Abstract
A new member of the family of periplasmic protein thiol:disulfide oxidoreductases, CcmG (also called DsbE), was characterized with regard to its role in cytochrome c maturation in Escherichia coli. The CcmG protein was shown to be membrane bound, facing the periplasm with its C-terminal, hydrophilic domain. A chromosomal, nonpolar in-frame deletion in ccmG resulted in the complete absence of all c-type cytochromes. Replacement of either one or both of the two cysteine residues of the predicted active site in CcmG (WCPTC) led to low but detectable levels of Bradyrhizobium japonicum holocytochrome c550 expressed in E. coli. This defect, but not that of the ccmG null mutant, could be complemented by adding low-molecular-weight thiol compounds to growing cells, which is in agreement with a reducing function for CcmG.
Covalent ligation of heme to apocytochrome c is a common characteristic of all c-type cytochromes. The binding occurs via two thioether bonds formed between the vinyl groups of heme and the cysteine residues of the conserved motif CXYCH of apocytochrome c. Although maturation of c-type cytochromes takes place in the oxidizing environment of the periplasm, the cysteines of apocytochrome c are expected to be in the reduced dithiol form before heme is attached.
Studies devoted to cytochrome c biogenesis in Escherichia coli led to the identification of the ccm (cytochrome c maturation) genes (8, 27), which are located in the aeg-46.5 operon (19, 21) together with the nap genes encoding the periplasmic nitrate reductase (Fig. 1A). The ccm genes encode eight proteins (CcmA, CcmB, CcmC, CcmD, CcmE, CcmF, CcmG, and CcmH) with high sequence similarity to other, previously identified bacterial proteins involved in cytochrome c biogenesis (1–7, 11, 16–18, 20, 23, 24, 30, 31). Their relevance for cytochrome c biogenesis in E. coli was shown by construction and analysis of a ccm null mutant that failed to produce mature cytochrome c (8, 27). The Ccm proteins include the subunits of an ATP-binding cassette transporter and of a putative cytochrome c-heme lyase complex and a thioredoxin-like protein. The E. coli thioredoxin-like protein is encoded by ccmG and has also been designated DsbE (14, 21). An involvement in cytochrome c maturation has been shown experimentally for the homologous proteins HelX of Rhodobacter capsulatus (2), CycY of Rhizobium leguminosarum (30), CycY of Bradyrhizobium japonicum (5), and CcmG of Paracoccus denitrificans (17).
FIG. 1.
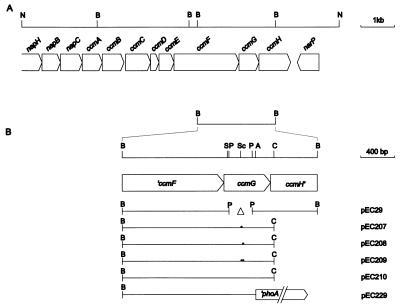
Physical map of the E. coli ccmABCDEFGH region. (A) Entire ccm gene cluster. (B) Plasmids used for deletion, point mutation, and phoA fusion analysis. They are derivatives of the ccmG-containing BamHI fragment that is shown enlarged. Plasmid pEC29 contains an internal deletion of a 272-bp PvuII fragment, which was replaced by an ApaI linker to restore the reading frame of the remaining ccmG coding region; plasmids pEC207, pEC208, pEC209, and pEC210 are the constructs for the active-site mutations (marked with dots) in ccmG produced by PCR; plasmid pEC229 contains a translational ccmG′-′phoA fusion; pEC207, pEC208, pEC209, pEC210, and pEC229 are pACYC184 derivatives carrying the ccmG gene under the control of the tetracycline promoter. Restriction sites: A, AscI; B, BamHI; C, ClaI; N, NcoI; P, PvuII; S, SacII; Sc, ScaI.
Characteristic features of the CcmG subfamily of thioredoxin-like proteins are not only the WCXYC motif representing the active site of the protein but also a hydrophobic N terminus and the well-conserved segment GVYGAPETF in the C-terminal part of the protein (residues 139 to 147 [5, 26]). Because of their thioredoxin-type active site and their involvement in cytochrome c maturation, CcmG homologs have been suggested to function as protein thiol:disulfide oxidoreductases in a cytochrome c-specific dithiol reduction pathway that reduces apocytochrome c for heme attachment.
