Abstract
Pseudomonas strain ADP metabolizes the herbicide atrazine via three enzymatic steps, encoded by the genes atzABC, to yield cyanuric acid, a nitrogen source for many bacteria. Here, we show that five geographically distinct atrazine-degrading bacteria contain genes homologous to atzA, -B, and -C. The sequence identities of the atz genes from different atrazine-degrading bacteria were greater than 99% in all pairwise comparisons. This differs from bacterial genes involved in the catabolism of other chlorinated compounds, for which the average sequence identity in pairwise comparisons of the known members of a class ranged from 25 to 56%. Our results indicate that globally distributed atrazine-catabolic genes are highly conserved in diverse genera of bacteria.
Atrazine [2-chloro-4-(ethylamino)-6-(isopropylamino)- 1,3,5-triazine] is a herbicide used for controlling broad-leaf and grassy weeds and is relatively persistent in soils (51). Atrazine and other s-triazine compounds have been detected in ground and surface waters at levels exceeding the Environmental Protection Agency’s maximum contaminant level of 3 ppb (30).
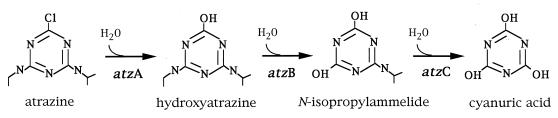
Microbial populations exposed to synthetic chlorinated compounds, such as atrazine, often respond by producing enzymes that degrade these molecules. Most of our current understanding of the genes and enzymes involved in atrazine degradation derives from studies using Pseudomonas strain ADP, in which the first three enzymatic steps in atrazine degradation have been defined (6, 14, 15, 48). The genes atz A, -B, and -C, which encode these enzymes, have been cloned and sequenced. Atrazine chlorohydrolase (AtzA), hydroxyatrazine ethylaminohydrolase (AtzB), and N-isopropylammelide isopropylaminohydrolase (AtzC) sequentially convert atrazine to cyanuric acid (6, 14, 15, 48) (Fig. 1). Cyanuric acid and related compounds are catabolized by many soil bacteria (10, 11, 17, 24, 26, 61), and by Pseudomonas sp. ADP, to carbon dioxide and ammonia (35). This provides the evolutionary pressure for the atzA, -B, and -C genes to permit bacterial growth on the more than one billion pounds of atrazine that have been applied to soils globally (20). Here we used a knowledge of the atzA, -B, and -C gene sequences to investigate the presence of homologous genes in other atrazine-degrading bacteria. In this study, we report that five atrazine-degrading microorganisms, which were recently isolated from geographically separated sites exposed to atrazine, contained nearly identical atzA, -B, and -C genes.
FIG. 1.
Pathway for atrazine catabolism to cyanuric acid in Pseudomonas sp. strain ADP.
Atrazine-catabolizing bacteria used in this study.
Until recently, attempts at isolating bacteria (18) or fungi (27) that completely degrade atrazine to carbon dioxide, ammonia, and chloride were unsuccessful. While several microorganisms were shown to dealkylate atrazine, they were unable to displace the chlorine atom (41, 54). Since 1994, several research groups have independently isolated atrazine-degrading bacteria that displaced the chlorine atom and mineralized atrazine (3, 7, 13, 35, 39, 46). Six of these bacterial cultures, listed in Table 1, were studied here, and the Clavibacter strain had been investigated previously (13).
TABLE 1.
Recently isolated atrazine-catabolizing bacteria
| Genus | Strain | Location where isolated | Yr reported (reference) |
|---|---|---|---|
| Pseudomonasa | ADP | Agricultural-chemical dealership site, Little Falls, Minn. | 1995 (35) |
| Ralstoniaa | M91-3 | Agricultural soil, Ohio | 1995 (46, 55) |
| Mixed culture | Basel, Switzerland | 1995 (57) | |
| Clavibacter | Agricultural soil, Riverside, Calif. | 1996 (13) | |
| Agrobacterium | J14a | Agricultural soil, Nebraska | 1996 (39) |
| NDb | 38/38 | Atrazine-contaminated soil, Indiana | 1996 (3) |
| Alcaligenesa | SG1 | Industrial settling pond, San Gabriel, La. | 1997 (7) |
Isolate identity based on 16S rRNA sequence analysis.
