Summary
Background
Sensory impairment has been related to age-associated cognitive decline. While these associations were investigated primarily in the auditory and visual domain, other senses such as touch have rarely been studied. Thus, it remains open whether these results are specific for particular sensory domains, or rather point to a fundamental role of sensory deficits in cognitive decline.
Methods
Data from 31 participants with mild cognitive impairment (MCI), 46 participants with frailty, and 23 non-clinical control participants (NCCs) were included. We assessed sensory function using visual acuity and contrast sensitivity, hearing threshold, and mechanical detection threshold. Cognitive function in participants with MCI was assessed using associative memory performance. Group differences on sensory thresholds were tested using analyses of covariance with age, sex, and years of education as covariates. Associations between measures within participants with MCI were evaluated using Spearman correlations.
Findings
We found a significant difference in mechanical detection threshold between the groups (p < 0.001, η2 = 0.18). Participants with MCI showed significantly reduced tactile sensitivity compared to participants with frailty and NCCs. In participants with MCI, lower associative memory performance was significantly related to reduced tactile sensitivity (rs = 0.39, p = 0.031) and auditory acuity (rs = 0.41, p = 0.022).
Interpretation
Our results indicate that reduced tactile sensitivity is related to cognitive decline. Prospective studies should investigate the age-related alterations of multimodal sensory processes and their contribution to dementia-related processes.
Funding
Deutsche Forschungsgemeinschaft (FL 156/41–1) and a grant of the Hector-Stiftung II, Weinheim, Germany.
Keywords: Mild cognitive impairment, Sensory deficits, Tactile sensitivity, Aging
Research in context.
Evidence before this study
Previous studies revealed an association between sensory deficits and age-associated cognitive decline. However, these studies mainly assessed the visual and auditory domain. Therefore, it remains open, whether these associations are specific to these modalities or whether impairments in other senses, such as touch, are also related to cognitive decline.
Added value of this study
Results of this study revealed lower tactile sensitivity in participants with mild cognitive impairment, compared to participants with physical impairment and participants without cognitive or physical impairment. Furthermore, a lower tactile sensitivity was associated with a lower memory performance in participants with mild cognitive impairment. Thus, the results of this study highlight that sensory deficits other than in the auditory and visual domain are related to cognitive decline.
Implications of all the available evidence
If validated in larger longitudinal studies, assessment of sensory functioning, including touch, might help to identify patients at high risk of cognitive decline. Furthermore, sensory training could also have positive effects on cognitive abilities and future research should investigate whether such types of training have preventive or stabilizing effects on cognitive decline.
Introduction
Global prevalence of dementia is expected to increase from 57 million cases in 2019 up to 153 million cases in 2050,1 placing a great need to identify potentially modifiable risk factors for disease prevention. One recent focus has been on the relationship between sensory deficits and cognitive decline. Longitudinal studies2, 3, 4, 5 have found that sensory impairments such as hearing loss and reduced visual contrast sensitivity are associated with increased risk of dementia or mild cognitive impairment (MCI), a syndrome of cognitive decline that often precedes neurodegenerative dementia. However, the mechanisms underlying this relationship are still not fully understood. Sensory and cognitive decline may be a sign for a shared neuropathological or neurodegenerative origin.2,6 Alternatively, sensory impairments may mediate and accelerate cognitive decline through the effects of sensory impairment on social isolation, depression, or physical inactivity as well as reduced brain plasticity.7,8 While the majority of prior studies focused on individual sensory impairments in the visual and auditory domain, one study has incorporated other senses such as touch.9 The study reported an increased risk of dementia for multisensory impairment in comparison to single or no sensory impairment.9 Investigating the association between cognitive and sensory impairment across multiple sensory systems may provide important information on whether the results are specific for the visual and auditory domain, or rather point to a fundamental role of sensory deficits in cognitive decline irrespective of the particular sensory domain.
Apart from age-related cognitive impairment, sensory decline has also been associated with nonpathological aging10,11 as well as other conditions of pathological aging such as frailty,12,13 a clinical syndrome characterised by an age-related physiological decline increasing the organism's vulnerability to stressors.14 Investigating sensory abilities across different groups of pathological and nonpathological aging may therefore help to identify potential specificities in sensory deficits and may shed light on the mechanisms underlying sensory and cognitive decline.
The objective of the present study was to examine whether visual, hearing, and somatosensory function were associated with cognitive impairment. We compared measures of sensory acuity between participants diagnosed with MCI, participants without cognitive impairment suffering from age-related physical frailty, and participants without cognitive or physical impairments. Relationships between sensory and cognitive measures were evaluated.