In this work, we present direct biochemical evidence that ccmG codes for a periplasmic, membrane-bound thioredoxin. Having constructed a nonpolar ccmG deletion mutant, we show that CcmG is essential for cytochrome c maturation. We also report on the characterization of mutants containing replacements of the cysteines in the active-site motif WCXYC, which indicates a reducing function for the two cysteine residues in a pathway for maturation of c-type cytochromes.
Construction and characterization of a mutant carrying a nonpolar in-frame deletion in ccmG.
It was shown previously that a deletion removing the genes ccmA to ccmH from the E. coli chromosome resulted in the loss of mature c-type cytochromes (8, 27). Here, we investigated the role of ccmG in cytochrome c biogenesis. A chromosomal deletion of 91 codons (L24 to S114) was constructed by gene replacement mutagenesis of the wild-type strain E. coli MC1061 (9), resulting in the nonpolar in-frame ccmG deletion mutant EC29 (Fig. 1B). The mutant failed to synthesize both indigenous and foreign c-type cytochromes (Fig. 2B, lane 1 [29]). Even overexpression of the soluble B. japonicum cytochrome c550 from plasmid pRJ3268 (28) did not allow detection of the holocytochrome in the ccmG mutant. The phenotype was complemented when ccmG was provided on plasmid pEC210 (Fig. 1B and 2B, lane 5), indicating that the ccmG deletion was the sole cause of the observed mutant phenotype. This is in contrast to the phenotype of a dsbE mutant that was found to be able to produce low levels of c-type cytochromes (12). However, the precise physical location of its Tn10 insertion is not known and may have led to incomplete inactivation of the gene.
FIG. 2.
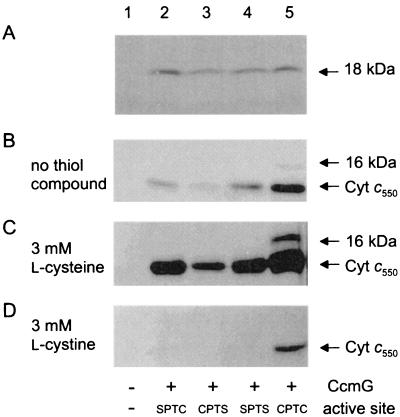
Phenotypic characterization of ccmG deletion and point mutants. E. coli cells were grown anaerobically in the presence of fumarate and nitrite. Membrane and periplasmic fractions (5) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: ΔccmG/pRJ3268 (lane 1), ΔccmG/pRJ3268/pEC207 (CcmGC80S) (lane 2), ΔccmG/pRJ3268/pEC208 (CcmGC83S) (lane 3), ΔccmG/pRJ3268/pEC209 (CcmGC80S/C83S) (lane 4), and ΔccmG/pRJ3268/pEC210 (wild-type CcmG) (lane 5). (A) Western blot analysis of membrane proteins (40 μg per lane) with polyclonal anti-CcmG immunoglobulins. (B to D) Heme stain of periplasmic proteins (30 μg per lane): (B) no addition of thiol compound to growing cells; (C) addition of 3 mM l-cysteine; (D) addition of 3 mM l-cystine. The position of B. japonicum cytochrome c550 is indicated in the right margin. The 16-kDa c-type cytochrome is most likely the NapB protein (8).
Subcellular localization of CcmG.
CcmG contains an N-terminal, hydrophobic sequence (residues 5 to 25) that might serve as a signal sequence. CcmG was detected in membranes of the wild type but not in those of the ΔccmG mutant by Western blot analysis with a purified, polyclonal antiserum against a CcmG-specific synthetic peptide (N103 to E118). Even if CcmG was expressed from the multicopy plasmid pEC210 under the control of the pACYC184-derived tetracycline promoter, CcmG could be detected only in the membrane (Fig. 2A, lane 5), not in the soluble fraction (data not shown). Analysis of the activity of a CcmG143-PhoA fusion protein encoded by pEC229 (Fig. 1B) revealed the periplasmic location of the C-terminal CcmG domain. The alkaline phosphatase activity of strain CC118(ΔphoA)/pEC229 was 56.31 ± 8.37 U as opposed to the control strain carrying ccmG without a phoA fusion (CC118/pEC210), for which 0.21 ± 0.09 U was obtained. These results show that CcmG is a periplasmic membrane-anchored protein and contradict the finding of Missiakas and Raina, who reported that DsbE (which is identical to CcmG) is a soluble, periplasmic protein (14).
