Abstract
PURPOSE:
To assess differences in baseline and longitudinal quality of life among Black and White individuals in the US with advanced prostate cancer.
METHODS:
Secondary analysis of data from the International Registry for Men with Advanced Prostate Cancer (IRONMAN) including US participants newly diagnosed with advanced prostate cancer and identifying their race as Black or White from 2017-2023. Participants completed the EORTC QLQ-C30 Quality of Life (QoL) Survey at study enrollment and every three months thereafter for up to one year of follow-up reporting 15 scale scores ranging from 0-100 (higher functioning and lower symptom scores represent better quality of life). Linear mixed effects models with race and month of questionnaire completion were fit for each scale, and model coefficients were used to assess differences in baseline and longitudinal QoL by race.
RESULTS:
879 participants were included (20% identifying as Black) at 38 US sites. Compared to White participants at baseline, Black participants had worse constipation (mean 6.3 percentage points higher; 95% CI 2.9-9.8), financial insecurity (5.7 (1.4-10.0)), and pain (5.1 (0.9-9.3)). QoL decreased over time similarly by race; most notably, role functioning decreased by 0.7 percentage points (95% CI −0.8, −0.5) per month.
CONCLUSION:
There are notable differences in quality of life at new diagnosis of advanced prostate cancer for Black and White individuals, and quality of life declines similarly in the first year for both groups. Interventions that address specific aspects of quality of life in these patients could meaningfully improve the overall survivorship experience.
Keywords: prostate cancer, epidemiology, racial disparities, quality of life
Introduction
In the US, Black individuals have a 1.7 times higher incidence and 2.1 times higher mortality from prostate cancer compared to White individuals.(1) Patients with advanced disease experience the highest prostate cancer-associated morbidity and mortality, and Black individuals have the highest incidence of advanced disease resulting from cultural factors (e.g. mistrust of the medical system and more stigma around prostate cancer) and economic factors (e.g. poorer access to care and increased financial burden of treatment) driven by institutional racism.(2,3) There are two major categories of advanced prostate cancer: metastatic hormone-sensitive (mHSPC) and castration resistant prostate cancer (CRPC), both representing incurable states of disease. (4)
Quality of life (QoL) is a multidimensional construct that comprises several aspects of the human experience including health and psychological status, independence, social relationships, environment, and personal beliefs. Integration of patient-reported outcome measures of QoL into routine oncology care represents a potential point for intervention to improve survivorship in prostate cancer populations. In a randomized trial of individuals with metastatic solid tumors, the group assigned to complete electronic patient-reported symptom measures with notifications sent to the care team for severe/worsening symptoms had a median overall survival time that was 20% longer than the control group. (5) Further, a 10-point increase in global QoL (on a scale of 0 to 100) on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) was associated with a 17% lower risk of death in a population of 1,097 prostate cancer patients with primarily localized disease.(6)
Several population-based studies of racial disparities in QoL in individuals with localized prostate cancer have found poorer QoL in Black patients compared to White patients. (7–10) For example, in 1,178 patients newly diagnosed with prostate cancer in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE), Black patients had poorer QoL at baseline and slower improvement over time compared to White patients for numerous QoL scales from pain to mental health and physical functioning. (7) Importantly, these studies all focused on individuals with localized disease, neglecting to include those with advanced prostate cancer who face the most severe QoL burdens due to progressed disease and aggressive therapies.
Studies of QoL in advanced prostate cancer suffer from a lack of racial diversity, primarily because most previous studies have focused on the association between a specific disease-directed therapy and QoL in overwhelmingly White randomized controlled trial (RCT) populations. (11–14) Black individuals are underrepresented in RCT populations due to factors such as fewer clinical trials being offered at institutions where they receive care and bias in health professionals in recommending trial enrollment; as such, investigation of racial disparities in QoL is typically not possible in this setting.(3)
Observational studies represent a promising means to investigate QoL independent of a specific disease-directed therapy; however, recent observational studies of QoL in populations with advanced prostate cancer have also only focused on White individuals. (15,16) For example, a study of 280 White patients with advanced prostate cancer across 7 countries in 2007 found a steady decline in QoL over the first year after study enrollment.(15) As Black individuals are the population that is most affected by advanced prostate cancer, there is a need to extend this work into understanding QoL into this population. Identifying specific unmet QoL needs in Black individuals with advanced prostate cancer would meaningfully improve the survivorship experience for this population and also has the potential to decrease racial disparities in overall survival with prostate cancer through intervention on specific QoL detriments.
This study used patient-reported measures to examine racial differences in QoL among individuals newly diagnosed with mHSPC or CRPC in the International Registry for Men with Advanced Prostate Cancer (IRONMAN). We assessed differences in baseline and longitudinal QoL at 3-month intervals over the first year after new diagnosis with mHSPC or CRPC using the EORTC QLQ-C30 questionnaire. We described overall group differences in trajectories of functional status and symptom domains of QoL among IRONMAN participants identifying as Black or White in the US.
Patients and Methods
Study participants
Study participants included individuals enrolled in the IRONMAN registry (NCT03151629) between July 21, 2017 and January 23, 2023. Participants are recruited through IRONMAN-affiliated clinicians at approximately 100 study sites in 16 countries, and detailed data is collected at study enrollment (corresponding to those newly diagnosed with mHSPC or CRPC with no more than 90 days of systemic therapy prior to enrollment for patients with CRPC and no more than 90 days of active therapy for patients with mHSPC) and throughout a follow-up period of at least 5 years. (17) Study sites span academic, private practice, and government health centers and are primarily located in urban centers in regions with high prostate cancer mortality. All study participants gave written informed consent prior to study enrollment and were able to withdraw from the study at any time. Because race has different social and historical contexts with different classifications in different global regions, this analysis focuses specifically on participants enrolled in the US (38 study sites located in 21 states).
Outcome measure: Quality of life (EORTC QLQ-C30 version 3)
QoL was measured with the EORTC QLQ-C30 over a period of up to 12 months, with assessments performed at study enrollment and 3, 6, 9, and 12 months afterwards. Surveys were self-administered using a web-based platform (TrueNth) or paper questionnaires. (18) The EORTC QLQ-C30 survey consists of 2 questions on global health status (1-7 Likert scale, “very poor” to “excellent”), 15 questions on functional status (1-4 Likert scale, “not at all” to “very much”), and 13 questions on symptom status (1-4 Likert scale, “not at all” to “very much”). The 30 questions are combined and linearly transformed to 15 scale scores with a range of 0 to 100. The instrument covers five functional scales (physical, role, emotional, cognitive, and social) with higher scale scores representing better functioning in the domain. In addition, nine symptom scales were assessed (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties) with higher scale scores representing more severe symptoms.
The EORTC QLQ-C30 questionnaire has shown high construct validity and good reliability in a population of cancer patients and a racially diverse population over the age of 50 years with similar factor structures by race.(19,20) The EORTC recommends using a minimally important difference (MID) of 5 to 10 points for interpreting both group differences in QLQ-C30 scale scores as well as changes in scores over time;(21) a study of EORTC outcomes in clinical trials of disease-directed therapies validated this estimate in a population of patients with prostate cancer.(22) Prior to February 20, 2020, IRONMAN participants could complete the questionnaire two weeks before or after the target date; this was subsequently adjusted to be +/− three months of the target date to improve study feasibility.
Demographic and clinical characteristics
Demographic information (age, highest level of education, employment status, marital status, military status, race, ethnicity) was collected through a patient-reported questionnaire at study enrollment. Clinical variables (disease state at enrollment, Gleason score, prostate-specific antigen (PSA) level, treatment at baseline, metastatic status at baseline, and type of health center) were abstracted from patient medical records and entered by study sites.
