Abstract
Background
Ischemic stroke, caused neurological dysfunction due to inadequate blood supply to brain, has a high morbidity and mortality. Ethyl pyruvate (EP), a simple aliphatic ester derived from pyruvic acid, has the advantages of safety and stability. Studies have confirmed that EP has anti-oxidative, anti-inflammation, anti-tumor, and other pharmacological effects, and it demonstrates significant therapeutic effects on multiple diseases. GAS6 and its high affinity Axl receptor play an important role in cell adhesion, anti-apoptosis, proliferation and migration by activating downstream signal transduction pathways. Previous studies have demonstrated the neuroprotective effects of the GAS6/Axl axis.
Methods
A series of experimental methods were employed to confirm the effect of EP against cerebral hypoxia/reoxygenation (HR) injury.
Results
In this study, the protective effect and mechanism of EP on HR injury in N2a cells was explored. The results found that treatment with EP could increase HR-injured neuronal viability, improve cell morphology, and reduce LDH release and ROS accumulation, thereby exhibiting a neuroprotective effect. Furthermore, EP treatment restored the down-regulated expression of GAS6, Axl, NQO1, PGC-1α, NRF1, and UCP2 caused by HR injury. Specifically, it was observed that the neuroprotective effect of EP was partially inhibited by GAS6 siRNA.
Conclusion
In conclusion, these results suggest that EP treatment attenuates HR-induced oxidative stress injury in neuroblastoma cells via activating GAS6/Axl signaling.
Keywords: Ethyl pyruvate, GAS6, Axl, Hypoxia/Reoxygenation injury, Neuroblastoma cells
1. Introduction
Ischemic stroke is an acute vascular disease caused by ischemic brain injury, which is characterized by high incidence, high disability rate and high mortality [1]. The development of ischemic stroke has been proven to involve multiple pathways, oxidative stress, inflammation, and apoptosis [2,3]. Thrombolytic therapy is a critical treatment methods in modern medicine, but it unavoidably leads to the ischemia/reperfusion injury (IRI) [4,5]. Cerebral IRI induces neuronal death and apoptosis and even severely impairs neurological function [6]. Therefore, there is an urgent need to explore novel and effective neuroprotective agents. However, few studies have found agents with profound beneficial effect and low side effect.
Ethyl pyruvate (EP), a simple aliphatic ester derived from pyruvic acid, possesses the advantages of safety and stability [7]. Several studies have confirmed that EP has anti-oxidative, anti-inflammation, anti-tumor, and other pharmacological effects [8]. Notably, there is compelling evidence supporting the beneficial role of EP in various models of acute injury, including myocardial IRI, kidney IRI, and liver IRI [9,10]. However, the associated mechanisms and specific molecular targets involved in the protection are not to be elucidated.
Growth arrest-specific gene 6 (GAS6) is a vitamin K-dependent protein and a ligand for the TAM receptor (Tyro3, Axl and Mer) [11,12], among which Axl has the highest affinity for GAS6 [13]. GAS6 binds to Axl to regulate cell proliferation, apoptosis, migration and angiogenesis through multiple signaling pathways [[14], [15], [16]]. Recently, the role of GAS6/Axl pathway in organs protection has been confirmed [17]. GAS6/Axl signaling contributes to the immune restoration after intracerebral hemorrhage mouse model, and it can attenuate brain injury and enhance neuroprotective effect [18]. Recombinant GAS6 has been shown to upregulated Axl expression and improved short-term and long-term effects after middle cerebral artery occlusion in a rat model of stroke, suggesting its significantly neuroprotective effect [19]. In addition, it is reported that some natural substances (dihydroartemisinin and curcumin) can regulate GAS6/Axl axis [20,21]. However, whether EP can regulate GAS6/Axl signaling and it's upstream and downstream regulatory signaling remains unknown.
In this study, the protective effect of EP against hypoxia/reoxygenation (HR) injury in neuroblastoma cells was examined. To further explored the neuroprotective effect of GAS6/Axl axis in EP treatment and its downstream targets to elucidate the potential protective mechanism of EP.
2. Methods
2.1. Cell culture and treatment
The mouse neuroblastoma N2a cell (purchased from ATCC, Rockville, MD, United States) culture and hypoxic reoxygenation (HR) injury model, please refer to our previous study [22]. With regard to EP (Macklin biochemical Co., Ltd, shanghai China) administration, the specific steps are as follows: cells were treated with different concentrations of EP (0.1, 1, 2, 5 or 10 mM) for 24 h to detect the toxic effects of EP. Then, in the absence or presence of GAS6 siRNA (Sangon Biotechnology Co., Ltd., Shanghai, China), the cells are treated with different concentrations of EP (0.1, 1, 2, 5 or 10 mM) for 3 h and then exposed to HR, unless otherwise indicated. After the treatment, the cells were collected for further analysis.
2.2. Cell viability and apoptosis assay
The treated cells were tested for cell viability and apoptosis using Muse ™ cell analyzer (Merck KGaA, Darmstadt, Germany) according to the manufacturer's protocol [23]. Moreover, Hoechst 33342 staining is another technique that reveals the morphological changes of the cells before and after treating with the drug administration [24].
2.3. LDH release, and ROS production assay
LDH release in the culture medium was determined using a commercially available kit according to the manufacturer's protocol (Nanjing Jiancheng Bioengineering Institute). For measurement of ROS production (Beyotime Biotechnology, Shanghai, China), please refer to Lei et al. [25].
2.4. Western blot analysis
Protein expression was determined by Western blot analysis as previously described [26]. The anti-bodies information used can be found in Table S1.
