Abstract
Background
Respiratory failure due to lung immaturity is a major cause of mortality in preterm infants. Although the use of intermittent positive pressure ventilation (IPPV) in neonates with respiratory failure saves lives, its use is associated with lung injury and chronic lung disease. A newer form of ventilation called high frequency oscillatory ventilation has been shown in experimental studies to result in less lung injury.
Objectives
The objective of this review was to determine the effect of the elective use of high frequency oscillatory ventilation (HFOV) as compared to conventional ventilation (CV) on the incidence of chronic lung disease (CLD), mortality and other complications associated with prematurity and assisted ventilation in preterm infants who were mechanically ventilated for respiratory distress syndrome (RDS).
Search methods
Searches were made of the Oxford Database of Perinatal Trials, MEDLINE, EMBASE, previous reviews including cross references, abstracts, conference and symposia proceedings; and from expert informants and handsearching of journals by The Cochrane Collaboration, mainly in the English language. The search was updated in January 2009 and again in November 2014.
Selection criteria
Randomised controlled trials comparing HFOV and CV in preterm or low birth weight infants with pulmonary dysfunction, mainly due to RDS, who required assisted ventilation. Randomisation and commencement of treatment needed to be as soon as possible after the start of CV and usually in the first 12 hours of life.
Data collection and analysis
The methodological quality of each trial was independently reviewed by the review authors. The standard effect measures were relative risk (RR) and risk difference (RD). From 1/RD the number needed to benefit (NNTB) to produce one outcome was calculated. For all measures of effect, 95% confidence intervals (CIs) were used. For interpretation of subgroup analyses, a P value for subgroup differences as well as the I2 statistic for between‐subgroup heterogeneity were calculated. Meta‐analysis was performed using both a fixed‐effect and a random‐effects model. Where heterogeneity was over 50%, the random‐effects model RR was also reported.
Main results
Nineteen eligible studies involving 4096 infants were included. Meta‐analysis comparing HFOV with CV revealed no evidence of effect on mortality at 28 to 30 days of age or at approximately term equivalent age. These results were consistent across studies and in subgroup analyses. The risk of CLD in survivors at term equivalent gestational age was significantly reduced with the use of HFOV but this effect was inconsistent across studies, even after the meta‐analysis was restricted to studies that applied a high lung volume strategy with HFOV. Subgroup analysis by HFOV strategy showed a similar effect in trials with a more strict lung volume recruitment strategy, targeting a very low fraction of inspired oxygen (FiO2), and trials with a less strict lung volume recruitment strategy and with a somewhat higher or unspecified target FiO2. Subgroup analyses by age at randomisation, routine surfactant use or not, type of high frequency ventilator (oscillator versus flow interrupter), inspiratory to expiratory (I:E) ratio of high frequency ventilator (1:1 versus 1:2) and CV strategy (lung protective or not) could not sufficiently explain the heterogeneity. Pulmonary air leaks, defined as gross air leaks or pulmonary interstitial emphysema, occurred more frequently in the HFOV group, whereas the risk of severe retinopathy of prematurity was significantly reduced.
Although in some studies an increased risk of severe grade intracranial haemorrhage and periventricular leukomalacia was found, the overall meta‐analysis revealed no significant differences in effect between HFOV and CV. The short‐term neurological morbidity with HFOV was only found in the subgroup of two trials not using a high volume strategy with HFOV. Most trials did not find a significant difference in long‐term neurodevelopmental outcome, although one recent trial showed a significant reduction in the risk of cerebral palsy and poor mental development.
Authors' conclusions
There is evidence that the use of elective HFOV compared with CV results in a small reduction in the risk of CLD, but the evidence is weakened by the inconsistency of this effect across trials. Probably many factors, both related to the intervention itself as well as to the individual patient, interact in complex ways. In addition, the benefit could be counteracted by an increased risk of acute air leak. Adverse effects on short‐term neurological outcomes have been observed in some studies but these effects are not significant overall. Most trials reporting long‐term outcome have not identified any difference.
Keywords: Humans; Infant, Newborn; High‐Frequency Ventilation; Chronic Disease; Infant, Premature; Infant, Premature, Diseases; Infant, Premature, Diseases/prevention & control; Lung Diseases; Lung Diseases/prevention & control; Lung Injury; Lung Injury/prevention & control; Randomized Controlled Trials as Topic; Respiration, Artificial; Respiratory Distress Syndrome, Newborn; Respiratory Distress Syndrome, Newborn/therapy
Plain language summary
Elective high frequency ventilation compared to conventional mechanical ventilation in the early stabilization of infants with respiratory distress
Review question. Does the elective use of high frequency oscillatory ventilation as compared to conventional ventilation reduce lung damage and other complications associated with prematurity and assisted ventilation in preterm infants who are mechanically ventilated for respiratory distress syndrome (RDS)?
Background. Respiratory failure due to lung immaturity is a major cause of deaths in preterm infants. Although the use of intermittent positive pressure ventilation in newborns with respiratory failure saves lives, its use is associated with lung injury and chronic lung disease. A newer form of ventilation called high frequency oscillatory ventilation has been shown in experimental studies to result in less lung injury.
Study characteristics. Nineteen eligible studies involving 4096 infants met our inclusion criteria.
Results. Insufficient evidence exists to support the routine use of high frequency oscillatory ventilation instead of conventional ventilation for preterm infants with lung disease who are given positive pressure ventilation. High frequency oscillatory ventilation is a way of providing artificial ventilation of the lungs that theoretically may produce less injury to the lungs and therefore reduce the rate of chronic lung disease. This review of the evidence from 19 randomised controlled trials showed that although a small protective effect towards the lungs can be seen, this moderate benefit is highly variable between studies and should be weighed against possible harm.
Background
Pulmonary disease continues to be the major cause of morbidity and mortality in very preterm infants. Although assisted ventilation with intermittent positive pressure ventilation (IPPV) has decreased mortality, morbidity from lung injury is high. Acute injury such as pulmonary air leak was common prior to the availability of surfactant. Chronic lung disease (CLD) develops in up to one third of preterm infants with respiratory distress syndrome (RDS) who receive IPPV (Ehrenkrantz 1992; Northway 1992). In addition to immaturity, over distention of the lung and oxygen toxicity are thought to be important factors in the pathogenesis of CLD (Jobe 2000).
In order to avoid distortion of the lung caused by the large swings in pulmonary pressures during conventional ventilation (CV) at rates of 30 to 80 breaths per minute, high frequency oscillatory ventilation (HFOV) at rates of 600 to 800 breaths per minute was developed. In animal models, the use of HFOV results in more uniform lung inflation, improves oxygenation and reduces the severity of lung pathology produced by IPPV (Truog 1984; de Lemos 1987).
As discussed by Clark 2000, there are strategies that reduce lung injury with both HFOV and CV. Animal studies show that lung volume maintenance with HFOV prevents lung injury (McCulloch 1988). The effectiveness of HFOV might also be enhanced by the use of more powerful piston driven ventilators compared with those that generate the oscillations by flow interruption (Jouvet 1997) and even by certain settings with the same type of ventilator (inspiratory to expiratory ratio of 1:1 versus 1:2) (Pillow 1999). Various strategies with CV appear to reduce acute lung injury. These include avoiding high tidal volumes, using positive end expiratory pressure (PEEP) and using short inspiratory times and faster rates. Allowing carbon dioxide blood levels to rise (permissive hypercapnia) rather than increasing ventilation may also reduce lung injury in preterm infants (Woodgate 2006). Many of these treatment strategies and their effects on lung injury are based on pathophysiological studies in animal models (increased cytokine release with higher tidal volumes and reduced PEEP) (Meredith 1989) or trials in adults with RDS (Petrucci 2007). There is evidence in preterm infants that strategies to synchronise ventilation (higher rates and patient triggered ventilation) reduce the rate of pneumothorax and the duration of ventilation, although there is no evidence that these strategies reduce CLD at 36 weeks postmenstrual age (Greenough 2008).
Objectives
The objective of this review was to determine the effect of the elective use of high frequency oscillatory ventilation (HFOV) when compared to conventional ventilation (CV) on the incidence of chronic lung disease (CLD), mortality and other complications associated with prematurity and assisted ventilation in preterm infants who were mechanically ventilated for respiratory distress syndrome (RDS).
The following subgroup analyses were pre‐specified.
(1) Management of HFOV: a strategy to maintain lung volume has the potential for better alveolar recruitment compared to a strategy to maintain low volume and thus might result in better outcomes in terms of CLD. A 'high volume strategy' (HVS) with HFOV was defined as one in which two or more of the following treatment approaches were explicitly stated in the methods: initial use of a higher mean airway pressure than on CV; initial weaning of fractional inspired oxygen concentration (FiO2) before weaning mean airway pressure; or use of alveolar recruitment manoeuvres. The FiO2 is considered as being a useful clinical parameter for lung volume recruitment. Optimal alveolar recruitment is reflected by the ability to wean the FiO2 below 0.30 or even 0.25. Therefore, trials were classified either as 'no high lung volume strategy', 'high lung volume strategy with a target FiO2 > 0.3 or not specified', and 'high lung volume strategy with a target FiO2 ≤ 0.30'.
(2) Surfactant replacement: surfactant replacement therapy would increase alveolar recruitment, attenuate RDS, and lead to less lung injury and CLD. A similar pulmonary benefit could occur in infants whose mothers received antenatal corticosteroids.
(3) Birth weight and gestational age: outcomes might differ in groups of infants born at different weights and gestational ages. Infants born at very low gestation or with very low birth weight, or both, have a higher incidence of CLD and may benefit more from HFOV. On the other hand, these infants are more susceptible to neurological complications such as intraventricular haemorrhage (IVH) and periventricular leukomalacia (PVL).
In order to explain persisting heterogeneity in the meta‐analyses in previous versions of this review, the following subgroup analyses were added for the 2007 update.
(4) Type of HFOV ventilator: true (piston) HFO ventilators might be more effective in maintaining lung volume and lead to different effects compared with those ventilators that use flow interruption. Also, differences in inspiratory to expiratory times on HFOV may affect the incidence of lung injury.
(5) Management of CV: lung protective strategies on CV (short inspiratory times, rates of ≥ 60/minute, PEEP of 4 to 6 cm H20, limiting tidal volume, patient triggering or permissive hypercapnia) may affect the differences between HFOV and CV.
(6) Duration of ventilation prior to randomisation or age at randomisation: the treatment that infants receive prior to randomisation could alter outcomes and this could be measured by duration of ventilation prior to randomisation or age at randomisation, or both.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials.
Types of participants
Preterm or low birth weight infants with pulmonary dysfunction, mainly due to RDS, who were considered to require IPPV.
Types of interventions
Elective HFOV versus CV: randomisation was accomplished early in the course of RDS soon after mechanical ventilation was begun. Such trials were classified as ' elective'. Trials were classified as 'rescue', and therefore excluded from this review when patients were randomised after failure to adequately ventilate on CV or when complications of CV developed or were likely to develop. The use of HFOV as rescue therapy and the use of elective high frequency jet ventilation are the subject of other reviews (Bhuta 2003; Henderson‐Smart 2005). Trials were not eligible if cross‐over of interventions was mandatory.
Types of outcome measures
Outcomes from trials were not eligible if there was a 20% or greater rate of missing or unreported data.
Primary
1. Mortality at 28 to 30 days and at term equivalent age.
2. Chronic lung disease (CLD):
oxygen dependency at 28 to 30 days (with and without chest x‐ray changes);
oxygen dependency or use of assisted ventilation at 36 to 37 weeks postmenstrual age (PMA) or at discharge.
3. Death or CLD.
Secondary
4. Failure of allocated treatment to maintain gas exchange, leading to cross‐over to alternate treatment.
5. Pulmonary air leak syndromes: all, including pulmonary interstitial emphysema (PIE) and gross extrapulmonary air leak (such as pneumothorax).
6. Intraventricular haemorrhage:
all grades;
grades 3 (ventricles distended with blood) or 4 (parenchymal involvement).
7. Periventricular leukomalacia. 8. Retinopathy of prematurity (ROP) ≥ grade 2. 9. Use of hospital resources (length of hospital stay, duration of IPPV). 10. Long‐term growth and neurodevelopment.
Search methods for identification of studies
Searches were made of the Oxford Database of Perinatal Trials; Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library 2008, Issue 4; MEDLINE and EMBASE (using MeSH headings 'high‐frequency‐ventilation' and 'infant, preterm') from 1983 to January 2009; previous reviews including cross references; abstracts conference and symposia proceedings; and from expert informants and handsearching of journals by The Cochrane Collaboration, mainly in the English language. An expert informant's search in the Japanese language was made by Professor Y Ogawa in 1996. Abstracts of the annual meetings of the Society for Pediatric Research (1996 to 2009 inclusive) were also searched. The searches in MEDLINE, EMBASE and CENTRAL were updated in November 2014.
Data collection and analysis
The standard methods of The Cochrane Collaboration and the Cochrane Neonatal Review Group (CNRG) were used to evaluate the methodological quality of each trial. Trials were reviewed independently by each author for eligibility. Data were extracted separately by each author, then compared and any differences resolved.
Additional information was obtained from Ogawa 1993; Gerstmann 1996; Rettwitz‐Volk 1998; Thome 1998; Plavka 1999; Moriette 2001; and Johnson 2002 regarding trial methodology. Schreiber 2003 re‐analysed their trial data on the use of nitric oxide to evaluate outcomes related to HFOV and CV to which the infants were also randomised. Clark 1992 and Plavka 1999 provided information on infants excluded post‐randomisation, which allowed for an intention‐to‐treat analysis. Plavka 1999; Moriette 2001; and Van Reempts 2003 provided additional outcome information from their trials (see the table 'Characteristics of included studies' for details).
Results for outcomes requiring survival to a given age were reported with survivors as the denominator (IPPV, CLD). Survival was used as the denominator for ROP, where the number examined was not given (HIFI 1989; Schreiber 2003; Van Reempts 2003).
The standard method of the CNRG was used to analyse the data. Treatment effects were expressed using relative risk (RR) and risk difference (RD). From 1/RD the number needed to benefit or to harm (NNTB or NNTH, respectively) to produce one outcome was calculated. For each measure the 95% confidence interval (CI) was routinely given. Between‐study heterogeneity was considered statistically significant if the P value for heterogeneity was < 0.10. Since some degree of variation between studies can be due to chance alone, the I2 statistic (%) was used to express the proportion of the observed variation between studies that was due to true between‐study differences instead of chance. Subgroup analyses were interpreted in a similar way, using a P value and I2 for between‐subgroup heterogeneity. Meta‐analysis was performed using both a fixed‐effect and a random‐ effects model. Where considerable true heterogeneity (I2 > 50%) was present, the random‐effects model RR was also reported.
Because lung volume recruitment (high volume strategy or HVS) with HFOV is now considered as being the most appropriate strategy for HFOV in preterm infants, meta‐analyses were performed both including all trials as well as after exclusion of trials that failed to use an HVS. For the same reason, trials that did not use an HVS were not included in the subgroup analyses, except for the subgroup analysis by HFOV strategy.
Results
Description of studies
Included studies
Overall, 28 randomised controlled trials of HFOV versus CV were found, of which 19 met the eligibility criteria and full trial data were available. Details of each of these included studies (HIFI 1989; Clark 1992; Ogawa 1993; Gerstmann 1996; Rettwitz‐Volk 1998; Thome 1998; Plavka 1999; Durand 2001; Moriette 2001; Courtney 2002; Johnson 2002; Craft 2003; Schreiber 2003; Van Reempts 2003; Vento 2005; Dani 2006; Lista 2008; Salvo 2012; Sun 2014) are given in the table 'Characteristics of included studies'.
Participants
All but six of the included studies (Clark 1992; Plavka 1999; Van Reempts 2003; Vento 2005; Dani 2006; Lista 2008) were multicentre studies. The total number of infants randomised in each study varied from 25 (Dani 2006) to 797 (Johnson 2002). All studies included preterm infants, although the upper limit for birth weight or gestation differed. This upper limit for birth weight was 1001 grams in one study (Craft 2003), 1200 grams in two (Durand 2001; Courtney 2002), 1500 grams in five (Rettwitz‐Volk 1998; Plavka 1999; Vento 2005; Salvo 2012, Sun 2014), 1750 grams in one (Clark 1992) and 2000 grams in three (HIFI 1989; Ogawa 1993; Schreiber 2003). Upper gestational age limits were 36 weeks in one study (Gerstmann 1996), 35 weeks in one (Clark 1992), 34 weeks in two (Craft 2003; Schreiber 2003), 32 weeks in three (Van Reempts 2003; Lista 2008; Sun 2014), 31 weeks in one (Plavka 1999), 30 weeks in four (Thome 1998, Moriette 2001; Dani 2006; Salvo 2012) and 29 weeks in two (Johnson 2002; Vento 2005). The average age at randomisation varied from less than one hour (Thome 1998; Johnson 2002; Vento 2005; Dani 2006) to 12 hours (Schreiber 2003). Each trial stratified infants at randomisation by weight or gestational age, although few data were reported by these subgroups.
Prenatal corticosteroid use was not reported in two trials (HIFI 1989; Ogawa 1993); they were used in a minority of women in two studies (Clark 1992; Gerstmann 1996), were an exclusion criterion in one (Salvo 2012) and used in 50% to 100% of women in the remaining 14 studies.
Interventions
Different ventilators were used to deliver HFOV. Eight trials used the Sensormedics 3100 (Clark 1992; Gerstmann 1996; Plavka 1999; Durand 2001; Courtney 2002; Schreiber 2003; Dani 2006; Salvo 2012), two used the Hummingbird (HIFI 1989; Ogawa 1993), one used a Stephan piston oscillator (Rettwitz‐Volk 1998), one used an Infant Star ventilator (Thome 1998), one used a French piston oscillator (Moriette 2001), two trials used the Dräger Babylog ventilator (Vento 2005; Lista 2008) and one trial used the SLE5000 (Sun 2014). Two trials used more than one type of ventilator: Van Reempts used either the Sensormedics 3100 (83%) or Infant Star (17%) (Van Reempts 2003) and in the United Kingdom Oscillation Study (UKOS) (Johnson 2002) a variety of ventilators (Sensormedics, SLE, Dräger) were used. HFOV was delivered at 10 to 15 Hz in 12 trials and at 15 to 20 Hz in one (Rettwitz‐Volk 1998).
The three criteria used to define a high volume strategy (HVS) with HFOV are given in the objectives. All 17 trials with a HVS used a higher mean airway pressure (MAP) on HFOV than on CV. In addition, three trials (Thome 1998; Moriette 2001; Salvo 2012) used both alveolar recruitment manoeuvres and weaning of FiO2 prior to weaning MAP, while Gerstmann 1996; Clark 1992; and Van Reempts 2003 used weaning of FiO2 first and Ogawa 1993 used alveolar recruitment manoeuvres. In one trial (Sun 2014) a lung volume recruitment manoeuvre was applied to reach optimal alveolar recruitment as described in detail by De Jaegere et al (De Jaegere 2006). In eight trials, lung volume recruitment was defined as the ability to wean to an FiO2 to 0.30 or less (Gerstmann 1996; Thome 1998; Johnson 2002; Vento 2005; Dani 2006; Lista 2008; Salvo 2012, Sun 2014), whereas in the other trials the targeted FiO2 was higher than 0.30 or not specified. Two trials (HIFI 1989; Rettwitz‐Volk 1998) did not use a HVS for HFOV.
In all trials, CV was administered using time cycled, pressure limited ventilators. There was a large variation in the specific methods of administration of CV that might provide lung protection. Details are given in the table 'Characteristics of included studies'.
Surfactant therapy with animal derived extracts was used as therapy for RDS in the majority of participants in all but two trials (HIFI 1989; Clark 1992).
Postnatal corticosteroids for CLD were used in 41% to 61% of infants in three trials (Rettwitz‐Volk 1998; Thome 1998; Courtney 2002), in 20% of infants in one trial (Johnson 2002) and in less than 8% of infants in three trials (Moriette 2001; Van Reempts 2003; Salvo 2012). Plavka 1999 reported cumulative dosage and Courtney 2002 reported mean days of therapy in each group. In all studies, the usage of postnatal steroids was similar in the two treatment groups, with the exception of the trial of Veemto and colleagues (Vento 2005), in which corticosteroids were administered to 35% of survivors in the HFOV group and to 60% of survivors in the CV group.
In Durand 2001 and Courtney 2002 prophylactic indomethacin was given routinely to all infants.
Outcomes
Not all outcomes were reported in each study. The definitions of CLD at 28 days differed between studies. CLD was assessed at 28 days of age in six studies (HIFI 1989; Ogawa 1993; Rettwitz‐Volk 1998; Thome 1998; Moriette 2001; Van Reempts 2003; Schreiber 2003) and 30 days of age in the other two (Clark 1992; Gerstmann 1996). In five studies, the definition of CLD at 28 days of age was based on oxygen therapy alone (Rettwitz‐Volk 1998; Thome 1998; Plavka 1999; Moriette 2001; Schreiber 2003) while in the remainder both oxygen therapy and an abnormal chest x‐ray were required.
Late CLD at term equivalent age varied from 36 weeks PMA (Clark 1992; Thome 1998; Plavka 1999; Moriette 2001; Courtney 2002; Johnson 2002; Salvo 2012 ; Sun 2014) or 37 weeks PMA (Rettwitz‐Volk 1998) to at discharge (Gerstmann 1996) (mean PMA 37.1 (95% CI 36.5 to 37.9) weeks in the HFOV group and 37.5 (95% CI 36.6 to 38.0) weeks in the CV group). The criterion for CLD at term equivalent age was based on use of oxygen therapy in nine trials, on clinical score (oxygen plus signs) in one trial (Plavka 1999), on oxygen or use of assisted ventilation in two trials (Courtney 2002; Van Reempts 2003) and on oxygen use plus an abnormal chest radiograph in one trial (Schreiber 2003).
In most trials, cross‐over to the other treatment was allowed when predetermined failure criteria were reached. These criteria (hypoxaemia or hypercarbia, or both) were similar in each trial and for each treatment group, but the decision to cross over was left to the clinician. In two trials (Clark 1992; Rettwitz‐Volk 1998) the additional criterion for cross‐over of severe pulmonary interstitial emphysema was applied only to the CV group. Because of the variable definition of 'failure of assigned treatment' between treatment groups, this outcome has not been included in the meta‐analysis. When cross‐over occurred, the participants were analysed in the groups as randomised. In the Sun 2014 trial, cross‐over was not allowed.
Excluded studies
The study by Froese 1987 has not been included because after randomisation of infants (unknown gestation range) with presumed RDS, 5 of 11 in the HFOV group and an unknown number from the CV group were excluded from the comparisons between treatments. Only data on infants < 29 weeks with RDS were reported. Lombet 1996 has been excluded because there was a 22% loss after randomisation. Pardou 1993 reported results for only 13 (54%) of the 24 infants randomised to the high frequency flow interrupter or CV.
Cambonie 2003 was not included as the trial only examined haemodynamic status during HFOV compared to CV and clinical outcomes were not reported.
HiFO 1993 was excluded since HFOV was used as rescue therapy. This study was included in a separate review of HFOV (Henderson‐Smart 2005). The study by Ramanathan 1995, which has only been published in abstract form, was excluded because there was a mandatory cross‐over from HFOV to CV at 96 hours of age. Some information from these latter two trials concerning rates of IVH is considered in the discussion.
Nazarchuk 2010 was excluded because the study was restricted to a population of very low birth weight infants with omphalocoele. Singh 2012 was excluded because 27% of infants were excluded post‐randomisation and because the main outcome was the oxygenation index in the first 24 hours.
Prashanth 2012 was excluded because it was not a randomised controlled study.
Risk of bias in included studies
Details of the methodological quality of each study are available in the table 'Characteristics of included studies' and summarized in Figure 1 and Figure 2.
1.
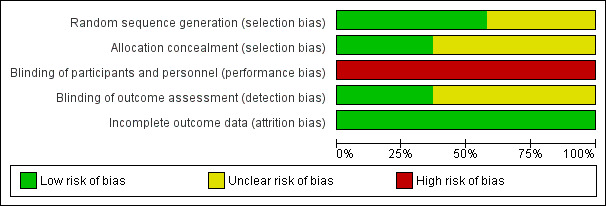
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
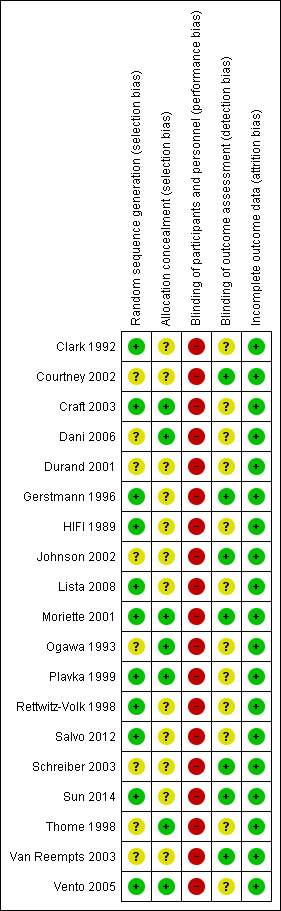
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Randomisation: the majority of the studies had an adequate, documented method of random sequence generation. In only seven studies sufficient information was provided to assess the quality of allocation concealment.
