Abstract
Zebrafish larvae are a model organism increasingly used in the study of the effect of neuroactive chemicals on vertebrate sleep/wake cycles. Sleep disturbances have a negative impact on mood, cognition and overall health. Here we present a protocol to assess over 24 h sleep/wake cycles in zebrafish larvae subjected to 12 h light/dark periods in 48-well plates, using video-tracking technologies. The protocol can be used to determine if the exposure to environmental pollutants or drugs can lead to sleep disturbances. The results on the effect of the tire rubber-derived 6PPD-quinone on zebrafish sleep/wake cycles presented here demonstrate the suitability of using this protocol in fish neurotoxicity studies. This protocol provides a new relevant tool to be used in the pharmacology and toxicology fields.
Keywords: Sleep disturbance, Sleep/wake cycles, Zebrafish larvae, Video-tracking technologies
Method name: Analysis of sleep/wake cycles in zebrafish larvae
Graphical abstract
Specifications table
| Subject area: | Neuroscience |
| More specific subject area: | Sleep disturbances |
| Name of your protocol: | Analysis of sleep/wake cycles in zebrafish larvae |
| Reagents/tools: | 6PPD-quinone analytical standard (purity>97%, TRC Canada) |
| Daniovision observation chamber (Noldus, Wageningen, The Netherlands) | |
| Temperature Control Unit (Noldus, Wageningen, The Netherlands) | |
| EthoVision XT 14 software (Noldus, Wageningen, The Netherlands) | |
| Experimental design: | 7 days post-fertilization zebrafish larvae, control and exposed to the neuroactive compounds, are videorecorded during a 24 h (6 h light:12 h dark:6 h light) period in 48-well microplates (1 larva/well), and then, bouts of mobility and immobility are determined. |
| Trial registration: | na |
| Ethics: | All procedures were approved by the Institutional Animal Care and Use Committees (IACUC) at the CID-CSIC and conducted under a license from the local government (agreement number 11336). |
| Value of the Protocol: |
|
Description of protocol
Sleep disturbances increase the risk of cardiovascular disease and neurobehavioral and cognitive impairment in humans, and air pollution has been identified as a potential factor leading to this adverse health outcome [1]. Zebrafish (Danio rerio) is a vertebrate model increasingly used in different areas of biomedical research, including neurobiology and neurotoxicology. Recently, this diurnal species has been also proposed as a powerful model in chronobiology [2,3], with numerous studies analyzing circadian rhythms, the sleep/wake cycles and hypocretin-melatonin interactions in zebrafish larvae [4], [5], [6]. In larval zebrafish, sleep has been defined as a period of immobility of at least 1 min, as larvae exhibiting immobility bouts with this duration also exhibit increased arousal threshold [4]. The endoints analyzed in each larva in sleep/wake cycles studies commonly include distance moved per hour or period, time (min) sleep per hour, number of sleep bouts, length of the sleep bouts, and the sleep latency at night (time between lights off and the first sleep bout [4].
Zebrafish model is used for determining toxicity associated to air pollution [7], [8], [9], the toxicodynamic mechanisms of the pollutants found in the air used to be conserved through evolution. Althought zebrafish model has been intensively used for determining developmental toxicity associated to air pollutants [7], [8], [9], information on the effects of these pollutants on the sleep/wake cycles in zebrafish is, however, still scarce, probably because of the difficulty for the environmental toxicology labs to find medium- and high-throughput protocols suitable for assessing this endpoint.
The present protocol [10] was developed to address a specific biological problem, the potential neurotoxicity of an environmental pollutant after 24 h of exposure. Although zebrafish embryos are commonly used in both developmental neurotoxicity and neurotoxicity research, in our opinion embryos do not have a fully mature nervous system and, therefore, this model should only be used for developmental neurotoxicity studies. At 7-8 dpf, however, neuronal proliferation is limited to a few specific regions, with the majority of brain areas composed of postmitotic neurons with well elaborated neuronal arbors [11].). Therefore, we have proposed to use 7 and 8 dpf zebrafish larvae for the development of the assay because, while they are still suitable for high-throughput screening of chemical libraries, the potential confounding factor of neurodevelopmental processes is strongly reduced at this developmental stage. Finally, there is a lot of basal information on the sleep/wake cycles in zebrafish larvae. However, the age of the larvae, photoperiod, type of microplates (96 wells instead of 48 wells, for instance), video-tracking platforms or video-tracking period (1, 2 or 3 days) proposed here can be modified accordingly to the needs of the different research groups. Therefore, the most relevant information provided by this protocol regardless of the differences in husbandry, experimental setups or behavioral testing, is probably the data analysis section.