2.5. GAS6 knockdown by small interfering RNA (siRNA)
The selection of the optimal sequence and concentration of siRNA was based on cell viability, mRNA and protein expressions (data not shown). Sangon Biotechnology (Shanghai, China) synthesized the siRNA sequences targeting murine GAS6, with the sense sequence being 5′-GGACACACUUAAGACACAUTT-3′ and the antisense sequence being 5′-AUGUGUCUUAAGUGUGUCCTT-3′. N2a cells were utilized to knockdown GAS6 using the synthesized siRNA. To initiate transfection, cells were initially seeded in 60 mm culture dishes and allowed to reach approximately 60–70 % confluence. Following the manufacturer's instructions, Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA, United States) was used to transfect cells with siRNA molecules targeting GAS6 for a minimum of 4 h in medium without antibiotics. Subsequently, the cells were incubated for 24 h.
2.6. Quantitative real-time PCR
We conducted quantitative real-time PCR as outlined in our previous study [23]. The primers used can be found in Table S2. The expression levels of the transcripts analyzed were compared to β-actin and normalized against the average value of the control samples.
2.7. Mitochondrial membrane potential assay
In this study, JC-1 was used to monitor mitochondrial membrane potential. Briefly, after treatment and washing three times with PBS, culture medium (1 mL) and JC-1 staining solution (1 mL) are added; after being fully mixed, the cells are incubated at 37 °C for 20 min. During incubation, according to 1 mL JC-1 staining buffer (5×) with 4 mL distilled water, JC-1 staining is mixed with JC-1 staining buffer (1×). After incubation at 37 °C, the supernatant was removed, then washed twice with JC-1 staining buffer (1×), 2 mL cell culture medium was added, and observed with fluorescence microscope (EVOSM5000, Thermo Fisher Scientific, Carlsbad, CA, USA).
2.8. Statistical analyses
All values are expressed as mean ± standard deviation (SD). According to the experimental design, significant differences were evaluated using student t-tests and ANOVA analysis. When the analysis of variance shows significance, use Tukey's HSD post hoc test for multiple comparisons. P < 0.05 indicates a statistically significant difference.
3. Results
3.1. Toxic effect of EP on N2a cells
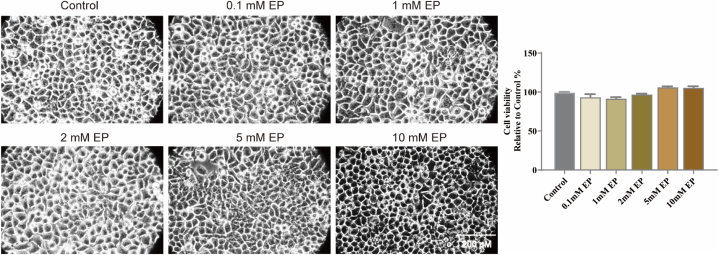
To assess the toxic effects of EP, we treated N2a cells with different concentrations of EP (0.1, 1, 2, 5, or 10 mM) for 24 h. As shown in Fig. 1, cell viability and morphology did not decrease significantly after EP treatment (vs. control group, p > 0.05). Notably, even the maximum concentration of EP (reached 10 mM) is non-toxic to the neuron cell.
Fig. 1.
Toxic effect of EP on N2a cells. N2a cells was treated with EP at the indicated concentrations for 24 h. Cell morphology was observed under an inverted/phase contrast microscope, and images were obtained. The data are presented as the mean ± SD, n = 3.
3.2. EP protected N2a cells against HR-induced injury
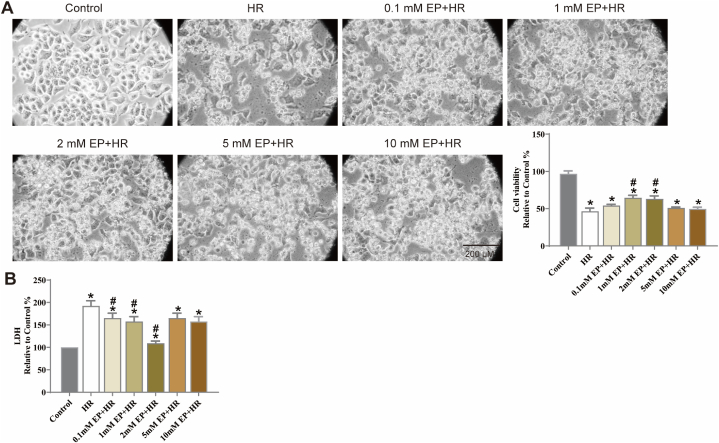
Subsequently, N2a cells were treatment with EP for 3 h, then expose to HR injury. The results showed that HR overtly reduced cells viability by more than 50 % (Fig. 2A, vs. control group, p < 0.05). In addition, cell atrophy and fragmentation were observed, along with considerable morphological changes. EP treatment reversed this changes. Furthermore, EP effectively reduced the release of LDH (Fig. 2B, vs. HR group, p < 0.05).
Fig. 2.
Effects of EP treatment on cell viability and LDH release in N2a cells injured by HR. (A) The representative cell morphologies of N2a cells is shown. Viability is expressed as a percentage of viability in the standard control group. (B) LDH release in the culture medium of N2a cells. The results are expressed as the mean ± SD, n = 3. *p < 0.05 vs. the control group. #p < 0.05 vs. the HR group.