Methods
Participants
Data of participants with MCI and frailty were collected in two separate interventional studies.15,16 The respective study designs are described in Bekrater-Bodmann et al.16 for participants with MCI and Beier et al.15,17 for participants with frailty. The aim of the interventional studies (randomised controlled trials) was to examine the effectiveness of a 90-day computerised neuroplasticity-based sensorimotor training in reversing maladaptive alterations of pathological aging. For participants with MCI, the sensorimotor training was compared to a validated cognitive training while primary and secondary outcomes consisted in episodic memory function as well as hippocampal volume and function during memory tasks, respectively.16 For participants with frailty, the sensorimotor training was compared to a computerised relaxation training. While both groups showed a reduction in frailty, the sensorimotor training demonstrated a trend towards a stronger reduction in frailty as well as a reduction in bodily pain.15,17
For participants with MCI, the diagnostic procedure followed current guidelines18 and included the German version of the Mini-Mental State Examination (MMSE),19 the Consortium to Establish a Registry for Alzheimer's Disease (CERAD+) neuropsychological test battery,20 the logical memory subtests of the Wechsler Memory Scale-Revised (WMS-R), an expert rating on the Clinical Dementia Rating scale (CDR),21 an expert evaluation of T1-, T2-weighted and fluid attenuated inversion recovery 3T MRI images with semi-quantitative ratings of medial-temporal lobe atrophy (Scheltens score, MTA)22 and subcortical white matter lesions (Fazekas scale),23 as well as analyses of cerebrospinal fluid to determine total tau (tTau), phosphorylated tau (pTau), Amyloid ß42 (Aß42), Amyloid ß40 (Aß40), their ratio (Aß42/Aß40, Aß-ratio), and neuron-specific enolase. Participants with MCI were included if they were rated with a score of 0.5 on the CDR21 and fulfilled one of the following criteria: (a) at least one z-score <−1.2 of the CERAD + word list recall or retention, figures recall or retention performance, WMS-R immediate or delayed recall performance24; (b) at least one altered amyloid/tau/neurodegenerative (ATN) biomarker: reduced Aß42 (<600) or Aß-ratio (<0.55), or increased tTau (>450) or pTau (>61)25, 26, 27; and (c) age >60 years and MTA ≥2, or age ≤60 years and MTA ≥1.22
The frailty phenotype model was used to determine the frailty status of all participants by using the criteria described by Fried et al.28: unintentional weight loss, exhaustion, low levels of physical activity, slow gait speed, and poor grip strength. Unintentional weight loss was evaluated based on self-reports asking the participant if they unintentionally lost 4.5 kg or more in weight within the past year. Exhaustion was assessed using two items from the German version of the Center for Epidemiologic Studies Depression Scale (CES-D)29,30: “I could not get going”, and “I felt that everything I did was an effort”. Exhaustion was classified as present if a response of “occasionally” (3–4 days) or “most of the time” (5–7 days) regarding the past week was given to either question. Physical activity was measured asking participants how much time they spent during the past two weeks doing 18 different leisure activities. The amount of time was converted into an estimate of the weekly energy expenditure in kilocalories and low physical activity was classified as present if the kilocalories expended per week fell below a cut-off value, stratified by sex. Gait speed was determined by measuring the time taken to walk 4.57 m at usual pace, using walking aids if needed. Cut-off points were stratified by sex and height. Grip strength was measured in kg using a Jamar hand dynamometer (Patterson Medical, Cedarburg, WI, USA). Maximal grip strength at the dominant hand was averaged across three trials and cut-off scores were stratified by sex and body mass index. According to Fried et al.,28 participants were classified as robust if they did not fulfil any of the criteria, prefrail if they fulfilled one or two criteria, and frail if they fulfilled three or more of the criteria.
To screen for cognitive deficits in participants with frailty and NCC, we used the German version of the MMSE.19 This commonly used screening test assesses cognitive functioning in different cognitive domains (e.g. memory, language, attention, executive function). We just included data of participants with frailty and NCC without cognitive deficits (i.e., MMSE score ≤1 SD below the norm).20 Sex was self-reported by the participants.
For the present analyses, we first selected from the original samples those participants who had complete baseline data sets prior to randomization to any intervention. Of n = 52 participants with MCI, we excluded those who met the criteria for frailty or the precursor state of prefrailty, resulting in a final sample of n = 31 participants with MCI but without physical impairments. Of n = 55 participants with prefrailty or frailty, we excluded those with a cognitive deficit, resulting in a final sample of n = 46 participants with prefrailty or frailty but without cognitive deficits. Participants with prefrailty (n = 33) and frailty (n = 13), did not significantly differ in auditory (t (44) = 0.46, p = 0.65; independent samples t-test), visual (t (44) = 0.68, p = 0.50; independent samples t-test), or tactile thresholds (Z = −0.33, p = 0.74; Mann-Whitney-Test) and were thus included into the same group. We further recruited n = 23 non-clinical control participants (NCC) not meeting the criteria for prefrailty or frailty as assessed with the frailty phenotype criteria28 or for a cognitive deficit. Further general exclusion criteria were life-time prevalence of severe mental disorders (schizophrenia and other psychotic disorders, bipolar disorder, obsessive-compulsive disorder, post-traumatic stress disorder), current severe major depression and other axis I mental disorders, severe tinnitus symptomatology, stroke, or myocardial infarction within the last 6 months, as well as current intake of benzodiazepines, highly potent neuroleptics or tricyclic and anticholinergic antidepressants. Demographics and sample characteristics are provided in Table 1 (see Table S1 for demographic and sample characteristics, separated by sex). According to the inclusion criteria, there was a significant difference in cognitive performance between the groups, with the participants with MCI showing the lowest performance. In addition, only the participants with frailty, but not the participants in either of the other two groups, met at least one frailty phenotype criterion. However, there were also significant differences between the groups in demographic characteristics such as age, sex, and years of education that could potentially influence the results. Therefore, in our analyses, we statistically controlled for the effects of age, sex, and years of education when groups were compared statistically.
Table 1.