ND, not determined.
Detection of atzA, -B, and -C homologs in atrazine-degrading microorganisms by PCR analysis.
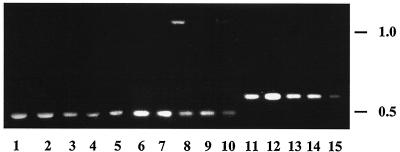
Recently isolated atrazine-degrading bacteria were screened for the presence of DNA homologous to the Pseudomonas strain ADP atzABC genes, which encode enzymes transforming atrazine to cyanuric acid (Fig. 1). Total genomic DNA was isolated from each of these bacteria as described elsewhere (49), and the PCR technique was used to amplify sequences internal to the atzA, -B, and -C genes as described elsewhere (13). Custom primers were designed specifically for atzA (5′CCATGTGAACCAGATCCT3′ and 5′TGAAGCGTCCACATTACC3′), atzB (5′TCACCGGGGATGTCGCGGGC3′ and 5′CTCTCCCGCATGGCATCGGG3′), and atzC (5′GCTCACATGCAGGTACTCCA3′ and 5′GTACCATATCACCGTTTGCCA3′) by using the Primer Designer package, version 2.01 (Scientific and Educational Software, State Line, Pa.), and were synthesized by Gibco BRL (Gaithersburg, Md.). PCR fragments were amplified by using Taq DNA polymerase (Gibco BRL) (22) and were separated from primers on a 1.0% agarose gel. The results of these studies (Fig. 2) indicated that PCR amplification consistently produced DNA fragments of 0.5 kb for all organisms when the atzA or -B primers were used and fragments of 0.6 kb when the atzC primers were used.
FIG. 2.
PCR analysis with primers designed to amplify internal regions of atzA (lanes 1 to 5), atzB (lanes 6 to 10), and atzC (lanes 11 to 15). The atrazine-degrading bacteria analyzed were Pseudomonas strain ADP (35) (lanes 1, 6, and 11), Alcaligenes strain SGI (7) (lanes 2, 7, and 12), Ralstonia strain M91-3 (46) (lanes 3, 8, and 13), Agrobacterium strain J14a (39) (lanes 4, 9, and 14), and isolate 38/38 (3) (lanes 5, 10, and 15). Values to the right of the gel are sizes (in kilobase pairs).
Southern hybridization analyses were performed on the PCR-amplified DNA as described elsewhere (49) to confirm the presence of homologous DNA. We used a 0.6-kb ApaI/PstI fragment from pMD4 (15), a 1.5-kb BglII fragment from pATZB-2 (6), and a 2.0-kb EcoRI/AvaI fragment from pTD2.5 (48) as probes for atzA, -B, and -C genes, respectively. DNA probes were labeled with [α-32P]dCTP by using the Rediprime Random Primer Labeling Kit (Amersham Life Science, Arlington Heights, Ill.) according to the manufacturer’s instructions. Southern hybridization analyses, performed under stringent conditions, confirmed that each strain contained DNA homologous to atzA, -B, and -C (data not shown). With strain M91-3 and isolate 38/38, however, in addition to the expected 0.5-kb atzB PCR product (Fig. 2, lanes 8 and 10), a 1.2-kb fragment was also obtained. However, no hybridization to this fragment was seen with the atzB probe. Similar investigations showed that a mixed culture obtained from Switzerland (Table 1), capable of degrading atrazine, also contained DNA homologous to all three atz genes (12).
As a negative control, bacteria known not to degrade atrazine were analyzed. PCR analyses were carried out with genomic DNA from the following randomly chosen laboratory strains: Rhodococcus chlorophenolicus (1), Flavobacterium sp. (47), Streptomyces coelicolor M145 (21), Amycolatopsis mediterranei (19), Agrobacterium strain A136 and strain A348 (A136/pTiA6NC) (60), Arthrobacter globiformis MN1 (45), Bradyrhizobium japonicum (33), Rhizobium sp. strain NGR 234 (44), Pseudomonas NRRLB12228, and Klebsiella pneumoniae 99 (16). None of these strains contained DNA that was amplified by PCR using the primers designed to identify the atzA, -B, or -C gene (data not shown).
