Abstract
Background
Campylobacteriosis is a common cause of bacterial gastroenteritis worldwide. This study aimed to investigate the potential risk factors, clinical and laboratory manifestations of children with campylobacteriosis under five years old in Taiwan.
Methods
This retrospective case–control study was conducted in ten major hospitals in Taiwan from 2014 to 2017. Laboratory tests and stool specimen were collected and analyzed together with questionnaire survey. Multivariate stepwise logistic regression model was used for identification of risk factors.
Results
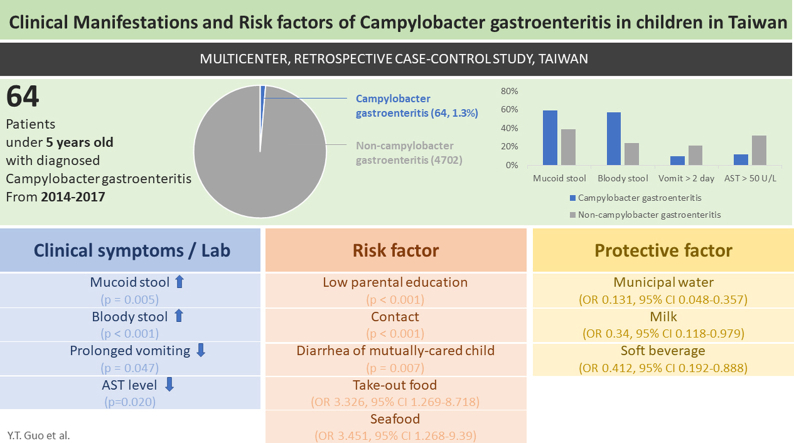
A total of 64 campylobacteriosis cases were included with a median age of 25 months. We observed a less prolonged vomiting (p = 0.047), more bloody (p < 0.001) and mucoid (p = 0.005) stools, and lower AST levels (p = 0.020) in patients with campylobacteriosis. Lower parental educational attainment (p < 0.001), direct contact with acute gastroenteritis patients (p < 0.001), as well as diarrhea in the mutually cared children (p = 0.007) were linked to campylobacteriosis. Consumption of municipal water (p < 0.001), milk (OR 0.34, 95% CI 0.118–0.979), and soft beverages (OR 0.41, 95% CI 0.192–0.888) were identified as protective factors, while consuming takeout food (p = 0.032) and seafood (p = 0.019) increased risk of campylobacteriosis.
Conclusions
Shorter vomiting duration, bloody and mucoid stool, and less elevated AST levels are manifestations suggestive of campylobacteriosis. Risk factors of campylobacteriosis were low parental educational attainment, direct contact with acute gastroenteritis patients, diarrhea in mutually cared children, takeout food and seafood intake. Potential protective factors include municipal water, milk, and soft beverage intake.
Keywords: Acute gastroenteritis, Campylobacter, Children
Graphical abstract
Introduction
Acute gastroenteritis is a common pediatric illness, resulting in significant childhood morbidity and mortality globally [1]. In 2016, diarrhea was the fifth leading cause of death among children under five years old [2]. Campylobacteriosis is a common bacterial gastroenteritis worldwide, with incidence and prevalence rising in many parts of the world [3].
Viruses are the predominant pathogens of diarrhea in Taiwan, but Campylobacter spp. still accounted for 16.7% of patients with acute diarrhea with identified pathogens in community clinics [4]. A study done in northern Taiwan revealed that Campylobacter spp. was identified in 6.8% of children with diarrhea [5]. More than half of campylobacteriosis patients were less than five years old [6].
The Campylobacteraceae family consists of more than 20 species of gram-negative, microaerobic Campylobacter bacteria. Morphologically they are diverse being spiral, curved, or rod shape depending on the species [3,7]. Campylobacter gastroenteritis is usually caused by Campylobacter jejuni or Campylobacter coli. Infections with other Campylobacter species are less common and tend to be milder [3,8]. Clinical presentations of campylobacteriosis include diarrhea, fever, vomiting, abdominal pain, and weight loss, with incubation period ranging from 24 to 72 h and could be as long as seven days [3,7,8]. Diarrhea can present as watery or bloody, and lasts for a median duration of six to seven days [3,9]. As for laboratory tests, fecal leukocytes and mild neutrophilic leukocytosis with bandemia may be found in campylobacteriosis patients, while electrolytes and liver function were mostly within normal ranges [[10], [11], [12]].
A number of risk factors have been identified for campylobacteriosis. Campylobacter spp. could be found in more than 40% of food-producing animals such as broilers, hens, pigs; hence, posing significant health impact on the agri-food chain [13,14]. Eating undercooked poultry and poultry cooked outside the home were linked to campylobacteriosis. International travel has been recognized as an important risk factor for Campylobacter infection as well [15,16]. Other risk factors include direct contact with farm animals, drinking untreated water, food preparation with poor hygiene, and consumption of unpasteurized dairy products [16]. As for younger children, similar risk factors for campylobacteriosis have been investigated. A meta-analysis conducted in children under five years old showed that contact with domestic animals and consumption of animal products significantly increased the risk of Campylobacter infection. The study also identified illiterate mothers and mothers with poor personal hygiene as risk factors [17].
In Taiwan, there have been several studies regarding the epidemiology and clinical characteristics of Campylobacter spp [5,6,18]. To the best of our knowledge however, comprehensive studies examining the risk factors and manifestations of campylobacteriosis as compared with other pathogens in young children are lacking. Therefore, we conducted this case–control study to investigate the potential risk factors, clinical symptoms and laboratory manifestations, hoping to provide more information in preventing and identifying the disease.
Material and methods
This retrospective, matched case–control study was conducted in ten major hospitals across Taiwan. A hospital-based acute gastroenteritis (AGE) surveillance system was built in conjunction with the Taiwan Centers for Disease Control (CDC) for study design and development of the letter of consent, questionnaire, as well as standard operation procedure of data and specimen management. Experts from the U.S. Centers for Disease Control and Prevention were consulted for questionnaire development, which collects demographic data, contact history, food consumption, domestic hygiene, immunization history, and clinical symptoms of all the cases and controls. The institutional review board of Mackay Memorial Hospital (13MMHIS285), National Taiwan University Hospital (201310064RINA), Chang Gung Memorial Hospital (102–4349A3), Show-Chwan Memorial Hospital (1,040,606), National Cheng Kung University Hospital (B-ER-102-359), China Medical University Hospital (CMUH102-REC2-136), Buddhist Tzu-Chi General Hospital (IRB102-155), Taiwan National Health Research Institutes (EC1021101-R5) approved this study.