Blinding of treatment: due to the nature of the intervention blinding of the experimental and control interventions was not possible for care‐givers. This could possibly introduce bias in all the trials. Blinding of participants was not relevant.
Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). For outcomes that needed interpretation of images, such as head ultrasound or chest x‐ray, and for long‐term neurodevelopmental assessment, blinding was variable between outcomes and studies. Assessment of chest x‐rays for the diagnosis of CLD was blinded as to treatment group in studies by Clark 1992; Ogawa 1993; Plavka 1999; Moriette 2001; and Schreiber 2003. Blinded head ultrasound assessments were carried out in the HIFI 1989; Clark 1992; Ogawa 1993; Durand 2001; Moriette 2001; Courtney 2002; Johnson 2002; Van Reempts 2003; and Schreiber 2003 trials.
Exclusions after randomisation were minimal for primary outcomes (all less than 8%), so low risk of bias. For long‐term neurological assessment follow‐up rates were lower (for some studies as low as 51% to 57%).
Effects of interventions
Nineteen trials involving 4096 infants were included.
Mortality
There were no significant differences in the rates of mortality by 28 to 30 days (Analysis 1.1) (2148 infants in 10 trials; summary RR 1.09, 95% CI 0.88 to 1.34) or in the rates of mortality by 36 to 37 weeks PMA or discharge (Analysis 1.6) (3329 infants in 17 trials; summary RR 0.95, 95% CI 0.81 to 1.10). There was no heterogeneity between studies for this outcome (I² = 0%). Only one individual trial (Sun 2014) showed a significant reduction in mortality before discharge (RR 0.31, 95% CI 0.10 to 0.94). Subgroup analyses including use of volume recruitment on HFOV, routine use of surfactant, use of piston oscillators, use of lung protective strategies on CV, and inspiratory to expiratory ratio on HFOV also failed to show any significant differences in effect on mortality rates between subgroups.
1.1. Analysis.
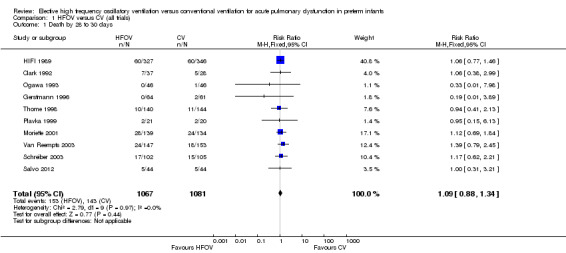
Comparison 1 HFOV versus CV (all trials), Outcome 1 Death by 28 to 30 days.
1.6. Analysis.
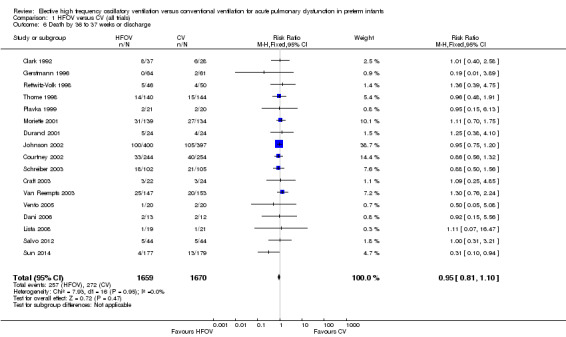
Comparison 1 HFOV versus CV (all trials), Outcome 6 Death by 36 to 37 weeks or discharge.
Chronic lung disease (CLD) at 28 to 30 days
The use of oxygen therapy at 28 to 30 days was reported for 1043 infants in six trials. There was no significant difference between the HFOV and CV groups in the individual trials or in the meta‐analysis (Analysis 1.3) (summary RR 0.98, 95% CI 0.88 to 1.10).
1.3. Analysis.
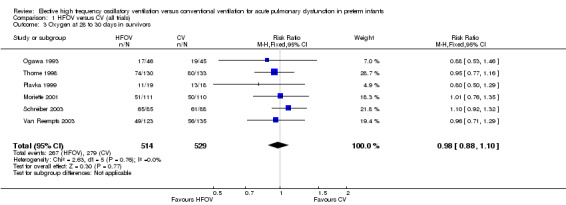
Comparison 1 HFOV versus CV (all trials), Outcome 3 Oxygen at 28 to 30 days in survivors.
CLD in survivors at 28 to 30 days of age, based on the use of oxygen or mechanical ventilation and the presence of an abnormal chest x‐ray, was reported for 820 infants in four trials (Analysis 1.4). Two trials (Clark 1992; Gerstmann 1996) showed a significantly lower incidence of this outcome in the HFOV group and there was a trend towards a reduced incidence in the overall analysis (summary RR 0.86, 95% CI 0.74 to 1.01). This latter meta‐analysis showed significant heterogeneity (P = 0.02; I2 = 71.3%), and when a random‐effects model was used the summary RR was 0.66 (95% CI 0.41 to 1.07). When only trials that used HVS were considered for analysis (thereby excluding the High Frequency Ventilation in Premature Infants (HIFI) trial), heterogeneity disappeared (I² = 0%) and a significant reduction in the need for oxygen at 28 to 30 days was shown (summary RR 0.53, 95% CI 0.36 to 0.76).
1.4. Analysis.
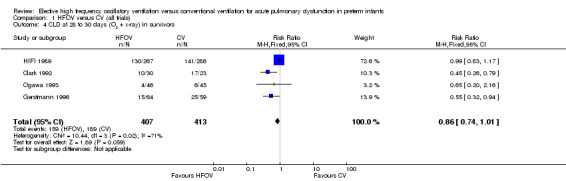
Comparison 1 HFOV versus CV (all trials), Outcome 4 CLD at 28 to 30 days (O2 + x‐ray) in survivors.
Five trials involving 1160 infants reported both mortality and CLD at 28 to 30 days (Analysis 1.5). Two showed a significant decrease of this combined outcome in the HFOV group (Clark 1992; Gerstmann 1996). In the overall analysis, there was a non‐significant trend towards a reduced risk of death or CLD at 28 to 30 days in the HFOV group (summary RR 0.94, 95% CI 0.85 to 1.04). Again, significant heterogeneity existed for this outcome (I² = 74%), which persisted when only trials that used HVS were considered (I² = 83%). The random effects model summary RR for this combined outcome was 0.83 (95% CI 0.65 to 1.07).
1.5. Analysis.
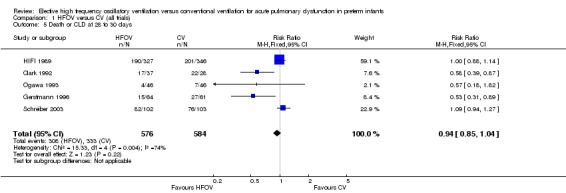
Comparison 1 HFOV versus CV (all trials), Outcome 5 Death or CLD at 28 to 30 days.
CLD at 36 to 37 weeks postmenstrual age (PMA) in survivors
CLD in survivors at 36 to 37 weeks PMA or at discharge was reported for 2786 infants in 17 trials (Analysis 1.7). Five trials (Clark 1992; Gerstmann 1996; Durand 2001; Vento 2005; Sun 2014) found a significant decrease in the HFOV group. In the overall analysis using a fixed‐effect model, there was a significant reduction of CLD in the HFOV group (summary RR 0.86, 95% CI 0.78 to 0.96; summary RD ‐0.05, 95% CI ‐0.08 to ‐0.02; NNTB 20, 95% CI 12 to 50). There was significant heterogeneity in this meta‐analysis (P = 0.002; I2 = 59%), and using a random‐effects model the summary RR was 0.80 (95% CI 0.65 to 0.99), which was borderline significant. Heterogeneity persisted if only trials that used HVS were considered.
1.7. Analysis.
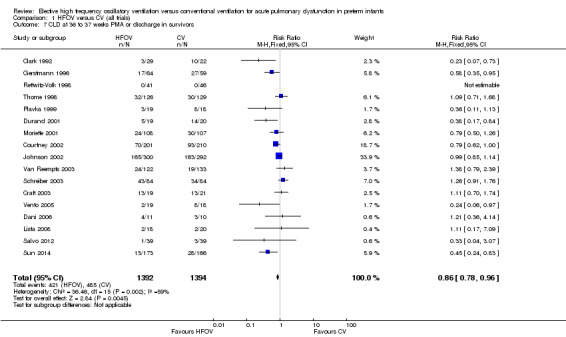
Comparison 1 HFOV versus CV (all trials), Outcome 7 CLD at 36 to 37 weeks PMA or discharge in survivors.
Subgroup analyses
The subgroup analysis by high volume strategy (HVS) on HFOV (Analysis 2.2) showed similar results in the subgroups: HVS with target FiO2 ≤ 0.30 (8 trials, 1483 infants; summary RR 0.87, 95% CI 0.76 to 0.99) and HFV with target FiO2 > 0.30 or unspecified (8 trials, 1216 infants; summary RR 0.86, 95% CI 0.73 to 1.00). Only one trial not using HVS reported CLD at 36 weeks PMA and had no cases of CLD to contribute to the overall analysis (Rettwitz‐Volk 1998). The test for subgroup difference was not significant (P = 0.50; I2 = 0%) and heterogeneity persisted within the subgroups.
2.2. Analysis.
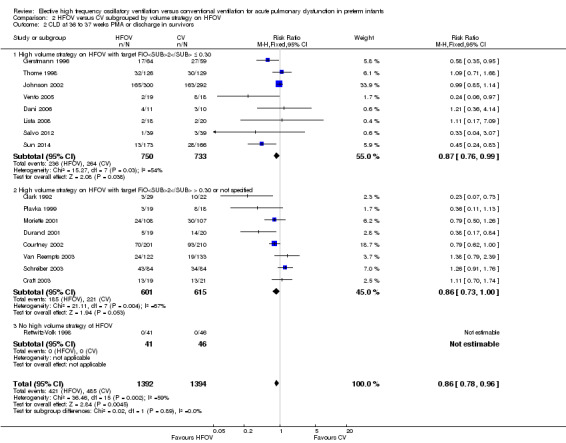
Comparison 2 HFOV versus CV subgrouped by volume strategy on HFOV, Outcome 2 CLD at 36 to 37 weeks PMA or discharge in survivors.
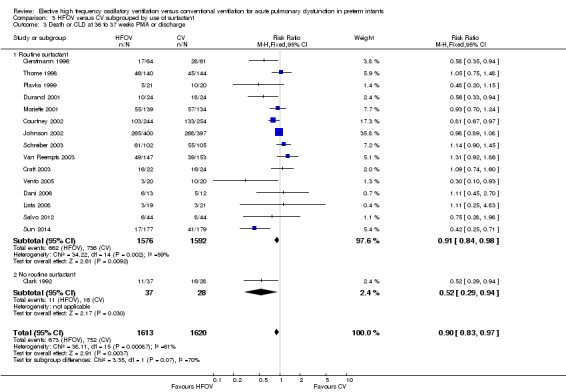
In the subgroup analysis by use of routine surfactant (Analysis 3.2) only 1 small trial of 51 infants (Clark 1992) reporting CLD at 36 weeks did not use surfactant. This trial showed a significant and marked reduction in the HFOV group (RR 0.23, 95% CI 0.07 to 0.73). This result was significantly different from the subgroup analysis of the 15 trials involving 2648 infants in which surfactant was used. The latter result is similar to the overall analysis of CLD at 36 weeks PMA (summary RR using fixed‐effect model 0.88, 95% CI 0.80 to 0.97) with persisting heterogeneity (I2 = 48%).
3.2. Analysis.
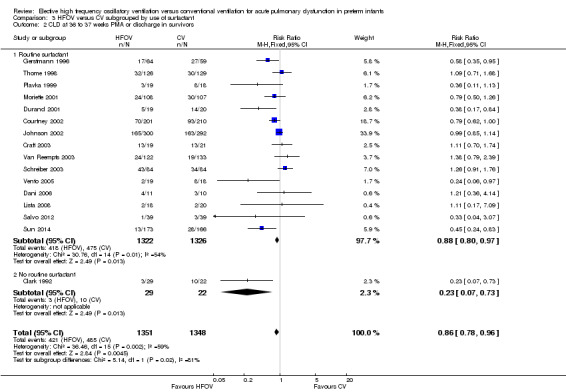
Comparison 3 HFOV versus CV subgrouped by use of surfactant, Outcome 2 CLD at 36 to 37 weeks PMA or discharge in survivors.
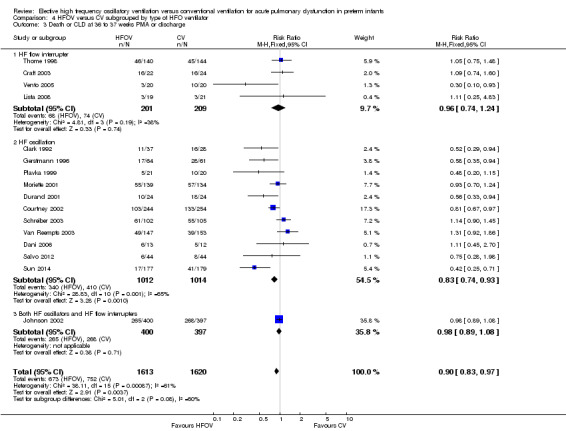
Subgroups by use of different types of oscillator (flow interrupters, true piston oscillators, or both) only showed a statistically significant difference between the summary RRs of the subgroups when a fixed‐effect model was used (test for subgroup difference P = 0.06; I2 = 64.4%), not when a random‐effects model was used (test for subgroup difference P = 0.14; I² = 50%) (Analysis 4.2). For the subgroup of 11 trials involving 1737 infants that used HF piston oscillators (including the Van Reempts trial, where 83% of study patients were ventilated with an HF oscillator and 17% were ventilated with a flow interrupter) there was a significant reduction in CLD in the HFOV group (summary RR 0.77, 95% CI 0.67 to 0.90; summary RD ‐0.07, 95% CI ‐0.11 to ‐0.03; NNTB 14, 95% CI 9 to 33). However, significant heterogeneity persisted within this subgroup (P = 0.003; I2 = 63%), and using a random‐effects model the summary RR was 0.77 (95% CI 0.67 to 0.90).
4.2. Analysis.
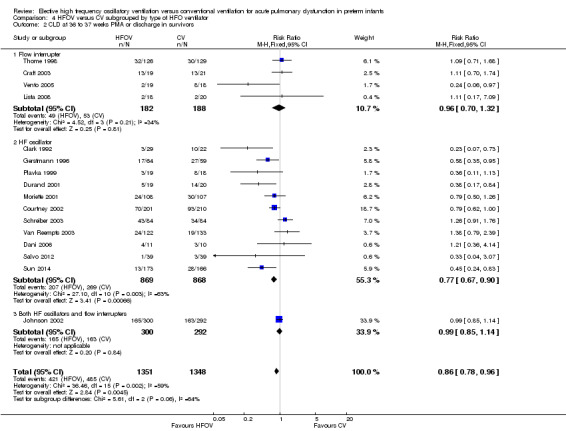
Comparison 4 HFOV versus CV subgrouped by type of HFO ventilator, Outcome 2 CLD at 36 to 37 weeks PMA or discharge in survivors.
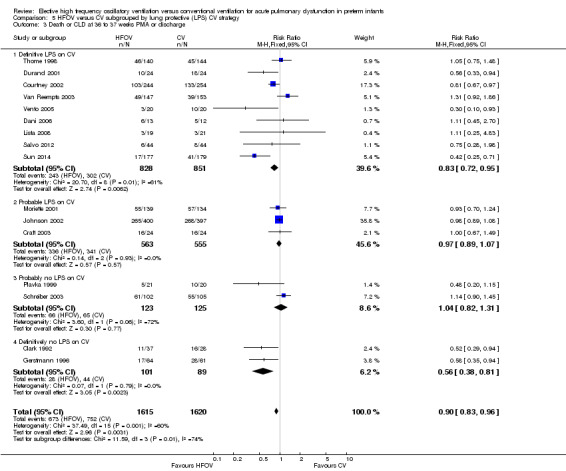
Similarly, for the subgroup analysis based on lung protective strategies (LPS) on CV (Analysis 5.2), the outcome was significantly different between subgroups when using a fixed‐effect model (test for subgroup difference P = 0.008; I² = 74.9%) but not when using a random‐effects model (test for subgroup difference P = 0.15; I2 = 43.5%). The largest benefit was seen in the subgroup of trials that definitively did not use LPS (fixed‐effect model summary RR 0.48, 95% CI 0.31 to 0.75; summary RD ‐0.24, 95 CI ‐0.37 to ‐0.10; NNTB 4, 95% CI 4 to 10; random‐effects model summary RR 0.42, 95% CI 0.18 to 1.02). There was persisting heterogeneity within the subgroups, with I² varying from 52% up to 77%.
5.2. Analysis.
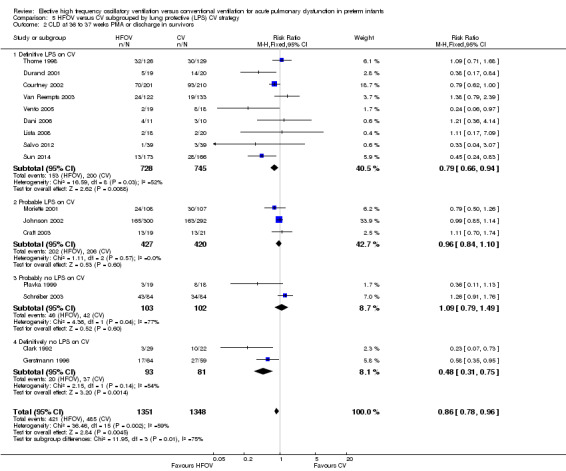
Comparison 5 HFOV versus CV subgrouped by lung protective (LPS) CV strategy, Outcome 2 CLD at 36 to 37 weeks PMA or discharge in survivors.
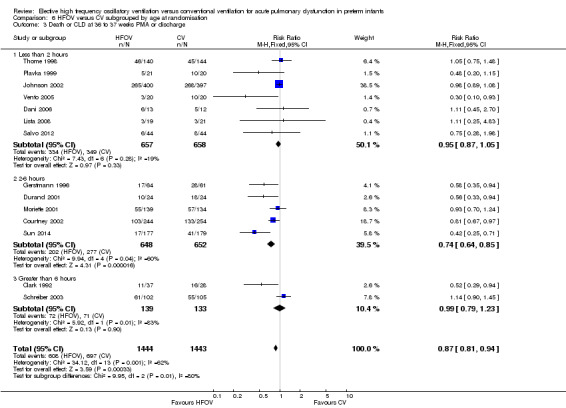
The subgroup analysis by age at randomisation (less than 2 hrs, 2 to 6 hrs, greater than 6 hrs) showed a significant difference between the summary RRs of the subgroups using the fixed‐effect model (P = 0.01; I2 = 78.3%) but not using the random‐effects model (P = 0.29; I2 = 18.2%) (Analysis 6.2).
6.2. Analysis.
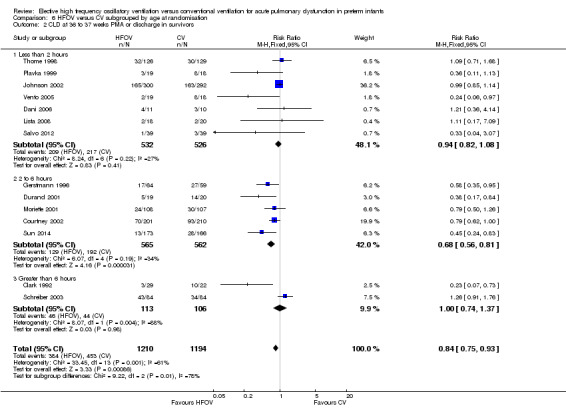
Comparison 6 HFOV versus CV subgrouped by age at randomisation, Outcome 2 CLD at 36 to 37 weeks PMA or discharge in survivors.
Subgroups by inspiratory:expiratory time ratio on HFOV (I:E = 1:1, 1:2 or variable or unknown) showed no statistically significant difference (test for subgroup difference P = 0.49; I² = 0% using fixed‐effect model and P = 0.77; I2 = 0% using random‐effects model) between the summary RRs of the subgroups (Analysis 7.2).
7.2. Analysis.
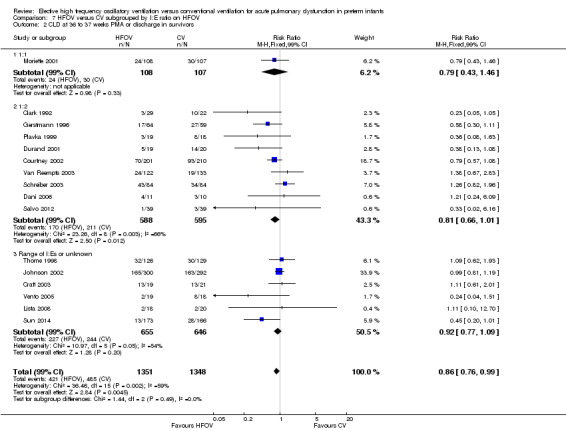
Comparison 7 HFOV versus CV subgrouped by I:E ratio on HFOV, Outcome 2 CLD at 36 to 37 weeks PMA or discharge in survivors.
Death or CLD at 36 weeks postmenstrual age
There was a small reduction in the risk of the combined outcome of death or CLD at 36 to 37 weeks PMA or at discharge in the HFOV group (Analysis 1.8) (summary RR 0.90, 95% CI 0.84 to 0.97; summary RD ‐0.05, 95% CI ‐0.08 to ‐0.01; NNTB 20, 95% CI 12 to 100; 17 trials, 3329 infants) using the fixed‐effect model. There was significant heterogeneity for this outcome (P = 0.001; I2 = 58%). The summary RR using a random‐effects model was RR 0.85 (95% CI 0.73 to 0.99).
1.8. Analysis.
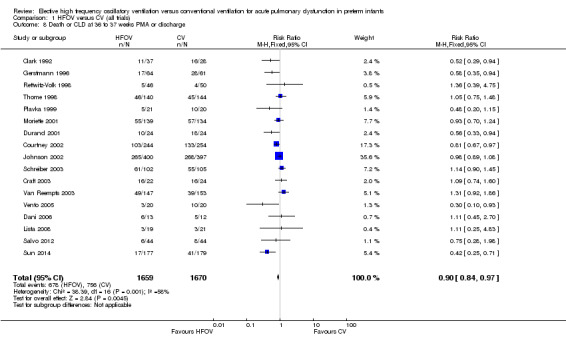
Comparison 1 HFOV versus CV (all trials), Outcome 8 Death or CLD at 36 to 37 weeks PMA or discharge.
Subgroup analyses
The subgroup analysis by high lung volume strategy for HFOV showed no significant differences between subgroups for this outcome (test for subgroup difference P = 0.76; I2 = 0%) (Analysis 2.3). In the subgroup analysis by surfactant use (Analysis 3.3), the one trial that did not use surfactant routinely (Clark 1992) showed a significantly larger reduction in the risk for death or CLD at 36 weeks PMA (RR 0.52, 95% CI 0.29 to 0.94) as compared to the subgroup of trials that used surfactant (summary RR 0.87, 95% CI 0.75 to 1.01; 15 trials, 3168 infants) (test for subgroup difference using fixed‐effect model P = 0.07; I2 = 70%). For the subgroup analysis by type of HFO ventilator (Analysis 4.3) subgroup differences were not statistically significant when a random‐effects model was used (test for subgroup difference P = 0.15; I2 = 46.7%). In the subgroup analysis by CV strategy (Analysis 5.3) results were significantly different between subgroups both when using a fixed‐effect model and a random‐effects model (test for subgroup difference for random‐effects model P = 0.02; I2 = 69.6%). The largest benefit from HFOV was seen in the subgroup of trials that definitively did not use an LPS (summary RR 0.55, 95% CI 0.38 to 0.81). For the subgroup analysis by age at randomisation (Analysis 6.3), the summary RRs of subgroups were only significantly different when using the fixed‐effect model (test for subgroup difference P = 0.007; I2 = 79.9%), and not when using the random‐effects model (test for subgroup difference P = 0.19; I2 = 40.7%). In the subgroup analysis by I:E ratio (Analysis 7.3), there were no significant between‐subgroup differences.