DNA sequences of atzA, -B, and -C homologs in atrazine-degrading microorganisms.
DNAs amplified from the five strains in Table 1 with the atzA, -B, and -C primers were purified from gel slices by using the GeneClean II System (Bio 101, Inc., Vista, Calif.) and sequenced with a PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing kit (Perkin-Elmer Corp., Norwalk, Conn.) and an ABI model 373A DNA sequencer (Applied Biosystems, Foster City, Calif.). The GCG sequence analysis software package (Genetics Computer Group, Inc., Madison, Wis.) was used for all DNA and protein sequence comparisons and alignments. Table 2 summarizes these data. The PCR-amplified genes were ≥99% identical to the Pseudomonas strain ADP atzA, -B, and -C genes in all pairwise comparisons of DNA sequences. This remarkable sequence identity suggested that each atz gene in the different genera was derived from a common ancestor and that they have diverged evolutionarily only to a limited extent.
TABLE 2.
Sequence identities of atzABC homologs from different atrazine-degrading bacteria
| Strain | % DNA sequence identitya
|
||
|---|---|---|---|
| atzA | atzB | atzC | |
| Pseudomonas ADP | 100 | 100 | 100 |
| Alcaligenes SG1 | 99.2 | 100 | 100 |
| Ralstonia M91-3 | 99.0 | 100 | 100 |
| Agrobacterium J14a | 99.1 | 100 | 100 |
| Isolate 38/38 | 99.3 | 100 | 99.8 |
DNA sequences obtained from each strain by using the ataA, -B, and -C primers were compared with the atzABC gene sequences from Pseudomonas strain ADP.
A review of the literature on other bacterial catabolic pathways indicated a much greater degree of divergence when genes encoding enzymes for the catabolism of other commercially relevant chlorinated compounds were compared (Table 3). As with atrazine, multiple bacterial strains that catabolize 1,2-dichloroethane, chloroacetic acid, 2,4-dichlorophenoxyacetate, dichloromethane, and 4-chlorobenzoate have been isolated. A comparison of the gene sequences encoding the initiating reactions in the catabolism of each of those compounds revealed that sequence divergence was comparatively high. In pairwise comparisons within each gene class, the average sequence identities ranged from 25 to 56% (divergence was 46 to 75%). With the atzABC genes, by contrast, there is at most a 1% sequence difference within the sequenced gene region (Table 2). Moreover, the atzB sequences were completely identical, and the atzC genes diverged by only 1 bp in one of the five strains tested. This suggests that the atz genes recently arose from a single origin and have become distributed globally. Similarly, identical parathion hydrolase genes were isolated from two bacteria representing different genera and global locations (40, 52, 53).
TABLE 3.
Sequence comparisons of isofunctional bacterial enzymes that catabolize chlorinated compounds
| Gene | Enzyme | Average % protein sequence identitya (no. of pairwise comparisons) | References |
|---|---|---|---|
| dhlA, dhaA | Haloalkane dehalogenase | 25.0 (1) | 23, 31 |
| dehC, hadL, dehH, dehH1, dehH2, dhlB, dehCI, dehCII | 2-Haloacid dehalogenase | 36.6 ± 3.9 (36) | 5, 25, 28, 29, 42, 43, 50, 59 |
| tfdA | 2,4-Dichlorophenoxyacetate monooxygenase | 43.2 ± 4.6 (21)b | 34, 37, 38, 56, 58 |
| dcmA | Dichloromethane dehalogenase | 56.0 (1) | 4, 32 |
| atzA | Atrazine chlorohydrolase | 98.6 ± 0.12 (15)c | This study |
| atzB | Hydroxyatrazine ethylaminohydrolase | 100 (10)c | This study |
| atzC | N-Isopropylammelide isopropylaminohydrolase | 99.0 ± 0.43 (10)c | This study |
All possible pairwise alignments of translated gene sequences were made. The average percent identity is the mean of the percent identity values for all pairwise alignments ± standard error of the mean.
Includes full protein sequences as well as partial protein sequences of ≥100 amino acids.
Sequence identity within a 0.5-kb PCR product for atzA and -B and within a 0.6-kb PCR product for atzC. Six sequences were analyzed for atzA, and five were analyzed for atzB and -C.
