Summary
Background
Pain is the leading cause of disability worldwide among adults and effective treatment options remain elusive. Data harmonization efforts, such as through core outcome sets (COS), could improve care by highlighting cross-cutting pain mechanisms and treatments. Existing pain-related COS often focus on specific conditions, which can hamper data harmonization across various pain states.
Methods
Our objective was to develop four overarching COS of domains/subdomains (i.e., what to measure) that transcend pain conditions within different pain categories. We hosted a meeting to assess the need for these four COS in pain research and clinical practice. Potential COS domains/subdomains were identified via a systematic literature review (SLR), meeting attendees, and Delphi participants. We conducted an online, three step Delphi process to reach a consensus on domains to be included in the four final COS. Survey respondents were identified from the SLR and pain-related social networks, including multidisciplinary health care professionals, researchers, and people with lived experience (PWLE) of pain. Advisory boards consisting of COS experts and PWLE provided advice throughout the process.
Findings
Domains in final COS were generally related to aspects of pain, quality of life, and physical function/activity limitations, with some differences among pain categories. This effort was the first to generate four separate, overarching COS to encourage international data harmonization within and across different pain categories.
Interpretation
The adoption of the COS in research and clinical practice will facilitate comparisons and data integration around the world and across pain studies to optimize resources, expedite therapeutic discovery, and improve pain care.
Funding
Innovative Medicines Initiative 2 Join Undertaking; European Union Horizon 2020 research innovation program, European Federation of Pharmaceutical Industries and Associations (EFPIA) provided funding for IMI-PainCare. RDT acknowledges grants from Esteve and TEVA.
Keywords: Core outcome sets, Delphi method, Acute pain, Transition from acute to chronic pain, Recurrent/episodic pain, Chronic pain
Research in context.
Evidence before this study
Core outcome sets (COS) have been developed for many health and pain-related conditions, but COS capturing shared experiences across pain conditions do not yet exist for the field of pain. We conducted a literature review to identify all articles that developed, updated, or implemented a COS for pain conditions or health conditions where pain is a major symptom in adults. Published articles were identified from PubMed (including Medline) and from the COMET database, and results of the literature review were used to inform the present Delphi study, which aimed to develop COS for: acute pain, acute to chronic pain, recurrent/episodic pain, and chronic pain.
Added value of this study
This study demonstrates consensus on pain domains for four overarching core outcome sets of acute, acute to chronic, recurrent/episodic, and chronic pain. These COS were developed with expert stakeholders across a range of disciplines, countries, and backgrounds, and uniquely included equal participation of people with lived experience (PWLE). The results of the study support outcome measures that are holistic in nature, covering biological, psychological, and social aspects of the pain experience.
Implications of all the available evidence
Clinicians and researchers should consider measuring these outcomes to holistically understand their patient's experiences with pain and gain an understanding of treatment outcomes. Further, the COS can facilitate data harmonization in research and clinical settings, which can spark improvements in scientific and clinical knowledge, which could improve future treatment approaches.
Introduction
Pain is the leading cause of disability worldwide, and negatively impacts individuals’ well-being and carries significant financial burden.1 The United Nations and international medical societies view access to effective pain management as a fundamental right. Yet, pain is grossly undertreated,2 causing public health challenges and ethical problems. Like the causes of pain themselves, reasons for its undertreatment are multifactorial. One contributing factor is the lack of rigorous tools to synthesize data across research and clinical practice, especially data encompassing the biopsychosocial nature of pain. Core outcome sets (COS) hold promise for improving data synthesis and subsequently identifying clinically meaningful, biopsychosocial treatment outcome measures.
COS of domains provide researchers with standardized outcome domains that should be assessed and reported in all clinical trials, studies and practices, in specific areas of health or health care.3 COS development entails a consensus process, which first identifies what to measure (i.e., domains/subdomains) and then identifies how they should be measured (i.e., patient-reported outcome measures; PROMs).3 COS can be used with additional outcome domains and offer the potential to boost rigor and generalizability by improving data harmonization across studies, facilitating meta-analyses, improving systematic reviews, reducing risks of bias in reporting, and using stakeholder consensus to increase the likelihood that measured outcomes are meaningful.3, 4, 5, 6 Numerous COS have been developed for specific pain conditions.7, 8, 9 However, pain categories (i.e., acute, transition from acute to chronic, episodic/recurrent, and chronic),2,10 which can differ in their biopsychosocial mechanisms and treatment approaches, are often not captured separately.
Although previous initiatives represent important efforts toward data harmonization within diagnostic areas, the heterogeneity across existing COS makes it difficult to compare treatment effects across pain conditions. This limits progress in several ways. First, chronic pain is increasingly understood as a standalone health condition with shared biopsychosocial mechanisms across separate diagnoses.10 Separating findings based on diagnosis risks missing the discovery of cross-cutting treatments that could broadly reduce the burden of pain. Second, the rate of chronic overlapping pain conditions (COPC) is high,11 so that the existing condition-specific COS might not fully capture the pain experience for a substantial proportion of individuals with COPC. Third, besides the considerable number of existing COS recommendations for chronic pain,12 other pain categories (e.g., acute pain) are underrepresented in COS development. Finally, existing COS for pain have variably engaged stakeholders13 – specifically people with lived experience (PWLE) of pain – and have inconsistently used a biopsychosocial perspective.14
To address these limitations, the Innovative Medicines Initiative (IMI)-National Institutes of Health (NIH) Transatlantic Emphasis Group on Research And Translation-to-care Efforts for Pain (INTEGRATE-Pain) Consortium15 aimed to develop COS of domains for pain. The specific objective and scope of this initiative was to develop consensus on stakeholder-driven COS across pain conditions within four categories (acute, transition from acute to chronic, recurrent/episodic, and chronic pain) for use with adults in clinical research and practice. Stakeholders included people with lived experience of pain (PWLE), researchers, clinicians, and other stakeholders (e.g., regulators, governmental agencies, payers) to best capture biopsychosocial dimensions of pain in the COS. The present COS initiative was unique in that we aimed to develop separate COS based on pain categories across the spectrum for acute to chronic pain. Given the unique experiences, mechanisms, and treatments needed to address each of the categories, we hypothesized that the resulting domains/subdomains for each pain category would be uniquely different.
Methods
An overview of the methods used in the present initiative is provided below. The Supplemental Materials contain detailed information about each step. Importantly, PWLE's perspectives were integrated across procedures. The study began in December 2020 and ended in January 2023.
Protocol and registry entry
The protocol was developed according to COMET Handbook recommendations3 and guidance from two advisory committees (ACs) (researchers and PWLE). The proposed COS was registered in the COMET database (https://www.comet-initiative.org/Studies/Details/2083) and is reported here according to COS-STAR standards.16
Study design and participants
The development of the COS included PWLE, researchers with pain research and/or COS experience, pain clinicians (e.g., physicians, psychologists, nurses, physiotherapists), pain health technology assessment (HTA) experts, representatives of related organizations, and national health policy staff from participating countries. These stakeholders were experienced in COS development, pain management, and/or the four defined categories on the pain continuum, and they represented many nationalities, largely from North America, Europe, and Oceania (due to the geographic makeup of existing COS studies and PWLE networks and advocacy groups). Stakeholders served on one of the initiative's ACs, attended and contributed to the virtual consensus meeting, and/or voted in the Delphi surveys. Respondents who voted in the Delphi process were identified through the SLR and snowball sampling. Researchers and PWLE who attended the pain domain meeting were asked to recruit respondents for the Delphi study, but PWLE were offered the opportunity to recruit more individuals from their networks to ensure equal representation among researcher/clinician perspectives and PWLE perspectives. See Supplemental Material (S1.1 and S1.2) for more information.
