Abstract
Background & Aims
Pathologists quantify liver steatosis as the fraction of lipid droplet-containing hepatocytes out of all hepatocytes, whereas the magnetic resonance-determined proton density fat fraction (PDFF) reflects the tissue triacylglycerol concentration. We investigated the linearity, agreement, and correspondence thresholds between histological steatosis and PDFF across the full clinical spectrum of liver fat content associated with non-alcoholic fatty liver disease.
Methods
Using individual patient-level measurements, we conducted a systematic review and meta-analysis of studies comparing histological steatosis with PDFF determined by magnetic resonance spectroscopy or imaging in adults with suspected non-alcoholic fatty liver disease. Linearity was assessed by meta-analysis of correlation coefficients and by linear mixed modelling of pooled data, agreement by Bland–Altman analysis, and thresholds by receiver operating characteristic analysis. To explain observed differences between the methods, we used RNA-seq to determine the fraction of hepatocytes in human liver biopsies.
Results
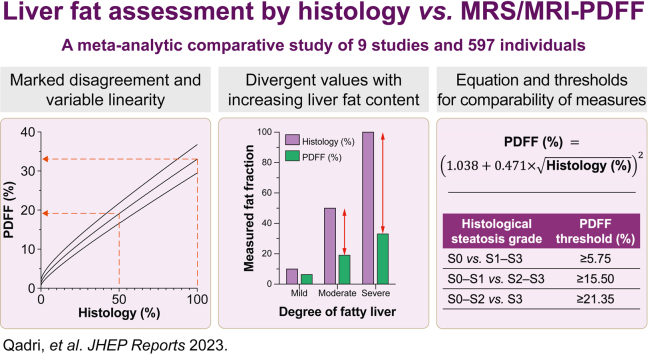
Eligible studies numbered 9 (N = 597). The relationship between PDFF and histology was predominantly linear (r = 0.85 [95% CI, 0.80–0.89]), and their values approximately coincided at 5% steatosis. Above 5% and towards higher levels of steatosis, absolute values of the methods diverged markedly, with histology exceeding PDFF by up to 3.4-fold. On average, 100% histological steatosis corresponded to a PDFF of 33.0% (29.5–36.7%). Targeting at a specificity of 90%, optimal PDFF thresholds to predict histological steatosis grades were ≥5.75% for ≥S1, ≥15.50% for ≥S2, and ≥21.35% for S3. Hepatocytes comprised 58 ± 5% of liver cells, which may partly explain the lower values of PDFF vs. histology.
Conclusions
Histological steatosis and PDFF have non-perfect linearity and fundamentally different scales of measurement. Liver fat values obtained using these methods may be rendered comparable by conversion equations or threshold values.
Impact and implications
Magnetic resonance-proton density fat fraction (PDFF) is increasingly being used to measure liver fat in place of the invasive liver biopsy. Understanding the relationship between PDFF and histological steatosis fraction is important for preventing misjudgement of clinical status or treatment effects in patient care. Our analysis revealed that histological steatosis fraction is often significantly higher than PDFF, and their association varies across the spectrum of fatty liver severity. These findings are particularly important for physicians and clinical researchers, who may use these data to interpret PDFF measurements in the context of histologically evaluated liver fat content.
Keywords: Fatty liver, Metabolic dysfunction-associated steatotic liver disease, Magnetic resonance imaging, Magnetic resonance spectroscopy, Biopsy, Histology, Hepatocytes, Pathologists, Triglycerides, Transcriptome, Systematic review, Meta-analysis
Graphical abstract
Highlights
-
•
Histology and PDFF are fundamentally different methods of liver fat quantification.
-
•
The relationship between histological steatosis and PDFF is predominantly linear.
-
•
Percentage liver fat by histology is often markedly higher compared to PDFF.
-
•
Differences between histological steatosis and PDFF increase with higher liver fat.
-
•
A formula or threshold values enable comparison of histological steatosis and PDFF.
Introduction
In histological evaluation of liver fat, the pathologist visually estimates the fraction of lobular hepatocytes containing macrovesicular lipid droplets.1 To diagnose non-alcoholic fatty liver disease (NAFLD), the recommended steatosis cut-off in American,2 European,3 and Asian-Pacific guidelines,4 as well as in textbooks,5 is 5%. Although pathologist scoring is generally the most concordant for macrovesicular steatosis as compared with other features of NAFLD, it is nevertheless subject to significant inter-rater variability and often graded using a four-point scale ranging from S0 to S3 (S0: <5%; S1: 5–33%; S2: 34–66%; S3: >66%).1
In lieu of histology, magnetic resonance (MR)-based techniques are increasingly used to measure liver fat accurately and non-invasively.6,7 Within this domain, in vivo proton MR spectroscopy (1H-MRS, later MRS) is the reference standard, as it enables direct calculation of the tissue proton density fat fraction (PDFF) from signal intensities of spectral peaks originating from mobile protons in hepatic triacylglycerols and water.7 However, as MRS requires specialised equipment and expertise to both acquire and analyse spectral data, it has in part been superseded by MR imaging (MRI)-based indirect quantification of PDFF.6 A recent meta-analysis with 23 studies and 1,679 patients showed MRS-PDFF and MRI-PDFF to be essentially in complete agreement, with an R2 of 0.96 between the modalities.8
In subjects of the Dallas Heart Study without a liver biopsy, the upper limit of normal for liver fat by MRS-PDFF was considered 5.56%—a cut-off closely approximating the histological definition of NAFLD.9 However, the exact relationship between PDFF and histologically determined steatosis fraction remains enigmatic. Although there generally exists a high correlation between PDFF and histology, use of crude scoring systems instead of more granular pathologist-reported steatosis fractions in most comparative studies has obscured their numerical relationship.[10], [11], [12], [13], [14], [15] Importantly, the theoretical basis of the methods suggests them to be fundamentally different. PDFF measures the volumetric tissue concentration of triacylglycerol, calculated as the ratio of MR-visible triacylglycerol protons to the sum of protons in triacylglycerol and water.7 On the other hand, pathologists estimate on the proportion of hepatocytes containing macrovesicular lipid droplets, out of all hepatocytes within a histological cross-section. While previous authors have acknowledged these differences, the likely effect on the methods’ concordance has not been systematically examined.7,[16], [17], [18], [19] Additionally, as MRS and MRI probe the liver without discriminating signal from different cell types, the sole consideration of hepatocytes by pathologists may act as an additional confounder. To the best of our knowledge, the proportion of hepatocytes out of all cells in human liver tissue remains undetermined.
With the increasing popularity of PDFF, knowledge by clinicians as to how it corresponds to histological steatosis fraction is important to prevent misjudgement of the clinical status or treatment effect in patient care. Most guidelines and expert recommendations on non-invasive assessment of NAFLD have, however, failed to acknowledge the potential differences between these key methods of steatosis assessment.2,3,20 This may be because of the lack of studies formally comparing their characteristics in sufficiently large populations.