Race was self-reported by participants at study enrollment, allowing multiple selection from the following categories: White/Caucasian, African/African American/Black/Black British/Caribbean, Asian/Asian American/Asian British, Native Hawaiian or Other Pacific Islander, American Indian/Alaska Native, Middle Eastern, and other. Ethnicity was self-reported by participants choosing between “Hispanic/Latino” and “not Hispanic/Latino” categories. This study focuses specifically on individuals self-identifying their race as only Black or White regardless of their ethnicity.
Age at study enrollment (years) was included as a continuous variable. Disease state at enrollment was categorized as mHSPC (de novo metastatic disease at diagnosis or progressed to metastasis after localized prostate cancer diagnosis) and m0 or m1 CRPC (progression of disease while on androgen deprivation therapy or with castrate level of testosterone as determined by the investigator).
Statistical analysis
We summarized demographic and clinical participant characteristics, stratified by self-reported race. We calculated the 15 EORTC scale scores described above for each individual at each time point if the questionnaire and all required questions for each scale were complete.(23) Missing individual covariates and EORTC scale scores on completed questionnaires were imputed using multiple imputation by chained equations (MICE)(24) with the data in wide format. Scale scores in which the entire questionnaire was missing were temporarily given a value of −250 as a numerical, proxy missing indicator to ensure that these scores would not be imputed. MICE was conducted using classification and regression trees with 10 iterations for each of 10 imputations and with the scale scores constrained between 0 and 100 (inclusive of the bounds). Subsequently, scale scores for missing questionnaires were re-marked as missing. Sensitivity analyses were conducted with different values for the missing indicator.
Longitudinal missingness in questionnaires was assessed, and an in-depth description of missing data exploration is included in the Supplementary Methods. As no clear patterns of reasons for missing whole questionnaires arose from these analyses, we fit linear mixed effects models for each of the fifteen scale scores with timepoints clustered within participant who were then clustered within study site. Initial models included only race, month of follow-up questionnaire time point (continuous), and their interaction, assuming a linear relationship between the outcome scale and time. As we were interested in overall differences by self-reported race, adjusted models additionally controlled for time-invariant variables (age at study enrollment and disease state at enrollment). We did not control for variables that may mediate the association between self-reported race and QoL (e.g. employment, marital status, etc.) in the statistical models. (25) Baseline differences and differences in longitudinal trajectories in each scale by race were estimated and pooled using Rubin’s Rules.(26) A sensitivity analysis excluding individuals who were censored due to being off-study was conducted. Figures depicting these trajectories by race using model coefficients were created to visualize trends. The longitudinal analyses were additionally stratified by disease state at enrollment to determine differences for participants with mHSPC and CRPC.
Additional methods information can be found in the Supplementary Methods. All analyses were completed using R version 4.1.0 with statistical significance assessed at the 0.05 level.
Patient involvement
A Black advanced prostate cancer survivor and long-time patient of one of the IRONMAN lead investigators with decades of experience as an advocate in his community was involved in setting the research question, study design, interpretation of the research findings, and review of the manuscript. With the goal of increasing the accessibility of this manuscript to patients and individuals outside of academia, we have included a glossary of technical terms in the supplementary material.
Results
Participant characteristics
This study included 879 participants from IRONMAN self-identifying as White (N=704, 80%) or Black (N=175, 20%) and receiving care at 38 study sites across the US (Figure 1, Supplementary Table S1). IRONMAN participants enrolled outside of the US (N=1979), missing data on disease state (N=7), and missing race data or identifying with a race that was not Black or White (N=227) were excluded from the analysis to specifically focus on QoL disparities within the US context of race. Demographic and clinical characteristics for the sample, stratified by self-reported race, are shown in Table 1. For the entire cohort, the mean age at enrollment was 69.1 years with a standard deviation of 8.9 years, and most of the participants (65.2%) had mHSPC compared to CRPC (34.8%).
Figure 1:
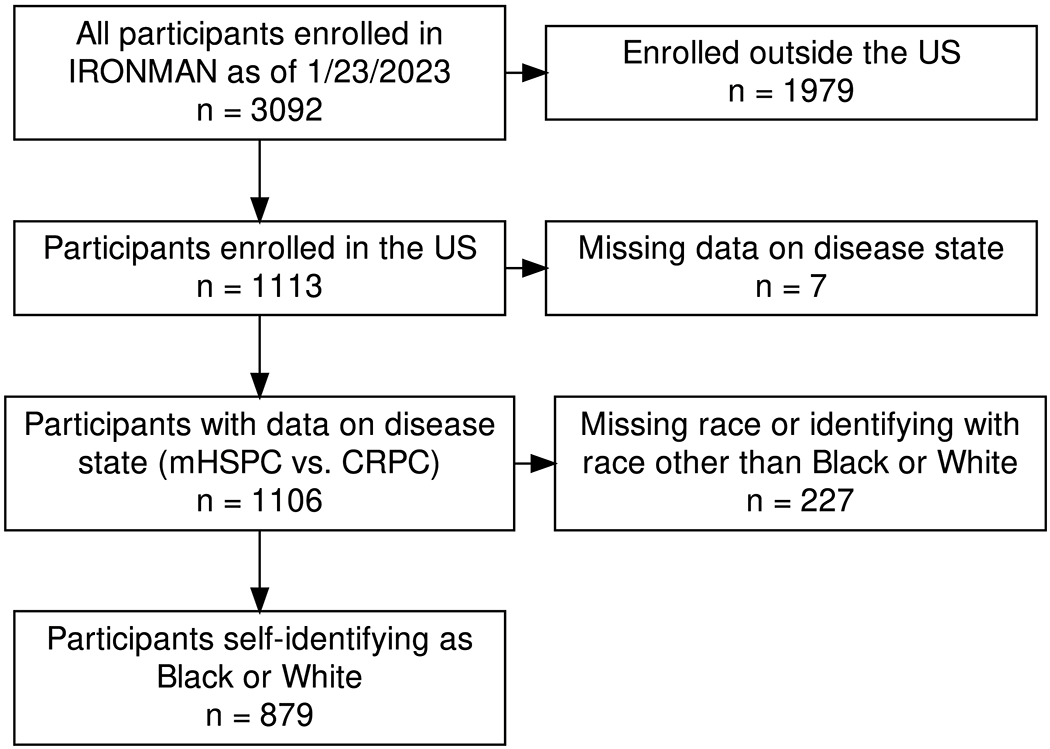
Selection of study population for analysis of patient-reported outcome measures (IRONMAN, 2017-2023)
Table 1:
Cohort demographic and clinical characteristics by self-reported race (N = 879), 2017-2023
| White (N=704) |
Black (N=175) |
|
|---|---|---|
| Age at study entry, years | ||
| Mean (SD) | 69.5 (9.0) | 67.2 (8.7) |
| Hispanic/Latino | ||
| No | 657 (97%) | 156 (96%) |
| Yes | 22 (3%) | 6 (4%) |
| Missing | n = 25 | n = 13 |
| Disease state at enrollment | ||
| CRPC | 241 (34%) | 65 (37%) |
| mHSPC | 463 (66%) | 110 (63%) |
| Highest education level at baseline | ||
| Less than College | 29 (14%) | 18 (43%) |
| Some College or Bachelor’s Degree | 72 (35%) | 13 (31%) |
| Vocational School/Program | 2 (1%) | 1 (2%) |
| Graduate Degree | 101 (49%) | 9 (21%) |
| Other | 3 (1%) | 1 (2%) |
| Missing | n = 497 | n = 133 |
| Marital status at baseline | ||
| Married | 545 (78%) | 88 (51%) |
| In a Civil Partnership | 20 (3%) | 2 (1%) |
| Widowed | 29 (4%) | 12 (7%) |
| Divorced/Separated | 76 (11%) | 43 (25%) |
| Never Married | 28 (4%) | 26 (15%) |
| Missing | n = 6 | n = 4 |
| Employment status at baseline | ||
| Retired | 408 (58%) | 82 (48%) |
| Working Full-Time | 200 (29%) | 45 (26%) |
| Working Part-Time | 58 (8%) | 11 (6%) |
| Unemployed | 12 (2%) | 16 (9%) |
| Disabled | 22 (3%) | 17 (10%) |
| Missing | n = 4 | n = 4 |
| Member of national military at baseline | ||
| Yes, currently or previously | 182 (33%) | 37 (28%) |
| No, I have never served in the national military | 364 (67%) | 97 (72%) |
| Missing | n = 158 | n = 41 |
| Prostatectomy or biopsy gleason score | ||
| 6 or less | 26 (5%) | 3 (2%) |
| 7 | 163 (28%) | 45 (34%) |
| 8 | 106 (18%) | 20 (15%) |
| 9-10 | 278 (49%) | 63 (48%) |
| Missing | n = 131 | n = 44 |
| First on-study PSA (ng/mL) | ||
| Mean (SD) | 88.6 (484.8) | 156.9 (396.3) |
| Missing | n = 33 | n = 8 |
| Metastases at baseline | ||
| No | 65 (9%) | 12 (7%) |
| Yes | 639 (91%) | 163 (93%) |
| Type of health center | ||
| Clinic | 30 (4%) | 7 (4%) |
| Hospital | 125 (18%) | 21 (12%) |
| NCI-designated | 535 (76%) | 131 (75%) |
| VA | 14 (2%) | 16 (9%) |
| Time on study (months) | ||
| Mean (SD) | 28.9 (17.4) | 24.8 (17.2) |
mHSPC = metastatic hormone-sensitive prostate cancer
CRPC = castration resistant prostate cancer
PSA = prostate-specific antigen
Differences by self-reported race were noted across many of the demographic and clinical characteristics (Table 1). Black participants were diagnosed with advanced prostate cancer at a younger age, reported lower education, were less likely to be married, were more likely to be disabled, had higher first on-study PSA levels, and had a shorter time on study on average compared to White participants. The most commonly received therapies at any point in the first year on study were androgen deprivation therapy (ADT) (88.9%), androgen receptor signaling inhibitors (ARSIs) (61.7%), and chemotherapy (17.5%) (Supplementary Table S2).