The data presented here provide further support for previous studies suggesting that hydroxyatrazine in the environment derives from biological processes (36), and not solely from abiotic reactions (2, 9). The present data, and a recent report by Bouquard et al. (8), indicate that the gene encoding atrazine chlorohydrolase is widespread in the United States and Europe.
Our observations argue for a single, recent evolutionary origin of the atz genes and their subsequent global distribution. We have recently localized the atzA, -B, and -C genes to a large, self-transmissible plasmid in Pseudomonas strain ADP (12), and possible mechanisms of transfer of the atzABC genes are currently under investigation.
Acknowledgments
This work was supported in part by a grant from Novartis Crop Protection, Greensboro, N.C. (formerly Ciba-Geigy Corporation), and by grant 94-34339-1122 from the U.S. Department of Agriculture BARD program (as grant US-2364-93 from BARD, the United States-Israel Binational Agricultural Research and Development Fund).
We thank Janis Mcfarland and Steven Dumford of Novartis Crop Protection for providing s-triazine compounds, and David Gartner for technical assistance. We also thank David Crowley, Dave Newcombe, Gerhard Stucki, Mark Radosevich, Thomas Moorman, Ron Turco, Kyria Boundy-Mills, and Zhaoukun Tong for providing atrazine-degrading microorganisms or plasmids.
REFERENCES
- 1.Apajalahti J H A, Kärpänoja P, Salkinoja-Salonen M S. Rhodococcus chlorophenolicus sp. nov., a chlorophenol-mineralizing actinomycete. Int J Syst Bacteriol. 1986;36:246–251. [Google Scholar]
- 2.Armstrong D E, Chesters G. Adsorption catalysed chemical hydrolysis of atrazine. Environ Sci Technol. 1968;2:683–689. [Google Scholar]
- 3.Assaf N A, Turco R F. Accelerated biodegradation of atrazine by a microbial consortium is possible in culture and soil. Biodegradation. 1994;5:29–35. doi: 10.1007/BF00695211. [DOI] [PubMed] [Google Scholar]
- 4.Bader R, Leisinger T. Isolation and characterization of the Methylophilus sp. strain DM11 gene encoding dichloromethane dehalogenase/glutathione S-transferase. J Bacteriol. 1994;176:3466–3473. doi: 10.1128/jb.176.12.3466-3473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth P T, Bolton L, Thompson J C. Cloning and partial sequencing of an operon encoding two Pseudomonas putida haloalkanoate dehalogenases of opposite stereospecificity. J Bacteriol. 1992;174:2612–2619. doi: 10.1128/jb.174.8.2612-2619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boundy-Mills K, de Souza M L, Mandelbaum R M, Wackett L P, Sadowsky M J. The atzB gene of Pseudomonas sp. strain ADP encodes the second enzyme of a novel atrazine degradation pathway. Appl Environ Microbiol. 1997;63:916–923. doi: 10.1128/aem.63.3.916-923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boundy-Mills, K. L., L. P. Wackett, and M. J. Sadowsky. Unpublished data.
- 8.Bouquard C, Ouazzani J, Prome J-C, Michel-Briand Y, Plesiat P. Dechlorination of atrazine by a Rhizobium sp. isolate. Appl Environ Microbiol. 1997;63:862–866. doi: 10.1128/aem.63.3.862-866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkhard N, Guth J A. Chemical hydrolysis of 2-chloro-4,6-bis(alkylamino)-1,3,5-triazine herbicides and their breakdown in soil under the influence of adsorption. Pestic Sci. 1981;17:241–245. [Google Scholar]
- 10.Cook A M. Biodegradation of s-triazine xenobiotics. FEMS Microbiol Rev. 1987;46:93–116. [Google Scholar]
- 11.Cook A M, Beilstein P, Grossenbacher H, Huetter R. Ring cleavage and degradative pathway of cyanuric acid in bacteria. Biochem J. 1985;231:25–30. doi: 10.1042/bj2310025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Souza, M. L. Unpublished data.