Husbandry and experimental setup
Note: All procedures described in this protocol should be approved by the corresponding Institutional Animal Care and Use Committee (IACUC).
Note: Larvae are not fed during the experimental period.
-
1.
Rearing zebrafish embryos in fish water [reverse-osmosis purified water containing 90 mg/L Instant Ocean (Aquarium Systems, Sarrebourg, France) and 0.58 mM CaSO4·2H2O] at 28.5°C on a 12 L:12 D photoperiod, with lights on at 8 am and off at 8 pm.
Note: The proposed photoperiod is not mandatory and can be modified accordingly to the requirements of the experimental design.
-
2.
At 7 days post-fertilization (8:00 am), transfer the larvae to a 48-well microplate (1 larva per well).
-
3.
Transfer the microplate with the larvae to a zebrafish observation chamber, at 28 °C and white-light conditions (1600 lx), for acclimatation.
-
4.
Replace the fish water by the different experimental solutions immediately before starting to video-record the larvae, at 2 pm (6 h after light on).
Note: Only one microplate can be tested by each video-tracking system in 24 h, and therefore, each microplate should contain all the experimental conditions. It is possible to increase the throughput of the method by using 96-well instead of 48-well microplates, or by increasing the number of video-tracking systems working in parallel.
Behavioral testing
Note: the instructions below are set following Ethovision software display, so other video-tracking software may present differences.
-
1.
Launch the multi-well tracking software.
-
2.
Create a new experiment.
-
3.
In “Experiment settings”, configure the parameters. Select the video source as Live tracking, define 48 as the number of arenas and one subject per arena, also select detection by center-point. In trial control hardware, select the Use of Trial Control hardware.
-
4.
Name the experiment as a template. The template will be created as an experiment folder with several subdivisions containing all the setup information.
-
5.
Under “Arena settings” click on the center of the screen and select “Grab Background Image”. Calibrate the image generating a calibrate rule. Use the width of the plate as a scale (12.5 cm). Draw the area of one well and click on “Copy Arena” to make the other arenas from the remaining 47 wells and arrange them to match the wells from the plate. Change the name of the arenas accordingly. In this experiment there is no need to draw zones, as the measurement of the larvae movement is analyzed in the whole arena.
Note: If a template is available, make sure that the areas delimiting each well are lined up with the current plate. If it does not line up, the arenas can be adjusted by clicking on the option Arrange Arenas. This allows to move or rotate the grid, to scale the arenas, or modify the spacing between them.
-
6.Under “Trial Control Settings” set the behavior battery (Fig 1):
-
(a)The first command is to start the trial:
-
-Rule Begin; Start-stop trial.
-
-
-
(b)After that, the track is set to start:
-
-Action; Start track.
-
-
-
(c)Immediately after the device is commanded to turn the light on (without fading):
-
-Action; Hardware act; Device: Device A; Command: Light on; Fade duration: 60.000 s.Note: the intensity level can differ between hardware, thus it should be measured and set at 1600 lux.
-
-
-
(d)After 6 h the light should be turned off:
-
-Condition; Time; after a time of 6.0 h.
-
-Action; Hardware act; Device: Device A; Command: Light off; Fade duration: 60.000 s.
-
-
-
(e)After 12 h the light should be turned on:
-
-Condition; Time; after a time of 12.0 h.
-
-Action; Hardware act; Device: Device A; Command: Light on; Fade duration: 60.000 s.Note: the intensity level can differ between hardware, thus it should be measured and set at 1600 lux.