Overview of missing data
The proportion of participants completing questionnaires, missing questionnaires, or being off-study is represented in Figure 2A. Eighty-seven percent of participants completed the baseline questionnaire, declining to 74% completion by individuals on-study at month 12.
Figure 2: Overview of longitudinal missing data of cohort in first year of enrollment.
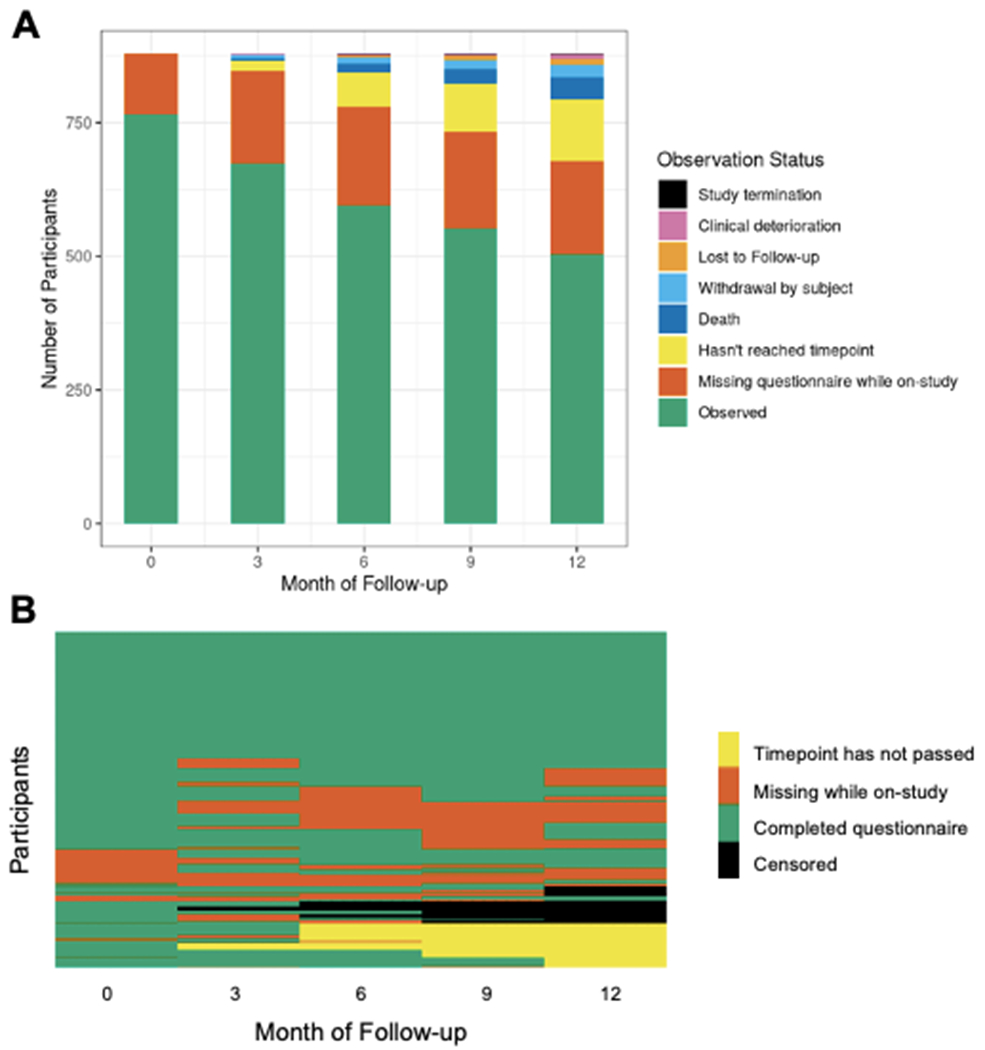
Figure 2A: Proportion of questionnaire completeness and reasons for incompleteness at each timepoint. Figure 2B: Longitudinal completion of questionnaires for each participant (N=897).
The longitudinal missing data patterns for full questionnaires are depicted in Figure 2B. Thirty-nine percent of participants completed questionnaires at all five timepoints. The remainder of study participants exhibited 30 distinct missing non-monotone data patterns. Participants who completed the baseline questionnaire were older, had lower education, were more likely to be retired, and had lower first on-study PSA compared to those who did not complete the baseline questionnaire (Supplementary Table S3). Amongst participants who were on study at month 12 and reached that timepoint, participants who completed the month 12 questionnaire had higher education, were more likely to be retired, and had lower first on-study PSA compared to those who did not complete the month 12 questionnaire (Supplementary Table S4).
Baseline differences in QoL by race
Baseline results from the longitudinal analyses are shown in Table 2. Overall, participants tended to have high functioning and low symptom burden across each of the scales. White participants had mean functioning scale scores ranging from 80.4 (95% CI 77.7, 83.2) for role functioning to 84.2 (95% CI 82.3, 86.2) for cognitive functioning (on a scale of 0-100; higher score is indicative of higher QoL) at the baseline questionnaire. Symptom burden for White participants was generally low, and the most disruptive symptoms for White participants were sleep problems with a mean scale score of 30.3 (95% CI 27.6, 33.0) and fatigue with a mean scale score of 30.1 (95% CI 27.7, 32.6) (on a scale of 0-100; higher score is indicative of worse QoL).