Demographic and sample characteristics of participants with mild cognitive impairment, frailty, and non-clinical controls.
| MCI [n = 31] | Frailty [n = 46] | NCC [n = 23] | Statistics | |
|---|---|---|---|---|
| Age | ||||
| M (SD) | 69.22 (9.08) | 80.45 (5.81) | 73.17 (5.58) | F2,97 = 25.58, p < 0.001 |
| min/max | 49/81 | 68/92 | 64/86 | |
| Years of education | ||||
| M (SD) | 13.90 (2.96) | 12.83 (2.99) | 15.87 (3.52) | F2,97 = 7.36, p < 0.05 |
| min/max | 8/20 | 8/22 | 10/21 | |
| Sex | ||||
| n f/m | 15/16 | 35/11 | 15/8 | Χ22 = 6.28, p < 0.05 |
| Number of frailty phenotype criteria (0–5) | ||||
| M(SD) | 0.00 (0.00) | 2.09 (0.81) | 0.00 (0.00) | |
| MMSE (0–30) | ||||
| M (SD) | 27.19 (1.49) | 29.35 (0.80) | 29.48 (0.73) | F2,97 = 46.94, p < 0.001 |
MCI = mild cognitive impairment, NCC = non-clinical control sample, n = number, M = mean, SD = standard deviation, min = minimum, max = maximum, MMSE = mini mental state examination.
Ethics
Prior to study participation, all participants gave written informed consent. The study was approved by the ethics committee II of the Medical Faculty Mannheim, Heidelberg University under Approval No. 2015-543N-MA and 2015-544N-MA.
Visual sensitivity testing
The automated Freiburg Acuity and Contrast Test (FrACT, Version 3.9.831,32; https://michaelbach.de/fract/index.html) was used to assess visual acuity and contrast sensitivity. To assess visual acuity, black Landolt rings of different sizes and orientation were depicted on a computer screen with white background in a pre-defined distance of 1.5m and standardised lighting conditions. To assess contrast sensitivity, grey-scaled Landolt rings of the same size but different orientation were depicted on the same computer screen with grey scaled background. In both tests, participants had to indicate each Landolt ring's orientation in a forced-choice manner. All participants performed the test without glasses. Those participants who had glasses additionally performed the test with their glasses that best corrected the visual acuity at the given distance (n = 27 participants with MCI, n = 27 participants with (pre)frailty, n = 17 NCC). Visual acuity threshold was determined by the “Best Parameter Estimation by Sequential Testing”. We report the logMAR score which is defined as the negative logarithm of the decimal visual acuity score (logMAR = -log (VA)). Thus, lower logMAR scores indicate higher visual acuity. For contrast sensitivity, the program reported Weber contrast values (in %) which were used to calculate the log contrast sensitivity score logCS using the formula logCS = log (100/Weber%). Higher logCS scores indicate enhanced visual contrast sensitivity. Unless otherwise indicated, we report the results of the test without glasses because the quality of optical care may confound with group. More specifically, the two clinical groups may be less likely to attend optical check-ups because of their physical and cognitive limitations.
Auditory sensitivity testing
We used a screening Audiometer (MA 25, MAICO Diagnostics GmbH, Berlin, Germany) to create an audiogram covering the frequency range from 125 Hz to 8 kHz separately for the left and right ear. All participants performed the test without hearing aids. The threshold for each frequency was determined by presenting single tones via headphones and by using a staircase procedure. Starting with a volume of 30 db, volume was decreased in 10 db steps until a participant no longer heard a tone. Volume was then increased and decreased in 5 db steps. The threshold for each frequency was set to the lowest volume that a participant heard three times. Based on the score defined by the World Health Organization (WHO) for determining hearing loss,33 auditory threshold for each ear was determined by the mean threshold of 0.5 kHz, 1 kHz, 2 kHz, and 4 kHz, with higher scores indicating a lower hearing ability, and we report data of the better ear. Due to technical problems with the audiometer, data on auditory threshold are missing for one NCC.
Tactile sensitivity testing
As a measure of tactile sensitivity, the mechanical threshold of the tip of the right index finger was assessed by using the standard examination protocol for Quantitative Sensory Testing (QST) of the German Research Network on Neuropathic Pain.34 We used a standardised set of von-Frey filaments with forces between 0.25 mN and 512 mN (Opti-hair2, MARSTOCK-Nervtest, Schriesheim, Germany). A staircase procedure was implemented to ascertain the mechanical threshold, defined as the geometric mean of five below- and five above-threshold intensities, with higher scores indicating a lower tactile sensitivity. Due to an acute injury at the stimulation site, data on mechanical detection threshold are missing for one participant with MCI.
Cognitive testing in participants with MCI
In participants with MCI, we assessed associative memory performance using the paired associates learning (PAL) paradigm of the Cambridge Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition, www.cantab.com). During this test, white boxes are displayed on a screen and are “revealed” and “concealed” in randomised order. Depending on the difficulty level, one or more of the boxes “contain” a visual pattern and the task is to memorize which box “contains” which visual pattern. After all boxes were “revealed” and “concealed”, the visual patterns are displayed one after the other in the middle of the screen and the participant has to select the box in which the pattern was originally located. We used the “total errors adjusted score” (a measure that is adjusted for the level of difficulty reached) as a measure for associative memory performance with higher scores indicating lower memory performance.35
Statistics
Data were tested for normal distribution using the Kolmogorov–Smirnov test. If the assumption of normality was violated, non-parametric statistics were used. We compared samples for age and years of education by using analysis of variance and for sex by using a Chi–Square test. Due to significant differences between groups in all three variables, we controlled for these effects when comparing the groups in their sensory capabilities. To test for differences in auditory and visual threshold, we used an analysis of covariance (ANCOVA) with age, sex, and years of education entered as covariates, and post-hoc pairwise comparisons (Bonferroni-corrected). Data for tactile threshold showed a non-normal distribution and transformation did not correct this deviation. To test for differences among groups on tactile threshold, we therefore used an ANCOVA based on ranked data with age, sex, and years of education entered as covariates, and post-hoc pairwise comparisons (Bonferroni-corrected). We repeated these analyses separately for female and male participants and report their results together with the respective descriptive data in the supplement.