-
-
-
(f)After 6 h the light should be turned off in order to finish the experiment.
-
-Condition; Time; after a time of 6.0 h.
-
-Action; Hardware act; Device: Device A; Command: Light off; Fade duration: 0 s.
-
-
-
(g)To end the experiment:
-
-Action; Stop track.
-
-Rule End; Start-stop trial.
-
-
-
(a)
-
7.
Under “Detection settings” check detection quality and that all the larvae are being detected by the software. To adjust detection, click on Auto Detect.
-
8.
Under “Trial list” Add one trial.
-
9.
Under “Data Profiles” create “Results” dialog window. Create one Results window for data output by each minute. Link the Results window to the “Start” window with arrows.
-
10.Under “Analysis Profiles” select the parameters to analyze:
-
(a)The distance moved to the center-point (default).
-
(b)Mobility state: in the 1st display (“Mobility state”), select 1000 samples as averaging interval for Outlier filter; in Threshold, select Highly mobile above 100%, Mobile between 3% and 100%, and Immobile below 3%; calculate statistics only for mobile and immobile. In the second display (“Trial Statistics”), select “Cumulative Duration”.Note: In this case the Highly mobile state was included in the Mobile state. If you are interested in calculating the highly mobile state separately, it can be adjusted by changing the selection to: Highly mobile above 70%, and Mobile between 3 and 70%.
-
(a)
-
11.
Save the template and close it without acquiring any data.
-
12.
To use the template, make a copy or start a new experiment selecting the option New from template > Applied a custom template > From video file (choose template. EthXV file). Name the new experiment and select its location.
-
13.
Before starting the experiment, go to “Detection Settings” or “Acquisition” to check that the larvae are well detected.
-
14.
Under “Acquisition”, start the experiment.
-
15.
When acquisition is finished, under “Statistics”, click on “Calculate” and then “Export Data”. Data exportation is directly located in the experiment folder.
-
16.
Select the folder “Export Files” of the experiment to save the excel report.
-
17.
Note: If you have several equipment and software licenses, the same template copied to each computer can be used to conduct the experiment. However, it is important to readjust the template to each camera.
Fig. 1.
Behavior battery.
Data treatment
For each plate
-
1.
For the raw data obtained, create an excel template and assign it the name of the tested plate.
-
2.
When the data of that plate are copied to an excel sheet, create a dynamic table for each result (the sum of cm and immobile cumulative duration) representing the different wells in columns and the minutes in each row.
-
3.
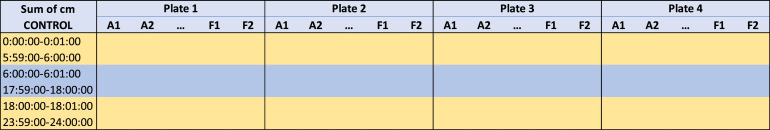
Check that the minutes are in order. If the dynamic table does not order the hours chronologically, copy the tables and paste them below in value format and put the hours (rows) in order (Fig. 2).
Note: if preferred, once ordered, change the color of the cells to distinguish when each of the different periods start. For example: the first 6 h are the same color (yellow) as the last six hours (daytime), and the 12 h in between (nighttime) are a different color (blue).
-
4.After ordering the data by time, make the same process as before:
-
a.Copy again the tables already ordered by time and paste them below.
-
b.Order the wells by treatment (Fig. 3).Note: it is recommended to separate the columns of different treatments with a line for further analysis.
-
a.
-
5.
Repeat this process for the excels with the data from the other plates tested.
Fig. 2.
Dynamic table obtained from raw data. The results need to be ordered chronologically.
Fig. 3.
Table obtained from reordering the columns by treatment; the rows are color-coded by period.
To unify results
-
1.
Create a new excel file (Name it as Review).
-
2.
Create a new sheet for each different treatment and paste the results of the different plates (Sum of cm and cumulative duration, next to each other) (Fig. 4).
-
3.
For the analysis of Sum of cm, calculate the sum of cm moved by the larvae in each well for daytime (period 1 and 3), and nighttime (period 2).
