Abstract
A vacuolar H+-ATPase-negative mutant of Saccharomyces cerevisiae was highly sensitive to nickel ion. Accumulation of nickel ion in the cells of this mutant of less than 60% of the value for the parent strain arrested growth, suggesting a role for this ATPase in sequestering nickel ion into vacuoles. An artificially imposed pH gradient (interior acid) induced transient nickel ion uptake by vacuolar membrane vesicles, which was inhibited by collapse of the pH difference but not of the membrane potential. Nickel ion transport into vacuoles in a pH gradient-dependent manner is thus important for its detoxification in yeast.
Nickel ion is a heavy metal ubiquitously distributed in nature and constitutes a trace element in most living cells (3); it is reported that this metal acts as a cofactor of several enzymes, such as hydrogenase, methyl coenzyme M reductase, CO dehydrogenase, and urease, in various organisms (7). At higher concentrations, nickel ion can be very toxic to both eukaryotic and prokaryotic cells. Nickel ion potentially inhibits synthesis of macromolecules such as RNA and protein (5). Toxicity is also exhibited as an alteration in the metabolism of carbohydrate and organic ions by interfering with the roles of other trace elements, such as Mg2+ and Fe3+, and excretion of pyruvate or potassium ions from the cell probably because of damage to membrane integrity (10). A series of homeostatic mechanisms has evolved to balance the internal metal contents with the need to acquire essential metals at low levels. In bacteria, homeostasis is exhibited as reduction of the internal ion levels by energy-dependent, metal-specific extrusion systems (21). In eukaryotes, metal detoxification is exhibited as metal-conjugate formation by binding of the ion not only internally with specific molecules such as metallothionein (6) but also externally with excreted small organic metabolites (14). In Saccharomyces cerevisiae, it has been reported that nickel ion detoxification was acquired as a decrease in free nickel ion by conjugation with excreted glutathione (16). Recently, Joho et al. (11) isolated a nickel ion-resistant mutant of S. cerevisiae, which could grow without any decrease in nickel ion uptake in nickel ion-supplemented medium. In this mutant, more than 70% of the internal nickel ion is distributed in the vacuolar fractions, suggesting a role of this organelle as the detoxifying compartment. Since it was estimated that the vacuoles of this mutant also contained large amounts of histidine (9), these authors suggested a possible role of histidine-nickel ion complex formation in sequestering excess amounts of the internal nickel ion into vacuoles. Various metabolites and ions are stored in vacuoles (17, 20). Active accumulation of most of these is driven by the electrochemical potential of protons across the vacuolar membranes, which is established by electrogenic proton influxes via the vacuolar H+-ATPase (13). Accumulation of histidine in vacuoles is likely mediated by a proton-linked transport system (20), but the route of nickel ion transport into vacuoles is still unknown. In this paper, we propose the pH gradient-driven system as the pathway for penetration of nickel ion into the vacuoles of S. cerevisiae.
