Abstract
Coronavirus disease 2019 (COVID-19) is associated with a high rate of thrombosis. Prolonged activated partial thromboplastin times (aPTT) and antiphospholipid antibodies (aPL) are reported in COVID-19 patients. The majority of publications have not reported whether patients develop clinically relevant persistent aPL, and the clinical significance of new aPL-positivity in COVID-19 is currently unknown. However, the reports of aPL-positivity in COVID-19 raised the question whether common mechanisms exist in the pathogenesis of COVID-19 and antiphospholipid syndrome (APS). In both conditions, thrombotic microangiopathy resulting in microvascular injury and thrombosis is hypothesized to occur through multiple pathways, including endothelial damage, complement activation, and release of neutrophil extracellular traps (NETosis). APS-ACTION, an international APS research network, created a COVID-19 working group that reviewed common mechanisms, positive aPL tests in COVID-19 patients, and implications of COVID-19 infection for patients with known aPL positivity or APS, with the goals of proposing guidance for clinical management and monitoring of aPL-positive COVID-19 patients. This guidance also serves as a call and focus for clinical and basic scientific research.
Keywords: Antiphospholipid antibodies, antiphospholipid syndrome, lupus anticoagulant, Hughes syndrome, thrombosis, anticoagulation, COVID-19
Scientific background
Since the initial cases of the coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulted in a worldwide pandemic. Venous thromboembolism (VTE) occurs more frequently in hospitalized COVID-19 patients, compared to those without COVID-19.1 Coagulation abnormalities include increased frequency of prolonged activated partial thromboplastin times (aPTT) and lupus anticoagulant (LA),2 as seen in antiphospholipid syndrome (APS) and its severe variant, catastrophic antiphospholipid syndrome (CAPS), which is characterized by inflammatory cytokine signals and microthrombi in multiple organs. Autopsies from patients with COVID-19 showed similar abnormalities.3
Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION), an international network created to design and conduct large-scale, multicenter studies and clinical trials in persistently aPL-positive patients, created a COVID-19 working group to examine the relationships between the two entities to propose guidance for clinical management and monitoring. This guidance also serves as a call and focus for clinical and basic scientific research. The APS ACTION COVID-19 working group started by asking the questions that follow.
What are the clinical similarities between APS and COVID-19?
Compared to non-COVID-19 ICU patients, who have VTE rates of 2–15%, COVID-19 ICU patients have VTE incidence rates of 24–49% despite prophylaxis.4,5 Events are mostly VTE, though ischemic strokes also occur.4,5 In ischemic stroke patients, the Global COVID-19 Stroke Registry (174 patients hospitalized at 28 sites in 16 countries) reported that COVID-19-associated ischemic strokes have worse functional outcomes and higher mortality than non-COVID-19 ischemic strokes. Possible reasons include viral-induced endotheliopathy, immune-mediated platelet activation, dehydration, infection-induced cardiac arrhythmias, and stay-at-home recommendations.
Although COVID-19 and APS are two different diseases, severe COVID-19 may result in a thrombotic syndrome with pulmonary, cardiovascular, renal, and central nervous system abnormalities, similar to CAPS. Elevated lactate dehydrogenase and D-dimer levels, and thrombocytopenia occur in both CAPS and COVID-19; most patients with COVID-19 have elevated fibrinogen levels. Thrombotic microangiopathy (TMA) in both conditions may occur through endothelial damage, complement activation, and release of neutrophil extracellular traps (NETosis).6–10
What common mechanisms are shared by APS and COVID-19?
Figure 1 summarizes common mechanisms shared by APS and COVID-19. SARS-CoV-2 enters cells by binding to angiotensin converting enzyme-2 (ACE-2) receptor, causing downregulation of ACE2 and over-activation of both the kallikrein–bradykinin pathways, cytokine release, and the renin-angiotensin system (RAS) pathways.11 The result is increased ACE2 substrate angiotensin II and decreased level of the product, Ang 1–7, which promotes vasodilation and has anti-inflammatory properties.12 By upregulating tissue factor, plasma activator inhibitor 1, and angiotensin II (Ang 1–8), SARS-CoV-2 has vasoconstrictive and prothrombotic effects. Similar to CAPS, autopsies in COVID-19 show severe endothelial injury and widespread thrombosis with microangiopathy (TMA).3
Figure 1.