Note: Additionally, these results can also be calculated in % regarding the total distance moved throughout the 24 h.
-
4.To calculate the minutes of sleep per hour and per period, make a new table in which every row is one whole hour of the experiment (Fig. 5).
-
a.To obtain the minutes of sleep for each hour, you have to count from the immobile cumulative duration table the number of cells that have 60 s of immobility. To do this you can use the excel formula “COUNTIF” and select the range of data corresponding to the according hour.
-
a.
Fig. 4.
Table with the results of Sum of cm for the CONTROL group. The results from the different plates are unified in this table. Next to it, another table should be placed with the results of immobile cumulative duration following the same display.
Fig. 5.
Table with the results of immobile cumulative duration for the CONTROL larvae by each hour and period.
For example:
For the 1st hour the formula would be: =COUNTIF(B6:B65;60).
For the 2nd hour the formula would be: =COUNTIF(B66:B125;60).
Note: B6 references the 1st cell of the table with data.
-
b.Calculate the total minutes of sleep for the 24 h, and for each period.
-
b.
-
5.To calculate the number of bouts per hour and per period the table with the data of the immobility cumulative duration needs to be modified. To do this, copy the table and paste it below or next to it to introduce the modifications.
-
a.Delete the data of the copy (Fig. 6).
-
a.
Fig. 6.
Table with the results of immobile cumulative duration for the CONTROL group. Next to it there is the copy without data.
Note: the cells with 60s of immobility have been highlighted in green for better visibility. For that create a custom conditional formatting rule.
-
b.
In the first cell of the copy where the results should start, use this formula:
=IF(B6<>60;"activity";IF(B5=60;"-";"IMM BOUT"))
Note: B6 references the 1st cell of the original table.
This formula is used to substitute the minutes with some level of activity by the text “activity”, the first minute of the immobility bout by the text “IMM BOUT”, and the following minutes with 60s of immobility are substituted by a dash (Fig. 7). This substitution allows to count the number of bouts using the same strategy as in the step 4a.
-
c.
Create a table as in step 4 (Fig. 5) and use the formula “COUNTIF”.
Fig. 7.
Copy of the original table showing the results of immobile cumulative duration for the CONTROL group with the corresponding substitutions specified in the formula used.
For the 1st hour the formula would be: =COUNTIF(B6:B65;“IMM BOUT”).
For the 2nd hour the formula would be: =COUNTIF(B66:B125;“IMM BOUT”).
Note: In this formula, B6 references the 1st cell of the table in which the formula of step 5b was used (Fig. 7).
-
6.
In order to calculate the number of bouts of specific duration for each period, several steps need to be conducted. To obtain this result it is necessary to know when the immobility bout starts and ends so that they can be subtracted to obtain the length of the bout. Afterwards, it will be necessary to count how many bouts of the same length are present in each period, and to classify them in the desired intervals (e.g. <5 min; 5–10 min; 11–30 min; >30 min….).
Note: the starting table used to calculate this result is the table created in step 5b (Fig. 7).
-
a.
Make a copy and place it next to the original table (Fig. 8).
-
b.
Delete the data of the copy. Now this table will be used to indicate the start of an immobility bout. For that, use this formula in the first cell and drag it to the bottom and to the other columns of the table:
=IF(B6="IMM BOUT";ROW(B6);0)
|Note: B6 references the 1st cell of the original table with data.
Fig. 8.
Original table, which shows the results of immobile cumulative duration with the corresponding substitutions (activity, IMM bout, -), and the copy next to it, used for calculating the start of each bout.
This formula will fill the cells with the number of the row only if in the starting table (original) the corresponding cell has the text “IMM BOUT”. The rest of cells will present a 0 (Fig. 8).
-
a.
Make another copy of the table and place it next to the Bout start table.
-
b.
Delete the data of the last copy. Use the following formula to obtain a similar table to the Bout start table but indicating the end of the immobility bout (Fig. 9):|
=IF(AND(B6=“IMM BOUT”;B7=“activity”);0;IF(AND(B6=“IMM BOUT”;B7=““);0;IF(AND(B6=”-”;B7=“activity”);ROW(B7);IF(AND(B6=“-”;B7=““);ROW(B7);”-”))))
Note: Formula explanation:
Fig. 9.