Phenotypic characterization of ccmG active-site mutants.
The two cysteines at positions 80 and 83 of the putative CcmG active site were changed to serines, either individually (CcmGC80S encoded by pEC207 and CcmGC83S encoded by pEC208) or together (CcmGC80S/C83S encoded by pEC209). The plasmids expressing the wild-type CcmG (pEC210) and the three mutant proteins (pEC207, pEC208, and pEC209 [Fig. 1B]) were used to complement the in-frame deletion mutant EC29, which in addition carried pRJ3268 for expression of the B. japonicum cytochrome c550. Cells were grown anaerobically in the presence of fumarate and nitrite and were analyzed for the presence of holocytochrome c by heme staining. Although the signals produced by the mutant strains (Fig. 2B, lanes 2 to 4) were considerably weaker than those of cells complemented with the wild-type gene (Fig. 2B, lane 5), it was obvious that these mutants are capable of producing some holocytochrome c. By contrast, cells lacking the entire ccmG gene completely failed to produce holocytochrome c (Fig. 2B, lane 1). Western blot analysis showed that the point mutations did not affect the stability of the CcmG polypeptide (Fig. 2A).
The phenotypic effects of thiol compounds with respect to c-type cytochrome biogenesis in an E. coli mutant lacking the protein-disulfide reductase DsbD have been analyzed previously (25). Cytochrome c maturation was restored by supplementing the medium with l-cysteine or mercaptoethanesulfonic acid but not with other thiol compounds. Therefore, we tested the influence of various thiol or disulfide compounds on the formation of holocytochrome c. The effect of l-cysteine, for example, was investigated in the three active-site mutants and in the ΔccmG in-frame deletion mutant. Addition of 3 mM l-cysteine to the medium led to a proportional increase in maturation of cytochrome c in the active-site mutants. As was also found for untreated cells, l-cysteine-treated EC29/pRJ3268/pEC207 (CcmGC80S) and EC29/pRJ3268/pEC209 (CcmGC80S/C83S) produced higher amounts of holocytochrome c than did EC29/pRJ3268/pEC208 (CcmGC83S) (Fig. 2C, lanes 2 to 4). By contrast, l-cysteine had no effect on cytochrome c maturation in the ΔccmG in-frame deletion mutant lacking the entire CcmG (Fig. 2C, lane 1). We conclude that, regardless of the redox state of the active site, the presence of the CcmG polypeptide is necessary for cytochrome c biogenesis. Interestingly, even the wild type could be stimulated for cytochrome c production when l-cysteine was added (Fig. 2C, lane 5).
An opposite effect was observed when the cells were treated with the corresponding, oxidized disulfide compound l-cystine. Upon addition of 3 mM l-cystine, the heme-stained bands of the wild type, and also of the active-site mutants, were much weaker than those of cells that had been grown without addition of any redox-active compound (Fig. 2D, lane 5; almost invisible in lanes 2 to 4). Again, no cytochrome c formation was observed in cells lacking the ccmG gene.
In addition, we analyzed the influence of various other thiol and disulfide compounds on cytochrome c maturation in EC29/pRJ3268/pEC208 (expressing CcmGC83S [data not shown]). The addition of the reducing agent mercaptoethanesulfonic acid or reduced glutathione resulted in cytochrome c formation increasing to a similar extent as was observed for the addition of l-cysteine. In contrast, the presence of oxidized glutathione and l-cystine in cultures led to a decrease of mature cytochrome c. Moreover, we found that addition of the reducing agent d-cysteine or reduced dithiothreitol seemed to have an inhibitory rather than a stimulating effect, as these agents led to weakly heme-stainable bands.