2.3. Analysis.
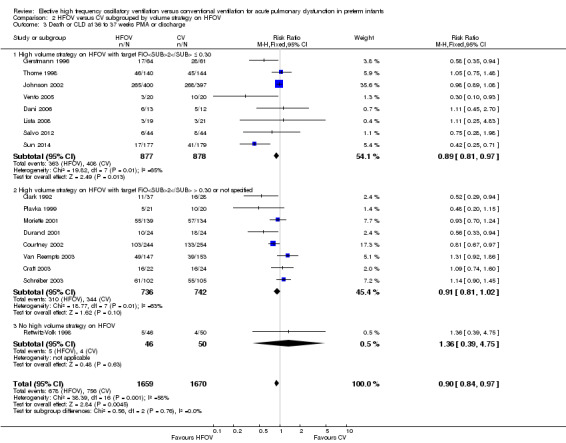
Comparison 2 HFOV versus CV subgrouped by volume strategy on HFOV, Outcome 3 Death or CLD at 36 to 37 weeks PMA or discharge.
3.3. Analysis.
Comparison 3 HFOV versus CV subgrouped by use of surfactant, Outcome 3 Death or CLD at 36 to 37 weeks PMA or discharge.
4.3. Analysis.
Comparison 4 HFOV versus CV subgrouped by type of HFO ventilator, Outcome 3 Death or CLD at 36 to 37 weeks PMA or discharge.
5.3. Analysis.
Comparison 5 HFOV versus CV subgrouped by lung protective (LPS) CV strategy, Outcome 3 Death or CLD at 36 to 37 weeks PMA or discharge.
6.3. Analysis.
Comparison 6 HFOV versus CV subgrouped by age at randomisation, Outcome 3 Death or CLD at 36 to 37 weeks PMA or discharge.
7.3. Analysis.
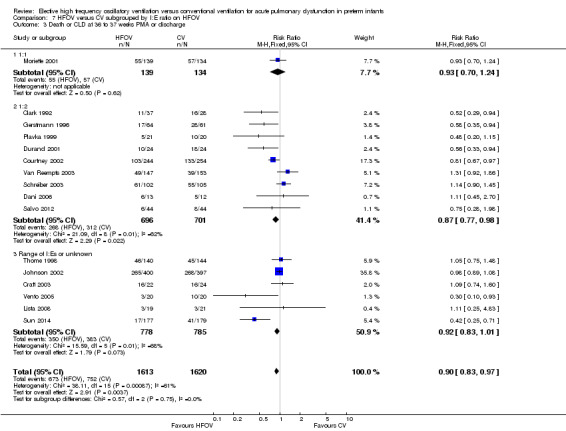
Comparison 7 HFOV versus CV subgrouped by I:E ratio on HFOV, Outcome 3 Death or CLD at 36 to 37 weeks PMA or discharge.
Duration of oxygen therapy
The duration of oxygen therapy was reported in nine trials. The statistical reporting of this outcome differed substantially between trials so meta‐analysis was not undertaken.
Gerstmann 1996 found no significant difference in the duration of oxygen therapy in infants with birth weights of one kilogram or less, but a shorter duration of oxygen therapy in HFOV infants with birth weights over one kilogram (median days 13.2, 95% CI 6.6 to 24.3 versus 27.6, 95% CI 14.3 to 37.7; P ≤ 0.05). Two studies reported means and standard deviations (SDs) for days of oxygen that were similar in the two groups: Van Reempts 2003, HFOV 23.6 (SD 28.2) versus CV 22.7 (SD 28.5); and Dani 2006, HFOV 20.3 (SD 14.6) versus CV 22.0 (SD 15.9). No significant difference in the median days of oxygen therapy between treatment groups was found in the three other studies: Thome 1998, 36 versus 39.5; Plavka 1999, 20 (95% CI 1 to 86) versus 29 (95% CI 4 to 107); Moriette 2001, 22 (interquartile range (IQR) 47) versus 22 (IQR 41). Craft 2003 reported mean (and range) for days of oxygen therapy for the two subgroups by birthweight; 500 to 750 grams, HFOV 75.5 (3 to 136) versus CV 95.1 (3 to 196); 751 to 1000 grams, HFOV 59.9 (1 to 119) versus CV 53.0 (27 to 93). These differences were not statistically different. Vento 2005 reported the mean (SD) hours of oxygen therapy, which was significantly lower in the HFOV group (mean 760, SD 473) compared with the CV group (mean 1445, SD 1297) (P = 0.03). Lista 2008 reported a significantly longer duration of oxygen dependency in the HFOV group (mean 36 days, SD 23) and in the CV group (mean 19 days, SD 11).
Use of mechanical ventilation
Twelve trials reported on the total duration of mechanical ventilation (MV). Overall, the trend was for shorter durations of ventilation in the HFOV groups, but only two trials (Salvo 2012; Sun 2014) showed a significant difference. Seven trials provided data that could be combined in a meta‐analysis but because of extreme heterogeneity between studies for this outcome (P = 0.00001; I2 = 97%) and strong dominance of the meta‐analysis by one trial (Sun 2014) with 89% of the total weight, the result of this meta‐analysis is not reported.
In the Gerstmann 1996 trial the median days on MV (95% CI) in those with a birth weight less than 1 kg was 24.7 days (95% CI 3.7 to 61.4) in the HFOV group and 53.7 days (95% CI 28.4 to 103) in the CV group, a trend that was not significantly different. In this trial there was also a similar median duration of MV in infants with birth weights over 1 kg; 4.1 days (95% CI 1.7 to 6) in the HFOV group versus 4.5 days (95% CI 3 to 6.1) in the CV group. Clark 1992 reported medians and ranges for the days on MV for all infants entered in the study, which were not significantly different between the HFOV group (16 days, 1.8 to 67) and the CV group (30.3 days, 0.5 to 222). Ogawa 1993 reported similar mean (± SD) days of mechanical ventilation in the HFOV group (17.3 ± 24.4) and CV group (13.5 ± 21). Plavka 1999 reported means with 95% CIs for duration of MV and no difference between the HFOV and CV groups (5, 95% CI 1 to 70 versus 7, 95% CI 3 to 52) was shown. Moriette 2001 found similar mean (IQR) durations of MV between HFOV and CV groups (9, IQR 17 versus 9, IQR 16). Van Reempts 2003 reported similar means and SDs for days of MV in the two groups (HFOV 7.7, SD 9.7) versus CV 4.9, SD 9.1). Craft 2003 reported mean (and range) for days of mechanical ventilation for the two subgroups by birthweight; 500 to 750 grams, HFOV 43.3 (range 1 to 136) versus CV 59 (range 3 to 133); 751 to 1000 grams, HFOV 37.7 (range 1 to 83) versus CV 20.1 (range 1 to 56). These differences were not statistically different. Vento 2005 reported the mean (SD) hours of MV, which were not significantly different between the HFOV group (mean 310, SD 313) and the CV group (mean 656, SD 981) (P = 0.15). Dani 2006 and Lista 2008 showed no significant differences in mean days (SD) of MV between the HFOV group and the CV group: 4.1 (SD 1.1) versus 4.5 (SD 2.2) respectively for Dani 2006; and 9.6 (SD 4) versus 10 (SD 2) respectively for Lista 2008. Salvo 2012 and Sun 2014 reported a significantly shorter duration of MV in the HFOV group as compared with the CV group (mean ± SD): 45 ± 17 hours versus 177 ± 84 hours (P < 0.01) for Salvo 2012; 4.0 ± 4.0 days versus 5.7 ± 5.0 days (P < 0.001) for Sun 2014.
Failed treatment
Two trials reported failure to maintain gas exchange with the allocated treatment. Thome 1998 reported a non‐significant trend towards more infants failing based on oxygenation index criteria in the HFOV group (7/140 versus 4/144), while Gerstmann 1996 reported more failures with CV (1/64 versus 9/64, P = 0.008).
Eight trials reported cross‐over to the alternate treatment, a decision that was left to the judgement of individual clinicians. In the HIFI 1989 trial there was a significant increase in treatment failures (failure to maintain adequate gas exchange) in the HFOV group leading to cross‐over of treatment (85/346 in the HFOV group and 60/327 in the CV group, P = 0.01). Moriette 2001 reported a switch in ventilator mode for fewer infants assigned to HFOV than to CV (15% versus 29%; OR 0.43, 95% CI 0.24 to 0.78). Johnson 2002 found the same rate of failure of assigned treatment (10% in each group), while Courtney 2002 reported that more infants exited the assigned mode of treatment in the CV group compared to the HFOV group (52/254 versus 31/244 respectively, P = 0.02). Van Reempts 2003 reported 17 (11.6%) failures in the HFOV group and 10 (6.5%) failures in the CV group, a non‐significant difference. In Durand 2001, two infants crossed over from HFOV and seven infants crossed over from CV at the discretion of clinicians. In Craft 2003, one infant crossed over from HFOV and none crossed over from SIMV. Salvo 2012 reported one treatment failure in each group; both of those infants subsequently died. These data have not been combined in a meta‐analysis as there were differences in definitions between trials and possibly in clinician uptake of the option to cross over.
Two trials had the additional failure criterion of PIE in the CV group. These trials reported cross‐over to be similar between groups (Rettwitz‐Volk 1998: 8/46 versus 9/50) or to be more common in the CV group (Clark 1992: 5/30 versus 9/26, P = 0.01).
Pulmonary air leak syndromes
Thirteen trials involving 2854 infants reported any pulmonary air leak (Analysis 1.9). Two trials showed a significant increase in any air leak in the HFOV group (Thome 1998: RR 1.38, 95% CI 1.01 to 1.89; Schreiber 2003: RR 1.67, 95% CI 1.15 to 2.43). Overall analysis of the 13 trials showed a small but significant increase in the risk of air leak in the HFOV group (fixed‐effect model summary RR 1.19, 95% CI 1.05 to 1.34; summary RD 0.04, 95% CI 0.01 to 0.07; NNTH 25, 95% CI 14 to 100), which remained similar when only HVS trials were considered (11 trials, 2085 infants; fixed‐effect model summary RR 1.19, 95% CI 1.01 to 1.40; summary RD 0.04, 95% CI 0.00 to 0.07). Results were consistent across trials (P for heterogeneity = 0.49; I² = 0% for all trials and 13% for HVS trials).
1.9. Analysis.
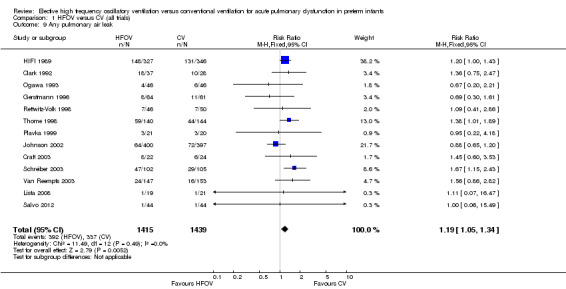
Comparison 1 HFOV versus CV (all trials), Outcome 9 Any pulmonary air leak.
Gross pulmonary air leak (excluding PIE alone) was reported for 2185 infants in 11 trials (Analysis 1.10). One trial (Sun 2014) showed a significant reduction with HFOV in the risk of gross air leak (RR 0.48, 95% CI 0.23 to 0.99). The meta‐analysis, however, showed a non‐significant trend towards an increased risk in the HFOV group (summary RR 1.13, 95% CI 0.88 to 1.45). When only HVS trials were considered, the summary RR was 1.15 (95% CI 0.90 to 1.49). There was no significant heterogeneity for this outcome (P = 0.27; I2 = 18%).
1.10. Analysis.
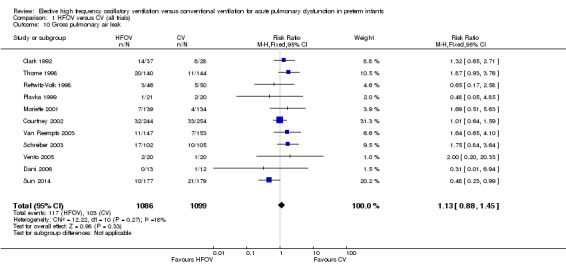
Comparison 1 HFOV versus CV (all trials), Outcome 10 Gross pulmonary air leak.
Subgroup analyses of gross pulmonary air leak
None of the subgroup analyses showed a significant difference between the summary RRs of the subgroups (P value for between‐subgroup heterogeneity > 0.10). However, in the subgroup analysis by type of HFO ventilator, a considerable proportion (I2 = 59.3% with fixed‐effect model) of the observed heterogeneity between the subgroups was due to true between‐subgroup differences rather than chance. In this subgroup analysis a clear trend towards an increased risk of gross pulmonary air leak was seen in the subgroup of trials using a high frequency flow interrupter (summary RR 1.88, 95% CI 0.96 to 3.67) as compared to the subgroup of trials using a true oscillator (summary RR 1.06, 95% CI 0.80 to 1.39).
Intraventricular haemorrhage (IVH)
Twelve trials involving 3084 infants reported all grades of IVH (Analysis 1.11). There was no significant difference in the rate of IVH (all grades) between the treatment groups in individual trials or in the overall analysis (summary RR 1.04, 95% CI 0.95 to 1.14). When only HVS trials (10 trials, 2315 infants) were included, the summary RR was 1.00 (95% CI 0.90 to 1.11). There was no heterogeneity between trials for this outcome.
1.11. Analysis.
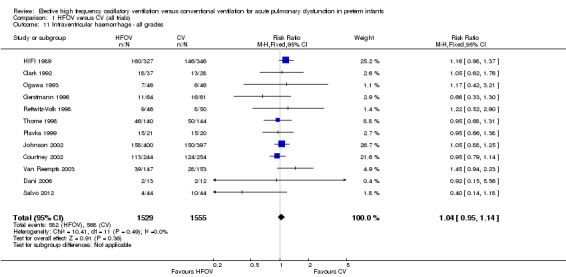
Comparison 1 HFOV versus CV (all trials), Outcome 11 Intraventricular haemorrhage ‐ all grades.
Eighteen trials involving 4069 infants reported the rates of the more severe grades of IVH, grade 3 or 4 (Analysis 1.12). Two trials reported significantly higher rates in the HFOV group: the large HIFI 1989 study, which contributed most weight in the overall analysis (RR 1.41, 95% CI 1.06 to 1.88); and the trial by Moriette 2001 (RR 1.73, 95% CI 1.04 to 2.87). Moriette 2001 reported an increased rate of severe IVH in the HFOV group, both in infants born at less than 28 weeks gestation (HFOV 26/81 versus CV 15/72) and in infants born at 28 or 29 weeks gestation (HFOV 8/58 versus CV 4/61). Overall, there was no significant difference in the rates of more severe grades of IVH between the HFOV and CV groups (summary RR 1.10, 95% CI 0.95 to 1.27). When only HVS trials (16 trials, 3300 infants) were included, the summary RR was 1.00 (95% CI 0.84 to 1.19). This finding was consistent across trials (P for heterogeneity = 0.30; I² = 13%).
1.12. Analysis.
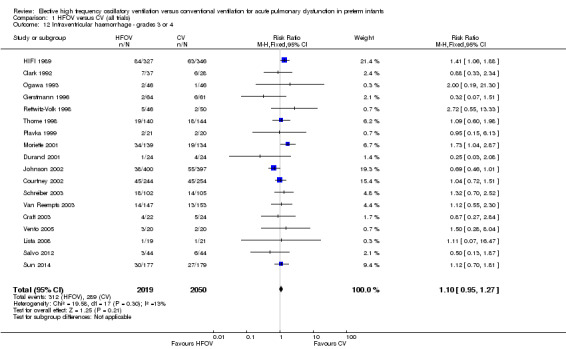
Comparison 1 HFOV versus CV (all trials), Outcome 12 Intraventricular haemorrhage ‐ grades 3 or 4.
A significantly different effect of HFOV on severe IVH was seen in the subgroup analysis by lung volume strategy: in the subgroup of trials not using a HVS the risk was significantly increased (summary RR 1.45, 95% CI 1.09 to 1.93; summary RD 0.07, 95% CI 0.02 to 0.13; NNTH 14, 95% CI 8 to 50) whereas in the subgroup of trials with a HVS targeting an FiO2 ≤ 0.30 the summary RR was 0.84 (95% CI 0.65 to 1.08) (test for between‐subgroup difference P = 0.02; I2 = 76%). No significant between‐subgroup differences were found for the subgroup analyses by surfactant use, type of HFO ventilator, CV strategy and age at randomisation. For the subgroup analysis by I:E ratio, a significantly increased risk was seen in the trial that used an I:E ratio of 1:1 (Moriette 2001) (RR 1.73, 95% CI 1.04 to 2.87; RD 0.10, 95% CI 0.01 to 0.20; NNTH 10, 95% CI 5 to 100) as compared to the other subgroups (I:E ratio of 1:2, and range of I:E ratios or unknown) (test for subgroup difference P = 0.08; I2 = 61%).
Periventricular leukomalacia (PVL)
PVL was reported for 3983 infants in 17 studies. There was a non‐significant trend towards an increased rate with HFOV in HIFI 1989 (RR 1.61, 95% CI 0.99 to 2.60) but no significant difference overall (summary RR 1.03, 95% CI 0.81 to 1.31) (Analysis 1.13).
1.13. Analysis.

Comparison 1 HFOV versus CV (all trials), Outcome 13 Periventricular leukomalacia.
In the subgroup analysis by HFOV strategy (Analysis 2.6), there was a significant between‐subgroup heterogeneity (test for subgroup difference P = 0.08; I2 = 61%) with a significantly increased risk for PVL in the subgroup of trials that did not use a HVS (summary RR 1.64, 95% CI 1.02 to 2.64). Both subgroups of trials using HVS showed no significant difference in the risk of PVL (summary RR 0.91, 95% CI 0.55 to 1.48 for subgroup HVS with target FiO2 ≤ 0.30; and 0.85, 95% CI 0.60 to 1.21 for subgroup HVS with target FiO2 > 0.30 or not specified).
2.6. Analysis.

Comparison 2 HFOV versus CV subgrouped by volume strategy on HFOV, Outcome 6 Periventricular leukomalacia.
For the subgroup analyses by type of HFO ventilator, CV strategy, age at randomisation and I:E ratio, where only trials that used an HVS with HFOV were included, no significant differences between subgroups were found.
Retinopathy of prematurity (ROP)
Twelve trials with 2781 surviving infants reported significant ROP (stage 2 or greater) (Analysis 1.14). The overall analysis showed a significant decrease in the HFOV group with no heterogeneity (summary RR 0.81, 95% CI 0.70 to 0.93; summary RD ‐0.04, 95% CI ‐0.07 to ‐0.01; NNTB 25, 95% CI 14 to 100) (I2 = 0%). When only trials that used HVS were considered, the summary RR was 0.75 (95% CI 0.63 to 0.89).
1.14. Analysis.
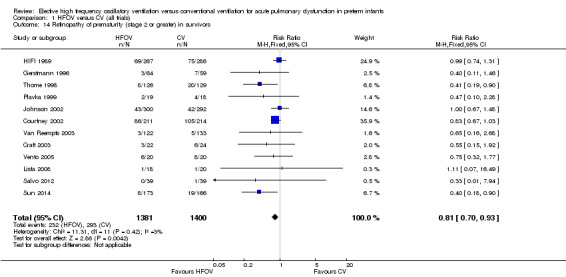
Comparison 1 HFOV versus CV (all trials), Outcome 14 Retinopathy of prematurity (stage 2 or greater) in survivors.
Pulmonary function tests, symptoms and growth at follow‐up
No significant differences in pulmonary function test results were found during the neonatal period (Abbasi 1991) or at discharge (Gerhardt 1989) in subgroups of infants from individual centres in the HIFI 1989 trial. Long‐term follow‐up assessments (in 82% of survivors), including pulmonary function tests (in 43% of survivors from 7 of the 10 centres), were carried out at nine months corrected age in infants from HIFI 1989 (The HIFI Study Group 1990a). There were no significant differences in respiratory function tests (compliance, resistance, lung volumes) or in the incidence of respiratory tract infections, hospital re‐admissions, respiratory symptoms and signs (retractions and episodes of wheezing) or in growth.
Twelve month follow‐up of patients in the Ogawa 1993 trial showed persistence of abnormal fibrous or emphysematous shadows on chest x‐ray in two of the infants in the HFOV group and four in the CV group.
Eighty‐seven per cent of the infants in Gerstmann 1996 were followed up at a mean age of 6.4 years. Improved respiratory function tests (decreased peak expiratory flow, increased residual lung volume, maldistribution of ventilation) were found in the HFOV group but there were no significant differences in symptoms (pulmonary illness, asthma, hospitalisation) between the groups.
Of 185 survivors from 12 centres in Johnson 2002, 149 were invited for respiratory function tests and these were successfully carried out in 76 at 11 to 14 months of age (Thomas 2004). No differences were found between the HFOV and CV groups in any of the measures (functional residual capacity, inspiratory and expiratory resistance, respirator rate). Respiratory symptoms, treatments and growth were assessed at two years of age (Marlow 2006) and there were no differences between the HFOV and CV groups. A larger cohort of adolescent survivors (n = 319) was followed by Zivanovic 2014. The HFOV group had superior results on a test of small‐airway function, forced expiratory volume in 1 second, forced vital capacity, peak expiratory flow, diffusing capacity, and impulse‐oscillometric findings. As compared with the conventional‐therapy group, the HFOV group had significantly higher ratings from teachers in three of eight school subjects assessed, but there were no other significant differences in functional outcomes.
A two years of age assessment of 138 (82%) of survivors in the NOVA study (Schreiber 2003), re‐analysed by type of ventilation, revealed no difference in the mean height, weight or head circumference of children in the HFOV and CV groups.
Neurodevelopmental outcomes at follow‐up
Neurodevelopmental status at follow‐up was reported for eight studies (HIFI 1989; Ogawa 1993; Gerstmann 1996; Moriette 2001; Johnson 2002; Schreiber 2003; Van Reempts 2003; Sun 2014). The age and methods of assessment varied between studies so the results were presented in the text and not included in a meta‐analysis.
Neurodevelopmental status was assessed at 16 to 24 months corrected age in 77% of survivors of the HIFI 1989 study (185 HFOV & 201 CV) using Bayley psychometric tests and central nervous system examinations (The HIFI Study Group 1990b). The rate of moderate to severe abnormality (Bayley's scores more than one SD below the mean, or neurological abnormality) was higher in the HFOV group (RR 1.28, 95% CI 1.02 to 1.60). The rate of cerebral palsy was 11% in both groups. There was an increase in the rate of hydrocephalus in the HFOV group (RR 2.08, 95% CI 1.07 to 4.06). Using logistic regression, abnormal neurological status was shown to be associated with the increased rate of severe grade 3 or 4 IVH in this study.
One year follow‐up in the trial by Ogawa 1993 showed no significant difference in motor or mental development, although the method of neurological assessment was not given.
Gerstmann 1996 reported neurodevelopmental status at a mean of 6.4 years for 87% of the infants. Assessment of mental function using the Wechsler Scale for Children, and motor function using the Bruinink‐Oseretsky test showed no significant difference in mean scores between the two groups.
Moriette 2001 assessed neuromotor outcome at the corrected age of two years in 192 of 212 survivors (90%) using a physician questionnaire. Despite a non‐significant increase in severe IVH rate in the HFOV group as compared with the CV group, the risk of spastic cerebral palsy was significantly lower for infants ventilated with HFOV (4% versus 17%; OR 0.87, 95% CI 0.79 to 0.96), even after adjustment for multiple factors. Survival without cerebral palsy was significantly more likely in the HFOV group than in the CV group (OR 1.89, 95% CI 1.04 to 3.44).
Johnson 2002 reported neurodevelopmental outcomes at 22 to 28 months (corrected for prematurity) based on paediatric report for 73% of survivors and on parent questionnaires for 49% of survivors. No differences between the HFOV and CV groups were found.
Van Reempts 2003 followed up a subgroup of infants who were less than 30 weeks gestation or 1250 grams at birth, or had intracranial lesions on ultrasound. This included 70 infants in the HFOV group and 68 in the CV group, representing 57% and 51% respectively of survivors in the whole trial. Bayley motor and mental developmental indices, as well as motor diagnoses, were assessed at 7 to 12 months corrected age. There was no significant difference between the groups, with 60% of HFOV infants and 70% of CV infants being completely normal. Follow‐up of only the 'abnormal' infants at 18 to 24 months corrected age revealed that none of the infants in the HFOV group and four of the infants in the CV group were persistently abnormal, which was not statistically different.
Schreiber 2003 re‐analysed the follow‐up data from the NOVA study according to mode of ventilation. Of the 168 survivors to two years of age (84 in each group), data were available for 66 (78.6%) of those in the HFOV group and 72 (85.7%) in the CV group. Based on blinded assessments using Bayley's Scales (mean scores and number with scores < 70) and Pediatric neurological assessment there were no differences between the groups.
