprised the full analysis set (Group 1, n = 99; Group 2, n = 177; Group 3, n = 36) (Supplementary Figure S1).
Mean (SD) age at screening was 3.4 (1.2), 8.6 (2.0), and 12.3 (2.7) years in Groups 1, 2 and 3, respectively, and 7.4 (3.5) years in the total population (Supplementary Figure S2). Mean (SD) age at initiation of GC therapy in Groups 1, 2 and 3 was 4.1 (0.5), 6.5 (1.9) and 8.1 (3.2) years, respectively, and 6.1 (2.3) years in the total population, with prednisone and prednisone acetate being the most common GC in this study. Mean (SD) duration of GC use was 11.7 (6.2), 29.9 (20.9) and 52.3 (36.5) months in Groups 1, 2 and 3, respectively, and 29.8 (24.8) months in the total population Mean (SD) NSAA total score at baseline was 21.2 (5.8) in Group 1 and 19.5 (8.3) in Group 2, and 20.0 (7.7) in the total ambulatory population (Table 1).
Table 1.
Baseline demographics and characteristics of the study population.
| Characteristic | Ambulatory, screening age <6 y |
Ambulatory, screening age ≥6 y |
Ambulatory |
Non-ambulatory of any age |
Total |
|---|---|---|---|---|---|
| n = 99a | n = 177a | n = 276a | n = 36a | N = 312 | |
| Age at screening, mean (SD), y | 3.4 (1.2) | 8.6 (2.0) | 6.7 (3.0) | 12.3 (2.7) | 7.4 (3.5) |
| Weight at baseline, mean (SD), kg | 16.5 (3.1) | 28.4 (8.0) | 24.1 (8.8) | 44.4 (11.7) | 26.5 (11.2) |
| Racial designation, n (%) | |||||
| Han Chinese | 94 (94.9) | 173 (97.7) | 267 (96.7) | 36 (100.0) | 303 (97.1) |
| Non-Han Chinese | 5 (5.1) | 4 (2.3) | 9 (3.3) | 0 | 9 (2.9) |
| Current residence setting, n (%) | |||||
| Urban | 77 (77.8) | 107 (60.5) | 184 (66.7) | 20 (55.6) | 204 (65.4) |
| Rural | 22 (22.2) | 70 (39.5) | 92 (33.3) | 16 (44.4) | 108 (34.6) |
| Prior GC use, n (%) | |||||
| Yes | 46 (46.5) | 177 (100.0) | 223 (80.8) | 36 (100.0) | 259 (83.0) |
| Duration of GC use, mean (SD), months | 11.7 (6.2) | 29.9 (20.9) | 26.2 (20.2) | 52.3 (36.5) | 29.8 (24.8) |
| Age at GC initiation, mean (SD), y | 4.1 (0.5) | 6.5 (1.9) | 5.8 (2.0) | 8.1 (3.2) | 6.1 (2.3) |
| Daily dose of GC, n; mean (SD) daily dose (mg/kg) | |||||
| Deflazacort | 6; 0.78 (0.09) | 22; 0.83 (0.12) | 28; 0.82 (0.12) | 2; 0.70 (0.16) | 30; 0.81 (0.12) |
| Methylprednisolone | 0; − (−) | 7; 0.48 (0.08) | 7; 0.48 (0.08) | 1; 0.53 (−) | 8; 0.49 (0.07) |
| Prednisolone | 1; 0.76 (−) | 3; 0.53 (0.05) | 4; 0.59 (0.12) | 0; − (−) | 4; 0.59 (0.12) |
| Prednisolone acetate | 5; 0.68 (0.05) | 1; 0.74 (−) | 6; 0.69 (0.05) | 0; − (−) | 6; 0.69 (0.05) |
| Prednisone | 22; 0.63 (0.09) | 70; 0.64 (0.35) | 92; 0.64 (0.31) | 23; 0.40 (0.12) | 115; 0.59 (0.29) |
| Prednisone acetate | 12; 0.61 (0.10) | 74; 0.56 (0.13) | 86; 0.57 (0.13) | 10; 0.42 (0.15) | 96; 0.55 (0.13) |
Data collected as the first visit were considered baseline assessments. Participants who were ≥4 years of age must have been receiving GC treatment. Daily dose (mg/kg) for each GC the participant received at baseline was calculated from the total daily dose and baseline weight.
GC, glucocorticoid; SD, standard deviation.
n denotes number of participants per group.
Primary assessments
Time to clinical milestones due to DMD disease progression
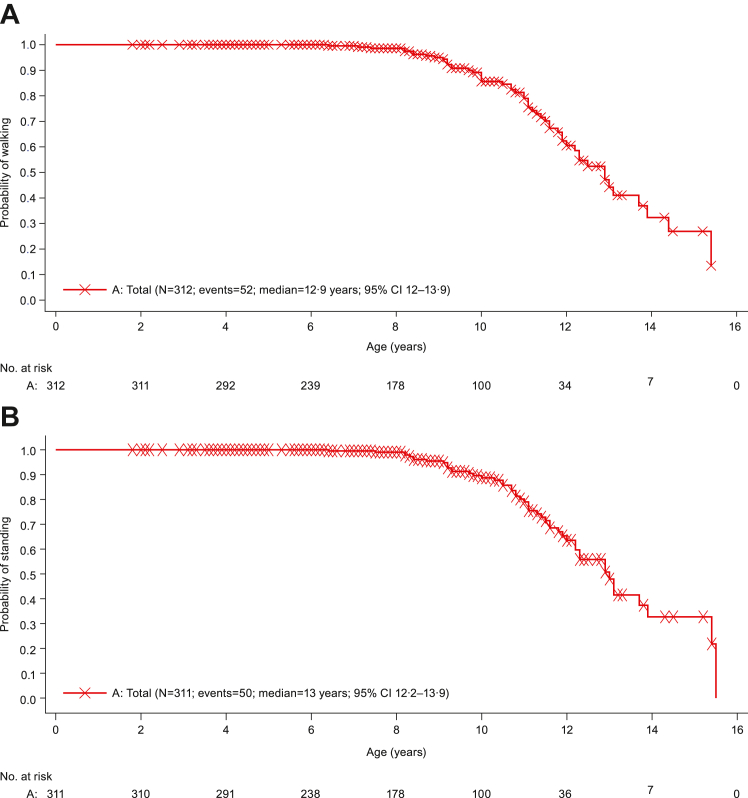
Kaplan–Meier plots of age at failure to walk and age at failure to stand are shown in Fig. 1A and B, respectively. Kaplan–Meier estimates of median (95% confidence interval [CI]) age at failure to walk was 15.4 (13.0, not evaluable) years in Group 2, 11.1 (10.0, 11.4) years in Group 3, and 12.9 (12.0, 13.9) years for total. All participants in Group 1 were able to walk. Kaplan–Meier estimate of median (95% CI) age at failure to stand was 15.4 (13.1, not evaluable) years in Group 2, 11.0 (10.5, 11.50) years in Group 3, and 13.0 (12.2, 13.9) years for total. All participants in Group 1 were able to stand. Kaplan–Meier estimate of median age at failure to self-feed was not evaluable (Supplementary Table S2). Data for age at failure to self-feed are not shown, as most participants were censored; only one participant from the non-ambulatory group experienced this event.