Ethics
An NIH Institutional Review Board (IRB) determined this initiative's activities did not qualify as research requiring protections for human participants (see 45 CFR 46 §46.104 subpart D5) and was exempt from IRB approval. Thus, informed consent was not required nor obtained. In compliance with the EU's General Data Protection Regulation, a data protection statement was shown to all voters, which indicated the plan for data storage, usage, and protection.
Procedures
Four separate COS were proposed for conditions within acute, transition from acute to chronic, recurrent/episodic, and chronic pain. These pain categories and their definitions were further informed by the International Association for the Study of Pain17 (https://www.iasp-pain.org/resources/terminology/), previous public health research,10,14 and the Acute to Chronic Pain Signatures Program,18 in combination with feedback from the initiative's advisory committees. Table 1 includes the definitions for the four pain categories.
Table 1.
Pain categories, and their associated definitions and examples, used in the present initiative.
| Pain category | Definition | Example condition(s) |
|---|---|---|
| Acute pain | Pain experiences and conditions lasting for a relatively limited time, up to a few weeks, and generally remitting when the underlying pathology resolves; often occurs after trauma, surgical interventions, and some disease processes | Acute post-operative pain, pain in labor, fracture, and ulcer |
| Transition from acute to chronic pain | Pain experiences and conditions lasting from a few weeks to three months | Post-operative recovery |
| Recurrent/episodic pain | Pain experiences and conditions lasting for a relatively short time but recurring across an extended period | Sickle cell-associated pain, migraine, polymyalgia rheumatica, calcium phosphate deposition, and dysmenorrhea |
| Chronic pain | Pain experiences and conditions lasting longer than three months | Chronic low back pain, chronic postsurgical pain, chronic pelvic pain, and diabetic neuropathy |
Members of the steering committee conducted a systematic literature review (SLR) (see S1.3) to generate the initial list of outcome domains/subdomains for the four pain categories prior to the virtual consensus meeting. The ACs, stakeholders at the consensus meeting, and participants in the first round of the Delphi were able to recommend additional domains/subdomains. The Steering Committee, with the assistance of the AC, evaluated these proposed domains/subdomains to identify those that were similar and could be combined.
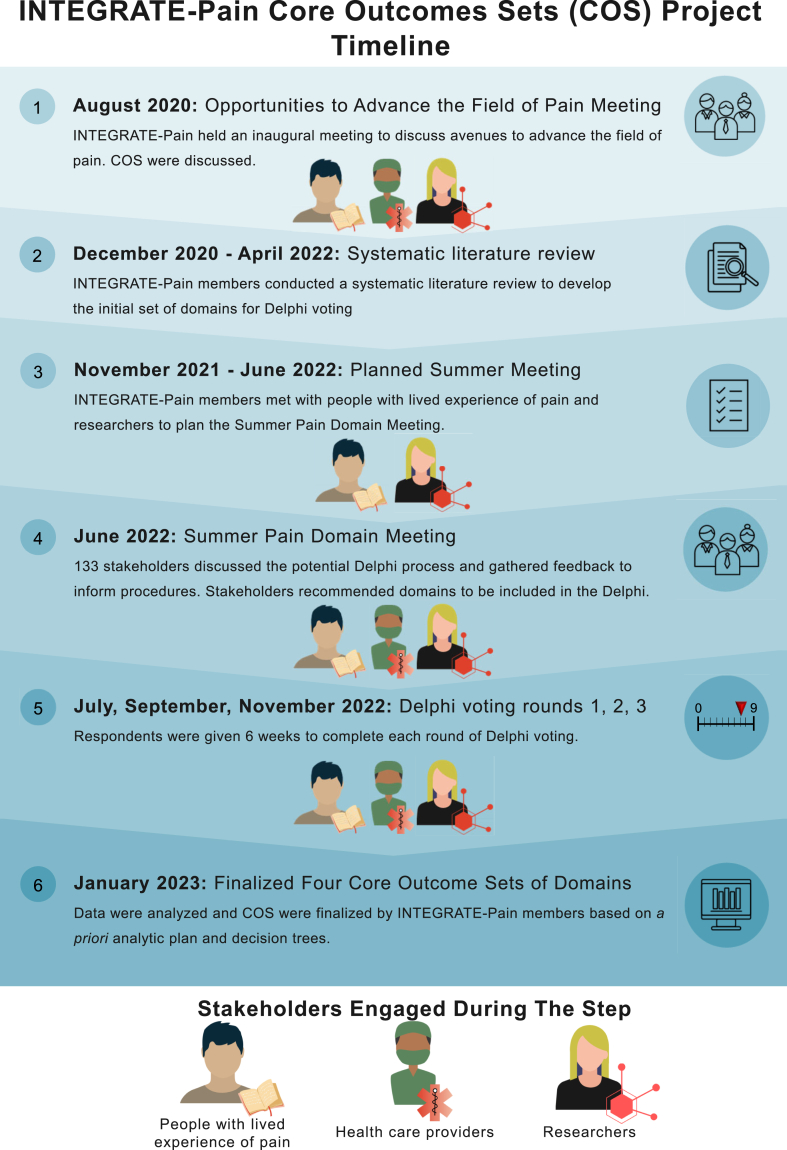
A consensus process was organized based on COMET Handbook guidelines3 (Fig. 1).
Fig. 1.
The timeline for the INTEGRATE-Pain core outcomes set initiative.
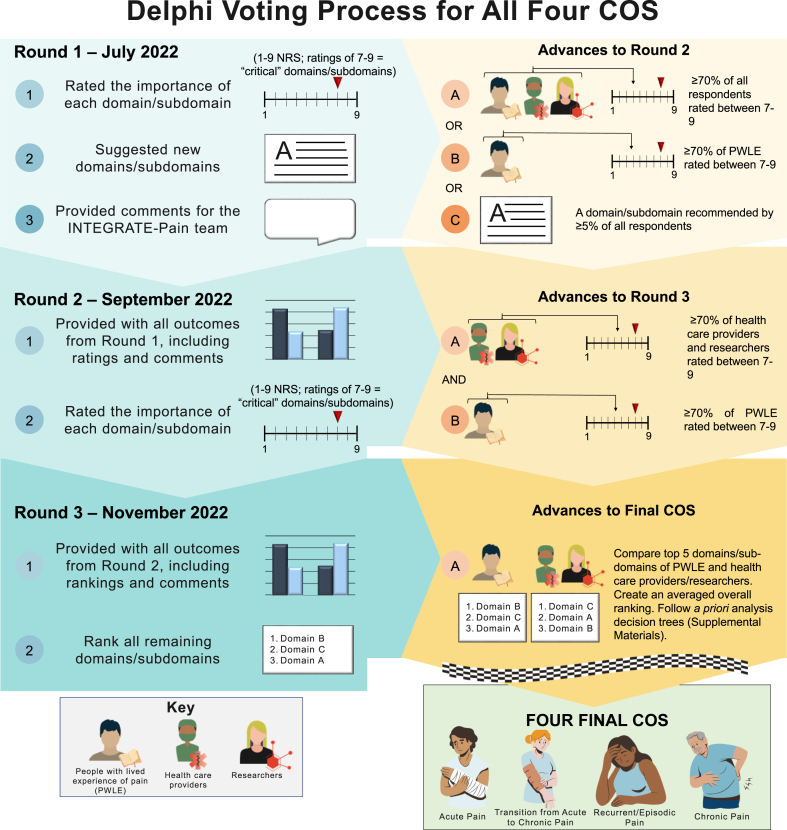
A virtual consensus meeting (June 2022) was held to discuss COS and vote on whether overarching COS should be created. A three-round Delphi method19 was subsequently performed between July 2022 and January 2023 with four separate Delphi surveys (one for each pain category) running in parallel. We chose a Delphi process as our consensus method for developing these COS due to its precedent in COS research.14 For this Delphi process, respondents were provided with lists of outcome domains (with definitions and citations) to rate over three rounds. Three rounds were chosen to offer respondents the opportunity to reflect on the groups’ responses each round and to reduce concerns that retention and respondent burden might be greater with more rounds. Domains/subdomains were presented together across rounds (rather than holding distinct rounds to vote on domains or subdomains separately) to limit the number of rounds.3 The decision to have respondents rate outcomes at the domain and subdomain level (rather than broader areas or specific measures) was also made to give respondents flexibility in prioritizing the level of specificity they felt most relevant to assessment, but not be too broad or too narrow for inclusion into a widely used COS. Fig. 2 shows the overall flow across the three rounds as well as the consensus definition.