Sun 2014 assessed neurodevelopmental outcomes at 18 months of corrected age in 145 infants of the HFOV group (84% of survivors) and in 143 infants of the CV group (86% of survivors). Cerebral palsy occurred significantly less in the HFOV group (3% versus 10% in the CV group, P = 0.03), and the risk of having a mental developmental index < 70 was significantly lower in the HFOV group as compared to the CV group (20% versus 31%, P = 0.03). The rate of visual impairment and severe hearing loss was comparable in both groups.
Length of stay and hospital costs
The total hospital costs from a subgroup of patients from one centre (Gerstmann 1996) suggested that the median hospital costs were less in 42 patients randomised to HFOV compared with 41 in the CV group. In this trial, similar differences were found for those infants with birth weights of one kilogram or less and those of more than one kilogram. There were no significant reductions in the median length of hospital stay or in median duration of IPPV in this trial, nor in Rettwitz‐Volk 1998 and Clark 1992, although the trend in each case was towards a reduction in the HFOV group. Johnson 2002 reported similar median and range of days of hospital stay in survivors between treatment groups (HFOV 94 days, range 73 to 114; CV 89 days, range 70 to 112). In the trial by Salvo 2012 a significantly shorter duration of hospital stay was found in the HFOV group (mean days ± SD): 53 ± 21 versus 77 ± 33 in the CV group, P < 0.05. In the Sun 2014 trial a significantly shorter duration of hospital stay was found in the HFOV group (mean days ± SD): 27.0 ± 20.2 versus 31.6 ± 21.7 in the CV group (P = 0.04).
Other outcomes
Subgroup analyses based on baseline patient risk factors have been performed in an individual patient data meta‐analysis reported elsewhere Cools 2010). No differences in effect were found between HFOV and CV on the outcomes of death or bronchopulmonary dysplasia (BPD) at 36 weeks and death or severe adverse neurological event for subgroups based on gestational age at birth (< 26 wk, 26 to 28 wk, 29 to 31 wk, ≥ 32 wk), oxygenation index at study entry (< 4, 4 to 9, > 9), treatment with antenatal corticosteroids or not, age at intubation (< 1 hr, 1 to 4 hr, > 4 hr) and age at randomisation (< 1 hr, 1 to 4 hr, > 4 hr).
Use of surfactant was not a pre‐specified outcome in this review. Four trials (Gerstmann 1996; Plavka 1999; Moriette 2001; Salvo 2012) reported less use of surfactant in the group receiving HFOV. In four trials there was no significant difference in surfactant use (Durand 2001; Courtney 2002; Johnson 2002; Van Reempts 2003).
Discussion
In this review, the search revealed 19 trials that met pre‐specified eligibility criteria and 10 trials that were excluded. It is possible that there are other trials that have not been published or were published in a language not covered by this systematic review. The review authors would be most interested in hearing of other published, unpublished or ongoing trials.
Limitations of this review
The studies have been carried out over a long time period (25 years), during which changing obstetric and neonatal practices may have influenced the conditions under study such as RDS, IVH and CLD. Participants in early trials could be up to 34 weeks gestational age or 2000 grams birth weight, whereas recent trials have been confined to more immature infants, for example of less than 30 weeks gestational age or less than 1200 grams birth weight, or to a more selected population of preterm infants who are at higher risk of developing CLD, for example preterm infants who did not receive antenatal corticosteroids or with more severe lung disease as expressed by an index of oxygenation.
Interventions varied markedly by type of ventilator and the strategy used for HFOV and CV. Over time it was more likely that HFOV was delivered using a HVS, which would be likely to improve the effect of HFOV, while CV was more likely to be delivered using lung protective strategies (LPS), which could reduce the comparative effectiveness of HFOV. In the light of the more recent discussion about the importance of obtaining an 'optimal alveolar recruitment' during HVS by aiming at a FiO2 below 0.30 or even 0.25 during the phase of lung volume recruitment, the subgroup analysis by HFOV strategy was further refined by looking separately at HVS trials that targeted a FiO2 ≤ 0.30 and HVS trials that targeted a FiO2 > 0.30. However, this subgroup analysis is based on the intended or prescribed ventilation strategy, which might not always reflect the actually used ventilation strategy in the trial. This might be further explored in the individual patient data meta‐analysis using multivariate analysis techniques. The LPS to prevent CLD in CV is difficult to define. There are variable manoeuvres that principally affect acute lung injury rather than CLD. In this review, four categories were used to evaluate trials that used most strategies ('definite LPS') compared with the other extreme of none ('definitively no LPS') and two intermediate groups. These summary data are based on mixed, soft evidence and might also be able to be explored further in the above mentioned individual patient data meta‐analysis.
The quality of the studies was generally high, as most attempted to conceal the randomisation process. However, the interventions were not blinded in any study and this could be associated with bias regarding the use of co‐interventions and the ascertainment of outcomes, such as duration of mechanical intervention and oxygen therapy. Ascertainment of outcomes was generally complete or made so by author clarification. Since the treatment could not be blinded in any study, outcomes that were care‐giver dependent, such as the duration of oxygen therapy or diagnosis of CLD, may be less valid.
Pulmonary outcomes
A ventilation strategy with HFOV aiming at optimal alveolar recruitment is now widely considered as being the most appropriate strategy for the use of HFOV in preterm infants in order to avoid lung injury. In this review, only two trials were identified that failed to use a high lung volume strategy (HIFI 1989; Rettwitz‐Volk 1998). Because of the clinical relevance of such a strategy, meta‐analyses were not only performed including all trials but also after excluding trials that failed to use a lung volume recruitment strategy with HFOV.
Possibly, the use of HFOV is associated with short‐term pulmonary benefits. Several studies report a shorter duration of mechanical ventilation, although not always statistically significant. Unfortunately, meta‐analysis was not possible for this outcome due to differences in reporting. The finding is supported by results from the individual patient data meta‐analysis, showing a significant reduction in postmenstrual age at final extubation for the HFOV group (9 trials, 2480 infants; weighted mean difference ‐0.35 weeks, 95% CI ‐0.57 to ‐0.12) (Cools 2010) and in the postmenstrual age at which CPAP could be discontinued in the HFOV group (4 trials, 737 infants; weighted mean difference ‐0.42 weeks, 95% CI ‐0.85 to 0.00) (Cools 2010).
This potential benefit, however, is counteracted by a possible increased risk of acute lung injury. A significantly increased risk in pulmonary air leaks was found in the HFOV group in the meta‐analysis when both pulmonary interstitial emphysema and gross pulmonary air leaks were considered in a combined outcome. When only gross pulmonary air leak is considered, which consists mainly of pneumothorax, a non‐significant trend towards an increased risk was found. This finding was consistent across trials.
Combining all trials that used a high volume strategy (HVS), a small but statistically significant reduction in the risk of CLD at term equivalent age (36 to 37 weeks gestation or at discharge) was seen with the use of HFOV. However, substantial heterogeneity existed between trials for this outcome, meaning that this benefit was not consistently reproducible in every trial. This was further explored with subgroup analyses.
Although the feasibility of achieving optimal alveolar recruitment with HFOV, thereby aiming at an FiO2 < 0.30, has been clearly demonstrated (De Jaegere 2006) and the importance of such a strict HVS has been advocated on many occasions, subgroup analysis by strictness of HFOV strategy ('strict' HVS with target FiO2 ≤ 0.30 versus 'less strict' HVS with target FiO2 > 0.30 or not specified) did not reduce this heterogeneity. This might be explained by the fact that the intended or prescribed ventilation strategy did not always reflect the strategy that was actually applied in the trial. Data from the individual patient data meta‐analysis support this idea by showing that in several trials more than 50% of infants in the HFOV group never reached the FiO2 that was set as a target for lung volume recruitment (unpublished data).
HFOV not only varied from trial to trial in terms of the ventilation strategy but also with regard to the type of ventilator (piston oscillators versus flow interrupters) and ventilator settings (I:E ratio of 1:1 versus 1:2). Experimental studies have shown that ventilators perform differently depending on the technique that is used (Pillow 2001) and that changes in I:E ratio can affect intrapulmonary pressures (Pillow 1999). In this review, however, no consistently significant between‐subgroup differences in effect were found in the subgroup analyses by type of ventilator or by I:E setting. In some trials (Johnson 2002) more than one type of ventilator was used, making classification of such trials problematic. Although the individual patient data meta‐analysis was able to overcome this limitation, it failed to show any significant subgroup interaction (Cools 2010).
In the subgroup analysis by CV strategy, no consistently significant difference in effect could be demonstrated between the subgroups, and considerable heterogeneity persisted between the trials within subgroups. Due to a lack of detailed information regarding the ventilation strategy, trials were difficult to classify. It should be noticed that the two trials that definitely did not use a lung protective ventilation strategy with CV (Clark 1992; Gerstmann 1996) both found a significant reduction in the risk for CLD at term equivalent age in the HFOV group.
The subgroup analysis by age at randomisation did not show a significant subgroup difference when the random‐effects model was used. The two trials in the subgroup > 6 hours, however, had contradictory results (a significant benefit in Clark 1992 versus a trend towards harm in Schreiber 2003) suggesting that these trials probably differed in many other aspects than just the age at randomisation. In contrast with the subgroup < 2 hours, in the subgroup 2 to 6 hours a significant reduction in the risk for CLD at term equivalent age was found, and this result was consistent across the four trials in this subgroup. Although hypothetical, the fact that randomisation at 2 to 6 hours of age compared with 0 to 2 hours may have included infants with more definite lung disease, rather than a mixed group including those with hypoventilation at birth, might be a plausible explanation.
Probably many factors have been interacting in those trials, such as the use of antenatal steroids, the use of surfactant and the ventilation strategy. The individual patient data meta‐analysis might be able to clarify the complex interaction of all these factors by using more elaborate multivariate analyses.
Neurodevelopmental outcomes
Increased rates of IVH or PVL occurred in some individual trials but not overall. The pathophysiological factors that might have led to an increased rate of IVH or PVL are not certain. The authors of HIFI 1989 suggested that the nearly constant high mean airway pressure during HFOV might restrict venous return, increase intracranial venous pressure, and decrease cerebral blood flow. However, animal studies (Kinsella 1991) and human studies (Laubscher 1996) failed to show these cardiovascular changes. The latter study reported that cardiac output fell when on HFOV. In a single centre involved in the study of Johnson 2002, echocardiography was carried out in 45 infants (Osborn 2003). Superior vena cava flow was reduced (< 50 ml/kg/min) in more HFOV infants (48%) than CV infants (20%) but this difference was not significant. Cambonie 2003 examined the haemodynamic changes during HFOV versus CV and found no difference in cardiac function but did find a lower end diastolic velocity and a higher resistance index in the anterior cerebral artery.
The tendency for higher rates of IVH or PVL found in this review in association with failure to use HVS was also shown in two other reviews of the evidence of effectiveness of HFV (both oscillation and jet ventilation) (Bollen 2003; Thome 2005) and in a review of elective jet ventilation versus CV (Bhuta 2003). Failure to recruit lung volume and the consequent cardiorespiratory instability has been implicated (Bryan 1991). Whether it was one of these mechanisms or just lack of experience with a new technology at the time is difficult to say. The large HIFI 1989 trial dominated this analysis.
In five of the seven trials reporting long‐term neurodevelopmental outcome no difference was apparent, although in some studies there was considerable loss to follow‐up. In only one trial (Sun 2014) a significant benefit was seen from HFOV on the risk of cerebral palsy or having a poor mental development at 18 months corrected age.
Research questions raised by this review
These include the following.
Is there a specific population of preterm infants at high risk of CLD that would benefit from early HFOV? The individual patient data meta‐analysis failed to identify such a group based on gestational age at birth, antenatal treatment with corticosteroids or not, early oxygenation index, or intrauterine growth but has limitations because of its retrospective design. The most recent trials that were included in this update also aimed at answering this question by including only infants who did not benefit from antenatal corticosteroids (Salvo 2012) or who had more severe lung disease (Sun 2014). Only one of those trials (Sun 2014) showed a significant benefit from HFOV, the other had a small sample size and the incidence of CLD in the control group was rather low (8%).
In the past decade, the early management of RDS in preterm infants has moved away from early invasive mechanical ventilation towards different forms of non‐invasive respiratory support (nasal continuous positive airway pressure (CPAP), high frequency nasal CPAP, nasal IPPV, synchronised nasal IPPV) combined or not with early surfactant administration (INSURE method, minimally invasive surfactant administration). What are the effects of elective HFOV compared to CV when used as early rescue intervention after failure of these initial non‐invasive respiratory interventions?
Are there differences in pulmonary outcomes and adverse effects by type of ventilator used to generate HFOV? Although the individual patient data meta‐analysis did overcome some limitations of the aggregate data meta‐analysis in addressing this question, possible confounding by other trial‐related factors cannot be excluded. The question would be answered best by a head to head trial of HFOV using a true oscillator versus a flow interrupter.
Long‐term neurological and pulmonary follow‐up data from the trials are still limited. Therefore, it remains uncertain what the long‐term growth and development of infants treated with HFOV versus CV are.
Are there differences in the costs compared to benefits of HFOV? Only one small study (Gerstmann 1996) suggested that HFOV reduced costs of care.
Authors' conclusions
Implications for practice.
There is evidence that the use of elective HFOV results in a small reduction in the risk of CLD when compared with CV, but the evidence is weakened by the inconsistency of this effect across trials. Probably many factors, both related to the intervention itself as well as to the individual patient, interact in a complex way. In addition, the benefit could be counteracted by an increased risk of acute air leak. So, presently the preference for a specific ventilation mode remains a matter of clinical judgement requiring a balance between a relatively small benefit and a possible short‐term harm. Adverse effects on short‐term neurological outcomes have been observed in some studies, but these effects are not significant overall. Most trials reporting long‐term outcome have not identified any difference.
Implications for research.
Any future trials on elective HFOV should target those infants who are at most risk of CLD (extremely preterm infants, infants who did not benefit from antenatal corticosteroids), compare different strategies for generating HFOV, examine the effects of elective HFOV as an early rescue intervention after failure of non‐invasive respiratory support, and report important long‐term pulmonary and neurodevelopmental outcomes. Economic analysis should also be incorporated.
What's new
| Date | Event | Description |
|---|---|---|
| 30 November 2014 | New search has been performed | Seach updated in November 2014. |
| 30 November 2014 | New citation required and conclusions have changed | Follow‐up study of pulmonary function in adolescents from the trial of Johnson 2002 identified. One new study from China identified (Sun 2014). Conclusions changed to note that the use of elective HFOV compared with CV resulted in a small reduction in the risk of CLD, but that the evidence is weakened by the inconsistency of this effect across trials. |
History
Protocol first published: Issue 1, 1996 Review first published: Issue 1, 1997
| Date | Event | Description |
|---|---|---|
| 11 February 2013 | New search has been performed | Search completed through February 2013. One additional study added (Salvo 2012). Three studies excluded. Results unchanged. Version not published. |
| 11 November 2009 | Amended | Minor reference edits |
| 12 August 2009 | Amended | Outcomes 1.6 and 1.7 ‐ X‐axis graph label corrected. |
| 1 February 2009 | New search has been performed | This updates the review 'Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants' published in The Cochrane Library, Issue 1, 2007 (Henderson‐Smart 2007). As a result of a search update to January 2009, data from two more trials have been added. Subgroup analyses have been further refined. |
| 1 February 2009 | New citation required but conclusions have not changed | New authorship; two trials added; subgroup analyses further refined. |
| 3 June 2008 | Amended | Converted to new review format. |
| 14 May 2007 | New search has been performed | This updates the review 'Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants' published in The Cochrane Library, Issue 1, 2003 (Henderson‐Smart 2003). As a result of a search update to November 2006, data from four trials have been added. One new trial is still awaiting assessment and another is yet to be published. Additional subgroup analyses have been added to explore heterogeneity. |
| 14 May 2007 | New citation required but conclusions have not changed | Substantive amendment |
Acknowledgements
Authors of the following trials: Clark 1992, Ogawa 1993, Gerstmann 1996, Rettwitz‐Volk 1998, Thome 1998, Plavka 1999, Moriette 2001, Johnson 2002, Van Reempts 2003 kindly provided additional information about their studies. Michael Schreiber kindly re‐analysed the data from the NOVA study (Schreiber 2003) and its follow‐up results according to type of ventilation.
The Cochrane Neonatal Review Group (CNRG) has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. HFOV versus CV (all trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by 28 to 30 days | 10 | 2148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.88, 1.34] |
| 2 Mechanical ventilation at 28 to 30 days in survivors | 3 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.35] |
| 3 Oxygen at 28 to 30 days in survivors | 6 | 1043 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.10] |
| 4 CLD at 28 to 30 days (O2 + x‐ray) in survivors | 4 | 820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 1.01] |
| 5 Death or CLD at 28 to 30 days | 5 | 1160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 6 Death by 36 to 37 weeks or discharge | 17 | 3329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.81, 1.10] |
| 7 CLD at 36 to 37 weeks PMA or discharge in survivors | 17 | 2786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.96] |
| 8 Death or CLD at 36 to 37 weeks PMA or discharge | 17 | 3329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 9 Any pulmonary air leak | 13 | 2854 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.05, 1.34] |
| 10 Gross pulmonary air leak | 11 | 2185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.45] |
| 11 Intraventricular haemorrhage ‐ all grades | 12 | 3084 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.95, 1.14] |
| 12 Intraventricular haemorrhage ‐ grades 3 or 4 | 18 | 4069 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.27] |
| 13 Periventricular leukomalacia | 17 | 3983 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.81, 1.31] |
| 14 Retinopathy of prematurity (stage 2 or greater) in survivors | 12 | 2781 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.93] |
1.2. Analysis.
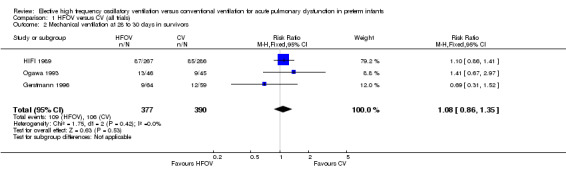
Comparison 1 HFOV versus CV (all trials), Outcome 2 Mechanical ventilation at 28 to 30 days in survivors.
Comparison 2. HFOV versus CV subgrouped by volume strategy on HFOV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by 36 to 37 weeks or discharge | 17 | 3329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.81, 1.10] |
| 1.1 High volume strategy on HFOV with target FiO2 ≤ 0.30 | 8 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.08] |
| 1.2 High volume strategy on HFOV with target FiO2 > 0.30 or not specified | 8 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.81, 1.28] |
| 1.3 No high volume strategy on HFOV | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.39, 4.75] |
| 2 CLD at 36 to 37 weeks PMA or discharge in survivors | 17 | 2786 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.96] |
| 2.1 High volume strategy on HFOV with target FiO2 ≤ 0.30 | 8 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 0.99] |
| 2.2 High volume strategy on HFOV with target FiO2 > 0.30 or not specified | 8 | 1216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.00] |
| 2.3 No high volume strategy of HFOV | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or CLD at 36 to 37 weeks PMA or discharge | 17 | 3329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 3.1 High volume strategy on HFOV with target FiO2 ≤ 0.30 | 8 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.97] |
| 3.2 High volume strategy on HFOV with target FiO2 > 0.30 or not specified | 8 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.02] |
| 3.3 No high volume strategy on HFOV | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.39, 4.75] |
| 4 Gross pulmonary air leak | 11 | 2185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.45] |
| 4.1 High volume strategy HFOV with target FiO2 ≤ 0.30 | 4 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.61, 1.51] |
| 4.2 High volume strategy on HFOV with target FiO2 > 0.30 or not specified | 6 | 1384 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.93, 1.71] |
| 4.3 No high volume strategy on HFOV | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.17, 2.58] |
| 5 Intraventricular haemorrhage ‐ grades 3 or 4 | 18 | 4069 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.27] |
| 5.1 High volume strategy on HFOV with target FiO2 ≤ 0.30 | 7 | 1730 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.08] |
| 5.2 High volume strategy on HFOV with target FiO2 > 0.30 or not specified | 9 | 1570 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.92, 1.48] |
| 5.3 No high volume strategy on HFOV | 2 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.09, 1.93] |
| 6 Periventricular leukomalacia | 17 | 3983 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.81, 1.31] |
| 6.1 High volume strategy on HFOV with target FiO2 ≤ 0.30 | 8 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.55, 1.48] |
| 6.2 High volume strategy with target FiO2 > 0.30 or not specified | 7 | 1459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 6.3 No high volume strategy on HFOV | 2 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.02, 2.64] |
2.1. Analysis.
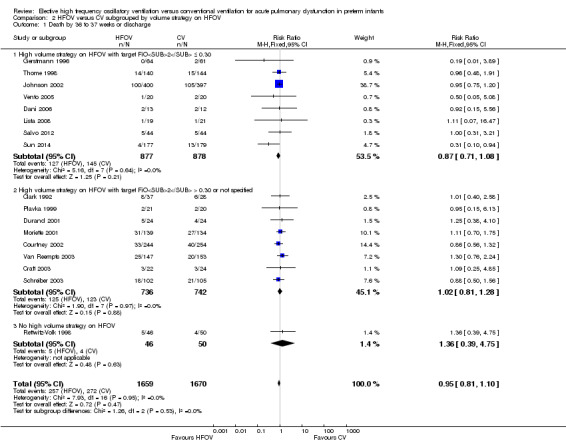
Comparison 2 HFOV versus CV subgrouped by volume strategy on HFOV, Outcome 1 Death by 36 to 37 weeks or discharge.
2.4. Analysis.

Comparison 2 HFOV versus CV subgrouped by volume strategy on HFOV, Outcome 4 Gross pulmonary air leak.
2.5. Analysis.
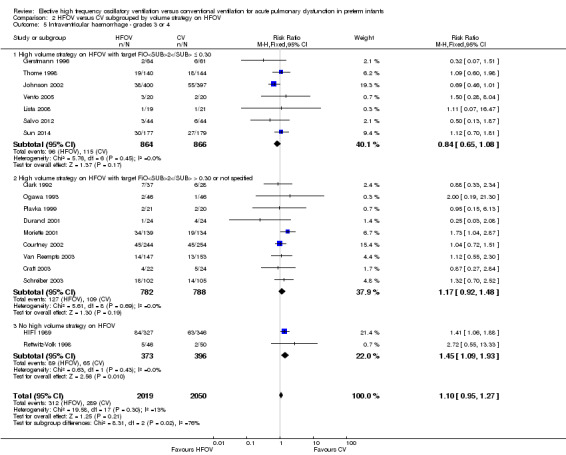
Comparison 2 HFOV versus CV subgrouped by volume strategy on HFOV, Outcome 5 Intraventricular haemorrhage ‐ grades 3 or 4.
Comparison 3. HFOV versus CV subgrouped by use of surfactant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by 36 to 37 weeks or discharge | 16 | 3233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 1.1 Routine surfactant | 15 | 3168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 1.2 No routine surfactant | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.40, 2.58] |
| 2 CLD at 36 to 37 weeks PMA or discharge in survivors | 16 | 2699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.96] |
| 2.1 Routine surfactant | 15 | 2648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.80, 0.97] |
| 2.2 No routine surfactant | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.73] |
| 3 Death or CLD at 36 to 37 weeks PMA or discharge | 16 | 3233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.97] |
| 3.1 Routine surfactant | 15 | 3168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.84, 0.98] |
| 3.2 No routine surfactant | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.29, 0.94] |
| 4 Gross pulmonary air leak | 10 | 2089 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.49] |
| 4.1 Routine surfactant | 9 | 2024 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.87, 1.49] |
| 4.2 No routine surfactant | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.65, 2.71] |
| 5 Intraventricular haemorrhage ‐ grades 3 or 4 | 16 | 3300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 5.1 Routine surfactant | 15 | 3235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 5.2 No routine surfactant | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.34] |
| 6 Periventricular leukomalacia | 15 | 3214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.65, 1.16] |
| 6.1 Routine surfactant | 15 | 3214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.65, 1.16] |
| 6.2 No routine surfactant | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
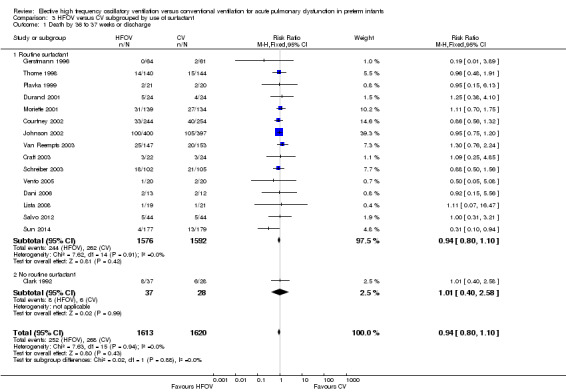
Comparison 3 HFOV versus CV subgrouped by use of surfactant, Outcome 1 Death by 36 to 37 weeks or discharge.