Fig. 1.
Kaplan–Meier plot of time to clinical milestones due to DMD disease progression: (A) Age at failure to walk and (B) Age at failure to stand (Group 1, 2, and 3 combined). Only included data until Month 12 visit. DMD, Duchene muscular dystrophy.
Function assessments
Motor function assessments
NSAA and performance of the upper limb 2.0
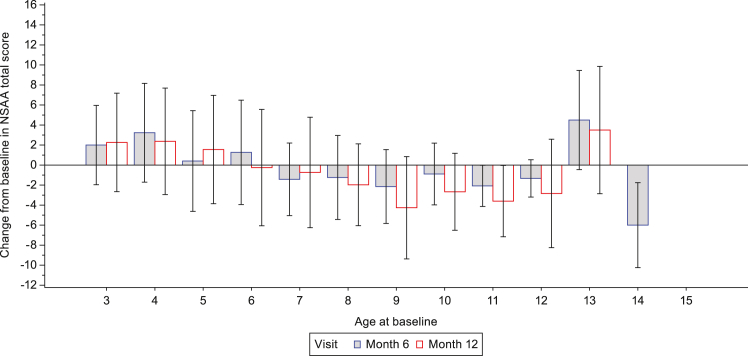
Mean (SD) change from baseline to Month 6 in NSAA total score was 1.7 (4.6) in Group 1, −1.0 (4.1) in Group 2 and −0.2 (4.4) in the total ambulatory population. Mean (SD) change from baseline to Month 12 in NSAA total score was 2.0 (5.1) in Group 1, −2.0 (5.0) in Group 2 and −0.9 (5.3) in the total ambulatory population (Table 2). The change from baseline in NSAA total score as a function of age at baseline is shown in Fig. 2.
Table 2.
Change from baseline in function assessments.
| Measure | Ambulatory, screening age <6 y |
Ambulatory, screening age ≥6 y |
Ambulatory |
Non-ambulatory of any age |
Total |
|---|---|---|---|---|---|
| n = 99a | n = 177a | n = 276 | n = 36a | N = 312 | |
| NSAA total score | |||||
| n | 70 | 176 | 246 | ||
| Mean (SD) at baseline | 21.2 (5.8) | 19.5 (8.3) | 20.0 (7.7) | ||
| n | 62 | 145 | 207 | ||
| Mean (SD) change at Month 6 | 1.7 (4.6) | −1.0 (4.1) | −0.2 (4.4) | ||
| n | 45 | 129 | 174 | ||
| Mean (SD) change at Month 12 | 2.0 (5.1) | −2.0 (5.0) | −0.9 (5.3) | ||
| PUL2.0 total score | |||||
| n | 51 | 51 | 31 | 82 | |
| Mean (SD) at baseline | 36.7 (4.9) | 36.7 (4.9) | 27.2 (9.3) | 33.1 (8.3) | |
| n | 43 | 43 | 4 | 47 | |
| Mean (SD) change at Month 6 | −0.2 (2.3) | −0.2 (2.3) | −0.8 (2.2) | −0.3 (2.3) | |
| n | 37 | 37 | 22 | 59 | |
| Mean (SD) change at Month 12 | −0.6 (2.1) | −0.6 (2.1) | −0.7 (2.3) | −0.6 (2.2) | |
| Rise from floor velocity,/s | |||||
| n | 80 | 177 | 257 | ||
| Mean (SD) at baseline | 0.2 (0.1) | 0.1 (0.1) | 0.2 (0.1) | ||
| n | 71 | 146 | 217 | ||
| Mean (SD) change at Month 6 | 0.0 (0.1) | −0.0 (0.1) | 0.0 (0.1) | ||
| n | 53 | 129 | 182 | ||
| Mean (SD) change at Month 12 | 0.0 (0.1) | −0.0 (0.1) | −0.0 (0.1) | ||
| 10 m w/r velocity, m/s | |||||
| n | 80 | 173 | 253 | ||
| Mean (SD) at baseline | 1.9 (0.5) | 1.7 (0.6) | 1.7 (0.6) | ||
| n | 71 | 143 | 214 | ||
| Mean (SD) change at Month 6 | 0.2 (0.4) | −0.1 (0.3) | 0.0 (0.3) | ||
| n | 53 | 124 | 177 | ||
| Mean (SD) change at Month 12 | 0.3 (0.4) | −0.1 (0.3) | −0.0 (0.4) | ||
| %pFVC | |||||
| n | 176 | 36 | 212 | ||
| Mean (SD) at baseline | 86.2 (17.1) | 68.2 (23.9) | 83.1 (19.6) | ||
| n | 139 | 27 | 166 | ||
| Mean (SD) change at Month 12 | −3.1 (161) | −6.7 (19.7) | −3.7 (16.7) | ||
| %pFEV1 | |||||
| n | 176 | 36 | 212 | ||
| Mean (SD) at baseline | 85.5 (18.4) | 65.4 (24.5) | 82.1 (20.9) | ||
| n | 139 | 27 | 166 | ||
| Mean (SD) change at Month 12 | −1.6 (18.8) | −5.0 (19.3) | −2.2 (18.8) | ||
| LVEF, % | |||||
| n | 177 | 36 | 213 | ||
| Mean (SD) at baseline | 66.2 (4.4) | 63.1 (7.4) | 65.7 (5.1) | ||
| n | 143 | 26 | 169 | ||
| Mean (SD) change at Month 12 | 4.7 (47.9) | −0.8 (6.3) | 3.9 (44.2) |
Pulmonary function tests and echocardiogram were only performed in participants ≥6 years of age.