Fig. 2.
Methods and consensus definition decisions involved in the Delphi method across all three rounds. Based on advice from the advisory committees and feedback from Delphi respondents, changes in procedures were made (as advised) and clearly communicated to Delphi respondents prior to the dissemination of the subsequent round.
Invited individuals were asked to respond in each of the three rounds, regardless of their participation in previous rounds. Surveys were administered with a web-based survey platform (SurveyMonkey, Advantage version, San Matteo, California). Prior to proceeding with domain ratings, respondents were required to watch a short educational video related to COS, Delphi methods, and the purpose and implications of this specific Delphi, and then correctly answer four comprehension questions (unlimited number of attempts).
For the first round, respondents rated all domains/subdomains from the initial list generated through the literature review, recommendations from the steering and advisory committees, and suggestions from attendees at the preliminary pain domain meeting. Definitions for domains/subdomains were taken or adapted from the literature and included citations. Respondents then rated or ranked domains/subdomains as instructed.
For subsequent rounds, respondents were asked to reflect on their own responses and the overall group responses. To facilitate this, we provided respondents with the previous round's ratings (broken down by stakeholder group), answers to frequently asked questions or comments, and the opportunity to receive a copy of their personal responses emailed to them upon request.
Criteria for including a domain/subdomain in the subsequent round or final COS were determined and communicated to the respondents prior to the beginning of each Delphi round.
Data analysis
In rounds 1 and 2, respondents rated each domain/subdomain according to its importance for inclusion in the respective pain category's COS. Domains/subdomains were rated using a 9-point Likert scale from the GRADE handbook (http://wwwcc-imsnet/gradepro) based on previous recommendations19,20: 1–3 = “not important,” 4–6 = “important, but not critical,” or 7–9 = “critical”. An additional option was 0 = “unable to rate”. In round 1, respondents could also recommend domains/subdomains that were not included to potentially advance to round 2. This was not an option after round 1 because we wanted respondents to have an opportunity to recommend domains, but needed to eliminate domains as we progressed each round. There is also precedent in COS research for allowing domain suggestions only during the first round.8
In round 3, respondents rank ordered all domains/subdomains that advanced from round 2 based on their importance for the respective pain category's COS. Flexibility in rating approach, such as switching from rating to rank ordering domains/subdomains, has been previously used to reduce respondent burden and facilitate consensus.21
Analytic plans and criteria to advance domains/subdomains and reach consensus were determined a priori and communicated to the respondents before each Delphi round (Fig. 2). Analyses were conducted and are reported separately for each of the four COS. Within a given COS, responses were divided into two groups: [1] PWLE and [2] researchers (R), clinicians (C), and others (O) or R/C/O. This approach served to weigh PWLE's responses equally with R/C/O, even if less than 50% of the respondents were PWLE in each round.
In round 1, domains/subdomains moved forward to round 2 if either [1] ≥70% of PWLE rated the domain/subdomain between 7 and 9 (i.e., “critical”), or [2] ≥70% of the entire group (i.e., PWLE + R/C/O combined) rated the domain/subdomain between 7 and 9. These thresholds were adapted from previous COS development and recommendations outlined by Williamson and colleagues.13 Our thresholds were slightly modified from the literature to simplify criteria for advancing domains since initial lists were comprehensive and we sought to eliminate domains each round. We also sought to ensure voices of PWLE were heard.13 We received consultation and guidance from members of our advisory committee (experienced in COS research and Delphi methods) on how to appropriately adapt the recommended guidelines for our study's specific aims and needs. New domains/subdomains were added to round 2 if ≥5% of respondents suggested it in round 1. This liberal threshold was set to encourage respondent input without advancing items suggested by only one respondent.
In round 2, domains/subdomains moved forward to round 3 if ≥70% of PWLE and ≥70% of R/C/O rated the domain/subdomain between 7 and 9. This stricter threshold was set to narrow the number of domains/subdomains to those that both groups found meaningful.
In round 3, the average rank orders of domains/subdomains were calculated separately for PWLE and R/C/O. The top five for each respondent group were retained. The items were then ordered based on their average ranking across both groups. For example, a domain/subdomain ranked highest in one group (ranking = 1) and fourth highest in the other group (ranking = 4) would have an averaged rank value of 2.5. The domains were then ordered according to these averaged rank values. The top five items were included in the final COS. Figure S3 shows the a priori decision tree analysis for final consensus. The final COS was restricted to the top five domains/subdomains to not be overburdensome to patients, researchers and clinicians. Domains/subdomains ranked within one group's top five that did not rank highly enough for the final COS are listed as items to be considered. We met with our advisory committees between each Delphi round. At the end of the Delphi, we met with the multidisciplinary group of PWLE and researchers on our advisory and steering committees to discuss and finalize the COS. We also communicated to respondents our plan to disseminate the results via webinars, conferences, and email communication.
Role of the funding source
Funders of the IMI-PainCare initiative (the NIH did not receive any funding) had no role in study design, data collection, data analyses, data interpretation, or writing of report.
Results
The main outcomes are reported below. The Supplemental Materials provide additional results.
Protocol deviations
The study team proposed a protocol that indicated how the team would move a domain/subdomain forward after each Delphi round. This information was disseminated to the respondents at the start of the Delphi. The AC recommended modifying the protocol before the start of ratings in round 2 and before the start of round 3 ranking to address issues related to overlapping/similar domains and ensure equity in respondent representation. The Delphi respondents were notified of the change in protocol prior to the beginning of each round. Please refer to Supplemental Material S1.4. and Figure S3 for more information.
Delphi respondents
In total, 446 unique individuals were identified as candidate respondents for the four pain groups. However, because some individuals were asked to vote in more than one pain category, a total of 627 survey invitations were sent. Of the invited individuals, 357 participated in at least one Delphi round. Table 2 shows respondents’ sociodemographic characteristics based on stakeholder group (PWLE or R/C/O) and pain COS category. Table 3 shows the response rates by pain group, which ranged from 74% for chronic pain to 81% for recurrent pain. Of the stakeholders invited to participate in the Delphi, 89 did not participate in any of the surveys, 45% of which were PWLE and 55% of which were R/C/O. Refer to the supplement for more details about respondents.
Table 2.