We further computed correlation coefficients between sensory thresholds and associative memory capacity as assessed with the PAL in participants with MCI by using Spearman correlations.
We report test statistics, p-values (in case of multiple testing we report Bonferroni-corrected p-values, i.e., pBonf), and absolute values of effect sizes using partial η2. All statistical analyses were conducted using SPSS Statistics (Version 27, IBM Corp., Armonk, USA).
To determine the achieved statistical power, a post-hoc power analysis was carried out using G∗Power for Windows (Version 3.1.9.4.).36 Within the software, the effect size f was calculated from the reported effect sizes partial η2 and used together with the given sample size, number of groups and covariates as well as an α error probability of 0.05 to calculate the achieved statistical power.
Role of funders
The funding source had no role in the study design; in the collection, analysis, and interpretation of data; in writing of the manuscript; and in the decision to submit the manuscript for publication.
Results
Descriptives for auditory, visual, and tactile thresholds are displayed in Table 2 and reported separately for female and male participants in Table S2 in the supplement.
Table 2.
Auditory, visual, and tactile thresholds of participants with mild cognitive impairment, frailty, and non-clinical controls.
| MCI [n = 31] |
Frailty [n = 46] |
NCC [n = 23] |
|
|---|---|---|---|
| M (SD) Mdn (IQR) |
M(SD) Mdn (IQR) | M (SD) Mdn (IQR) | |
| Auditory threshold [db] | 24.80 (11.86) 23.75 (17.50) |
32.58 (12.23) 29.38 (16.56) |
29.05 (12.59)a 28.13 (13.44)a |
| Visual acuity threshold [logMAR] | 0.30 (0.31) 0.23 (0.36) |
0.32 (0.26) 0.26 (0.31) |
0.35 (0.28) 0.33 (0.44) |
| Visual contrast sensitivity [logCS] | 1.47 (0.53) 1.60 (0.39) |
1.46 (0.40) 1.43 (0.24) |
1.51 (0.48) 1.49 (0.42) |
| Tactile threshold [mN] | 1.83 (2.08)b 1.23 (2.13)b |
0.71 (1.06) 0.31 (0.52) |
0.56 (0.49) 0.41 (0.43) |
MCI = mild cognitive impairment, NCC = non-clinical control sample, n = number, M = mean, SD = standard deviation, Mdn = median, IQR = interquartile range.
n = 22.
n = 30. Note: We report descriptives of original data for tactile threshold in the table while the statistical analysis in the main text was based on ranks.
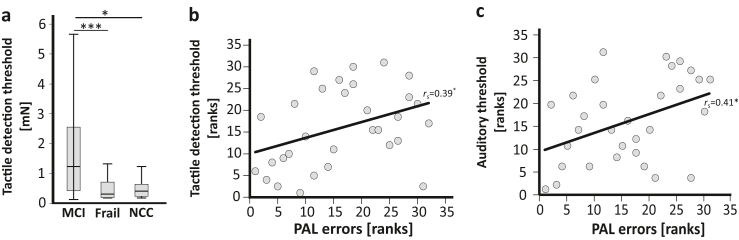
After controlling for age, sex, and years of education, there was a significant difference in tactile detection threshold between the groups (F2,93 = 10.35, p < 0.001, η2 = 0.18; ANCOVA based on ranked data), with participants with MCI showing significantly reduced tactile sensitivity compared to participants with (pre)frailty (pBonf < 0.001, MDiff = 33.17, 95%-CI [15.26, 51.08]; Bonferroni-corrected post-hoc pairwise comparison) and NCC (pBonf = 0.024, MDiff = 19.95, 95%-CI [1.99, 37.92]; Bonferroni-corrected post-hoc pairwise comparison; Fig. 1a). There was no significant difference between NCC and participants with (pre)frailty in tactile detection threshold (pBonf < 0.235, MDiff = 13.22, 95%-CI [−4.89, 31.33]; Bonferroni-corrected post-hoc pairwise comparison).
Fig. 1.
The association of tactile sensitivity and cognitive function. a) Medians and inter-quartile ranges of tactile detection thresholds as assessed with von-Frey filaments in n = 30 participants with mild cognitive impairment (MCI), n = 46 participants suffering from prefrailty or frailty (Frail), and n = 23 participants without physical or cognitive impairments (NCC). Note that higher tactile detection thresholds reflect reduced tactile sensitivity. There was a significant difference in tactile detection threshold between participants with MCI and participants with frailty (pBonf < 0.001) and between participants with MCI and NCC (pBonf = 0.024; Bonferroni-corrected post-hoc pairwise comparisons after significant ANCOVA based on ranked data with age, sex, and years of education as covariates). b) Association (Spearman correlation) between ranks of tactile detection thresholds and ranks of associative memory performance as assessed with the paired associate learning test (PAL; subtest of the Cambridge Neuropsychological Test Automated Battery, CANTAB, Cambridge Cognition, Cambridge, United Kingdom) in n = 30 participants with MCI. c) Association (Spearman correlation) between ranks of auditory threshold and ranks of associative memory performance as assessed with PAL in n = 31 participants with MCI. Whiskers indicate 1.5 inter-quartile range or minimum/maximum value. ∗p < 0.05, ∗∗∗p < 0.001.
For the Omnibus-tests, the pattern of results was similar for female and male participants. However, most post-hoc comparisons, which were significant for the whole sample, did not reach significance in the analysis by sex (Supplemental Data), which might be due to reduced power in smaller samples.