%pFEV1, percent predicted forced expiratory volume in 1 s; %pFVC, predicted forced vital capacity; 10 m w/r, 10 m walk/run; LVEF, left-ventricular ejection fraction, NSAA, the North Star Ambulatory Assessment; PUL2.0, performance of the upper limb; SD, standard deviation.
n denotes number of participants per group.
Fig. 2.
Change from baseline in NSAA total score as a function of age at baseline (ambulatory participants). For Month 6: Ambulatory (screening age <6 years) (n = 62); Ambulatory (screening age ≥6 years) (n = 145). For Month 12: Ambulatory (screening age <6 years) (n = 45); Ambulatory (screening age ≥6 years) (n = 129). ∗ NSAA total score was based on assessment of 17 ambulatory actions and ranges from 0 to 34 with higher score indicating greater function ability and was only performed in ambulatory participants ≥3 years of age. Data are mean (SD). North Star Ambulatory Assessment, SD, standard deviation.
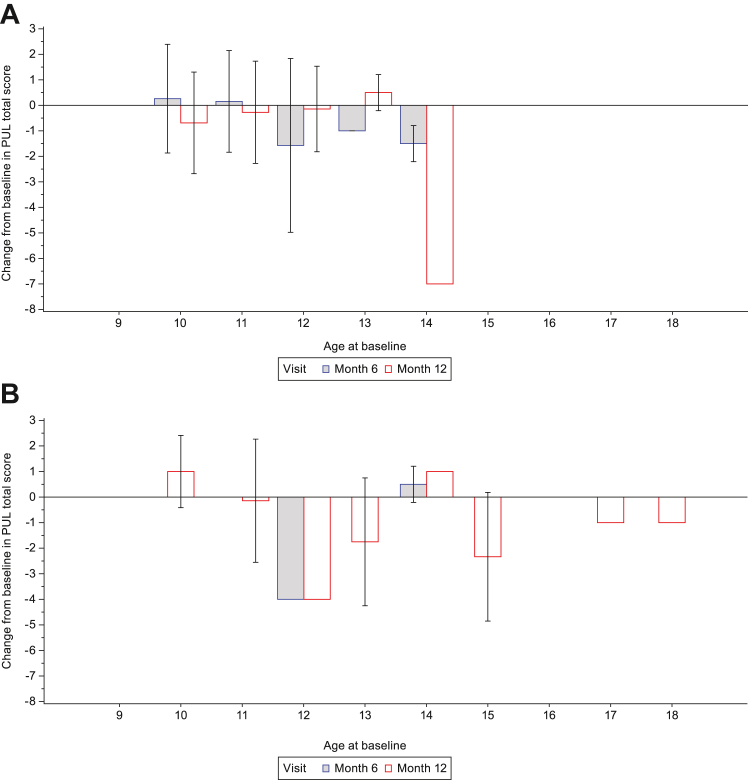
Mean (SD) change from baseline to Month 6 in PUL2.0 total score was −0.2 (2.3) in Group 2, −0.8 (2.2) in Group 3 and −0.3 (2.3) in the total population. Mean (SD) change from baseline to Month 12 in PUL2.0 total score was similar in both groups: −0.6 (2.1), −0.7 (2.3) and −0.6 (2.2) in Groups 2, 3 and the total population, respectively (Table 2). The change from baseline in PUL2.0 total score as a function of age at baseline is shown in Fig. 3.
Fig. 3.
Change from baseline in PUL2.0 total score as a function of age at baseline in (A) ambulatory participants and (B) non-ambulatory participants. For Month 6: Ambulatory (screening age ≥6 years) (n = 43); Non-ambulatory (n = 4). For Month 12: Ambulatory (screening age ≥6 years) (n = 37); Non-ambulatory (n = 22). Only data with the same preferred arm as baseline was included in the analysis. PUL2.0 total score was only performed in participants ≥10 years of age. Data are mean (SD). Missing error bars indicate n = 1. PUL, Performance of the Upper Limb; SD, standard deviation.
Strength assessments
There were numerical decreases in lower limb muscle strength; this was observed earlier than loss of strength in the upper limbs. Changes from baseline to Month 6 and Month 12 in strength of muscle groups (knee extension, elbow flexion, elbow extension, shoulder abduction) are shown in Supplementary Table S3. Overall, changes in strength of muscle groups were negligible over 6 and 12 months. However, at Month 6, there was a trend towards greater numerical changes from baseline in strength of muscle groups in participants from Group 1, than those in Groups 2 and 3 (Supplementary Table S3).
Range of motion
Changes from baseline to Month 6 and Month 12 in ROM are shown in Supplementary Table S4. Overall, lower limb ROM total score decreased from Month 6 in participants from Group 1, whereas changes in the upper limb were observed from Month 12. ROM decline in the upper and lower limbs was greater in participants from Group 3 than those in Groups 2 (Supplementary Table S4).
Timed motor function
There was no change from baseline to Month 6 or Month 12 in rise from floor velocity (Groups 1, 2 and the total ambulatory population) (Table 2). Mean (SD) change from baseline to Month 6 in 10 m w/r velocity was 0.2 (0.4) m/s in Group 1, −0.1 (0.3) m/s in Group 2 and 0.0 (0.3) in the total ambulatory population (Table 2). Mean (SD) change from baseline to Month 12 in 10 m w/r velocity was 0.3 (0.4) m/s in Group 1, −0.1 (0.3) m/s in Group 2 and −0.0 (0.4) in the total ambulatory population (Table 2).