From February, 2014 to December, 2017, all children in those hospitals were deemed eligible for enrollment as long as they met all the following inclusion criteria: 1) age less than 61 months on the day of stool specimen collection, 2) hospitalized in one of the 10 participating hospitals, 3) diagnosed with acute gastroenteritis under ICD-9-CM CODEa, 4) experienced diarrhea and/or vomiting within three days after seeking medical attention. Diarrhea was defined as three or more passages of loose stool within a 24-h time period.
Children who met the inclusion criteria were enrolled once informed consent signed by the legal guardian was obtained. Non-AGE children of the same sex and an age difference under 3 months with no diarrhea in the recent week was selected from the outpatient department or healthy children from the community based on each case, with informed consent required as well. Questionnaires were filled in by research assistants in the AGE group, and were filled in by the parent or the legal guardian in the non-AGE group. All the questionnaires were sent to Taiwan National Health Research Institutes (NHRI) for management and analysis.
Stool specimens of AGE cases and healthy children were collected. Stool specimens of AGE cases underwent enteric pathogens screening with traditional bacterial culture and rotavirus antigen test in sentinel hospitals. Then, specimens were kept frozen until sent to Taiwan CDC reference laboratory for further analysis, including multiplex real-time polymerase chain reaction (PCR) for detection of rotavirus, norovirus, Vibrio cholerae, Vibrio parahaemolyticus, Salmonella spp., Shigella spp., Clostridium perfringens, Clostridium difficile, Campylobacter spp., Listeria monocytogenes, and Bacillus cereus. In this study, only part of AGE cases underwent cultivation for Campylobacter spp. in sentinel hospitals. Thus, an AGE patient yielding a positive PCR result for Campylobacter spp. in the stool sample would be defined as a campylobacteriosis case. The use of PCR for detection of Campylobacter spp. has been widely use and proved to be selective and accurate [19,20]. AGE patients with a negative Campylobacter PCR result was matched by age, gender, and study site to be qualified as non-Campylobacter AGE controls. Patients with co-infections in the case group were not excluded, neither were those in the non-Campylobacter AGE control group.
The control group for analysis of laboratory tests and clinical manifestations were non-Campylobacter AGE control. As for risk factor analysis, the primary control group were non-AGE patients. However, to identify specific risk factors for campylobacteriosis, we not only compared the data with non-AGE control but also with non-Campylobacter AGE control.
Statistical analysis was performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina, USA). The case–control ratio was 1:4 for analysis of clinical symptoms and laboratory data, and 1:3 for analysis of risk factors. Comparison of risk factors, clinical symptoms and laboratory data was analyzed using the Student's t test for continuous variables and the Chi-square test for categorical variables. Univariable conditional logistic regression was also performed for identification of independent risk factors for Campylobacter gastroenteritis. Statistically significant variables, those with a P-value of less than 0.05, were selected into the multiple logistic regression model with stepwise selection.
Results
A total of 4766 cases of acute gastroenteritis (AGE) and 2501 non-AGE controls were qualified for further analysis. Among the 4766 children diagnosed with AGE, 64 (1.3%) were Campylobacter PCR positive, including eight cases with norovirus and Campylobacter spp. coinfection, one with rotavirus and Campylobacter spp. coinfection, three with Salmonella spp. and Campylobacter spp. coinfection, three with C. difficile and Campylobacter spp. infection, and one with C. difficile plus B. cereus plus Campylobacter spp. coinfection. As for underlying disease of the Campylobacter PCR positive patients, one had ventricular septal defect, and another had G6PD deficiency (Glucose-6-Phosphate Dehydrogenase Deficiency).
For analysis, we excluded those cases for whom no matched controls could be found. Of the 64 Campylobacter gastroenteritis cases, 62 were eligible for comparison with matched non-Campylobacter AGE control for clinical symptoms and laboratory data, and 53 cases were compared with matched non-AGE control for the risk factors.
Clinical symptoms and laboratory manifestations
Among the 62 Campylobacter gastroenteritis cases, 34 (65%) were male and the average age was 25 ± 16 months old. No obvious difference was found in sex or age between the case and the non-Campylobacter AGE control group (Table 1).
Table 1.
Clinical symptoms and laboratory manifestations of campylobacteriosis.
| Campylobacteriosis |
Non-campylobacter-AGE-control |
p-value | |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Total | 62 | 223 | |
| Male | 40 (64.52) | 150 (67.26) | 0.685 |
| Age (months) | |||
| Mean ± SD | 25.29 ± 16.47 | 23.29 ± 14.85 | 0.471 |
| Median | 21 (11.37) | 18 (11.35) | |
| Range | 0.669 | ||
| <6 | 4 (6.45) | 11 (4.93) | |
| 6-11 | 14 (22.58) | 46 (20.63) | |
| 12-23 | 16 (25.81) | 69 (30.94) | |
| 24-35 | 10 (16.13) | 48 (21.52) | |
| 36-47 | 11 (17.74) | 35 (15.7) | |
| 48-60 | 7 (11.29) | 14 (6.28) | |
| Symptoms | |||
| Vomiting alone | 0 (0) | 1 (0.45) | >0.99 |
| Diarrhea alone | 4 (6.45) | 9 (4.04) | 0.489 |
| Fever alone | 0 (0) | 4 (1.79) | 0.580 |
| Vomiting and Diarrhea | 0 (0) | 29 (13) | 0.003∗ |
| Vomiting and Fever | 0 (0) | 0 (0) | |
| Diarrhea and Fever | 27 (43.55) | 78 (34.98) | 0.216 |
| Vomiting + Diarrhea + Fever | 31 (50) | 102 (45.74) | 0.552 |
| Bloody stool | 35 (57.38) | 53 (24.2) | <0.001∗ |
| Mucoid stool | 36 (59.02) | 85 (38.99) | 0.005∗ |
| Duration of symptoms (days) | |||
| Vomiting, mean ± SD | 0.97 ± 1.4 | 1.36 ± 1.64 | 0.080 |
| >2 days | 6 (9.68) | 46 (20.72) | 0.047∗ |
| Diarrhea, mean ± SD | 6.06 ± 2.61 | 5.74 ± 3.34 | 0.321 |
| >5 days | 32 (51.61) | 108 (48.65) | 0.680 |
| >8 days | 10 (16.13) | 23 (10.36) | 0.210 |
| Fever, mean ± SD | 3.55 ± 2.02 | 3.56 ± 2.56 | 0.988 |
| >2 days | 41 (66.13) | 144 (64.86) | 0.854 |
| >39.0° (Before) | 30 (52.63) | 96 (51.34) | 0.864 |
| >39.0° (After) | 23 (47.92) | 73 (39.04) | 0.264 |
| Hospital stay (days) | |||
| Mean ± SD | 5.98 ± 2.24 | 5.67 ± 3.16 | 0.086 |
| >5 days | 31 (50) | 87 (40.65) | 0.190 |
| URI symptoms ( + ) | 21 (34.43) | 96 (43.24) | 0.216 |
| Lab data on admission | |||
| Hemoglobin (g/dL), mean ± SD | 12.07 ± 0.86 | 12.01 ± 1.28 | 0.683 |
| NA | 0 | 3 | |
| WBC (1000/μL), mean ± SD | 11.21 ± 5.09 | 11.19 ± 5.78 | 0.491 |
| <5000 | 5 (8.06) | 21 (9.55) | 0.722 |
| >15,000 | 10 (16.13) | 48 (21.82) | 0.328 |
| NA | 0 | 3 | |
| Platelet (1000/μL), mean ± SD | 284.98 ± 97.96 | 291.38 ± 107.85 | 0.623 |
| <150,000 | 2 (3.23) | 13 (5.94) | 0.535 |
| NA | 0 | 4 | |
| CRP (mg/L), mean ± SD | 43.8 ± 42.52 | 47.48 ± 69.13 | 0.150 |
| >10 | 49 (80.33) | 133 (69.27) | 0.094 |
| >20 | 37 (60.66) | 103 (53.65) | 0.337 |
| >40 | 26 (42.62) | 67 (34.9) | 0.276 |
| NA | 1 | 31 | |
| AST (U/L), mean ± SD | 36.24 ± 13.26 | 54.64 ± 71.91 | 0.020∗ |
| >50 | 4 (12.12) | 27 (31.76) | 0.030∗ |
| NA | 29 | 138 | |
Abbreviations: AST: aspartate aminotransferase; CRP: C-reactive protein; NA: not available; SD: standard deviation; URI: upper respiratory infection; WBC:white blood cells.