Our aim was to determine the degree of linearity and agreement between histological steatosis fraction and PDFF, across the full clinical spectrum of liver fat content associated with NAFLD. To this end, we performed a systematic review with meta-analytic assessment of pooled patient-level data, including unpublished data from our institution. Because we found the methods to be in considerable disagreement, we derived a conversion equation and correspondence thresholds for relating PDFF with histological steatosis. Finally, to explore the significance of the non-parenchymal hepatic cell fraction as a confounder of steatosis measurement, we determined the cell-type composition of human liver biopsies.
Materials and methods
Systematic review of the literature
Two investigators (SQ and HYJ) independently conducted a literature search to identify peer-reviewed articles and meeting abstracts of any language reporting associations between the pathologist-reported histological macrovesicular steatosis fraction and PDFF. We considered studies using either MRS or confounder-corrected chemical shift-encoded MRI, as the methods provide essentially identical measures of PDFF.8 Expert recommendations for appropriate confounder correction in PDFF acquisition have been published elsewhere.7 The target population was adults undergoing a liver biopsy either because of suspected NAFLD or in conjunction with routine work-up of living liver donor candidates, with the exclusion of other primary liver diseases (see below). We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.21 An institutional review board approval was not required for this systematic review. The review protocol was not publicly registered.
Search strategy
The literature search consisted of three main concepts: (1) liver fat or fatty liver disease; (2) biopsy or histology; and (3) MRI or MRS.
The MEDLINE (via PubMed), CENTRAL (via the Cochrane Library), Embase (via Scopus), and Web of Science Core Collection databases were searched from database inception until 16 August 2022. The search was initially built in PubMed and was subsequently translated to other databases as accurately as possible. Controlled vocabulary was used where appropriate, supplemented with (truncated) keywords. A detailed electronic search strategy is provided in Table S1.
Identification of eligible studies
Search results were exported from each database and imported to EndNote version 20.2 (Clarivate, Philadelphia, PA, USA) for deduplication. The deduplicated reference library was then exported from EndNote to the Rayyan web application (Rayyan Systems Inc., Cambridge, MA, USA) for screening of titles and abstracts for potential eligibility by the lead author (SQ).22 Bibliographic data of the potentially eligible studies were again imported to EndNote for reviewing of full-text records. After identification of all the eligible studies, their reference lists were reviewed to identify additional reports for inclusion. Additionally, relevant systematic reviews and meta-analyses were tagged for subsequent review of their reference lists to identify additional reports.
Study selection
Studies were selected if they fulfilled the following inclusion criteria:
-
(1)
Study design: any controlled trials, comparative studies, and observational studies.
-
(2)
Target population: adults undergoing a liver biopsy because of suspected NAFLD or during work-up as living liver donor candidates.
-
(3)
Reference standard: a pathologist’s assessment of histological steatosis fraction in liver biopsies, defined as the fraction of hepatocytes containing macrovesicular lipid droplets (out of all hepatocytes).
-
(4)
Index tests: liver fat content measured by MRS/MRI-PDFF within 180 days (on average) of undergoing liver biopsy.
In addition, the following exclusion criteria were exercised:
-
(1)
Not reporting data on associations between histological steatosis and PDFF.
-
(2)
Studies conducted in paediatric populations, with animals, or ex vivo.
-
(3)
Studies including fewer than 10 subjects.
-
(4)
Studies including patients with primary liver diseases other than NAFLD or with liver cancer or metastases, and studies with insufficient reporting to ascertain correct target population. Studies including patients with other primary liver diseases were considered if data for patients with NAFLD could be extracted separately.
-
(5)
Ordinal reference standard (i.e. steatosis grade instead of macrovesicular steatosis fraction) or incorrect index test, or insufficient reporting to ascertain eligibility.
-
(6)
Insufficient characterisation of the study population (at least the number of males/females, mean age, and mean BMI should be reported).
Data extraction
The lead author (SQ) extracted the following study-level data: author, year, country, study design, and index test. Regarding patient-level data, we extracted information about the target population, number of participants, sex distribution, mean age, mean BMI, histological diagnoses, and the average interval between imaging and biopsy. Additionally, we extracted the following information regarding the index test: scanner manufacturer, field strength, repetition time, echo time, number of echoes, number of voxels/regions of interest, dimensionality (for MRI), reconstruction method (for MRI), and pulse sequence (for MRS).
A requirement for study inclusion was access to individual patient-level data for histological and MR-based liver fat measurements. Corresponding authors of the selected studies were contacted by e-mail to request raw data for this meta-analysis, and the authors were given 60 days to respond. If no response was received within this timeframe, we digitised the data from published figures.
Quality and risk-of-bias assessment
We assessed the methodological quality and risk-of-bias of the included studies using the QUADAS-2 tool.23 With QUADAS-2, methodological quality is assessed across four domains: (1) patient selection; (2) index test; (3) reference standard; and (4) flow and timing. The tool was appropriately tailored for use in this systematic review. Because of the known poor inter-rater agreement in macrovesicular steatosis assessment,24 risk-of-bias for the reference standard was deemed high unless the study utilised a consensus reading of at least two pathologists.
The Helsinki University Hospital MRS-PDFF cohort
In the present meta-analysis, we included unpublished data from 71 eligible individuals who were studied at our institution. Detailed methodology regarding the Helsinki MRS-PDFF cohort is described in the Supplementary material, and clinical characteristics are shown in Table S2.
Hepatic cell-type composition analysis
To determine the fractional contributions of different cell types in human liver tissue, we used an RNA-seq-based computational approach (CIBERSORTx) and a previously published human liver single-cell RNA-seq dataset in a liver biopsy cohort consisting of 138 patients.25,26 The methods are described in the Supplementary material, and characteristics of the cohort are shown in Table S3.
Statistical methods
Analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) or GraphPad Prism version 9.3.1 (GraphPad Software, San Diego, CA, USA) for macOS. The R package ‘meta’ version 5.2-0 was used to derive all meta-analytic estimates,27 and the package ‘lme4’ version 1.1-28 was used for mixed-effects modelling.28 Data are shown as means ± standard deviations, medians (25th–75th percentiles), or counts (percentages). We considered p values of ≤0.05 as statistically significant.
Evaluation of publication bias
We assessed the possibility of underlying publication bias and other small-study effects by using funnel plots. Effect estimates included Fisher’s z transformed Pearson correlation coefficients and their standard errors (the main measure of linearity), and proportional Bland–Altman bias estimates and their standard errors (the main measure of agreement). We evaluated funnel plot asymmetry using the Egger’s test.
Linearity between histological steatosis and PDFF
Using a two-stage approach, Pearson correlation coefficients derived for each individual study underwent meta-analytic assessment after Fisher’s z transformation using a random-effects model and inverse variance weighting. Test statistics and confidence intervals were adjusted by using the method of Hartung and Knapp.
Agreement between histological steatosis and PDFF
Agreement was assessed using a one-stage approach. Because of a non-constant relationship between the measures, non-linear regression was used to fit curves in Bland–Altman plots describing bias over the full range of liver fat content. To describe the average relationship between histological steatosis and PDFF, a linear mixed model was fit in the pooled dataset. Heteroscedasticity and non-normality of residuals was rectified via square root transformation of the variables. The curve fit was then back-transformed for display. Study effects were considered as random effects in all analyses.