Table 2:
EORTC QLQ-C30 quality of life scale scores at study enrollment for White and Black participants (N = 879)
| Score for White participants Mean (95% CI), N = 704 |
Difference for Black participants Mean (95% CI), N = 175a |
Standard deviation of participant random effect | Standard deviation of site random effect | ||
|---|---|---|---|---|---|
| UNADJUSTED | ADJUSTED | ||||
| Global Quality of Life (QL) 1 | 70.9 (68.8, 73.0) | −0.6 (−4.0, 2.9) | −0.7 (−4.2, 2.8) | 15.4 | 3.3 |
| Functioning Scales 1 | |||||
| Physical (PF) | 84.1 (81.6, 86.6) | −1.0 (−4.3, 2.3) | −2.0 (−5.2, 1.2) | 15.0 | 4.9 |
| Emotional (EF) | 81.3 (79.3, 83.1) | 4.4 (1.4, 7.5) | 5.0 (2.0, 8.0) | 13.6 | 3.0 |
| Social (SF) | 80.5 (78.1, 83.0) | 0.7 (−3.1, 4.6) | 0.9 (−3.0, 4.7) | 16.3 | 4.6 |
| Role (RF) | 80.4 (77.7, 83.2) | −0.2 (−4.6, 4.2) | −0.5 (−4.9, 3.9) | 18.7 | 5.0 |
| Cognitive (CF) | 84.2 (82.3, 86.2) | 0.5 (−2.5, 3.6) | 0.4 (−2.6, 3.5) | 13.8 | 3.5 |
| Symptom Scales 2 | |||||
| Fatigue (FA) | 30.1 (27.7, 32.6) | −1.8 (−5.7, 2.0) | −1.8 (−5.7, 2.1) | 17.3 | 4.5 |
| Nausea/vomiting (NV) | 4.1 (3.3, 4.9) | 2.8 (1.0, 4.6) | 2.4 (0.6, 4.1) | 7.1 | 0.2 |
| Pain (PA) | 19.0 (16.4, 21.6) | 5.1 (0.9, 9.3) | 4.5 (0.3, 8.6) | 18.3 | 4.9 |
| Dyspnea (DY) | 13.8 (11.7, 15.9) | 0.6 (−3.2, 4.4) | 1.2 (−2.7, 5.0) | 17.4 | 2.9 |
| Sleep (SL) | 30.3 (27.6, 33.0) | −7.3 (−11.9, −2.7) | −7.9 (−12.5, −3.3) | 20.0 | 3.7 |
| Appetite (AP) | 10.7 (8.8, 12.6) | 2.3 (−1.1, 5.7) | 2.2 (−1.2, 5.6) | 13.9 | 2.8 |
| Constipation (CO) | 12.3 (10.6, 14.0) | 6.0 (2.5, 9.5) | 6.3 (2.9, 9.8) | 14.5 | 1.4 |
| Diarrhea (DI) | 10.3 (8.5, 12.1) | −1.4 (−4.4, 1.6) | −1.7 (−4.7, 1.3) | 11.4 | 3.1 |
| Financial insecurity (FI) | 16.4 (13.8, 19.0) | 7.0 (2.7, 11.4) | 5.7 (1.4, 10.0) | 20.2 | 4.2 |
Bolded represents statistical significance at the 0.05 level.
Unadjusted model includes race as the only covariate. Adjusted model additionally includes age and disease state (mHSPC or CRPC) at study enrollment.
All scale scores are rated on a scale of 0-100;
a higher score is indicative of higher quality of life for the global and functioning scales,
while a lower score is indicative of a higher quality of life for the symptom scales.
Interpretation: mean change in EORTC scale score at enrollment for Black participants compared to White participants. For the global and functioning scales, a positive number represents higher quality of life for Black participants compared to White participants. For the symptom scales, a positive number represents lower quality of life for Black participants compared to White participants.
Compared to White participants, Black participants reported several differences in QoL domains at baseline. Black participants had better emotional functioning (comprising questions about anxious and depressive symptoms) at baseline (increase of mean 4.4 percentage points, 95% CI 1.4, 7.5) compared to White participants. However, Black participants reported worse pain (5.1 point increase, 95% CI 0.9, 9.3) and financial insecurity (7.0 point increase, 95% CI 2.7, 11.4) compared to White participants. Scale scores varied substantially more between participants than between study sites.
Longitudinal differences in QoL by race
Trajectory results from the longitudinal analyses are provided in Table 3, with a graphical representation provided in Figure 3. For the majority of scales, White participants showed a decline in QoL over time. Role functioning (the ability to accomplish daily activities) declined most sharply of the functioning scales, with scores for White participants losing 0.7 percentage points on average per month (95% CI −0.8, −0.5). Fatigue worsened most sharply of the symptom scales, with scores for White participants gaining 0.6 percentage points on average per month (95% CI 0.5, 0.7).
Table 3:
Longitudinal changes in EORTC QLQ-C30 scale per month for White and Black participants during the first month after study enrollment (N = 879)
| Change per month for White participantsa Mean (95% CI), N = 704 |
Additional change for Black participantsb Mean (95% CI), N = 175 |
|||
|---|---|---|---|---|
|
|
||||
| UNADJUSTED | ADJUSTED | UNADJUSTED | ADJUSTED | |
| Global Quality of Life (QL) 1 | −0.4 (−0.5, −0.2) | −0.4 (−0.5, −0.2) | 0.1 (−0.2, 0.4) | −0.1 (−0.2, 0.1) |
| Functioning Scales 1 | ||||
| Physical (PF) | −0.6 (−0.7, −0.5) | −0.6 (−0.7, −0.5) | 0.1 (−0.1, 0.4) | −0.4 (−0.5, −0.3) |
| Emotional (EF) | −0.1 (−0.2, 0) | −0.1 (−0.2, 0) | 0.1 (−0.2, 0.4) | 0.3 (0.2, 0.4) |
| Social (SF) | −0.3 (−0.4, −0.1) | −0.3 (−0.4, −0.1) | 0.1 (−0.3, 0.5) | 0.1 (−0.1, 0.2) |
| Role (RF) | −0.7 (−0.8, −0.5) | −0.6 (−0.8, −0.5) | 0.3 (−0.1, 0.7) | −0.1 (−0.3, 0) |
| Cognitive (CF) | −0.3 (−0.5, −0.2) | −0.3 (−0.5, −0.2) | 0.3 (0, 0.7) | 0 (−0.1, 0.1) |
| Symptom Scales 2 | ||||
| Fatigue (FA) | 0.6 (0.5, 0.7) | 0.6 (0.5, 0.7) | −0.5 (−0.9, −0.2) | 0 (−0.1, 0.2) |
| Nausea/vomiting (NV) | 0 (−0.1, 0.1) | 0 (−0.1, 0.1) | −0.1 (−0.3, 0.1) | −0.2 (−0.2, −0.1) |
| Pain (PA) | 0.4 (0.2, 0.6) | 0.4 (0.2, 0.6) | −0.2 (−0.6, 0.2) | −0.3 (−0.4, −0.1) |
| Dyspnea (DY) | 0.6 (0.4, 0.7) | 0.6 (0.4, 0.7) | −0.1 (−0.4, 0.3) | 0.2 (0, 0.3) |
| Sleep (SL) | 0.1 (−0.1, 0.3) | 0.1 (−0.1, 0.3) | 0.2 (−0.3, 0.6) | −0.3 (−0.5, −0.2) |
| Appetite (AP) | 0.1 (−0.1, 0.2) | 0 (−0.1, 0.2) | 0 (−0.4, 0.4) | 0 (−0.1, 0.1) |
| Constipation (CO) | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.4) | −0.3 (−0.7, 0.1) | 0.1 (0, 0.2) |
| Diarrhea (DI) | 0.1 (0, 0.2) | 0.1 (−0.1, 0.2) | −0.1 (−0.5, 0.2) | −0.1 (−0.2, 0) |
| Financial insecurity (FI) | −0.1 (−0.3, 0.1) | −0.1 (−0.3, 0) | −0.2 (−0.6, 0.2) | −0.6 (−0.8, −0.4) |
Bolded represents statistical significance at the 0.05 level.
Unadjusted model includes race as the only covariate. Adjusted model additionally includes age and disease state (mHSPC or CRPC) at study enrollment.
All scale scores are rated on a scale of 0-100;
a higher score is indicative of higher quality of life for the global and functioning scales,
while a lower score is indicative of a higher quality of life for the symptom scales.