Diarrhea and fever (93.6%) were the most common clinical manifestations. Mucoid (59% versus 39%, p = 0.005) and bloody stool (57% versus 24%, p < 0.001) occurred more frequently in Campylobacter gastroenteritis patients. Fewer children experienced vomiting for more than two days with Campylobacter gastroenteritis, with 9.7% versus 21% in the control group (p = 0.047). Campylobacter gastroenteritis was associated with lower Aspartate Transaminase (AST) level, with a mean of 36 ± 13 (U/L) in 33 case-patients compared to 55 ± 72 (U/L) of the control group (p = 0.020). An AST level higher than 50 U/L were also less frequently seen in Campylobacter gastroenteritis patients (12% versus 32%, p = 0.030).
Risk factors (non-AGE control)
After 1:3 matching of the case and control group by age, gender, and study site, 53 Campylobacter gastroenteritis cases and 148 non-AGE controls were qualified for analysis (44 cases with 1:3 pairing, seven cases with 1:2 pairing, two cases with 1:1 pairing). No significant difference was found in sex, age, or geographic data between the two groups (Table 2). Children were more likely to have Campylobacter gastroenteritis if their parents had lower educational attainment (p < 0.001). Those with at least one parent having an academic degree had significantly lower odds to experience Campylobacter gastroenteritis (OR 0.224, 95% CI 0.101–0.498). Direct contact with gastroenteritis patients in the prior week led to higher possibility of Campylobacter gastroenteritis (20.75% versus 0%, p < 0.001). Children who had contact with other children have lower odds of developing Campylobacter gastroenteritis if the other children did not experience recent diarrhea (OR 0.079, 95% CI 0.008–0.757).
Table 2.
Risk factors for campylobacteriosis with non-AGE control.
| Campylobacterosis No. (%) | Non-AGE-control No. (%) | p-value | Univariate OR (95%CI) | Multivariate OR (95%CI) | |
|---|---|---|---|---|---|
| Total | 53 | 148 | |||
| Male | 34 (64.15) | 97 (65.54) | 0.855 | ||
| Age (months) | 25.36 ± 15.89 | 23.94 ± 14.92 | 0.545 | ||
| Geographic area | 0.994 | ||||
| Northern | 25 (47.17) | 71 (47.97) | |||
| Central | 5 (9.43) | 14 (9.46) | |||
| Southern | 23 (43.4) | 63 (42.57) | |||
| Eastern | 0 (0) | 0 (0) | |||
| Parental education attainment | < 0.001∗ | ||||
| Both high school or lower | 23 (43.4) | 25 (16.89) | 1 | ||
| At least one with college degree or above | 29 (54.72) | 122 (82.43) | 0.224 (0.101, 0.498)∗ | 0.167 (0.044, 0.63)∗ | |
| One high school or lower, the other unknown | 1 (1.89) | 1 (0.68) | - | - | |
| At least one of the parents is new immigrant | 2 (3.77) | 8 (5.48) | > 0.99 | 0.546 (0.113, 2.654) | |
| Rotavirus vaccination (yes vs. no) | 26 (50.98) | 93 (64.58) | 0.087 | 0.624 (0.316, 1.234) | |
| Contact with AGE patients within 1 week | 11 (20.75) | 0 (0) | < 0.001∗ | - | |
| Breastfeeding duration (months) | 6.71 ± 7.69 | 6.76 ± 7.35 | 0.515 | 0.999 (0.958, 1.042) | |
| Public place visited in the recent week | |||||
| Nursery/Kindergarten/After school club/School | 14 (26.42) | 39 (26.35) | 0.993 | 0.865 (0.38, 1.971) | |
| Clinic/Hospital/Nursing home | 22 (41.51) | 78 52.7) | 0.162 | 0.491 (0.226, 1.067) | |
| Contact with animals within 1 week | 19 (35.85) | 54 (36.49) | 0.934 | 0.956 (0.501, 1.825) | |
| Dogs | 17 (32.08) | 34 (22.97) | 0.191 | 1.61 (0.782, 3.314) | |
| Cats | 2 (3.77) | 16 (10.81) | 0.164 | 0.335 (0.074, 1.519) | |
| Family visit or travel abroad within 1 week | 0 (0) | 1 (0.68) | > 0.99 | - | |
| Hand wash before meals | 0.756 | ||||
| Never | 9 (16.98) | 23 (15.65) | 1 | ||
| Occasional | 36 (67.92) | 95 (64.63) | 0.958 (0.349, 2.634) | ||
| Always | 8 (15.09) | 29 (19.73) | 0.515 (0.132, 2.009) | ||
| Living environment | |||||
| Other children cared for by the mutual primary caregiver had diarrhea or not | 0.007∗ | ||||
| With diarrhea | 4 (8.33) | 2 (1.6) | 1 | ||
| Without diarrhea | 16 (33.33) | 69 (55.2) | 0.079 (0.008, 0.757)∗ | 0.069 (0.005, 0.98)∗ | |
| The primary caregiver did not care for other children | 28 (58.33) | 54 (43.2) | 0.201 (0.02, 1.988) | 0.18 (0.012, 2.63) | |
| Healthy primary caregiver | 52 (98.11) | 123 (89.13) | 0.045∗ | 7.104 (0.867, 58.235) | |
| Primary caregiver prepared food | 4 (10.53) | 20 (17.7) | 0.296 | 0.362 (0.094, 1.404) | |
| Care place: restroom occupants | 0.596 | ||||
| < = 5 occupants | 38 (76) | 113 (79.58) | 1 | ||