3.3. EP inhibited HR-induced ROS generation, cellular apoptosis, and mitochondrial function
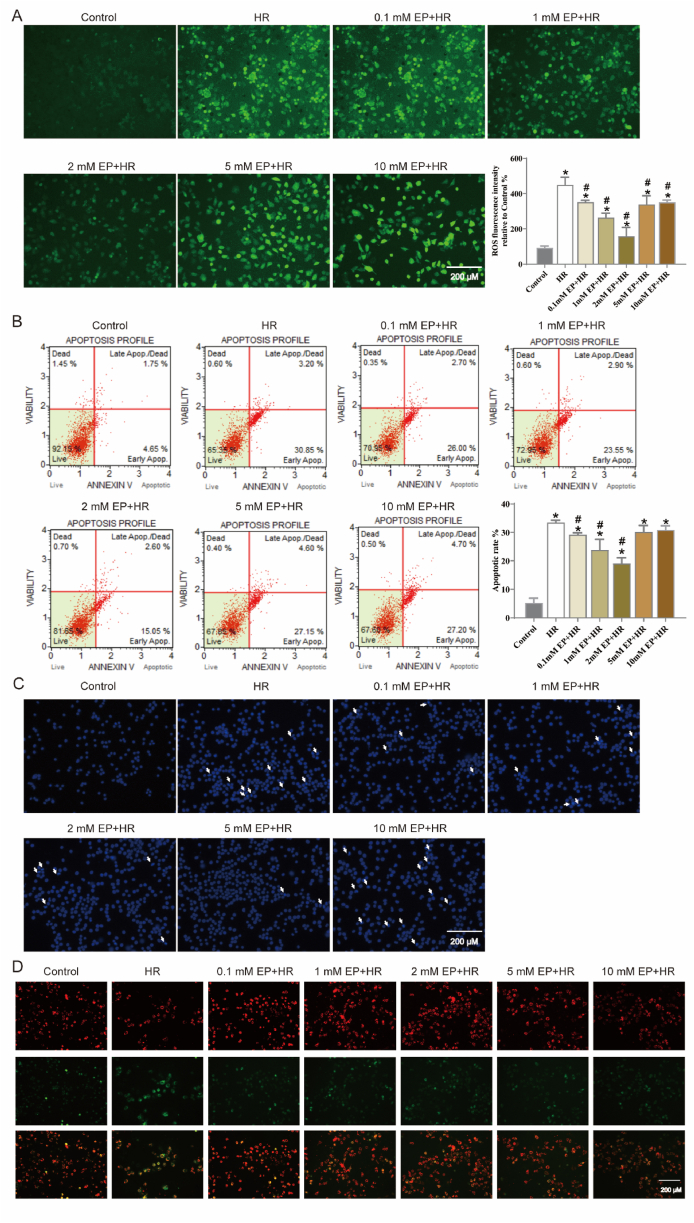
The DHE staining and apoptosis assay were performed to assess the effects of EP on ROS generation and apoptotic rate, (Fig. 3A and B). HR treatment obviously increased ROS production and apoptotic rate in N2a cells (vs. control group, p < 0.05); whereas treatment with EP reversed this trend (vs. HR group, p < 0.05). Then, Hoechst 33342 staining was also used to detect apoptosis. As exhibited in Fig. 3C, apoptotic cells increased significantly after HR injury, which can be marked by high light blue-fluorescence from Hoechst 33342, while EP treatment reversed this phenomena. Subsequently, we have performed additional studies for mitochondrial membrane potential. The results showed HR injured increased green fluorescence and decreased red fluorescence, indicating a decrease in mitochondrial membrane potential. However, the mitochondrial membrane potential was recovered in EP + HR group (Fig. 3D).
Fig. 3.
Effects of EP treatment on ROS generation, cellular apoptosis, and mitochondrial function in N2a cells injured by HR. (A) N2a cells was treated with EP at the different concentrations and then exposed to HR injury followed by incubation with 10 μM H2-DCF-DA for 20 min. ROS staining was evident as green fluorescence. (B) Representative flow cytometry results for N2a cells is shown. The following four subpopulations and their fractions are indicated: early apoptotic cells (lower right), late apoptotic cells (upper right), normal cells (lower left) and dead cells (upper left). The apoptotic index is expressed as the number of apoptotic cells/the total number of cells counted × 100 %. (C) Apoptotic cells was evident as blue fluorescence by Hoechst 33342 staining. (D) Mitochondrial membrane potential was detected using JC-1 staining, with red fluorescence indicating an increase in mitochondrial membrane potential and green indicating a decrease in mitochondrial membrane potential. The results are expressed as the mean ± SD, n = 3. *p < 0.05 vs. the control group. #p < 0.05 vs. the HR group.
