Abstract
Background –
Maternal distress is associated with an increased risk for adverse emotional development in the offspring, including difficulties with emotion regulation. Prenatal maternal distress has been associated with alterations in infant brain development. However, less is known about these associations with postnatal maternal distress, despite this being an important modifiable risk factor that can promote healthy brain development and emotional outcomes in infants.
Methods & Results -
Infants underwent magnetic resonance imaging (MRI) and mothers completed standardized questionnaires concerning their levels of perceived distress 2–5 months postpartum. Infant emotion regulation was assessed at 8–11 months via maternal report. When examining the associations between maternal distress and infant macrostructure, maternal anxiety was associated with infant right pallidum volume. Increased display of negative emotions at 8–11 months of age was associated with larger hippocampal volumes and this association was stronger in girls than boys.
Conclusion -
Findings suggest that postnatal maternal distress may be associated with early infant brain development and emphasize the importance of maternal mental health, supporting previous work. Furthermore, macrostructural properties of infant subcortical structures may be further investigated as potential biomarkers to identify infants at risk of adverse emotional outcomes.
1. Introduction
Maternal distress in the postpartum period, consisting of symptoms of anxiety and/or depression, is prevalent in 10–25% of pregnancies and is associated with reduced or dysregulated mother-infant bonding, representing a significant stressor to the infant (Essex et al., 2002; Lebel et al., 2020; Thomas et al., 2017). In turn, postnatal maternal distress poses risks to the emotional development of the offspring and is associated with a range of adverse emotional outcomes that may persist across the lifespan, such as internalizing and externalizing behavioral problems (Rees et al., 2019; Stein et al., 2014; Zou et al., 2019). Internalizing behavioral problems may include anxious, depressed, or withdrawn behaviors that affect one’s internal environment (Liu et al., 2004). In contrast, external behavioral problems may include disruptive or aggressive behaviors that affect one’s external environment (Liu et al., 2004).
Emotional regulation, defined as the ability to modulate emotional responses, including intensity, duration, or occurrence using age-appropriate strategies, is important for long-term emotional development (Blanchard-Fields & Coats, 2008; Blandon et al., 2008; Cai et al., 2021). Emotional regulation emerges in the first few months of life and continues to mature across the lifespan (Aktar & Pérez-Edgar, 2020; Rutherford et al., 2015). However, infancy through to early childhood is a sensitive period for its development (Aktar & Pérez-Edgar, 2020). During this time, emotion regulation is learned or achieved within the context of caregiver-child interactions. Disruptions in these interactions, such as lower-quality interactions or decreased sensitivity, have been observed in mothers experiencing maternal distress and can adversely affect an infant’s emotional development (Feldman., 2003; Thomas et al., 2017), increasing the risk of internalizing and externalizing disorders (Crugnola et al., 2016; Feng et al., 2008; Granat et al., 2017; Maughan et al., 2007). Previous work suggests that high-quality maternal-infant interactions help to regulate the infants stress response and thus support healthy brain development (Center on the Developing Child, 2009; Donnici et al., 2021; National Scientific Council on the Developing Child, 2014). However, low-quality interactions, characterized by decreased sensitivity and negative affect which have been observed in cases of maternal distress, may leave the infant’s stress response unregulated (Donnici et al., 2021; Feldman et al., 2003; Kaitz et al., 2009; Thomas, 2017).
Alterations in brain development may underlie associated risks to emotional development in offspring exposed to maternal distress (Lean, 2021). Infancy is a sensitive period for neural development. The brain undergoes rapid and complex structural and functional changes leaving developmental trajectories vulnerable to early experiences. In line with this, evidence suggests maternal distress may alter brain development in infants. For example, postnatal maternal anxiety and depression have been associated with amygdala volume in children (Donnici et al., 2021; Lupien et al., 2011). Furthermore, postnatal maternal anxiety has been negatively associated with left hippocampal volumes in infants (Qiu et al., 2013). However, associations between maternal distress and the developing infant brain have not been fully elucidated, with previous work focusing mainly on limbic structures. As such, research on infant subcortical structures more broadly in association with maternal distress is needed to identify potential early neural markers of risk.
Previous work has found variations in brain structure to predict developing emotional capabilities such as emotional regulation (Graham et al., 2017; Luby et al., 2016; Moog et al., 2021; Pagliaccio et al., 2014). For example, research has found associations between macrostructure (volume) of limbic areas and later emotion regulation at different stages of development from infants to adolescence (Blanton et al., 2010; Moog et al., 2021; Pagliaccio et al., 2014). This may suggest brain-behavior relationships may be useful predictors of emotional development. However, potential relationships between brain structure and subsequent emotional capabilities early in development during infancy have been limited. Previous research has emphasized the importance of infancy through to early childhood for emotional development, and indicates early interventions are key to promote healthy outcomes, as such, further research in elucidating neural markers to identify those at risk during this developmental period is needed.
Evidence suggests that biological sex of the infants should be considered in examination of these brain-behavior relationships. Sex differences have been found in emotional development including emotion regulation, as well as vulnerability to emotion-related pathologies (Chaplin & Aldao, 2013; Stein et al., 2014; Weinburg & Tronick, 1999). For example, internalizing disorders are more prevalent in girls, while externalizing disorders are more common in boys, both generally and in association with maternal distress (Chaplin et al., 2019; Hick et al., 2019). Together, these results suggest biological sex should be considered in examining relationships between brain structure and predictors of emotional development (Letourneau et al., 2019).
Given the evidence that infancy is a key period for brain development and maternal interactions are essential for healthy emotional development, in the present work we sought to better understand the associations between maternal distress, infant brain development and subsequent emotion regulation to identify risk and resilience factors. We hypothesized that high-quality maternal interactions support heathy brain development of the offspring. Low-quality interactions, characterized by decreased sensitivity and negative affect as observed in cases of maternal distress, may alter developing neural systems and could underlie the risks to emotional development (Feldman et al., 2003; Kaitz et al., 2009; Thomas, 2015). Furthermore, given sex-differences in the vulnerability to emotional outcomes, we hypothesized that biological sex would be an important moderator of this relationship. Based on previous findings, we hypothesized that postnatal maternal distress would be associated with infant subcortical macrostructure. We further predicted that infant subcortical macrostructure would be associated with emotion regulation abilities. Moreover, we hypothesized this association would be moderated by biological sex.
Infants underwent MRI scanning between 2–5 months of age. Subcortical brain structure volumes were extracted from anatomical MRI images. During this period (2–5 months postpartum), maternal distress was measured via self-report on anxiety and depression questionnaires. Infant emotion regulation was subsequently assessed at 8–11 months via maternal report. This study addressed the following questions: What is the association between postnatal maternal distress and infant subcortical macrostructure? Does infant subcortical macrostructure predict emotional regulation in infants in the first year of life? Does biological sex moderate this relationship?
2. Methods
2.1. Participants
Data for this project were obtained via the National Institute of Mental Health Data Archive (NDA identifier: 2340). Data collection occurred between September 2015 and July 2017 at the Children’s Hospital of Pittsburgh (Phillips et al., 2021). Inclusion criteria was defined as healthy mothers and full-term infants (> 37 weeks) around the age of 3-months. Exclusion criteria for infants was MRI contraindications (i.e., non-removable metal), premature birth (< 37 weeks), low birth weight (< 5.5lbs), low Apgar scores at 5 minutes (< 7) or prolonged hospital stay for physical illness (Phillips et al., 2021). Exclusion criteria for the mothers included substance use problems (either disorder, or illicit use), age (< 18 years), or cared for her infant less than two hours a day (Phillips et al., 2021). The study was originally approved by the University of Pittsburgh Institutional Review Board (Phillips et al., 2021). Informed consent was obtained from the mothers.
2.2. Procedures
Infants underwent scanning and mothers completed questionnaires regarding their perceived levels of distress 2–5 months postpartum. Infant temperament was later assessed at 8–12 months via maternal report. Participants were selected if mothers had completed questionnaire measures of interest and infant T1-weighted MRI scans were available.