| > 5 occupants | 12 (24) | 29 (20.42) | 1.097 (0.474, 2.537) | ||
| Care place: drinking water source | |||||
| Municipal water | 28 (52.83) | 106 (81.54) | < 0.001∗ | 0.131 (0.048, 0.357)∗ | 0.2 (0.044, 0.91)∗ |
| Bottled water | 4 (7.55) | 8 (6.15) | 0.747 | 1.323 (0.348, 5.02) | |
| Water refilling station | 10 (18.87) | 13 (10) | 0.101 | 2.374 (0.903, 6.239) | |
| Filtered and boiled water | 51 (100) | 113 (91.87) | 0.078 | - | |
| Food eaten in the recent week | |||||
| Dining out | 36 (67.92) | 101 (69.18) | 0.866 | 0.902 (0.437, 1.861) | |
| Take-out food | 38 (71.7) | 80 (54.79) | 0.032∗ | 3.326 (1.269, 8.718)∗ | 6.959 (1.395, 34.72)∗ |
| Milk | 7 (13.21) | 34 (23.13) | 0.125 | 0.34 (0.118, 0.979)∗ | |
| Breast milk | 9 (16.98) | 24 (16.33) | 0.912 | 1.14 (0.453, 2.871) | |
| Milk powder | 42 (79.25) | 113 (76.87) | 0.723 | 1.273 (0.543, 2.984) | |
| Goat milk | 2 (3.77) | 1 (0.68) | 0.172 | 5.998 (0.544, 66.14) | |
| Egg | 40 (75.47) | 101 (70.14) | 0.462 | 1.344 (0.57, 3.172) | |
| Cooked egg | 40 (75.47) | 99 (68.75) | 0.359 | 1.477 (0.624, 3.495) | |
| Raw or half-boiled egg | 0 (0) | 2 (1.39) | > 0.99 | - | |
| Iced products | 19 (35.85) | 60 (41.38) | 0.482 | 0.672 (0.337, 1.34) | |
| Cold soft drinks | 26 (49.06) | 92 (62.59) | 0.086 | 0.412 (0.192, 0.888)∗ | 0.188 (0.048, 0.73)∗ |
| Leftovers | 14 (26.42) | 48 (32.88) | 0.384 | 0.728 (0.354, 1.494) | |
| Vegetable | 44 (84.62) | 124 (84.93) | 0.957 | 1 (0.354, 2.822) | |
| Cabbage | 30 (57.69) | 89 (60.96) | 0.680 | 0.853 (0.402, 1.807) | |
| Carrot | 28 (53.85) | 85 (58.22) | 0.584 | 0.828 (0.413, 1.659) | |
| Corn | 15 (28.85) | 41 (28.08) | 0.916 | 1.117 (0.528, 2.363) | |
| Green broccoli | 13 (25) | 28 (19.18) | 0.374 | 1.485 (0.645, 3.423) | |
| Fruit | 47 (88.68) | 111 (76.03) | 0.051 | 2.619 (0.977, 7.021) | |
| Apple | 28 (52.83) | 75 (51.37) | 0.855 | 1.033 (0.56, 1.907) | |
| Guava | 12 (22.64) | 29 (19.86) | 0.668 | 1.261 (0.589, 2.698) | |
| Grape | 12 (22.64) | 37 (25.34) | 0.696 | 0.865 (0.407, 1.837) | |
| Banana | 23 (43.4) | 57 (39.04) | 0.580 | 1.274 (0.644, 2.522) | |
| Seafood | 47 (88.68) | 105 (72.92) | 0.019∗ | 3.451 (1.268, 9.39)∗ | 3.675 (0.749, 18.04) |
| Chicken | 29 (54.72) | 67 (45.89) | 0.271 | 1.582 (0.771, 3.247) | |
| Duck | 2 (3.77) | 6 (4.11) | > 0.99 | 0.851 (0.171, 4.241) | |
| Goose | 2 (3.77) | 5 (3.42) | > 0.99 | 1.128 (0.218, 5.835) | |
| Pork | 36 (67.92) | 104 (71.23) | 0.652 | 0.662 (0.259, 1.696) | |
| Beef | 8 (15.09) | 25 (17.12) | 0.734 | 0.904 (0.373, 2.192) | |
Risk of campylobacteriosis was reduced if the source of drinking water was municipal water (OR 0.131, 95% CI 0.048–0.357). Consuming take-out food in the prior week had increased odds of Campylobacter gastroenteritis (OR 3.326, 95% CI 1.269–8.718). Ingestion of seafood (OR 3.451, 95% CI 1.268–9.39) also was associated with Campylobacter gastroenteritis. Milk (OR 0.34, 95% CI 0.118–0.979) and soft drink (OR 0.412, 95% CI 0.192–0.888) consumption were protective factors. In the final stepwise regression model, takeout food consumption (OR 6.959, 95% CI 1.395–34.72) was a risk factor, while at least one of the parents having an academic degree (OR 0.167, 95% CI 0.044–0.63), healthy children cared for by the mutual caregiver (OR 0.069, 95% CI 0.005–0.98), consumption of municipal water (OR 0.2, 95% CI 0.044–0.91) and soft drink (OR 0.188, 95% CI 0.048–0.73) were statistically significant protective factors.
Risk factors (non-campylobacter AGE control)
After 1:4 matching of the case and control group by age, gender, and study site, 62 Campylobacter gastroenteritis cases and 223 non-Campylobacter AGE controls were qualified for analysis (51 cases with 1:4 pairing, one case with 1:3 pairing, six cases with 1:2 pairing, and four cases with 1:1 pairing). No significant difference was found in sex, age, or geographic data between the two groups (Table 3). Of the 223 non-Campylobacter AGE patients, 38 were detected with norovirus, 25 were detected with rotavirus, 50 were detected with Salmonella spp., 10 were detected with C. difficile, three were detected with B. cereus, one was detected with V. parahaemolyticus, and one was detected Giardia. When the control group was non-Campylobacter AGE instead of non-AGE patients, lower educational attainment of the parents (p = 0.003) remained as a significant risk factor.
Table 3.