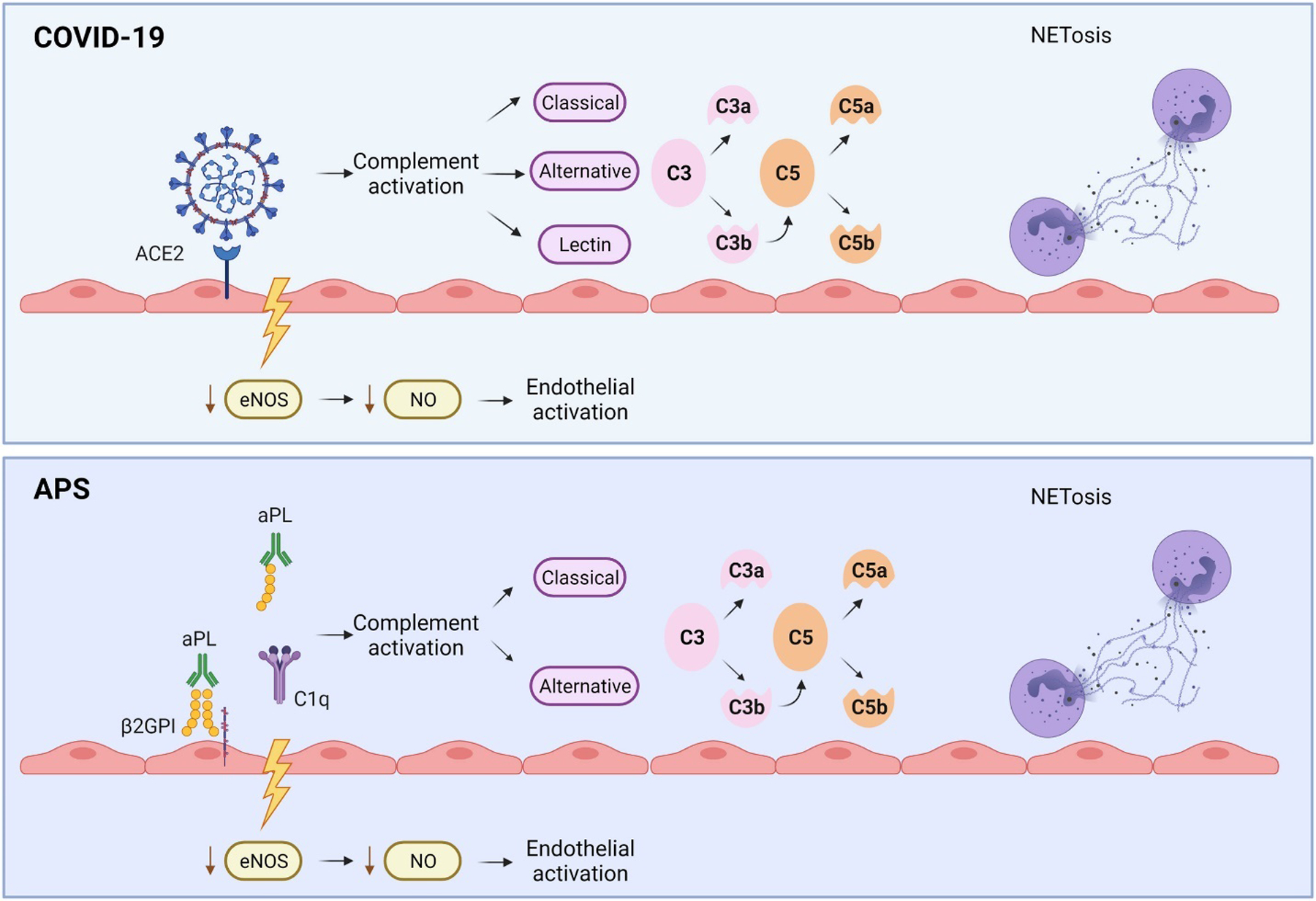
Common Mechanisms of Thrombosis Shared by Antiphospholipid Syndrome and COVID-19. SARS-CoV-2 enters cells by binding to angiotensin converting enzyme-2 (ACE-2) receptor, whereas in APS, endothelial injury is mediated through the exposure of endothelial cells to antiphospholipid antibodies (aPL). In both cases, inhibition of endothelial nitric oxide synthase (eNOS) production decreases production of nitric oxide (NO), an anti-inflammatory and vasodilatory agent, increasing susceptibility of the endothelium to injury. Complement activation plays a key role in both COVID-19 and CAPS. In COVID-19, unchecked inflammatory signals lead to the recruitment of neutrophils and excessive NETosis, resulting in microvascular occlusions, which have been also demonstrated in APS. Both COVID-19 and aPL induce proinflammatory and prothrombotic cytokines; a subgroup of both COVID-19 and APS patients may have cytokine storm, characterized by high levels of proinflammatory cytokines and chemokines.
Endothelial injury
The role of binding of SARS-CoV-2’s spike protein to ACE-2 receptors, thus activating endothelium, is controversial, as endothelial cells express low levels of the primary SARS-CoV-2 receptor, ACE2, and its cofactor, transmembrane protease serine 2 (TMPRSS2). In addition, primary cultures of endothelial cells are resistant to infection.13 The counter argument is that in vitro studies and autopsies from patients with COVID-19 demonstrate viral elements and inflammatory cells in the endothelium.11 In APS, endothelial injury is mediated through the exposure of endothelial cells to antiphospholipid antibodies (aPL).14 In both cases, inhibition of endothelial nitric oxide synthase (eNOS) production decreases production of nitric oxide (NO), an anti-inflammatory and vasodilatory agent, increasing susceptibility of the endothelium to injury. In COVID-19, markers of endothelial activation/damage including von Willebrand factor (vWF), tissue-type plasminogen activator (t-PA), and soluble thrombomodulin (sTM) correlate with disease severity.8 Endothelial injury causes activation of inflammatory cytokines and recruitment of neutrophils via increased neutrophil adhesion in the setting of decreased NO and complement activation,6 leading to loss of vascular integrity and increased platelet aggregation and thrombosis. Angiotensin II, which mediates endothelial dysfunction in patients with pre-eclampsia, is also implicated in endothelial damage in COVID-19, as it increases secretion of sFlt-1 (soluble FMS-like tyrosine kinase),15 and correlates with survival and thrombosis.