Pulmonary function assessments (aged ≥6 years)
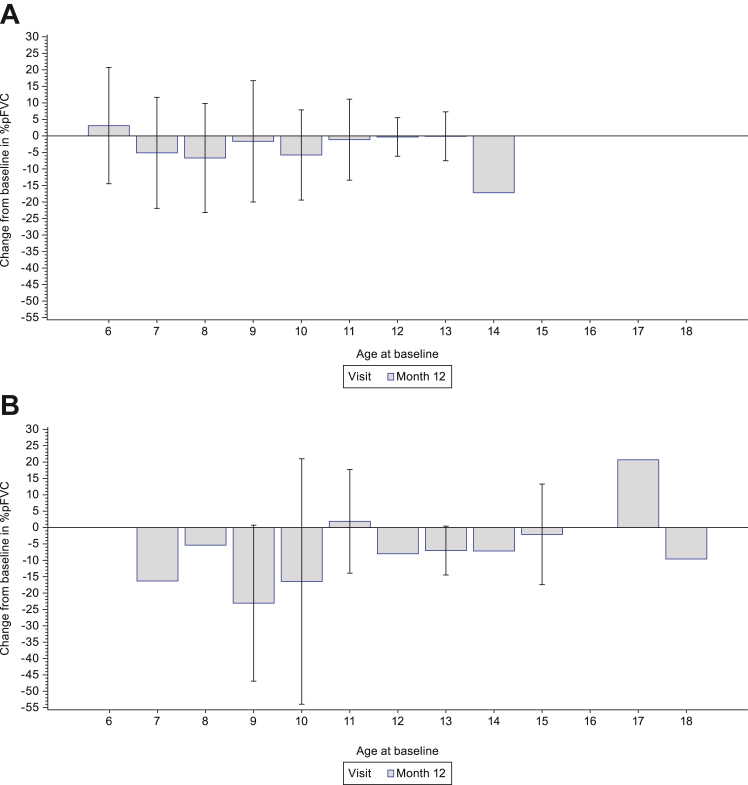
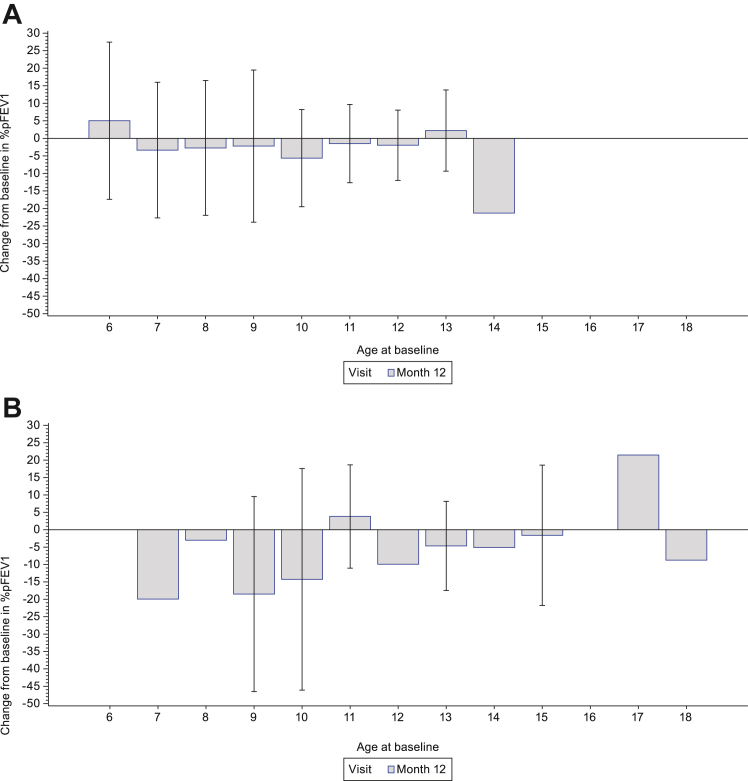
Mean (SD) change from baseline to Month 12 in %pFVC was −3.1% (16.1%) in Group 2, −6.7% (19.7%) in Group 3 and −3.7% (16.7%) in the total population (Table 2). The change from baseline in %pFVC as a function of age at baseline is shown in Fig. 4. Mean (SD) change from baseline to Month 12 in %pFEV1 was −1.6% (18.8%) in Group 2, −5.0% (19.3%) in Group 3 and −2.2% (18.8%) in the total population (Table 2). The change from baseline in %pFEV1 as a function of age at baseline is shown in Fig. 5. Change from baseline to Month 12 in MIP, MEP and PCF are presented in Supplementary Table S5.
Fig. 4.
Change from baseline in %pFVC as a function of age at baseline in (A) ambulatory participants and (B) non-ambulatory participants. For Month 12: Ambulatory (screening age ≥6 years) (n = 139); Non-ambulatory (n = 27). Data are mean (SD). Missing error bars indicate n = 1. pFVC, predicted forced vital capacity. SD, standard deviation.
Fig. 5.
Change from baseline in %pFEV1 as a function of age at baseline in (A) ambulatory participants and (B) non-ambulatory participants. For Month 12: Ambulatory (screening age ≥6 years) (n = 139); Non-ambulatory (n = 27). Pulmonary function tests were only performed in participants ≥6 years of age. Data are mean (SD). %pFEV1, percent predicted forced expiratory volume in 1 s; SD, standard deviation.
Echocardiogram (aged ≥6 years)
Mean (SD) change from baseline to Month 12 in LVEF was 4.7% (47.9%) in Group 2, –0.8% (6.3%) in Group 3 and 3.9% (44.2%) in the total population (Table 2).
Secondary assessments
Quality of life
A summary of index values and VAS scores from the EQ-5D at baseline and Month 12 is shown in Table 3. Mean (SD) index values of ambulatory participants aged <16 years, non-ambulatory participants aged <16 years, and non-ambulatory participants aged ≥16 years, respectively, were 0.9 (0.1), 0.6 (0.1), and 0.5 (0.2) at baseline, and 0.8 (0.2), 0.6 (0.1), and 0.5 (0.2) at Month 12. Mean (SD) VAS scores of ambulatory participants aged <16 years, non-ambulatory participants aged <16 years, and non-ambulatory participants aged ≥16 years, respectively, were 85.6 (18.7), 86.0 (16.4), and 90.7 (15.3) at baseline, and 83.5 (20.0), 82.5 (20.8), and 90.0 (0.0) at Month 12.