Conclusions.
The mutational analysis of the active-site cysteines revealed that, although they are not required for the stability of the CcmG protein, each of them is important for attachment of heme to apocytochrome c. Remarkably, cytochrome c biogenesis was drastically reduced, but not abolished completely, in these active-site mutants. Moreover, cells expressing the CcmG protein with the second cysteine of the active site exchanged (CcmGC83S) produced lower levels of mature cytochrome c than the CcmGC80S and CcmGC80S/C83S mutants (Fig. 2). This phenomenon might be explained by the reaction mechanism that has been proposed for thioredoxins (10). The first active-site cysteine attacks the target protein to form a covalently linked mixed disulfide transition state, which is subsequently resolved by the attack of the second cysteine. This finally leads to a dithiol form of the target molecule and a disulfide form of the thioredoxin. In mutants expressing either CcmGC80S or CcmGC80S/C83S, the formation of a mixed disulfide is presumably hampered. Thus, the target of CcmG remains accessible for reduction by other, perhaps less efficient reducing agents, whereby low levels of cytochrome c can still be formed. By contrast, the CcmGC83S protein might still be able to attack its target protein with C80, but it lacks the second cysteine (C83) that is necessary to resolve the mixed disulfide. In this case, the mixed disulfide is stabilized, and less CcmG target is released. Hence, less-mature cytochrome c might be produced.
Our finding that cytochrome c maturation in ccmG active-site mutants can be restored by certain thiol compounds such as l-cysteine and glutathione, but not by their oxidized counterparts l-cystine and oxidized glutathione, indicates that CcmG has a reducing role in vivo. This is in agreement with the rather negative redox potential (−0.217 V) that was measured for the CcmG homolog CycY of B. japonicum (5). Moreover, a reducing role of the R. capsulatus HelX protein was proposed recently based on an in vitro assay in which purified HelX was capable of reducing an 11-amino-acid apocytochrome c peptide including the CXYCH motif as well as the soluble, periplasmic domain of the cytochrome c biogenesis protein Ccl2, which contains a CXYC motif (15).
Why are ccmG active-site mutants still able to produce holocytochrome c, whereas a nonpolar, in-frame deletion mutant of ccmG is completely deficient in cytochrome c maturation? We speculate that the CcmG protein has a dual function. On the one hand, it is a periplasmic disulfide reductase, reducing apocytochrome c, CcmH, or an as-yet-unidentified component of the cytochrome c maturation pathway. On the other hand, it appears to stabilize one or several proteins involved in cytochrome c formation. Lack of the reducing function, as it occurs in the active-site mutants, can be compensated for by the addition of certain thiol compounds, whereas the stabilizing effect requires the presence of the CcmG polypeptide itself. Suggesting two CcmG-specific functions might also explain why thiol compounds can restore only cytochrome c oxidase activity and not cytochrome c biosynthesis in ΔccmG mutants of P. denitrificans (17).
Having proposed a reducing function of CcmG for cytochrome c maturation, we can speculate that CcmG receives reduction equivalents from a donor that might be involved more generally in redox control of the periplasm, such as DsbD (13, 22). Future experiments will address the question of which are the proteins that interact directly with CcmG.
Acknowledgments
We are grateful to F. Fischer, P. Künzler, and R. Zufferey for their help with the construction of plasmids and characterization of the ΔccmG mutant and to A. Hungerbühler for excellent technical assistance.
This work was supported by grants from the Swiss National Foundation for Scientific Research and from the Federal Institute of Technology, Zurich, Switzerland.