2.3. Maternal Distress
Maternal distress was measured via self-report using questionnaires. The state anxiety component of the state-trait anxiety scale (STAI-S; Spielberger et al., 1983) was used to measure anxiety symptoms and the Edinburgh postnatal depression scale (EPDS; Cox et al.,1987) was used to assess depressive symptoms in mothers during the postpartum period. These measures have been widely used in maternal mental health research (Spry et al., 2020; Meades & Ayers, 2011).
State-Trait Anxiety Inventory
The state subscale of the state-trait anxiety inventory (STAI-S) measures state anxiety, defined as situation-related anxiety or current anxiety level (Spielberger et al., 1983; Dennis et al., 2013). The STAI-S consists of 20 items on a 4-point scale (1=not at all; 4 very much so) (Spielberger et al., 1983). For example: “I am presently worrying over possible misfortunes” (Spielberger, et al.,1983). Scores range from 20–80, with higher scores indicating greater levels of state anxiety (Spielberger et al., 1983). There is no established cutoff score, however, perinatal maternal mental health research suggests scores above 40 may represent clinical significance (Dennis et al., 2013).
Edinburgh Postnatal Depression Scale
The Edinburgh Postnatal Depression Scale (EPDS) measures depression symptoms common in women during pregnancy and postpartum period (Cox et al., 1987). Participants respond based on the frequency of depressive feelings or behaviors experienced over the previous week. For example: “I have been so unhappy that I have had difficulty sleeping” (Cox et al., 1987). The EPDS consists of 10-items on a 4-point Likert scale (0 = never/not at all; 3 = yes, quite often/most of the time). Scores range from 0–30, with higher scores indicating greater levels of depression symptoms (Cox et al., 1987). There is no established cut-off, however, scores of 10 and greater have been previously used to indicate clinically relevant depression in mothers during the postpartum period (Kozinszky et al., 2015).
2.4. Infant Emotion Regulation
Infant temperament was measured at 8–11 months via maternal report on the Infant Behavior Questionnaire-Revised Short Form (IBQ-R-S) (Putnam et al., 2014). The IBQ-R-S consists of 91 items and 14 scales that assess various dimensions of infant temperament (Putnam et al., 2014). The IBQ-R-S temperament scales can be further divided into three broad temperament dimensions: surgency/extraversion, negative affectivity, orienting/regulation (Putnam et al., 2014). Caregivers respond on a 7-point Likert scale (1 = never; 7 = always) based on the frequency of infant behaviors over the previous 1–2 weeks (Putnam et al., 2014). For example: “after sleeping, how often did the baby cry if someone doesn’t come within a few minutes” (Putnam et al., 2014, p. 5). Scores range from 1–7 on each scale, with greater scores representing greater levels of that temperament dimension (Enlow et al., 2016).
The present study focuses on the negative affectivity dimension of the IBQ-R-S. The negative affectivity dimension of temperament reflects a propensity to respond to everyday situations with negative emotions (Scheper et al., 2017). This dimension encompasses the following scales: sadness, distress to limitations, fear, and falling reactivity or rate of recovery from distress (Putnam et al., 2014). This dimension was chosen as previous research has found negative affectivity is associated with difficulties or impairments in emotional regulation development (Bridgett et al., 2009; Zimmer-Gembeck & Skinner, 2016).
2.5. MRI Acquisition & Image Analysis
Infants underwent MRI scanning at 2–5 months during natural sleep. Anatomical MRI scans were acquired on a Siemens Magnetom Skyra 3T scanner (Hanford et al., 2018; Siemens Healthcare, Erlangen). Three dimensional T1-weighted images were acquired using a Magnetization-Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence (Field of view [FOV] = 240 × 224, 1.0 mm voxels, repetition time [TR]= 2.4s, echo time [TE] = 0.000316s, flip angle = 8°) using a 32-channel head coil (Hanford et al., 2018).
Infant T1-weighted images were automatically segmented using Infant FreeSurfer (Zöllei et al., 2020). Infant FreeSurfer is an automatic processing stream for T1-weighted MRI scans in infants (Zöllei et al., 2020). Automatic processing steps include intensity normalization, skull stripping, and segmentation of the cortex, white matter and subcortical structures (Zöllei et al., 2020). Intensity normalization involves standardizing MRI signal intensity which may differ across subjects, scanners, or visits (Sun et al., 2015). Skull-stripping is the process of removing all non-brain tissue from MRI images (Poldrack et al., 2011). Segmentation involves segmenting the brain into its anatomical features including cortical/subcortical brain structures and white/gray matter (Poldrack et al., 2011). It is performed using a multi-atlas approach, in which multiple atlases are first registered to subject space and structure labels are transferred. Labels are then fused into a single segmentation result, providing higher accuracy than single-atlas approaches (Iglesias & Sabuncu, 2015). Volumetric measurements for anatomical features can then be extracted (Zöllei et al., 2020).
Infant brain subcortical brain structure volumes were extracted as well as cerebral white matter and cerebral cortex volumes to compute total cerebral volumes (TCV). Subcortical brain structures included the left and right: cerebellum cortex, thalamus, caudate, putamen, pallidum, hippocampus, amygdala, accumbens area and ventral diencephalon. An example of an automatic segmentation of an infant T1-weighted MRI scan is provided in Figure 1. All brain parcellations were examined by an expert and found to be of high quality.
Figure 1.
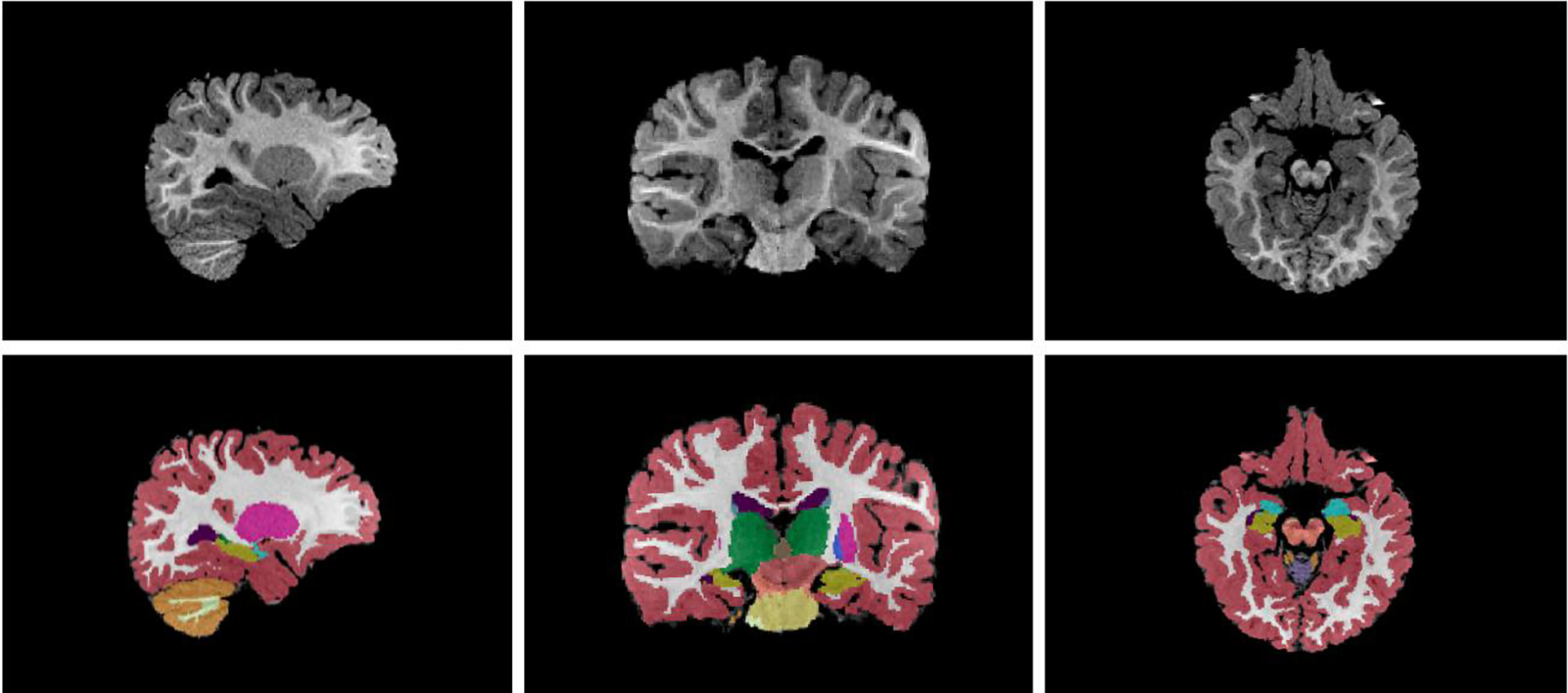
Example of an automatic segmentation of an infant scan via Infant FreeSurfer
Sagittal (left), coronal (middle), and axial (left) view of an infant T1-weighted MRI scan. Bottom shows segmentation produced by Infant FreeSurfer. Colors represent distinct subcortical regions.