3.4. Effects of EP on GAS6/Axl signaling in HR-injured N2a cells
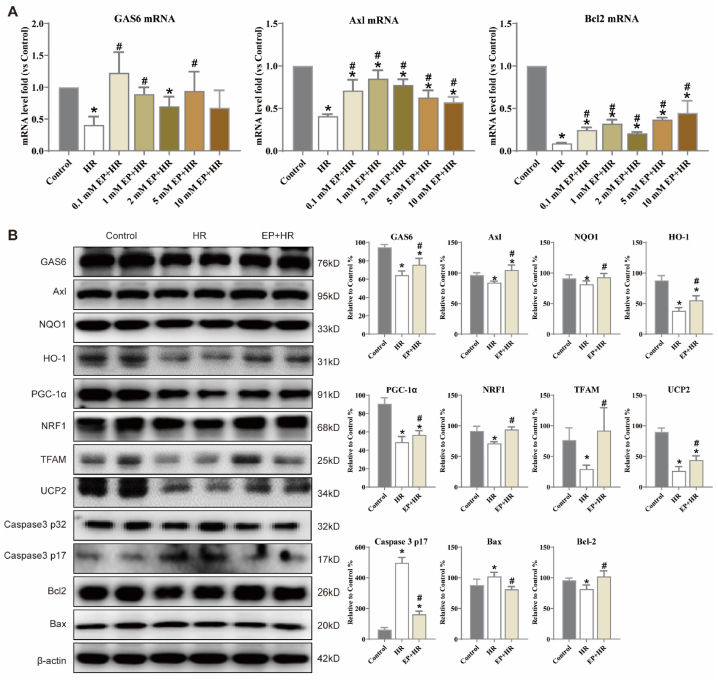
As shown in Fig. 4A, compared with the control groups, HR injury decrease the levels of GAS6, Axl, and Bcl2, whereas EP treatment reversed these molecules levels in EP + HR groups (p < 0.05). Based on the result above, the dose of 2 mM EP showed the most significant improvement, hence, this dose will be used in the next experiments. Subsequently, GAS6, Axl, NQO1, HO-1, PGC-1α, NRF1, TFAM, UCP2, Caspase3, Bax, and Bcl2 expression were detected by Western blot. HR injury decreased the expression of GAS6, Axl, NQO1, HO-1, PGC-1α, NRF1, TFAM, UCP2 and Bcl2, and increased the expression Caspase3 and Bax. However, treatment with EP could significantly increase these related protein expressions (Fig. 4B, vs. HR group, p < 0.05). These results indicated that EP might contribute to improve oxidative stress, mitochondrial function, apoptosis, and activate GAS6/Axl signaling in HR-injured N2a cells.
Fig. 4.
Effects of EP on GAS6/Axl signaling in N2a cells injured by HR. (A) The level of GAS6, Axl, Bcl2 in different concertation EP treatment HE-injured N2a cells. (B) Representative Western blot results for N2a cells are shown. Cytoplasmic extracts were isolated as described in the Materials and Methods section and subjected to Western blot analysis with antibodies specific for GAS6, Axl, NQO1, HO-1, PGC-1α, NRF1, UCP2, TFAM, Caspase3, Bax, and Bcl-2; β-actin served as a control. The statistics are representative of the bands for the control and treatment groups. The results are expressed as the mean ± SD, n = 3. *p < 0.05 vs. the control group. #p < 0.05 vs. the HR group.
3.5. Effects of GAS6 siRNA and EP on cell viability, apoptosis, ROS generation, and LDH release in HR-injured N2a cells
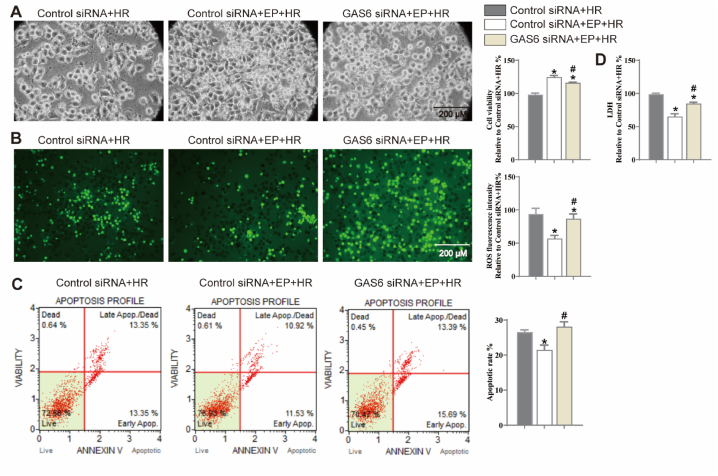
To investigate the role of GAS6/Axl signaling in the protective effects of EP, three siRNAs were used to silence GAS6 and determine the effective interference fragments. Initially, GAS6 siRNA treatment (100 pM, 24 h) showed no toxic effects on N2a cells (Supplementary Fig. 1A) but effectively decreased GAS6 and Axl mRNA levels (vs. control siRNA group, Supplementary Fig. 1B, p < 0.05). After being treated with GAS6 siRNA for 24 h, N2a cells were treated with EP (2 mM) and then exposed to HR injury. GAS6 siRNA exhibited distinctly reversed protective effects of EP against HR-induced cell death, ROS generation, cellular apoptosis, and LDH release (vs. control siRNA + EP + HR group, Fig. 5A–D, p < 0.05).
Fig. 5.
Effects of GAS6 siRNA and EP on cell viability, ROS generation, cellular apoptosis, and LDH release in N2a cells injured by HR. (A) The results of cell viability, (B) The results of ROS generation, (C) The results of cellular apoptosis, (D) The results of LDH release in N2a cells are shown. The results are expressed as the mean ± SD, n = 3. *p < 0.05 vs. the control siRNA + HR. #p < 0.05 vs. the control siRNA + EP + HR group.
3.6. Effects of GAS6 siRNA and EP on GAS6/Axl signaling in N2a cells injured by HR
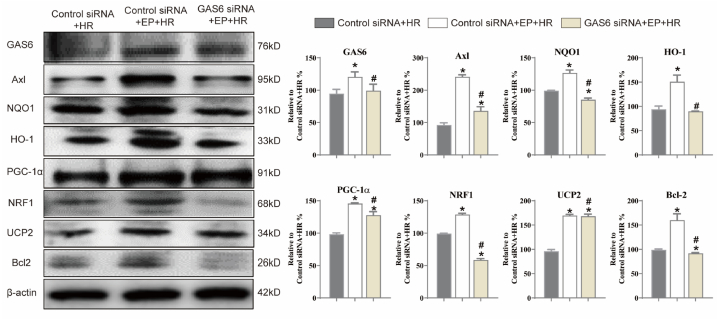
As shown in Fig. 6, compared to the control siRNA + HR group, EP treatment induced an obvious upregulation of GAS6, Axl, NQO1, HO-1, PGC-1α, NRF1, UCP2, and Bcl-2 (p < 0.05); however, GAS6 siRNA treatment reversed the upregulation of GAS6, Axl, NQO1, HO-1, PGC-1α, NRF1, UCP2, and Bcl-2 (vs. control siRNA + EP + HR group, p < 0.05). These results demonstrated that GAS6 deficiency prominently reversed the protective effect of EP against HR-injury N2a cells.