Interpretation: the mean change in EORTC scale score per month for White participants. For the global and functioning scales, a positive number represents an increase in quality of life over time. For the symptom scales, a positive number represents a decrease in quality of life over time.
Interpretation: the mean additional change in EORTC scale score per month for Black participants compared to White participants. Adding this number to the change in score per month for White participants gives the change in score per month for Black participants.
Figure 3: Longitudinal trajectories in EORTC QLQ-C30 scale scores.
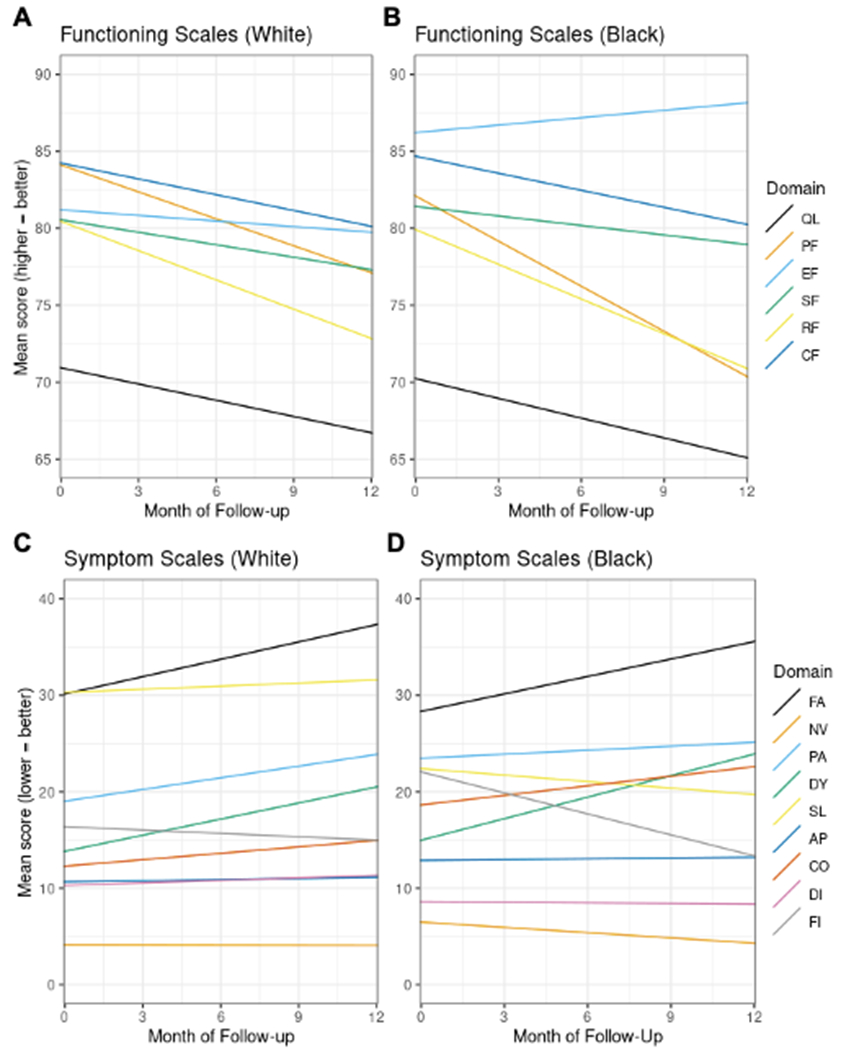
Figure 3A: Trajectories of functioning scales for White participants. Figure 3B: Trajectories of functioning scales for Black participants. Figure 3C: Trajectories of symptom scales for White participants. Figure 3D: Trajectories of symptom scales for Black participants. QL = global quality of life, PF = physical functioning, EF = emotional functioning, SF = social functioning, RF = role functioning, CF = cognitive function, FA = fatigue, NV = nausea/vomiting, PA = pain, DY = dyspnea, SL = sleep, AP = appetite, CO = constipation, DI = diarrhea, FI = financial insecurity
Black and White participants had similar trajectories in QoL over their first year after enrollment. In a few scales, Black participants had slower decline or faster improvement compared White participants. Most notably, reported financial security for Black participants improved by an additional 0.6 percentage points per month compared to White participants (95% CI −0.8, −0.4). The results for both baseline and longitudinal differences in the scale scores were robust to sensitivity analyses with different values for the missing indicator in the MICE procedure (Supplementary Table S5) as well as when individuals who were off study at any point in the first year were excluded (Supplementary Table S6). When stratified by disease state at study enrollment, results were largely the same across participants with mHSPC and CRPC (Supplementary Table S7 and S8).
Discussion
In a nationwide sample of 879 individuals with advanced prostate cancer in the US participating in the IRONMAN registry, we found that our study population had better functioning and fewer symptoms at study enrollment compared to the EORTC recurrent/metastatic prostate cancer reference population across all but two scales (diarrhea and financial insecurity).(27) For example, compared to our study population, the EORTC reference population had poorer global QoL (mean 62.1) and role functioning (mean 67.0) and worse pain (mean 38.6) and constipation (mean 27.1). These differences are likely due to the EORTC reference population being more diverse and representative of all individuals with advanced prostate cancer (rather than only those receiving care at highly-resourced IRONMAN study sites); additionally, the EORTC population only includes individuals who have not yet begun treatment for their prostate cancer, potentially resulting in lower QoL due to higher disease burden at the time of questionnaire completion.
At study enrollment, Black and White participants reported a number of differences in QoL, and the majority of statistically significant differences that we found by race at baseline also reach the 5-point minimally important difference recommended by the EORTC; thus, our findings represent clinically meaningful racial differences as well. Some previous studies in populations with localized prostate cancer suggested that Black individuals have poorer QoL in all domains at baseline compared to White individuals,(9,28) while others reported similar QoL across domains by race (29,30) or higher QoL in Black individuals.(31–33) Our study suggests that, at the time of new diagnosis with advanced prostate cancer, Black individuals have either similar or worse QoL compared to White individuals. One exception to this is that Black participants reported better baseline emotional functioning than White participants in our study. A 2014 study of 50,856 individuals with prostate cancer in the SEER-Medicare database similarly found that Black individuals had a lower risk of clinically-diagnosed mental health disorders compared to White individuals.(32) These findings could be a result of increased stigma around mental health disorders in Black communities leading to underreporting of true symptoms in our study population.(34) Assuming true symptoms were reported, one of many possible explanations for higher emotional functioning in Black participants is higher levels of spirituality leading to better QoL. Numerous studies have shown that spirituality and religiosity are positively associated with well-being in prostate cancer populations often due to increased hopefulness and positivity,(35) and this is particularly important for Black populations where spirituality tends to be an integral part of daily life.(36) Health professionals should be supportive of the role that spirituality can play to support overall QoL in Black populations.
Longitudinally, QoL generally declined for both Black and White participants in our study during their first year after enrollment in IRONMAN. A previous study of White individuals with advanced prostate cancer found declines in QoL corresponding to between 3 and 17 points on the EORTC scales over the first 9 months after enrollment.(15) We observed declines in the QoL scales by 3-7 points in our study population over the first year, indicating that, while both populations experienced declines in QoL over time, the IRONMAN population exhibited a less steep decline in QoL compared to this previous study. Again, this is likely due to the IRONMAN population most commonly receiving care at highly-resourced centers that can provide additional supports for patients when needed. Regardless, a monthly change of approximately 0.4 percentage points per month in our study correlates to the clinically meaningful change of 5 percentage points in a year,(22) demonstrating that the majority of statistically significant changes that we find in QoL over time are also clinically meaningful for the participant and their cancer care.