2.6. Statistical Analyses
Statistical analyses were performed using SPSS (version 27, IBM, Armonk, NY).
To examine the association between maternal distress (anxiety and depression) and infant brain subcortical structure volumes at 2–5 months, multivariate general linear models were used. Infant brain structure volumes (left and right: cerebellum, thalamus, caudate, putamen, pallidum [globus pallidus], hippocampus, amygdala, accumbens area and ventral diencephalon) were the dependent variables. Maternal anxiety (STAI-S scores) and maternal depression (CES-D or EPDS scores) were the independent variables. The models were adjusted for total cerebral volumes and infant age at scan by including them as covariates. Total cerebral volume (TCV) was included as covariates in each model to account for sex-difference between males and females in brain volumes (Barnes et al., 2010; Duerden et al., 2020). As one model was implemented to address our hypothesis, the alpha level was set at 0.05.
To investigate whether infant subcortical brain structure volumes (2–5 months) may predict later emotional regulation (8–11 months), a generalized linear model with an identity link function was used, with infant temperament (negative affectivity dimension scores) as the dependent variable and infant subcortical structure volumes as the independent variables. The model was adjusted for total cerebral volumes, infant age at scan and infant age at temperament assessment, by including them as covariates. As we had one hypothesis regarding the association between infant emotional regulation outcomes and brain volumes, the alpha level was set at 0.05.
Finally, to address our third aim to determine whether biological sex moderates the relationship between infant subcortical volumes and infant emotional regulation, the interaction variable (biological sex * subcortical structure volume) was included in the models. As we had 9 regions of interest (cerebellum, thalamus, caudate, putamen, pallidum [globus pallidus], hippocampus, amygdala, accumbens area and ventral diencephalon) in the left and right hemispheres, a single model was run to address our hypothesis and the alpha level was set at 0.003.
3. Results
3.1. Participant Demographics
A total of 36 mother-infant pairs were included. Participant demographics for infants are shown in Table 1. The average age of infants at time of MRI scan was 3.36 months (SD = 0.762), and ranged from 2–5 months. The average age of infants at time of temperament measure was 9.25 (SD = 0.692), and ranged from 8–11 months. The average age of mothers was 22.22 (SD = 1.22, range = 4.25). Maternal ethnicity, education, and use of public assistance is reported in Table 1.
Table 1.
Participant characteristics
| Infant Characteristics n | 36 |
| Female n (%) | 16 (44.4%) |
| Male n (%) | 20 (55.6%) |
| Age at scan, months M (SD) | 3.36 (0.762) |
| Age at IBQ-R-S M (SD) | 9.25 (0.692) |
| Maternal Characteristics n | 36 |
| Ethnicity n (%) | |
| Caucasian | 7 (19.4%) |
| African American | 28 (77%) |
| Multi-racial | 1 (2.8%) |
| Education | |
| No diploma or degree | 6 (16.7%) |
| General Educational Development (GED) | 3 (8.3%) |
| High school diploma | 22 (61.1%) |
| Other certificate of training | 4 (11.1%) |
| Bachelor’s degree | 1 (2.8%) |
| Use of public assistance | |
| No public assistance | 14 (38.9%) |
| Use of public assistance | 22 (61.1%) |
3.2. Maternal Anxiety and Depression
To examine maternal distress, perceived levels of anxiety and depression were assessed using self-report questionnaires. Descriptive information regarding maternal distress questionnaires is shown in Table 2. The average maternal depression score determined by EPDS was 5.81 (SD = 5.092) and 7 participants scored above the generally defined cut-off score (≥10) to indicate clinically significant depressive symptoms in this population. Average maternal anxiety scores (STAI-S) were 32.78 (SD = 10.10), and 4 participants scored above the generally defined cut-off score (> 40) in this population to indicate clinically significant anxiety.
Table 2.
Descriptive information for maternal distress measures
| Dataset 1 (n=36) | |
|---|---|
|
| |
| STAI-S (M, SD) | 32.78 (10.100) |
| EPDS (M, SD) | 5.81 (5.092) |
| Frequencies | |
| STAI-S cut off > 40 | 4 (11%) |
| EPDS cut off ≥ 10 | 7 (19.44%) |
Maternal depression was assessed using EPDS, Edinburg Postnatal Depression Scale (range 0–30). Maternal anxiety was measured using the State-Trait Anxiety Inventory - State Anxiety Subscale (range 20–80).
3.3. Association between Postnatal Maternal Distress and Infant Subcortical Macrostructure
We first examined the association between maternal distress (anxiety and depression) and infants’ subcortical brain structure volumes using a multivariate general linear model, adjusting for total cranial volumes, infant age, and biological sex. Maternal anxiety (STAI-S) was positively associated with infant right pallidum volume (F(1,30) = 4.519, p = 0.042, Figure 2), suggesting higher anxiety scores were associated with greater right pallidum volumes in infants. This association was only significant when maternal depression (EPDS) was included in the model. These results indicate that maternal anxiety is associated with infant subcortical brain structure, specifically within the right pallidum, and that maternal depression is an important moderator in this association.
Figure 2.
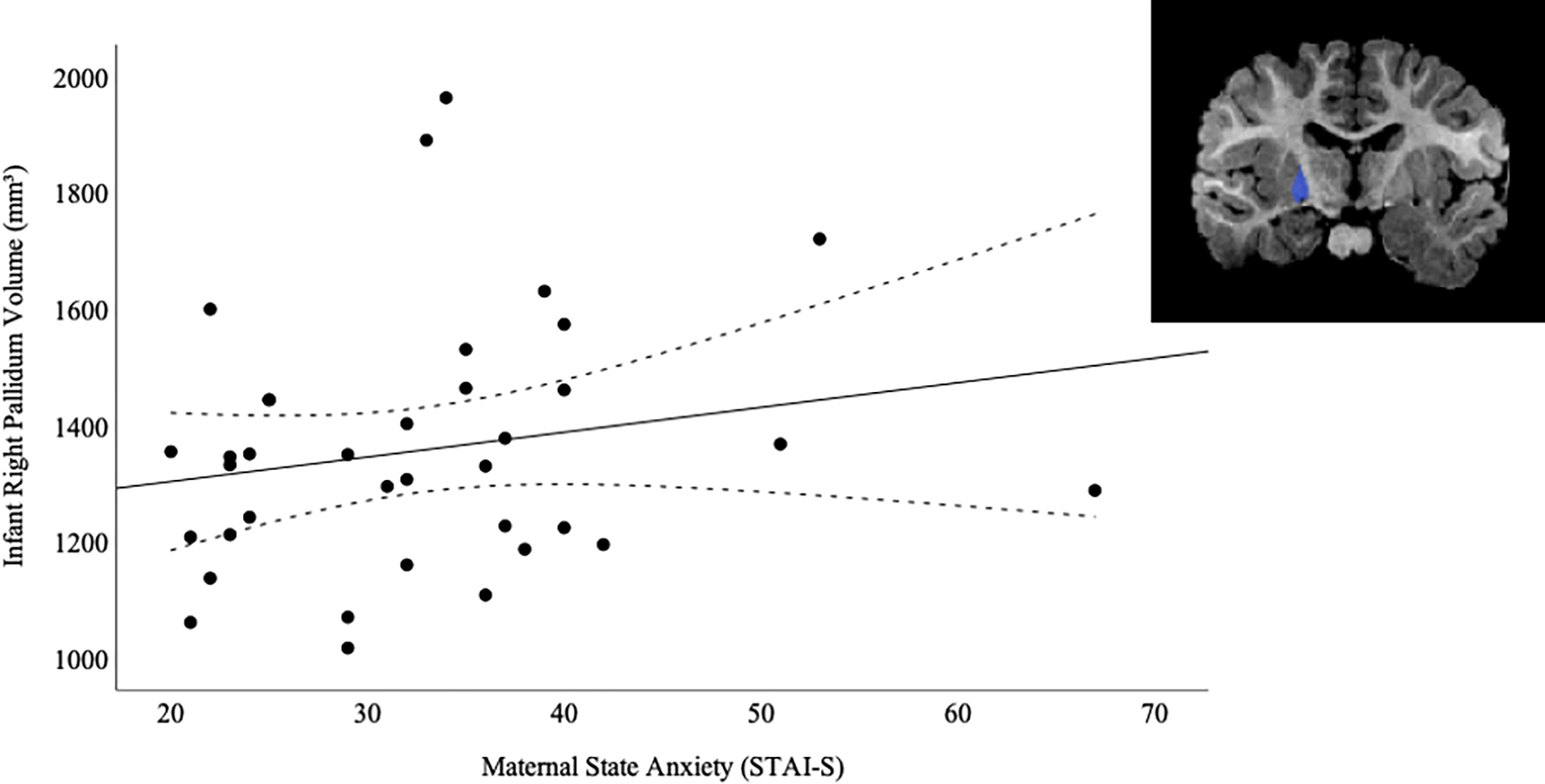
Association between Maternal State Anxiety and Infant Right Pallidum Volume (mm3)
Note. Scatterplot depicting association between postnatal maternal anxiety on State-Trait Anxiety Index State Subscale (STAI-S) at 2–5 months postpartum and infant right pallidum volume (mm3) at 2–5 months. Dashed lines represent 95% confidence interval.