Self-reported demographics and classifications for voters in the Delphi surveys, including researchers, clinicians, and others (R/C/O) and people with lived experience of pain (PWLE).
| Acute pain |
Transition from acute to chronic pain |
Recurrent pain |
Chronic pain |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 69) | R/C/O (n = 36) | PWLE (n = 33) | Overall (n = 88) | R/C/O (n = 40) | PWLE (n = 48) | Overall (n = 72) | R/C/O (n = 22) | PWLE (n = 50) | Overall (n = 254) | R/C/O (n = 156) | PWLE (n = 98) | |
| Gender | ||||||||||||
| Female | 41 | 16 | 25 | 52 | 20 | 32 | 54 | 13 | 41 | 162 | 87 | 75 |
| Male | 27 | 20 | 7 | 33 | 19 | 14 | 15 | 9 | 6 | 83 | 63 | 20 |
| Reported as Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Other | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 3 | 4 | 2 | 2 |
| Sex assigned at birth | ||||||||||||
| Female | 40 | 16 | 24 | 51 | 19 | 32 | 50 | 12 | 38 | 151 | 84 | 67 |
| Male | 26 | 19 | 7 | 29 | 17 | 12 | 14 | 8 | 6 | 74 | 56 | 18 |
| Intersex | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reported as Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Member of minority group | 26 | 12 | 14 | 35 | 10 | 25 | 34 | 5 | 29 | 76 | 31 | 45 |
| Identify as | ||||||||||||
| R/C/O | 31 | 31 | 0 | 25 | 25 | 0 | 15 | 14 | 1 | 127 | 125 | 2 |
| PWLE | 30 | 0 | 30 | 39 | 1 | 38 | 39 | 0 | 39 | 80 | 1 | 79 |
| Both | 7 | 5 | 2 | 22 | 12 | 10 | 15 | 7 | 8 | 41 | 26 | 15 |
| Years of clinical experience | ||||||||||||
| <1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1–5 | 2 | 2 | 0 | 3 | 2 | 1 | 1 | 1 | 0 | 12 | 8 | 4 |
| 6–10 | 3 | 2 | 1 | 4 | 3 | 1 | 5 | 2 | 3 | 16 | 13 | 3 |
| >10 | 30 | 28 | 2 | 29 | 24 | 5 | 22 | 18 | 4 | 129 | 116 | 13 |
| Years of research experience | ||||||||||||
| <1 | 1 | 0 | 1 | 1 | 0 | 1 | 3 | 0 | 3 | 7 | 4 | 3 |
| 1–5 | 12 | 5 | 7 | 12 | 3 | 9 | 14 | 4 | 10 | 27 | 16 | 11 |
| 6–10 | 7 | 5 | 2 | 7 | 6 | 1 | 3 | 1 | 2 | 36 | 24 | 12 |
| >10 | 31 | 25 | 6 | 36 | 29 | 7 | 21 | 15 | 6 | 117 | 101 | 16 |
| Years of COS experience | ||||||||||||
| None | 8 | 4 | 4 | 21 | 2 | 19 | 23 | 2 | 21 | 39 | 11 | 28 |
| <1 | 10 | 2 | 8 | 17 | 3 | 14 | 12 | 0 | 12 | 33 | 11 | 22 |
| 1–5 | 21 | 12 | 9 | 19 | 12 | 7 | 10 | 4 | 6 | 50 | 31 | 19 |
| 6–10 | 8 | 4 | 4 | 11 | 7 | 4 | 8 | 3 | 5 | 38 | 30 | 8 |
| >10 | 22 | 14 | 8 | 20 | 16 | 4 | 19 | 13 | 6 | 94 | 73 | 21 |
| Region of residence | ||||||||||||
| North America | 33 | 14 | 19 | 58 | 28 | 30 | 44 | 11 | 33 | 127 | 71 | 56 |
| Europe | 25 | 15 | 10 | 14 | 4 | 10 | 16 | 7 | 9 | 78 | 45 | 33 |
| Oceania | 6 | 3 | 3 | 10 | 6 | 4 | 6 | 2 | 4 | 26 | 19 | 7 |
| Middle East | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 7 | 6 | 1 |
| Africa | 1 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 4 | 0 | 0 | 0 |
| Asia | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 |
| South America | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 |
Total counts may be less than actual n because voters were not required to answer all demographic questions.
Table 3.
Response rates for Delphi by pain category.
| Acute pain | Transition from acute to chronic pain | Recurrent pain | Chronic pain | Total | |
|---|---|---|---|---|---|
| Invited to Vote, n | 86 | 108 | 89 | 344 | 627 |
| Total in pain group who voted, n (%) | 69 (80%) | 88 (81%) | 72 (81%) | 254 (74%) | 483 (77%) |
| R/C/O who voted, n (% of total voters) | 42 (49%) | 47 (44%) | 26 (29%) | 201 (58%) | 316 (50%) |
| PWLE who voted, n (% of total voters) | 44 (51%) | 61 (56%) | 63 (71%) | 143 (42%) | 311 (50%) |
Yes = Included in final COS; Consider = Not included in final COS but could be considered based final voting results; No = Voted out in final round of Delphi Survey; Dropped = domain was voted out in an earlier round of Delphi survey for that pain condition. N/A = Domain was not voted on for that pain condition.
The number of respondents for each group represents those who participated in at least one round of the Delphi. Some individuals were invited to vote in multiple groups due to having experience with multiple pain categories, therefore the totals have duplicate votes since some individuals voted in multiple categories.
Outcomes
All domains/subdomains presented to respondents during each round are displayed in the Supplemental Materials. Refer to Table S6 for respondent dropout rates.
There were n = 27 domains/subdomains considered in round 1 for acute pain. Of those, n = 9 advanced to round 2, and n = 8 were added because of respondent recommendations. For round 2, n = 17 total domains/subdomains were presented. Of those, n = 7 advanced to round 3.
There were n = 16 domains/subdomains considered in round 1 for transition from acute to chronic pain. Of those, n = 9 advanced to round 2, and n = 9 were added because of respondent recommendations. For round 2, n = 18 total domains/subdomains were presented. Of those, n = 7 advanced to round 3.
There were n = 24 domains/subdomains considered in round 1 for recurrent/episodic pain. Of those, n = 11 advanced to round 2, and n = 8 were added because of respondent recommendations. Two of the domains that advanced (pain frequency and pain recurrence) were folded into a recommended domain (pain temporality) that consolidated several similar/overlapping domains. For round 2, n = 17 total domains/subdomains were presented to respondents, of which n = 8 advanced to round 3.
There were n = 65 domains/subdomains considered in round 1 for chronic pain. Of those, n = 29 advanced to round 2, and n = 1 was added because of respondent recommendations. Two domains from round 1 (pain frequency and pain duration) were consolidated into another similar/overlapping domain (pain temporality) that had advanced. In round 2, n = 28 total domains/subdomains were presented to respondents, of which n = 12 advanced to round 3.