Risk factors for campylobacteriosis with non-Campylobacter AGE control.
| Campylobacteriosis No. (%) | Non-campylobacter AGE control No. (%) | p-value | Univariate (OR 95%CI) | Multivariate (OR 95%CI) | |
|---|---|---|---|---|---|
| Total | 62 | 223 | |||
| Male | 40 (64.52) | 150 (67.26) | 0.685 | ||
| Age (months) | 25.29 ± 16.47 | 23.29 ± 14.85 | 0.471 | ||
| Geographic area | 0.507 | ||||
| Northern | 29 (46.77) | 112 (50.22) | |||
| Central | 6 (9.68) | 24 (10.76) | |||
| Southern | 25 (40.32) | 85 (38.12) | |||
| Eastern | 2 (3.23) | 2 (0.9) | |||
| Parental education attainment | 0.003∗ | ||||
| Both high school or lower | 26 (41.94) | 50 (22.42) | 1 | ||
| At least one with college degree or above | 34 (54.84) | 170 (76.23) | 0.376 (0.203, 0.696)∗ | 0.575 (0.227, 1.450) | |
| One high school or lower, the other unknown | 2 (3.23) | 3 (1.35) | - | - | |
| At least one of the parents is new immigrant | 2 (3.23) | 19 (8.52) | 0.269 | 0.401 (0.091, 1.771) | |
| Rotavirus vaccination (yes vs. no) | 29 (49.15) | 116 (52.25) | 0.672 | 0.892 (0.482, 1.651) | |
| Contact with AGE patients within 1 week (no vs. yes) | 13 (20.97) | 34 (15.81) | 0.341 | 0.775 (0.361, 1.660) | |
| Breastfeeding duration (months) | 6.83 ± 7.60 | 6.87 ± 8.17 | 0.899 | 0.993 (0.957, 1.031) | |
| Public place visited in the recent week | |||||
| Nursery/Kindergarten/After school club/School | 16 (25.81) | 59 (26.58) | 0.903 | 0.764 (0.369, 1.583) | |
| Clinic/Hospital/Nursing home | 25 (40.32) | 132 (59.46) | 0.007∗ | 0.44 (0.234, 0.829)∗ | 0.53 (0.229, 1.230) |
| Contact with animals within 1 week | 23 (37.1) | 58 (26.01) | 0.087 | 1.636 (0.9, 2.974) | |
| Dogs | 19 (30.65) | 45 (20.18) | 0.081 | 1.76 (0.939, 3.3) | |
| Cats | 4 (6.45) | 9 (4.04) | 0.489 | 1.46 (0.387, 5.507) | |
| Family visit or travel abroad within 1 week | 0 (0) | 2 (0.9) | > 0.99 | - | |
| Hand wash before meals | 0.334 | ||||
| Never | 10 (16.13) | 25 (11.31) | 1 | ||
| Occasional | 43 (69.35) | 148 (66.97) | 0.507 (0.191, 1.346) | ||
| Always | 9 (14.52) | 48 (21.72) | 0.299 (0.089, 1.001) | ||
| Living environment | |||||
| Other children cared for by the mutual primary caregiver had diarrhea or not | 0.063 | ||||
| with diarrhea | 4 (7.14) | 31 (14.62) | 1 | ||
| without diarrhea | 18 (32.14) | 88 (41.51) | 2.192 (0.610, 7.884) | 0.785 (0.135, 4.59) | |
| the primary caregiver did not care for other children | 34 (60.71) | 93 (43.87) | 4.298 (1.215, 15.204)∗ | 2.881 (0.587, 14.13) | |
| Healthy primary caregiver | 61 (98.39) | 187 (85.39) | 0.005∗ | 11.993 (1.528, 94.16)∗ | 7.737 (0.841, 71.15) |
| Primary caregiver prepared food | 5 (11.11) | 52 (30.59) | 0.009∗ | 0.301 (0.109, 0.826)∗ | |
| Care place: restroom occupants | 0.596 | ||||
| < = 5 occupants | 46 (77.97) | 182 (84.65) | 1 | ||
| > 5 occupants | 13 (22.03) | 33 (15.35) | 1.469 (0.676, 3.192) | ||
| Care place: drinking water source | |||||
| Municipal water | 34 (54.84) | 167 (77.31) | < 0.001∗ | 0.292 (0.144, 0.594)∗ | 0.158 (0.049, 0.51)∗ |
| Bottled water | 5 (8.06) | 18 (8.33) | 0.946 | 0.947 (0.323, 2.776) | |
| Water refilling station | 11 (17.74) | 29 (13.43) | 0.393 | 1.432 (0.628, 3.262) | |
| Filtered and boiled water | 60 (100) | 190 (91.79) | 0.016∗ | - | |
| Food eaten in the recent week r | |||||
| Dining out | 41 (66.13) | 146 (65.47) | 0.923 | 1.004 (0.526, 1.914) | |
| Take-out food | 40 (64.52) | 130 (59.09) | 0.441 | 1.285 (0.647, 2.55) | |
| Milk | 10 (16.13) | 23 (10.41) | 0.215 | 1.378 (0.559, 3.393) | |
| Breast milk | 11 (17.74) | 36 (16.29) | 0.786 | 1.076 (0.466, 2.488) | |
| Milk powder | 49 (79.03) | 171 (77.38) | 0.782 | 1.280 (0.618, 2.652) | |
| Goat milk | 3 (4.84) | 4 (1.81) | 0.180 | 3 (0.671, 13.404) | |
| Egg | 46 (74.19) | 157 (71.04) | 0.626 | 1.121 (0.528, 2.381) | |
| Cooked egg | 45 (72.58) | 157 (71.04) | 0.813 | 1.04 (0.5, 2.164) | |
| Raw or half-boiled egg | 0 (0) | 0 (0) | - | - | |
| Iced products | 25 (40.32) | 76 (34.23) | 0.376 | 1.141 (0.617, 2.108) | |
| Cold soft drinks | 31 (50) | 114 (51.82) | 0.800 | 0.875 (0.468, 1.635) | |
| Leftovers | 15 (24.19) | 88 (39.82) | 0.024∗ | 0.355 (0.165, 0.761)∗ | 0.607 (0.214, 1.72) |
| Vegetable | 52 (85.25) | 176 (81.86) | 0.538 | 1.205 (0.49, 2.961) | |
| Cabbage | 37 (60.66) | 127 (59.07) | 0.824 | 0.932 (0.491, 1.77) | |
| Carrot | 35 (57.38) | 118 (54.88) | 0.730 | 1.036 (0.561, 1.914) | |
| Corn | 17 (27.87) | 34 (15.81) | 0.032∗ | 2.232 (0.988, 5.041) | 2.38 (0.671, 8.44) |
| Green broccoli | 15 (24.59) | 73 (33.95) | 0.166 | 0.553 (0.272, 1.125) | |
| Fruit | 54 (87.1) | 170 (77.27) | 0.091 | 2.076 (0.83, 5.19) | |
| Apple | 32 (51.61) | 126 (57.27) | 0.428 | 0.805 (0.451, 1.436) | |
| Guava | 15 (24.19) | 33 (15) | 0.089 | 1.758 (0.844, 3.665) | |
| Grape | 14 (22.58) | 37 (16.82) | 0.298 | 1.489 (0.725, 3.056) | |
| Banana | 27 (43.55) | 65 (29.55) | 0.038∗ | 1.760 (0.973, 3.184) | 1.131 (0.433, 2.95) |
| Seafood | 53 (85.48) | 157 (71.04) | 0.022∗ | 3.809 (1.383, 10.49)∗ | 1.968 (0.473, 8.19) |
| Chicken | 33 (53.23) | 113 (51.36) | 0.796 | 1.071 (0.582, 1.971) | |
| Duck | 2 (3.23) | 5 (2.24) | 0.648 | 1.535 (0.246, 9.572) | |
| Goose | 2 (3.23) | 3 (1.35) | 0.299 | 3.153 (0.426, 23.357) | |
| Pork | 43 (69.35) | 155 (69.82) | 0.944 | 0.833 (0.403, 1.72) | |
| Beef | 9 (14.52) | 37 (16.59) | 0.694 | 0.846 (0.363, 1.968) | |
Discussion
In this retrospective study, we aimed to understand the clinical manifestations of campylobacteriosis and distinguish it from diarrhea caused by other pathogens through a large-scale study. Among the 4766 AGE patients who are under five years old, 64 (1.3%) were Campylobacter PCR positive. The result was comparable to previous studies conducted in Taiwan. Lin CW et al. reported the isolation rate of Campylobacter spp. from stool specimens of diarrhea patients to be 2.5% in 1998 [21]. In children under five years old, Chen KT et al. reported an 0.7% isolation rate in 2005 [22], while Yu WJ et al. mentioned yield rates of at least 1.0% from 2007 to 2011 and at least 2.3% from 2012 to 2016 [23]. Forty of the 64 campylobacteriosis patients were male, accounting for 63% in accordance with previous reports of a slightly higher male dominance among infected people [6,19,24]. Compared with children infected with other pathogens, those with campylobacteriosis had a lower percentage of suffering from vomiting for more than two days in our study. However, 50% of case-patients experienced vomiting, which was slightly higher than figures ranging from 20% to 35.8% reported by studies conducted in Canada, England, and Egypt [[25], [26], [27]].