S. cerevisiae YPH499 (MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1), RH104 (MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 Δvma1::TRP1), or DV3T-A (MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 Δvma3::TRP1) was grown in a complex medium (yeast-peptone-dextrose [YPD]) or a completely synthetic medium (CSD) (15) containing 0.4 mM MgCl2; the medium pH was adjusted to 5.5 with 10 mM morpholinoethanesulfonic acid (MES)-NaOH (pH 5.5), since the growth of these vacuolar ATPase mutants was optimal at lower pH (8, 22). Cell growth was monitored by measuring the optical density at 540 nm with a spectrophotometer. Internal Ni2+ content was measured in cells grown in medium containing 63NiCl2 (67.8 MBq/mmol); the cells were washed twice with 50 mM potassium phosphate buffer (pH 5.8), and the radioactivity of the cells was counted. The uptake of 63Ni2+ by whole cells was assayed as described previously (15). Right-side-out vacuolar membrane vesicles were prepared from the cells grown in YPD medium as described previously (12, 17) and were suspended in 10 mM MES-Tris(hydroxymethyl)aminomethane (pH 6.9) containing 5 mM MgCl2 and 25 mM KCl, and the suspension was stored at −80°C. Artificially pH gradient (interior acidic)-dependent 63Ni2+ uptake by the vesicles was assayed as follows. The vesicles were washed four times with 5 mM MES-Tris (pH 6.9) containing 25 mM KCl and suspended in the same buffer (0.3 mg of protein/ml). After the addition of 50 μM 63NiCl2 (169.5 MBq/mmol) to the suspension, the medium pH was shifted from 6.9 to 9.0 by the addition of KOH. At intervals, the vesicles (100 μl of the suspension) were collected on the filter (Millipore [pore size, 0.4 μm]) and washed twice with 5 ml of the same buffer, and the radioactivity of the filters was counted. The intravesicular water space was estimated to be 5 μl per mg of protein as described previously (13) by the use of [3H]water and [14C]inulin.
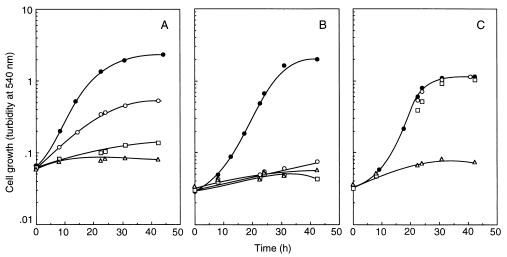
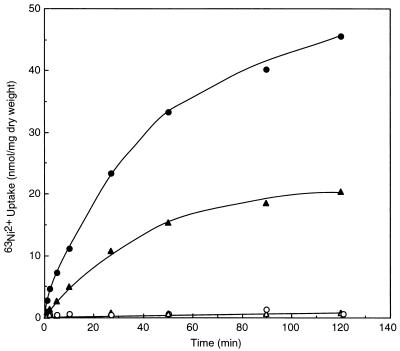
Figure 1 shows the effect of nickel ion on the growth of S. cerevisiae in CSD medium (pH 5.5) containing 0.4 mM MgCl2. The growth of strain YPH499 was nearly arrested by the addition of 0.5 mM NiCl2 (Fig. 1A). The growth of a vacuolar H+-ATPase mutant (strain RH104), in which the VMA1 gene was disrupted (8), was highly sensitive to nickel ion; 0.1 mM NiCl2 was enough for growth arrest (Fig. 1B). In the same medium, the growth of another vacuolar ATPase mutant (strain DV3T-A), in which the VMA3 gene was disrupted (22), was also inhibited by 0.1 mM NiCl2 (data not shown). Since it has been observed that the sizes and abundance of vacuoles in these vacuolar ATPase mutants did not differ so much from those of the parent strain (8, 22), the function of the vacuolar ATPase as a proton pump is linked to the sensitivity of yeast to nickel ion. Figure 2 shows the time course of 63Ni2+ uptake by whole cells. In YPH499, 63Ni2+ was taken up with a Km value of 13 μM and a Vmax value of 0.5 nmol/min/mg (dry weight). Defect of the vacuolar ATPase was exhibited by much slower velocity and a reduced level of 63Ni2+ accumulation; accumulation of 63Ni2+ by RH104 was about 50% of the value for YPH499. Nickel ion accumulation in these strains in different media was also examined. In the cells cultured in CSD medium containing 0.1 mM 63NiCl2, in which YPH499 grew but RH104 did not (Fig. 1), the amounts of the internal nickel ion of YPH499 and RH104 were 19.9 and 11.5 nmol/mg (dry weight), respectively. The vacuolar ATPase thus plays a role in vacuolar compartmentation of nickel ion; a role for the vacuole in toxic metal ion detoxification has been recently proposed by using other vacuolar mutants (19). The absolute requirement of this metal for the growth of yeast is obscure; nickel ion is usually omitted from the standard culture medium (Fig. 1). Although the significance of nickel ion in the cellular metabolism of yeast is unknown, vacuolar compartmentation of nickel ion is likely to be the method of removing the toxic metal from the cytoplasm.