REFERENCES
- 1.Beckman D L, Trawick D R, Kranz R G. Bacterial cytochrome c biogenesis. Genes Dev. 1992;6:268–283. doi: 10.1101/gad.6.2.268. [DOI] [PubMed] [Google Scholar]
- 2.Beckman D L, Kranz R G. Cytochromes c biogenesis in a photosynthetic bacterium requires a periplasmic thioredoxin-like protein. Proc Natl Acad Sci USA. 1993;90:2179–2183. doi: 10.1073/pnas.90.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y L, Knaff D B. Partial ccmF and complete ccmG sequences of Chromatium vinosum. GenBank accession no. L78437. 1996. [Google Scholar]
- 4.Delgado M-J, Yeoman K H, Wu G, Vargas C, Davies A E, Poole R K, Johnston A W B, Downie J A. Characterization of the cycHJKL genes involved in cytochrome c biogenesis and symbiotic nitrogen fixation in Rhizobium leguminosarum. J Bacteriol. 1995;177:4927–4934. doi: 10.1128/jb.177.17.4927-4934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabianek R A, Huber-Wunderlich M, Glockshuber R, Künzler P, Hennecke H, Thöny-Meyer L. Characterization of the Bradyrhizobium japonicum CycY protein, a membrane-anchored periplasmic thioredoxin that may play a role as a reductant in the biogenesis of c-type cytochromes. J Biol Chem. 1997;272:4467–4473. doi: 10.1074/jbc.272.7.4467. [DOI] [PubMed] [Google Scholar]
- 6.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 7.Gaballa A, Koedam N, Cornelis P. A cytochrome c biogenesis gene involved in pyoverdine production in Pseudomonas fluorescens ATCC 17400. Mol Microbiol. 1996;21:777–785. doi: 10.1046/j.1365-2958.1996.391399.x. [DOI] [PubMed] [Google Scholar]
- 8.Grove J, Tanapongpipat S, Thomas G, Griffiths L, Crooke H, Cole J. Escherichia coli K-12 genes essential for the synthesis of c-type cytochromes and a third nitrate reductase located in the periplasm. Mol Microbiol. 1996;19:467–481. doi: 10.1046/j.1365-2958.1996.383914.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallis G-B, Holmgren A. Differential reactivity of the functional sulfhydryl groups of cysteine-32 and cysteine-35 present in the reduced form of thioredoxin from Escherichia coli. J Biol Chem. 1980;255:10261–10265. [PubMed] [Google Scholar]
- 11.Kereszt A, Slaska-Kiss K, Putnoky P, Banfalvi Z, Kondorosi A. The cycHJKL genes of Rhizobium meliloti involved in cytochrome c biogenesis are required for “respiratory” nitrate reduction ex planta and for nitrogen fixation during symbiosis. Mol Gen Genet. 1995;247:39–47. doi: 10.1007/BF00425819. [DOI] [PubMed] [Google Scholar]
- 12.Metheringham R, Tyson K L, Crooke H, Missiakas D, Raina S, Cole J A. Effects of mutations in genes for proteins involved in disulphide bond formation in the periplasm on the activities of anaerobically induced electron transfer chains in Escherichia coli K12. Mol Gen Genet. 1996;253:95–102. doi: 10.1007/pl00013815. [DOI] [PubMed] [Google Scholar]
- 13.Missiakas D, Schwager F, Raina S. Identification and characterization of a new disulfide isomerase-like protein (DsbD) in Escherichia coli. EMBO J. 1995;14:3415–3424. doi: 10.1002/j.1460-2075.1995.tb07347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Missiakas D, Raina S. Protein folding in the bacterial periplasm. J Bacteriol. 1997;179:2465–2471. doi: 10.1128/jb.179.8.2465-2471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monika E M, Goldman B S, Beckman D L, Kranz R G. A thioredoxin pathway tethered to the membrane for periplasmic cytochromes c biogenesis; in vitro and in vivo studies. J Mol Biol. 1997;271:679–692. doi: 10.1006/jmbi.1997.1227. [DOI] [PubMed] [Google Scholar]
- 16.Page M D, Ferguson S J. Cloning and sequence analysis of cycH gene from Paracoccus denitrificans: the cycH gene product is required for assembly of all c-type cytochromes, including cytochrome c1. Mol Microbiol. 1995;15:307–318. doi: 10.1111/j.1365-2958.1995.tb02245.x. [DOI] [PubMed] [Google Scholar]