3.4. Examining Infant Emotion Regulation
Infant emotion regulation was assessed using the negative affectivity dimension of the IBQ-R-S. The average score for this dimension was 3.589 (SD=0.790). Higher scores on this dimension are associated with impaired emotion regulation (Bridgett et al., 2009; Zimmer-Gembeck & Skinner, 2016).
3.5. Association between Infant Subcortical Macrostructure and Emotion Regulation
We next examined whether infant brain subcortical macrostructure (2–5 months) predicted later infant emotion regulation (8–11 months) using a generalized linear model, adjusting for age at scan and IBQ-R-S assessment, total cranial volume, and biological sex. The results of the generalized linear model are shown in Table 3. Infant negative affectivity scores were positively predicted by left pallidum volume (B = 0.001, p = 0.002), with larger volumes predicting impaired emotion regulation. Furthermore, infant outcome scores were negatively predicted by left accumbens area (B = −0.011, p < 0.001), left hippocampus (B = −0.002, p < 0.001) and right hippocampus (B = −0.001, p = 0.019) volumes, indicating that smaller volumes of these structures predict impaired emotion regulation. The results for the associations of the left and right hippocampus are shown in Figure 3. To summarize, we observed infant subcortical macrostructure at 2–5 months, specifically the left pallidum, left accumbens area and hippocampus, predicted or were significantly associated with subsequent emotion regulation at 8–11 months.
Table 3.
Results of a generalized linear model examining the associations between infant subcortical macrostructure (2–5 months) and subsequent emotion regulation (8–11 months).
| 95% Confidence Interval | ||||
|---|---|---|---|---|
|
| ||||
| Beta Value | Lower | Upper | p value | |
|
| ||||
| Sex (Female) | −1.098 | −1.489 | −0.708 | <0.001 |
| IBQR Age in Months | 0.575 | 0.235 | 0.915 | 0.001 |
| Left Cerebellum Cortex | −0.0006 | 0.000 | 0.000 | 0.432 |
| Left Thalamus | 0.000 | −0.001 | 0.001 | 0.550 |
| Left Caudate | −0.001 | −0.003 | 0.000 | 0.116 |
| Left Putamen | 0.000 | 0.000 | 0.001 | 0.236 |
| Left Pallidum | 0.001 | 0.000 | 0.001 | 0.002 |
| Left Hippocampus | −0.002 | −0.003 | −0.001 | <0.001 |
| Left Amygdala | 0.000 | −0.002 | 0.003 | 0.810 |
| Left Accumbens Area | −0.011 | −0.017 | −0.005 | <0.001 |
| Left Ventral Diencephalon | 0.003 | −0.002 | 0.007 | 0.201 |
| Right Cerebellum Cortex | <0.001 | 0.000 | 0.000 | 0.846 |
| Right Thalamus | 0.000 | −0.001 | 0.000 | 0.555 |
| Right Caudate | 0.001 | −0.0006 | 0.002 | 0.071 |
| Right Putamen | 0.001 | −0.0004 | 0.002 | 0.064 |
| Right Pallidum | −0.001 | −0.003 | 0.001 | 0.161 |
| Right Hippocampus | −0.001 | −0.002 | 0.000 | 0.019 |
| Right Amygdala | 0.002 | −0.00008 | 0.004 | 0.059 |
| Right Accumbens area | 0.002 | −0.003 | 0.006 | 0.464 |
| Right Ventral Diencephalon | 0.001 | −0.003 | 0.005 | 0.638 |
| Baby Age at Scan | −0.210 | −0.793 | 0.373 | 0.479 |
| TCV | −0.00006 | −0.000001 | 0.000006 | 0.119 |
Figure 3.
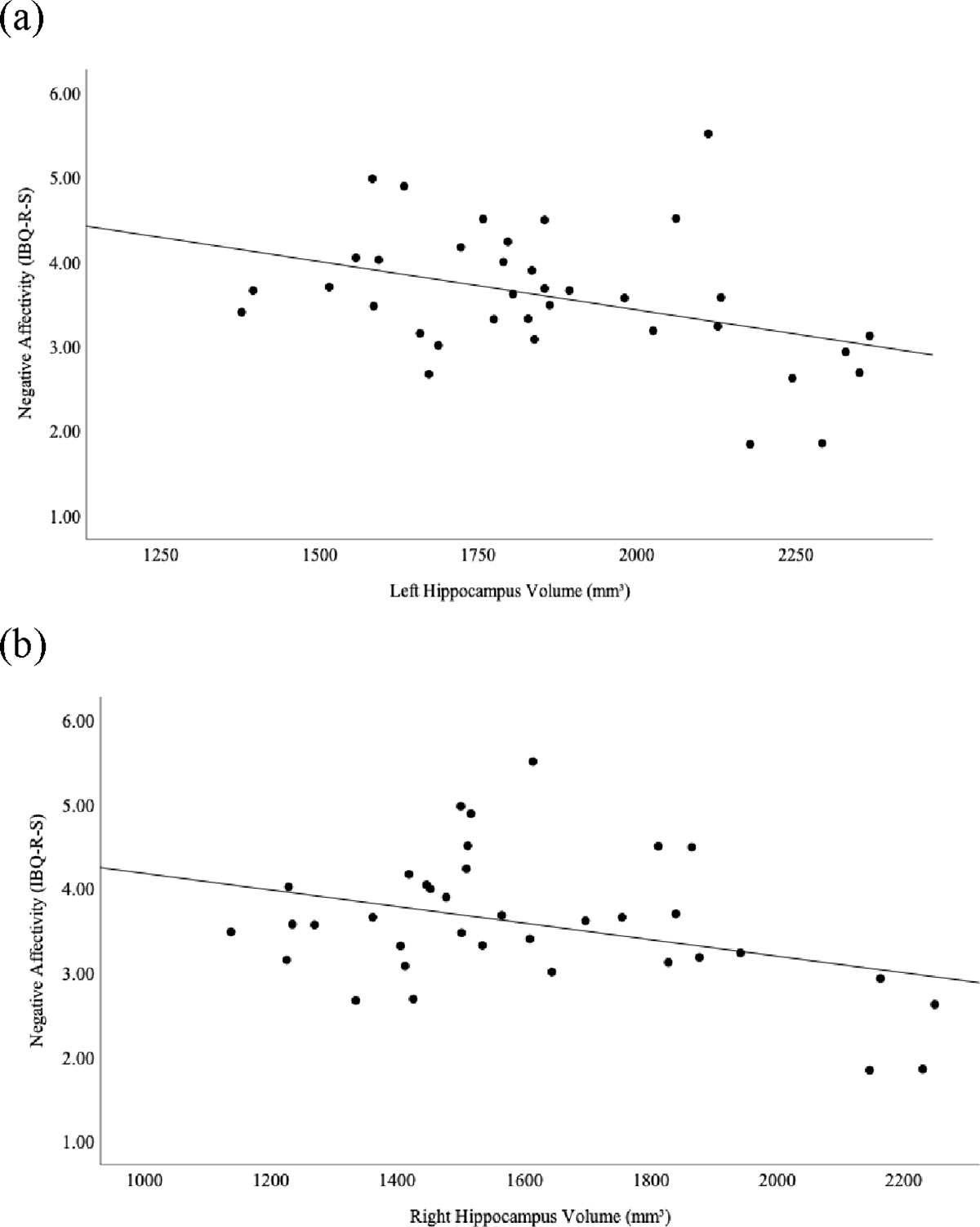
Associations between Infant Hippocampus Volumes and Subsequent Emotion Regulation (Negative Affectivity Dimension Scores)
Scatterplots depicting associations between infant (a) left and (b) right hippocampus volumes (mm3) at 2–5 months and subsequent emotion regulation (negative affectivity scores on Infant Behavior Questionnaire-Revised Short Form, IBQ-R-S) at 8–11 months in the high anxiety sample.