Core outcome sets
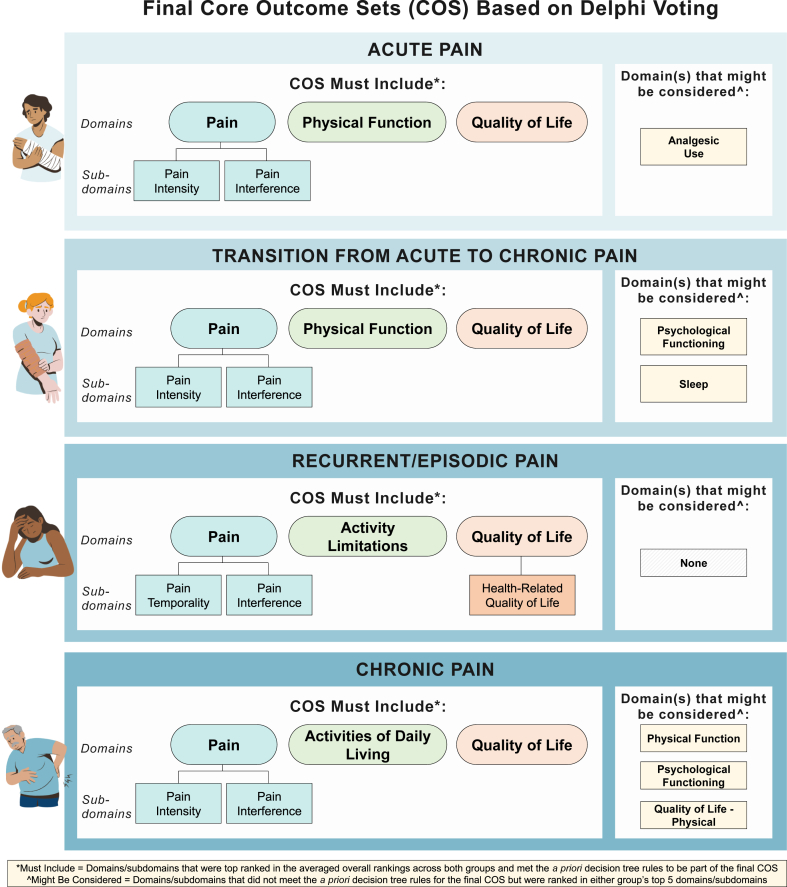
Fig. 3 shows the final domains included in the acute, transition from acute to chronic, recurrent/episodic, and chronic pain COS, and Table 4 provides their definitions and final results for their transition from round 2 to round 3 for all four pain categories.
Fig. 3.
Final core outcome sets across all four pain stage categories.
Table 4.
Final core outcome set (COS) domains/subdomains and their respective definitions.
| Domain | Definition | Included in final COS for |
|||
|---|---|---|---|---|---|
| Acute pain | Transition from acute to chronic pain | Recurrent pain | Chronic pain | ||
| Pain | An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage. | Yes | Yes | Yes | Yes |
| Pain Intensity | Magnitude of the pain sensations experienced (in the past week). | Yes | Yes | N/A | Yes |
| Pain Interference | The degree to which there are consequences of pain on aspects of a participant's life (in the past 24 h or past week for acute or chronic pain, respectively). | Yes | Yes | Yes | Yes |
| Quality of Life | Broad multidimensional concept that usually includes subjective evaluations of both positive and negative aspects of life. | Yes | Yes | Yes | Yes |
| Physical Function | Ability to carry out activities requiring physical actions. | Yes | Yes | No | Consider |
| Sleep | Perceptions of difficulty falling asleep, sleep quality, sleep depth, duration and restoration associated with sleep. | No | Consider | No | No |
| Analgesic Use | Use of a drug that reduces pain. | Consider | N/A | N/A | Dropped |
| Psychological Functioning | The ability to achieve one's own goals within themselves and the external environment. It includes an individual's behavior, emotion, social skills, and overall mental health. | Dropped | Consider | Consider | Consider |
| Activities of daily living | Fundamental skills required to independently care for oneself, such as eating, bathing, and mobility. | Dropped | Dropped | Dropped | Yes |
| Quality of life (physical) | Subjective evaluations of both positive and negative aspects of physical life (i.e., pain and discomfort, energy and fatigue, sexual activity, sleep and rest, sensory functions). | N/A | N/A | N/A | Consider |
| Health Related Quality of Life | At an individual level, HRQOL includes physical and mental health perceptions (e.g., energy level, mood) and their correlates—including health risks and conditions, functional status, social support, and socioeconomic status. | N/A | N/A | Yes | No |
| Disability | Any condition of the body or mind (impairment) that makes it more difficult for the person with the condition to do certain activities (activity limitation) and interact with the world around them (participation restrictions). | N/A | N/A | Dropped | No |
| Pain Affect | The distress caused by the pain, reflecting the emotional response to pain. | N/A | N/A | N/A | No |
| Medication Use | Use of correct (and/or incorrect) medication. | Dropped | N/A | No | N/A |
| Pain Temporality | Variability in intensity, time to onset of meaningful pain relief, durability of pain relief, and frequency, duration and intensity of episodes of breakthrough pain. | N/A | Dropped | Yes | Dropped |
| Activity Limitations | A dimension of disability, such as difficulty seeing, hearing, walking, or problem solving. | Dropped | N/A | Yes | Dropped |
Yes = Included in final COS; Consider = Not included in final COS but could be considered based final voting results; No = Voted out in final round of Delphi Survey; Dropped = domain was voted out in an earlier round of Delphi survey for that pain condition. N/A = Domain was not voted on for that pain condition.
The number of respondents for each group represents those who participated in at least one round of the Delphi. Some individuals were invited to vote in multiple groups due to having experience with multiple pain categories, therefore the totals have duplicate votes since some individuals voted in multiple categories.
The final COS for acute pain included pain (which must include measures of pain intensity and pain interference as subdomains), physical function, and quality of life (as a broad construct). Although not part of the final COS, investigators also might consider measuring analgesic use since it was among domains included in at least one group's top five ranking.
The final COS for transition from acute to chronic pain included pain (which must include measures of pain intensity and pain interference as subdomains), physical function, and quality of life. Although not part of the final COS, investigators might also consider measuring psychological functioning and sleep since they were among domains included in at least one group's top five ranking.
The final COS for recurrent/episodic pain included pain (which must include measures of pain interference and pain temporality as subdomains), quality of life (which should include a measure of the subdomain health-related quality of life), and activity limitations.
The final COS for chronic pain included pain (which must include measures of pain intensity and pain interference as subdomains), quality of life, and activities of daily living. Although not part of the final COS, investigators might also consider measuring physical function, psychological functioning, and physical quality of life since they were among domains included in at least one group's top five ranking.
Discussion
Existing pain-related COS focus on specific conditions, hampering cross-cutting integration of data to improve generalizability of research and clinical practice. The INTEGRATE-Pain Consortium aimed to develop overarching COS across pain conditions along the spectrum from acute to chronic pain. Our consortium led a comprehensive consensus process to develop four COS for acute pain, transition from acute to chronic pain, recurrent/episodic pain, and chronic pain. A virtual consensus meeting was held to determine whether the pain community believed these four COS were needed. Meeting participants decided it was important to proceed with a three-round Delphi, which was performed from July 2022 to January 2023. At the end, respondents reached consensus on the most important domains/subdomains for inclusion into four COS. We hypothesized that although similar wide-ranging sets of biopsychosocial outcomes would be relevant to conditions within a given pain category, there would be unique domains/subdomains perceived as critical by stakeholders across pain conditions within each category with little overlap across categories. This hypothesis was only partially supported, as there was substantial overlap across the four final COS. All final COS included pain, pain interference, and quality of life as domains/subdomains. Furthermore, all final COS included one domain/subdomain related to activities of daily living/activity limitations/physical function, supporting the multidimensional (biopsychosocial) nature of pain. Although we suggest that these COS be adopted for inclusion in research and clinical practice, they do not preclude the addition of other outcomes as appropriate to specific pain or health conditions.