Fig. 6.
Effects of GAS6 siRNA and EP on GAS6/Axl signaling in N2a cells injured by HR. N2a cells were transfected with siRNA targeting GAS6 or a negative control siRNA (NC) for 24 h, treated with 2 mM EP for 3 h, and then exposed to HR injury. Whole-cell extracts were isolated as described in the Materials and Methods and subjected to Western blot analysis with antibodies specific for GAS6, Axl, NQO1, HO-1, PGC-1α, NRF1, UCP2, and Bcl-2; β-actin served as a control. The statistics are representative of the bands for the control and treatment groups. The results are expressed as the mean ± SD, n = 3. *p < 0.05 vs. the control siRNA + HR, #p < 0.05 vs. the GAS6 siRNA + EP + HR group.
4. Discussion
Cerebral ischemia, a neurological disorder, is most often caused by blocking of blood vessels due to thrombosis, resulting in brain damage [27]. Although significant advances has been made in the treatment of cerebral ischemia, however, current therapies are still not fully satisfactory in reducing the severity of cerebral ischemia. EP is a stable simple lipophilic ester with anti-oxidative, anti-inflammatory, and anti-apoptotic properties, and it has been confirmed to have significant protective effects in animal models of multiple diseases, including severe sepsis, burns, and acute pancreatitis [7,28]. Zhong et al. found that EP treatment significantly alleviated cecal ligation and puncture (CLP)-induced cognitive decline, microglial activation and neurogenesis impairment, indicating that EP had a protective effect on sepsis-associated encephalopathy [29]. In the present study, treatment with different concentrations (up to 10 mM) of EP in neuroblastoma N2a cells found that it did not show significant cytotoxicity under normal culture conditions, suggesting that EP has good safety and tolerability. Then, HR-treated N2a cells served as in vitro cerebral IRI model. As expected, hypoxia (24 h)/reoxygenation (12 h) inhibited cell viability and increased LDH release of N2a cells. However, EP treatment achieved obvious protection under such conditions, as suggested by restored cells viability, morphology, and reduced LDH release.
Oxidative stress occurs due to an imbalance between ROS generation and antioxidants production [30]. Stress can induce the generation of oxygen free radicals in the body, which are mainly formed in mitochondria, peroxisomes, lysosomes, plasma membranes and cytoplasm [31]. Brain cells are highly susceptible to ROS damage due to the brain is a main metabolic organ of oxygen, and has relatively incapable protective antioxidant system. Therefore, the brain is particularly susceptible to oxidative stress [32]. Besides, modulation of key regulators of ROS production or scavenging has been shown to provide protection against cerebral IRI [33,34]. NAD(P)H: quinone oxidoreductase 1 (NQO1) is a multifunctional protein that exhibits antioxidant effect via reducing intracellular ROS levels and preventing multiple toxicities [35,36]. In vitro model of cerebral IRI, our study confirmed that HR obviously increased the fluorescence intensity of ROS in cells, suggesting an increase in ROS content. Moreover, HR treatment significantly decreased cellular NQO1 expression. However, EP treatment partially reversed the HR-induced ROS increased and promoted the expression of NQO1, demonstrating the antioxidant effects of EP on HR injury.
PGC-1α is a multifunctional protein that activates multiple nuclear receptors and functions, playing a pivotal role in various central nervous system diseases [37]. Study confirmed that the nuclear PGC-1α protein expression remarkably reduced in HR-injured neuronal [23]. Quercetin treatment directly induces the up-regulation of PGC-1α level in regions of the mouse brain [38]. NRF1 has been verified to be targets of PGC-1α and accounts for some of its beneficial actions [39]. In human neural stem cells treated with advanced glycosylation end products, rosiglitazone treatment activates PGC-1 to upregulate mitochondrial NRF1, leading to an increase in the oxidative defense Gpx1, superoxide dismutase 1 (SOD1), and SOD2 genes [40]. UCP2, a member of the mitochondrial uncoupling protein family, has been verified to be downstream target of PGC-1α, and is involved in PGC-1α′s various biological functions [41,42]. As expected, HR inhibited PGC-1α, NRF1 and UCP2 expression, while EP treatment upregulated these proteins expression.
Recently, Axl has been found to play a significant role in various biological processes, such as immune regulation, cellular signaling, apoptosis, oxidative stress, and mitochondrial function [43,44]. Axl has an intracellular (C-terminal) tyrosine kinase domain, which plays an essential role in signal transduction [45]. The vitamin k-dependent GAS6 serves as a high affinity ligand for Axl. GAS6 binding to Axl primes the homodimerization of receptor with another GAS6/Axl ligand-receptor complex and autophosphorylation of three tyrosine residues [46,47]. This series of reactions leads to the recruitment of the p85 subunit of phosphoinositide-3 kinase (PI3K), phospholipase C-γ (PLCγ), or growth factor receptor-bound protein 2 (Grb2), activating downstream signaling pathways associated with survival, proliferation, apoptosis, oxidative stress, and mitochondrial function [48,49]. It is worth noting that the activation of Axl is suppressed when its soluble form, sAxl, binds to GAS6 [48,50,51].