FIG. 1.
Effect of nickel chloride on the growth of S. cerevisiae. S. cerevisiae YPH499 (A) or RH104 (B and C) was cultured in CSD medium containing 0.4 mM MgCl2 (A and B) or 10 mM MgCl2 (C). Nickel chloride was added to the medium at various concentrations before starting the culture, and cell growth was monitored by measuring the optical density at 540 nm. •, control; ○, 0.1 mM NiCl2; □, 0.5 mM NiCl2; ▵, 5 mM NiCl2.
FIG. 2.
Nickel ion uptake by whole cells. S. cerevisiae cells grown in YPD medium (pH 5.5) were harvested at late logarithmic phase, washed, and suspended in 20 mM MES-NaOH (pH 5.5) containing 10 mM glucose at 0.3 mg (dry weight) per ml. 63NiCl2 uptake (67.8 MBq/mmol) by YPH499 (circles) or RH104 (triangles) was assayed at 30°C (closed symbols) or 0°C (open symbols) as described in Materials and Methods.
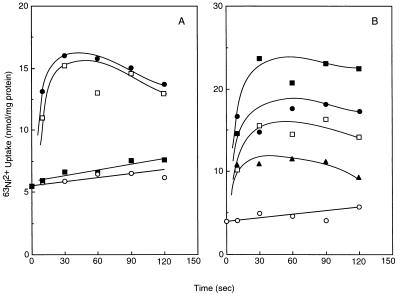
Several roles of the H+-ATPase in vacuolar compartmentation of various metabolites and ions have been reported (1). First, ATPase generates the electrochemical gradient of protons across the vacuolar membranes, which is utilized as the driving force of various proton-coupled secondary transport systems; for instance, the Ca2+/H+ antiporter plays an important role in calcium homeostasis in S. cerevisiae (1). Histidine probably taken up via the antiport system participates in complex formation with nickel ion in vacuoles (20). Second, it is reported that several cationic substances are accumulated in vacuoles by binding with polyphosphate (4); acidification of intravacuolar space by the ATPase may be important for polyphosphate synthesis (23). In our work, these possibilities with regard to the role of the ATPase in the sequestering of nickel ion into vacuoles were discriminated with the vacuolar membrane vesicles; the intravacuolar constituents were removed by disintegration of the intact vacuoles. First, we attempted to investigate Mg2+-ATP-driven 63Ni2+ uptake by vacuolar membrane vesicles; however, this was unsuccessful (data not shown). The following are several explanations for this failure: (i) free nickel ions could be decreased by chelating with ATP, (ii) nickel ion could inhibit the activity of ATPase, or (iii) magnesium ion, which is required as the substrate chelated with ATP, may interfere with Ni2+ transport. Instead, 63Ni2+ uptake of the vacuolar membrane vesicles was examined by imposing artificially a pH gradient (Fig. 3). Transient 63Ni2+ uptake was observed by shifting the external pH from 6.9 to 9.0 (Fig. 3A [closed circles]) with 3 mM KOH; the uptake was not observed by the addition of 3 mM KCl (Fig. 3A [open circles]). The membrane-permeative cation dibenzyldimethylammonium (DDA) (Fig. 3A [open squares]), collapsing the membrane potential, did not inhibit 63Ni2+ uptake, suggesting that nickel ion uptake did not result from passive uptake by the membrane potential generated by proton movements. Nickel ion uptake by a shift in pH was completely inhibited by the addition of 50 mM ammonium chloride, which collapses the pH gradient (Fig. 3A [closed squares]), suggesting that the nickel ion uptake is driven by the pH gradient across the membranes. Under experimental conditions, the established gradient of Ni2+ was calculated to be about 20 by imposing a pH gradient of 2. Assumption of an electroneutral Ni2+/2H+ antiport mechanism does not fit the data; explanation of these data and the mechanism needs further investigation.