Many factors can mediate the association between race and QoL in patients with prostate cancer and are worth investigating in future studies as potential points of intervention. One such potential mediator is therapy received, especially because Black individuals with advanced prostate cancer are less likely to receive aggressive treatments compared to White individuals.(37) The majority of participants in our study population received ADT and/or ARSIs in their first year on study. Multiple randomized controlled trials in advanced prostate cancer populations have shown changes in QoL after treatment with various combinations of ADT and ARSIs with and without other treatment options including chemotherapy and radiotherapy for advanced prostate cancer.(38–41) These therapies have the ability to improve QoL by decreasing disease burden; however, these therapies are also known to have side effect profiles that can worsen QoL including increased risk of inflammatory rheumatic diseases and back pain.(42,43) Health professionals should monitor treatment side effects and adjust regimens accordingly to ensure that QoL is negatively impacted as little as possible.
Other potential mediators are social factors that differ by race such as access to social determinants of health resources that support a nutritious diet, physical activity and dietary supplements, quiet living spaces to support high quality sleep, and access to healthcare resources to decrease treatment side effects, among others.(44) Health institutions should strive to support their patients’ socioeconomic needs outside the hospital as much as possible as these are fundamental for improving QoL and overall health. Our study lays the foundation for future studies to investigate the mechanisms underlying differences in QoL seen here and determine the impact of specific interventions on QoL and overall survival.
There are several potential limitations of this study. First, this study focuses specifically on participants self-identifying their race as either Black or White; it is important to expand this research amongst more diverse populations. Second, the EORTC QLQ-C30 questionnaire has not specifically been validated in this study population; however, the majority of scales have shown high reliability in a racially diverse population over the age of 50 years with similar factor structures in Black and White individuals that reflect our study population.(45,46) Finally, these results may not be generalizable to individuals who choose not to participate in IRONMAN or individuals receiving care at other health centers within or outside of the US. Centers participating in IRONMAN tend to be highly-resourced and located in urban environments; individuals living in more rural areas of the US or receiving care at urban centers with less clinical trial infrastructure could have different distributions and trajectories of QoL.
Our study expands QoL research into a more racially diverse population of individuals diagnosed with advanced prostate cancer. We found that Black participants tended to report poorer QoL at diagnosis compared to White participants, and QoL decreased similarly over time for both groups. We identified clinically meaningful increased pain, constipation, and financial insecurity for Black participants; health professionals should ask about and address these symptoms as needed with clinical treatments and support navigating healthcare costs to improve QoL for this population. As QoL decreased over the first year on study for both Black and White participants, there is a need for health professionals to monitor QoL longitudinally and adjust support as needed to improve survivorship. Our study additionally highlights opportunities for deeper investigation into QoL interventions to improve the prostate cancer survivorship experience and potentially improve overall survival for this patient population, particularly for Black individuals who face the greatest burden of advanced prostate cancer.
Supplementary Material
Acknowledgments
The authors would like to thank the participants enrolled in the IRONMAN registry, without whom this research would not be possible. We would also like to thank the IRONMAN investigators and study teams at the 38 sites included in this study for their efforts to enroll and support the IRONMAN participants.
Funding
E.M. Rencsok was supported by the National Cancer Institute [F30CA264965] and the National Institute of General Medical Sciences [T32GM007753, T32GM144273]; L.A. Mucci is a Prostate Cancer Foundation Young Investigator; D.J. George and L.A. Mucci are recipients of the Prostate Cancer Foundation Challenge Award; the International Registry for Men with Advanced Prostate Cancer (IRONMAN, NCT 03151629) is funded by Amgen, AstraZeneca, Astellas, Bayer, Janssen, Merck, and Sanofi. This project is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Competing Interests
AM consults for Astellas, AstraZeneca, AAA, Bayer, Dendreon, Exelixis, Janssen, Pfizer, Myovant, Novartis, Myriad Genetics, Lantheus, Telix, Sanofi and receives research funding from Astellas, Bayer, Pfizer, Myovant, Sanofi, SeaGen.
PB consults for Astellas, AVEO Oncology, Bayer, BMS, Dendreon, Eisai, Exelixis, Janssen, EMD Serono, Guardant Health, Pfizer, Seattle Genetics; receives research support from AstraZeneca, AVEO Oncology, BlueEarth Diagnostics, Merck, Natera, Caris Life Sciences; and is part of the speaker’s bureau for Bayer, Caris Life Sciences, Pfizer, Myovant, Natera.
HHC receives institutional research support from Astellas, Clovis Oncology, Color Genomics, Janssen, Medivation, Promontory Therapeutics, Sanofi; consults for AstraZeneca; and receives royalties from UpToDate. RD consults for Astellas, Aveo, Bayer, Exelixis, Gilead, Hinova, Janssen, Merck, Pfizer, Sanofi Genzyme, Tavanta.
EH consults for Astellas Pharma, Bayer, Janssen Research & Development LLC, Sanofi; receives paid travel from Astellas Pharma, Caris Life Sciences, Sanofi, Seattle Genetics; receives research funding from Astellas Pharma, Arvinas, AstraZeneca, BioXcel Therapeutics, Bristol-Myers Squibb, Calibr, Calithera Biosciences Inc, Caris Life Sciences, Corcept Therapeutics, Corvis Pharmaceuticals, Daiichi Sankyo Inc, Eisai Inc, Exelixis, Five Prime Therapeutics, Fortis Therapeutics, GlaxoSmithKline, Gilead Sciences Inc, Harpoon Therapeutics, Hoffman-La Roche, Infinity Pharmaceuticals, iTeos Therapeutics, Janssen Research & Development LLC, Merck Sharp & Dohme Merck, Mirati Therapeutics, Modra Pharmaceuticals, Oncolys BioPharma, Peloton Therapeutics Inc, Pfizer, Pharmacyclics LLC, POINT Biopharma, Seattle Genetics; and receives honoraria from Bayer, Sanofi, and Seattle Genetics.
RRM consults for Aveo, AstraZeneca, Bayer, BMS, Calithera, Caris, Dendreon, Exelixis, JNJ, Lilly, Myovant, Merck, Novartis, Pfizer, Sanofi, Sorrento Therapeutics, Telix, Tempus; receives research funding from Bayer, Tempus, AstraZeneca, Oncternal Therapeutics.
DR receives institutional research support from Janssen, Bayer, AstraZeneca, Genentech, BMS/Celgene, Taiho, Promontory; serves on the board for Janssen, AstraZeneca, Bayer, Myovant, Genentech, Promontory, BMS/Celgene.
ST receives institutional research support from Sanofi, Medivation, Astellas, Janssen, Amgen, Progenics, Dendreon, Lilly, Genentech, Newlink, BMS, Inovio, AstraZeneca, Immunomedics, Aveo, Rexahn, Atlab, Boehringer Ingelheim, Millennium, Bayer, Merck, Abbvie, Karyopharm, Endocyte, Clovis, Seattle Genetics, Novartis, Gilead, POINT Biopharma, Ambrx; consults for Sanofi, Medivation/Astellas, Dendreon, Janssen, Genentech, Bayer, Endocyte, Eisai, Immunomedics, Karyopharm, Abbvie, Tolmar, Seattle Genetics, Amgen, Clovis, QED, Pfizer, AAA/Novartis, Clarity, Genomic Health, POINT Biopharma, Blue Earth, AIkido Pharma, Telix Pharma, Convergent Therapeutics, EMD Serono, Myovant, Merck, Atlab Pharma, Phosplatin Therapeutics, Amgen, Ambrx; has a patent on biomarkers for sacituzumab govitecan therapy (Immunomedics / Gilead / Weill Cornell).
YW receives institutional research support from Arvinas, Clovis Oncology; consults for Janssen.
PWK has investment interest in Convergent Therapeutics, Context Therapeutics LLC, ESSA Pharma; serves on the board for Convergent Therapeutics, Context Therapeutics, ESSA Pharma; consults for ImmunisAI, PrognomIQ.
LAM serves on the board of Convergent Therapeutics; consults for Bayer; receives institutional research support from Janssen, AstraZeneca. All other authors report no conflicts of interest.