Complement activation
Complement activation plays a key role in both COVID-19 and CAPS. SARS-CoV-2 activates the three complement pathways. Viral antigens activate the classical pathway through immune complexes, while the binding of the mannose-binding lectin (MBL) with the spike protein of SARS-CoV-2 triggers the lectin pathway,16 generating C3 convertase. F-spike proteins (subunit 1 and 2) activate the alternative complement pathway on cell surfaces, an effect that can be blocked by a Factor D inhibitor.17 The alternative pathway can also be activated when its component binds to the surface of a pathogen, resulting in C3 convertase production. Activation of any of these pathways results in C5 convertase production, catalyzing the splitting of C5 to C5a, which increases leukocyte recruitment, and C5b, which initiates the formation of C5b-9 (membrane attack complex/MAC). Histologic samples of both lung and skin tissues,18 and elevated MAC levels in serum of patients implicate these pathways in COVID-19 as in thrombotic APS.8 In APS, anti-β2-glycoprotein-I (aβ2GPI) complexed with β2GPI activates the classical complement pathway by binding to C1q, activating C3b and engaging the alternative pathway through the amplification loop,7 demonstrated, for instance, by high serum levels of soluble MAC in APS patients with strokes and CAPS.7 Addition of phospholipid vesicles to APS plasma causes formation of macroimmune complexes that activate complement.19 A higher rate of mutations in complement regulating genes occurs in CAPS patients than in APS patients without CAPS.20 A variant in regulatory genes may be associated with enhanced complement activation and severe COVID-19 symptoms.21 Heparin, an established drug for APS treatment with anticoagulant as well as complement inhibiting activities, may improve prognosis in COVID-19 patients with moderately severe disease.22
NETosis
Neutrophil extracellular traps (NETs) are extracellular webs of chromatin and microbicidal proteins that serve as a first line of defense against infections by corralling and killing pathogens. However, NETosis may result in endothelial damage and accelerated thrombosis when it occurs in the intravascular space. In COVID-19, unchecked inflammatory signals lead to the recruitment of neutrophils and excessive NETosis, resulting in microvascular occlusions.9 The involvement of NETs in COVID-19 is supported by increased myeloperoxidase-DNA (MPO-DNA) complexes and citrullinated histone H3 (Cit-H3) in blood, which track closely with disease severity.23,24 By expressing tissue factor and promoting thrombin generation,24 circulating neutrophils/NETs are thrombogenic. Tissue samples from COVID-19 patients demonstrate NET-containing microthrombi with neutrophil-platelet infiltrates in both pulmonary and coronary tissues. Both APS patient sera and purified IgG increase neutrophil adhesion and NETosis.9,10,25
Coagulopathy in COVID-19 and crosstalk between inflammation and coagulation
Mild to moderate thrombocytopenia occurs in severe COVID-19 patients, with mildly prolonged prothrombin time in a minority, and high fibrinogen levels in the majority of COVID-19 patients. Very elevated D-dimer levels, possibly explained by systemic thrombin generation with subsequent fibrin formation and breakdown and/or enhanced fibrin turnover in the lung due to severe inflammation, are associated with high 28-day mortality.22,26
COVID-19 initiates complex systemic inflammatory response as part of innate immunity, resulting in crosstalk between inflammation and coagulation. The raised levels of factor VIII and fibrinogen reflect a marked acute phase response. Severe COVID-19 induces proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukins (IL), including IL-1 and IL-6.26,27 IL-6 can induce tissue factor expression on mononuclear cells, leading to coagulation activation and thrombin generation.28 A subgroup of patients with severe COVID-19 may have cytokine storm, characterized by high levels of proinflammatory cytokines and chemokines.
Do COVID-19 patients have increased frequency of antiphospholipid antibodies?
Initial studies in hospitalized COVID-19 patients with thrombosis despite DVT prophylaxis noted prolonged aPTT. Subsequent testing showed positive aPL, especially in critically ill patients. In some studies, LA was positive in up to half of ICU patients.2,29 However, these results are difficult to interpret due to limitations of LA tests in the critical care setting.