FIG. 3.
Nickel ion uptake by vacuolar membrane vesicles. Vacuolar vesicles were prepared from cells of YPH499 grown in YPD medium as described by Ohsumi and Anraku (17). The vesicles were suspended in a buffer (5 mM MES-Tris [pH 6.9], 25 mM KCl) at 0.3 mg of protein/ml. After the addition of 50 μM 63NiCl2 (169.5 MBq/mmol) to the suspension, the external pH was shifted from 6.9 to 9.0 by the addition of 3 mM KOH. As a control, 3 mM KCl (○) instead of KOH was added. Several reagents and cations were added 30 s before the pH shift. (A) Effect of ammonia and DDA (•, control; □, 25 mM DDA; ▪, 50 mM ammonium chloride); (B) effect of divalent cations at 250 μM (•, control; □, MgCl2; ▪, CaCl2; ▴, ZnCl2).
When considering the toxicity of nickel ion in yeast, it is important to understand the pathway of nickel ion accumulation. The presence of a nickel ion-preferential transport system in yeast may be dubious, since nickel ion is not essential for the growth of yeast under standard culture conditions. It is reported that 63Ni2+ uptake by the intact cells of S. cerevisiae was competitively inhibited by Mg2+ (2, 15). 63Ni2+ uptake by whole cells in this work was also preferentially inhibited by Mg2+ but not as much by other metal ions such as Zn2+, Co2+, and Ca2+ (data not shown). In addition, the toxicity of nickel ion to S. cerevisiae cells was rescued by supplementation with higher concentrations of magnesium ion (Fig. 1C); the internal Ni2+ amount was negligible in the presence of 10 mM Mg2+. Nickel ion is probably taken up into the cells via the putative Mg2+ transport system in the plasma membrane. It is noteworthy that uptake of 63Ni2+ in the vacuolar membrane vesicles by the pH gradient was inhibited more intensely by Zn2+ than by Mg2+ and Ca2+ (Fig. 3B). Inhibition by Zn2+ was reduced at the higher concentrations of external Ni2+. Although no zinc-specific transport systems in the vacuolar membranes of S. cerevisiae cells have been identified, the presence of zinc is quite probable, since a zinc transporter in the endosomal-lysosomal compartment in mammalian cells has been reported (18). We plan to investigate this possibility.
Acknowledgments
We thank Y. Anraku and R. Hirata for providing the vacuolar ATPase mutants RH104 and DV3T-A and T. Michael for critical reading of the manuscript.
This work was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Anraku Y, Umemoto N, Hirata R, Wada Y. Structure and function of the yeast vacuolar membrane proton ATPase. J Bioenerg Biomembr. 1989;21:589–603. doi: 10.1007/BF00808115. [DOI] [PubMed] [Google Scholar]
- 2.Borst-Pauwels G W F H. Ion transport in yeast. Biochim Biophys Acta. 1981;650:88–127. doi: 10.1016/0304-4157(81)90002-2. [DOI] [PubMed] [Google Scholar]
- 3.Boyle R W, Robinson H A. Nickel ion in the natural environment. In: Sigel H, Sigel A, editors. Nickel ion and its role in biology. Vol. 23. New York, N.Y: Marcel Dekker; 1988. pp. 123–164. [Google Scholar]
- 4.Cramer C L, Davis R H. Polyphosphate-cation interaction in the amino acid-containing vacuole of Neurospora crassa. J Biol Chem. 1984;259:5152–5157. [PubMed] [Google Scholar]
- 5.Guha C, Mookerjee A. Effect of nickel ion on macromolecular synthesis in Escherichia coli K-12. Nucleus. 1979;22:45–47. [Google Scholar]