3.4. Analysis.

Comparison 3 HFOV versus CV subgrouped by use of surfactant, Outcome 4 Gross pulmonary air leak.
3.5. Analysis.
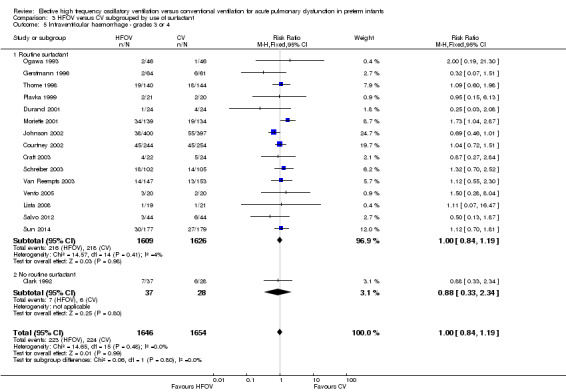
Comparison 3 HFOV versus CV subgrouped by use of surfactant, Outcome 5 Intraventricular haemorrhage ‐ grades 3 or 4.
3.6. Analysis.
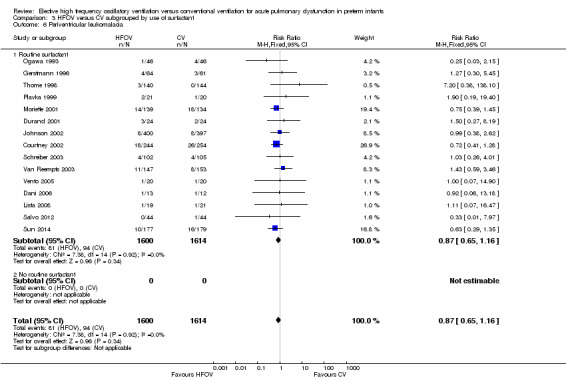
Comparison 3 HFOV versus CV subgrouped by use of surfactant, Outcome 6 Periventricular leukomalacia.
Comparison 4. HFOV versus CV subgrouped by type of HFO ventilator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by 36 to 37 weeks or discharge | 16 | 3233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 1.1 Flow interrupter | 4 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.52, 1.69] |
| 1.2 HF oscillator | 11 | 2026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.16] |
| 1.3 Both HF oscillation and flow interruptors | 1 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.20] |
| 2 CLD at 36 to 37 weeks PMA or discharge in survivors | 16 | 2699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.96] |
| 2.1 Flow interrupter | 4 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.32] |
| 2.2 HF oscillator | 11 | 1737 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.90] |
| 2.3 Both HF oscillators and flow interrupters | 1 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.85, 1.14] |
| 3 Death or CLD at 36 to 37 weeks PMA or discharge | 16 | 3233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.97] |
| 3.1 HF flow interrupter | 4 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.24] |
| 3.2 HF oscillation | 11 | 2026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.93] |
| 3.3 Both HF oscillators and HF flow interrupters | 1 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.08] |
| 4 Gross pulmonary air leak | 10 | 2089 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.49] |
| 4.1 HF flow interrupter | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.96, 3.67] |
| 4.2 HF oscillation | 8 | 1765 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.80, 1.39] |
| 4.3 Both HF oscillators and HF flow interrupters | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Intraventricular haemorrhage ‐ grades 3 or 4 | 16 | 3300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 5.1 HF flow interrupter | 4 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.65, 1.78] |
| 5.2 HF oscillator | 11 | 2093 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.90, 1.36] |
| 5.3 Both HF oscillators and HF flow interrupters | 1 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.46, 1.01] |
| 6 Periventricular leukomalacia | 16 | 3216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.65, 1.16] |
| 6.1 HF flow interrupter | 3 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [0.52, 10.04] |
| 6.2 HF oscillator | 12 | 2055 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.11] |
| 6.3 Both HF oscillators and HF flow interrupters | 1 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.38, 2.62] |
4.1. Analysis.
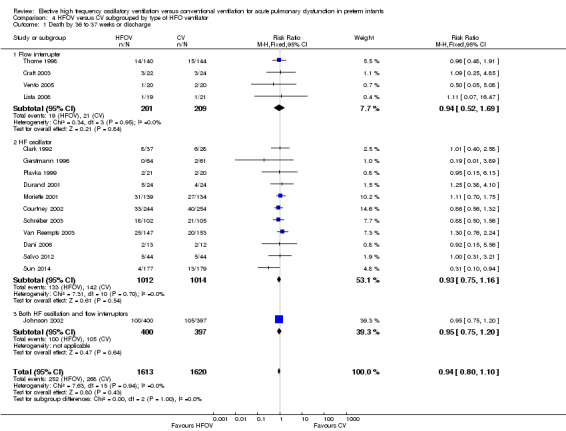
Comparison 4 HFOV versus CV subgrouped by type of HFO ventilator, Outcome 1 Death by 36 to 37 weeks or discharge.
4.4. Analysis.
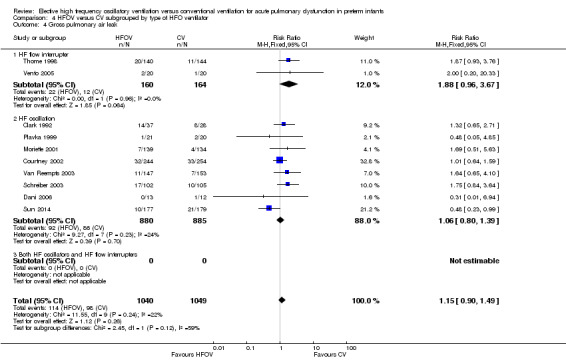
Comparison 4 HFOV versus CV subgrouped by type of HFO ventilator, Outcome 4 Gross pulmonary air leak.
4.5. Analysis.
Comparison 4 HFOV versus CV subgrouped by type of HFO ventilator, Outcome 5 Intraventricular haemorrhage ‐ grades 3 or 4.
4.6. Analysis.
Comparison 4 HFOV versus CV subgrouped by type of HFO ventilator, Outcome 6 Periventricular leukomalacia.
Comparison 5. HFOV versus CV subgrouped by lung protective (LPS) CV strategy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by 36 to 37 weeks or discharge | 16 | 3233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 1.1 Definitive LPS on CV | 9 | 1679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.18] |
| 1.2 Probable LPS on CV | 3 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 1.3 Probably no LPS on CV | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.52, 1.53] |
| 1.4 Definitively no LPS on CV | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.33, 1.88] |
| 2 CLD at 36 to 37 weeks PMA or discharge in survivors | 16 | 2699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.96] |
| 2.1 Definitive LPS on CV | 9 | 1473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.66, 0.94] |
| 2.2 Probable LPS on CV | 3 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.10] |
| 2.3 Probably no LPS on CV | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.49] |
| 2.4 Definitively no LPS on CV | 2 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.31, 0.75] |
| 3 Death or CLD at 36 to 37 weeks PMA or discharge | 16 | 3235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.96] |
| 3.1 Definitive LPS on CV | 9 | 1679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.95] |
| 3.2 Probable LPS on CV | 3 | 1118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.07] |
| 3.3 Probably no LPS on CV | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.31] |
| 3.4 Definitively no LPS on CV | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.38, 0.81] |
| 4 Gross pulmonary air leak | 10 | 2089 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.49] |
| 4.1 Definitive LPS on CV | 6 | 1503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.41] |
| 4.2 Probable LPS on CV | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.51, 5.63] |
| 4.3 Probably no LPS on CV | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.77, 3.04] |
| 4.4 Definitively no LPS on CV | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.65, 2.71] |
| 5 Intraventricular haemorrhage ‐ grades 3 or 4 | 16 | 3300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 5.1 Definitive LPS on CV | 8 | 1654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.81, 1.30] |
| 5.2 Probable LPS on CV | 3 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.71, 1.27] |
| 5.3 Probably no LPS on CV | 3 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.73, 2.37] |
| 5.4 Definitively no PLS on CV | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.39] |
| 6 Periventricular leukomalacia | 15 | 3214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.65, 1.16] |
| 6.1 Definitive LPS on CV | 9 | 1679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.28] |
| 6.2 Probable LPS on CV | 2 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.48, 1.42] |
| 6.3 Probably no LPS on CV | 3 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.30, 2.06] |
| 6.4 Definitively no LPS on CV | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.30, 5.45] |
5.1. Analysis.

Comparison 5 HFOV versus CV subgrouped by lung protective (LPS) CV strategy, Outcome 1 Death by 36 to 37 weeks or discharge.
5.4. Analysis.
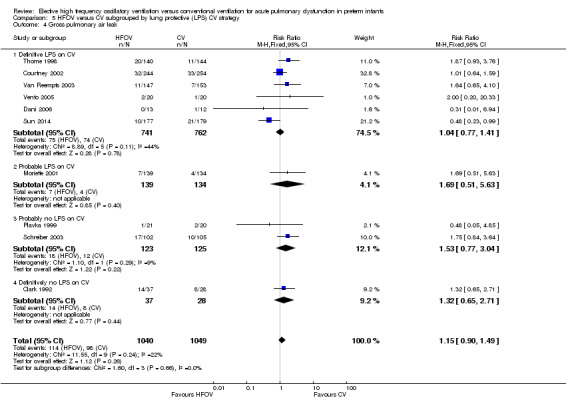
Comparison 5 HFOV versus CV subgrouped by lung protective (LPS) CV strategy, Outcome 4 Gross pulmonary air leak.
5.5. Analysis.
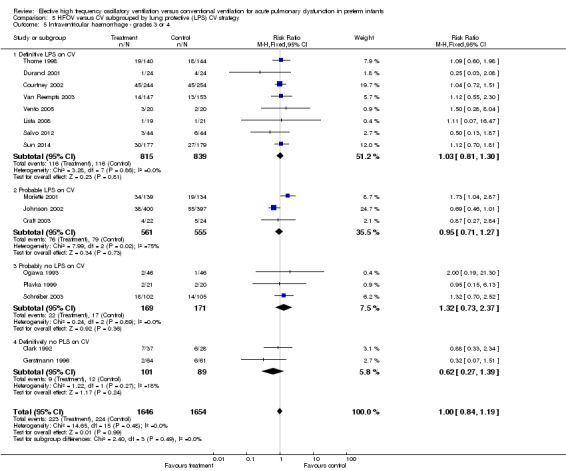
Comparison 5 HFOV versus CV subgrouped by lung protective (LPS) CV strategy, Outcome 5 Intraventricular haemorrhage ‐ grades 3 or 4.
5.6. Analysis.
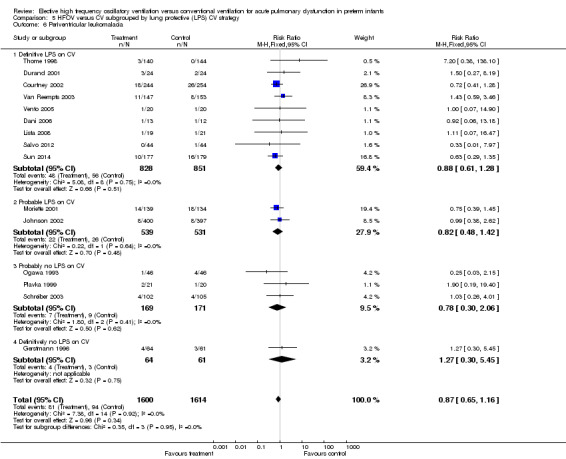
Comparison 5 HFOV versus CV subgrouped by lung protective (LPS) CV strategy, Outcome 6 Periventricular leukomalacia.
Comparison 6. HFOV versus CV subgrouped by age at randomisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by 36 to 37 weeks or discharge | 14 | 2887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.07] |
| 1.1 Less than 2 hours | 7 | 1315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.18] |
| 1.2 2 to 6 hours | 5 | 1300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.14] |
| 1.3 Greater than 6 hours | 2 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.56, 1.48] |
| 2 CLD at 36 to 37 weeks PMA or discharge in survivors | 14 | 2404 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.75, 0.93] |
| 2.1 Less than 2 hours | 7 | 1058 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 2.2 2 to 6 hours | 5 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.81] |
| 2.3 Greater than 6 hours | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.74, 1.37] |
| 3 Death or CLD at 36 to 37 weeks PMA or discharge | 14 | 2887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.81, 0.94] |
| 3.1 Less than 2 hours | 7 | 1315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.87, 1.05] |
| 3.2 2‐6 hours | 5 | 1300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.64, 0.85] |
| 3.3 Greater than 6 hours | 2 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.23] |
| 4 Gross pulmonary air leak | 9 | 1789 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.86, 1.46] |
| 4.1 Less than 2 hours | 4 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.84, 2.82] |
| 4.2 2 ‐ 6 hours | 3 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.24] |
| 4.3 Greater than 6 hours | 2 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.92, 2.59] |
| 5 Intraventricular haemorrhage ‐ grades 3 or 4 | 15 | 3050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.21] |
| 5.1 less than 2 hours | 7 | 1382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.08] |
| 5.2 2 ‐ 6 hours | 6 | 1396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.46] |
| 5.3 Greater than 6 hours | 2 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.69, 2.01] |
| 6 Periventricular leukomalacia | 15 | 2916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.11] |
| 6.1 Less than 2 hours | 8 | 1407 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.52, 1.90] |
| 6.2 2 ‐ 6 hours | 5 | 1300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.53, 1.08] |
| 6.3 Greater than 6 hours | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.26, 4.01] |
6.1. Analysis.
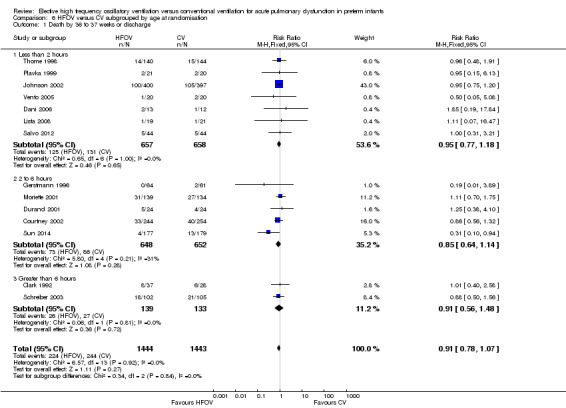
Comparison 6 HFOV versus CV subgrouped by age at randomisation, Outcome 1 Death by 36 to 37 weeks or discharge.
6.4. Analysis.
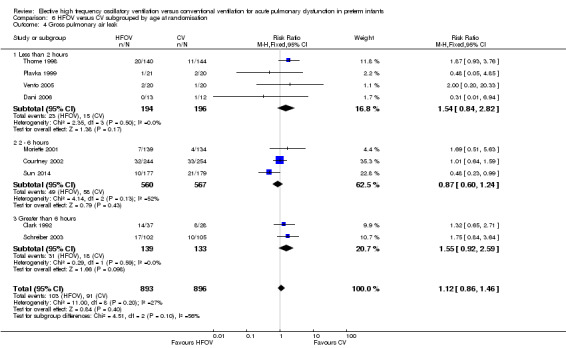
Comparison 6 HFOV versus CV subgrouped by age at randomisation, Outcome 4 Gross pulmonary air leak.
6.5. Analysis.
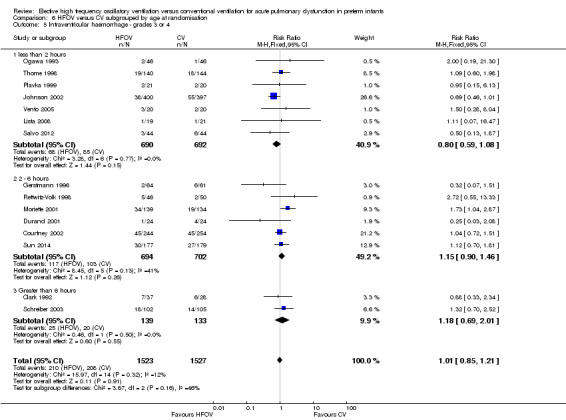
Comparison 6 HFOV versus CV subgrouped by age at randomisation, Outcome 5 Intraventricular haemorrhage ‐ grades 3 or 4.
6.6. Analysis.
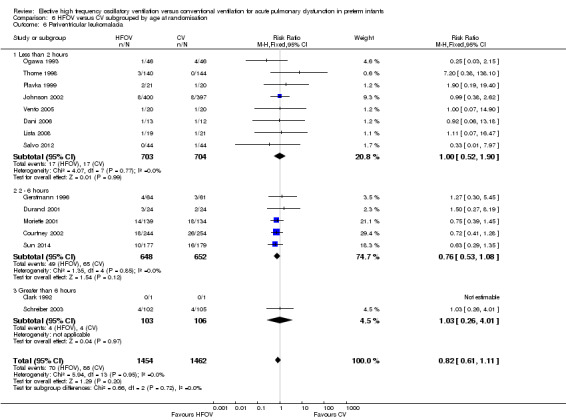
Comparison 6 HFOV versus CV subgrouped by age at randomisation, Outcome 6 Periventricular leukomalacia.
Comparison 7. HFOV versus CV subgrouped by I:E ratio on HFOV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death by 36 to 37 weeks or discharge | 16 | 3233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 1.1 1:1 | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.70, 1.75] |
| 1.2 1:2 | 9 | 1397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.75, 1.25] |
| 1.3 Range of I:Es or unknown | 6 | 1563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.10] |
| 2 CLD at 36 to 37 weeks PMA or discharge in survivors | 16 | 2699 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.86 [0.76, 0.99] |
| 2.1 1:1 | 1 | 215 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.79 [0.43, 1.46] |
| 2.2 1:2 | 9 | 1183 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.66, 1.01] |
| 2.3 Range of I:Es or unknown | 6 | 1301 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.92 [0.77, 1.09] |
| 3 Death or CLD at 36 to 37 weeks PMA or discharge | 16 | 3233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.97] |
| 3.1 1:1 | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.24] |
| 3.2 1:2 | 9 | 1397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.77, 0.98] |
| 3.3 Range of I:Es or unknown | 6 | 1563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.01] |
| 4 Gross pulmonary air leak | 11 | 2185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.45] |
| 4.1 1:1 | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.51, 5.63] |
| 4.2 1:2 | 7 | 1232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.86, 1.58] |
| 4.3 Range of I:Es or unknown | 3 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| 5 Intraventricular haemorrhage ‐ grades 3 or 4 | 15 | 3259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 5.1 1:1 | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.04, 2.87] |
| 5.2 1:2 | 7 | 1331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.26] |
| 5.3 Range of I:Es or unknown | 7 | 1655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.16] |
| 6 Periventricular leukomalacia | 15 | 3214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.65, 1.16] |
| 6.1 1:1 | 1 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.39, 1.45] |
| 6.2 1:2 | 8 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.64, 1.43] |
| 6.3 Range of I:Es or unknown | 6 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.36] |
7.1. Analysis.
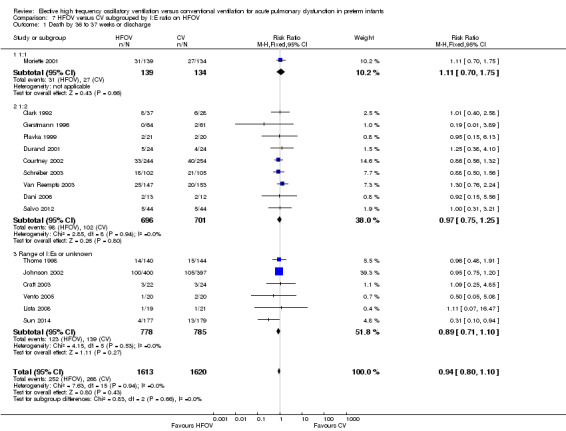
Comparison 7 HFOV versus CV subgrouped by I:E ratio on HFOV, Outcome 1 Death by 36 to 37 weeks or discharge.
7.4. Analysis.
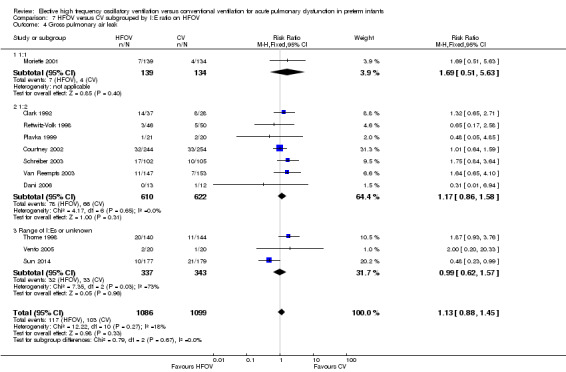
Comparison 7 HFOV versus CV subgrouped by I:E ratio on HFOV, Outcome 4 Gross pulmonary air leak.
7.5. Analysis.
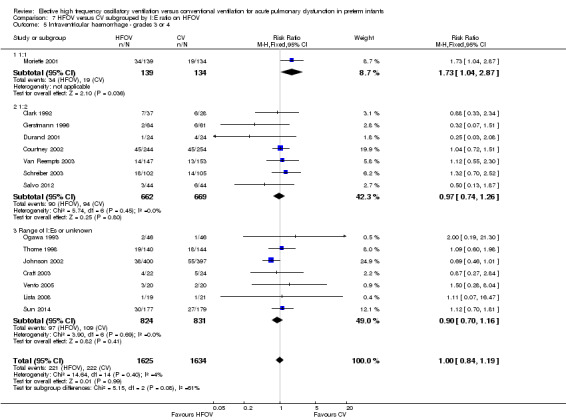
Comparison 7 HFOV versus CV subgrouped by I:E ratio on HFOV, Outcome 5 Intraventricular haemorrhage ‐ grades 3 or 4.
7.6. Analysis.
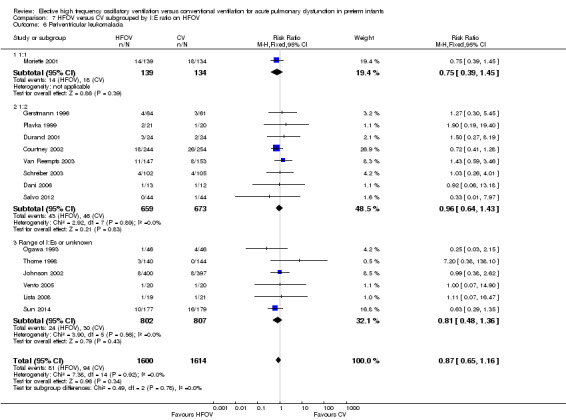
Comparison 7 HFOV versus CV subgrouped by I:E ratio on HFOV, Outcome 6 Periventricular leukomalacia.