Original table, which shows the results of immobile cumulative duration with the corresponding substitutions (activity, IMM bout, -), and the copy used for calculating the end of each bout.
The formula needs to indicate the end of the immobility bout, which in the original table corresponds to a dash or to the text “IMM BOUT” when the length is only 1 minute. To do so it is necessary to define all the possibilities that could be encountered and the desired output for each of them.
There are three different outputs:
-
▪
Dash (-): indicates that it does not correspond to the end of a bout.
-
▪
Number of row: indicates the end of an immobility bout longer than 1 minute.
-
▪
0: indicates the end of a one-minute-long immobility bout.
Note: it is necessary to differentiate between these two types of bouts (length=1 min; length>1 min) for following steps.
Here is the formula represented with different colors to better understand each part:
=IF(AND(B6="IMM BOUT";B7="activity");0;IF(AND(B6="IMM BOUT";B7="");0;IF(AND(B6="-";B7="activity");ROW(B7);IF(AND(B6="-";B7="");ROW(B7);"-"))))
-
•
IF(AND(B6="IMM BOUT";B7="activity");0;…) → if the corresponding cell of the original table to the current cell has the text "IMM BOUT" and the cell below presents the text “activity”, displaysa 0 (output for END of immobility bout of 1 min).
-
•
IF(AND(B6="IMM BOUT";B7="");0;…) → if the corresponding cell of the original table to the current cell has the text "IMM BOUT" and the cell below is blank (end of the table), displaysa 0 (output for END of immobility bout of 1 min).
-
•
IF(AND(B6="-";B7="activity");ROW(B7);…) → if the corresponding cell of the original table to the current cell presents a dash and the cell below has the text “activity”, displaysthe number of the row (output for END of immobility bout longer than 1 min).
-
•
IF(AND(B6="-";B7="");ROW(B7);"-") → if the corresponding cell of the original table to the current cell presents a dash and the cell below is blank (end of the table), show the number of the row (output for END of immobility bout longer than 1 min). If the last condition does not happen, displaysa dash (output for not an END of immobility bout)
Note: The output of the row corresponds to the cell below for the following steps (to subtract the start row form the end row and have the correct number of minutes).
-
e.
At this point, you have the three tables next to each other. The next step is to subtract the start row from the end row of the immobility bout. However, if the tables are compared, the rows are not aligned to do the subtraction. For that, copy each table and paste it below.
-
f.
Delete the data of both copies. Now to align the start and end rows use the function “FILTER” (Fig. 10). As the data corresponds to 3 different periods, the formula needs to be used 3 times in each column, indicating the range for each different period.
Note: since the number of bouts can be different in each experiment, it is recommended to leave a good number of blank rows between each period, just in case they are needed.
-
g.
After filtering each period (and after the blank rows) add a row (SUM) with the formula “COUNT” including all the cells of the period in the range. Afterwards this row will be used for verification.
Fig. 10.
Bout start and Bout end tables showing the number of row in which each immobility bout starts and ends. The tables beneath them show the same results but filtering the cells that are relevant, aligning the results to be able to do the subtraction.
Note: In the Filtered Bout end at the start of the nighttime period it can be noticed that, in all the columns except for F1 and A2 (from the 2nd plate), there is an additional end of bout compared to the number of starts. This additional bout end (highlighted in red) corresponds to the end of a bout that started in the daytime period. To correct this, the range of the “FILTER” function of the previous period must be increased until the last bout has ended, even if it ends in the following period. It has to be taken into account that this may also happen in the transition from nighttime period to the last daytime period. So probably, the range of the nighttime period should be increased at the bottom too.
-
h.
After filtering all the columns for each period and making the necessary corrections, create a new table following the same method as before (copy, paste and delete data). Use the following formula to obtain the length of each immobility bout (Fig. 11):
Fig. 11.