Among those campylobacteriosis patients, 59% had mucoid stool and 57% suffered from bloody stool, both significantly higher than those infected with other pathogens. The results were compatible with the consensus that mucoid and bloody stool are commonly seen in Campylobacter gastroenteritis [[28], [29], [30]]. On the other hand, the average AST level of our case-patients was 36 ± 13 (U/L), significantly lower than the non-Campylobacter AGE control group. We also found that campylobacteriosis children were less likely to have an AST over 50 (U/L). These results matched previous reports of a relatively normal liver function test in campylobacteriosis patients [12].
To demonstrate whether the differences in laboratory findings and clinical manifestations are of bacterial origin or are specific to Campylobacter origin, we also performed the analysis excluding all the virus infected cases (Supplement Table 1). When comparing 53 Campylobacter bacterial AGE cases with 137 non-Campylobacter bacterial AGE controls, mucous and bloody stool remained significantly more frequent in the case group, and prolonged vomiting was less frequently seen. AST level were lower but did not reach significance (p = 0.0599) likely due to the scale of the study. This demonstrated that the differences were specific to Campylobacter origin.
Lower educational attainments of parents were linked to higher risk of campylobacteriosis in the current study. Previous studies were divided in this finding. Diriba, K. et al. reported that an illiterate mother was a significant risk factor for campylobacteriosis [17]. However, Bhattarai, V. et al. reported that there was no association between parental education level and Campylobacter infection in Nepal. The same study also revealed that parental occupation was not linked to campylobacteriosis [31], which was consistent with the results in Canada [25]. While more studies are needed to clarify the impact of parental educational attainment, parental occupation, and household income on Campylobacter infection, it is conceivable that geographical and cultural differences contributed to this dissimilarity.
Person-to-person contact is a recognized route of transmission for Campylobacter gastroenteritis. Little, CL et al. reported that person-to-person transmission accounted for 3% of 143 campylobacteriosis cases in England and Wales [32]. Studies from New Zealand and Australia showed similar rates of 4% of 364 patients and 1% by expert elicitation [33,34]. In our study, 21% of the Campylobacter patients had contact with acute gastroenteritis patients within one week prior to the diagnosis. However, without genotype data, we cannot confirm infection caused by direct person-to-person contact, since they might share same food sources or environment with the contact. For the same reason, although we observed an increased odds for campylobacteriosis if other children taken care of by the same primary caregiver were experiencing diarrhea, the contribution of individual risk factors such as food, water source, or hygiene still awaits further studies.
As for water source, drinking untreated water has been recognized as an important risk factor for Campylobacter infection, while consumption of filtered water has been described as a protective factor [29,[35], [36], [37]]. In our study, water sources included municipal water, bottled water, and water refilling stations. We observed that consumption of municipal water was a protective factor for campylobacteriosis when compared with both non-AGE control and non-campylobacter AGE control, but there were no significant differences between those who consumed filtered and boiled the water and those who did not. This result may be associated with modernized municipal water supply systems in Taiwan, which leads to less contamination. Drinking bottled water has also been claimed as a risk factor, but we did not have similar finding [38].
Cindy R. Friedman et al. claimed having meals prepared at a restaurant was a significant risk factor in univariate analysis but not multivariate analysis [29]. Eating out within a week prior to infection did not increase the odds of having Campylobacter gastroenteritis in our study. We suspect this may be associated with the improving hygiene and food-processing of current restaurants. However, we identified take-out food consumption as a risk factor (OR 3.326), which may be due to prolonged time between food preparation and consumption. Soft drink consumption was found as a protective factor for campylobacteriosis in our study. According to Azeredo, D. R., addition of preservatives and sometimes CO2 as well as generally acidic nature of soft drinks may be major barriers for bacterial growth [39].
While Osbjer, K. et al. reported an association between campylobacteriosis and eating undercooked meat [40], our study did not show significantly increased odds of infection in those who consumed poultry or meat except for seafood. Education for storing and processing of seafood might be needed to further reduce Campylobacter infection in Taiwan.
There are some limitations to this study. First, since the case-patients were selected from ten major hospitals in Taiwan, those with milder symptoms who did not require hospitalization were not included. Second, although ten hospitals participated in the study, sample size were still relatively small. Therefore, sensitivity analysis was conducted, which confirmed the main finding (Supplement Table 1). Third, due to the fact that the questionnaire was not specifically designed for Campylobacter gastroenteritis but multiple pathogens instead, some factors specific to Campylobacter gastroenteritis might not be included in our questionnaire. Forth, our questionnaire contained numerous aspects and details, many of which tracing back to a week ago, such as food consumption or contact history for risk factor analysis. It is possible that some details were lost or not correctly filled in. Fifth, due to the difficulty of thorough analysis of stool sample in the 10 hospitals participating in this study, the sample were kept frozen and sent to Taiwan CDC for further analysis. This might decrease the yield rate of pathogens. Thus PCR were used as the main detection tool. Nevertheless, the use of culture-independent diagnostic tests (CIDT) such as PCR for surveillance and diagnosis has been discussed in previous studies and was used by CDC of the United States [19,20]. Lastly, due to the research methodology, we did not acquire genotype data, nor did we follow the cases through a period of time. This made us unable to gain more information, such as further investigation of the spreading routes in each case-patient.