There are some domains/subdomains, such as physical function (included in the acute and transition from acute to chronic pain categories), activity limitations (recurrent/episodic pain), activities of daily living (chronic pain) and pain interference (all categories), that are similar to each other, but also have unique attributes that can be distinctly measured. For example, physical function is the ability to carry out activities requiring physical actions.14 Activity limitations is a dimension of disability, such as difficulty seeing, hearing, walking, or problem solving.22 Pain interference is the degree to which there are consequences of pain on aspects of a participant's life (in the past 24 h or past week for acute or chronic pain, respectively).23 With respect to the quality of life domain, Delphi respondents were provided with outcomes at different levels of specificity. We included outcomes at the domain and subdomain level to be inclusive for the initial list of domains in round one. For rounds two and three, we sent an FAQ (responses to Frequently Asked Questions/Comments) along with the Delphi survey that asked respondents to consider the level of specificity in their voting choices. Some pain groups (acute, transition, chronic) consented on the broader domain quality of life, while the recurrent group consented on the subdomain health-related quality of life. These differences are reflected in the final COS, and we recommend researchers and clinicians consider the tension between generalizability and specificity in their application of these COS as appropriate to their research and clinical settings. Example measures for these domains/subdomains are, respectively, Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function scale,24 the World Health Organization Disability Assessment Schedule 2.0,25 and the PROMIS Pain Interference scale.23 However, the “how” to measure the outcomes/subdomains needs further systematic research followed by a final consensus process on the COS of PROMs for each pain category. The result of our Delphi provides COS with lists of domains achieved by respondent consensus. Pain intensity was included in three out of the four COS. However, pain intensity did not reach consensus for the recurrent/episodic pain COS; instead, pain temporality was included by Delphi participants, indicating the importance of frequency and duration of pain.
There are two intended principal uses for the present COS. First, they can be used in research to supplement measures that researchers already collect for certain conditions. In this way, the COS can advance the pain field through data harmonization across studies without limiting innovation within specific projects. Second, these four COS can be used as a minimum set of measures in clinical practice with the goals of applying a more holistic framework in clinical decision-making, benchmarking, and addressing common limitations in clinical practice.26 The COS are not meant for diagnostic purposes; rather, the outcomes should be measured in research and clinical practice where the diagnosis of the health or pain condition has already been determined. Finally, they are not intended to be used as the only measures. Therefore, our COS are limited to five domains because burden on patients should be carefully considered when choosing the number and length of measures included in a COS.26
Our approach had several strengths. The domains/subdomains in our COS were agreed upon by a broad range of respondents, including PWLE, respondents from many countries, and respondents with previous experience in COS research related to pain, including from a wide range of disease backgrounds. As COS should be meaningful and accessible to PWLE, our initiative heavily incorporated their views while addressing challenges faced by COS developers, such as aligning researchers'/clinicians' research efforts to PWLE's concerns, preferences, and respondent burden, securing international input, and achieving consensus among disparate stakeholder groups.27 Another strength of our approach is that we sought feedback from PWLE advisors throughout the Delphi process. We also prioritized inviting PWLE to participate in the Delphi surveys and structured our analytic plan to promote equal representation among PWLE and R/C/O. The diversity of views represented in the final COS and the robust inclusion of PWLE could increase their uptake and acceptance.
These strengths are balanced with several limitations. First, because the COS literature is concentrated in North America, Europe, and Oceania, our process of using a SLR to invite stakeholders and participants contributed to limited representation of individuals outside of these regions, and the Delphi was only offered in English. Greater representation from underrepresented world regions, as well as minoritized racial/ethnic groups, is critical for promoting health equity in future COS development. The use of snowball sampling (asking stakeholders to identify additional participants from their networks) in recruiting PWLE for participation also hampered diverse representation among PWLE in the study. Despite this limitation, we found snowball sampling helped to facilitate the inclusion of many PWLE to enable equal representation between R/C/O and PWLE, a unique facet of this Delphi. Second, although these COS were designed for clinical research and practice, we recognize that these two applications have different demands and requirements. We tried addressing this concern by including researchers, clinicians, and PWLE as respondents. However, we anticipated that this diverse group of stakeholders would have disparate experiences with and opinions on clinical research and practice, which can impede clarity on what consensus means.28 Therefore, all respondents completed a short training video with comprehension questions related to COS and the purpose and implications of this Delphi and its resulting COS. Future work is needed to evaluate these COS across settings to determine potential implementation barriers. Third, a barrier across COS development is variable uptake.29 Previous work has shown that this might be largely due to researchers' preferences to prioritize other outcome measures and limited knowledge about the COS’ existence.30 Fourth, the flexible and complex nature of Delphi studies (which asks for voting over multiple rounds and reflection on group responses) can lead to drop-outs and introduce bias towards majority views.28 To mitigate turnover, we allowed registered participants to vote in any round, regardless of their participation in previous rounds. To reduce risk of the majority views influencing individual responses, we reiterated the purpose of the study each round, providing clear instructions and responses to frequently asked questions. Lastly, a barrier to uptake is the lack of recommendations regarding how to measure the outcomes. To encourage effective and widespread adoption of these COS, INTEGRATE-Pain will engage professional organizations to gather endorsements for the COS and facilitate dissemination. We will emphasize the use of these COS alongside disease-specific COS. For research where pain is only one symptom of the disease under study, we also will encourage researchers to use these COS. To inform PWLE globally, our PWLE advisors asked to partner with INTEGRATE-Pain to develop materials that will communicate key findings and implications among PWLE.
The present initiative follows recommended guidance for COS development describing that a consensus process should first identify what to measure and then identify how they should be measured.3 Since the aim of this Delphi was to obtain agreement on what to measure, the next step for the field of pain will be to reach consensus on how to measure these domains. However, recent research has shown that many PROMs for acute and chronic pain lack validity and reliability among various populations.14 Therefore, a challenge for future researchers will be to determine how to create or adopt PROMs for the domains in these COS that can be generalized across cultures, languages, backgrounds, and disorders. In the meantime, researchers and clinicians can apply the present COS in their work by using PROMs that measure the consensus-driven domains/subdomains.
The INTEGRATE-Pain Consortium brought together a large and diverse group of stakeholders to develop COS for acute, transition from acute to chronic, recurrent/episodic, and chronic pain. Our final COS have the potential to improve data harmonization and allow for comparisons across pain conditions and intervention approaches. For these implications to be realized, however, the present COS must be effectively incorporated in research and clinical practice. Moving forward, INTEGRATE-Pain will work with stakeholders to disseminate these COS and promote their uptake in the field of pain.
Contributors
Conceptualization: Esther Pogatzki-Zahn; Ulrike Kaiser; Laura Wandner; Giulia Bova, Barbara Karp, Jan Vollert, Laura Wandner, Hiltrud Liedgens, Rolf-Detlef Treede, Peter Tugwell, Ulrike Kaiser, Esther Pogatzki-Zahn, Leah Pogorzala.
Resources: Kate Nicholson, Rolf-Detlef Treede, Deirdre Ryan, Winfried Meißner, Esther Pogatzki-Zahn, Katie Golden.
Funding Acquisition: Rolf-Detlef Treede, Winfried Meißner, Esther Pogatzki-Zahn, Katy Vincent, Winfried Meissner, Ralf Baron, Didier Bouhassira.
Data Curation: Andrew Siddons; Giulia Bova; Anthony Domenichiello; Laura Wandner; Daniela Rosenberger, Laura Wandner, Anthony Domenichiello, Sarah Woller, Smriti Iyengar, Esther Pogatzki-Zahn, Leah Pogorzala.
Literature Review: Barbara Karp, Laura Wandner, Sara Woller, Smriti Iyengar, Ulrike Kaiser, Esther Pogatzki-Zahn.
Study Design: Giulia Bova, Laura Wandner, Paula Williamson, Dennis Turk, Smriti Iyengar, Ulrike Kaiser, Esther Pogatzki-Zahn.
Formal Analysis and Interpretation: Andrew Siddons, Giulia Bova, Jan Vollert, Laura Wandner, Paula Williamson, Daniela Rosenberger, Anthony Domenichiello, Smriti Iyengar, Ruth Zaslansky, Esther Pogatzki-Zahn.