Criteria antiphospholipid antibodies in COVID-19
Three universally accepted APS classification criteria tests to detect aPL are LA, aβ2GPI IgG/M, and anticardiolipin antibodies (aCL) IgG/M.30 The LA test was positive in 5–90% of patients infected with COVID-19,29,31 the wide range possibly due to the test being a functional coagulation assay influenced by multiple factors, particularly elevated FVIII and C-reactive protein,32 use of anticoagulants, including heparin, vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs),33,34 and pregnancy.32
Positive tests for anticardiolipin IgM occur in 3–23% and IgG in 5–13% of critically ill patients with COVID-19, while aβ2GPI IgM occurs in 2–16% and IgG in 3–18%.31,33,35–37 However, patients with clinically meaningful/relevant aPL profiles (high aCL/aβ2GPI titers or triple positive aPL (LA, aCL, and aβ2GPI)) are rarely reported.29 Two studies of ICU patients with prolonged aPTT reported positive LA rates of 53–77% but did not describe results of aCL or aβ2GPI.38
In most studies, aPL were measured at a single time point, and without specific information provided about the methods and quantitative results. Studies that retested patients noted a change from positive aPL to negative or different levels of positive aPL at retesting.29,35 Studies that reported titer levels showed that all criteria aPL tests except for aCL IgG were lower in COVID-19 patients than in APS patients,33,36 and they differed in reactivity to the domains of β2GPI.33
Non-criteria antiphospholipid antibodies in COVID-19
Several other antibodies target anionic phospholipids, phospholipid-binding plasma proteins, or phospholipid-protein complexes, for instance, antibodies directed against prothrombin (aPT) or phosphatidylserine/prothrombin complex (aPS/PT), IgA aCL or aβ2GPI, and antibodies against specific domains of β2GPI.
Severe COVID-19 patients have increased phosphatidylserine expression on platelets promoting sustained inflammation, which may trigger aPL. One study stated that high titer IgM and IgG aPS/PT associate with markers of endothelial activation in moderate-to-severe COVID-19 patients.39 In another study, anti-PS/PT IgG were detected in 24% of COVID-19 hospitalized patients, whereas the IgM in 18%,37 but only 12% had both IgM and IgG titers ≥40 units.37 In this study, aPS/PT was not associated with thrombosis and was transient in nature, but the IgG fractions from high and low titer aPS/PT COVID-19 patients accelerated thrombosis in vivo.37 Similar proportion of critically ill COVID-19 patients was found positive for aPS/PT in a different study, most of which had the IgM isotype at a low titer.33
Although IgA antibodies are not part of APS classification criteria, both aβ2GPI IgA and aCL IgA have occurred in COVID-19.29,31 In one cohort, both IgA aCL and aβ2GPI were significantly higher in severely ill patients on both screening and confirmatory tests 40; and IgA aCL and aβ2GPI were positive at low titer in hospitalized COVID-19 patients but negative in non-hospitalized patients. A different cohort reported only IgA aβ2GPI positivity.33 Except for a few cases with VTE occurring in combination to other criteria aPL,29 there has been no direct cause-effect relationship between IgA aβ2GPI or IgA aCL and thrombosis in COVID-19 patients.
Is antiphospholipid antibody positivity in COVID-19 associated with increased risk of thrombosis?
The clinical significance of positive aPL tests in COVID-19 patients remains undefined. Antiphospholipid antibody tests, elevated in viral and bacterial infections such as hepatitis B/C, HIV, and syphilis, are typically transient and are not associated with thrombosis. Antiphospholipid antibodies in COVID-19 may also be transient and mostly of low titers.36 One study reported that aPL in COVID-19 is against the D4–5 domain of aβ2GPI, rather than the D1 domain, which is associated with thrombosis risk and pregnancy complications in APS.33 Anti-D5 lacks thrombogenicity in rat models. Thus, published studies do not yet support a correlation between thrombosis and positive aPL tests.33
However, the pathogenic nature of COVID-19-associated aPL may be supported by the fact that, as with traditional APS patients,10 COVID-19-associated IgG fractions isolated from patients induce thrombosis in mouse models.37 Also, some clinical studies note correlation between positive aPL and thrombosis.29,31 Furthermore, the possible transient nature of aPL in COVID-19 does not preclude pathogenicity: transient aPL in hepatitis C correlates with increased risk of thrombotic events in a meta-analysis.
In summary, it remains unclear whether aPL-positivity is just an epiphenomenon of an upregulated inflammatory state triggered by COVID-19, or a true player in the thrombotic storm of severe COVID-19 patients.
Is COVID-19 infection in aPL-positive patients (with or without APS) associated with increased risk of complications?
Data on COVID-19 outcomes in patients with aPL (with or without APS) are limited to a few case reports and subject to publication bias. In primary APS, a case report described a patient on therapeutic anticoagulation who developed adrenal hemorrhage and arterial thrombosis after COVID-19; another had suspected recurrent diffuse alveolar hemorrhage complicated by fungal pneumonia. A patient with systemic lupus erythematosus (SLE) and APS developed severe thrombocytopenia during COVID-19 infection; another SLE patient with triple aPL-positivity but no APS developed VTE during a mild infection with COVID-19. Two cases described new diagnosis of APS in patients with COVID-19 infection, one also with new SLE diagnosis, and another with history of miscarriages who presented with adrenal hemorrhage and had a triple positive aPL profile. Outcomes in patients with established APS and COVID-19 infection remain to be reported from established large cohorts.
APS ACTION management guidance recommendations
Risk assessment and prognosis in patients with APS and COVID-19 infection is extrapolated from data on patients with other rheumatic diseases or the general public. There are no specific treatments recommended for COVID-19 in patients with APS. The guidance below is based on expert consensus; recommendations should be provided on a case-by-case basis by clinicians. Patients with APS should continue to practice general COVID-19 precautions even if previously diagnosed with COVID-19 and communicate with their doctors should they develop recurrent APS symptoms.