The groups did not significantly differ in auditory (F2,93 = 0.50, p = 0.608, η2 = 0.01; ANCOVA) or visual thresholds (visual acuity: F2,94 = 0.57, p = 0.569, η2 = 0.01; contrast sensitivity: F2,94 = 2.04, p = 0.136, η2 = 0.04; ANCOVAs). The latter was still true when we included the results of the visual threshold tests with glasses in the analysis for participants who achieved a better test result with glasses (visual acuity: F2,94 = 0.26, p = 0.771, η2 = 0.01; contrast sensitivity: F2,94 = 2.55, p = 0.083, η2 = 0.05; ANCOVAs). For results of sex-specific analyses, please refer to the supplement.
In participants with MCI, associative memory performance as assessed by PAL was significantly positively related with tactile sensitivity (rs = 0.39, p = 0.031, Spearman correlation; Fig. 1b) and auditory acuity (rs = 0.41, p = 0.022, Spearman correlation; Fig. 1c) but not with visual thresholds (visual acuity: rs = 0.04, p = 0.814; contrast sensitivity rs = −0.17, p = 0.342; Spearman correlations).
Discussion
In this study, we compared auditory, visual, and tactile perception thresholds between participants with MCI (pre)frailty, and NCC. After controlling for age, sex, and years of education, we found a significantly reduced tactile sensitivity in participants with MCI compared to both other groups. Moreover, we found a significant correlation between associative memory performance and tactile as well as auditory threshold in participants with MCI, indicating that lower memory performance was associated with lower tactile sensitivity and auditory acuity.
Our work extends previous studies on sensory perception in persons with cognitive impairments which have rarely assessed the association between somatosensation and cognitive decline. Our present results are in line with results of a previous longitudinal study, which reported reduced touch perception as predictor of dementia in older adults.9 Previous studies have also provided some evidence of structural and functional alterations in the somatosensory system that may underlie reduced tactile sensitivity in MCI. In an autopsy study on brains of individuals with Alzheimer's Disease (AD), Suvà et al.37 found senile plaques, a neuropathological marker of MCI,38 not only in brain areas well known to be affected in AD such as the entorhinal cortex and hippocampus,39,40 but also in the primary somatosensory cortex. From a functional perspective, an MEG study revealed larger somatosensory evoked fields in response to electrical stimulation of participants with MCI compared to participants with AD or a non-clinical control sample,41 indicating that changes in somatosensory cortex might be an early marker for the transition to AD.
While reduced tactile sensitivity has previously been associated with an age-related decrease in the number of peripheral mechanoreceptors,42,43 it is important to note that we observed reduced tactile sensitivity for participants with MCI while statistically controlling for age. Thus, our observations suggest that the relationship between somatosensory and cognitive impairments is not due to age per se, but additionally due to pathological aging mechanisms specific for MCI.
The mechanisms underlying the relationship between sensory impairment and an increased risk for dementia or MCI are still a matter of debate.2, 3, 4, 5 Neuroscientific models of aging consider the brain to play a major role in determining age-related sensory and cognitive decline.8,44 As a result of aging, the representation of complex sensory inputs becomes more inaccurate and manifests as temporally and spatially “noisy processing” of sensory stimuli.8 It is assumed that noisy processing of sensory input affects cognitive processing such as memory performance.45,46 Given that impaired tactile sensitivity was found to be related to enlarged hand representations in somatosensory cortex,47 we may assume that the significantly reduced tactile sensitivity found in participants with MCI to be a behavioural correlate of altered cortical sensory representations and noisy neuronal processing. Our results could thus provide support for the hypothesis that altered and less specific sensory representations are related to cognitive decline.
Besides, other mechanisms have also been hypothesised to underlie the link between sensory deficits and cognitive decline. Sensory dysfunction may augment social withdrawal and isolation and thereby drive cognitive decline,7 e.g. when hearing ability is reduced.3 However, deficits in tactile sensitivity may be less associated with social interaction compared to deficits in the auditory domain. Therefore, our results might support previous research which suggested that in MCI, deficits in sensory systems are not only subject to shared48 but also independent underlying mechanisms such as detrimental environmental effects or differences in the decline of peripheral receptors.49 For instance, Fischer et al.2 found that sensory deficits, in terms of hearing, visual, and olfactory impairment, independently increased the risk to develop MCI.
While we did not observe significant group differences in hearing thresholds, we found a significant association between increased hearing thresholds and reduced associative memory performance in participants with MCI. These findings are in line with previous research reporting associations between hearing loss and cognitive decline in older adults.3,4,50 With respect to potential underlying mechanisms, these relationships have been associated with neuroplastic changes in the brain in MCI. For instance, participants with MCI showed greater neuronal activation during speech recognition compared to healthy individuals, presumably reflecting a compensatory process to overcome possible deficits in auditory performance and speech perception.51 However, these compensatory strategies are likely to fail with the progression of the degenerative disease, due to structural atrophies and grey matter reductions as well as the accumulation of misfolded proteins in brain regions involved in hearing.4,51 Additionally, hearing loss was found to be associated with reduced hippocampal volume in individuals with perceived cognitive decline but normal cognitive performance.52 Thus, our findings are consistent with the hypothesis that hearing impairment may reflect the risk for cognitive decline and subsequent dementia.4
We did not find a significant relationship between cognition and visual impairment for either visual acuity or contrast sensitivity. Previous research revealed heterogeneous results with some studies reporting associations between cognition and vision2,5 while others did not.9 Moreover, the relationship between vision and cognition was found to differ by measure of vision and that impaired contrast sensitivity is related to declines across more cognitive domains than visual acuity.53 Notably, participants with MCI in the present study were younger by six to eight years and had higher cognitive performance (mean MMSE score 27 vs. 25) compared to participants examined in a prior study.5 This suggests that associations between cognitive and visual impairment might manifest at older ages or with temporally more advanced disease symptomatology at the transition to AD.38
While deficits in auditory or visual abilities can be compensated by means of hearing and visual aids to reduce the risk of dementia or to slow down cognitive decline in MCI,54 compensation of tactile deficits is difficult. Moreover, tactile deficits are less often recognised than auditory and visual deficits.49 In fact, intensive somatosensory stimulation and sensory discrimination training were shown to have the potential to improve sensorimotor performance and promote cortical changes in somatosensory brain areas.55,56 Notably, high tactile acuity in terms of tactile spatial resolution was shown to be retained into old age for blind braille readers compared to sighted individuals, suggesting that intensive use of active touch in daily activities could preserve tactile acuity across the life span.57 The transfer of the effect to nonreading fingers suggests an underlying central nervous mechanism.57 While braille reading performance was found to be associated with certain cognitive functions,58,59 the relationship between the maintenance of braille reading performance and cognitive performance in old age is still unclear. Future research should therefore examine whether diagnostic and prognostic predictions for patients can be improved by including somatosensory assessments and whether interventions targeted to improve multisensory impairment may have the potential to modulate the risk for age-related cognitive decline and subsequent dementia.