Table 3.
Summary of EQ-5D index values and VAS scores.
| EQ-5D-Y |
EQ-5D-3L |
|||||
|---|---|---|---|---|---|---|
| Ambulatory, screening age <6 y |
Ambulatory, screening age ≥6 y |
Ambulatory total |
Non-ambulatory, age <16 y |
Total |
Non-ambulatory, age ≥16 y |
|
| n = 99a | n = 177a | n = 276a | n = 32a | N = 308a | n = 4a | |
| Index value | ||||||
| n | 57 | 175 | 232 | 32 | 264 | 3 |
| Baseline | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.6 (0.1) | 0.8 (0.2) | 0.5 (0.2) |
| n | 49 | 142 | 191 | 22 | 213 | 2 |
| Month 12 | 0.9 (0.1) | 0.8 (0.2) | 0.8 (0.2) | 0.6 (0.1) | 0.8 (0.2) | 0.5 (0.2) |
| VAS scores | ||||||
| n | 57 | 175 | 232 | 32 | 264 | 3 |
| Baseline | 89.5 (12.2) | 84.3 (20.3) | 85.6 (18.7) | 86.0 (16.4) | 85.6 (18.4) | 90.7 (15.3) |
| n | 49 | 142 | 191 | 22 | 213 | 2 |
| Month 12 | 89.2 (15.9) | 81.6 (21.0) | 83.5 (20.0) | 82.5 (20.8) | 83.4 (20.1) | 90.0 (0.0) |
Data are mean (SD).
EQ-5D-3L, EuroQoL 5 Dimension three-level; EQ-5D-Y, EuroQoL 5 Dimension Youth; SD, standard deviation; VAS, visual analogue scale.
n denotes number of participants per group.
Of the five domains, the most commonly reported problem in ambulatory participants aged <16 years at Month 12 was self-care (57.6%), followed by usual activities (51.8%) and pain/discomfort (38.7%). The most commonly reported problem in non-ambulatory participants aged <16 years at Month 12 was mobility (100%), followed by self-care (86.4%) and usual activities (81.8%). All participants aged ≥16 years reported problems with mobility, self-care, and usual activities at Month 12. There were no consistent trends across the groups; however, participants of any age generally had poorer outcomes at Month 12 compared with their domain scores at baseline (Table 4).
Table 4.
Summary of EQ-5D reported health problems.
| Parameter visit | Result | EQ-5D-Y |
EQ-5D-3L |
||||
|---|---|---|---|---|---|---|---|
| Ambulatory, screening age <6 y |
Ambulatory, screening age ≥6 y |
Ambulatory total |
Non-ambulatory, age <16 y |
Total |
Non-ambulatory, age ≥16 y |
||
| n = 99a | n = 177a | n = 276a | n = 32a | n = 308a | n = 4a | ||
| Mobility | |||||||
| Baseline | na | 57 | 175 | 232 | 32 | 264 | 3 |
| No problems | 48 (84.2) | 95 (54.3) | 143 (61.6) | 0 | 143 (54.2) | 0 | |
| Some problems | 9 (15.8) | 75 (42.9) | 84 (36.2) | 1 (3.1) | 85 (32.2) | 3 (100.0) | |
| Extreme problems | 0 | 5 (2.9) | 5 (2.2) | 31 (96.9) | 36 (13.6) | 0 | |
| Month 12 | na | 49 | 142 | 191 | 22 | 213 | 2 |
| No problems | 39 (79.6) | 80 (56.3) | 119 (62.3) | 0 | 119 (55.9) | 0 | |
| Some problems | 10 (20.4) | 46 (32.4) | 56 (29.3) | 0 | 56 (26.3) | 2 (100.0) | |
| Extreme problems | 0 | 16 (11.3) | 16 (8.4) | 22 (100) | 38 (17.8) | 0 | |
| Self-care | |||||||
| Baseline | na | 57 | 175 | 232 | 32 | 264 | 3 |
| No problems | 18 (31.6) | 99 (56.6) | 117 (50.4) | 3 (9.4) | 120 (45.5) | 0 | |
| Some problems | 26 (45.6) | 63 (36.0) | 89 (38.4) | 11 (34.4) | 100 (37.9) | 1 (33.3) | |
| Extreme problems | 13 (22.8) | 13 (7.4) | 26 (11.2) | 18 (56.3) | 44 (16.7) | 2 (66.7) | |
| Month 12 | na | 49 | 142 | 191 | 22 | 213 | 2 |
| No problems | 19 (38.8) | 62 (43.7) | 81 (42.4) | 3 (13.6) | 84 (39.4) | 0 | |
| Some problems | 21 (42.9) | 65 (45.8) | 86 (45.0) | 7 (31.8) | 93 (43.7) | 1 (50.0) | |
| Extreme problems | 9 (18.4) | 15 (10.6) | 24 (12.6) | 12 (54.5) | 36 (16.9) | 1 (50.0) | |
| Usual Activities | |||||||
| Baseline | n | 57 | 175 | 232 | 32 | 264 | 3 |
| No problems | 41 (71.9) | 82 (46.9) | 123 (53.0) | 5 (15.6) | 128 (48.5) | 0 | |
| Some problems | 14 (24.6) | 87 (49.7) | 101 (43.5) | 16 (50.0) | 117 (44.3) | 1 (33.3) | |
| Extreme problems | 2 (3.5) | 6 (3.4) | 8 (3.4) | 11 (34.4) | 19 (7.2) | 2 (66.7) | |
| Month 12 | na | 49 | 142 | 191 | 22 | 213 | 2 |
| No problems | 32 (65.3) | 60 (42.3) | 92 (48.2) | 4 (18.2) | 96 (45.1) | 0 | |
| Some problems | 17 (34.7) | 67 (47.2) | 84 (44.0) | 5 (22.7) | 89 (41.8) | 1 (50.0) | |
| Extreme problems | 0 | 15 (10.6) | 15 (7.9) | 13 (59.1) | 28 (13.1) | 1 (50.0) | |
| Pain/Discomfort | |||||||
| Baseline | na | 57 | 175 | 232 | 32 | 264 | 3 |
| No problems | 40 (70.2) | 124 (70.9) | 164 (70.7) | 19 (59.4) | 183 (69.3) | 3 (100.0) | |
| Some problems | 17 (29.8) | 49 (28.0) | 66 (28.4) | 11 (34.4) | 77 (29.2) | 0 | |
| Extreme problems | 0 | 2 (1.1) | 2 (0.9) | 2 (6.3) | 4 (1.5) | 0 | |
| Month 12 | na | 49 | 142 | 191 | 22 | 213 | 2 |
| No problems | 30 (61.2) | 87 (61.3) | 117 (61.3) | 17 (77.3) | 134 (62.9) | 2 (100.0) | |
| Some problems | 19 (38.8) | 53 (37.3) | 72 (37.7) | 5 (22.7) | 77 (36.2) | 0 | |
| Extreme problems | 0 | 2 (1.4) | 2 (1.0) | 0 | 2 (0.9) | 0 | |
| Anxiety/Depression | |||||||
| Baseline | na | 57 | 175 | 232 | 32 | 264 | 3 |
| No problems | 50 (87.7) | 133 (76.0) | 183 (78.9) | 22 (68.8) | 205 (77.7) | 3 (100.0) | |
| Some problems | 7 (12.3) | 33 (18.9) | 40 (17.2) | 10 (31.3) | 50 (18.9) | 0 | |
| Extreme problems | 0 | 9 (5.1) | 9 (3.9) | 0 | 9 (3.4) | 0 | |
| Month 12 | na | 49 | 142 | 191 | 22 | 213 | 2 |
| No problems | 37 (75.5) | 96 (67.6) | 133 (69.6) | 13 (59.1) | 146 (68.5) | 2 (100.0) | |
| Some problems | 8 (16.3) | 39 (27.5) | 47 (24.6) | 8 (36.4) | 55 (25.8) | 0 | |
| Extreme problems | 4 (8.2) | 7 (4.9) | 11 (5.8) | 1 (4.5) | 12 (5.6) | 0 | |
Data are n (%).
Only includes data until Month 12 visit.