- 17.Page M D, Ferguson S J. Paracoccus denitrificans CcmG is a periplasmic protein-disulphide oxidoreductase required for c- and aa3-type cytochrome biogenesis; evidence for a reductase role in vivo. Mol Microbiol. 1997;24:977–990. doi: 10.1046/j.1365-2958.1997.4061775.x. [DOI] [PubMed] [Google Scholar]
- 18.Page M D, Pearce D A, Norris H A C, Ferguson S J. The Paracoccus denitrificans ccmA, B and C genes: cloning and sequencing, and analysis of the potential of their products to form a haem or apo-c-type cytochrome transporter. Microbiology. 1997;143:563–567. doi: 10.1099/00221287-143-2-563. [DOI] [PubMed] [Google Scholar]
- 19.Rabin R S, Stewart V. Dual response regulators (NarL and NarP) interact with dual sensors (NarX and NarQ) to control nitrate- and nitrite-regulated gene expression in Escherichia coli K-12. J Bacteriol. 1993;175:3259–3268. doi: 10.1128/jb.175.11.3259-3268.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramseier T M, Winteler H V, Hennecke H. Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J Biol Chem. 1991;266:7793–7803. [PubMed] [Google Scholar]
- 21.Richterich P, Lakey N, Gyran G, Jaehn L, Mintz L, Robinson K, Church G M. Centisome 49 region of E. coli K-12 BHB2600. GenBank accession no. U00008. 1993. [Google Scholar]
- 22.Rietsch A, Belin D, Martin N, Beckwith J. An in vivo pathway for disulfide bond isomerization in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:13048–13053. doi: 10.1073/pnas.93.23.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritz D, Bott M, Hennecke H. Formation of several bacterial c-type cytochromes requires a novel membrane-anchored protein that faces the periplasm. Mol Microbiol. 1993;9:729–740. doi: 10.1111/j.1365-2958.1993.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 24.Ritz D, Thöny-Meyer L, Hennecke H. The cycHJKL gene cluster plays an essential role in the biogenesis of c-type cytochromes in Bradyrhizobium japonicum. Mol Gen Genet. 1995;247:27–38. doi: 10.1007/BF00425818. [DOI] [PubMed] [Google Scholar]
- 25.Sambongi Y, Ferguson S J. Specific thiol compounds complement deficiency in c-type cytochrome biogenesis in Escherichia coli carrying a mutation in a membrane-bound disulphide isomerase protein. FEBS Lett. 1994;353:235–238. doi: 10.1016/0014-5793(94)01053-6. [DOI] [PubMed] [Google Scholar]
- 26.Thöny Meyer L. Biogenesis of respiratory cytochromes in bacteria. Microbiol Mol Biol Rev. 1997;61:337–376. doi: 10.1128/mmbr.61.3.337-376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thöny-Meyer L, Fischer F, Künzler P, Ritz D, Hennecke H. Escherichia coli genes required for cytochrome c maturation. J Bacteriol. 1995;177:4321–4326. doi: 10.1128/jb.177.15.4321-4326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thöny-Meyer L, Künzler P, Hennecke H. Requirements for maturation of Bradyrhizobium japonicum cytochrome c550 in Escherichia coli. Eur J Biochem. 1996;235:754–761. doi: 10.1111/j.1432-1033.1996.00754.x. [DOI] [PubMed] [Google Scholar]
- 29.Throne-Holst M, Thöny-Meyer L, Hederstedt L. Escherichia coli ccm in-frame deletion mutants can produce periplasmic cytochrome b but not cytochrome c. FEBS Lett. 1997;410:351–355. doi: 10.1016/s0014-5793(97)00656-x. [DOI] [PubMed] [Google Scholar]
- 30.Vargas C, Wu G, Davies A E, Downie J A. Identification of a gene encoding a thioredoxin-like product necessary for cytochrome c biosynthesis and symbiotic nitrogen fixation in Rhizobium leguminosarum. J Bacteriol. 1994;176:4117–4123. doi: 10.1128/jb.176.13.4117-4123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C-H, Azad H R, Cooksey D A. A chromosomal locus required for copper resistance, competitive fitness, and cytochrome c biogenesis in Pseudomonas fluorescens. Proc Natl Acad Sci USA. 1996;93:7315–7320. doi: 10.1073/pnas.93.14.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]