Crucially, reduced tactile sensitivity was found in participants with MCI not only compared to NCCs, but also compared to participants with frailty. These results suggest that sensory impairment may be differentially associated with different conditions of pathological aging. The syndrome of frailty is determined by decline in body composition, such as a loss of muscle mass and function,60 resulting in reduced motor function and performance.61,62 While positive relationships between frailty and sensory impairment were found for multiple sensory domains,12,13,63 it could be that sensory impairment per se may play a differential role in frailty, due to the multidimensional physiological nature of the syndrome. Also, it could be that the extent of sensory deficits in frailty was underestimated in our sample. Given that participants consented to participate in a multi-month intervention study requiring several onsite visits, it could be that we primarily included participants with relatively mild forms of pre-frailty and frailty (mean number of frailty phenotype criteria [0–5] = 2.09, range 1–4) and sensory decline. The effects observed in the present study must therefore be replicated in participants spanning a wider range of the extent of pathological aging.
Our study is subject to limitations. Due to the cross-sectional nature of the analyses, the results do not provide information on the temporal relationship and causal or mechanistic pathways underlying the relationship between cognitive and somatosensory impairment. The sample size was small, yet post-hoc power analysis revealed a high power of 0.99 for the significant effect between groups in tactile threshold. However, the power for the non-significant effects in visual and auditory thresholds were lower (≤0.51), indicating that the sample size was not sufficient to detect differences between groups for these variables. Replication studies on larger samples are therefore necessary to confirm the robustness of our results and to investigate potential sex-specific effects. Moreover, the fact that the analyses were performed on baseline data from participants with MCI and frailty participating in an intervention study might have promoted selection and exclusion bias in the sample. In sum, the small sample size, the adjustment for covariates as well as the fact that the comparison groups were taken from three separate studies together limit the generalizability of the present findings.
In conclusion, our results suggest that somatosensory impairment is associated with reduced cognitive function in participants with MCI. Our study therefore extends previous research focusing on the association of cognitive impairment with auditory and visual ability. Future studies are required to investigate the mechanistic basis of this observed association and to determine whether these mechanisms may be amenable to treatment. Thus, assessment of sensory functioning in multiple domains, including somatosensation, may help identify patients at high risk of cognitive decline and may provide useful targets for preventive interventions.
Contributors
HF conceived the main studies. HF and LF obtained funding. AL, FB, RBB, LH, SD, SS, DK, ML, FN, LF, and HF contributed to conception and design of the main studies. AL, FB, RBB, and LH contributed to data collection. AL, FB, RBB, and HF developed research question and the statistical analysis plan for the current analyses. AL, and FB analysed and verified the underlying data. AL created the figure. AL, and FB wrote the first draft of the manuscript. AL, and FB contributed equally. All authors critically revised and edited the manuscript and approved the final version for submission. All authors had full access to all the data.
Data sharing statement
Data is available upon reasonable request from the corresponding author (florian.beier@zi-mannheim.de) or herta.flor@zi-mannheim.de.
Declaration of interest
We declare no competing interests.
Acknowledgements
We thank Lea Hild for her support with data collection. This work was supported by the Reinhart Koselleck Programme of the Deutsche Forschungsgemeinschaft to HF (FL 156/41–1) and by a grant of the Hector-Stiftung II, Weinheim, Germany to HF and LF.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104896.