Footnotes
Compliance with Ethical Standards
1) The original project underwent ethics review and was approved by the Institutional Review Board of the Harvard T. H. Chan School of Public Health.
2) Informed consent was obtained from all participants in the IRONMAN Registry and included consent to access their personal demographic and health information in addition to use of their de-identified data for analyses using IRONMAN Registry data.
3) The authors received access to de-identified IRONMAN registry data for the analyses presented here through an arrangement between the Harvard T. H. Chan School of Public Health and the Prostate Cancer Clinical Trials Consortium (the Clinical and Data Coordination Center for the IRONMAN Registry).
References
- 1.National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer stat facts: Prostate cancer [Internet] 2021. [cited 2022 Apr 3]. Available from: https://seer.cancer.gov/statfacts/html/prost.html
- 2.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011. Jun 15;71(9):985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lillard JW, Moses KA, Mahal BA, George DJ. Racial disparities in Black men with prostate cancer: A literature review. 2022. [cited 2023 May 15]; Available from: 10.1002/cncr.34433. [DOI] [PMC free article] [PubMed]
- 4.Aggarwal RR, Feng FY, Small EJ. Emerging categories of disease in advanced prostate cancer and their therapeutic implications [Internet]. Cancer Network. 2017. [cited 2022 May 5]. Available from: https://www.cancernetwork.com/view/emerging-categories-disease-advanced-prostate-cancer-and-their-therapeutic-implications [PubMed] [Google Scholar]
- 5.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA [Internet]. 2017. Jul 11 [cited 2022 Aug 23];318(2):197–8. Available from: https://jamanetwork.com/journals/jama/fullarticle/2630810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husson O, Rooij BH de, Kieffer J, Oerlemans S, Mols F, Aaronson NK, van der Graaf WTA, van de Poll-Franse LV. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the “Real-World”: Results from the Population-Based PROFILES Registry. Oncologist [Internet]. 2020. Apr 1 [cited 2022 Aug 23];25(4):e722. Available from: /pmc/articles/PMC7160310/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubeck DP, Kim H, Grossfeld G, Ray P, Penson DF, Flanders SC, Carroll PR. HEALTH RELATED QUALITY OF LIFE DIFFERENCES BETWEEN BLACK AND WHITE MEN WITH PROSTATE CANCER: DATA FROM THE CANCER OF THE PROSTATE STRATEGIC UROLOGIC RESEARCH ENDEAVOR. 2001; [PubMed]
- 8.Eton DT, Lepore SJ, Helgeson VS, Schulz R, Hrebinko R, Cohen J, Hakala R, Salup R ,Bonci L, Trump D, Lutins J, Brufsky A, Franz J, Camp-anella S, Long S, Schwartz R, Miller R, Benoit R. Early Quality of Life in Patients with Localized Prostate Carcinoma An Examination of Treatment-Related, Demographic, and Psychosocial Factors. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice K, Hudak J, Peay K, Elsamanoudi S, Travis J, Lockhart R, Cullen J, Black L, Houge S, Brassell S. Comprehensive quality-of-life outcomes in the setting of a multidisciplinary, equal access prostate cancer clinic. Urology. 2010;76(5):1231–8. [DOI] [PubMed] [Google Scholar]
- 10.Chhatre S, Wein AJ, Malkowicz SB, Jayadevappa R. Racial differences in well-being and cancer concerns in prostate cancer patients. Journal of Cancer Survivorship. 2011;5(2):182–90. [DOI] [PubMed] [Google Scholar]
- 11.Cella D, Ivanescu C, Holmstrom S, Bui CN, Spalding J, Fizazi K. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Annals of Oncology. 2015. Jan 1;26(1):179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saad F, Cella D, Basch E, Hadaschik BA, Mainwaring PN, Oudard S, Graff JN, Mcquarrie K, Li S, Hudgens S, Lawson J, Lopez-Gitlitz A, Yu MK, Smith MR, Small EJ. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Articles Lancet Oncol [Internet]. 2018. [cited 2023 May 15];19:1404–20. Available from: www.thelancet.com/oncology [DOI] [PubMed] [Google Scholar]
- 13.Agarwal N, Mcquarrie K, Bjartell A, Chowdhury S, Pereira AJ, Gomes S, Chung H, Ozguroglu M, Soto AJ, Merseburger AS, Uemura H, Ye D, Given R, Cella D, Basch E, Miladinovic B, Dearden L, Deprince K, Naini V, Lopez-Gitlitz A, Chi KN. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. www.thelancet.com/oncology [Internet]. 2019. [cited 2023 May 15];20. Available from: www.thelancet.com/oncology [DOI] [PubMed] [Google Scholar]
- 14.Feyerabend S, Saad F, Li T, Ito T, Diels J, Van Sanden S, De Porre P, Roiz J, Abogunrin S, Koufopoulou M, Fizazi Kl. Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: A network meta-analysis. Eur J Cancer. 2018. Nov 1;103:78–87. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan PW, Mulani PM, Fishman M, Sleep D. Quality of life findings from a multicenter, multinational, observational study of patients with metastatic hormone-refractory prostate cancer. Quality of Life Research [Internet]. 2007. May 10 [cited 2022 Aug 23];16(4):571–5. Available from: 10.1007/s11136-006-9156-2 [DOI] [PubMed] [Google Scholar]
- 16.Zajdlewicz L, Hyde MK, Lepore SJ, Gardiner RA, Chambers SK. Health-Related Quality of Life After the Diagnosis of Locally Advanced or Advanced Prostate Cancer: A Longitudinal Study. Cancer Nurs [Internet]. 2017. Sep 1 [cited 2022 Aug 23];40(5):412–9. Available from: https://pubmed.ncbi.nlm.nih.gov/28282307/ [DOI] [PubMed] [Google Scholar]
- 17.Mucci LA, Vinson J, Gold T, Gerke T, Filipenko J, Green RM, Anderson SG, Badal S, Bjartell A, Chi KN, Davis ID, Enting D, Fay AP, Lazarus J, Mateo J, McDermott R, Odedina FT, Olmos D, Omlin A, et al. IRONMAN: A Novel International Registry of Men With Advanced Prostate Cancer. JCO Glob Oncol. 2022. Nov 4;(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Organization for Research and Treatment of Cancer. EORTC QLQ-C 30 Specimen [Internet]. [cited 2022 Oct 9]. Available from: https://www.eortc.org/app/uploads/sites/2/2018/08/Specimen-QLQ-C30-English.pdf
- 19.Shih CL, Chen CH, Sheu CF, Lang HC, Hsieh CL. Validating and Improving the Reliability of the EORTC QLQ-C30 Using a Multidimensional Rasch Model. Value in Health. 2013. Jul 1;16(5):848–54. [DOI] [PubMed] [Google Scholar]
- 20.Ford ME, Havstad SL, Kart CS. Assessing the reliability of the EORTC QLQ-C30 in a sample of older African American and Caucasian adults. [DOI] [PubMed]
- 21.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. https://doi.org/101200/JCO1998161139. 2016. Sep 21;16(1):139–44. [DOI] [PubMed] [Google Scholar]
- 22.Gamper EM, Musoro JZ, Coens C, Stelmes JJ, Falato C, Groenvold M, Velikova G, Cocks K, Flechtner HH, King MIT, Bottomley A. Minimally important differences for the EORTC QLQ-C30 in prostate cancer clinical trials. BMC Cancer [Internet]. 2021. [cited 2022 Dec 29];21(1):1083. Available from: https://pubmed.ncbi.nlm.nih.gov/34620124/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EORTC Data Center. EORTC QLQ-C30 Scoring Manual. 2001;3rd edition. [Google Scholar]