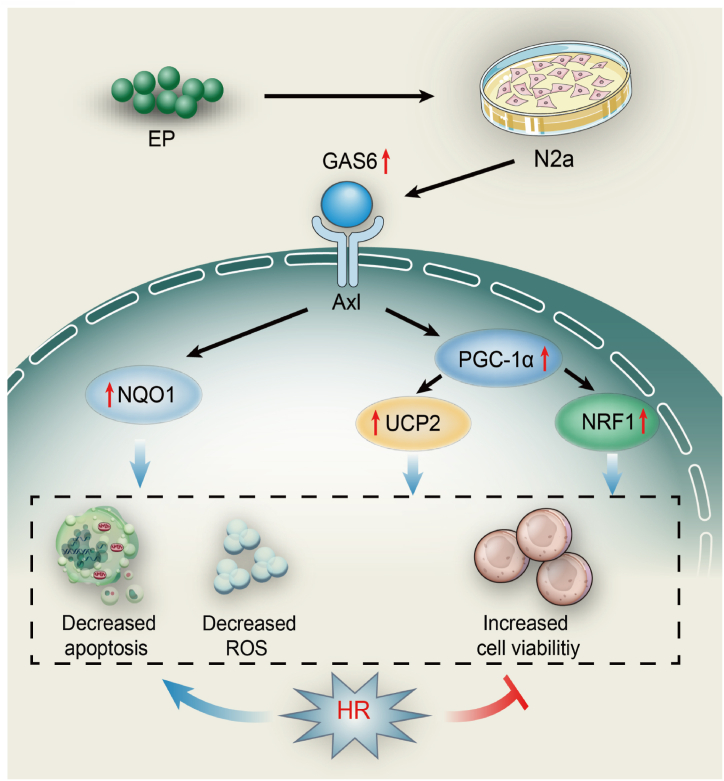
GAS6/Axl is participated in survival and growth processes during tissue repair and development [52,53]. In the acute phase, GAS6/Axl axis exhibits protective effects after IRI on various organs such as heart [54], kidney [55], and liver [56]. Importantly, the GAS6/Axl axis also has a protective effect on the neurons [18,19]. In the present study, HR treatment of N2a cells significantly decreased GAS6 and Axl expression, whereas EP treatment reversed this effect. Besides, compared with the Control siRNA EP + HR group, GAS6 siRNA could partially downregulate cell viability, increase LDH release, ROS production, and cell apoptotic rate. These results demonstrate that the GAS6/Axl axis may play a vital role in the neuroprotective effects of EP. Furthermore, Studies have confirmed that GAS6/Axl axis can interact with multiple pathways to exert various biological effects [[57], [58], [59]]. In this study, GAS6 siRNA partially reversed the effect of EP on PGC-1α/UCP2 and PGC-1α/NRF1 pathways, indicating that these pathways take part in benefits effects of GAS6/Axl signaling (Fig. 7).
Fig. 7.
GAS6/Axl signaling-dependent mechanism of EP-induced neuroprotective effects against HR injury. EP treatment promotes GAS6/Axl axis activation, which further upregulates PGC-1α and UCP2 expression, thus elevating their downstream targets, such as NQO1 and NRF1, which further inhibit HR-induced oxidative stress and the subsequent apoptosis of neurons. These results suggest that EP treatment may be a valuable therapeutic strategy against cerebral IRI.
5. Conclusion
Taken together, our current study demonstrates that EP treatment promotes GAS6/Axl axis activation, further upregulates PGC-1α expression, thereby promoting the expression of downstream targets NQO1, NRF1 and UCP2, ultimately inhibiting HR-induced oxidative stress and neuronal apoptosis. These findings suggest that EP may be a valuable treatment strategy for cerebral IRI. Additionally, it provides in vitro evidence for the profound neuroprotective effect of EP and its potential mechanisms.
Ethics approval and consent to participate
Not applicant.
Funding
Youth Science and Technology Rising Star Project of Shaanxi Province (2020KJXX-036), High-end Foreign Expert Introduction Program of National Science and Technology (G2022040014L), and Innovation Capability Strong Foundation Plan of Xi'an City (Medical Research Project, 21YXYJ0037).
Data availability statement
Data associated with this study has not been deposited into any publicly available repository. Data will be made available on request.
CRediT authorship contribution statement
Ying Chen: Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Data curation. Junmin Chen: Writing – original draft, Validation, Methodology, Investigation, Formal analysis. Lin Zhao: Methodology, Investigation, Formal analysis, Data curation. Xin Zhang: Methodology, Investigation. Xue Wu: Writing – review & editing, Methodology, Investigation. Xin Wang: Methodology, Investigation. Zhe Zhang: Methodology, Investigation. Yang Yang: Writing – review & editing, Validation, Project administration, Funding acquisition. Chao Deng: Writing – review & editing, Visualization, Validation, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e22787.
Contributor Information
Yang Yang, Email: yang200214yy@nwu.edu.cn.