EQ-5D-3L, EuroQoL 5 Dimension three-level; EQ-5D-Y, EuroQoL 5 Dimension Youth.
n denotes number of participants per group.
Discussion
Data from this prospective natural history study in Chinese participants with DMD demonstrated the age at failure to walk for the total population (median 12.9 [min, max 12.0, 13.9] years) was similar to previous findings in Chinese individuals with DMD.13 This was consistent with data reported by Li and colleagues, with loss of ambulation occurring at a median age of 13 years in DMD individuals who had been treated with corticosteroids.24
The age of failure to stand for the total population (median 13.0 [min, max 12.2, 13.9] years) was first reported in this study, and the age at failure to self-feed was not evaluable in this study.
A previous study has shown that the median age of loss of ambulation (unable to ambulate 10 m w/r) was 10.0 years in individuals who were using GC for <1 month compared with 13.4 years for >1 year.25 These findings are similar to the results of the current study, although our work did not analyse the effect of GC on age of loss of ambulation. Further analysis may be conducted after the study is complete, as several outcomes have indicated that GC treatment is a key variable in natural history studies in DMD and could reduce the risk of milestone-related disease progression and mortality.25, 26, 27 The rate of GC treatment in participants in clinical practice in China was relatively low compared with developed countries.13 This was largely due to the lack of awareness of physicians who specialize in treating patients with muscular dystrophies, although this is evolving with the implementation of the TREAT-NMD guidelines.28,29 Moreover, all participants ≥4 years in this study received GC treatment for at least 6 months before enrolment, and the age of GC initiation was later in non-ambulatory participants than those who were ambulatory. This is in line with clinical practice in China in recent years.30,31 In addition, although the dosing frequency was not collected in this study, the daily dose of GC was similar to the treatment recommendations for DMD.28,29
In this study, decreases in NSAA and timed motor function were observed from the age of 6 years for the total population, with the largest reductions reported after age 6, and were evident over 12 months of the study. This may be, at least in part, due to the combined effects of disease progression and growth, since the role of growth is particularly important when participants were younger than 6 years. However, as the disease progressed, muscle injury was more dominant than the role of growth. PUL2.0 total scores were greater in the ambulatory group than non-ambulatory participants of the same age. A similar trend was observed with rise from floor velocity and 10 m w/r velocity.
Overall, motor function increased in participants aged ≤6 years, and subsequently declined. However, motor function decreased over 12 months in participants aged ≥6 years. The PUL2.0 total score was assessed in participants aged ≥10 years and declined from around age 10–11 years in the ambulatory group. This was consistent with findings from a study that assessed the reliability of the PUL assessment in individuals with DMD by Pane et al.,32 although data were limited in some age groups in the non-ambulatory group and could not show a clear trend in line with our findings. However, this could be further evaluated after our study is complete, but overall, the findings are consistent with the characteristics of disease progression, whereby the decline in upper limb function typically occurs after decline in lower limb function. There were no clear changes or trends in changes in muscular strength and ROM over 12 months in this study.
Greater reductions in pulmonary function assessments, including %pFVC and %pFEV1, were noted in non-ambulatory participants compared with the ambulatory group. Furthermore, decreases in %pFVC and %pFEV1 were typically observed at 7 years of age. The sharp decline in pulmonary function in this study was consistent with disease progression. Overall, cardiac function was similar between ambulatory and non-ambulatory participants, which may be due to the improvement of disease management, such as early initiation of GC treatment or other medications for the protection of cardiac function. These findings are consistent with other studies in DMD, which have also shown no substantial differences in cardiac function between ambulatory and non-ambulatory participants.33
In the current study, mean index values for DMD participants ranged from 0.5 to 0.9 across the groups, and, together with VAS scores and health problems, declined after 12 months. This was indicative of the inverse association between disease progression and QoL, and was consistent with other studies.34,35 The index values in this study were slightly higher than those reported in the literature (Europe: 0.2436; multiple countries: 0.15–0.7535; Portugal: −0.05 to 0.5134; Hungary: 0.31037; Netherlands: 0.4438). This may be due to the “ceiling effect” of the EQ-5D-3L questionnaire39 with the varying preferences of the younger participants in this study. Participants in this study experienced problems with mobility, self-care, and usual activities, with those aged <16 years also experiencing pain/discomfort and anxiety/depression.