Appendix A. Supplementary data
References
- 1.Nichols E., Steinmetz J.D., Vollset S.E., et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer M.E., Cruickshanks K.J., Schubert C.R., et al. Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc. 2016;64(10):1981–1987. doi: 10.1111/jgs.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin F.R., Yaffe K., Xia J., et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H.F., Zhang W., Rolls E.T., et al. Hearing impairment is associated with cognitive decline, brain atrophy and tau pathology. eBioMedicine. 2022;86 doi: 10.1016/j.ebiom.2022.104336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward M.E., Gelfand J.M., Lui L.Y., et al. Reduced contrast sensitivity among older women is associated with increased risk of cognitive impairment. Ann Neurol. 2018;83(4):730–738. doi: 10.1002/ana.25196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindenberger U., Baltes P.B. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9(3):339–355. doi: 10.1037/0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 7.Fischer M.E., Cruickshanks K.J., Klein B.E.K., Klein R., Schubert C.R., Wiley T.L. Multiple sensory impairment and quality of life. Ophthalmic Epidemiol. 2009;16(6):346–353. doi: 10.3109/09286580903312236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahncke H.W., Bronstone A., Merzenich M.M. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 9.Brenowitz W.D., Kaup A.R., Lin F.R., Yaffe K. Multiple sensory impairment is associated with increased risk of dementia among black and white older adults. J Gerontol A Biol Sci Med Sci. 2019;74(6):890–896. doi: 10.1093/gerona/gly264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rigters S.C., Bos D., Metselaar M., et al. Hearing impairment is associated with smaller brain volume in aging. Front Aging Neurosci. 2017;9:2. doi: 10.3389/fnagi.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer S.W., Harrison A.L. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87(2):193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- 12.Kamil R.J., Li L., Lin F.R. Association between hearing impairment and frailty in older adults. J Am Geriatr Soc. 2014;62(6):1186–1188. doi: 10.1111/jgs.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira A.I., Nogueira D., de Azevedo Reis E., da Lapa Rosado M., Vânia Nunes M., Castro-Caldas A. Hand tactile discrimination, social touch and frailty criteria in elderly people: a cross sectional observational study. Arch Gerontol Geriatr. 2016;66:73–81. doi: 10.1016/j.archger.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Clegg A., Young J., Iliffe S., Rikkert M.O., Rockwood K. Frailty in elderly people. Lancet (London, England) 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beier F., Löffler M., Nees F., Hausner L., Frölich L., Flor H. Promoting neuroplasticity and neuropsychological functioning in frailty through an app-based sensorimotor training: study protocol for a randomized trial. BMC Geriatr. 2021;21(1):343. doi: 10.1186/s12877-021-02293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekrater-Bodmann R., Löffler A., Silvoni S., et al. Tablet-based sensorimotor home-training system for amnestic mild cognitive impairments in the elderly: design of a randomised clinical trial. BMJ Open. 2019;9(8) doi: 10.1136/bmjopen-2018-028632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beier F., Löffler M., Nees F., et al. Effects of an app-based sensorimotor training in promoting neuroplasticity and neuropsychological functioning in frailty: a randomized controlled trial. Arch Gerontol Geriatr. 2023;115 doi: 10.1016/J.ARCHGER.2023.105202. [DOI] [PubMed] [Google Scholar]
- 18.Deuschl G., Maier W. S3-Leitlinie demenzen. DGPPN, DGN. Springer; Berlin, Heidelberg: 2017. [DOI] [Google Scholar]
- 19.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Welsh K.A., Butters N., Mohs R.C., et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 21.Morris J.C. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 22.Scheltens P., Launer L.J., Barkhof F., Weinstein H.C., van Gool W.A. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol. 1995;242(9):557–560. doi: 10.1007/BF00868807. [DOI] [PubMed] [Google Scholar]
- 23.Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. 1987;149(2):351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 24.Wolfsgruber S., Jessen F., Koppara A., et al. Subjective cognitive decline is related to CSF biomarkers of AD in patients with MCI. Neurology. 2015;84(12):1261–1268. doi: 10.1212/WNL.0000000000001399. [DOI] [PubMed] [Google Scholar]
- 25.Jack C.R., Bennett D.A., Blennow K., et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer’s Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansson O., Lehmann S., Otto M., Zetterberg H., Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's Disease. Alzheimer's Res Ther. 2019;11(1):1–15. doi: 10.1186/s13195-019-0485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumurgier J., Schraen S., Gabelle A., et al. Cerebrospinal fluid amyloid-β 42/40 ratio in clinical setting of memory centers: a multicentric study. Alzheimer's Res Ther. 2015;7(1):30. doi: 10.1186/s13195-015-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 29.Hautzinger M., Bailer M., Hofmeister D., Keller F. 2nd ed. Hogrefe; Göttingen: 2012. Allgemeine depressionsskala. [Google Scholar]
- 30.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 31.Bach M. The Freiburg visual acuity test - automatic measurement of visual acuity. Optom Vis Sci. 1996;73(1):49–53. doi: 10.1097/00006324-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Bach M. The Freiburg visual acuity test-variability unchanged by post-hoc re-analysis. Graefe’s Arch Clin Exp Ophthalmol. 2007;245(7):965–971. doi: 10.1007/s00417-006-0474-4. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization . 1991. Report of the informal working group on prevention of deafness and hearing impairment programme planning: Geneva; pp. 18–21. Geneva. [Google Scholar]
- 34.Rolke R., Baron R., Maier C., et al. Quantitative sensory testing in the German research Network on neuropathic pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 35.Barnett J.H., Blackwell A.D., Sahakian B.J., Robbins T.W. The paired associates learning (PAL) test: 30 years of CANTAB translational neuroscience from laboratory to bedside in dementia research. Curr Top Behav Neurosci. 2016;28:449–474. doi: 10.1007/7854_2015_5001. [DOI] [PubMed] [Google Scholar]