As part of exploratory analysis, we further investigated the finer grain temperament dimensions: 1. fear, 2. sadness, 3. distress to limitations, and 3. falling reactivity or rate of recovery from distress. We investigated whether infant macrostructure (2–5 months) predicted subsequent parent-based reports of fearfulness/sadness/distress to limitations/falling reactivity (9–12 months) using separate generalized linear models, adjusting for age at scan and IBQ-R-S assessment, total cranial volume, and biological sex. Of note, significant associations between the volumes of several subcortical structure and subsequent fear (Supplementary Data Table S1) were evident. Infant fear was positively predicted by right amygdala volume (B = 0.007, p = 0.001) with larger volumes predicting greater fearfulness (Supplementary Data Figure S1). The results for other finer grain temperament dimensions are in Supplementary Tables 2–4.
3.6. Moderating Role of Biological Sex
Finally, we examined the moderating role of sex on the associations between infant subcortical macrostructure and emotion regulation. Associations between infant subcortical macrostructure and subsequent emotion regulation demonstrated regionally-specific associations with biological sex (Table 4). In boys, increased difficulties with emotional regulation were associated with larger left pallidum volumes (B = 0.01, p = < 0.001). However, a reverse association was seen for girls, whereby infant scores were negatively predicted by the left accumbens area (B = −0.012, p < 0.001) and left hippocampus (B = −0.002, p <0.001;), when correcting for multiple comparisons. The associations between left hippocampus volume and emotion regulation by sex are shown in Figure 4, with females showing a stronger relationship than males.
Table 4.
Results of generalized linear models examining moderating role of biological sex on the associations between infant subcortical macrostructure (2-5 months) and subsequent emotion regulation (8-11 months).
| 95% Confidence Interval |
||||
|---|---|---|---|---|
|
| ||||
| Beta Value | Lower | Upper | p value | |
|
| ||||
| [Sex=F] * Left Pallidum | 0.01 | 0.0001 | 0.001 | 0.032 |
| [Sex=M] * Left Pallidum | 0.01 | 0.0001 | 0.002 | < 0.001 |
| [Sex=F] * Left Accumbens Area | −0.012 | −0.018 | −0.006 | <0.001 |
| [Sex=M] * Left Accumbens Area | −0.009 | −0.015 | −0.003 | 0.003 |
| [Sex=F] * Left Hippocampus | −0.002 | −0.003 | −0.001 | <0.001 |
| [Sex=M] * Left Hippocampus | −0.002 | −0.003 | −0.001 | 0.004 |
| [Sex=F] * Right Hippocampus | −0.002 | −0.003 | −0.001 | 0.001 |
| [Sex=M] * Right Hippocampus | −0.001 | −0.002 | −0.001 | 0.043 |
F, female, M, male
Figure 4.
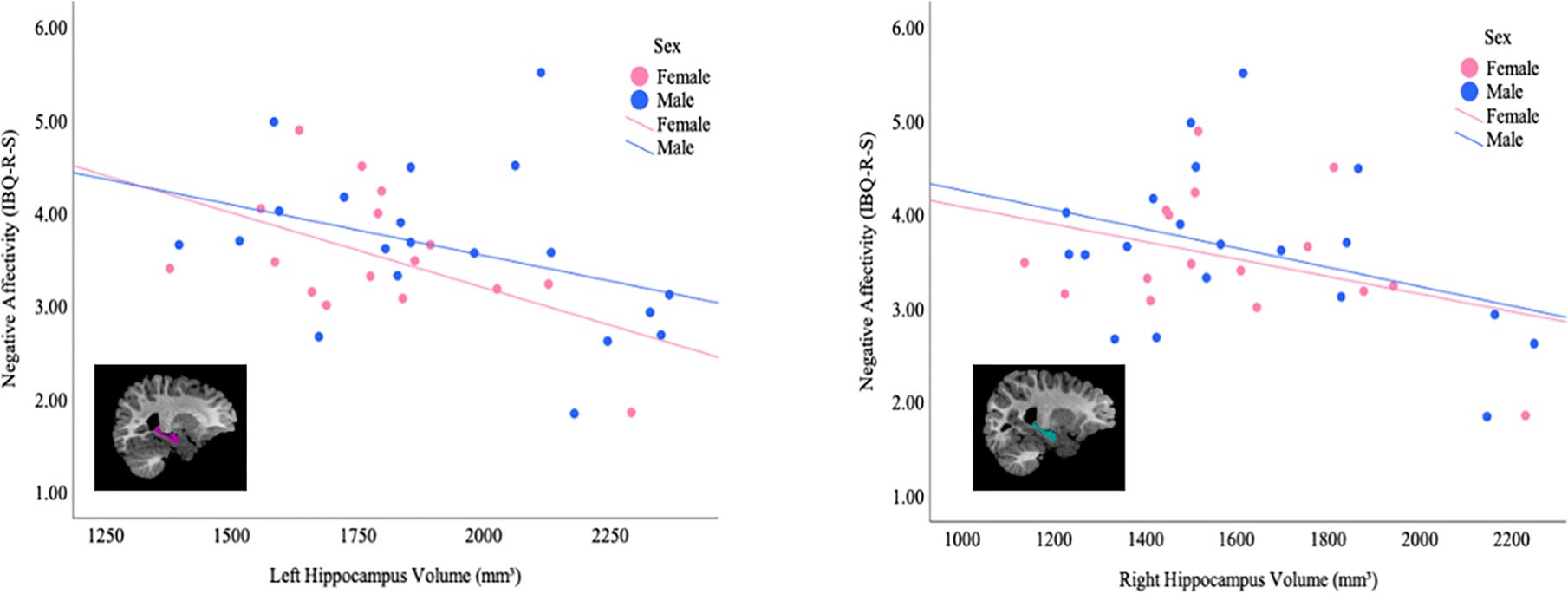
Associations between Infant Hippocampus Volume and Emotion Regulation (IBQ-R-S Negative Affectivity Dimension Scores) by Biological Sex
Scatterplots depicting the associations between infant (a) left and (b) right hippocampus volumes (mm3) at 2–5 months and subsequent emotion regulation (negative affectivity dimension scores on Infant Behavior Questionnaire-Revised Short Form IBQ-R-S) at 8–11 months in males and females.
4. Discussion
The association between postnatal maternal distress and infant subcortical macrostructure were examined in a cohort of mother-baby dyads, as well as the associations between infant subcortical macrostructure and subsequent emotion regulation abilities, taking into consideration the moderating role of biological sex. In support of our prediction, we found an association between postnatal maternal distress and infant subcortical brain structure at 2–5 months. Specifically, maternal anxiety was associated with infant right pallidum volume when accounting for maternal depression.