A. Management of antiphospholipid antibody positive (with or without APS) COVID-19 patients
-
1.
To guide long-term management in COVID-19 patients with newly detected aPL, patients should be retested after 12 weeks, and management decisions should be based on the clinical presentation and assessment of their full aPL profile.
Comment: Newly detected aPL in patients with COVID-19 infection may be false positive and/or transient; published data on the frequency of persistent LA and/or moderate-to-high titers of aCL/aβ2GPI are limited.29 Low aCL/aβ2GPI titers are generally not clinically relevant for thrombosis. Persistent triple aPL-positivity (LA, aCL, and aβ2GPI) provides better confidence that aPL profile is clinically relevant.
-
2a.
Hospitalized COVID-19 patients with newly detected aPL should receive at least prophylactic dose anticoagulation with low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH) unless there are contraindications.
-
2b.Hospitalized COVID-19 patients known to have persistently positive aPL:
- but not on anticoagulation should receive at least prophylactic dose anticoagulation with LMWH or UFH unless there are contraindications.
- and on oral anticoagulation (warfarin, alternative VKA, or DOAC) should be transitioned to therapeutic dose LMWH or UFH.
Comment: Hospitalized patients have an increased frequency of thrombosis; thus, VTE prophylaxis is recommended for all patients, including those with COVID-19. Ongoing studies are examining the benefit of therapeutic or intermediate dose versus prophylactic dose anticoagulation; one trial has shown no benefit of intermediate dose over prophylactic dose anticoagulation in ICU patients with COVID-19. The role of continued thromboprophylaxis after hospital discharge for aPL-negative COVID-19 patients remains under investigation; although it is generally recommended for at least 14 days for patients at high thrombotic risk,41 the decision should be individualized after balancing risks and benefits.
-
3.Non-hospitalized patients with COVID-19 and known to have persistent aPL:
- Not on anticoagulation, should be considered for prophylactic dose anticoagulation with LMWH, especially in the setting of additional VTE risk factors, for example, reduced mobility, high body mass index, or malignancy) unless there are contraindications.
- On anticoagulation (warfarin, alternative VKA, LWMH, or DOAC), should keep their prescribed anticoagulation.
Comment: The benefit of anticoagulation and anti-platelet agents is uncertain in non-hospitalized patients without aPL; medical specialty societies provide conflicting guidance. The topic is currently being researched. Given that prophylactic anticoagulation is recommended for asymptomatic aPL-positive patients during high-risk periods such as surgeries, physicians should assess the risks and benefits of prophylactic dose anticoagulation on a case-by-case basis.
-
4.
All COVID-19 pregnant patients known to have persistently positive aPL should follow the recommendations above with strong consideration of prophylactic dose LMWH in those who are not on anticoagulation, and a low threshold to investigate suspected thrombosis.
Comment: Pregnancy is an independent risk factor for severe COVID-19, with increased risk for ICU admissions, mechanical ventilation, extracorporeal membrane oxygenation, and death.42 The absolute risk of the above adverse events during pregnancy is small and substantially lower than those reported during the H1N1 influenza pandemic, with generally good outcomes in patients with mild to moderate COVID-19.
Venous thromboembolism prophylaxis with LMWH or UFH should be given to all hospitalized pregnant women with suspected or confirmed COVID-19 unless delivery is anticipated within 12–24 h; prophylaxis should be considered on a case-by-case basis in the outpatient setting. In pregnant aPL-positive patients with COVID-19 infection and on prophylactic dose anticoagulation, there is no evidence that increasing the prophylactic dose to therapeutic dose anticoagulation improves outcomes.
-
5.
All aPL-positive patients with or without APS are recommended to receive a COVID-19 vaccine given the critical importance for public health.
Comment: Reports of thrombosis with thrombocytopenia following two adenoviral vaccines (AstraZeneca and Johnson & Johnson) are rare. These cases bear a resemblance to autoimmune heparin-induced thrombocytopenia with positive antibodies to platelet factor 4–polyanion complexes (PF4).43 Based on the suggested pathogenetic mechanism, the use of non-heparin anticoagulation and IVIG is recommended, while platelet transfusion should be avoided.
Currently, no evidence exists that patients with a history of thrombosis or known risk factors for thrombosis (including APS) are at higher risk of developing this complication following vaccination. Regulatory agencies both in the United States and Europe confirm that the benefits of the vaccines significantly outweigh the extremely low risk of thrombosis. Therefore, APS patients should be vaccinated with any of the available vaccines in line with national policies. These policies depend on a variety of factors and may well be subject to change with time.
Given that some APS patients receive immunosuppression, mostly for non-thrombotic or microvascular diseases, clinicians should be aware that (a) it is unknown if one vaccine is superior or safer over another in patients on immunosuppressive medications; (b) hydroxychloroquine use should not have any effect on the vaccine response; (c) rituximab may correlate with severe COVID-19 manifestations and persistent viremia44; thus, the American College of Rheumatology (ACR) recommends scheduling the vaccine dose 4 weeks prior to rituximab dose and delaying rituximab dose by 2–4 weeks after vaccination; and (d) reductions in vaccine response have been noted in patients on potent immunosuppression, for example, mycophenolate mofetil45; thus, ACR recommends holding the dose for 1 week after each vaccine dose if the disease is stable.