- 24.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman JS, Cooper RS. Commentary: considerations for use of racial/ethnic classification in etiologic research. Am J Epidemiol [Internet]. 2001. Aug 15 [cited 2022 May 16];154(4):291–8. Available from: https://pubmed.ncbi.nlm.nih.gov/11495850/ [DOI] [PubMed] [Google Scholar]
- 26.Little RJ, Rubin DB. Statistical analysis with missing data. 2nd ed. Hoboken: John Wiley & Sons; 2014. [Google Scholar]
- 27.EORTC Quality of Life Group. EORTC QLQ-C30 Reference Values [Internet]. 2008. [cited 2023 Jan 22]. Available from: https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf
- 28.Jayadevappa R, Johnson JC, Chhatre S, Wein AJ, Malkowicz SB. Ethnic variation in return to baseline values of patient-reported outcomes in older prostate cancer patients. Cancer. 2007;109(11):2229–38. [DOI] [PubMed] [Google Scholar]
- 29.Litwin MS, Mcguigan KA, Shpall AI, Dhanani N. RECOVERY OF HEALTH RELATED QUALITY OF LIFE IN THE YEAR AFTER RADICAL PROSTATECTOMY: EARLY EXPERIENCE. Vol. 161. 1999. [PubMed] [Google Scholar]
- 30.Johnson TK, Gilliland FD, Hoffman RM, Deapen D, Penson DF, Stanford JL, Albertsen PC, Hamilton AS. Racial/ethnic differences in functional outcomes in the 5 years after diagnosis of localized prostate cancer. Journal of Clinical Oncology. 2004;22(20):4193–201 [DOI] [PubMed] [Google Scholar]
- 31.Halbert CH, Coyne J, Weathers B, Mahler B, Delmoor E, Vaughn D, Malkowicz SB, Lee D, Troxel A. Racial differences in quality of life following prostate cancer diagnosis. Urology. 2010;76(3):559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravi P, Karakiewicz PI, Roghmann F, Gandaglia G, Choueiri TK, Menon M, McKay RR, Nguyen PL, Sammon JD, Sukumar S, Varda B, Chang SL, Kibel AS, Sun M, Trinh QD. Mental health outcomes in elderly men with prostate cancer. Urologic Oncology: Seminars and Original Investigations. 2014;32(8):1333–40. [DOI] [PubMed] [Google Scholar]
- 33.Erim DO, Bensen JT, Mohler JL, Fontham ETH, Song L, Farnan L, Delacroix SE, Peters ES, Erim TN, Chen RC, Gaynes BN. Patterns and predictors of self-reported clinical diagnosis and treatment for depression in prostate cancer survivors. Cancer Med. 2019;8(8):3648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eylem O, Eylem O, De Wit L, Van Straten A, Steubl L, Melissourgaki Z, Danlsman GT, De Vries R, Kerkhof AJFM, Bhui K, Cuijpers P. Stigma for common mental disorders in racial minorities and majorities a systematic review and meta-analysis. BMC Public Health [Internet]. 2020. Jun 8 [cited 2023 May 19];20(1):1–20. Available from: 10.1186/s12889-020-08964-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickey SL, Ogunsanya ME. Quality of Life Among Black Prostate Cancer Survivors: An Integrative Review. Am J Mens Health [Internet]. 2018. Sep 1 [cited 2023 May 19];12(5):1648. Available from: /pmc/articles/PMC6142144/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatters LM, Taylor RJ, Bullard KM, Jackson JS. Race and Ethnic Differences in Religious Involvement: African Americans, Caribbean Blacks and Non-Hispanic Whites. :48109–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beebe-Dimmer JL, Ruterbusch JJ, Cooney KA, Bolton A, Schwartz K, Schwartz AG, Heath E. Racial differences in patterns of treatment among men diagnosed with de novo advanced prostate cancer: A SEER-Medicare investigation. Cancer Med [Internet]. 2019. Jun 1 [cited 2023 May 20];8(6):3325. Available from: /pmc/articles/PMC6558501/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalaf DJ, Sunderland K, Eigl BJ, Kollmannsberger CK, Ivanov N, Finch DL, Oja C, Vergidis J, Zulfiqar M, Gleave ME, Chi KN. Health-related Quality of Life for Abiraterone Plus Prednisone Versus Enzalutamide in Patients with Metastatic Castration-resistant Prostate Cancer: Results from a Phase II Randomized Trial. Eur Urol [Internet]. 2019. [cited 2022 Dec 5];75:940–7. Available from: 10.1016/j.eururo.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 39.Stenzl A, Dunshee C, De Giorgi U, Alekseev B, Iguchi T, Szmulewitz RZ, Flaig TW, Tombal B, Morlock R, Ivanescu C, Ramawamy K, Saad F, Armstrong AJ. Effect of Enzalutamide plus Androgen Deprivation Therapy on Health-related Quality of Life in Patients with Metastatic Hormone-sensitive Prostate Cancer: An Analysis of the ARCHES Randomised, Placebo-controlled, Phase 3 Study. Eur Urol. 2020. Oct 1;78(4):603–14. [DOI] [PubMed] [Google Scholar]
- 40.Boevé L, Hulshof MCCM, Verhagen PCMS, Twisk JWR, Witjes WPJ, de Vries P, van Moorselaar RJA, van Andel G, Vis AN. Patient-reported Quality of Life in Patients with Primary Metastatic Prostate Cancer Treated with Androgen Deprivation Therapy with and Without Concurrent Radiation Therapy to the Prostate in a Prospective Randomised Clinical Trial; Data from the HORRAD Trial. Eur Urol [Internet]. 2021. Feb 1 [cited 2022 Dec 5];79(2):188–97. Available from: https://research.vumc.nl/en/publications/patient-reported-quality-of-life-in-patients-with-primary-metasta [DOI] [PubMed] [Google Scholar]
- 41.Rush HL, Murphy L, Morgans AK, Clarke NW, Cook AD, Attard G, Macnair A, Dearnaley DP, Parker CC, Russell JM, Gillessen S, Matheson D, Millman R, Brawley CD, Pugh C, Tanguay JS, Jones RJ, Wagstaff J, Rudman S, et al. Quality of Life in Men with Prostate Cancer Randomly Allocated to Receive Docetaxel or Abiraterone in the STAMPEDE Trial. Journal of Clinical Oncology. 2022. Mar 10;40(8):825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drevinskaite M, Dadoniene J, Miltiniene D, Patasius A, Smailyte G. Clinical Medicine Association between Androgen Deprivation Therapy and the Risk of Inflammatory Rheumatic Diseases in Men with Prostate Cancer: Nationwide Cohort Study in Lithuania. J Clin Med [Internet]. 2022. [cited 2023 Mar 24]; Available from: 10.3390/jcm11072039 [DOI] [PMC free article] [PubMed]
- 43.Zobniw CM, Causebrook A, Fong MK. Clinical use of abiraterone in the treatment of metastatic castration-resistant prostate cancer. 2014. [cited 2023 Mar 24]; Available from: 10.2147/RRU.S29003 [DOI] [PMC free article] [PubMed]
- 44.Richardson LD, Norris M. Access to health and health care: how race and ethnicity matter. Mt Sinai J Med [Internet]. 2010. Mar [cited 2023 Mar 13];77(2):166–77. Available from: https://pubmed.ncbi.nlm.nih.gov/20309927/ [DOI] [PubMed] [Google Scholar]
- 45.Morgans AK, Van Bommel ACM, Stowell C, Abrahm JL, Basch E, Bekelman JE, Berry DL, Bossi A, Davis ID, de Reijke TM, Denis LJ, Evans SM, Fleshner NE, George DJ, Kiefert J, Lin DW, Matthew AG, McDermott R, Payne H, et al. Development of a Standardized Set of Patient-centered Outcomes for Advanced Prostate Cancer: An International Effort for a Unified Approach. Eur Urol. 2015;68(5):891–8. [DOI] [PubMed] [Google Scholar]
- 46.Ford ME, Havstad SL, Kart CS. Assessing the reliability of the EORTC QLQ-C30 in a sample of older African American and Caucasian adults. Quality of Life Research. 2001;10:533–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