Chao Deng, Email: chaodeng888@126.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Broderick J., et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American heart association/American stroke association stroke council, high blood pressure Research council, and the quality of care and outcomes in Research interdisciplinary working group. Circulation. 2007;116:e391–e413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 2.Thom T., et al. Heart disease and stroke statistics--2006 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 3.Chen B., Wu Z., Xu J., Xu Y. Calreticulin binds to fas ligand and inhibits neuronal cell apoptosis induced by ischemia-reperfusion injury. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/895284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y., et al. SIRT1 activation by pterostilbene attenuates the skeletal muscle oxidative stress injury and mitochondrial dysfunction induced by ischemia reperfusion injury. Apoptosis. 2016;21:905–916. doi: 10.1007/s10495-016-1258-x. [DOI] [PubMed] [Google Scholar]
- 5.Wang J., Cao B., Han D., Sun M., Feng J. Long non-coding RNA H19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging Dis. 2017;8:71–84. doi: 10.14336/AD.2016.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo E.H., Dalkara T., Moskowitz M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 7.Jung S.M., et al. Ethyl pyruvate ameliorates inflammatory arthritis in mice. Int. Immunopharm. 2017;52:333–341. doi: 10.1016/j.intimp.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Lu C., et al. Ethyl pyruvate: a newly discovered compound against ischemia-reperfusion injury in multiple organs. Pharmacol. Res. 2021;171 doi: 10.1016/j.phrs.2021.105757. [DOI] [PubMed] [Google Scholar]
- 9.Shen H., et al. Ethyl pyruvate protects against hypoxic-ischemic brain injury via anti-cell death and anti-inflammatory mechanisms. Neurobiol. Dis. 2010;37:711–722. doi: 10.1016/j.nbd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong Z., Pan R., Chang L., Lee W. Combination treatment with ethyl pyruvate and IGF-I exerts neuroprotective effects against brain injury in a rat model of neonatal hypoxic-ischemic encephalopathy. Int. J. Mol. Med. 2015;36:195–203. doi: 10.3892/ijmm.2015.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider C., King R.M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 12.Happonen K.E., Dahlback B. Gas6 fueling tumor-mediated thrombosis. Blood. 2016;127:672–673. doi: 10.1182/blood-2015-12-683474. [DOI] [PubMed] [Google Scholar]
- 13.Wu G., et al. Molecular insights of Gas6/TAM in cancer development and therapy. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Q., Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 15.Hafizi S., Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006;273:5231–5244. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- 16.Weinger J.G., et al. Loss of the receptor tyrosine kinase Axl leads to enhanced inflammation in the CNS and delayed removal of myelin debris during experimental autoimmune encephalomyelitis. J. Neuroinflammation. 2011;8:49. doi: 10.1186/1742-2094-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giangola M.D., Yang W.L., Rajayer S.R., Nicastro J., Coppa G.F., Wang P. Growth arrest-specific protein 6 attenuates neutrophil migration and acute lung injury in sepsis. Shock. 2013;40:485–491. doi: 10.1097/SHK.0b013e3182a588c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong L.S., et al. Recombinant Gas6 augments Axl and facilitates immune restoration in an intracerebral hemorrhage mouse model. J. Cerebr. Blood Flow Metabol. 2017;37:1971–1981. doi: 10.1177/0271678X16658490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu G., McBride D.W., Zhang J.H. Axl activation attenuates neuroinflammation by inhibiting the TLR/TRAF/NF-kappaB pathway after MCAO in rats. Neurobiol. Dis. 2018;110:59–67. doi: 10.1016/j.nbd.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K.C., Baek S.H., Lee C. Curcumin-induced downregulation of Axl receptor tyrosine kinase inhibits cell proliferation and circumvents chemoresistance in non-small lung cancer cells. Int. J. Oncol. 2015;47:2296–2303. doi: 10.3892/ijo.2015.3216. [DOI] [PubMed] [Google Scholar]
- 21.Paccez J.D., Duncan K., Sekar D., Correa R.G., Wang Y. Dihydroartemisinin inhibits prostate cancer via JARID2/miR-7/miR-34a-dependent downregulation of Axl. Oncogenesis. 2019;8:14. doi: 10.1038/s41389-019-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu X., et al. Neuroprotective effects of omentin-1 against cerebral hypoxia/reoxygenation injury via activating GAS6/axl signaling pathway in neuroblastoma cells. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.784035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M., et al. SIRT1/PGC-1α signaling activation by mangiferin attenuates cerebral hypoxia/reoxygenation injury in neuroblastoma cells. Eur. J. Pharmacol. 2021;907 doi: 10.1016/j.ejphar.2021.174236. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H.K., Ye Y., Li K.J., Zhao Z.N., He J.F. Gypenosides prevent H(2)O(2)-induced retinal ganglion cell apoptosis by concurrently suppressing the neuronal oxidative stress and inflammatory response. J. Mol. Neurosci. 2020;70:618–630. doi: 10.1007/s12031-019-01468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei W., et al. Psoralidin protects against cerebral hypoxia/reoxygenation injury: role of GAS6/Axl signaling. Phytother Res. 2022;36:2628–2640. doi: 10.1002/ptr.7481. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y., et al. A new flavonoid glycoside (APG) isolated from Clematis tangutica attenuates myocardial ischemia/reperfusion injury via activating PKCepsilon signaling. Biochim. Biophys. Acta. 2017;1863:701–711. doi: 10.1016/j.bbadis.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Saiardi A., Guillermier C., Loss O., Poczatek J.C., Lechene C. Quantitative imaging of inositol distribution in yeast using multi-isotope imaging mass spectrometry (MIMS) Surf. Interface Anal. 2014;46:169–172. doi: 10.1002/sia.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang G.H., Lin D.J., Xiao W., Jia C.Q., Li Y., Wang A.H., et al. Ethyl pyruvate reduces mortality in an endotoxin-induced severe acute lung injury mouse model. Respir. Res. 2009;10:91. doi: 10.1186/1465-9921-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong X., et al. Ethyl pyruvate protects against sepsis-associated encephalopathy through inhibiting the NLRP3 inflammasome. Mol. Med. 2020;26:55. doi: 10.1186/s10020-020-00181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Jiang S., Yan J., Li Y., Xin Z., Lin Y., et al. An overview of the molecular mechanisms and novel roles of Nrf2 in neurodegenerative disorders. Cytokine Growth Factor Rev. 2015;26:47–57. doi: 10.1016/j.cytogfr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Djordjevic J., Djordjevic A., Adzic M., Niciforovic A., Radojcic M.B. Chronic stress differentially affects antioxidant enzymes and modifies the acute stress response in liver of Wistar rats. Physiol. Res. 2010;59:729–736. doi: 10.33549/physiolres.931862. [DOI] [PubMed] [Google Scholar]