- 13.de Souza M L, Newcombe D, Alvey S, Crowley D E, Hay A, Sadowsky M J, Wackett L P. Molecular basis of a bacterial consortium: interspecies catabolism of atrazine. Appl Environ Microbiol. 1998;64:178–184. doi: 10.1128/aem.64.1.178-184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza M L, Sadowsky M J, Wackett L P. Atrazine chlorohydrolase from Pseudomonas sp. strain ADP: gene sequence, enzyme purification, and protein characterization. J Bacteriol. 1996;178:4894–4900. doi: 10.1128/jb.178.16.4894-4900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza M L, Wackett L P, Boundy-Mills K L, Mandelbaum R T, Sadowsky M J. Cloning, characterization, and expression of a gene region from Pseudomonas sp. strain ADP involved in the dechlorination of atrazine. Appl Environ Microbiol. 1995;61:3373–3378. doi: 10.1128/aem.61.9.3373-3378.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton R W, Karns J S. Cloning and comparison of the DNA encoding ammelide aminohydrolase and cyanuric acid amidohydrolase from three s-triazine-degrading bacterial strains. J Bacteriol. 1991;173:1363–1366. doi: 10.1128/jb.173.3.1363-1366.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson E L, Lee K H. Degradation of atrazine and related s-triazines. Crit Rev Environ Contam. 1989;19:1–13. [Google Scholar]
- 18.Geller A. Studies on the degradation of atrazine by bacterial communities enriched from various biotopes. Arch Environ Contam Toxicol. 1980;9:289–305. doi: 10.1007/BF01057409. [DOI] [PubMed] [Google Scholar]
- 19.Ghisalba O, Nuesch J. A genetic approach to the biosynthesis of the rifamycin-chromophore in Nocardia mediterranei. IV. Identification of 3-amino-5-hydroxybenzoic acid as a direct precursor of the seven-carbon amino started-unit. J Antibiot. 1981;34:64–71. doi: 10.7164/antibiotics.34.64. [DOI] [PubMed] [Google Scholar]
- 20.Gianessi L P. Lack of data stymies informed decisions on agricultural pesticides. Resources. 1987;89:1–4. [Google Scholar]
- 21.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H S. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: John Innes Institute; 1985. [Google Scholar]
- 22.Innis M A, Gelfand D H, Sinsky J J, White T J. PCR protocols: a guide to methods and applications. New York, N.Y: Academic Press, Inc.; 1990. [Google Scholar]
- 23.Janssen D B, Pries F, van der Ploeg J, Kazemier B, Terpstra P, Witholt B. Cloning of 1,2-dichloroethane degradation genes of Xanthobacter autotrophicus GJ10 and expression and sequencing of the dhlA gene. J Bacteriol. 1989;171:6791–6799. doi: 10.1128/jb.171.12.6791-6799.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jessee J A, Benoit R E, Hendricks A C, Allen G C, Neal J L. Anaerobic degradation of cyanuric acid, cysteine, and atrazine by a facultatively anaerobic bacterium. Appl Environ Microbiol. 1983;45:97–102. doi: 10.1128/aem.45.1.97-102.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones D H A, Barth P T, Byrom D, Thomas C M. Nucleotide sequence of the structural gene encoding a 2-haloalkanoic acid dehalogenase of Pseudomonas putida strain AJ1 and purification of the encoded protein. J Gen Microbiol. 1992;138:675–683. doi: 10.1099/00221287-138-4-675. [DOI] [PubMed] [Google Scholar]
- 26.Jutzi K, Cook A M, Hutter R. The degradative pathway of the s-triazine melamine. Biochem J. 1982;208:679–684. doi: 10.1042/bj2080679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman D D, Blake J. Degradation of atrazine by soil fungi. Soil Biol Biochem. 1970;2:73–80. [Google Scholar]
- 28.Kawasaki H, Toyama T, Maeda T, Nishino H, Tonomura K. Cloning and sequence analysis of a plasmid-encoded 2-haloacid dehalogenase gene from Pseudomonas putida no. 109. Biosci Biotechnol Biochem. 1994;58:160–163. doi: 10.1271/bbb.58.160. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki H, Tsuda K, Matsushita I, Tonomura K. Lack of homology between two haloacetate dehalogenase genes encoded on a plasmid from Moraxella sp. strain B. J Gen Microbiol. 1992;138:1317–1323. doi: 10.1099/00221287-138-7-1317. [DOI] [PubMed] [Google Scholar]