B. Basic Science and Clinical Research Agenda
-
Basic science studies should investigate the mechanisms leading to aPL positivity in COVID-19 patients, and how these aPL may contribute to increased thrombosis risk.
Comment: The clinical significance and the duration of aPL positivity, as well as the pathogenicity of aPL in COVID-19 remains controversial. Further research is necessary to determine whether and how aPL emerging during COVID-19 infection increases risk for thrombosis. Determination of persistence of such aPL tests at 12 weeks and beyond, and long-term outcomes in these patients are necessary to understand whether infection with SARS-CoV-2 triggers long-lasting effects on the hemostasis and immune systems and could be responsible for development of autoimmune syndromes such as APS.
The observation of positive LA in patients with severe COVID-19 has emphasized weaknesses of current LA assays. Projects addressing the interference of anticoagulation with LA assays are underway by APS ACTION.
-
Clinical research should focus on defining poor prognostic factors, optimal management, and potential therapeutic targets in aPL-positive COVID-19 patients
Comment: The literature suggests common mechanisms between CAPS and severe COVID-19, which represent possible targets of intervention in the treatment of both COVID-19 and APS; treatment strategies used in CAPS patients, for example, IVIG or plasma exchange, may have a role in the management of COVID-19 patients; clinical trials are in progress.
Numerous opportunities exist to investigate the role of complement in both APS and COVID-19. Case reports suggest eculizumab as an effective therapy for both COVID-19 and APS.46 In addition, the role of blockade of C3, given its position at convergence point of all complement activation pathways, is investigated in COVID-19; early clinical data indicate that compstatins (small peptides that bind C3 preventing its activation) are associated with rapid normalization of inflammatory markers, reduction in circulating NETs, and respiratory improvement in COVID-19.47 Mechanistic studies showed that complement C3 inhibition with compstatin Cp40 disrupted neutrophil-derived tissue factor expression at both the mRNA and protein levels.24
Restraining NET formation may be a therapeutic approach in both conditions. Heparin neutralizes NET-derived cytotoxic histones and also potentiates NET clearance via DNase I.9 At the same time, glucocorticoids such as dexamethasone reduce NETosis in vivo,48 most likely via suppression of various mediators of neutrophil activation. In terms of novel therapies, recombinant DNase I dismantles NETs and thereby has the potential to mitigate both airway obstruction and intravascular thrombosis.9 Another strategy might be the activation of surface adenosine receptors, which suppresses NETosis via cyclic AMP-dependent signaling.49 Dipyridamole is an inexpensive drug with a favorable safety profile that potentiates adenosine receptor signaling by both inhibition of nucleoside reuptake and stabilization of intracellular cyclic AMP. In models of APS, dipyridamole restrains NET release in vitro, while suppressing NET-dependent thrombosis in mice.49 In a small study, dipyridamole suppressed D-dimer levels in patients with COVID-19.
-
Clinical research should define the outcomes in pregnant aPL-positive COVID-19 patients.
Comment: The effect of COVID-19 on pregnancy requires further research. In this context, prior coronavirus infections, such as Middle East Respiratory Syndrome (MERS), are associated with preterm birth.50
-
Clinical research should also focus on COVID-19 vaccine outcomes in persistently aPL-positive patients, including thrombosis and thrombocytopenia.
Comment: Given rare reports of vaccine-induced immune thrombocytopenia and thrombosis (VITT), independent of a history of APS, it is important to monitor vaccination-related thrombotic and non-thrombotic adverse outcomes, particularly in patients with rheumatic diseases, and to elucidate the mechanism of these adverse effects. Studies are underway.
Conclusion
The role of aPL in SARS-CoV-2-related thrombosis remains to be elucidated. While the COVID-19 pandemic is devastating, it provides a unique opportunity to better characterize the short- and long-term effects of the disease on the immune and hemostatic systems, and the common mechanisms, with the potential to guide targeted strategies in both COVID-19 and APS. APS ACTION recommendations for the management of aPL-positive COVID-19 patients are summarized in the Table 1. As these recommendations are extrapolated from limited existing literature, particularly for COVID-19 infection in APS patients, clinicians should tailor the management based on each patient’s COVID-19 disease severity, aPL profile, and APS clinical phenotype, as guided by clinical judgment and discussion with the patient.
Table 1 -.