Recent studies have characterised the natural history of DMD; however, these analyses have primarily focused on the progression of lower extremity motor function and ambulation.17,18 Moreover, few studies have characterised the progression of upper extremity weakness or pulmonary disease,19,20 and despite ongoing efforts to identify predictors of disease progression, these data are sparse.40 Of note, over the past decades, the survival of individuals with DMD has improved. A recent study in France found that the median life expectancy was 26 years for individuals born before 1970 and 41 years for those born after 1970.41
This is the first prospective, well-designed, natural history study in the Chinese DMD population with a large sample size. This is of interest as many natural history studies are retrospective in design. Furthermore, this is the first study to provide a holistic view of the function assessments of DMD within the Chinese population, with a comprehensive assessment of motor and pulmonary assessment, cardiac function, and QoL. Indeed, these findings may inform the design of future studies in DMD for a Chinese population.
One potential limitation of the current study is that the percent predicted values of change in MIP, MEP and PCF were not used. This approach has limitations, as pulmonary function is associated with age, height, gender and ethnicity. However, there is currently no consensus to apply the percent predicted values across broad ranges of ethnicity, age, height, and gender, or Chinese population reference data. Another potential limitation of this study is that the duration of GC use varied across the groups of participants, which may have influenced the disease course and the natural history of DMD. In this study, the disease course was not analysed by the duration of GC usage but was pooled for the overall study population. Furthermore, the small numbers of participants in some age groups may have influenced the results; for example, there were only four non-ambulatory participants aged ≥16 years who completed the EQ-5D-3L, which may have impacted the results of the index values and VAS, as well as the domains of the questionnaires. However, the overall trends observed were unaffected. No statistical testing was conducted on the results of this study; therefore, the data should be interpreted with caution.
This natural history study improves the understanding of the progression of DMD according to the current standards of care in the Chinese DMD population. Moreover, it may inform the design of clinical trials in terms of the selected endpoints and patient populations.
Contributors
YG, YZ and XL were involved in study design. YG, CJ, XC, YZ, JF, TP, XL, JL, SH, ZW and WZ were involved in data analysis. YG, CJ, YZ, JF and TP ensured accuracy of the data. All authors had full access to the study data, were involved in data interpretation, reviewed and revised each draft of the manuscript, and approved the final manuscript for submission. NM (medical writer) developed drafts of the manuscript under direct supervision of the authors and formatted the final version of the manuscript for publication.
Data sharing statement
Upon request, and participant to review, Pfizer will provide the data (from study C3391004) that support the findings of this study. Pursuant to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Declaration of interests
This study (NCT03760029; C3391004) was sponsored by Pfizer Inc. YG, YZ, CJ and JF are employees of Pfizer, and may hold stock and/or stock options. TP was an employee at the time of initiation of the manuscript and may hold stock and/or stock options. XL, JL, WZ, SH, ZW and XC have no conflicts of interest.
Acknowledgements
Medical writing support was provided by Neel Misra, MSc, of Engage Scientific Solutions and was funded by Pfizer.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100944.
Appendix A. Supplementary data
Supplementary Figure S1.
Supplementary Figure S2.
References
- 1.Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Gao Q.Q., McNally E.M. The dystrophin complex: structure, function, and implications for therapy. Compr Physiol. 2015;5(3):1223–1239. doi: 10.1002/cphy.c140048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald C.M., Henricson E.K., PTC GD-DMD Study Group, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve. 2013;48(3):343–356. doi: 10.1002/mus.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald C.M., Henricson E.K., Abresch R.T., et al. The cooperative international neuromuscular research group duchenne natural history study–a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used. Muscle Nerve. 2013;48(1):32–54. doi: 10.1002/mus.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henricson E.K., Abresch R.T., Cnaan A., et al. The cooperative international neuromuscular research group duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve. 2013;48(1):55–67. doi: 10.1002/mus.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzur A.Y., Kuntzer T., Pike M., Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;1:CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Ricotti V., Ridout D.A., Scott E., et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 2013;84(6):698–705. doi: 10.1136/jnnp-2012-303902. [DOI] [PubMed] [Google Scholar]
- 8.Kieny P., Chollet S., Delalande P., et al. Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann Phys Rehabil Med. 2013;56(6):443–454. doi: 10.1016/j.rehab.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Jeppesen J., Green A., Steffensen B.F., Rahbek J. The Duchenne muscular dystrophy population in Denmark, 1977-2001: prevalence, incidence and survival in relation to the introduction of ventilator use. Neuromuscul Disord. 2003;13(10):804–812. doi: 10.1016/s0960-8966(03)00162-7. [DOI] [PubMed] [Google Scholar]
- 10.Anthony K., Arechavala-Gomeza V., Ricotti V., et al. Biochemical characterization of patients with in-frame or out-of-frame DMD deletions pertinent to exon 44 or 45 skipping. JAMA Neurol. 2014;71(1):32–40. doi: 10.1001/jamaneurol.2013.4908. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration . 2019. Rare diseases: natural history studies for drug development. Guidance for industry.https://www.fda.gov/media/122425/download [Google Scholar]