Our results align broadly with literature that similarly found maternal psychological distress is associated with offspring brain structure. For example, postnatal maternal anxiety has been previously associated broadly with alterations in cerebral white and cortical gray matter (Lebel et al., 2016; Zou et al., 2019). However, in contrast to previous work, we did not find associations specifically within limbic structures such as the hippocampus or amygdala (Donnici et al., 2021; Lupien et al., 2011; Qiu et al., 2013). Differences in results may be due to variations in the measures, severity, or timing of maternal distress across study samples. For example, previous research investigated maternal trait anxiety rather than maternal state anxiety (Qiu et al., 2013). Furthermore, maternal distress was quite low for both samples in the present study, as opposed to previous research, in which average anxiety scores neared clinical cut-off (Qiu et al., 2013). Moreover, differences may also arise due to differences in the age of offspring at the time of MRI scanning (Tottenham & Sheridan, 2010). The development of subcortical structures spans from infancy into adulthood and, therefore, associations may differ depending on age of offspring. Furthermore, previous work suggests there may be temporal delays for structural alterations of the brain in association with various forms of early life stress (Lee et al., 2020).
The right pallidum has not been previously associated with maternal distress to our knowledge. The pallidum is a structure of the basal ganglia broadly involved in components of movement and cognition, and, recently, it has also been implicated in early child-parent interactions (Abraham et al., 2020). Differences in these results may be due to the focus of previous work which examined limbic structures as opposed to subcortical structures of the basal ganglia.
Our results support previous research that suggests maternal distress may be associated with infant brain structure, emphasizing the importance of maternal mental health for healthy offspring brain development. Given the findings regarding the right pallidum, subcortical structures of the basal ganglia should be considered when examining associations between maternal mental health and infant brain development to gain a better understanding of the relationships between maternal distress and infant brain development.
In support of our second prediction, we found infant subcortical structure volumes at 2–5 months were associated with emotion regulation at 8–11 months. Specifically, our findings suggest larger left pallidum and smaller left accumbens, and left and right hippocampus may predict subsequently impaired emotion regulation. These results are broadly in line with previous work on brain-behavior relationships that have found variations in subcortical structure volumes to predict outcomes relating to emotional development, including emotional regulation. More specifically, our findings that smaller left and right hippocampal volumes may predict impaired emotional regulation is similar to those observed in newborns in association with prenatal maternal distress, as well as adolescence, at risk for depression (Barch et al., 2019; Moog et al., 2021). The hippocampus is a key brain region implicated in emotion regulation as well as stress response modulation. Variations in its structure have been reported across various emotional problems/disorders in both children and adolescence such as anxiety and depression, albeit these relationships have been inconsistent (De Bellis et al., 2000; Gold et al., 2017; Hanson et al., 2015; Rao et al., 2010). Further research is needed to elucidate the role of structural alterations in the hippocampus and emotional problems.
To our knowledge, previous research has not implicated structural alterations in either the left pallidum or nucleus accumbens and emotion regulation. The pallidum and nucleus accumbens are structures of the basal ganglia. The nucleus accumbens is part of the ventral striatum, and is involved in a range of functions, such as emotional and motivational processing (Hyde & Garcia-Rill, 2019). The observed differences in results may be due to the focus of previous work, which has focused on limbic regions.
Given that infant subcortical macrostructure specifically within the left pallidum, left nucleus accumbens, and bilateral hippocampus predicted subsequent emotion regulation, these structures could be further explored as potential predictors or neural biomarkers of risk for adverse emotional development in infants exposed to symptoms of maternal distress. The ability to identify infants at risk could provide the opportunity for early interventions (Savage-McGlynn et al., 2015).
Regarding the role of biological sex, we found the associations between subcortical structure and subsequent emotion regulation were observed in both males and females. However, trajectories of these associations differed by sex. These results align with literature that similarly did not find sex differences in the relationships between subcortical structures and later socioemotional development in infants (Moog et al., 2021; Gold et al., 2017). However, the sex differences in the trajectories of the associations may suggest or emphasizes the importance of examining sex differences in research on brain-behavior relationships. Furthermore, it has previously been suggested that sex differences may depend upon the stage of development and, therefore, may become apparent at a later age (Kaczkurkin et al., 2019; Ortiz-Mantilla et al., 2010). As such, and in line with recommendations by Letourneau and colleagues (2020), biological sex should be considered when evaluating brain-behavior relations between subcortical macrostructure and emotional development.
With regards to our exploratory analysis investigating the finer grain temperament dimension fear, infant macrostructure was associated with later infant fearfulness. Interestingly we found a positive association between right amygdala volumes and subsequent fearfulness. This result aligns well with previous literature which has widely implicated the amygdala in fear processing and fear-related pathology such as anxiety (Ressler, 2010). For example, a similar positive relationship between amygdala volume and fear was observed in children, albeit only in girl and more broadly greater amygdala volumes have been associated with generalized anxiety disorder in children (De Bellis et al., 2000; Qin et al., 2014; van der Plas et al., 2010). Given that fearful temperament is associated with later risk for internalizing disorders such as anxiety it may be useful to further explore these potential early biomarkers (Kochanska et a., 1997).
Relative to other studies, the present study explored how stress relates to a number of subcortical structures within a unique developmental period. Previous work has focused mainly on limbic structures, including the hippocampus and amygdala, when examining associations with both maternal distress and emotional development, with studies targeting children and adolescence. Given that there is currently insufficient identification of young children with emotional development risks and the importance of the early period (infancy-early childhood) for interventions to promote healthy outcomes, identifying potential neural markers of risk is essential in maximizing outcomes for these individuals (Cooper et al., 2009). In the present study we investigated numerous subcortical structures, linking stress to neural development beyond the limbic system. Moreover, our work suggests that early infancy could be a reasonable period at which to identify children at risk for adverse emotional development (Cooper et al., 2009).
Limitations
A limitation of the present study is the use of maternal reports to assess infant emotion regulation. Although caregiver reports provide insight into an infant’s behavior across a variety of contexts, some research suggests psychological distress may bias a mother’s perceptions of her infant’s temperament (Gartstein & Rothbart, 2003; Leerkes et al., 2003; Liu et al., 2021). As such, future work may benefit from a combination of measures to assess infant emotion regulation, including both lab and caregiver-based assessments.
A second limitation includes the lack of maternal distress measures during the antenatal period. Mothers experiencing psychological distress in the postnatal period are likely to have also experienced distress symptoms during pregnancy (Heron et al., 2004). Moreover, prenatal maternal distress has also been associated with offspring brain structure; therefore, our results may reflect both prenatal and postnatal maternal distress or may be confounded by maternal distress in the prenatal period. Future longitudinal research should consider anxiety and depression symptoms both antenatally and postnatally to better reflect the chronicity of maternal distress during this period (Fitzgerald et al., 2021).
Finally, due to the nature of our data collection (database), several other potential confounding variables were unable to be controlled for in the present project. These include depression and anxiety medication use by the mothers, infant sleep, parental behaviors, genetics, and other forms of early life stress (Brito & Noble, 2014; Choi et al., 2022; Hanson et al., 2015; Kocevska et al., 2017; Rice et al., 2010). These variables have been found to be associated with brain development and as such, should be considered in future work.
5. Conclusions
In conclusion, our findings support an association between postnatal maternal anxiety and infant subcortical brain structure and add to the literature that emphasizes the importance of maternal mental health during this period. Furthermore, the observed associations between infant subcortical macrostructure and subsequent emotion regulation suggest it may be important to examine these relationships further to potentially identify early predictors of emotional development risk. This may, allow for early interventions and, thus, promotion of healthy development in offspring.