Classifying histological steatosis grades by PDFF
We used receiver operating characteristic (ROC) analysis and area under the ROC curve (AUROC) for studying the discriminatory ability of PDFF for dichotomised histological steatosis grades (one-stage approach). Optimal rule-in thresholds were selected at the lowest value of PDFF to provide 90% specificity. For the selected thresholds, we calculated sensitivities, specificities, positive predictive values (PPV), negative predictive values (NPV), and their CIs. The AUROCs and performance parameters of the rule-in thresholds underwent 10-fold cross-validation to generate more robust, cross-validated parameters and their CIs.
Evaluation of heterogeneity and sensitivity analysis
We evaluated statistical heterogeneity using the I2 statistic obtained from meta-analysis of Pearson correlation coefficients, in combination with Cochran’s Q test. Additionally, heterogeneity was assessed in the pooled dataset using intraclass correlation coefficient, which was calculated based on the linear mixed model (see above). To evaluate different MR modalities as a potential source of between-study heterogeneity, we performed sensitivity analyses by assessing the relationship between histological steatosis and PDFF in subgroups stratified by the modality used (MRS or MRI).
Results
Study selection and risk-of-bias assessment
Fig. S1 shows the PRISMA flow diagram for study selection. We identified 3,094 potentially eligible records, which underwent screening for titles and abstracts. Out of the 293 records that finally underwent full-text screening, eight were eligible. Of these studies, two compared histology with MRI-PDFF (n = 159) and six with MRS-PDFF (n = 386). We additionally included unpublished data from 71 eligible individuals studied at our institution (the Helsinki MRS-PDFF cohort; see Materials and methods). Table 1 shows the characteristics of the studies included, and details regarding the MR protocols are shown in Table S4. The nine studies comprised 616 individuals (334 [54.2%] males, 282 [45.8%] females) out of which 19 had missing data (Pavlides et al.,30 n = 3 because of unavailable MRS-PDFF and n = 3 as a result of unreported macrovesicular steatosis; Hwang et al.,34 n = 12 and Parente et al.,35 n = 1 for unknown reasons). The final dataset comprised 597 unique subjects.
Table 1.
Characteristics of the included studies.
| Author, year, country, ref. | Index method | Study design | Target population | Number of participants (m/f) | Patient demographics | Histological diagnosis | Interval between imaging and biopsy |
|---|---|---|---|---|---|---|---|
| Qadri, 2022, Finland∗ | MRS-PDFF | Prospective | Patients undergoing liver biopsy to evaluate NAFLD during metabolic surgery | 21/50 | Age: 52 ± 11 yr BMI: 37.6 [32.9, 41.2] kg/m2 |
No NAFLD: 23 NAFL: 29 NASH: 19 |
7.2 [2.8, 15.7] d |
| Runge, 2018, The Netherlands29 | MRS-PDFF | Prospective | Patients undergoing liver biopsy because of suspected NAFLD | 40/15 | Age: 52.3 [43.7, 57.6] yr BMI: 27.8 [26.0, 33.1] kg/m2 |
No NAFLD: 5 NAFL: 30 NASH: 20 |
27 [7, 44] d |
| Pavlides, 2017, UK30 | MRS-PDFF | Prospective | Patients with known or suspected NAFLD undergoing liver biopsy | 43/28 (65)† | Age: 53 ± 12 yr BMI: 32.7 [28.1, 38.1] kg/m2 |
NAFL: 25 NASH: 46 |
13 [5, 27] d |
| Traussnigg, 2017, Austria31 | MRS-PDFF | Prospective | Patients undergoing liver biopsy because of suspected NAFLD | 18/12 |
Patients with NAFL: Age: 48.0 ± 9.6 yr BMI: 27.3 ± 5.2 kg/m2 Patients with NASH: Age 48.0 ± 12.5 yr BMI 31.4 ± 4.1 kg/m2 |
NAFL: 8 NASH: 22 |
Performed on the same day |
| Rastogi, 2016, India32 | MRS-PDFF | Retrospective | Living liver donor candidates undergoing preoperative or intraoperative liver biopsy | 59/14 |
Males: Age: 33 (20–55) yr BMI: 24.6 (17.2–34.8) kg/m2 Females: Age: 33 (19–55) yr BMI: 24.7 (17.9–29.8) kg/m2 |
No NAFLD: 39 NAFL: 34 |
≤20 d |
| Tang, 2015, USA33 | MRI-PDFF | Prospective | Patients with known or suspected NAFLD undergoing liver biopsy | 38/51 | Age: 51.0 ± 13.0 yr BMI: 30.6 ± 5.0 kg/m2 |
No NAFLD: 6 NAFLD: 83 |
Median 35 (range 0–173) d |
| Hwang, 2014, Republic of Korea34 | MRS-PDFF | Retrospective | Living liver donor candidates undergoing preoperative or intraoperative liver biopsy | 62/22 (72)† | Age: 33 (17–61) yr BMI: 24.1 (17.1–31.5) kg/m2 |
No NAFLD: 59 NAFLD: 25 |
13 (0–55) d |
| Parente, 2014, Brazil35 | MRS-PDFF | Prospective | Patients with type 2 diabetes undergoing liver biopsy because of suspected NAFLD | 13/60 (72)† | Age: 54 ± 9 yr BMI: 31.4 (23.2–42.7) kg/m2 |
No NAFLD: 6 NAFL: 40 NASH: 27 |
≤90 d |
| Idilman, 2013, Turkey36 | MRI-PDFF | Retrospective | Patients undergoing liver biopsy because of suspected NAFLD | 40/30 | Age: 44.7 ± 13.1 yr BMI: 29.9 ± 4.3 kg/m2 |
No NAFLD: 7 NAFLD: 63 |
Median 14.5 (range 0–259) d |
Unless otherwise specified, data are shown as means ± standard deviations, means (range), medians [25th, 75th percentiles], or as counts.
d, days; f, females; m, males; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NAFL, non-alcoholic fatty liver; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PDFF, proton density fat fraction.
Previously unpublished data from the Helsinki MRS-PDFF cohort (see Materials and methods and Supplementary material).
Number of participants with complete data.
Most studies had a low risk of bias regarding flow and timing, index test, and patient selection (Fig. S2 and Table S5). However, reference standard risk-of-bias was deemed high for seven studies, as only Pavlides et al.30 used consensus histological readings by two pathologists. Funnel plots of Pearson correlation coefficients and Bland–Altman bias estimates were symmetric and did not point to significant underlying small-study effects, with respective Egger’s test p values of 0.28 and 0.28 (Fig. S3).
The relationship between histological steatosis and PDFF is highly linear
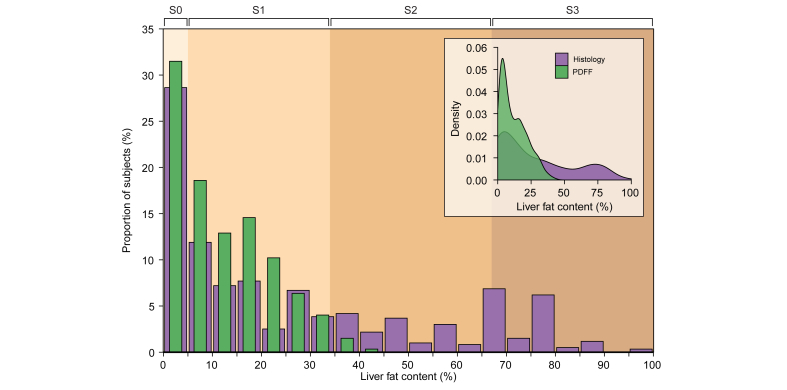
Fig. 1 shows the distribution of all histological and PDFF liver fat measurements in the pooled dataset. Histological steatosis ranged from 0% to 100%, whereas PDFF was distributed within a significantly narrower range and varied from 0% to 42.8%. Both distributions were positively skewed and had a numerically similar skewness and kurtosis (data not shown).