Investigation: Andrew Siddons, Barbara Karp, Giulia Bova, Jan Vollert, Anthony Domenichiello, Esther Pogatzki-Zahn, Leah Pogorzala.
Methodology: Giulia Bova, Jan Vollert, Paula Williamson. Anthony Domenichiello, Hiltrud Liedgens, Peter Tugwell, Esther Pogatzki-Zahn.
Project administration: Andrew Siddons, Giulia Bova, Jan Vollert, Daniela Rosenberger, Rolf-Detlef Treede, Winfried Meißner, Esther Pogatzki-Zahn.
Supervision: Giulia Bova, Laura Wandner, Rolf-Detlef Treede, Esther Pogatzki-Zahn.
Validation: Anthony Domenichiello.
Visualization/figure creation: Janelle Letzen; Anthony Domenichiello, Giulia Bova, Daniela Rosenberger, Smriti Iyengar.
Writing—original draft: Giulia Bova, Janelle Letzen, Laura Wandner, Daniela Rosenberger, Anthony Domenichiello, Hiltrud Liedgens, Ulrike Kaiser, Esther Pogatzki-Zahn.
Writing—review & editing: Andrew Siddons, Barbara Karp, Giulia Bova, Janelle Letzen, Jan Vollert, Laura Wandner, Paula Williamson, Daniela Rosenberger, Sara Woller, Dennis Turk, Judy Birch, George Casey, Ralf Baron, Adam Anicich, Smriti Iyengar, Katy Vincent, Kate Nicholson, Ruth Zaslansky, Rolf-Detlef Treede, Didier Bouhassira, Peter Tugwell, Deirdre Ryan, Winfried Meißner, Ulrike Kaiser, Esther Pogatzki-Zahn, Katie Golden.
All authors read and approved the final version of the manuscript.
All authors had access to the deidentified data used in this study and accept responsibility for the decision to submit the manuscript for publication.
Giulia Bova, Andrew Siddons, Laura Wandner, Daniela Rosenberger, Janelle Letzen, Esther Pogatzki-Zahn, and Ulrike Kaiser have directly accessed and verified the underlying data reported in the manuscript.
Data sharing statement
Individual participant data that was collected throughout the research process from Delphi participants is not available to others. De-identified participant data was aggregated for analysis and presented in an anonymized format through tables in the article and supplement. All other research data is unavailable.
Declaration of interests
Giulia Bova has nothing to disclose.
Anthony Domenichiello has nothing to disclose.
Janelle Letzen has nothing to disclose.
Daniela C. Rosenberger has nothing to disclose.
Andrew Siddons has nothing to disclose.
Ulrike Kaiser is the principal investigator of VAPAIN.
Adam Anicich has nothing to disclose.
Ralf Baron reports grants from EU Projects: „Europain“ (115,007). DOLORisk (633,491). IMI Paincare (777,500). Ðerman Federal Ministry of Education and Research (BMBF): Verbundprojekt: Frühdetektion von Schmerzchronifizierung (NoChro) (13GW0338C). Ðerman Research Network on Neuropathic Pain (01EM0903). Ðfizer Pharma GmbH, Sanofi Genzyme GmbH, Grünenthal GmbH, Mundipharma Research GmbH und Co. KG., Alnylam Pharmaceuticals Inc., Zambon GmbH, Bayer AG, Sanofi Aventis GmbHÐ personal fees from Pfizer Pharma GmbH, Sanofi Genzyme GmbH, Grünenthal GmbH, Mundipharma, Lilly GmbH, Desitin Arzneimittel GmbH, Teva GmbH, Bayer AG, MSD GmbH, Seqirus Australia Pty. Ltd, Novartis Pharma GmbH, TAD Pharma GmbH, Grünenthal SA Portugal, Grünen¬thal Pharma AG Schweiz, Grünenthal B.V. Niederlande, Evapharma, Takeda Pharmaceuticals International AG Schweiz, Ology Medical Education Netherlands, Ever Pharma GmbH, Amicus Therapeutics GmbH, Novo Nordisk Pharma GmbH, Chiesi GmbH, Stada Mena DWC LLC Dubai, Hexal AG, Viatris, personal fees from Pfizer Pharma GmbH, Sanofi Genzyme GmbH, Grünenthal GmbH, Lilly, Novartis Pharma GmbH, Bristol-Myers Squibb, Biogenidec, AstraZeneca GmbH, Daiichi Sankyo, Glenmark Pharmaceuticals S.A., Seqirus Australia Pty. Ltd, Teva Pharmaceuticals Europe Niederlande, Teva GmbH, Genentech, Mundipharma International Ltd. UK, Galapagos NV, Kyowa Kirin GmbH, Vertex Pharmaceuticals Inc., Biotest AG, Celgene GmbH, Desitin Arzneimittel GmbH, Regeneron Pharmaceuticals Inc. USA, Theranexus DSV CEA Frankreich, Abbott Products Operations AG Schweiz, Bayer AG, Grünenthal Pharma AG Schweiz, Akcea Therapeutics Germany GmbH, Asahi Kasei Pharma Corporation, AbbVie Deutschland GmbH & Co. KG, Air Liquide Sante International Frankreich, Alnylam Germany GmbH, Lateral Pharma Pty Ltd, Hexal AG, Angelini, Janssen, SIMR Biotech Pty Ltd Australien, Confo Therapeutics N. V. Belgium, Merz Pharmaceuticals GmbH, Neumentum Inc., F. Hoffmann-La Roche Ltd. Switzerland, AlgoTherapeutix SAS France, Nanobiotix SA France, AmacaThera Inc. Canada, outside the submitted work.
Judy Birch has nothing to disclose.
Didier Bouhassira reports personal fees from Grunenthal, personal fees from Bayer, outside the submitted work.
George Casey has nothing to disclose.
Katie Golden has nothing to disclose.
Smriti Iyengar has nothing to disclose.
Barbara Karp has nothing to disclose.
Hiltrud Liedgens has nothing to disclose.
Winfried Meissner reports grants from European Commission, during the conduct of the study; personal fees from Grünenthal, grants from Mundipharma, grants from Pfizer, personal fees from Ethypharm, personal fees from Kyowa, personal fees from Spectrum Therapeutics, outside the submitted work.
Kate Nicholson has no conflicts or disclosures. She is employed by the National Pain Advocacy Center, which takes no industry funding and advocates for the health and rights of people living with pain. She was a member on the patient Advisory Committee for this project and received an honorarium for attending early organizational strategic planning for the project. She also received support for attending the European Pain Federation (EFIC) conference to present on concepts from this project.
Leah Pogorzala has nothing to disclose.
Deidre Ryan has nothing to disclose.
Rolf-Detlef Treede reports grants from Innovative Medicines Initiative EU and EFPIA, during the conduct of the study; grants from Deutsche Forschungsgemeinschaft, grants from Esteve, TEVA, personal fees from Bayer, Grünenthal, GSK, Merz, Saluda Medical, Sanofi, Cered, and Vertex outside the submitted work.
Peter Tugwell has nothing to disclose.
Dennis Turk has received in the past 5 years research grants and contracts from the US Food and Drug Administration and the US National Institutes of Health, U.S. Patient-Centered Outcome Research Institute, and US National Center for Occupational Health and Safety; received compensation for serving on advisory boards from Eli Lilly, GlaxoSmithKline, Novartis, and Pfizer. He received an honorarium from Wolters Kluwer in his role as editor-in-chief of The Clinical Journal of Pain.