Supplementary Material
Acknowledgements
We would like to thank the families who participated in this research. Funding for this study was provided by the Canada First Research Excellence Fund (CFREF), the Molly Towell Perinatal Research Foundation and the Natural Sciences and Engineering Council of Canada (NSERC). We thank the investigators who were involved in the original data collection for conducting the study and making the data available: Mary L. Philips, Alison E. Hipwell, Ashley Stiller, Ricki Stiffler, and Genna Bebko. Data used in the preparation of this manuscript were obtained from the NIMH Data Archive (NDA), and were funded by R21 MH106570 (Impact of Maternal Caregiving on Brain-Behavior Relationships in Infants), R01 MH056630 (Pittsburgh Girls Study), and R01 MH115466 (Caregiving Effects on the Early Development of Infant Brain-Behavior Relationships). The raw data are available at nda.nih.gov/edit_collection.html?id=2340.
References
- Abraham E, Posner J, Wickramaratne PJ, Aw N, van Dijk MT, Cha J, ... & Talati A. (2020). Concordance in parent and offspring cortico-basal ganglia white matter connectivity varies by parental history of major depressive disorder and early parental care. Social cognitive and affective neuroscience, 15(8), 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktar E, & Pérez-Edgar K (2020). Infant Emotion Development and Temperament. In Lockman J & Tamis-LeMonda C (Eds.), The Cambridge Handbook of Infant Development: Brain, Behavior, and Cultural Context (Cambridge Handbooks in Psychology, pp. 715–741). Cambridge: Cambridge University Press. doi: 10.1017/9781108351959.026 [DOI] [Google Scholar]
- Barch DM, Harms MP, Tillman R, Hawkey E, & Luby JL (2019). Early childhood depression, emotion regulation, episodic memory, and hippocampal development. Journal of abnormal psychology, 128(1), 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, ... & Fox NC. (2010). Head size, age and gender adjustment in MRI studies: a necessary nuisance?. Neuroimage, 53(4), 1244–1255. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F, & Coats AH (2008). The experience of anger and sadness in everyday problems impacts age differences in emotion regulation. Developmental psychology, 44(6), 1547. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, & O’Brien M (2008). Individual differences in trajectories of emotion regulation processes: the effects of maternal depressive symptomatology and children’s physiological regulation. Developmental psychology, 44(4), 1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton RE, Chaplin TM, & Sinha R (2010). Sex differences in the correlation of emotional control and amygdala volumes in adolescents. Neuroreport, 21(14), 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgett DJ, Gartstein MA, Putnam SP, McKay T, Iddins E, Robertson C, ... & Rittmueller, A. (2009). Maternal and contextual influences and the effect of temperament development during infancy on parenting in toddlerhood. Infant Behavior and Development, 32(1), 103–116. [DOI] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in neuroscience, 8, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, ... & Birn RM. (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature neuroscience, 15(12), 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai RY, Hardan AY, Phillips JM, Frazier TW, & Uljarević M (2021). Brief Report: Emotion Regulation Influences on Internalizing and Externalizing Symptoms Across the Normative-Clinical Continuum. Frontiers in Psychiatry, 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center on the Developing Child at Harvard University. (2009). Maternal depression can undermine the development of young children. Working Paper No. 8. [Google Scholar]
- Chaplin TM, & Aldao A (2013). Gender differences in emotion expression in children: a metaanalytic review. Psychological bulletin, 139(4), 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Poon JA, Thompson JC, Hansen A, Dziura SL, Turpyn CC, ... & Ansell EB. (2019). Sex-differentiated associations among negative parenting, emotion-related brain function, and adolescent substance use and psychopathology symptoms. Social Development, 28(3), 637–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Son SJ, & Park B (2022). Shared genetic effects of emotion and subcortical volumes in healthy adults. NeuroImage, 118894. [DOI] [PubMed] [Google Scholar]
- Cooper JL, Masi R, & Vick J (2009). Social-emotional development in early childhood: What every policymaker should know. [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. The British journal of psychiatry, 150(6), 782786. [DOI] [PubMed] [Google Scholar]
- Crugnola CR, Ierardi E, Ferro V, Gallucci M, Parodi C, & Astengo M (2016). Mother-infant emotion regulation at three months: the role of maternal anxiety, depression and parenting stress. Psychopathology, 49(4), 285–294. [DOI] [PubMed] [Google Scholar]
- Dean DC, Planalp EM, Wooten W, Schmidt CK, Kecskemeti SR, Frye C, ... & Davidson RJ. (2018). Investigation of brain structure in the 1-month infant. Brain Structure and Function, 223(4), 1953–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, ... & Ryan ND. (2000). A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological psychiatry, 48(1), 51–57. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Coghlan M, & Vigod S (2013). Can we identify mothers at-risk for postpartum anxiety in the immediate postpartum period using the State-Trait Anxiety Inventory?. Journal of affective disorders, 150(3), 1217–1220. [DOI] [PubMed] [Google Scholar]
- Dick F, Lloyd-Fox S, Blasi A, Elwell C, Mills D, & Elwell C (2014). Neuroimaging methods. Educational neuroscience, 13–45. [Google Scholar]
- Donnici C, Long X, Dewey D, Letourneau N, Landman B, Huo Y, & Lebel C (2021). Prenatal and postnatal maternal anxiety and amygdala structure and function in young children. Scientific reports, 11(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Scaramella L, & Zeanah CH (2016). The neurobiological impact of postpartum maternal depression: prevention and intervention approaches. Child and Adolescent Psychiatric Clinics, 25(2), 179–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Chakravarty MM, Lerch JP, & Taylor MJ (2020). Sex-based differences in cortical and subcortical development in 436 individuals aged 4–54 years. Cerebral Cortex, 30(5), 2854–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufford AJ, Salzwedel AP, Gilmore JH, Gao W, & Kim P (2021). Maternal trait anxiety symptoms, frontolimbic resting-state functional connectivity, and cognitive development in infancy. Developmental psychobiology, 63(6), e22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, & Eggum ND (2010). Emotion-related self-regulation and its relation to children’s maladjustment. Annual review of clinical psychology, 6, 495–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow MB, Kitts RL, Blood E, Bizarro A, Hofmeister M, & Wright RJ (2011). Maternal posttraumatic stress symptoms and infant emotional reactivity and emotion regulation. Infant Behavior and Development, 34(4), 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, & Kalin NH (2002). Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological psychiatry, 52(8), 776–784. [DOI] [PubMed] [Google Scholar]
- Feldman R, & Klein PS (2003). Toddlers’ self-regulated compliance to mothers, caregivers, and fathers: implications for theories of socialization. Developmental psychology, 39(4), 680. [DOI] [PubMed] [Google Scholar]
- Feng X, Shaw DS, Kovacs M, Lane T, O’Rourke FE, & Alarcon JH (2008). Emotion regulation in preschoolers: The roles of behavioral inhibition, maternal affective behavior, and maternal depression. Journal of Child Psychology and Psychiatry, 49(2), 132–141. [DOI] [PubMed] [Google Scholar]
- Feng X, Shaw DS, Kovacs M, Lane T, O’Rourke FE, & Alarcon JH (2008). Emotion regulation in preschoolers: The roles of behavioral inhibition, maternal affective behavior, and maternal depression. Journal of Child Psychology and Psychiatry, 49(2), 132–141. [DOI] [PubMed] [Google Scholar]
- Fitzgerald E, Parent C, Kee MZ, & Meaney MJ (2021). Maternal distress and offspring neurodevelopment: Challenges and opportunities for pre-clinical research models. Frontiers in Human Neuroscience, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the revised infant behavior questionnaire. Infant behavior and development, 26(1), 64–86. [Google Scholar]
- Gold AL, Steuber ER, White LK, Pacheco J, Sachs JF, Pagliaccio D, ... & Pine, D. S. (2017). Cortical thickness and subcortical gray matter volume in pediatric anxiety disorders. Neuropsychopharmacology, 42(12), 2423–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS, Aksan N, & Buss KA (2000). Temperamental substrates of personality. In Molfese VJ & Molfese DL (Eds.), Temperament and personality development across the life span (pp. 1–32). Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, ... & Buss C. (2018). Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biological psychiatry, 83(2), 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granat A, Gadassi R, Gilboa-Schechtman E, & Feldman R (2017). Maternal depression and anxiety, social synchrony, and infant regulation of negative and positive emotions. Emotion, 17(1), 11. [DOI] [PubMed] [Google Scholar]