- 30.Kello, D. 1989. WHO drinking water quality guidelines for selected herbicides. Food Addit. Contam. 6(Suppl.):S79–S85. [DOI] [PubMed]
- 31.Kulakova A N, Larkin M J, Kulakov L A. The plasmid-located haloalkane dehalogenase gene from Rhodococcus rhodochrous NCIMB 13064. Microbiology. 1997;143:109–115. doi: 10.1099/00221287-143-1-109. [DOI] [PubMed] [Google Scholar]
- 32.LaRoche S D, Leisinger T. Sequence analysis and expression of the bacterial dichloromethane dehalogenase structural gene, a member of the glutathione S-transferase supergene family. J Bacteriol. 1990;172:164–171. doi: 10.1128/jb.172.1.164-171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohrke S M, Orf J H, Martinez-Romero E, Sadowsky M J. Host-controlled restriction of nodulation by Bradyrhizobium japonicum strains in serogroup 110. Appl Environ Microbiol. 1995;61:2378–2383. doi: 10.1128/aem.61.6.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maltseva O, McGowan C, Fulthorpe R, Oriel P. Degradation of 2,4-dichlorophenoxyacetic acid by haloalkaliphilic bacteria. Microbiology. 1996;142:1115–1122. doi: 10.1099/13500872-142-5-1115. [DOI] [PubMed] [Google Scholar]
- 35.Mandelbaum R T, Allan D L, Wackett L P. Isolation and characterization of a Pseudomonas sp. that mineralizes the s-triazine herbicide atrazine. Appl Environ Microbiol. 1995;61:1451–1457. doi: 10.1128/aem.61.4.1451-1457.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandelbaum R T, Wackett L P, Allan D L. Rapid hydrolysis of atrazine to hydroxyatrazine by soil bacteria. Environ Sci Technol. 1993;27:1943–1946. [Google Scholar]
- 37.Matheson V G, Forney L J, Suwa Y, Nakatsu C H, Sexsone A J, Holben W E. Evidence for acquisition in nature of a chromosomal 2,4-dichlorophenoxyacetic acid/α-ketoglutarate dioxygenase gene by different Burkholderia spp. Appl Environ Microbiol. 1996;62:2457–2463. doi: 10.1128/aem.62.7.2457-2463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGowan C, Fulthorpe R, Wright A, Tiedje J, Maltseva O. Interspecies gene transfer in the evolution of 2,4-D degrading bacteria. GenBank accession number U43196. 1996. [Google Scholar]
- 39.Moscinski J K, Jayachandran K, Moorman T B. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Mineralization of the herbicide atrazine by Agrobacterium radiobacter, abstr. Q-414; p. 458. [Google Scholar]
- 40.Mulbry W W. Parathion hydrolase specified by the Flavobacterium opd gene: relationship between gene and protein. J Bacteriol. 1989;171:6740–6746. doi: 10.1128/jb.171.12.6740-6746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy I, Compernolle F, Ghys K, Vanderleyden J, De Mot R. A single cytochrome P-450 system is involved in degradation of the herbicides EPTC (S-ethyl dipropylthiocarbamate) and atrazine by Rhodococcus sp. strain NI86/21. Appl Environ Microbiol. 1995;61:2056–2060. doi: 10.1128/aem.61.5.2056-2060.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nardi-Dei V, Kurihara T, Okamura T, Liu J-Q, Koshikawa H, Ozaki H, Terashima Y, Esaki N, Soda K. Comparative studies of genes encoding thermostable l-2-halo acid dehalogenase from Pseudomonas sp. strain YL, other dehalogenases, and two related hypothetical proteins from Escherichia coli. Appl Environ Microbiol. 1994;60:3375–3380. doi: 10.1128/aem.60.9.3375-3380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nardi-Dei V, Kurihara T, Park C, Esaki N, Soda K. Bacterial dl-2-haloacid dehalogenase from Pseudomonas sp. strain 113: gene cloning and structural comparison with d- and l-2-haloacid dehalogenases. J Bacteriol. 1997;179:4232–4238. doi: 10.1128/jb.179.13.4232-4238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price N P J, Relic B, Talmont E, Lewin A, Prome D, Pueppke S G, Maillet F, Denarie J, Prome J C, Broughton W J. Broad-host-range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulfated. Mol Microbiol. 1992;6:3575–3584. doi: 10.1111/j.1365-2958.1992.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 45.Qi B. M.S. thesis. St. Paul: University of Minnesota; 1991. [Google Scholar]