Katy Vincent reports grants from Innovative Medicines Initiative 2 Joint Undertaking (GA No. 777500) (Horizon 2020 and EFPIA), during the conduct of the study; grants and personal fees from Bayer Healthcare (paid to institution), and personal fees from Reckitts (paid to institution), outside the submitted work. She received payment from Gedeon Richter for consultancy and talks, paid to the institution.
Jan Vollert reports personal fees from Vertex Pharmaceuticals, personal fees from Embody Orthopaedics, personal fees from Casquar, outside the submitted work.
Paula Williamson chairs the COMET Initiative Management Group.
Sarah Woller has nothing to disclose.
Ruth Zaslansky has nothing to disclose.
Laura Wandner has nothing to disclose.
Esther Pogatzki-Zahn reports grants from the German Research Foundation (DFG), the Federal Ministry of Education and Research (BMBF), the Federal Joint Committee (G-BA) and the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777500. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA. All money goes to the institutions (WWU/UKM) EPZ is working for. EPZ received personal fees (money went to her institution) from Gruenenthal, Medtronic and Novartis unrelated to the current work. She received an honorarium in her role as debuty-editor-in-chief of the European Journal of Anaesthesiology.
Acknowledgements
The authors would like to thank all stakeholders who participated in the August 2020 Opportunities to Advance the Field of Pain meeting and June 2022 virtual consensus meeting. We would also like to thank all pain researchers, clinicians, people with lived experience of pain, and organization representatives who played a role in the Delphi, including those who reached out to their social networks to help advertise the initiative and those who voted in the surveys. Additionally, we would like to thank the NIH and EU leadership who helped initiate this effort. The NIH and NIH staff did not receive any funding for the INTEGRATE-Pain Consortium. This work was conducted as part of IMI PainCare PROMPT (an Innovative Medicines Initiative 2 Joint undertaking under grant agreement No 777500). This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA. www.imi.europa.eu; www.imi-paincare.eu. The statements and opinions presented here reflect the authors' view and neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein. RDT acknowledges grants from Esteve and TEVA. None of the funders of the IMI-PainCare initiative (the NIH did not receive any funding) were involved in the study design, data collection, data analyses, interpretation, or writing of report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102340.
Appendix A. Supplementary data
References
- 1.Rice A.S.C., Smith B.H., Blyth F.M. Pain and the global burden of disease. Pain. 2016;157:791–796. doi: 10.1097/j.pain.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg D.S., McGee S.J. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson P.R., Altman D.G., Bagley H., et al. The COMET handbook: version 1.0. Trials. 2017;18:280. doi: 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker E., Hernandez A.V., Kattan M.W. Meta-analysis: its strengths and limitations. Cleve Clin J Med. 2008;75:431. doi: 10.3949/ccjm.75.6.431. [DOI] [PubMed] [Google Scholar]
- 5.Webbe J., Sinha I., Gale C. Core outcome sets. Arch Dis Child Educ Pract Ed. 2018;103:163. doi: 10.1136/archdischild-2016-312117. [DOI] [PubMed] [Google Scholar]
- 6.Saldanha I.J., Dodd S., Gorst S.L., Williamson P.R. More than half of systematic reviews have relevant core outcome sets. J Clin Epidemiol. 2021;136:168–179. doi: 10.1016/j.jclinepi.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiarotto A., Deyo R.A., Terwee C.B., et al. Core outcome domains for clinical trials in non-specific low back pain. Eur Spine J. 2015;24:1127–1142. doi: 10.1007/s00586-015-3892-3. [DOI] [PubMed] [Google Scholar]
- 8.Duffy J.M.N., Hirsch M., Vercoe M., et al. A core outcome set for future endometriosis research: an international consensus development study. BJOG. 2020;127:967–974. doi: 10.1111/1471-0528.16157. [DOI] [PubMed] [Google Scholar]
- 9.Haywood K., Potter R., Froud R., et al. Core outcome set for preventive intervention trials in chronic and episodic migraine (COSMIG): an international, consensus-derived and multistakeholder initiative. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-043242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treede R.-D., Rief W., Barke A., et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11) Pain. 2019;160:19–27. doi: 10.1097/j.pain.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 11.Maixner W., Fillingim R.B., Williams D.A., Smith S.B., Slade G.D. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain. 2016;17:T93–T107. doi: 10.1016/j.jpain.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turk D.C., Dworkin R.H., Allen R.R., et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Williamson P.R., Altman D.G., Blazeby J.M., et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:1–8. doi: 10.1186/1745-6215-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogatzki-Zahn E., Schnabel K., Kaiser U. Patient-reported outcome measures for acute and chronic pain: current knowledge and future directions. Curr Opin Anesthesiol. 2019;32:616–622. doi: 10.1097/ACO.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 15.Wandner L.D., Bloms-Funke P., Bova G., et al. INTEGRATE-Pain: a transatlantic consortium to advance development of effective pain management. Pain Med. 2023;24(6):730–733. doi: 10.1093/pm/pnad033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkham J.J., Gorst S., Altman D.G., et al. Core outcome set–STAndards for reporting: the COS-STAR statement. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Association for the Study of Pain . Terminology. 2011. https://www.iasp-pain.org/resources/terminology/ [Google Scholar]
- 18.A2CPS Acute to chronic pain signatures. https://a2cps.org/
- 19.De Meyer D., Kottner J., Beele H., et al. Delphi procedure in core outcome set development: rating scale and consensus criteria determined outcome selection. J Clin Epidemiol. 2019;111:23–31. doi: 10.1016/j.jclinepi.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Crew R., Williamson P. COMET initiative Delphi manager brochure. https://www.comet-initiative.org/delphimanager/docs/DelphiManagerBrochureV5.0.pdf
- 21.Park J.K., Mecoli C.A., Alexanderson H., et al. Advancing the development of patient-reported outcomes for adult myositis at OMERACT 2016: an international Delphi study. J Rheumatol. 2017;44:1683–1687. doi: 10.3899/jrheum.161252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DIN PCB . 2001. International classification of functioning, disability and health. [Google Scholar]
- 23.Amtmann D., Cook K.F., Jensen M.P., et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose M., Bjorner J.B., Gandek B., Bruce B., Fries J.F., Ware J.E., Jr. The PROMIS physical function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol. 2014;67:516–526. doi: 10.1016/j.jclinepi.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold L.H. DSM-5 and the assessment of functioning: the world health organization disability assessment schedule 2.0 (WHODAS 2.0) J Am Acad Psychiatry Law. 2014;42:173–181. [PubMed] [Google Scholar]
- 26.Chiarotto A., Ostelo R.W., Turk D.C., Buchbinder R., Boers M. Core outcome sets for research and clinical practice. Braz J Phys Ther. 2017;21:77–84. doi: 10.1016/j.bjpt.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young B., Bagley H. Including patients in core outcome set development: issues to consider based on three workshops with around 100 international delegates. Res Involv Engagem. 2016;2:1–13. doi: 10.1186/s40900-016-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett D., Heale R. What are Delphi studies? Evid Based Nurs. 2020;23:68–69. doi: 10.1136/ebnurs-2020-103303. [DOI] [PubMed] [Google Scholar]
- 29.Hughes K.L., Clarke M., Williamson P.R. A systematic review finds core outcome set uptake varies widely across different areas of health. J Clin Epidemiol. 2021;129:114–123. doi: 10.1016/j.jclinepi.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matvienko-Sikar K., Avery K., Blazeby J.M., et al. Use of core outcome sets was low in clinical trials published in major medical journals. J Clin Epidemiol. 2022;142:19–28. doi: 10.1016/j.jclinepi.2021.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.