- 12.Straub V., Mercuri E., DMD Outcome Measure Study Group Report on the workshop: meaningful outcome measures for Duchenne muscular dystrophy, London, UK, 30-31 January 2017. Neuromuscul Disord. 2018;28(8):690–701. doi: 10.1016/j.nmd.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Zhao L., Zhou S., et al. A comprehensive database of Duchenne and Becker muscular dystrophy patients (0-18 years old) in East China. Orphanet J Rare Dis. 2015;10:5. doi: 10.1186/s13023-014-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling C., Dai Y., Fang L., et al. Exonic rearrangements in DMD in Chinese Han individuals affected with Duchenne and Becker muscular dystrophies. Hum Mutat. 2020;41(3):668–677. doi: 10.1002/humu.23953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong X., Zhong X., Liu L., Cui S., Yang Y., Kong L. Genetic analysis of 1051 Chinese families with duchenne/becker muscular dystrophy. BMC Med Genet. 2019;20(1):139. doi: 10.1186/s12881-019-0873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan F. Occurrence rule of major lifetime events of children with Duchenne muscular dystrophy. J Appl Clin Pediatr. 2012;27:1866–1868. [Google Scholar]
- 17.Mazzone E.S., Pane M., Sormani M.P., et al. 24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0052512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bello L., Morgenroth L.P., Gordish-Dressman H., et al. DMD genotypes and loss of ambulation in the CINRG duchenne natural history study. Neurology. 2016;87(4):401–409. doi: 10.1212/WNL.0000000000002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly A.M., Florence J.M., Zaidman C.M., et al. Clinical trial readiness in non-ambulatory boys and men with Duchenne muscular dystrophy: MDA-DMD network follow-up. Muscle Nerve. 2016;54(4):681–689. doi: 10.1002/mus.25089. [DOI] [PubMed] [Google Scholar]
- 20.Mayer O.H., Finkel R.S., Rummey C., et al. Characterization of pulmonary function in Duchenne muscular dystrophy. Pediatr Pulmonol. 2015;50(5):487–494. doi: 10.1002/ppul.23172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzone E.S., Messina S., Vasco G., et al. Reliability of the North Star ambulatory assessment in a multicentric setting. Neuromuscul Disord. 2009;19(7):458–461. doi: 10.1016/j.nmd.2009.06.368. [DOI] [PubMed] [Google Scholar]
- 22.Liu G.G., Guan H., Jin X., Zhang H., Vortherms S.A., Wu H. Rural population's preferences matter: a value set for the EQ-5D-3L health states for China's rural population. Health Qual Life Outcomes. 2022;20(1):14. doi: 10.1186/s12955-022-01917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z., Jiang J., Wang P., et al. Estimating an EQ-5D-Y-3L value set for China. Pharmacoeconomics. 2022;40(Suppl 2):147–155. doi: 10.1007/s40273-022-01216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koeks Z., Bladen C.L., Salgado D., et al. Clinical outcomes in duchenne muscular dystrophy: a study of 5345 patients from the TREAT-NMD DMD global database. J Neuromuscul Dis. 2017;4(4):293–306. doi: 10.3233/JND-170280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald C.M., Henricson E.K., Abresch R.T., et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet. 2018;391(10119):451–461. doi: 10.1016/S0140-6736(17)32160-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S., Qin D., Wu L., et al. Genotype characterization and delayed loss of ambulation by glucocorticoids in a large cohort of patients with Duchenne muscular dystrophy. Orphanet J Rare Dis. 2021;16(1):188. doi: 10.1186/s13023-021-01837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S., Campbell K.A., Fox D.J., Matthews D.J., Valdez R., M. D. STARnet Corticosteroid treatments in males with Duchenne muscular dystrophy: treatment duration and time to loss of ambulation. J Child Neurol. 2015;30(10):1275–1280. doi: 10.1177/0883073814558120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bushby K., Finkel R., Birnkrant D.J., et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 29.Bushby K., Finkel R., Birnkrant D.J., et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9(2):177–189. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]
- 30.Yuanyun Chinese DMD guidelines. Nat Med J China. 2018;98(35):2803. [Google Scholar]
- 31.Pumchcuily Chinese DMD consensus statement. China J Neurol. 2016;49:1. [Google Scholar]
- 32.Pane M., Mazzone E.S., Fanelli L., et al. Reliability of the performance of upper limb assessment in Duchenne muscular dystrophy. Neuromuscul Disord. 2014;24(3):201–206. doi: 10.1016/j.nmd.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Buddhe S., Cripe L., Friedland-Little J., et al. Cardiac management of the patient with Duchenne muscular dystrophy. Pediatrics. 2018;142(Suppl 2):S72–S81. doi: 10.1542/peds.2018-0333I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreozzi V., Labisa P., Mota M., et al. Quality of life and informal care burden associated with duchenne muscular dystrophy in Portugal: the COIDUCH study. Health Qual Life Outcomes. 2022;20(1):36. doi: 10.1186/s12955-022-01941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landfeldt E., Lindgren P., Bell C.F., et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology. 2014;83(6):529–536. doi: 10.1212/WNL.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavazza M., Kodra Y., Armeni P., et al. Social/economic costs and health-related quality of life in patients with Duchenne muscular dystrophy in Europe. Eur J Health Econ. 2016;17:19–29. doi: 10.1007/s10198-016-0782-5. [DOI] [PubMed] [Google Scholar]
- 37.Pentek M., Baji P., Pogany G., Brodszky V., Boncz I., Gulacsi L. Health related quality of life of patients and their caregivers in rare diseases results of the burqol-rd project in Hungary. Value Health. 2014;17(7):A538. doi: 10.1016/j.jval.2014.08.1724. [DOI] [PubMed] [Google Scholar]
- 38.Pangalila R.F., van den Bos G.A., Stam H.J., van Exel N.J., Brouwer W.B., Roebroeck M.E. Subjective caregiver burden of parents of adults with Duchenne muscular dystrophy. Disabil Rehabil. 2012;34(12):988–996. doi: 10.3109/09638288.2011.628738. [DOI] [PubMed] [Google Scholar]
- 39.Buchholz I., Janssen M.F., Kohlmann T., Feng Y.S. A systematic review of studies comparing the measurement properties of the three-level and five-level versions of the EQ-5D. Pharmacoeconomics. 2018;36(6):645–661. doi: 10.1007/s40273-018-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzone E.S., Coratti G., Sormani M.P., et al. Timed rise from floor as a predictor of disease progression in Duchenne muscular dystrophy: an observational study. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan D., Goemans N., Takeda S., Mercuri E., Aartsma-Rus A. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021;7(1):13. doi: 10.1038/s41572-021-00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.