- 36.Faul F., Erdfelder E., Lang A.G., Buchner A.G. ∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 37.Suvà D., Favre I., Kraftsik R., Esteban M., Lobrinus A., Miklossy J. Primary motor cortex involvement in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58(11):1125–1134. doi: 10.1097/00005072-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Stephan B.C.M., Hunter S., Harris D., et al. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry. 2012;17(11):1056–1076. doi: 10.1038/mp.2011.147. [DOI] [PubMed] [Google Scholar]
- 39.Devanand D.P., Pradhaban G., Liu X., et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68(11):828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 40.Scahill R.I., Schott J.M., Stevens J.M., Rossor M.N., Fox N.C. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A. 2002;99(7):4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephen J.M., Montaño R., Donahue C.H., et al. Somatosensory responses in normal aging, mild cognitive impairment, and Alzheimer's disease. J Neural Transm. 2010;117(2):217–225. doi: 10.1007/s00702-009-0343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowden J.L., McNulty P.A. Age-related changes in cutaneous sensation in the healthy human hand. Age. 2013;35(4):1077–1089. doi: 10.1007/s11357-012-9429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Piqueras J., García-Mesa Y., Cárcaba L., et al. Ageing of the somatosensory system at the periphery: age-related changes in cutaneous mechanoreceptors. J Anat. 2019;234(6):839–852. doi: 10.1111/joa.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinse H.R. Cortical reorganization in the aging brain. Prog Brain Res. 2006;157:57–80. doi: 10.1016/S0079-6123(06)57005-0. [DOI] [PubMed] [Google Scholar]
- 45.Goh J.S.O. Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis. 2011;2(1):30–48. [PMC free article] [PubMed] [Google Scholar]
- 46.Koen J.D., Hauck N., Rugg M.D. The relationship between age, neural differentiation, and memory performance. J Neurosci. 2019;39(1):149–162. doi: 10.1523/JNEUROSCI.1498-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalisch T., Ragert P., Schwenkreis P., Dinse H.R., Tegenthoff M. Impaired tactile acuity in old age is accompanied by enlarged hand representations in somatosensory cortex. Cereb Cortex. 2009;19(7):1530–1538. doi: 10.1093/cercor/bhn190. [DOI] [PubMed] [Google Scholar]
- 48.Stevens J.C., Cruz L.A., Marks L.E., Lakatos S. A multimodal assessment of sensory thresholds in aging. J Gerontol B Psychol Sci Soc Sci. 1998;53(4):P263–P272. doi: 10.1093/geronb/53B.4.P263. [DOI] [PubMed] [Google Scholar]
- 49.Cavazzana A., Röhrborn A., Garthus-Niegel S., Larsson M., Hummel T., Croy I. Sensory-specific impairment among older people. An investigation using both sensory thresholds and subjective measures across the five senses. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panza F., Solfrizzi V., Logroscino G. Age-related hearing impairment - a risk factor and frailty marker for dementia and AD. Nat Rev Neurol. 2015;11(3):166–175. doi: 10.1038/nrneurol.2015.12. [DOI] [PubMed] [Google Scholar]
- 51.Bidelman G.M., Lowther J.E., Tak S.H., Alain C. Mild cognitive impairment is characterized by deficient brainstem and cortical representations of speech. J Neurosci. 2017;37(13):3610–3620. doi: 10.1523/JNEUROSCI.3700-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giroud N., Pichora-Fuller M.K., Mick P., et al. Hearing loss is associated with gray matter differences in older adults at risk for and with Alzheimer's disease. Aging Brain. 2021;1 doi: 10.1016/j.nbas.2021.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varadaraj V., Munoz B., Deal J.A., et al. Association of vision impairment with cognitive decline across multiple domains in older adults. JAMA Netw Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bucholc M., McClean P.L., Bauermeister S., et al. Association of the use of hearing aids with the conversion from mild cognitive impairment to dementia and progression of dementia: a longitudinal retrospective study. Alzheimer’s Dement Transl Res Clin Interv. 2021;7(1) doi: 10.1002/trc2.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dinse H.R., Kattenstroth J.C., Gatica Tossi M.A., Tegenthoff M., Kalisch T. In: Augmenting cognition lausanne. Markram H., Segev I., editors. EPFL Press; 2011. Sensory stimulation for augmenting perception, sensorimotor behavior and cognition; pp. 11–39. [DOI] [Google Scholar]
- 56.Flor H., Denke C., Schaefer M., Grüsser S. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet. 2001;357(9270):1763–1764. doi: 10.1016/S0140-6736(00)04890-X. [DOI] [PubMed] [Google Scholar]
- 57.Legge G.E., Madison C., Vaughn B.N., Cheong A.M.Y., Miller J.C. Retention of high tactile acuity throughout the life span in blindness. Percept Psychophys. 2008;70(8):1471–1488. doi: 10.3758/PP.70.8.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martiniello N., Wittich W. The association between tactile, motor and cognitive capacities and braille reading performance: a scoping review of primary evidence to advance research on braille and aging. Disabil Rehabil. 2022;44(11):2515–2536. doi: 10.1080/09638288.2020.1839972. [DOI] [PubMed] [Google Scholar]
- 59.Martiniello N., Barlow M., Wittich W. Exploring correlates of braille reading performance in working-age and older adults with visual impairments. Sci Stud Read. 2022;26(4):267–286. doi: 10.1080/10888438.2021.1969402. [DOI] [Google Scholar]
- 60.Buchman A.S., Leurgans S.E., Wang T., et al. Motor function is the primary driver of the associations of sarcopenia and physical frailty with adverse health outcomes in community-dwelling older adults. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0245680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castell M.V., Sánchez M., Julián R., Queipo R., Martín S., Otero Á. Frailty prevalence and slow walking speed in persons age 65 and older: implications for primary care. BMC Fam Pract. 2013;14:86. doi: 10.1186/1471-2296-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis D.H.J., Rockwood M.R.H., Mitnitski A.B., Rockwood K. Impairments in mobility and balance in relation to frailty. Arch Gerontol Geriatr. 2011;53(1):79–83. doi: 10.1016/j.archger.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Swenor B.K., Lee M.J., Tian J., Varadaraj V., Bandeen-Roche K. Visual impairment and frailty: examining an understudied relationship. J Gerontol A Biol Sci Med Sci. 2020;75(3):596–602. doi: 10.1093/gerona/glz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.