General management recommendations for persistently antiphospholipid antibody positive patients with new COVID-19 infection.
| Inpatient | Outpatient | |
|---|---|---|
| No history of thrombosis and/or not on long-term anticoagulation | Start at least prophylactic dose anticoagulation with LMWH or UFH. | Consider prophylactic dose anticoagulation depending on additional non-aPL VTE risk factors |
| History of thrombosis on long-term anticoagulation | Switch to therapeutic dose LMWH or intravenous UFH. | Continue anticoagulation with regular INR monitoring if on warfarin with INR checks as appropriate |
| Pregnant—not on anticoagulation | Start at least prophylactic dose anticoagulation with LMWH or UFH. | Consider prophylactic dose anticoagulation, given the increased risk of thrombosis during pregnancy and postpartum (at least 6 weeks after delivery) |
| Pregnant—on anticoagulation (LMWH or UFH) with or without low dose aspirin | Continue anticoagulation: At least prophylactic dose LMWH or UFH for patients on prophylactic dose anticoagulation; and therapeutic dose LMWH or UFH for patients on therapeutic dose anticoagulation | Continue anticoagulation based on the original dosing strategy |
Prophylactic dose LMWH is preferred over UFH as a) once daily versus twice or three times daily injections: more convenient for the patient and fewer subcutaneous administrations required from nursing staff (hence less avoidable direct contact with a patient with COVID-19 infection) and b) lower risk of heparin-induced thrombocytopenia. LMWH is generally preferable to VKAs in hospitalized patients because of potential variability of the INR due to acute illness-related factors. LMWH is also preferable to oral anticoagulants (VKAs and DOACs) in patients with thrombocytopenia or coagulopathy, in view of its shorter half-life. For all patients, there should be a low threshold to investigate suspected thrombosis. VTE prevention patient information should be provided for inpatients and outpatients.
aPL: antiphospholipid antibodies, DOACs: direct oral anticoagulants, LMWH: low-molecular-weight heparin, UFH: unfractionated heparin, VTE: venous thromboembolism, INR: international normalized ratio.
Acknowledgments
AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking Members: Argentina: Santa Fe (Guillermo Pons-Estel); Australia: Sydney (Bill Giannakopoulos, Steve Krilis); Brazil: Rio de Janeiro (Guilherme de Jesus, Roger Levy), São Paulo (Gustavo Balbi, Danieli Andrade); Canada: Calgary (Ann Clarke, Leslie Skeith), Quebec (Paul F. Fortin); China: Beijing (Lanlan Ji, Zhouli Zhang), Shanghai (Hui Shi, Chengde Yang); France: Nancy (Stephane Zuily, Denis Wahl); Greece: Athens (Maria Tektonidou); Italy: Brescia (Cecilia Nalli, Laura Andreoli, Angela Tincani), Milan (Cecilia B. Chighizola, Maria Gerosa, Pierluigi Meroni), Padova (Chunyan Cheng, Alessandra Banzato, Vittorio Pengo), Turin (Silvia Foddai, Massimo Radin, Savino Sciascia); Jamaica: Kingston (Stacy Davis); Japan: Sapporo (Olga Amengual, Tatsuya Atsumi); Lebanon: Beirut (Imad Uthman); Netherlands: Utrecht (Maarten Limper, Philip de Groot); Spain: Barakaldo (Guillermo Ruiz Irastorza, Amaia Ugarte), Barcelona (Jose Pardos-Gea, Ignasi Rodríguez-Pinto, Roberto Ríos-Garcés, Ricard Cervera), Madrid (Esther Rodriguez, Maria Jose Cuadrado), Cordoba (Maria Angeles Aguirre Zamorano, Rosario Lopez-Pedrera); Turkey: Istanbul (Bahar Artim-Esen, Murat Inanc); United Kingdom: London (Maria Laura Bertolaccini, Hannah Cohen, Maria Efthymiou, Munther Khamashta, Ian Mackie, Giovanni Sanna); USA: Ann Arbor (Jason S. Knight), Baltimore (Michelle Petri), Boston (Rebecca Karp-Leaf), Chapel Hill (Robert Roubey), Durham (Tom Ortel), Galveston (Emilio Gonzalez, Rohan Willis), New Hyde Park (Nina Kello), New York City (H. Michael Belmont, Steven Levine, Jacob Rand, Medha Barbhaiya, Doruk Erkan, Jane Salmon, Michael Lockshin), Rochester (Ali Duarte), Salt Lake City (D. Ware Branch).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MP (Hopkins Lupus Cohort) is supported by a grant from the NIH RO1 AR069572.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Porfidia A, Valeriani E, Pola R, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res 20202020; 196: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. New Engl J Med 2020; 383: 288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020; 173: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020; 191: 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middeldorp S, Coppens M, Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020; 18: 1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubes P, Suzuki M and Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci 1991; 88: 4651–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi S, Brodsky RA and McCrae KR. Complement in the pathophysiology of the antiphospholipid syndrome. Front Immunol 2019; 10: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cugno M, Meroni PL, Gualtierotti R, et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Autoimmun 2021; 116: 102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leppkes M, Knopf J, Naschberger E, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 2020; 58: 102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng H, Yalavarthi S, Kanthi Y, et al. In vivo role of neutrophil extracellular traps in antiphospholipid antibodymediated venous thrombosis. Arthritis Rheumatol 2017; 69: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Ortega M, Lorenzo O, Rupérez M, et al. Renin- angiotensin system and renal damage: emerging data on angiotensin II as a proinflammatory mediator. Contrib Nephrol 2001; 135: 123–137. [DOI] [PubMed] [Google Scholar]
- 13.Ahmetaj-Shala B, Peacock TP, Baillon L, et al. Resistance of endothelial cells to SARS-CoV-2 infection in vitro. BioRxiv Immunology 2020. DOI: 10.1101/2020.11.08.372581. [DOI] [Google Scholar]
- 14.Corban MT, Duarte-Garcia A, McBane RD, et al. Antiphospholipid syndrome. J Am Coll Cardiol 2017; 69: 2317–2330. [DOI] [PubMed] [Google Scholar]