Characteristics of studies
Characteristics of included studies [ordered by year of study]
HIFI 1989.
| Methods | Multicentre (11) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ birth weight 750 to 2000 g ‐ respiratory failure in first 24 hrs of life; those at 1250 to 2000 g only eligible if severe RDS ‐ receiving < 12 hrs of mechanical ventilation Exclusion criteria: meconium aspiration; neuromuscular disease; hydrops fetalis; major congenital malformations; hypoplastic lungs Stratification: by centre; by birth weight (from 750 to 1500 g in 250 g strata, 1501 to 2000 g in single stratum) 673 infants enrolled (12 withdrawals, died or consent withdrawn before treated) Mean gestational age: 28.4 ± 2.3 wk in HFOV, 28.3 ± 2.2 in CV Mean birth weight: 1092 ± 294 g in HFOV, 1087 ± 281 g in CV Antenatal corticosteroids: not reported Mean age at randomisation: 6.1 ± 4.6 hrs in HFOV, 5.8 ± 4.0 hrs in CV |
|
| Interventions |
HFOV: OSC using Hummingbird. Settings: initial MAP same or below MAP on CV, 15 Hz HVS: no. Hypoxaemia first treated by increasing FiO2, and thereafter by increasing MAP CV: IMV. Settings: rate 20 to 40/min, IT 0.3 to 1.0 sec, PIP 20 to 25 cmH2O, PEEP 2 to 5 cm H2O. LPS: no. Target PCO2: 35 to 60 mmHg Duration of assigned treatment: until extubation, unless infant meets failure criteria Cross‐over: if infants meet failure criteria (failure to oxygenate or ventilate adequately with assigned ventilator) |
|
| Outcomes | Chronic lung disease (CLD) = oxygen therapy at 28 days and abnormal chest x‐ray; mortality at 28 days; pulmonary air leak (± pneumothorax); all IVH; grade 3 and 4 IVH; PVL; mechanical ventilation at 28 days; failure of assigned treatment (PaO2 < 45 mmHg or PaCO2 > 65 mmHg; NEC; use of vasopressors; pulmonary function at 9 months (432, 82% of survivors); neurodevelopmental outcome at 16 to 24 months (386, 74% of survivors) | |
| Notes | Surfactant: not used (not available) Cross‐over: 26% in HFOV, 17% in CV Postnatal corticosteroids: 12% in HFOV, 9% in CV |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomisation scheme" |
| Allocation concealment (selection bias) | Unclear risk | No information on randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). No information on blinding for other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcome (CLD, 98.3%); completeness of follow‐up for longer‐term outcomes: 74% to 82% |
Clark 1992.
| Methods | Single centre randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ gestational age < 35 weeks ‐ birth weight < 1751 g ‐ requiring mechanical ventilation ‐ younger than 24 hours Exclusion criteria: positive blood culture; lethal congenital anomaly; hydrops fetalis; congenital diagrammatic hernia Stratification: by birth weight: < 1001 versus 1001 to 1750 g; by age: 0 to 12 versus 12 to 24 hrs 152 infants eligible, 98 (63%) enrolled: 78% inborn; RDS chest x‐ray score at entry HFOV > CV; 15 post‐randomisation exclusions in publication ‐ data retrieved from author Mean gestational age: 28 ± 3 wk in HFOV and in CV Mean birth weight: 1080 ± 310 g in HFOV, 1080 ± 340 g in CV Antenatal corticosteroids: 12% in HFOV and 13% in CV Mean age at randomisation: 7 hrs in HFOV, 9 hrs in CV |
|
| Interventions |
HFOV: OSC using Sensormedics 3100. Settings: MAP 1 to 2 cm H2O higher than CV, 10 Hz, I:E ratio 1:2 HVS: yes; increase MAP to optimise oxygenation, wean FiO2 first, once FiO2 < 0.6 priority to wean MAP CV: time‐cycled, pressure‐limited ventilation; no synchronisation. Settings: IT 0.3 to 0.6 sec, rate 25 to 40/min, PEEP 4 to 6 cm H2O, PIP 20 to 27 cm H2O LPS: no Target PCO2: 35 to 55 mmHg Duration of assigned treatment: HFOV until extubation Cross‐over: balanced crossover design, offering patients failing to respond to the assigned mode a trial of the alternative mode. Failure criteria: failure to maintain adequate oxygenation (PO2 > 50 mmHg) or ventilation (PCO2 > 60 mmHg) for 3 hrs |
|
| Outcomes | Chronic lung disease (CLD) = oxygen therapy at 30 days + abnormal chest x‐ray; oxygen therapy at 36 weeks postmenstrual age; failure of assigned treatment to maintain PaO2 > 50 mmHg or PaCO2 < 60 mmHg or in CV group development of pulmonary air leak; pulmonary air leak (± pneumothorax); all IVH; grades 3 or 4 IVH; mortality at 30 days | |
| Notes | Surfactant: no (not available) Cross‐over: 9% in HFOV, 35% in CV Postnatal corticosteroids: not reported Additional arm to the trial consisted of HFOV for 72 hrs then switch to CV (not analysed here) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random cards" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "blind draw" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). No information on outcomes such as brain ultrasound |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up (after additional data from author) |
Ogawa 1993.
| Methods | Multicentre (9) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ birth weight 750 to 2000 g ‐ requiring mechanical ventilation soon after birth Exclusion criteria: if > 12 hrs old; presence of IVH within 1 hr after birth for inborns and within 6 hrs for transferred babies Stratification: by birth weight (750 to 1249 g, 1250 to 1999 g) 92 infants enrolled and analysed Mean gestational age: 29 ± 2.3 wk in HFOV, 29 ± 2.1 in CV Mean birth weight: 1243 ± 322 g in HFOV, 1250 ± 318 g in CV Antenatal corticosteroids: not reported Mean age at randomisation: 2.0 ± 1.6 hrs in HFOV, 1.7 ± 1.5 hrs in CV |
|
| Interventions |
HFOV: OSC using Hummingbird. Settings: high initial MAP, 15 Hz HVS: yes. Alveolar recruitment by manual bagging and use of high MAP. Target FiO2 not reported CV: pressure‐limited time‐cycled, method not stated, using Bear Cub or Sechrist Target PCO2: 35 to 50 mmHg Duration of assigned treatment: not reported Cross‐over: allowed if infant meets failure criteria (same as in HIFI trial) |
|
| Outcomes | Primary outcome all IVH and grade 3 or 4 IVH; chronic lung disease (CLD) = oxygen therapy at 28 days and abnormal chest x‐ray; mortality at 28 days; failure of assigned treatment (as for HIFI 1989); pulmonary air leak; PVL; mechanical ventilation at 28 days; duration of mechanical ventilation; neurodevelopmental outcome at 12 months (all survivors) | |
| Notes | Surfactant: bovine surfactant given in case of "clinical diagnosis of RDS"; 78% of infants received surfactant in both groups Cross‐over: 9% in HFOV, 2% in CV Postnatal corticosteroids: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible for randomisation" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation with opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). No information on blinding of assessment of head ultrasound or chest x‐ray |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcome and for long‐term follow‐up |
Gerstmann 1996.
| Methods | Multicentre (3) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ gestational age < 36 wk ‐ requiring mechanical ventilation shortly after birth ‐ at least moderate respiratory insufficiency (Pa/AO2 < 0.5; poor ventilation) Exclusion criteria: patients > 12 hrs old; severe congenital defects; pre‐existing air leak Stratification: by birth weight (≤ 1000 g, >1000 g); by age at randomisation (≤ 4 hrs, > 4hrs) 125 infants enrolled Mean gestational age: 30.8 ± 2.2 wk in HFOV, 30.1 ± 2.7 wk in CV Mean birth weight: 1560 ± 460 g in HFOV, 1460 ± 470 in CV Antenatal corticosteroids: 30% in HFOV, 18% in CV Mean age at randomisation: mean 2.9 hrs (range 2.4 to 3.3) in HFOV, mean 2.0 hrs (range 1.4 to 3.0) in CV |
|
| Interventions |
HFOV: OSC using Sensormedics 3100(A). Settings: initial MAP 1 to 2 cm H2O > with CV, I:E ratio 0.33, 10 to 15 Hz HVS: yes. Increase MAP to improve oxygenation with target FiO2 < 0.30 CV: IMV using Sechrist. Synchronisation: no. Settings: IT 0.35 to 0.55 sec, rate < 60/min, PEEP 3 to 7 cm H2O, PIP up to 30 cm H2O if < 1 kg and up to 35 cm H2O if > 1 kg LPS: no Target PCO2: 35 to 45 mmHg Duration of assigned treatment: HFOV until extubation or switched to CV if insufficient respiratory drive Cross‐over: if infants meet failure criteria (insufficient oxygenation or ventilation for > 2 hrs; persistent haemodynamic problems; destabilizing problem of air leak; requiring hand ventilation) |
|
| Outcomes | Chronic lung disease (CLD) = oxygen therapy at 30 days and abnormal chest x‐ray; oxygen at discharge (mean age 37 weeks PMA); mortality at 30 days; failure of assigned treatment (PaO2 < 50 or PaCO2 > 60 mmHg for > 2 hrs, or excessive pressures of IPPV); pulmonary air leak; all IVH; grade 3 or 4 IVH; PVL; mechanical ventilation at 28 days; NEC; use of vasopressors; PDA (treated); ROP; BAER; hospital cost | |
| Notes | Surfactant: all infants received at least one dose of bovine surfactant after enrolment in the trial. Infants in the HFOV group received significantly fewer surfactant doses than infants in the CV group Cross‐over: 2% in HFOV, 15% in CV Postnatal corticosteroids: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was by blind card draw from separate sets of..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information regarding concealment procedures |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). Neurodevelopmental assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcome. Long‐term follow‐up of infants of 1 centre: 87% completeness of follow‐up |
Rettwitz‐Volk 1998.
| Methods | Multicentre (3) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ birth weight 750 to 1500 g ‐ respiratory distress syndrome Exclusion criteria: congenital anomalies; hydrops fetalis Stratification: by birth weight (750 to 1000 g, 1001 to 1500 g) 96 infants enrolled and analysed Mean gestational age: 28.5 ± 0.9 wk in HFOV, 28.4 ± 0.95 wk in CV Mean birth weight: 1107 ± 112 g in HFOV, 1111 ± 116 g in CV Antenatal corticosteroids: 24% in HFOV, 18% in CV Mean age at randomisation: 1.16 ± 0.50 hrs in HFOV, 0.66 ± 0.33 hrs in CV |
|
| Interventions |
HFOV: OSC using Stephan SHF 3000 piston oscillator. Settings: initial MAP and amplitude to show good chest movements, 15 to 20 Hz, I:E ratio 1:1 HVS: no. Weaning of MAP first CV: IMV using Stephan HF 300 or Dräger Babylog 8000. Synchronisation: not reported. Settings: PIP to show good chest expansion, IT 0.25 to 0.45 sec, I:E at least = 1:2, PEEP 3 to 4 cm H2O LPS: no Target PCO2: 35 to 48 mmHg Duration of assigned treatment: until extubation or allowed switched to CV if FiO2 < 0.30 and MAP 3 to 4 cm H2O Cross‐over: allowed if infant meets failure criteria (inadequate oxygenation of ventilation with assigned mode; for patients on CV: development of PIE) |
|
| Outcomes | Mortality before discharge, CLD (O2 at 37 weeks PMA), failure of assigned treatment (PaO2 < 45 mmHg or PaCO2 > 60 mmHg), pulmonary ALS ± pneumothorax, IVH, PVL | |
| Notes | Surfactant: bovine surfactant (Survanta) was administered after randomisation when chest x‐ray showed RDS grade II and FiO2 > 0.60 was necessary to achieve PO2 > 50 mmHg Cross‐over: 17% in HFOV, 18% in CV Postnatal corticosteroids: 43% in HFOV, 60% in CV (all patients still on oxygen therapy at 7 days of age received a 21 day course of dexamethasone) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "balanced block randomization scheme" |
| Allocation concealment (selection bias) | Unclear risk | No information on procedures to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). No information on assessment of head ultrasound or chest x‐ray |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary endpoint |
Thome 1998.
| Methods | Multicentre (6) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ inborn ‐ gestational age 24 to 29 wk ‐ requiring mechanical ventilation within 6 hrs of birth Exclusion criteria: major congenital or chromosomal anomalies, hydrops fetalis Stratification: by centre; by gestational age (24 to 25 wk, 26 to 27 wk, 28 to 29 wk) 289 infants enrolled; 4 infants excluded because of congenital malformation and 1 infant excluded because of pneumothorax before randomisation; 284 infants analysed Median (range) gestational age: 27.0 wk (23.4 to 29.9 wk) in HFOV, 27.3 wk (24.0 to 29.9 wk) in CV Median (range) birth weight: 888 g (420 to 1830 g) in HFOV, 870 g (370 to 1395 g) in CV Antenatal corticosteroids: 81% in HFOV, 86% in CV Median (range) age at intubation: 12 minutes (0 to 360) both in HFOV and in CV Median (range) duration of positive pressure ventilation before randomisation: 28 minutes (0 to 90) in HFOV, 0 minutes (0 to 90) in CV |
|
| Interventions |
HFOV: HFFI using Infant Star ventilator (software version 83). Settings: initial MAP 1 to 2 cm H2O higher than with CV or 10 to 12 cm H2O if HFV started immediately, 10 Hz HVS: yes. Stepwise increase of MAP until target FiO2 < 0.30 is reached. Mild sustained inflations (MAP +5 cm H2O during 15 sec) after tracheal suctioning CV: IPPV time‐cycled pressure‐limited ventilation using various ventilators (Dräger Babylog 8000, Stephan HF300, Infant Star, Sechrist IV‐100B). Settings: initial rates 60 to 80/min, aimed at lower PIP and PEEP ≥ 3 cm H2O LPS: yes Target PCO2: 40 to 60 mmHg, up to 70 mmHg from day 7 Duration of assigned treatment: until extubation or for 10 days Cross‐over: in first 10 days allowed if infant meets failure criteria (air leak, oxygenation index as defined in primary outcome), decision left to the attending physician |
|
| Outcomes | "Treatment failure" (ALS < 10 days, oxygenation index > 35 , 40 or 45 in the 3 gestation strata, CLD at 36 weeks or death before discharge), CLD = oxygen or ventilatory support at 36 weeks, ALS = PIE or gross air leaks; IVH; PVL; ROP | |
| Notes | Surfactant: if FiO2 was > 0.30 in HFOV group, or > 0.40 in CV group. Bovine or porcine surfactant given to 68% of HFOV and 71% of CV Cross‐over: not reported Postnatal corticosteroids: 39% of HFOV, 41% of CV |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutively numbered computer‐printed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). Assessment of chest x‐ray was blinded, but not reported for head ultrasound |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up (98.3%) |
Plavka 1999.
| Methods | Single centre randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ birth weight 500 to 1500 g ‐ gestational age < 31 wk ‐ respiratory insufficiency Exclusion criteria: small for gestational age infants; major congenital anomalies or neuromuscular disease; ventilated for CNS disorder or circulatory reason Stratification: no 43 infants enrolled and analysed Mean gestational age: Mean birth weight: Antenatal corticosteroids: Mean age at randomisation: selective intubation in the delivery room with immediate randomisation and transfer to the NICU to start assigned ventilation mode. Initiation of ventilation within 20 minutes after birth |
|
| Interventions |
HFOV: OSC using Sensormedics 3100A. Settings: MAP stepwise increased, 15 Hz, I:E ratio 1:2 HVS: yes. MAP stepwise increased to reach optimal lung inflation; target FiO2 not reported CV: (S)IMV time‐cycled, pressure‐limited using Bearcub 2100 or Infant Star. Synchronisation: not in all patients. Settings: rate 30 to 60/min, IT 0.3 to 0.5 sec, PEEP 3 to 5 cm H2O, PIP to reach adequate chest rise LPS: probably not Target PCO2: not specified ("normocapnia") Duration of assigned treatment: HFOV until extubation Cross‐over: from HFOV to CV if inadequate oxygenation or ventilation despite optimum lung inflation (confirmed by chest x‐ray) and arterial normotension; from CV to HFOV if inadequate oxygenation or ventilation with MAP ≥ 15 cm H2O, PIP ≥ 35 cm H2O and FiO2 ≥ 80% |
|
| Outcomes | Mortality at 30 days and at 36 weeks PMA, any air leak, pneumothorax, CLD 30 days and 36 weeks PMA, any IVH and severe grade 3 or 4 IVH, PVL, ROP > grade 2 | |
| Notes | Surfactant: administered within first 3 hrs of life if criteria for surfactant treatment (not reported) are fulfilled; 42% of infants in HFOV group, and 94% of infants in CV group received surfactant Cross‐over: 0% in HFOV, 10% in CV Postnatal corticosteroids: median (range) cumulative dose of dexamethasone 1.6 mg/kg (0 to 11.3) in HFOV, 2.75 mg/kg (0 to 17.5) in CV Author provided additional data on rates of IVH and pulmonary air leaks in excluded early deaths |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). Chest x‐rays reviewed by blinded observers. No information on assessment of head ultrasound |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up; 2 excluded (1 CNS abnormality in HFOV group and 1 congenital heart disease in CV) |
Durand 2001.
| Methods | Multicentre (7) randomised controlled pilot study | |
| Participants |
Inclusion criteria: ‐ birth weight 501 to 1200 g ‐ mechanically ventilated within 4 hrs after birth ‐ received 1 dose of surfactant ‐ FiO2 ≥ 0.25 ‐ expected to need ventilation > 24 hrs Exclusion criteria: growth not appropriate for gestational age; 5 minute Apgar < 3; base deficit > 14; severe hypotension Stratification: by weight (501 to 800 g, 801 to 1200 g), by antenatal steroids 48 infants enrolled Mean gestational age: 25.9 ± 2.1 in HFOV, 26.1 ± 1.7 in CV Mean birth weight: 823 ± 215 g in HFOV, 856 ± 206 in CV Antenatal corticosteroids: 42% in HFOV, 50% in CV Mean age at randomisation: 2.8 ± 1.2 hrs in HFOV, 2.4 ± 1.0 in CV |
|
| Interventions |
HFOV: OSC using Sensormedics 3100A. Settings: initial MAP 2 cm H2O higher than with CV, 15 Hz, I/T 0.33 HVS: yes. Increase MAP to optimise oxygenation with target FiO2 < 0.40 CV: SIMV using Dräger Babylog, Bearcub, VIP Bird. Settings: Rate < 60/min, PEEP 4 to 6 cm H2O, Ti 0.25 to 0.35 sec, target Vt 5 to 6 ml/kg LPS: yes. Target PCO2: 40 to 55 mmHg (45 to 65 mmHg for infants with CLD) Duration assigned treatment: until death or extubation or development of CLD Cross‐over: no |
|
| Outcomes | Death by 36 weeks, CLD at 36 weeks in survivors, IVH grades 3 or 4, PVL, mean number of doses of surfactant | |
| Notes | Surfactant: all infants received Survanta before enrolment Cross‐over: 8% in HFOV, 29% in CV Postnatal corticosteroids: 42% in HFOV, 62% in CV Pilot study for Courtney 2003 ‐ subjects not include in later study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No information on randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). No information on blinding of other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ 2 infants withdrawn from HFOV arm at parental request |
Moriette 2001.
| Methods | Multicentre (10) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ gestational age 24 to 29 wk ‐ requiring mechanical ventilation before 6 hrs of life ‐ PaO2/FiO2 < 200 ‐ chest x‐ray compatible with RDS Exclusion criteria: IVH grade 3 or 4; pre‐existing pneumothorax; ROM before 24 wk gestational age; severe congenital malformation or hydrops fetalis Stratification: by centre; by gestational age (24 to 27 wk, 28 to 29 wk) 292 infants randomised; 7 inclusion errors, 8 withdrawals of consent 273 infants analysed Mean gestational age: 27.5 ± 1.4 wk in HFOV, 27.6 ± 1.5 wk in CV Mean birth weight: 976 ± 219 g in HFOV, 997 ± 245 g in CV Antenatal corticosteroids: 52% in HFOV, 55% in CV Mean age at randomisation: 2.42 ± 1.58 hrs in HFOV, 2.37 ± 1.97 hrs in CV |
|
| Interventions |
HFOV: OSC using OHF1 piston oscillator (Dufour, France). Settings: initial MAP 2 cm H2O > than on CV, I:E ratio 1:1, 15 Hz, high volume strategy (higher mean airway pressure, sighs) HVS: yes. Increase MAP to optimise oxygenation; use of 'sighs'; target FiO2 < 0.40 CV: SIMV using Dräger babylog 8000. Synchronisation: yes. Settings: TI < 0.45 sec, PEEP 4 to 5 cm H2O, minimal PIP to achieve target PCO2 LPS: probably yes. Target PCO2: 40 to 50 mmHg Duration of assigned treatment: 10 days Cross‐over: allowed during first 10 days if infant meets failure criteria (criteria for ventilatory failure, criteria for radiographic failure such as air leak) |
|
| Outcomes | Death (neonatal and before discharge) Use of > 1 dose of surfactant Pulmonary air leak (PIE and pneumothorax) ROP (? grade) CLD (oxygen at 28 days and oxygen at 36 weeks Duration of IPPV, O2 therapy, hospitalisation Grade 3/4 IVH (7 to 10 day ultrasound (U/S)) PVL (28 day U/S) | |
| Notes | Surfactant: all infants received first dose of surfactant (Curosurf, 200 mg/kg) after randomisation Cross‐over: 15% in CV, 29% in CV Postnatal corticosteroids: 54% in HFOV, 52% in CV |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization" |
| Allocation concealment (selection bias) | Low risk | Quote: "using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). Assessment of head ultrasound and chest x‐rays was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes (7% loss) |
Johnson 2002.
| Methods | Multicentre (25) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ gestational age 23 to 28 wk ‐ inborn ‐ requiring mechanical ventilation from birth Exclusion criteria: transfer to another hospital shortly after birth; congenital malformations Stratification: by centre; by gestational age (23 to 25 wk, 26 to 28 wk) 870 infants randomised; 66 infants were ineligible and excluded; 7 infants withdrawn (5 deemed ineligible, 2 at parent's request. 797 infants included in analyses Mean gestational age: 26.5 wk for total study population Mean birth weight: 853 ± 185 g for total study population Antenatal corticosteroids: 91% in HFOV, 92% in CV Mean age at randomisation: all infants were randomised within the first 60 minutes after birth |
|
| Interventions |
HFOV: mix of OSC using SLE2 2000 (187 infants) or Sensormedics 3100A (38 infants), and HFFI using Dräger Babylog 8000 (165 infants). Settings: 10 Hz, MAP 6 to 8 cm H2O; I:E 1:1 or 1:2, FiO2 weaned before MAP (high volume strategy) HVS: yes. Increase MAP until FiO2 < 0.30 CV: (S)IMV using different ventilators: SLE 2000 (193 infants), Drager Babylog 8000 (192 infants), other ventilators (12 infants). Settings: IT 0.4 sec, initial rate 60/min LPS: probably yes Target PCO2: 34 to 53 mmHg Duration of assigned treatment: after 120 hrs the clinician could choose the ventilation mode Cross‐over: if infants meet failure criteria (failure to achieve adequate oxygenation or ventilation during > 1 hr) |
|
| Outcomes | Death by 36 weeks PMA, chronic lung disease at 36 weeks PMA (oxygen therapy or other assisted ventilation), failure of assigned treatment, IVH, PVL, pulmonary air leak (not defined), ROP grade 2 or more, NEC, length of hospital stay | |
| Notes | Surfactant: protocol recommended surfactant treatment as soon as possible after birth; 97% of HFOV group and 99% of CV group received surfactant Cross‐over: 10% in HFOV, 10% in CV Postnatal corticosteroids: 31% in HFOV, 28% in CV Follow‐up of pulmonary function: Thomas 2004 and Zivanovic 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "infants were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No information on randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). Assessment of brain ultrasound was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcome |
Courtney 2002.
| Methods | Multicentre (26) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ birth weight 601 to 1200 g ‐ appropriate growth for gestational age ‐ received 1 dose of surfactant ‐ requiring mechanical ventilation with FiO2 ≥ 0.25 and MAP ≥ 6 cm H2O ‐ less than 4 hours old ‐ expected to require ventilation for > 24 hrs Exclusion criteria: 5 minute Apgar < 3; base deficit ≥ 15; severe hypotension; chromosomal or congenital anomalies, neuromuscular disease Stratification: by centre; by birth weight (601 to 700 g, 701 to 800 g, 801 to 1000 g, 1001 to 1200 g); by antenatal steroids 498 infants enrolled Mean gestational age: 26.0 ± 1.6 wk in HFOV, 26.1 ± 1.6 in CV Mean birth weight: 859 ± 161 in HFOV, 848 ± 160 in CV Antenatal corticosteroids: 74% in HFOV, 71% in CV Mean age at randomisation: 2.7 hrs in both groups |
|
| Interventions |
HFOV: OSC using SensorMedics 3100A. Settings: initial MAP 2 cm H2O > CV, 10 to 15 Hz, IT 0.33 (I:E ratio 1:2) HVS: yes; increase MAP to optimise oxygenation in order to keep FiO2 ≤ 0.40 CV: SIMV using VIP Bird, Dräger Babylog 800, Bear Cub with volume monitor or Bear Cub 750vs. Setting: Vt 4 to 7 ml/kg, IT 0.25 to 0.40 sec, rate < 60/min LPS: yes PaCO2 target: 45 to 60 mmHg Duration of assigned treatment: HFOV until extubation or for 28 days Crossover: if infants met "clear exit criteria". Analysis according to intention‐to‐treat |
|
| Outcomes | Death by 36 weeks PMA, chronic lung disease at 36 weeks PMA (oxygen therapy or other assisted ventilation), IVH, PVL, pneumothorax, ROP grade 2 or more, NEC, duration of IPPV | |
| Notes | Surfactant: first dose before study entry; subsequent doses if FiO2 ≥ 0.30 Prophylactic indomethacin Cross‐over: 10% in HFOV, 19% in CV Postnatal corticosteroids: 46% in HFOV, 55% in CV |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No information on randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). Radiologist assessing cranial ultrasound was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% loss in HFOV and 2% loss in CV by 36 weeks PMA |
Craft 2003.