- 32.Dringen R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 33.Spescha R.D., et al. Deletion of the ageing gene p66(Shc) reduces early stroke size following ischaemia/reperfusion brain injury. Eur. Heart J. 2013;34:96–103. doi: 10.1093/eurheartj/ehs331. [DOI] [PubMed] [Google Scholar]
- 34.Xu P., et al. Breast cancer susceptibility protein 1 (BRCA1) rescues neurons from cerebral ischemia/reperfusion injury through NRF2-mediated antioxidant pathway. Redox Biol. 2018;18:158–172. doi: 10.1016/j.redox.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinkova-Kostova A.T., Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 37.Wang R., et al. Metabolic stress modulates Alzheimer's beta-secretase gene transcription via SIRT1-PPARgamma-PGC-1 in neurons. Cell Metabol. 2013;17:685–694. doi: 10.1016/j.cmet.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X., Wang H. Neuroprotection by quercetin via mitochondrial function adaptation in traumatic brain injury: PGC-1alpha pathway as a potential mechanism. J. Cell Mol. Med. 2018;22:883–891. doi: 10.1111/jcmm.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan H., et al. A PGC-1alpha-mediated transcriptional network maintains mitochondrial redox and bioenergetic homeostasis against doxorubicin-induced toxicity in human cardiomyocytes: implementation of TT21C. Toxicol. Sci. 2016;150:400–417. doi: 10.1093/toxsci/kfw006. [DOI] [PubMed] [Google Scholar]
- 40.Chiang M.C., Cheng Y.C., Nicol C.J., Lin C.H. The neuroprotective role of rosiglitazone in advanced glycation end product treated human neural stem cells is PPARgamma-dependent. Int. J. Biochem. Cell Biol. 2017;92:121–133. doi: 10.1016/j.biocel.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Jezek P., Engstova H., Zackova M., Vercesi A.E., Costa A.D., Arruda P., et al. Fatty acid cycling mechanism and mitochondrial uncoupling proteins. Biochim. Biophys. Acta. 1998;1365:319–327. doi: 10.1016/s0005-2728(98)00084-x. [DOI] [PubMed] [Google Scholar]
- 42.Skulachev V.P. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 43.Du W., Brekken R.A. Does Axl have potential as a therapeutic target in pancreatic cancer? Expert Opin. Ther. Targets. 2018;22:955–966. doi: 10.1080/14728222.2018.1527315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemke G. Biology of the TAM receptors. Cold Spring Harbor Perspect. Biol. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korshunov V.A. Axl-dependent signalling: a clinical update. Clin. Sci. (Lond.) 2012;122:361–368. doi: 10.1042/CS20110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manfioletti G., Brancolini C., Avanzi G., Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol. Cell Biol. 1993;13:4976–4985. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki T., et al. Structural basis for Gas6-Axl signalling. EMBO J. 2006;25:80–87. doi: 10.1038/sj.emboj.7600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scaltriti M., Elkabets M., Baselga J. Molecular pathways: AXL, a membrane receptor mediator of resistance to therapy. Clin. Cancer Res. 2016;22:1313–1317. doi: 10.1158/1078-0432.CCR-15-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braunger J., et al. Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene. 1997;14:2619–2631. doi: 10.1038/sj.onc.1201123. [DOI] [PubMed] [Google Scholar]
- 50.Ekman C., Stenhoff J., Dahlbäck B. Gas6 is complexed to the soluble tyrosine kinase receptor Axl in human blood. J. Thromb. Haemostasis. 2010;8:838–844. doi: 10.1111/j.1538-7836.2010.03752.x. [DOI] [PubMed] [Google Scholar]
- 51.Pidkovka N., Belkhiri A. Altered expression of AXL receptor tyrosine kinase in gastrointestinal cancers: a promising therapeutic target. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1079041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemke G., Rothlin C.V. Immunobiology of the TAM receptors. Nat. Rev. Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linger R.M., Keating A.K., Earp H.S., Graham D.K. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurtado B., et al. Association study between polymorphims in GAS6-TAM genes and carotid atherosclerosis. Thromb. Haemostasis. 2010;104:592–598. doi: 10.1160/TH09-11-0787. [DOI] [PubMed] [Google Scholar]
- 55.Nagai K., et al. Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in diabetic nephropathy. Kidney Int. 2005;68:552–561. doi: 10.1111/j.1523-1755.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- 56.Llacuna L., et al. Growth arrest-specific protein 6 is hepatoprotective against murine ischemia/reperfusion injury. Hepatology. 2010;52:1371–1379. doi: 10.1002/hep.23833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melaragno M.G., Fridell Y.W., Berk B.C. The Gas6/Axl system: a novel regulator of vascular cell function. Trends Cardiovasc. Med. 1999;9:250–253. doi: 10.1016/s1050-1738(00)00027-x. [DOI] [PubMed] [Google Scholar]
- 58.Kariolis M.S., et al. Inhibition of the GAS6/AXL pathway augments the efficacy of chemotherapies. J. Clin. Invest. 2017;127:183–198. doi: 10.1172/JCI85610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim H., et al. alpha-Lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J. Cell Mol. Med. 2012;16:273–286. doi: 10.1111/j.1582-4934.2011.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has not been deposited into any publicly available repository. Data will be made available on request.