On top, there are two tables showing the filtered results from the start and end of each immobility bout. The table showing the end of each bout has already been corrected adjusting the range of the “FILTER” formula to include the end of the bouts that start in the previous period. The table beneath shows the product of the subtraction of the tables above (following the formula explained in step 6h).
=IF(AB6="";"";IF(AB6=0;1;AB6-B6))
Note: AB6 references 1st cell with data from the Filtered Bout End. B6 references 1st cell with data from the Filtered Bout Start.
This formula subtracts the row-number of the START of an immobility bout to the row-number of the END of the same immobility bout. However, if the cell showing the END of a bout is 0, meaning that the length of the bout is of only 1 min (as defined in the step 6d), the formula will show 1 as a result instead of doing the subtraction. Additionally, if the cell is blank, it will not show anything. To make sure that everything has been done correctly, the cells of the rows added at the end of each period (SUM) should all be equal to 0 in the table product of the subtraction.
-
i.
The next step is to know the frequency of each bout length. For that, make a new table in which the rows indicate the length of the bout (Fig. 12). And use the following formula to calculate the frequency of each specific duration:
=COUNT.IF(B$6:B$99;$AA6)
-
▪
B6 references the first cell with data from the Subtraction result Table.
-
▪
B99 references the first cell of the last row for the 1st period from the Subtraction result Table.
-
▪
AA6 references the first cell indicating the length of the bout from the Bout length frequency (new table created) (Fig. 12).
Fig. 12.
Table indicating the frequency of the bouts of a specific length for each larva in every period.
Note: This formula needs to be adjusted for each period. The formula counts the number of cells with the duration specified in the row of the new table (cells different than 0 are colored for better visibility). The number of rows will be different for each period depending on the maximum bout length. The number of rows necessary for each period can be known by using the “MAX” function for every column of each period (this can be calculated below the Subtraction result table).
-
j.Next to the last column of the table do the sum for all the columns of each row. After these steps, it is possible to calculate the frequency of bouts for a specific interval of time (e.g. <5 min; 5–10 min; 10–30 min; >30 min) by summing the frequency of the minutes included in the interval for each period.
-
j.
-
7.
To calculate the latency for the transition between periods make a new table (Fig. 13, right) and use the following formula:
=XMATCH("IMM BOUT";B$366:B$400;0;1)-1 → use this to calculate the latency for the transition between Day 1 period and Nighttime period. This corresponds to how much do they take to begin an immobility bout.
Fig. 13.
The table on the left there shows the results of immobile cumulative duration with the corresponding substitutions (activity, IMM bout, -). Next to it there is a table at the level of the row where the transition between periods happens. These table is used to calculate the latency between daytime and nighttime periods.
Note: the table used to calculate this result is the one generated in step 5b (Fig. 7).
Note: the cell B366 references the first cell of the first column in the nighttime period. The formula does not need to include all the cells of the column for the whole period, therefore B400 was chosen as an example for the end of the range.
-
8.
Return to step 2, where a new sheet of excel was created for each treatment, and continue from this point to obtain the same results for the other treatments.
Statistical analysis
After obtaining the results for each treatment, analyze the normality (Shapiro–Wilk test) and homocedasticity (Levene test) of the data for each experimental group. If data fulfil these two criteria, use one-way ANOVA followed by Dunnett's multiple comparison test to check for differences between the different groups with the control. If the data do not fit any of these two criteria, use Kruskal-Wallis test folowed by a pairwise comparison using the Bonferroni correction. Afterwards, plot the results with a software for graphics.
Protocol validation
First of all, the main parameters of the sleep/wake cycle in control 7 dpf zebrafish larvae, using our experimental conditions (12:12 L:D, wild-type larvae growth at 28 °C) are presented in Table 1, Table 2, Table 3.
Table 1.
Distance moved (cm) and minutes of sleep per period (day/night) in 7dpf control zebrafish.
| Control | DAY | NIGHT | |
|---|---|---|---|
| Distance (cm) | Mean | 5021.28 | 1104.76 |
| Deviation | 1143.36 | 318.47 | |
| Error | 208.75 | 58.14 | |
| Minutes of Sleep | Mean | 274.93 | 679.23 |
| Deviation | 91.41 | 17.97 | |
| Error | 16.69 | 3.28 | |
Table 2.