Fig. 1.
Distribution of liver fat measurements by histology and PDFF.
Distribution of liver fat values in the pooled dataset of nine studies (N = 597). Purple bars denote histological steatosis, and the superimposed green bars denote PDFF. The colour-shaded background of the plot illustrates division of the x-axis into histological steatosis grades S0–S3 (S0: <5%; S1: 5–33%; S2: 34–66%; S3: >66%). The inset shows a density plot using the same data, depicting the distribution of histological steatosis and PDFF on a continuous scale (probability density function). The purple distribution denotes histology, and the green distribution denotes PDFF. PDFF, proton density fat fraction.
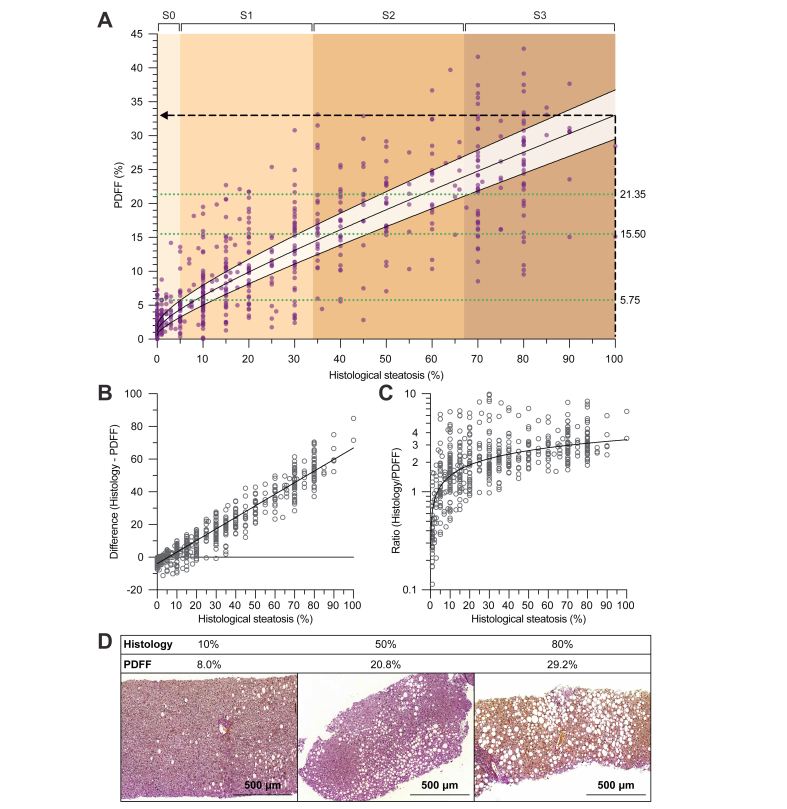
Fig. 2A shows the relationship between histological steatosis and PDFF. Except for at the lower end of liver fat content (approximately 0–10% by histology), PDFF increased highly linearly as a function of histological steatosis. The individual studies also demonstrated a considerably linear relationship, with Pearson correlation ranging from 0.72 to 0.92 (Fig. S4). Meta-analytic assessment of correlation coefficients yielded a combined estimate of 0.85 (95% CI, 0.80–0.89) (Fig. S5).
Fig. 2.
Relationship between histological steatosis fraction and PDFF.
(A) Association between histological steatosis and PDFF in the pooled dataset of nine studies (N = 597). The best-fit line was determined using a linear mixed model, with study effects considered as random effects. Both variables underwent square root transformation before model fitting, and the curve fit was then back-transformed for display. The shaded area around the curve denotes 95% CI. The colour-shaded background of the plot illustrates division of the x-axis into histological steatosis grades S0–S3 (S0: <5%; S1: 5–33%; S2: 34–66%; S3: >66%). The horizontal dotted green lines denote optimal rule-in thresholds for PDFF to predict dichotomised steatosis grades at 90% specificity (see Table 2). The dashed black lines are drawn for illustrative purposes. (B) Bland–Altman plots showing the absolute differences and (C) ratios between histological steatosis and PDFF, as a function of histological steatosis. The best-fit lines were determined using linear regression, and variables in (C) underwent logarithmic transformation before model fitting (the curve fit was then back-transformed for display). (D) Representative liver histological sections of three individuals in the Helsinki MRS-PDFF cohort. Above each image, the corresponding pathologist-reported histological steatosis fraction and PDFF are shown. Histological sections of formalin-fixed and paraffin-embedded liver biopsies underwent Herovici staining and digitisation using Pannoramic Scan 150 (3DHISTECH Ltd.; Budapest, Hungary). The images were acquired at 10 × magnification. PDFF, proton density fat fraction.
The general relationship between histological steatosis and PDFF in the pooled dataset was best described by a square root function, using the following equation (see curve in Fig. 2A):
Corresponding values of liver fat by PDFF are markedly lower as compared with histology
At nearly every value of steatosis by histology, the corresponding PDFF was considerably lower (Fig. 2A). The histological diagnostic threshold for NAFLD at 5% represented an important inflection point below which PDFF exceeded histology and, above this point, values of PDFF were lower (Fig. 2A). At 5% histological steatosis, average PDFF was 4.4% (95% CI, 3.2–5.7%). Absolute differences between the measures increased linearly as a function of liver fat content (Fig. 2B). Relative differences increased sharply up to approximately 10–20% histological steatosis and remained more constant at higher degrees of liver fat, with histological steatosis exceeding PDFF by up to 3.4-fold (Fig. 2C). On average, 100% histological steatosis corresponded to a PDFF of 33.0% (95% CI, 29.5–36.7%) (Fig. 2A). Fig. 2D shows representative histological sections from three individuals with corresponding pathologist-reported and PDFF liver fat values.