| Methods | Multicentre (2) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐gestational age between 23 and 34 weeks ‐ birth weight < 1000 g ‐ requiring mechanical ventilation Stratification: by birth weight (500 to 750 g versus 751 to 1000 g) 46 infants enrolled Mean birth weight: 22 infants in group '500 to 751 g' and 24 infants in group '751 to 1000 g' Antenatal corticosteroids (full course): 45% in HFOV, 54% in CV Mean age at randomisation: nor reported |
|
| Interventions |
HFOV: HFFI using Infant Star. Settings: MAP to obtain optimal lung volume, 10 to 12 Hz, amplitude set to meet goal PCO2 HVS: yes; increase MAP to improve oxygenation with target FiO2 < 0.40 CV: SIMV using Infant Star. Synchronisation: yes. Settings: PEEP 4 to 6 cm H2O, PIP 16 to 24 cm H2O, rate adjusted to reach target PCO2 LPS: probably yes Target PCO2: 50 to 60 mmHg Duration of assigned treatment: HFOV until extubation or until MAP < 7 cm H2O Crossover: if inability to ventilate (pH < 7.20) or to oxygenate (PO2 < 50) |
|
| Outcomes | CLD at 36 weeks PMA ‐ oxygen required to maintain SaO2 > 92%, death, IVH grades 3 or 4, air leak, ROP, failure leading to cross‐over of ventilation type | |
| Notes | Surfactant: all infants received Survanta either in delivery room or within 20 minutes after intubation Cross‐over: not reported Postnatal corticosteroids: not reported January 1999 to May 2000: trial stopped because of lack of effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "envelopes with a previously generated random number sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results on outcomes available for 100% of included infants |
Van Reempts 2003.
| Methods | Single centre randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ gestational age < 32 wk ‐ less than 6 hrs old ‐ requiring mechanical ventilation ‐ FiO2 > 0.40 or MAP x FiO2 > 3.8 ‐ chest x‐ray compatible with RDS Exclusion criteria: active infection at birth, congenital anomalies Stratification: not reported 300 infants enrolled and analysed. Mean gestational age: 28.5 ± 1.8 wk in HFOV, 28.8 ± 1.9 in CV Mean birth weight: 1173 ± 346 g in HFOV, 1217 ± 363 g in CV Antenatal corticosteroids: 63% in HFOV, 66% in CV Median (range) age at randomisation: 0.93 hrs (0.33 to 24.9 hrs) in HFOV, 0.88 hrs (0.33 to 24.8 hrs) in CV |
|
| Interventions |
HFOV: mix of OSC using Sensormedics 3100A (122 infants) and HFFI using Infant Star (25 infants). Settings: initial MAP 8 cm H2O if < 29 weeks and 10 cm H2O if 29 to 31 weeks, 10 Hz. HVS: yes. Increase MAP to improve oxygenation and wean FiO2. Target FiO2 not reported CV: IMV using Dräger Babylog 8000 (73 infants) or Infant Star (80 infants). Synchronisation: not reported. Settings: PIP 20 cm H2O (aim low), PEEP 4 cm H2O, IT < 0.35 sec, rate 80/min, I:E ratio1: 1.1 LPS: yes Target PCO2: 35 to 45 mmHg Duration of assigned treatment: preferentially HFOV until extubation, unless failure of weaning (criteria described) in which case infants were switched to CV Crossover: infant was changed to alternative mode if failure criteria were met (one of the following: 1) inadequate oxygenation or ventilation, as described in the trial, in the first 7 days of life, 2) uncontrollable air leak, 3) cardiovascular dysfunction, 4) need for hand ventilation to maintain adequate gas exchange) |
|
| Outcomes | Chronic lung disease (CLD ‐ on O2 or assisted ventilation) at 36 weeks, death before discharge, failure of assigned treatment, CLD at 28 days, pulmonary interstitial air, pneumothorax, intraventricular haemorrhage, periventricular leukomalacia, retinopathy of prematurity, days of IPPV or CPAP or O2, developmental outcome in early childhood for infants < 30 weeks or with an abnormal head ultrasound | |
| Notes | Surfactant: after initial stabilization either on CV or on HFOV, all infants were given surfactant (Alveofact or Survanta) Cross‐over: 12% in HFOV, 7% in CV Postnatal corticosteroids: not reported Author provided additional information on grades of ROP, prenatal steroids and neonatal mortality |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "using sealed folded papers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). Blinding was applied for grading of chronic lung disease, intracranial haemorrhage, periventricular leukomalacia and retinopathy of prematurity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcome For long‐term outcome: only 57% follow‐up for HFOV, and 51% follow‐up for CV |
Schreiber 2003.
| Methods | Single centre randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ birth weight < 2000 g ‐ gestational age < 34 wk ‐ < 72 hrs old ‐ RDS requiring mechanical ventilation and surfactant treatment Exclusion criteria: major congenital anomalies; hydrops fetalis 2 by 2 factorial design Stratification: by birth weight in 250 g categories 207 infants enrolled and analysed Mean gestational age: 27.2 ± 2.6 wk for total study population Mean birth weight: 983 ± 378 g for total study population Antenatal corticosteroids: 53% for total study population Mean age at randomisation: not reported |
|
| Interventions | Trial randomised infants to nitric oxide versus placebo and to HFOV versus CV
HFOV: OSC using Sensormedics 3100A. Settings: initial MAP 2 cm H2O higher than on CV, 10 to 15 Hz HVS: yes. Use of higher MAP to optimise oxygenation, target FiO2 not reported CV: IMV. Synchronisation: not reported. Settings: rate 40/min, PEEP 4 to 6 cm H2O, PIP to inflate chest LPS: probably not Target PCO2: 35 to 55 mmHg Duration of assigned treatment: not reported Cross‐over: allowed if patient's condition was considered to be critical by attending physician |
|
| Outcomes | Death, CLD at 28 days and 36 week PMA, pulmonary air leak, severe IVH grades 3 or 4, PVL, ROP | |
| Notes | Surfactant: all infants received Survanta before enrolment (inclusion criterion) Prophylactic indomethacin for infants with birth weight < 1250 g Cross‐over: not reported Postnatal corticosteroids: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" "according to permuted block design" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). Blinding of outcome assessment for chest x‐ray, head ultrasound, ROP, neurodevelopment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary analysis, 82% for developmental follow‐up |
Vento 2005.
| Methods | Single centre randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ inborn ‐ birth weight 500 to 1500 g ‐ gestational age 24 to 29 wk ‐ requiring intubation at birth and ongoing intensive care Exclusion criteria: congenital malformations, prenatal infections Stratification: not reported 42 infants eligible; 2 infants excluded with congenital pneumonia; 40 infants enrolled and analysed Mean gestational age: 27.1 ± 1.4 wk in HFOV, 27.4 ± 1.2 wk in CV Mean birth weight: 882 ± 157 g in HFOV, 936 ± 285 g in CV Antenatal corticosteroids: any steroids in 100% in HFOV and 90% in CV, complete course of steroids in 55% in HFVO and 60% in CV Mean age at randomisation: all infants were randomised within 30 minutes of life |
|
| Interventions |
HFOV: HFFI using Dräger Babylog 8000+. Settings: initial MAP 2 cm H2O higher than with CV or at 10 cm H2O, 10 Hz HVS: yes. Increase MAP to improve oxygenation and wean FiO2 with target < 0.25 CV: SIMV using Dräger Babylog 8000+. Setting: Vt 4‐6 ml/kg, PEEP 4 to 5 cm H2O, TI 0.30 to 0.40 sec, maximum rate 60/min, PIP weaned first LPS: yes. Target PCO2: 45 to 55 mmHg Duration of assigned treatment: HFOV until extubation Cross‐over: no |
|
| Outcomes | Death before discharge, CLD (O2 therapy at 36 weeks PMA), pneumothorax, PIE, IVH grades 3 or 4, PVL, ROP > stage 2 | |
| Notes | Surfactant: 75% of infants in both groups received surfactant. Criteria are not described Cross‐over: 0% in both groups Postnatal corticosteroids: 35% in HFOV, 60% in CV |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number allocation" |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque numbered sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). No information on blinding of chest x‐ray or head ultrasound |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up is 95%: two infants (one from each group) excluded after randomisation due to diagnosis of congenital pneumonia |
Dani 2006.
| Methods | Single centre randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ gestational age < 30 wk ‐ requiring mechanical ventilation ‐ clinical signs and chest x‐ray compatible with RDS Exclusion criteria: major congenital malformation; IVH > grade 3; ventilation for < 24 hrs Stratification: no 25 infants enrolled Mean gestational age: 28.3 ± 1.5 wk in HFOV, 28.2 ± 0.8 wk in CV Mean birth weight: 1126 ± 170 g in HFOV, 1075 ± 313 g in CV Antenatal corticosteroids: 85% in HFOV, 75% in CV Mean age at randomisation: 0.75 ± 0.15 hrs in HFOV, 0.78 ± 0.13 hrs in CV |
|
| Interventions |
HFOV: OSC using SensorMedics 3100A. Settings: 10 Hz, initial MAP 8 cm H2O, amplitude 30 cm H2O HVS: yes; no additional information on strategy or target FiO2 CV: pressure support ventilation with volume guarantee (PSV + VG) with Dräger Babylog 8000 plus. Synchronisation: yes. Settings: Vt 5 ml/kg, back‐up rate 60/min, PEEP 3 to 4 cm H2O LPS: yes. PaCO2 target: < 65 mmHg Duration of assigned treatment: presumably until extubation (not explicitly reported) Cross‐over: not reported |
|
| Outcomes | Mortality, CLD (defined as oxygen dependency at 36 weeks postmenstrual age), pneumothorax, any IVH, PVL, duration of mechanical ventilation, duration O2 therapy, duration of CPAP, length of hospital stay | |
| Notes | Surfactant: all infants received Curosurf (200 mg/kg) after lung volume recruitment was obtained Cross‐over: not reported Postnatal corticosteroids: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Enrolled patients were randomly assigned..." |
| Allocation concealment (selection bias) | Low risk | Quote: "...using the opening of sealed opaque envelopes balanced in blocks of four." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). No information on blinding of assessment of head ultrasound or chest x‐ray |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/32 infants were excluded post randomisation for the analyses (3 in HFOV group and 4 in CV group) because of mechanical ventilation less than 24 hours, although this was not a pre‐specified exclusion criterion. Final analysis was done on 25 infants |
Lista 2008.
| Methods | Single centre randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ gestational age 25 to 32 wk ‐ received at least one course of antenatal steroids ‐ requiring mechanical ventilation in the first hour of life ‐ severe RDS with a/A ratio of < 0.2 Exclusion criteria: lethal congenital anomalies; IVH > grade 2; suspected infection Stratification: by gestational age (25 to 28 wk, 29 to 32 wk) 40 infants enrolled Mean gestational age: 27.3 ± 2 in HFOV, 27.4 ± 2 in CV Mean birth weight: 1015 ± 200 g in HFOV, 1006 ± 185 g in CV Antenatal corticosteroids: all infants had at least 1 course Mean age at randomisation: all infants randomised at 1 hr of life |
|
| Interventions |
HFOV: HFFI using Babylog 8000 plus. Settings: 10 Hz, initial MAP 8 to 10 cm H2O, amplitude 40% HVS: yes. Increase MAP to maintain FiO2 below 0.30 CV: assist/control + volume guarantee using Babylog 8000 plus. Settings: Vt 5 ml/kg, PEEP 5 cm H2O, rate 60/min, inspiratory time 0.35 sec LPS: yes Target PCO2: 40 to 65 mmHg Duration of assigned treatment: infants on HFOV were switched to CV if MAP < 8 cm H2O and FiO2 < 0.30 Cross‐over: no |
|
| Outcomes | Death at 36 wk PMA, CLD at 36 wk PMA, air leak severe IVH, PVL, severe ROP, duration of mechanical ventilation, duration of oxygen dependency | |
| Notes | All infants underwent a lung recruitment manoeuvre at birth (20 to 25 cm H2O during 2 sec followed by PEEP 5 cm H2O) Surfactant: all infants received surfactant (Curosurf) within first 2 hours after birth All infants received prophylactic ibuprofen Crossover: 0% in both groups Postnatal corticosteroids: 10% in HFOV, 9% in CV |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "following a sequence of random numbers..." |
| Allocation concealment (selection bias) | Unclear risk | No information on concealment of allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). No information on blinding of assessment of head ultrasound or chest x‐ray |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 5/45 eligible infants were excluded before randomisation. All enrolled infants were analysed |
Salvo 2012.
| Methods | Multicentre (3) randomised controlled trial | |
| Participants |
Inclusion criteria: ‐ birth weight < 1500 g ‐ gestational age < 30 wk ‐ no antenatal corticosteroids ‐ requiring mechanical ventilation for RDS within first 2 hrs of life Exclusion criteria: major congenital malformation; hydrops fetalis; congenital diaphragmatic hernia; congenital pneumonia; multiple pregnancies; congenital heart disease Stratification: not reported 112 infants eligible; 24 infants excluded (3 no consent, 18 not ventilated, 3 congenital pneumonia or malformation); 88 infants enrolled and analysed Mean gestational age: 26.4 ± 2.2 wk in HFOV, 26.5 ± 3.2 wk in CV Mean birth weight: 869 ± 266 g in HFOV, 913 ± 224 g in CV Antenatal corticosteroids: 0% (exclusion criterion) Mean age at randomisation: not reported; all infants were randomised within 2 hrs of birth |
|
| Interventions |
HFOV: OSC using Sensormedics 3100A. Settings: initial MAP 6 to 8 cm H2O, 15 Hz, I:E ratio 1:2, amplitude producing visible chest vibrations HVS: yes. Lung volume recruitment as described by De Jaegere 2006. Target FiO2 < 0.30 CV: SIMV using Bear Cub 750 PSV. Settings: PIP 18 to 24 cm H2O, PEEP 5 to 8 cm H2O, IT 0.30 to 0.40 sec, rate 40 to 60/min LPS: yes Target PCO2: < 65 mmHg Duration of assigned treatment: HFOV until extubation Crossover: switch to alternative mode permitted but not mandatory if failure criteria are met (inadequate oxygenation or ventilation as described in trial protocol; signs of decreased cardiac output) |
|
| Outcomes | Primary outcomes were: the length of ventilatory support, the need of reintubation, and the length of nasal continuous positive airway pressure support in the postextubation period. Secondary outcomes were: the length of stay in neonatal intensive care unit and in hospital, death before discharge, adverse short‐ and long‐term pulmonary and neonatal outcomes, and the need for a second dose of surfactant and of postnatal glucocorticoid treatment | |
| Notes | Surfactant: all infants received surfactant (Curosurf, 200 mg/kg) at a mean postnatal age of 47 min for the HFOV group and 44 min for the CV group Cross‐over: 1 infant out of 44 in each group Postnatal corticosteroids: 1 of 39 infants (2.5%) in HFOV, 4 of 39 infants (10.2%) in CV |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). No information on assessment of chest x‐ray and head ultrasound |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up of enrolled infants: although it is mentioned that infants who crossed over would be excluded from the analyses ('as treated' instead of 'intention‐to‐treat' analysis), all 78 survivors (39 in each group) are represented in the table results. One patient crossed over in each arm |
Sun 2014.
| Methods | Multicentre (2) randomised controlled trial | |
| Participants |
Inclusion Criteria: ‐ gestational age < 32 wks ‐ birth weight < 1500 g ‐ less than 24 hours of age ‐ requiring mechanical ventilation for RDS ‐ PaO2/FiO2 ratio < 200 mmHg ‐ Radiograph criteria of severe RDS Exclusion criteria: genetic metabolic diseases, congenital abnormalities, pneumothorax, IVH grade 3 to 4 Stratification: by centre; by gender; by gestational age (< 28 wk, ≥ 28 wk) 366 infants eligible and randomised; 3 infants excluded post‐randomisation (congenital heart disease); 7 dropouts; 356 infants analysed Mean gestational age: 29.3 ± 2.5 wk in HFOV, 29.5 ± 2.3 in CV Mean birth weight: 1129 ± 199 g in HFOV, 1117 ± 241 g in CV Antenatal corticosteroids: 77% in HFOV, 73% in CV Mean age at randomisation: 5.8 ± 5.0 hrs in HFOV, 5.9 ± 5.1 in CV |
|
| Interventions |
HFOV: OSC using SLE5000. Settings: initial MAP 6 to 8 cm H2O and progressively increased, 10 Hz, IT set at default level of ventilator (I:E ratio not reported) HVS: yes. Lung volume recruitment as described by De Jaegere 2006. Target FiO2 < 0.25 CV: SIMV with pressure‐support (SIMV‐PS) using Servo‐i‐Maquet. Settings: Vt 4 to 6 ml/kg, PIP needed to achieve chest expansion, PEEP 4 to 6 cm H2O, IT 0.25 to 0.40 sec, rate ≤ 60/min (typically 30 to 40/min plus PS) LPS: yes Target PCO2: 40 to 55 mmHg Duration of assigned treatment: HFOV until extubation Cross‐over: no |
|
| Outcomes | Primary outcomes were mortality or incidence of BPD. Secondary outcomes were duration of ventilation and hospitalisation, surfactant requirements, pneumothorax, retinopathy of prematurity ≥ stage 2, and neurodevelopment at 18 months of corrected age. Survival and complete outcome data were available for 288 infants at 18 months of corrected age | |
| Notes | Surfactant: rescue surfactant (Curosurf, 200 mg/kg) was administered after randomisation and only if, after 2 hours of ventilation, PaO2/FiO2 was < 200, and if parental consent was given (parents have to pay for the surfactant). A subsequent dose was administered 12 hours after the first dose if PaO2/FiO2 was < 200 Cross‐over: 0% in both groups Postnatal corticosteroids: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomization plan" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for care‐givers and not relevant for patients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not relevant for most outcomes (death, oxygen dependency). Unclear whether assessment of head ultrasound was blinded Long‐term follow‐up was blinded. Quote: "doctors were blind as to group allocation during follow‐up until 18 months of corrected age" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: 98% for the primary outcome, 82% for neurodevelopmental outcome at 18 months |
HFOV = high‐frequency oscillatory ventilation; OSC = true oscillator; HFFI : high frequency flow interrupter; CV = conventional ventilation; (S)IMV = (synchronised) intermittent mandatory ventilation; IT = inspiratory time; PIP = positive inspiratory pressure; PEEP = positive end‐expiratory pressure; MAP = mean airway pressure; CLD = chronic lung disease; ALS = air leak syndrome; IVH = intraventricular haemorrhage; PVL = periventricular leukomalacia; PMA = postmenstrual age; HVS = high volume strategy; LPS = lung protective strategy (for CV); Vt = tidal volume
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cambonie 2003 | No relevant outcomes |
| Froese 1987 | After randomisation of infants (unknown gestation range), 5 of 11 in the HFOV group and an unknown number from the CV group were excluded from the comparisons between treatments |
| HiFO 1993 | Rescue treatment with primary aim of preventing pulmonary air leak |
| Iscan 2014 | Cross‐over trial comparing two modes of HFOV |
| Lombet 1996 | A total of 22% excluded after randomisation, mixed population of preterm and term infants |
| Nazarchuk 2010 | Prospective randomised trial comparing early HFOV to synchronised intermittent mandatory ventilation (SIMV) in very low birth weight (VLBW) premature infants with omphalocoele: 15 infants weighing 501 to 1000 g less than 4 hours of age, who had received one dose of surfactant and required ventilation with mean airway pressure > 4 to 6 cm H2O and FiO2 > 0.25, and had an anticipated duration of ventilation greater than 48 hours. Newborns were stratified by birth weight and prenatal steroid status, and then randomised to either HFOV or SIMV with tidal volume monitoring. Ventilator management for patients in both study arms was strictly governed by protocols that included optimising lung inflation and blood gases, weaning strategies and extubation criteria. Not included because of unique underlying condition of enrolled infants (presence of omphalocoele) |
| Pardou 1993 | Results reported for only 13 (54%) of the 24 subjects randomised to high frequency flow interrupter or CV |
| Prashanth 2012 | Prospective, non‐randomised comparison of HFOV versus synchronized intermittent mandatory ventilation (SIMV) in 52 preterm infants 26 to 36 weeks' gestation. The study was excluded because it was not a randomised controlled trial |
| Ramanathan 1995 | Mandatory cross‐over of treatments at 96 hrs |
| Singh 2012 | Randomised controlled trial comparing HFOV (Dräger Babylog Plus) with synchronised intermittent mandatory ventilation (SIMV) in preterm infants with birth weight > 750 g with RDS. The primary outcome was the oxygen index in the first 24 hours. In all 53 out of the 215 eligible infants could not be included because of unavailability of the designated ventilator. A total of 150 preterm infants (mean gestational age of 32 weeks) were randomised to receive either HFOV (66 infants) or SIMV (84 infants). After randomisation 40 infants were excluded from the analyses because ventilation was discontinued within 24 hours (17 in the HFOV group and 23 in the SIMV group). The reason for discontinuation was death in 15 cases and "left against medical advice" in 25 cases. HFOV strategy was aimed at recruiting lung volume, and adequate lung inflation was assessed by counting posterior ribs on chest x‐ray. SIMV was aimed at keeping tidal volumes low. The study was excluded because of concerns about both selection bias (25% of eligible infants were not included) as well as attrition bias (post‐randomisation exclusion of 27% of infants). In addition, clinically relevant outcomes were not measured |
Characteristics of studies awaiting assessment [ordered by study ID]
Elazeez 2010.
| Methods | Elazeez 2010 conducted a randomised controlled trial to determine differences in regional cerebral blood flow velocity (CBFV) in preterm infants receiving conventional ventilation (CV) versus high frequency oscillatory ventilation (HFOV) using a high‐volume strategy |
| Participants | Preterm infants admitted to the NICU at Mansoura University Children's Hospital before 12 hours of age. Those requiring ventilator support were randomly allocated to HFOV (n = 23) or CV (n= 24) |
| Interventions | HFOV versus CV |
| Outcomes | Doppler cranial ultrasound and echocardiography were performed on all subjects on day 1 and day 4 with measurements of CBFV in the anterior cerebral (ACA) and middle cerebral arteries (MCA) and assessment of the ductus arteriosus |
| Notes | Published as abstract PAS 2010 |
Sarafidis 2011.
| Methods | Sarafidis 2011 conducted a single centre randomised controlled trial to evaluate the effect of optimised synchronized intermittent mandatory ventilation (SIMV) versus high‐frequency oscillatory ventilation (HFOV) on circulating CC16 and IL‐6 levels |
| Participants | Preterm neonates (gestational age < 30 weeks) requiring mechanical ventilation within the first 2 hrs of life. Of the 30 neonates studied, 24 (gestational age 27.1 ± 1.7 weeks, birth weight (942 ± 214 g) were finally analysed |
| Interventions | Synchronised intermittent mandatory ventilation (SIMV) versus high frequency oscillatory ventilation (HFOV) used as the initial ventilation methods |
| Outcomes | Serum CC16 and IL‐6 were measured on establishment of the assigned ventilation mode after admission, at days 3 and 14 of life as well as at 36 weeks postmenstrual age. Demographic‐perinatal data and clinical parameters were also recorded |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Texas Infant Star.
| Trial name or title | Texas infant Star |
| Methods | |
| Participants | Eligible infants include < 1250 g birth weight or < 29 weeks gestation |
| Interventions | Randomisation to 3 modes of ventilation: conventional, high frequency oscillation, and combination (HFOV + 2 to 5 bpm CV) |
| Outcomes | |
| Starting date | Study commenced in late 1996 ‐ has been completed but not yet published |
| Contact information | Contact: Dr Anthony L Talbert, Texas Tech University School of Medicine, Odessa, Texas, USA. Phone +1 915 335 5270 |
| Notes |
Contributions of authors
An earlier version of this review was developed by Bhuta and Henderson‐Smart and published in 1996. Both authors were involved at all stages in the review.
In 1999 the review was reformatted by Henderson‐Smart with inclusion of two new review authors, Cools and Offringa. Each author evaluated the trials and extracted data independently. Henderson‐Smart entered the data and wrote the text while all the co‐reviewers contributed to data checking and editing.
The update in 2007 included a search and data extraction from four new trials, by Henderson‐Smart and Cools. Henderson‐Smart entered the data and edited the review. The review was evaluated by all review authors.
The update in 2009 included a search by Cools and Offringa. Data from the two new trials were extracted by Cools, Offringa and Henderson‐Smart. Cools entered the data and edited the review. The review was evaluated by all authors.
The update in 2012 included a search by the CNRG Editorial Office through December 2012. Drs Cools and Offringa reviewed the data from the new trial of Salvo 2012 and edited the review. The review was evaluated by all authors.
The updated search in 2014 identified a follow‐up study of pulmonary function from the original trial of Johnson 2002 (Zivanovic. N Engl J Med. 2014 Mar 20;370 (12):1121‐30) and a new trial from Sun 2014. Drs Cools and Offringa reviewed the data from the Sun 2014 trial and edited the review.
Sources of support
Internal sources
Royal Prince Alfred Hospital, Sydney, Australia.
Department of Neonatology, Royal North Shore Hospital, Sydney, Australia.
Department of Neonatology, Academic Medical Centre, Amsterdam, Netherlands.