Bout length frequency per period (day/night) in 7dpf control zebrafish.
| Control | DAY | NIGHT | ||
|---|---|---|---|---|
| Bout length frequency | <=5 min | Mean | 57.78 | 4.86 |
| Deviation | 24.63 | 4.97 | ||
| Error | 4.11 | 0.83 | ||
| 6–10 min | Mean | 9.11 | 2.50 | |
| Deviation | 5.53 | 2.81 | ||
| Error | 0.92 | 0.47 | ||
| 11–30 min | Mean | 4.00 | 6.17 | |
| Deviation | 4.45 | 4.79 | ||
| Error | 0.74 | 0.80 | ||
| 31–60 min | Mean | 0.39 | 3.31 | |
| Deviation | 1.08 | 2.28 | ||
| Error | 0.18 | 0.38 | ||
| >60 min | Mean | 0.08 | 3.11 | |
| Deviation | 0.28 | 1.70 | ||
| Error | 0.05 | 0.28 | ||
Table 3.
Latency during the transition between day and night periods in 7dpf control zebrafish.
| Control | day to night | |
|---|---|---|
| Latency | Mean | 6.94 |
| Deviation | 3.86 | |
| Error | 0.64 | |
Moreover, the protocol was validated by analyzing how the 24 h exposure to environmental levels of 6PPD-quinone affected the sleep/wake cycle in zebrafish larvae [2]. Zebrafish larvae (7 dpf) were exposed for 24 h to three different concentrations of 6PPD-quinone (20, 200 and 2000 ng/L) prepared in ethanol to a final solvent concentration of 0.002% for all conditions, including the control group. All procedures were approved by the CID-CSIC Institutional Animal Care and Use Committees at and conducted in accordance with the institutional guidelines under the local government license number 11336.
After conducting the behavioral test and processing the data, the results for Sum of cm were presented as a linear graph for each condition over the 24 h duration of the experiment (Fig. 14 A). Additionally, results were also presented as a scatter plot with the distance moved in 12 h for each period and condition (Fig. 14 B). The results for minutes of sleep by hour were presented as a linear graph for each condition for the 24 h of the experiment (Fig. 14 C). And the graph for minutes of sleep by period was plotted as a scatter plot (Fig. 14 D). The results for the number of sleep bouts per period were presented as a scatter plot for each condition (Fig. 14 E). Likewise, the results from sleep latency were represented by a scatter plot (Fig. 14 F).
Fig. 14.
Effects of 24 h of exposure to environmental concentrations of 6PPD-quinone on the wake/sleep cycle of zebrafish larvae. (A) Locomotor activity of zebrafish larvae, both control and those exposed to the three concentrations of 6PPD-quinone, entrained and tested under 12:12 h light–dark conditions. The period in which activity was recorded, from 7 to 8 dpf, corresponds to the 24 h exposure period. The bars at the bottom of the graph on the left indicate the light/dark/light periods (white bar on the left (D1): 2 pm to 8 pm / black bar in the middle (N): 8pm to 8 am / white bar on the right (D2): 8 am to 2 pm). The results are presented as the mean ± SE. (B) Total distance moved during the daytime (D1+D2) and nighttime (N) periods. Data are shown as scatter plots with the median (n=34–35; one-way ANOVA with Dunnett's multiple comparison test). (C) Time spent by the larvae in the sleep state per hour over 24 h. (D) Time spent by the larvae in the sleep state during D1 (6 h), N (12h), and D2 (6 h). Data are shown as scatter plots with the median (D1: n=31, Student's t-test; N and D2: n=31, Mann–Whitney U test). (E) Number of sleep bouts during the D1, N, and D2 periods (n=31, Student's t-test). (F) Sleep latency at night (n=30, Mann–Whitney U test).