To summarize, acute childhood campylobacteriosis had a lower rate of vomiting for over two days, a higher rate of bloody and mucoid stool, and a lower AST level compared to those infected with other pathogens. We also found that direct contact with AGE patients could increase the risk of campylobacteriosis. Higher parental educational attainment, consumption of municipal water, milk, and soft beverages were identified as a protective factor, while consuming takeout food and seafood were risk factors for campylobacteriosis.
Declaration of competing interest
None.
Acknowledgements
We thank the research staff and all the children and families who have participated in this study. This work was supported by grants (03D9-PHCDC01, 04D9-PHCDC01, 05D9-PHCDC01, 06D9-PHCDC02) from National Health Research Institutes, Taiwan, and grants (MOHW103-CDC-C-114-000802, MOHW104-CDC-C-114-113701, MOHW105-CDC-C-114-123302, MOHW106-CDC-C-114-133302) from Center of Disease Control, Taiwan. We gratefully acknowledge the Taiwan Pediatric ID Alliance (TPIDA) for the study paticipation. The individual authors and affiliations within the TPIDA are the following: Luan-Yin Chang, Chun-Yi Lu, Pei-Lan Shao, Ting-Yu Yen, Li-Min Huang (Department of Pediatrics, National Taiwan University Hospital, Taipei, Taiwan); Nan-Chang Chiu, Hsin Chi, Daniel Tsung-Ning Huang (Department of Pediatrics, Mackay Memorial Hospital, Taipei, Taiwan); Hsiao-Chuan Lin, Kao-Pin Hwang, Tsung-Hsueh Hsieh (Department of Pediatrics, China Medical University Hospital, Taichung, Taiwan); Ching-Chuan Liu, Ching-Fen Shen, Shih-Min Wang (Department of Pediatrics, National Cheng-Kung University Hospital, Tainan, Taiwan); Shun-Cheng Yang (Department of Pediatrics, Changhua Christian Hospital, Changhua, Taiwan); Yu-Chia Hsieh, Yhu-Chering Huang, Cheng-Hsun Chiu (Department of Pediatrics, Chang Gung Hospital, Taoyuan, Taiwan); Yu-Huai Ho (Department of Pediatrics, Tzu Chi University, Hualien, Taiwan); Jung–Jung Mu (Research and Diagnostic Center, Centers for Disease Control, R.O.C (Taiwan)); and Yi-Chuan Huang (Department of Pediatrics, Chang Gung Hospital, Kaohsiung, Taiwan).
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2023.03.003.
Patient diagnosed with ICD-9-CM code 001–005 (003.2 excluded), 008.0–008.5, 027.0, 008.6–008.8, 006–007 (006.3–006.6 excluded), 009.0–009.3, 558.9, 787.91 met the third inclusion criteria, but V30–V39 were excluded.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Lin FJ, Huang YC, Huang YC, Huang LM, Liu CC, Chi H, et al. Clinical and epidemiological features in hospitalized young children with acute gastroenteritis in Taiwan: a multicentered surveillance through 2014-2017. J Formos Med Assoc. 2022;121(2):519–528. doi: 10.1016/j.jfma.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev. 2015;28(3):687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi CY, Liao LN, Ho CM, Chou CH, Ho MW, Wang JH. Epidemiology, clinical features, and microbiology of patients with diarrhea in community clinics in Taiwan. J Microbiol Immunol Infect. 2018;51(4):527–534. doi: 10.1016/j.jmii.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Yang JR, Wu HS, Chiang CS, Mu JJ. Pediatric campylobacteriosis in northern Taiwan from 2003 to 2005. BMC Infect Dis. 2008;8:151. doi: 10.1186/1471-2334-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang SC, Chang LY, Hsueh PR, Lu CY, Lee PI, Shao PL, et al. Campylobacter enteritis in children in northern Taiwan--a 7-year experience. J Microbiol Immunol Infect. 2008;41(5):408–413. [PubMed] [Google Scholar]
- 7.Man SM. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol. 2011;8(12):669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 8.Allos BM. Clinical manifestations, diagnosis, and treatment of Campylobacter infection. In: UpToDate, post TW (Ed), UpToDate, Waltham, MA. [accessed on December 2, 2021].