- 46.Radosevich M, Traina S J, Hao Y, Tuovinen O H. Degradation and mineralization of atrazine by a soil bacterial isolate. Appl Environ Microbiol. 1995;61:297–302. doi: 10.1128/aem.61.1.297-302.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saber D L, Crawford R L. Isolation and characterization of Flavobacterium strains that degrade pentachlorophenol. Appl Environ Microbiol. 1985;50:1512–1518. doi: 10.1128/aem.50.6.1512-1518.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadowsky M J, Tong Z, de Souza M L, Wackett L P. AtzC is a new member of the amidohydrolase protein superfamily and is homologous to other atrazine-metabolizing enzymes. J Bacteriol. 1998;180:152–158. doi: 10.1128/jb.180.1.152-158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Schneider B, Muller R, Frank R, Lingens F. Complete nucleotide sequences and comparison of the structural genes of two 2-haloalkanoic acid dehalogenases from Pseudomonas sp. strain CBS3. J Bacteriol. 1991;173:1530–1535. doi: 10.1128/jb.173.4.1530-1535.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seiler A, Brenneisen P, Green D H. Benefits and risks of plant protection products—possibilities of protecting drinking water: case atrazine. Water Supply. 1992;10:31–42. [Google Scholar]
- 52.Serdar C M, Murdock D C, Rohde M F. Parathion hydrolase gene from Pseudomonas diminuta MG: subcloning, complete nucleotide sequence, and expression of the mature portion of the enzyme in Escherichia coli. Bio/Technology. 1989;7:1151–1155. [Google Scholar]
- 53.Sethunathan N, Yoshida T. A Flavobacterium sp. that degrades diazinon and parathion. Can J Microbiol. 1973;19:873–875. doi: 10.1139/m73-138. [DOI] [PubMed] [Google Scholar]
- 54.Shao Z Q, Behki R. Cloning of the genes for degradation of the herbicides EPTC (S-ethyl dipropylthiocarbamate) and atrazine from Rhodococcus sp. strain TE1. Appl Environ Microbiol. 1995;61:2061–2065. doi: 10.1128/aem.61.5.2061-2065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamper D M, Hallberg K B, Radosevich M, Traina S J, Tuovinen O H. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. Phylogenetic and biochemical characterization of an atrazine-mineralizing bacterial isolate, abstr. R-4; p. 525. [Google Scholar]
- 56.Streber W R, Timmis K M, Zenk M Z. Analysis, cloning, and high-level expression of 2,4-dichlorophenoxyacetate monooxygenase gene tfdA of Alcaligenes eutrophus JMP134. J Bacteriol. 1987;169:2905–2955. doi: 10.1128/jb.169.7.2950-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stucki G, Yu C W, Baumgartner T, Gonsalez-Valero J F. Microbial atrazine mineralization under carbon limited and denitrifying conditions. Water Res. 1995;1:291–296. [Google Scholar]
- 58.Suwa Y, Wright A D, Fukimori F, Nummy K A, Hausinger R P, Holben W E, Forney L J. Characterization of a chromosomally encoded 2,4-dichlorophenoxyacetic acid/α-ketoglutarate dioxygenase from Burkholderia sp. strain RASC. Appl Environ Microbiol. 1996;62:2464–2469. doi: 10.1128/aem.62.7.2464-2469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Ploeg J, van Hall G, Janssen D B. Characterization of the haloacid dehalogenase from Xanthobacter autotrophicus GJ10 and sequencing of the dhlB gene. J Bacteriol. 1991;173:7925–7933. doi: 10.1128/jb.173.24.7925-7933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watson B, Currier T C, Gordon M P, Chilton M D, Nester E W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975;123:255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolf D C, Martin J P. Microbial decomposition of ring-14C atrazine, cyanuric acid and 2-chloro-4,6-diamino-s-triazine. J Environ Qual. 1975;4:134–139. [Google Scholar]