Centre for Perinatal Health Services Research, University of Sydney, Australia.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
None
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Clark 1992 {published and unpublished data}
- Clark RH, Gerstmann DL, Null DM, deLemos RA. Prospective randomized comparison of high‐frequency oscillatory and conventional ventilation in respiratory distress syndrome. Pediatrics 1992;89(1):5‐12. [PUBMED: 1728021] [PubMed] [Google Scholar]
Courtney 2002 {published data only}
- Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT, Neonatal Ventilation Study Group. High‐frequency oscillatory ventilation versus conventional mechanical ventilation for very‐low‐birth‐weight Infants. New England Journal of Medicine 2002;347(9):643‐52. [PUBMED: 12200551] [DOI] [PubMed] [Google Scholar]
- Durand DJ, Asselin JM, Hudak ML, Aschner JL, McArtor RD, Cleary JP, et al. Early high frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation in very low birth weight infants: a pilot study of two ventilation protocols. Journal of Perinatology 2001;21(4):221‐9. [PUBMED: 11533838] [DOI] [PubMed] [Google Scholar]
Craft 2003 {published data only}
- Craft SP, Bhandari V, Finer NN. The sy‐fi study: a randomized prospective trial of synchronized intermittent mandatory ventilation versus a high‐frequency flow interrupter in infants less than 1000 g. Journal of Perinatology 2003;23(1):14‐9. [PUBMED: 12556921] [DOI] [PubMed] [Google Scholar]
Dani 2006 {published data only}
- Dani C, Bertini G, Pezzati M, Filippi L, Pratesi S, Caviglioli C, et al. Effects of pressure support ventilation plus volume guarantee vs. high‐frequency oscillatory ventilation on lung inflammation in preterm infants. Pediatric Pulmonology 2006;41(3):242‐9. [PUBMED: 16397875] [DOI] [PubMed] [Google Scholar]
Durand 2001 {published data only}
- Durand DJ, Asselin JM, Hudak ML, Aschner JL, McArtor RD, Cleary JP, et al. Early high‐frequency oscillatory ventilation versus synchronized mandatory ventilation in very low birth weight infants: a pilot study of two ventilation protocols. Journal of Perinatology 2001;21(4):221‐9. [PUBMED: 11533838] [DOI] [PubMed] [Google Scholar]
Gerstmann 1996 {published and unpublished data}
- Gerstmann DR, Minton SD, Stoddard RA. Results of the Provo multicenter surfactant high frequency oscillatory ventilation controlled trial. Pediatric Research 1995;37:333A. [Google Scholar]
- Gerstmann DR, Minton SD, Stoddard RA, Meredith KS. The use of early high‐frequency oscillatory ventilation in respiratory distress syndrome. Clinical Research 1994;42:109A. [Google Scholar]
- Gerstmann DR, Minton SD, Stoddard RA, Meredith KS, Bertrand JM. Results of the Provo multicenter surfactant high frequency oscillatory ventilation controlled trial. Pediatric Research 1995;37:333A. [PubMed] [Google Scholar]
- Gerstmann DR, Minton SD, Stoddard RA, Meredith KS, Monarco F, Bertrand JM, et al. The Provo multicenter early high‐frequency oscillatory ventilation trial: improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics 1996;98(6 Pt 1):1044‐57. [PUBMED: 8951252] [PubMed] [Google Scholar]
- Gerstmann DR, Wood K, Miller A, Steffen M, Ogden B, Stoddard RA, et al. Childhood outcome after early high‐frequency oscillatory ventilation for neonatal respiratory distress syndrome. Pediatrics 2001;108(3):617‐23. [PUBMED: 11533327] [DOI] [PubMed] [Google Scholar]
HIFI 1989 {published data only}
- Abbasi S, Bhutani VK, Spitzer AR, Fox WW. Pulmonary mechanics in preterm neonates with respiratory failure treated with high‐frequency compared with conventional mechanical ventilation. Pediatrics 1991;87(4):487‐93. [PUBMED: 2011425] [PubMed] [Google Scholar]
- Gerhardt T, Reifenberg L, Goldberg RN, Bancalari E. Pulmonary function in preterm infants whose lungs were ventilated conventionally or by high‐frequency oscillation. Journal of Pediatrics 1989;115(1):121‐6. [PUBMED: 2738780] [DOI] [PubMed] [Google Scholar]
- The HIFI study group. High frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants. New England Journal of Medicine 1989;320(2):88‐93. [PUBMED: 2643039] [DOI] [PubMed] [Google Scholar]
- The HIFI study group. High frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants: assessment of pulmonary function at 9 months of corrected age. Journal of Pediatrics 1990a;116(6):933‐41. [PUBMED: 2112188] [DOI] [PubMed] [Google Scholar]
- The HIFI study group. High frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants: neurodevelopmental status at 16 to 24 months of postterm age. Journal of Pediatrics 1990b;117(6):939‐46. [PUBMED: 1701005] [DOI] [PubMed] [Google Scholar]
Johnson 2002 {published data only}
- Greenough A, Peacock J, Zivanovic S, Alcazar‐Paris M, Lo J, Marlow N, et al. United Kingdom Oscillation Study: long‐term outcomes of a randomised trial of two modes of neonatal ventilation. Health Technology Assessment 2014;18(41):1‐95. [PUBMED: 24972254] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AH, Peacock JL, Greenough A, Marlow N, Limb ES, Marston L, et al. United Kingdom Oscillation Study Group. High‐frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. New England Journal of Medicine 2002;347(9):633‐42. [PUBMED: 12200550] [DOI] [PubMed] [Google Scholar]
- Osborn DA, Evans N. Randomized trial of high‐frequency oscillatory ventilation versus conventional ventilation: effects on systemic blood flow in very preterm infants. Journal of Pediatrics 2003;143(2):192‐8. [PUBMED: 12970631] [DOI] [PubMed] [Google Scholar]
- Thomas MR, Rafferty GF, Limb ES, Peacock JL, Clavert SA, Marlow N, et al. Pulmonary function at follow‐up of very preterm infants from the United Kingdom oscillation study. American Journal of Respiratory and Critical Care Medicine 2004;169(7):868‐72. [PUBMED: 14693671] [DOI] [PubMed] [Google Scholar]
- Zivanovic S, Peacock J, Alcazar‐Paris M, Lo JW, Lunt A, United Kingdom Oscillation Study Group. Late outcomes of a randomized trial of high‐frequency oscillation in neonates. New England Journal of Medicine 2014;370(12):1121‐30. [PUBMED: 24645944] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lista 2008 {published data only}
- Lista G, Castoldi F, Bianci S, Battaglioli M, Cavigioli F, Bosoni MA. Volume guarantee versus high‐frequency ventilation: lung inflammation in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(4):F252‐6. [PUBMED: 17405870] [DOI] [PubMed] [Google Scholar]
Moriette 2001 {published data only}
- Moriette G, Paris‐Llado J, Walti H, Escande B, Magny JF, Cambonie G, et al. Prospective randomized multicenter comparison of high‐frequency oscillatory ventilation and conventional ventilation in preterm infants of less than 30 weeks with respiratory distress syndrome. Pediatrics 2001;107(2):363‐72. [PUBMED: 11158471] [DOI] [PubMed] [Google Scholar]
- Moriette G, Walti H, Salanave B, Chagnot D, Magny JF, Cambonie G, et al. Prospective randomized multicenter comparison of high‐frequency oscillatory ventilation (HFOV) and conventional ventilation (CV) in preterm infants < 30 weeks gestational age (GA) with RDS. Pediatric Research 1999;45:212A. [DOI] [PubMed] [Google Scholar]
- Truffert P, Paris‐Llado J, Escande B, Magny JF, Cambonie G, Saliba E, et al. Neuromotor outcome at 2 years of very preterm infants who were treated with high‐frequency oscillatory ventilation or conventional ventilation for neonatal respiratory distress syndrome. Pediatrics 2007;119(4):e860‐5. [PUBMED: 17339385] [DOI] [PubMed] [Google Scholar]
Ogawa 1993 {published data only}
- Ogawa Y, Miyasaka K, Kawano T, Imura S, Inukai K, Okuyama K, et al. A multicentre randomised trial of high frequency oscillatory ventilation as compared with conventional mechanical ventilation in preterm infants with respiratory failure. Early Human Development 1993;32:1‐10. [DOI] [PubMed] [Google Scholar]
Plavka 1999 {published data only}
- Plavka R, Kopecky P, Sebron V, Svihovec P, Zlatohlavkova B, Janus V. A prospective randomized comparison of conventional mechanical ventilation and early high frequency oscillatory ventilation in extremely premature newborns with respiratory distress syndrome. Intensive Care Medicine 1999;25(1):68‐75. [PUBMED: 10051081] [DOI] [PubMed] [Google Scholar]
Rettwitz‐Volk 1998 {published and unpublished data}
- Rettwitz‐Volk W, Veldman A, Roth B, Vierzig A, Kachel W, Varnholt V, et al. A prospective, randomized, multicentre trial of high‐frequency oscillatory ventilation compared with conventional ventilation in preterm infants with respiratory distress syndrome receiving surfactant treatment. Journal of Pediatrics 1998;132(2):249‐54. [PUBMED: 9506636] [DOI] [PubMed] [Google Scholar]
Salvo 2012 {published data only}
- Salvo V, Zimmermann LJ, Gavilanes AW, Barberi I, Ricotti A, Abella R, et al. First intention high‐frequency oscillatory and conventional mechanical ventilation in premature infants without antenatal glucocorticoid prophylaxis. Pediatric Critical Care Medicine 2012;13(1):72‐9. [PUBMED: 21499177] [DOI] [PubMed] [Google Scholar]
Schreiber 2003 {published and unpublished data}
- Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. New England Journal of Medicine 2005;353(1):23‐32. [PUBMED: 16000353] [DOI] [PubMed] [Google Scholar]
- Schreiber MD, Gin‐Mestan K, Marks JD, Hou D, Lee L, Srisuparp P. Inhaled nitric oxide in premature infants with respiratory distress. New England Journal of Medicine 2003;349(22):2099‐107. [PUBMED: 14645637] [DOI] [PubMed] [Google Scholar]
- Schreiber MD, Gin‐Mestan K, Srisuparp P, Marks J. Inhaled nitric oxide decreases BPD, death and IVH/PVL in premature infants with respiratory distress syndrome. Pediatric Research 2003;53:31. [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Sun H, Cheng R, Kang W, Xiong H, Zhou C, Zhang Y, et al. High‐frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation plus pressure support in preterm infants with severe respiratory distress syndrome. Respiratory Care 2014;59(2):159‐69. [PUBMED: 23764865] [DOI] [PubMed] [Google Scholar]
Thome 1998 {published and unpublished data}
- Thome U, Kossel H, Lipowsky G, Porz F, Furste H, Genzel‐Boroviczeny O, et al. HFOV Study Group. [Hochfrequenzoszillationsbeatmung (HFOV) im Vergleich mit hochfrequenter intermittierender positiver Druckbeatmung (IPPV) als Ersttherapie bei Fruhgeborenen (FG) mit Atemnotsyndrom. Eine propektive randomisierte multizentrische Studie]. Proceedings of the German‐Austrian Society for Neonatology and Pediatric Critical Care, Munster. October 1997.
- Thome U, Kossel H, Lipowsky G, Porz F, Furste H, Genzel‐Boroviczeny O, et al. HFOV Study Group. High frequency oscillatory ventilation (HFOV) compared with high rate intermittent positive pressure ventilation (IPPV) as first line therapy for premature infants with respiratory insufficiency. A prospective randomized multicenter trial. Pediatric Research 1998;43:300A. [Google Scholar]
- Thome U, Kossel H, Lipowsky G, Porz F, Furste HO, Genzel‐Boroviczeny O, et al. Randomized comparison of high‐frequency ventilation with high‐rate intermittent positive pressure ventilation in preterm infants with respiratory failure. Journal of Pediatrics 1999;135(1):39‐46. [PUBMED: 10393602] [DOI] [PubMed] [Google Scholar]
Van Reempts 2003 {published data only}
- Reempts P, Borstlap C, Laroche S, Auwer JC. Early use of high frequency ventilation in the premature neonate. Eurpean Journal of Pediatrics 2003;162(4):219‐26. [PUBMED: 12647193] [DOI] [PubMed] [Google Scholar]
- Reempts P, Borstlap K, Laroche S. Randomised controlled trial comparing high frequency ventilation versus conventional ventilation in preterm infants. Pediatric Research 2000;47:379A. [Google Scholar]
Vento 2005 {published data only}
- Vento G, Matassa PG, Ameglio F, Capoluongo E, Zecca E, Martelli M, et al. HFOV improves lung mechanics and late pulmonary outcome in extremely low gestational age infants (<30 wks) with respiratory distress syndrome. Pediatric Research 2002;51:393A. [Google Scholar]
- Vento G, Matassa PG, Ameglio F, Capoluongo E, Zecca E, Tortorolo L, et al. HFOV in premature neonates: effects on pulmonary mechanics and epithelial lining fluid cytokines. A randomized controlled trial. Intensive Care Medicine 2005;31(3):463‐70. [PUBMED: 15717206] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cambonie 2003 {published data only}
- Cambonie G, Guillaumont S, Luc F, Vergnes C, Milesi C, Voisin M. Haemodynamic features during high‐frequency oscillatory ventilation in preterms. Acta Paediatrica 2003;92(9):1068‐73. [PUBMED: 14599072] [DOI] [PubMed] [Google Scholar]
Froese 1987 {published data only}
- Froese AB, Butler PO, Fletcher WA, Byford LJ. High‐frequency oscillatory ventilation in premature infants with respiratory failure: a preliminary report. Anesthesia and Analgesia 1987;66(9):814‐24. [PUBMED: 3304021] [PubMed] [Google Scholar]
HiFO 1993 {published data only}
- HiFO study group. Randomised study of high‐frequency oscillatory ventilation in infants with severe respiratory distress syndrome. Journal of Pediatrics 1993;122(4):609‐19. [PUBMED: 8463913] [DOI] [PubMed] [Google Scholar]
Iscan 2014 {published data only}
- Iscan B, Duman N, Kumral A, Ozkan H. Crossover trial comparing high‐frequency oscillatory ventilation versus volume guarantee plus high‐frequency oscillatory ventilation: a preliminary report. Archives Diseases of Childhood 2014;99(Suppl 2):A497‐8. [DOI: 10.1136/archdischild-2014-307384.1380] [DOI] [Google Scholar]
Lombet 1996 {published data only}
- Lombet J, Claris O, Debauche C, Putet G, Rigo J, Verellen G, et al. High frequency oscillation (HFO) versus conventional mechanical ventilation (CMV) for respiratory distress syndrome (RDS). Pediatric Research 1996;40:540. [DOI: 10.1203/00006450-199609000-00172] [DOI] [Google Scholar]
Nazarchuk 2010 {published data only}
- Nazarchuk O, Bertsun K, Dmytriiev D, Palamarchuk Y, Katilov O. Early high‐frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation in very low birth weight infants with omphalocoele. Early Human Development 2010;86:S67‐8. [Google Scholar]
Pardou 1993 {published data only}
- Pardou A, Vermeylen D, Muller MF, Detemmerman D. High‐frequency ventilation and conventional mechanical ventilation in newborn babies with respiratory distress syndrome: a prospective, randomized trial. Critical Care Medicine 1993;19(7):406‐10. [PUBMED: 8270721] [DOI] [PubMed] [Google Scholar]
Prashanth 2012 {published data only}
- Prashanth GP, Malik GK, Singh SN. Elective high‐frequency oscillatory ventilation in preterm neonates: a preliminary investigation in a developing country. Paediatrics and International Child Health 2012;32(2):102‐6. [PUBMED: 22595219] [DOI] [PubMed] [Google Scholar]
Ramanathan 1995 {published data only}
- Ramanathan R, Ruiz I, Tantivit P, Cayabyab R, deLemos R. High frequency oscillatory ventilation compared to conventional mechanical ventilation in preterm infants with respiratory distress syndrome. Pediatric Research 1995;37:347A. [Google Scholar]
Singh 2012 {published data only}
- Singh SN, Malik GK, Prashanth GP, Singh A, Kumar M. High frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation in preterm neonates with hyaline membrane disease: a randomized controlled trial. Indian Pediatrics 2012;49(5):405‐8. [PUBMED: 22700666] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Elazeez 2010 {published data only}
- Shabaan, AEA, Elsallab SM, Bassiouny MR, Abd‐Elhady HE. Randomized trial of high frequency oscillatory ventilation (HFOV) versus conventional ventilation (CV): effect on cerebral hemodynamics in preterm infants. Pediatric Academic Societies Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010:E‐PAS20103748.547.
Sarafidis 2011 {published data only}
- Sarafidis K, Stathopoulou T, Agakidou E, Taparkou A, Soubasi V, Diamanti E, et al. Comparable effect of conventional ventilation versus early high‐frequency oscillation on serum CC16 and IL‐6 levels in preterm neonates. Journal of Perinatology 2011;31(2):104‐11. [PUBMED: 20671716] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Texas Infant Star {unpublished data only}
- Talbert AL. Texas Infant Star. Randomization to 3 modes of ventilation: conventional, high frequency oscillation, and combination (HFOV + 2 ‐ 5 bpm CV). Contact: Dr Anthony L Talbert, Texas Tech University School of Medicine, Odessa, Texas, USA Phone +1 915 335 5270.
Additional references
Bancalari 1992
- Bancalari E, Sinclair JC. Mechanical ventilation. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:200‐20. [Google Scholar]
Bhuta 2003
- Bhuta T, Henderson‐Smart DJ. Elective high frequency jet ventilation versus conventional ventilation in preterm infants mechanically ventilated for RDS. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD000328] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bollen 2003
- Bollen CW, Uiterwaal CS, Vught AJ. Cumulative meta‐analysis of high‐frequency versus conventional ventilation in premature neonates. American Journal of Respiratory Critical Care Medicine 2003;168(10):1150‐5. [PUBMED: 14607823] [DOI] [PubMed] [Google Scholar]
Bryan 1991
- Bryan AC, Froese AB. Reflections on the HIFI trial. Pediatrics 1991;87(4):565‐7. [PUBMED: 2011435] [PubMed] [Google Scholar]
Clark 2000
- Clark RH, Slutsky AS, Gerstmann DR. Lung protective strategies of ventilation in the neonate: what are they?. Pediatrics 2000;105(1 Pt 1):112‐4. [PUBMED: 10617711] [DOI] [PubMed] [Google Scholar]
Cools 2010
- Cools F, Askie LM, Offringa M, Asselin JM, Calvert SA, Courtney SE, et al. PreVILIG collaboration. Elective high‐frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta‐analysis of individual patients' data. Lancet 2010;375(9731):2082‐91. [PUBMED: 20552718] [DOI] [PubMed] [Google Scholar]
De Jaegere 2006
- Jaegere A, Veenendaal MB, Michiels A, Kaam AH. Lung recruitment using oxygenation during open lung high‐frequency ventilation in preterm infants. American Journal of Respiratory and Critical Care Medicine 2006;174(6):639‐45. [PUBMED: 16763218] [DOI] [PubMed] [Google Scholar]
de Lemos 1987
- Lemos RA, Coalson JS, Gerstmann DR, et al. Ventilatory management of infant baboons with hyaline membrane disease; the use of high frequency ventilation. Pediatric Research 1987;21(6):594‐602. [PUBMED: 3299231] [DOI] [PubMed] [Google Scholar]
DeJaegere 2006
- Jaegere A, Veenendaal MB, Michiels A, Kaam AH. Lung recruitment using oxygenation during open lung high‐frequency ventilation in preterm infants. American Journal of Respiratory and Critical Care Medicine 2006;174(6):639‐45. [PUBMED: 16763218] [DOI] [PubMed] [Google Scholar]
Ehrenkrantz 1992
- Ehrenkranz RA, Mercurio MR. Bronchopulmonary dysplasia. In: Sinclair JC, Bracken MB editor(s). Effective Care of theNewborn Infant. Oxford: Oxford University Press, 1992:399‐424. [Google Scholar]
Greenough 2008
- Greenough A, Milner AD, Dimitriou G. Synchronised mechanical ventilation in neonates. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000456.pub3] [DOI] [PubMed] [Google Scholar]
Halliday 2003
- Halliday H, Ehrenkranz R. Moderately early (7‐14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001144] [DOI] [Google Scholar]
Henderson‐Smart 2005
- Henderson‐Smart DJ, Bhuta T. Rescue high frequency oscillatory ventilation versus conventional ventilation for pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000438] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2000
- Jobe AH, Ikegami M. Lung development and function in preterm infants in the surfactant treatment era. Annual Review of Physiology 2000;62:825‐46. [PUBMED: 10845113] [DOI] [PubMed] [Google Scholar]
Jouvet 1997
- Jouvet P, Hubert P, Isabey D, Oinquier D, Dahan E, et al. Assessment of high‐frequency neonatal ventilator performances. Intensive Care Medicine 1997;23(2):208‐13. [PUBMED: 9069008] [DOI] [PubMed] [Google Scholar]
Kinsella 1991
- Kinsella JP, Gerstmann DR, Clark RH, Null DM Jr, Morrow WR, Taylor AF, et al. High frequency oscillatory ventilation versus intermittent mandatory ventilation: early hemodynamic effects in the premature baboon with hyaline membrane disease. Pediatric Research 1991;29(2):160‐6. [PUBMED: 2014152] [DOI] [PubMed] [Google Scholar]
Laubscher 1996
- Laubscher B, Melle G, Fawer CL, Sekarski N, Calame A. Haemodynamic changes during high frequency oscillation for respiratory distress syndrome. Archives of Diseases in Childhood. Fetal and Neonatal Edition 1996;74(3):F172‐6. [PUBMED: 8777679] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCulloch 1988
- McCulloch PR, Forkert PG, Froese AB. Lung volume maintenance prevents lung injury during high‐frequency oscillatory ventilation in surfactant‐deficient rabbits. American Review of Respiratory Disease 1988;137(5):1185‐92. [PUBMED: 3195813 ] [DOI] [PubMed] [Google Scholar]
Meredith 1989
- Meredith KS, deLemos RA, Coalson JJ, King RJ, Gerstmann DR, Kumar R, et al. Role of lung injury in the pathogenesis of hyaline membrane disease in premature baboons. Journal of Applied Pysiology 1989;66(5):2150‐8. [PUBMED: 2745284] [DOI] [PubMed] [Google Scholar]
Northway 1992
- Northway WH Jr. An introduction to bronchopulmonary dysplasia. Clinics in Perinatology 1992;19(3):489‐95. [PUBMED: 1526068] [PubMed] [Google Scholar]
Petrucci 2007
- Petrucci N, Iacovelli W. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003844.pub3] [DOI] [PubMed] [Google Scholar]
Pillow 1999
- Pillow JJ, Neil H, Wilkinson MH, Ramsden CA. Effect of I/E ratio on mean alveolar pressure during high‐frequency oscillatory ventilation. Journal of Applied Physiology 1999;87(1):407‐14. [PUBMED: 10409602] [DOI] [PubMed] [Google Scholar]
Pillow 2001
- Pillow JJ, Wilkinson MH, Neil HL, Ramsden CA. In vitro performance characteristics of high‐frequency oscillatory ventilators. American Journal of Respiratory and Critical Care Medicine 2001;164(6):1019‐24. [PUBMED: 11587990] [DOI] [PubMed] [Google Scholar]
Roberts 2006
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Thome 2005
- Thome UH, Carlo WA, Pohlandt F. Ventilation strategies and outcome in randomized trials of high frequency ventilation. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(6):F466‐73. [PUBMED: 15941826] [DOI] [PMC free article] [PubMed] [Google Scholar]
Truog 1984
- Truog WE, Standaert TA, Murphy JH, Woodrum DE, Hodson WA. Effect of prolonged high frequency oscillatory ventilation in premature primates with experimental hyaline membrane disease. American Review of Respiratory Disease 1984;130(1):76‐80. [PUBMED: 6564845] [DOI] [PubMed] [Google Scholar]
Woodgate 2006
- Woodgate PG, Davies WW. Permissive hypercapnia for the prevention of morbidity and mortality in mechanically ventilated newborn infants. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002061] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Henderson‐Smart 1999
- Henderson‐Smart DJ, Bhuta T, Cools F, Offringa M. Elective high frequency oscillatory ventilation vs conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD000104.pub2] [DOI] [Google Scholar]
Henderson‐Smart 2001
- Henderson‐Smart DJ, Bhuta T, Cools F, Offringa M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000104.pub2] [DOI] [PubMed] [Google Scholar]
Henderson‐Smart 2003
- Henderson‐Smart DJ, Bhuta T, Cools F, Offringa M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000104.pub2] [DOI] [PubMed] [Google Scholar]
Henderson‐Smart 2007
- Henderson‐Smart DJ, Cools F, Bhuta T, Offringa M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD000104.pub2] [DOI] [PubMed] [Google Scholar]