CRediT authorship contribution statement
Marina Ricarte: Investigation, Data curation, Writing – original draft, Writing – review & editing. Eva Prats: Data curation, Formal analysis, Investigation, Writing – review & editing. Juliette Bedrossiantz: Data curation, Methodology, Writing – review & editing. Demetrio Raldúa: Conceptualization, Data curation, Formal analysis, Investigation, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Grant PID2020-113371RB-C21 and CEX2018-000794-S, funded by MCIN/AEI/10.13039/501100011033. Juliette Bedrossiantz was supported by grant PRE2018-083513, funded by MCIN/AEI/10.13039/501100011033 and by “ESF Investing in your future”.
Footnotes
Related research article: M. Ricarte, E. Prats, N. Montemurro, J. Bedrossiantz, M. Bellot, C. Gómez-Canela, D. Raldúa, Environmental concentrations of tire rubber-derived 6PPD-quinone alter CNS function in zebrafish larvae, Sci. Total Environ. 896 (2023), 165240. 10.1016/J.SCITOTENV.2023.165240
Contributor Information
Marina Ricarte, Email: mratam@cid.csic.es.
Juliette Bedrossiantz, Email: jbdqam@cid.csic.es.
Demetrio Raldúa, Email: drpqam@cid.csic.es.
Data availability
Data will be made available on request.
References
- 1.Liu J., Wu T., Liu Q., Wu S., Chen J.C. Air pollution exposure and adverse sleep health across the life course: a systematic review. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhdanova I.V. Sleep and its regulation in zebrafish. Rev. Neurosci. 2011;22:27–36. doi: 10.1515/RNS.2011.005. [DOI] [PubMed] [Google Scholar]
- 3.Chiu C.N., Prober D.A. Regulation of zebrafish sleep and arousal states: current and prospective approaches. Front. Neural Circuits. 2013;7:1–14. doi: 10.3389/fncir.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prober D.A., Rihel J., Onah A.A., Sung R.J., Schier A.F. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J. Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aston-Jones G., Gonzalez M., Doran S. Role of the locus coeruleus-norepinephrine system in arousal and circadian regulation of the sleep–wake cycle. Brain Norepinephrine Neurobiol. Ther. 2007:157–195. doi: 10.1017/CBO9780511544156.007. [DOI] [Google Scholar]
- 6.Appelbaum L., Wang G.X., Maro G.S., Mori R., Tovin A., Marin W., Yokogawa T., Kawakami K., Smith S.J., Gothilf Y., Mignot E., Mourrain P. Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21942–21947. doi: 10.1073/pnas.906637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesquita S.R., Van Drooge B.L., Oliveira E., Grimalt J.O., Barata C., Vieira N., Guimarães L., Piña B. Differential embryotoxicity of the organic pollutants in rural and urban air particles. Environ. Pollut. 2015;206:535–542. doi: 10.1016/J.ENVPOL.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Mesquita S.R., van Drooge B.L., Dall'Osto M., Grimalt J.O., Barata C., Vieira N., Guimarães L., Piña B. Toxic potential of organic constituents of submicron particulate matter (PM1) in an urban road site (Barcelona) Environ. Sci. Pollut. Res. 2017;24:15406–15415. doi: 10.1007/S11356-017-9201-4/FIGURES/4. [DOI] [PubMed] [Google Scholar]
- 9.Olivares A., Van Drooge B.L., Casado M., Prats E., Serra M., Van Der Ven L.T., Kamstra J.H., Hamers T., Hermsen S., Grimalt J.O., Piña B. Developmental effects of aerosols and coal burning particles in zebrafish embryos. Environ. Pollut. 2013;178:72–79. doi: 10.1016/J.ENVPOL.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Ricarte M., Prats E., Montemurro N., Bedrossiantz J., Bellot M., Gómez-Canela C., Raldúa D. Environmental concentrations of tire rubber-derived 6PPD-quinone alter CNS function in zebrafish larvae. Sci. Total Environ. 2023;896 doi: 10.1016/j.scitotenv.2023.165240. [DOI] [PubMed] [Google Scholar]
- 11.Fero K., Yokogawa T., Burgess H.A. Zebrafish Models in Neurobehavioral Research. Springer; 2011. The behavioral repertoire of larval zebrafish; pp. 249–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.