- 9.Nelson JM, Smith KE, Vugia DJ, Rabatsky-Ehr T, Segler SD, Kassenborg HD, et al. Prolonged diarrhea due to ciprofloxacin-resistant campylobacter infection. J Infect Dis. 2004;190(6):1150–1157. doi: 10.1086/423282. [DOI] [PubMed] [Google Scholar]
- 10.Blaser MJ, Berkowitz ID, LaForce FM, Cravens J, Reller LB, Wang WL. Campylobacter enteritis: clinical and epidemiologic features. Ann Intern Med. 1979;91(2):179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- 11.DeWitt TG, Humphrey KF, Doern GV. White blood cell counts in patients with Campylobacter-induced diarrhea and in controls. J Infect Dis. 1985;152(2):427–428. doi: 10.1093/infdis/152.2.427. [DOI] [PubMed] [Google Scholar]
- 12.Allos BM. Campylobacter jejuni Infections: update on emerging issues and trends. Clin Infect Dis. 2001;32(8):1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 13.Rossler E, Signorini ML, Romero-Scharpen A, Soto LP, Berisvil A, Zimmermann JA, et al. Meta-analysis of the prevalence of thermotolerant Campylobacter in food-producing animals worldwide. Zoonoses Public Health. 2019;66(4):359–369. doi: 10.1111/zph.12558. [DOI] [PubMed] [Google Scholar]
- 14.Osimani A, Aquilanti L, Pasquini M, Clementi F. Prevalence and risk factors for thermotolerant species of Campylobacter in poultry meat at retail in Europe. Poultry Sci. 2017;96(9):3382–3391. doi: 10.3382/ps/pex143. [DOI] [PubMed] [Google Scholar]
- 15.Varrone L, Glass K, Stafford RJ, Kirk MD, Selvey L. A meta-analysis of case-control studies examining sporadic campylobacteriosis in Australia and New Zealand from 1990 to 2016. Aust N Z J Publ Health. June 2020;44(3):313–319. doi: 10.1111/1753-6405.12998. [DOI] [PubMed] [Google Scholar]
- 16.Domingues AR, Pires SM, Halasa T, Hald T. Source attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infections. Epidemiol Infect. 2012;140(6):970–981. doi: 10.1017/S0950268811002676. [DOI] [PubMed] [Google Scholar]
- 17.Diriba K, Awulachew E, Anja A. Prevalence and associated factor of Campylobacter species among less than 5-year-old children in Ethiopia: a systematic review and meta-analysis. Eur J Med Res. 2021;26(1):2. doi: 10.1186/s40001-020-00474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng CF, Chiu NC, Huang CY, Huang DT, Chang L, Kung YH, et al. The epidemiology of non-typhoidal Salmonella gastroenteritis and Campylobacter gastroenteritis in pediatric inpatients in northern Taiwan. J Microbiol Immunol Infect. 2019;52(3):449–455. doi: 10.1016/j.jmii.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Imdad A, Retzer F, Thomas LS, McMillian M, Garman K, Rebeiro PF, et al. Impact of culture-independent diagnostic testing on recovery of enteric bacterial infections. Clin Infect Dis. 2018;66(12):1892–1898. doi: 10.1093/cid/cix1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwamoto M, Huang JY, Cronquist AB, Medus C, Hurd S, Zansky S, et al. Bacterial enteric infections detected by culture-independent diagnostic tests--FoodNet, United States, 2012-2014. MMWR Morb Mortal Wkly Rep. 2015;64(9):252–257. [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CW, Yin PL, Cheng KS. Incidence and clinical manifestations of Campylobacter enteritis in central Taiwan. Zhonghua Yixue Zazhi. 1998;61(6):339–345. [PubMed] [Google Scholar]
- 22.Chen KT, Chen PY, Tang RB, Huang YF, Lee PI, Yang JY, et al. Sentinel hospital surveillance for rotavirus diarrhea in Taiwan, 2001-2003. J Infect Dis. 2005;192(Suppl 1):S44–S48. doi: 10.1086/431495. [DOI] [PubMed] [Google Scholar]
- 23.Yu WJ, Chen SY, Tsai CN, Chao HC, Kong MS, Chang YJ, et al. Long-term impact of suboptimal rotavirus vaccines on acute gastroenteritis in hospitalized children in Northern Taiwan. J Formos Med Assoc. 2018;117(8):720–726. doi: 10.1016/j.jfma.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Altekruse SF. Campylobacter, 3rd edition. Emerg Infect Dis. 2008;14(12):1977. [Google Scholar]
- 25.Karmali MA, Fleming PC. Campylobacter enteritis in children. J Pediatr. 1979;94(4):527–533. doi: 10.1016/s0022-3476(79)80004-9. [DOI] [PubMed] [Google Scholar]
- 26.Gillespie IA, O’Brien SJ, Frost JA, Tam C, Tompkins D, Neal KR, et al. Investigating vomiting and/or bloody diarrhoea in Campylobacter jejuni infection. J Med Microbiol. 2006;55(Pt 6):741–746. doi: 10.1099/jmm.0.46422-0. [DOI] [PubMed] [Google Scholar]
- 27.Sainato R, ElGendy A, Poly F, Kuroiwa J, Guerry P, Riddle MS, et al. Epidemiology of Campylobacter infections among children in Egypt. Am J Trop Med Hyg. 2018;98(2):581–585. doi: 10.4269/ajtmh.17-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Switaj TL, Winter KJ, Christensen SR. Diagnosis and management of foodborne illness. Am Fam Physician. 2015;92(5):358–365. [PubMed] [Google Scholar]
- 29.Friedman CR, Hoekstra RM, Samuel M, Marcus R, Bender J, Shiferaw B, et al. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin Infect Dis. 2004;38(Suppl 3):S285–S296. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- 30.Fischer GH, Paterek E. StatPearls [internet] StatPearls Publishing; Treasure Island (FL): 2021 Jan. Campylobacter.https://www.ncbi.nlm.nih.gov/books/NBK537033/ Available from: [Google Scholar]
- 31.Bhattarai V, Sharma S, Rijal KR, Banjara MR. Co-infection with Campylobacter and rotavirus in less than 5 year old children with acute gastroenteritis in Nepal during 2017-2018. BMC Pediatr. 2020;20(1):68. doi: 10.1186/s12887-020-1966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little CL, Gormley FJ, Rawal N, Richardson JF. A recipe for disaster: outbreaks of campylobacteriosis associated with poultry liver pâté in England and Wales. Epidemiol Infect. 2010;138(12):1691–1694. doi: 10.1017/S0950268810001974. [DOI] [PubMed] [Google Scholar]
- 33.Gilpin BJ, Walshe G, On SL, Smith D, Marshall JC, French NP. Application of molecular epidemiology to understanding campylobacteriosis in the Canterbury region of New Zealand [published correction appears in Epidemiol Infect. 2014;142(4):893. Walsh, G [corrected to Walshe, G]] Epidemiol Infect. 2013;141(6):1253–1266. doi: 10.1017/S0950268812001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vally H, Glass K, Ford L, Hall G, Kirk MD, Shadbolt C, et al. Proportion of illness acquired by foodborne transmission for nine enteric pathogens in Australia: an expert elicitation. Foodb Pathog Dis. 2014;11(9):727–733. doi: 10.1089/fpd.2014.1746. [DOI] [PubMed] [Google Scholar]
- 35.Hopkins RS, Olmsted R, Istre GR. Endemic Campylobacter jejuni infection in Colorado: identified risk factors. Am J Publ Health. 1984;74(3):249–250. doi: 10.2105/ajph.74.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adak GK, Cowden JM, Nicholas S, Evans HS. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of campylobacter infection. Epidemiol Infect. 1995;115(1):15–22. doi: 10.1017/s0950268800058076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obaidat MM. Seroprevalence and risk factors for Campylobacter jejuni seropositivity in Jordan. Inf Disp. 2019;51(2):140–146. doi: 10.1080/23744235.2018.1540883. [DOI] [PubMed] [Google Scholar]
- 38.Evans MR, Ribeiro CD, Salmon RL. Hazards of healthy living: bottled water and salad vegetables as risk factors for Campylobacter infection. Emerg Infect Dis. 2003;9(10):1219–1225. doi: 10.3201/eid0910.020823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azeredo DR, Alvarenga V, Sant’Ana AS, Srur AUS. An overview of microorganisms and factors contributing for the microbial stability of carbonated soft drinks. Food Res Int. 2016;82:136–144. [Google Scholar]
- 40.Osbjer K, Boqvist S, Sokerya S, Chheng K, San S, Davun H, et al. Risk factors associated with Campylobacter detected by PCR in humans and animals in rural Cambodia. Epidemiol Infect. 2016;144(14):2979–2988. doi: 10.1017/S095026881600114X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.