- 15.Giardini V, Carrer A, Casati M, et al. Increased sFLT-1/PlGF ratio in COVID −19: a novel link to angiotensin II -mediated endothelial dysfunction. Am J Hematol 2020; 95. DOI: 10.1002/ajh.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortensen SA, Sander B, Jensen RK, et al. Structure and activation of C1, the complex initiating the classical pathway of the complement cascade. Proc Natl Acad Sci 2017; 114: 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Yuan X, Chen H, et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood 2020; 136: 2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020; 220: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rand JH, Wu XX, Wolgast LR, et al. A novel 2-stage approach that detects complement activation in patients with antiphospholipid antibody syndrome. Thromb Res 2017; 156: 119–125. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi S, Braunstein EM, Yuan X, et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood 2020; 135: 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenti L, Griffini S, Lamorte G, et al. Chromosome 3 cluster rs11385942 variant links complement activation with severe COVID-19. J Autoimmun 2021; 117: 102595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020; 18: 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020;5(11):e138999. DOI: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skendros P, Mitsios A, Chrysanthopoulou A, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest 2020; 130: 6151–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sule G, Kelley WJ, Gockman K, et al. Increased adhesive potential of antiphospholipid syndrome neutrophils mediated by β2 integrin Mac-1. Arthritis Rheumatol 2020; 72: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo XH, Zhu Y, Mao J, et al. T cell immunobiology and cytokine storm of COVID-19. Scand J Immunol 2021; 93. DOI: 10.1111/sji.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stouthard JML, Levi M, Hack CE, et al. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost 1996; 76: 738–742. [PubMed] [Google Scholar]
- 29.Devreese KMJ, Linskens EA, Benoit D, et al. Antiphospholipid antibodies in patients with COVID-19: A relevant observation? J Thromb Haemost 2020; 18: 2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Cao W, Jiang W, et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis 2020; 50: 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devreese KMJ, Groot PG, Laat B, et al. Guidance from the scientific and standardization committee for lupus anticoagulant/antiphospholipid antibodies of the international society on thrombosis and haemostasis. J Thromb Haemost 2020; 18: 2828–2839. [DOI] [PubMed] [Google Scholar]
- 33.Borghi MO, Beltagy A, Garrafa E, et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front Immunol 2020; 11: 584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripodi A, Chantarangkul V, Clerici M, et al. Laboratory control of oral anticoagulant treatment by the INR system in patients with the antiphospholipid syndrome and lupus anticoagulant. Results of a collaborative study involving nine commercial thromboplastins. Br J Haematol 2001; 115: 672–678. [DOI] [PubMed] [Google Scholar]
- 35.Xiao M, Zhang Y, Zhang S, et al. Antiphospholipid antibodies in critically Ill patients with COVID-19. Arthritis Rheumatol 2020; 72: 1998–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gatto M, Perricone C, Tonello M, et al. Frequency and clinical correlates of antiphospholipid antibodies arising in patients with SARS-CoV-2 infection: findings from a multicentre study on 122 cases. Clin Exp Rheumatol 2020; 38: 754–759. [PubMed] [Google Scholar]
- 37.Zuo Y, Estes SK, Ali RA, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Trans Med 2020; 12: eabd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenzel C, Stoiser B, Locker GJ, et al. Frequent development of lupus anticoagulants in critically ill patients treated under intensive care conditions. Crit Care Med 2002; 30: 763–770. [DOI] [PubMed] [Google Scholar]
- 39.Shi H, Zuo Y, Navaz S, et al. Endothelial cell-activating antibodies in COVID-19. MedRxiv: 2021: 21250041. DOI: 10.1101/2021.01.18.21250041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasan Ali O, Bomze D, Risch L, et al. Erratum to: severe coronavirus disease 2019 (COVID-19) is associated with elevated serum immunoglobulin (Ig) A and antiphospholipid IgA antibodies. Clin Infect Dis 2020; 73(9):1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020; 18: 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1641–1647. DOI: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. New Engl J Med 2021; 384: 2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loarce-Martos J, García-Fernández A, López-Gutiérrez F, et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int 2020; 40: 2015–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021; 80: 1098–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strakhan M, Hurtado-Sbordoni M, Galeas N, et al. 36-yearold female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol 20142014; 2014: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mastellos DC, Pires da Silva BGP, Fonseca BAL, et al. Complement C3 vs C5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin Immunol 2020; 220: 108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vargas A, Boivin R, Cano P, et al. Neutrophil extracellular traps are downregulated by glucocorticosteroids in lungs in an equine model of asthma. Respir Res 2017; 18: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali RA, Gandhi AA, Meng H, et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun 2019; 10: 1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alfaraj SH, Al-Tawfiq JA and Memish ZA. Middle east respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect 2019; 52: 501–503. [DOI] [PMC free article] [PubMed] [Google Scholar]