- 6.Hamer D H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- 7.Hausinger R P. Nickel ion utilization by microorganisms. Microbiol Rev. 1987;51:22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirata R, Ohsumi Y, Nakano A, Kawasaki H, Suzuki K, Anraku Y. Molecular structure of a gene, VMA1, encoding the catalytic subunit of H+-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1990;265:6726–6733. [PubMed] [Google Scholar]
- 9.Joho M, Inouhe M, Tohoyama H, Murayama T. A possible role of histidine in a nickel ion resistant mechanism of Saccharomyces cerevisiae. FEMS Microbiol Lett. 1990;66:333–338. doi: 10.1016/0378-1097(90)90308-d. [DOI] [PubMed] [Google Scholar]
- 10.Joho M, Inouhe M, Tohoyama H, Murayama T. Nickel ion resistance mechanisms in yeasts and other fungi. J Ind Microbiol. 1995;14:164–168. doi: 10.1007/BF01569899. [DOI] [PubMed] [Google Scholar]
- 11.Joho M, Ishikawa Y, Kunikane M, Inouhe M, Tohoyama H, Murayama T. The subcellular distribution of nickel ion in Ni-sensitive and Ni-resistant strains of Saccharomyces cerevisiae. Microbios. 1992;71:149–159. [PubMed] [Google Scholar]
- 12.Kakinuma Y, Masuda N, Igarashi K. Proton potential-dependent polyamine transport system in vacuolar membrane vesicles of Saccharomyces cerevisiae. Biochim Biophys Acta. 1993;1107:126–130. doi: 10.1016/0005-2736(92)90337-l. [DOI] [PubMed] [Google Scholar]
- 13.Kakinuma Y, Ohsumi Y, Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1981;256:10859–10863. [PubMed] [Google Scholar]
- 14.Lee J, Reevews R D, Brooks R R, Jaffré T. Isolation and identification of a citrate-complex of nickel ion from nickel ion-accumulating plants. Phytochemistry. 1977;16:1503–1505. [Google Scholar]
- 15.Maruyama T, Masuda N, Kakinuma Y, Igarashi K. Polyamine-sensitive magnesium ion transport in Saccharomyces cerevisiae. Biochim Biophys Acta. 1993;1194:289–295. doi: 10.1016/0005-2736(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 16.Murata K, Fukuda Y, Shimosaka M, Watanabe K, Saikusa T, Kimura A. Phenotypic character of the methylglyoxal resistance gene in Saccharomyces cerevisiae: expression in Escherichia coli and application to breeding wild-type yeast strains. Appl Environ Microbiol. 1985;50:1200–1207. doi: 10.1128/aem.50.5.1200-1207.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohsumi Y, Anraku Y. Active transport of basic amino acids driven by a protonmotive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981;256:2079–2082. [PubMed] [Google Scholar]
- 18.Palmiter R D, Cole T B, Findley S D. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 1996;15:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsay L M, Gadd G M. Mutants of Saccharomyces cerevisiae defective in vacuolar function confirm a role for the vacuole in toxic metal ion detoxification. FEMS Microbiol Lett. 1997;152:293–298. doi: 10.1111/j.1574-6968.1997.tb10442.x. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Ohsumi Y, Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984;259:11505–11508. [PubMed] [Google Scholar]
- 21.Silver S, Walderhaug M. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol Rev. 1992;56:195–228. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umemoto N, Yoshihisa T, Hirata R, Anraku Y. Roles of the VMA3 gene product, subunit c of the vacuolar membrane H+-ATPase on vacuolar acidification and protein transport. A study with VMA3-disrupted mutants of Saccharomyces cerevisiae. J Biol Chem. 1990;265:18447–18453. [PubMed] [Google Scholar]
- 23.Wurst H, Shiba T, Kornberg A. The gene for a major exopolyphosphatase of Saccharomyces cerevisiae. J Bacteriol. 1995;177:898–906. doi: 10.1128/jb.177.4.898-906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]