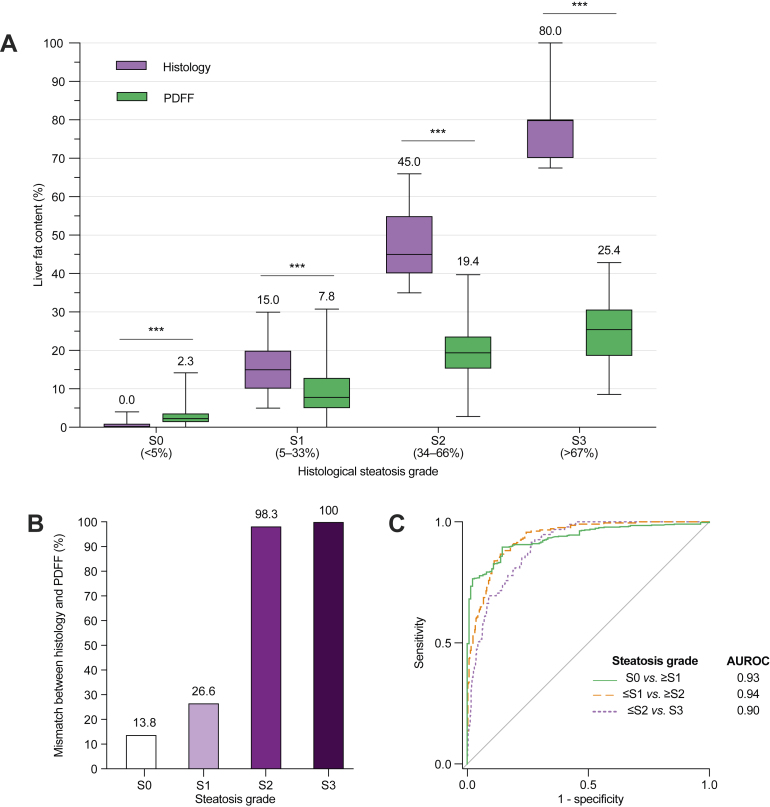
Use of PDFF to classify steatosis grades requires distinct thresholds
Steatosis grades S0–S3 are frequently used to quantify histological liver fat (S0: <5%; S1: 5–33%; S2: 34–66%; S3: >66%). Consistent with our findings above, PDFF was significantly higher compared with histological steatosis fraction in individuals with grade S0, while being significantly lower in subjects with grades S1 to S3 (Fig. 3A). Median PDFF values in individuals with histological steatosis grades S0, S1, S2, and S3 were 2.3%, 7.8%, 19.4%, and 25.4%, respectively (Fig. 3A). In accordance, use of PDFF to predict steatosis grades with the thresholds that are commonly used for histology led to a gross mismatch between the actual and predicted steatosis grades, especially for individuals with grades S2–S3 (Fig. 3B and Table S6).
Fig. 3.
Relationship between histological steatosis grades and PDFF.
(A) Distribution of histological steatosis fraction (purple boxes) and PDFF (green boxes) with respect to histological steatosis grades S0–S3. Horizontal lines within the boxes denote medians and whiskers denote minimum and maximum values. The Mann-Whitney U test was used. ∗∗∗p ≤0.001. (B) Proportion of individuals with steatosis grade mismatch between histology and PDFF, when PDFF is interpreted with the same grading thresholds that are conventionally used for histology (S0: <5%; S1: 5–33%; S2: 34–66%; S3: >66%). (C) Receiver operating characteristic (ROC) curves for PDFF to classify the subjects into dichotomised histological steatosis grades. Areas under the ROC curves (AUROC) are shown. PDFF, proton density fat fraction.
Despite the significant disagreement between histological steatosis and PDFF in terms of absolute values, ROC analysis revealed a remarkably high discriminatory ability for PDFF to classify dichotomised steatosis grades (Fig. 3C). Cross-validated AUROCs (± standard errors) were 0.94 ± 0.02 for S0 vs. S1–S3, 0.94 ± 0.03 for S0–S1 vs. S2–S3, and 0.91 ± 0.04 for S0–S2 vs. S3. Targeting at a specificity of 90%, optimal PDFF rule-in thresholds to classify steatosis grades were ≥5.75% for S1 or higher (i.e. a diagnosis of NAFLD), ≥15.50% for S2 or higher (moderate-to-severe steatosis), and ≥21.35% for S3 (severe steatosis). Table 2 shows cross-validated diagnostic performance parameters for these thresholds in the pooled dataset. Raw performance parameters, and additional rule-in and rule-out thresholds for 90/95% sensitivity/specificity, are shown in Table S7.
Table 2.
Thresholds and 10-fold cross-validated diagnostic performance parameters for PDFF to predict dichotomised histological steatosis grades at 90% specificity in the pooled cohort.
| Steatosis grade classification | Threshold | Se, % (95% CI) | Sp, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| S0 vs. S1–S3 | ≥5.75 | 79.5 (77.2–81.8) | 90.1 (85.7–96.1) | 96.6 (94.8–98.4) | 57.1 (52.9–61.2) |
| S0–S1 vs. S2–S3 | ≥15.50 | 78.8 (73.6–83.9) | 90.1 (87.2–93.1) | 81.7 (77.0–86.4) | 88.6 (85.7–91.5) |
| S0–S2 vs. S3 | ≥21.35 | 69.0 (59.9–78.1) | 90.0 (88.6–91.5) | 56.7 (52.8–60.5) | 94.0 (92.5–95.6) |
NPV, negative predictive value; PDFF, proton density fat fraction; PPV, positive predictive value; Se, sensitivity; Sp, specificity.
Between-study heterogeneity and sensitivity analysis
The included studies demonstrated a moderate-to-substantial degree of heterogeneity with respect to observed linearity between histological steatosis and PDFF (I2 = 67.0% [95% CI, 33.3–83.7%], p <0.01; Fig. S5). In the linear mixed model of pooled data (Fig. 2A), the proportion of variance attributable to between-study differences in the relationship between histological steatosis and PDFF was 28.9% (intraclass correlation coefficient). Regression lines fit to individual study data showed variable slopes, but this variability was random across the different MR modalities (MRS or MRI) (Fig. S6). In a sensitivity analysis, the data for MRS-PDFF and MRI-PDFF showed a complete overlap, with best-fit lines having a near-identical association with histological steatosis (Fig. S7). Thus, heterogeneity likely originated from interrater variability related to histological steatosis assessment.
The non-hepatocyte cell fraction as a potential confounder of liver fat measurement
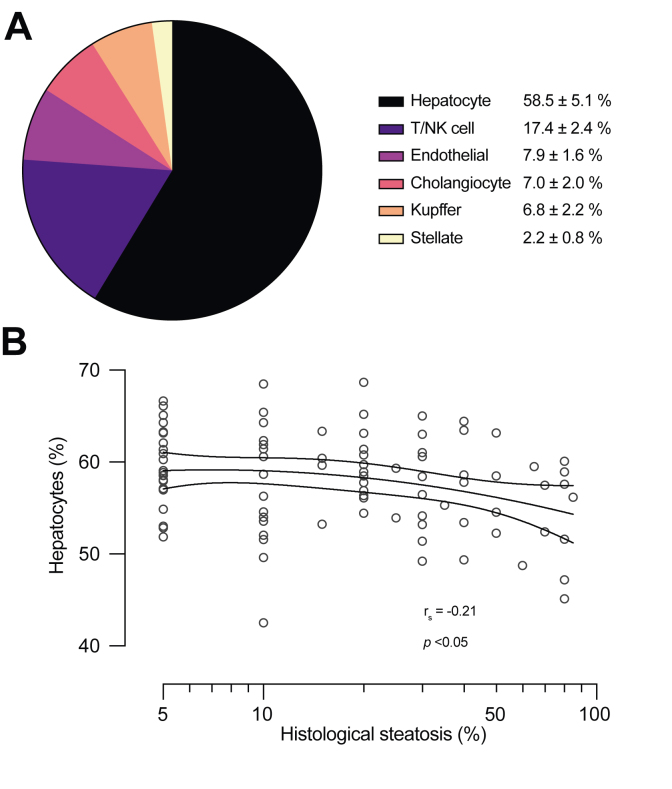
To determine whether a significant non-hepatocyte cell fraction may act as a confounder with respect to liver fat measurement by histology vs. PDFF, we determined the size of this fraction in liver biopsies from 138 individuals. The RNA-seq-based analysis of hepatic cell-type composition identified six distinct cell populations. The average proportion of hepatocytes was 58.5 ± 5.2% (Fig. 4A), and the fraction of hepatocytes had a significantly negative correlation with liver fat content (rs = -0.21, p <0.05) (Fig. 4B). This finding provides one explanation as to why PDFF values are lower compared with histopathology, as the latter only considers hepatocytes in deriving the steatosis fraction.
Fig. 4.
The human liver cell-type composition.
(A) Average proportions of the major hepatic cell-type fractions, as determined by the RNA-seq-based CIBERSORTx analysis in 138 human liver biopsies. Data are shown as mean ± SD. (B) Association between histological steatosis and the fraction of hepatocytes in human liver biopsies. Regression line was fit using a quadratic model after log-transforming the liver fat fraction. The dashed lines denote 95% CI. The Spearman correlation coefficient is shown. NK, natural killer.
Discussion
We pooled patient-level measurements of liver fat assessed by histology and PDFF from 597 individuals across nine studies. Our principal finding was that, as a function of steatosis, both absolute and relative differences between the two methods increased markedly. Compared with histological steatosis fraction, values of PDFF for the same individuals were in general significantly lower (Fig. 2A–D). The highest recorded value of histological steatosis was 100%, whereas the highest PDFF was only 42.8%. This was despite the methods having considerable (albeit non-perfect) linearity and seemingly measuring liver fat content in the same units, that is, percentages.
PDFF slightly exceeded histological steatosis in the lowest range of liver fat below 5% (Fig. 3A). In the normal human liver without histologically visible lipid droplets, biochemically measured triacylglycerols constitute 2–6% of wet tissue weight.[37], [38], [39] This amount of lipid is quantifiable by PDFF but would be invisible to the pathologist. Thus, as we observed, PDFF would predictably be higher in the <5% range. At 5% liver fat, which fortuitously is the histological diagnostic threshold for NAFLD, histology and PDFF approximately coincided. Above the inflection point of 5%, however, histological steatosis was consistently and up to over threefold higher. On average, 100% steatosis by histology corresponded to a PDFF of 33%.
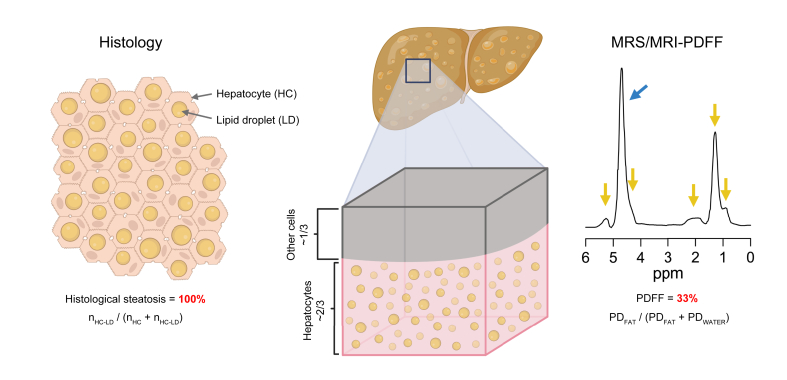
Fig. 5 illustrates how the principles underlying liver fat assessment by histology and PDFF are fundamentally different. The pathologist visualises a histological cross-section and estimates the proportion of macrovesicular lipid droplet-containing hepatocytes out of all hepatocytes, which can range from 0% to 100%. This scale is inherently semi-quantitative and disregards changes in size of the lipid droplets. In contrast, PDFF quantifies fat within a sampled liver volume, based on the measured density of mobile protons in fatty acids out of the total mobile proton densities of fatty acids and water (Fig. 5). Protons originating from membrane lipid-incorporated fatty acids are opaque to MR, and thus the MR-visible fat-attributable protons mainly represent triacylglycerols.40 Because the denominator in PDFF includes tissue water residing in all cells and within the extracellular space—and because excess triacylglycerol only accumulates inside of hepatocytes—liver PDFF should never reach 100%. The highest PDFF of 42.8% in the present analysis is similar to the maximum of 47.5% reported in the Dallas Heart Study with 2,287 individuals.9 Even in the most severe cases of fatty liver in which most or all hepatocytes contain macrovesicular lipid droplets in histology, biochemically measured lipid content rarely exceeds 40%.37
Fig. 5.
Simplified illustration of key differences in liver fat measurement between histology and PDFF.
In fatty liver disease, lipid droplets accumulate in the cytoplasm of hepatocytes, which represent the principal tissue cell type. However, cells other than hepatocytes comprise approximately one-third of the total hepatic cell population (Fig. 4A). In histological analysis of steatosis, a pathologist visually estimates liver fat as the fraction of macrovesicular lipid droplet-containing hepatocytes (nHC-LD), out of all visible hepatocytes (nHC + nHC-LD) within a histological cross-section. A 100% steatosis is reached when all the visible hepatocytes contain macrovesicular lipid droplets (exemplified on the left). However, liver fat as measured by MRS/MRI-PDFF is calculated by dividing the MR-visible proton density of tissue fat (PDFAT) by the sum of proton densities of tissue fat and water (PDFAT + PDWATER). PDFF takes into account the spectral complexity of fat, including the smaller fat peaks (arrows in yellow) relative to that of water (arrow in blue). In the present study, 100% histological steatosis corresponded to an average PDFF of 33%. A representative MR spectrum from a patient with a PDFF of 33% is shown on the right. In addition to hepatocytes, the tissue volume fraction probed by MRS/MRI contains other cell types as well as components of the extracellular matrix, influencing the MR-visible water-to-fat ratio and presumably contributing to the numerical difference between PDFF and histological steatosis. HC, hepatocyte; LD, lipid droplet; MR, magnetic resonance; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; PD, proton density; PDFF, proton density fat fraction.
In addition to hepatocytes, the hepatic volume fraction probed by MRS and MRI contains a variety of other cell types, which also contain water and presumably affect PDFF by contributing to the denominator. Using a state-of-the-art RNA-seq method to estimate the human liver cell-type composition, we found that hepatocytes comprised less than 60% of all cells on average (Fig. 4A). Although this analysis discounts volume differences between cells (hepatocytes are among the largest hepatic cells) and extracellular water was not measured, the high proportion of non-parenchymal cells may partly explain the discrepancy between histological steatosis and PDFF. Interestingly, and despite the low prevalence of advanced liver fibrosis in the RNA-seq cohort, higher liver fat was associated with a slight but significant decrease in the proportion of hepatocytes (Fig. 4B). This finding is novel and may point to an early degradation of hepatocyte viability already in the initial stages of NAFLD.
Owing to poor agreement between the absolute values of PDFF and histological steatosis, the standard thresholds to classify steatosis grades were unapplicable for PDFF (Fig. 3B). We successfully derived optimal thresholds for PDFF to classify dichotomised histological steatosis grades (Table 2 and Table S7). The PDFF rule-in threshold to predict steatosis grade ≥S1 (i.e. histological steatosis ≥5%, or NAFLD) at a specificity of 90.1% and PPV of 96.6% was ≥5.75%. This finding is in line with the currently widely adopted PDFF definition of ≥5.56% for NAFLD, which was derived in the population-based Dallas Heart Study without liver histology information, based on the 95th percentile PDFF in normal-weight individuals without a history of liver disease or metabolic risk factors.9 Comparable albeit variable PDFF thresholds have been found previously in small NAFLD liver biopsy cohorts.[14], [15], [16],29,33 Our large multi-centre analysis is the first to provide robust and likely well-generalisable estimates. It is, however, challenging to accurately define the upper limit of normal for PDFF. Use of pathologists’ interpretation as the reference standard is problematic, as inter-rater variability likely introduces some bias in all estimates.24 The relationship between histology and PDFF was also less linear in the 0–10% range (Fig. 2A). An alternative approach could be to determine a level of PDFF associated with a clinically significant increase in adverse liver-related outcomes.
The main limitation of this study relates to methodological variability in liver fat assessment. Compared with histology, PDFF represents an inherent physical tissue property, is observer-independent, and is measured within a much larger volume compartment. It does, however, lack standardisation, as is evident from variability in the reported MR protocol-related parameters (Table S4). We carefully examined the MR protocols of each study to ascertain that the most important sources of bias were likely accounted for.7 In a sensitivity analysis, MRS-PDFF and MRI-PDFF showed strikingly concordant results (Fig. S7), which is in keeping with the meta-analysis by Yokoo et al.8 Moreover, PDFF has been found to be consistent across different imaging centres, scanner manufacturers, field strengths, and reconstruction methods.41 Individual-related factors such as age, sex, or BMI do not significantly influence PDFF quantification.42
Histological assessment of steatosis is subjective and inherently semiquantitative, bearing several well-known limitations such as inter-rater error and the biopsy-associated sampling error.43,44 Across the included studies, liver histology was analysed by nine different pathologists. This likely introduced the greatest degree of bias in our analysis by manifesting as between-study heterogeneity. In the pooled data, PDFF exhibited moderate variance at each degree of histological steatosis (Fig. 2A), which was less pronounced at the individual-study level (Fig. S4). Despite of this variability, differences between the two methods consistently increased as a function of liver fat in both absolute (Fig. 2B) and relative (Fig. 2C) terms. This phenomenon was readily observable in all individual study data (Fig. S4). In recent years, digital image analysis of histology has gained popularity in quantifying steatosis, especially in clinical trials.45 These methods usually quantify steatosis as the percentage image area occupied by lipid droplets and are thereby expected to deviate from the semi-quantitative assessment by pathologists. Because computerised analysis eliminates human variability, it would likely render the relationship of histological steatosis and PDFF more comparable across different centres. Future studies should investigate whether this is the case and determine the linearity and agreement between PDFF and image analysis-acquired histological steatosis fraction.
Given that histological steatosis and PDFF share a similar diagnostic threshold for NAFLD, what, then, are the clinical implications of our findings? In longitudinal studies with registry-based outcome data, the only baseline feature of NAFLD consistently predicting liver-related mortality is fibrosis.46 However, paired-biopsy studies have shown that the higher the degree of liver fat is at baseline, the more likely is fibrosis onset or progression during follow-up.[47], [48], [49] On the other hand, a ≥30% decrease in PDFF predicts fibrosis regression, which may be a useful marker in cases where liver biopsy is not clinically indicated and non-invasive measures of fibrosis, such as MR elastography, are unavailable.50 Therefore, steatosis, while perhaps not prognostic by itself, is a relevant predictor of disease progression and regression. We found that disregarding the differences between PDFF and histology would lead to a gross misclassification of especially those patients with severe steatosis. The future clinician is likely to be confronted with information from different types of exams, as liver biopsy and PDFF may be used in parallel or sequentially during diagnosis and follow-up. This adds a layer of complexity in clinical decision-making. For example, if PDFF is used to assess treatment effect after an initial liver biopsy, lack of consideration of methodological differences may lead to an illusion of significant improvement in liver fat. However, if biopsy and imaging were performed in parallel, their results could appear conflicting. Future guidelines for NAFLD should emphasise that histology and PDFF represent fundamentally different methods of liver fat quantification, while underlining that the former may yield values in excess of three times higher. This is important considering the near-term increase in the use of MRI-PDFF in particular, in routine patient care.
Financial support
HYJ was supported by grants from the Academy of Finland (no. 309263), the Novo Nordisk Foundation, and the Sigrid Jusélius Foundation. SQ was supported by grants from the Orion Research Foundation, the Yrjö Jahnsson Foundation, the Maud Kuistila Memorial Foundation, the Emil Aaltonen Foundation, and the Finnish Medical Foundation. TT was supported by grants from the Academy of Finland (no. 315589 and no. 320129) and the Sigrid Jusélius Foundation. RL receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019). AT was supported by a Senior Clinical Research Scholarship from the Fonds de recherche du Québec en Santé and Fondation de l'association des radiologistes du Québec Clinical Research Scholarship (FRQS-ARQ #298509). MP acknowledges support from the Oxford NIHR Biomedical Research Centre.
Authors’ contributions
Designed the study, performed the literature review, and screened records for inclusion: SQ, HYJ. Acquired data for the meta-analysis, interpreted the data, and wrote the manuscript: SQ. Performed statistical analysis: SQ, ML. Performed RNA-seq analysis: EV, TT. Performed clinical investigation: KP, PA. Performed liver biopsies: AJ, AKP. Analysed liver histology: JA. Analysed and interpreted magnetic resonance data: JD, TEL, WS. Provided data for the United States cohort: AT, RL, CBS. Provided data for the United Kingdom cohort: MP. Provided data for the Turkey cohort: ISI, MK. Provided data for the Netherlands cohort: JHR, JS. Supervised the study: HYJ. Critically revised the manuscript draft for important intellectual content and approved the final manuscript: all authors.
Data availability statement
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding authors on reasonable request.
Conflict of interest
RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharm, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics. In addition, RL’s institutions received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Pfizer, Sonic Incytes, and Terns Pharmaceuticals. RL is a co-founder of LipoNexus Inc. CBS reports research grants from ACR, Bayer, GE, Gilead, Pfizer, Philips, Siemens; equipment loans from GE; lab service agreements with Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva, Takeda; institutional consulting for BMS, Exact Sciences, IBM-Watson, Pfizer; Personal consulting for Blade, Boehringer, Epigenomics, and Guerbet; receipt of royalties and/or honoraria from Medscape and Wolters Kluwer; ownership of stock options in Livivos; unpaid advisory board position in Quantix Bio. CBS serves as Chief Medical Officer for Livivos (unsalaried position). Unrelated to this paper, MP declares stock ownership in Perspectum Ltd. All other authors state that no conflicts of interest regarding this manuscript exist.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We gratefully acknowledge the volunteers for their help and thank Aila Karioja-Kallio and Päivi Ihamuotila for their excellent technical assistance. Fig. 5 was created with BioRender.com. Open access funded by Helsinki University Library.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100928.
Contributor Information
Sami Qadri, Email: sami.qadri@helsinki.fi.
Hannele Yki-Järvinen, Email: hannele.yki-jarvinen@helsinki.fi.
Supplementary data
The following are the supplementary data to this article:
:
References
- 1.Tiniakos D.G., Vos M.B., Brunt E.M. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N., Younossi Z., Lavine J.E., et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Eslam M., Sarin S.K., Wong V.W., et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 5.Burt A.D., Portmann B.C., Ferrell L.D. 5 edn. Churchill Livingstone/Elsevier; London: 2007. MacSween’s Pathology of the liver. [Google Scholar]
- 6.Caussy C., Reeder S.B., Sirlin C.B., et al. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018;68:763–772. doi: 10.1002/hep.29797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeder S.B., Cruite I., Hamilton G., et al. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoo T., Serai S.D., Pirasteh A., et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta-analysis. Radiology. 2018;286:486–498. doi: 10.1148/radiol.2017170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szczepaniak L.S., Nurenberg P., Leonard D., et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 10.Noureddin M., Lam J., Peterson M.R., et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loomba R., Neuschwander-Tetri B.A., Sanyal A., et al. Multicenter validation of association between decline in MRI-PDFF and histologic response in NASH. Hepatology. 2020;72:1219–1229. doi: 10.1002/hep.31121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPherson S., Jonsson J.R., Cowin G.J., et al. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol. 2009;51:389–397. doi: 10.1016/j.jhep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Permutt Z., Le T.A., Peterson M.R., et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease – MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park C.C., Nguyen P., Hernandez C., et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152:598–607.e592. doi: 10.1053/j.gastro.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middleton M.S., Heba E.R., Hooker C.A., et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology. 2017;153:753–761. doi: 10.1053/j.gastro.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang A., Tan J., Sun M., et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoo T., Shiehmorteza M., Hamilton G., et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258:749–759. doi: 10.1148/radiol.10100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeder S.B., Sirlin C.B. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2010;18:337–357. doi: 10.1016/j.mric.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoo T., Bydder M., Hamilton G., et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021;75:659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouzzani M., Hammady H., Fedorowicz Z., et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiting P.F., Rutjes A.W., Westwood M.E., et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 24.El-Badry A.M., Breitenstein S., Jochum W., et al. Assessment of hepatic steatosis by expert pathologists: the end of a gold standard. Ann Surg. 2009;250:691–697. doi: 10.1097/SLA.0b013e3181bcd6dd. [DOI] [PubMed] [Google Scholar]
- 25.Newman A.M., Steen C.B., Liu C.L., et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37:773–782. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacParland S.A., Liu J.C., Ma X.Z., et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balduzzi S., Rucker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates D., Machler M., Bolker B.M., et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 29.Runge J.H., Smits L.P., Verheij J., et al. MR spectroscopy-derived proton density fat fraction is superior to controlled attenuation parameter for detecting and grading hepatic steatosis. Radiology. 2018;286:547–556. doi: 10.1148/radiol.2017162931. [DOI] [PubMed] [Google Scholar]
- 30.Pavlides M., Banerjee R., Tunnicliffe E.M., et al. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int. 2017;37:1065–1073. doi: 10.1111/liv.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traussnigg S., Kienbacher C., Gajdosik M., et al. Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis. Liver Int. 2017;37:1544–1553. doi: 10.1111/liv.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rastogi R., Gupta S., Garg B., et al. Comparative accuracy of CT, dual-echo MRI and MR spectroscopy for preoperative liver fat quantification in living related liver donors. Indian J Radiol Imaging. 2016;26:5–14. doi: 10.4103/0971-3026.178281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang A., Desai A., Hamilton G., et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274:416–425. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang I., Lee J.M., Lee K.B., et al. Hepatic steatosis in living liver donor candidates: preoperative assessment by using breath-hold triple-echo MR imaging and 1H MR spectroscopy. Radiology. 2014;271:730–738. doi: 10.1148/radiol.14130863. [DOI] [PubMed] [Google Scholar]
- 35.Parente D.B., Rodrigues R.S., Paiva F.F., et al. Is MR spectroscopy really the best MR-based method for the evaluation of fatty liver in diabetic patients in clinical practice? PLoS One. 2014;9 doi: 10.1371/journal.pone.0112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idilman I.S., Aniktar H., Idilman R., et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767–775. doi: 10.1148/radiol.13121360. [DOI] [PubMed] [Google Scholar]
- 37.Gillman J., Gillman T. Grune & Stratton; New York: 1951. Perspectives in human malnutrition: a contribution to the biology of disease from a clinical and pathological study of chronic malnutrition and pellagra in the african. [Google Scholar]
- 38.Roldan-Valadez E., Favila R., Martinez-Lopez M., et al. In vivo 3T spectroscopic quantification of liver fat content in nonalcoholic fatty liver disease: correlation with biochemical method and morphometry. J Hepatol. 2010;53:732–737. doi: 10.1016/j.jhep.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Li M., Song J., Mirkov S., et al. Comparing morphometric, biochemical, and visual measurements of macrovesicular steatosis of liver. Hum Pathol. 2011;42:356–360. doi: 10.1016/j.humpath.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakumaki J.M., Kauppinen R.A. 1H NMR visible lipids in the life and death of cells. Trends Biochem Sci. 2000;25:357–362. doi: 10.1016/s0968-0004(00)01614-5. [DOI] [PubMed] [Google Scholar]
- 41.Hu H.H., Yokoo T., Bashir M.R., et al. Linearity and bias of proton density fat fraction as a quantitative imaging biomarker: a multicenter, multiplatform, multivendor phantom study. Radiology. 2021;298:640–651. doi: 10.1148/radiol.2021202912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heba E.R., Desai A., Zand K.A., et al. Accuracy and the effect of possible subject-based confounders of magnitude-based MRI for estimating hepatic proton density fat fraction in adults, using MR spectroscopy as reference. J Magn Reson Imaging. 2016;43:398–406. doi: 10.1002/jmri.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Younossi Z.M., Gramlich T., Liu Y.C., et al. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998;11:560–565. [PubMed] [Google Scholar]
- 44.Ratziu V., Charlotte F., Heurtier A., et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 45.Dinani A.M., Kowdley K.V., Noureddin M. Application of artificial intelligence for diagnosis and risk stratification in NAFLD and NASH: the state of the art. Hepatology. 2021;74:2233–2240. doi: 10.1002/hep.31869. [DOI] [PubMed] [Google Scholar]
- 46.Ekstedt M., Hagström H., Nasr P., et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 47.Ekstedt M., Franzen L.E., Mathiesen U.L., et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 48.Pais R., Charlotte F., Fedchuk L., et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 49.Ajmera V., Park C.C., Caussy C., et al. Magnetic resonance imaging proton density fat fraction associates with progression of fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155:307–310.e302. doi: 10.1053/j.gastro.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamaki N., Munaganuru N., Jung J., et al. Clinical utility of 30% relative decline in MRI-PDFF in predicting fibrosis regression in non-alcoholic fatty liver disease. Gut. 2022;71:983–990. doi: 10.1136/gutjnl-2021-324264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
:
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding authors on reasonable request.