- Guadagno A, Belliveau C, Mechawar N, & Walker CD (2021). Effects of early life stress on the developing basolateral amygdala-prefrontal cortex circuit: the emerging role of local inhibition and perineuronal nets. Frontiers in Human Neuroscience, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanford LC, Schmithorst VJ, Panigrahy A, Lee V, Ridley J, Bonar L, … & Phillips ML. The Impact of Caregiving on the Association Between Infant Emotional Behavior and Resting State Neural Network Functional Topology. Front Psychol. 2018. Oct 15;9:1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, ... & Davidson RJ. (2015). Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological psychiatry, 77(4), 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks LM, Swales DA, Garcia SE, Driver C, & Davis EP (2019). Does prenatal maternal distress contribute to sex differences in child psychopathology?. Current Psychiatry Reports, 21(2), 1–9. [DOI] [PubMed] [Google Scholar]
- Heron J, O’Connor TG, Evans J, Golding J, Glover V, & ALSPAC Study Team. (2004). The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of affective disorders, 80(1), 65–73. [DOI] [PubMed] [Google Scholar]
- Hyde J, & Garcia-Rill E (2019). Autism and arousal. In Arousal in Neurological and Psychiatric Diseases (pp. 83–114). Academic Press. [Google Scholar]
- Iglesias JE, & Sabuncu MR (2015). Multi-atlas segmentation of biomedical images: A survey. Medical Image Analysis, 24(1), 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Dufoix R, Laplante DP, Elgbeili G, Patel R, Chakravarty MM, ... & Pruessner JC. (2019). Larger amygdala volume mediates the association between prenatal maternal stress and higher levels of externalizing behaviors: sex specific effects in project ice storm. Frontiers in human neuroscience, 13, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Raznahan A, & Satterthwaite TD (2019). Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology, 44(1), 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitz M, Maytal HR, Devor N, Bergman L, & Mankuta D (2010). Maternal anxiety, mother–infant interactions, and infants’ response to challenge. Infant Behavior and Development, 33(2), 136–148. [DOI] [PubMed] [Google Scholar]
- Kocevska D, Muetzel RL, Luik AI, Luijk MP, Jaddoe VW, Verhulst FC, ... & Tiemeier H. (2017). The developmental course of sleep disturbances across childhood relates to brain morphology at age 7: the Generation R Study. Sleep, 40(1). [DOI] [PubMed] [Google Scholar]
- Kochanska G (1997). Multiple pathways to conscience for children with different temperaments: from toddlerhood to age 5. Developmental psychology, 33(2), 228. [DOI] [PubMed] [Google Scholar]
- Kozinszky Z, & Dudas RB (2015). Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. Journal of affective disorders, 176, 95–105. [DOI] [PubMed] [Google Scholar]
- Lean RE (2021). Links Between Caregiver Postpartum Internalizing Symptoms and Infant Brain and Behavior Raise New Questions. Biological Psychiatry, 90(5), e27–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, & Giesbrecht G (2020). Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. Journal of affective disorders, 277, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walton M, Letourneau N, Giesbrecht GF, Kaplan BJ, & Dewey D (2016). Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biological psychiatry, 80(11), 859–868. [DOI] [PubMed] [Google Scholar]
- Lee KH, Yoo JH, Lee J, Kim SH, Han JY, Hong SB, ... & Brent, D. A. (2020). The indirect effect of peer problems on adolescent depression through nucleus accumbens volume alteration. Scientific reports, 10(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, & Crockenberg SC (2003). The impact of maternal characteristics and sensitivity on the concordance between maternal reports and laboratory observations of infant negative emotionality. Infancy, 4(4), 517–539. [Google Scholar]
- Letourneau N, Dewey D, Kaplan BJ, Ntanda H, Novick J, Thomas JC, ... & APrON Study Team. (2019). Intergenerational transmission of adverse childhood experiences via maternal depression and anxiety and moderation by child sex. Journal of developmental origins of health and disease, 10(1), 88–99. [DOI] [PubMed] [Google Scholar]
- Liu J (2004). Childhood externalizing behavior: Theory and implications. Journal of child and adolescent psychiatric nursing, 17(3), 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Snidman N, & Kagan J (2020). The Effect of Maternal Distress on Perceptions of Infant Behavior May Differ in Chinese-and European American Mothers and Infants. Journal of developmental and behavioral pediatrics: JDBP, 41(3), 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden A, Harms MP, Tillman R, & Barch DM (2016). Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proceedings of the National Academy of Sciences, 113(20), 5742–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, ... & Séguin JR. (2011). Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences, 108(34), 14324–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan A, Cicchetti D, Toth SL, & Rogosch FA (2007). Early-occurring maternal depression and maternal negativity in predicting young children’s emotion regulation and socioemotional difficulties. Journal of abnormal child psychology, 35(5), 685–703. [DOI] [PubMed] [Google Scholar]
- Meades R, & Ayers S (2011). Anxiety measures validated in perinatal populations: a systematic review. Journal of affective disorders, 133(1–2), 1–15. [DOI] [PubMed] [Google Scholar]
- Moog NK, Nolvi S, Kleih TS, Styner M, Gilmore JH, Rasmussen JM, ... & Buss C. (2021). Prospective association of maternal psychosocial stress in pregnancy with newborn hippocampal volume and implications for infant social-emotional development. Neurobiology of stress, 15, 100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Choe MS, Flax J, Grant PE, & Benasich AA (2010). Associations between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. Neuroimage, 49(3), 2791–2799. [DOI] [PubMed] [Google Scholar]
- National Scientific Council on the Developing Child. (2014). Excessive Stress Disrupts the Architecture of the Developing Brain. Working Paper 3. [Google Scholar]
- Pagliaccio D, Luby JL, Luking KR, Belden AC, & Barch DM (2014). Brain–behavior relationships in the experience and regulation of negative emotion in healthy children: Implications for risk for childhood depression. Development and psychopathology, 26(4pt2), 1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Schmithorst VJ, Banihashemi L, Taylor M, Samolyk A, Northrup JB, ... & Hipwell, A. E. (2021). Patterns of Infant Amygdala Connectivity Mediate the Impact of High Caregiver Affect on Reducing Infant Smiling: Discovery and Replication. Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, & Nichols TE (2011). Handbook of functional MRI data analysis. Cambridge University Press. [Google Scholar]
- Potapova NV (2019). Infant emotion regulation patterns and electroencephalography (EEG) asymmetry in response to still face procedure. Washington State University. [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, & Leerkes E (2014). Development and assessment of short and very short forms of the Infant Behavior Questionnaire–Revised. Journal of personality assessment, 96(4), 445–458. [DOI] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, & Menon V (2014). Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biological psychiatry, 75(11), 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Rifkin-Graboi A, Chen H, Chong YS, Kwek K, Gluckman PD, ... & Meaney MJ. (2013). Maternal anxiety and infants’ hippocampal development: timing matters. Translational psychiatry, 3(9), e306–e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement, 1(3), 385–401. [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, & Hammen CL (2010). Hippocampal changes associated with early-life adversity and vulnerability to depression. Biological psychiatry, 67(4), 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees S, Channon S, & Waters CS (2019). The impact of maternal prenatal and postnatal anxiety on children’s emotional problems: a systematic review. European child & adolescent psychiatry, 28(2), 257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ (2010). Amygdala activity, fear, and anxiety: modulation by stress. Biological psychiatry, 67(12), 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, Van den Bree M, Hay DF, & Thapar A (2010). The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychological medicine, 40(2), 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJ, Wallace NS, Laurent HK, & Mayes LC (2015). Emotion regulation in parenthood. Developmental Review, 36, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacrey LAR, Zwaigenbaum L, Brian JA, Smith IM, Armstrong V, Raza S, ... & Schmidt LA. (2021). Affect and gaze responses during an Emotion-Evoking Task in infants at an increased likelihood for autism spectrum disorder. Molecular autism, 12(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber EL, Lopez de Armentia M, & Power JMJPR (2003). The amygdaloid complex: anatomy and physiology. Physiological reviews, 83(3), 803–834. [DOI] [PubMed] [Google Scholar]
- Savage-McGlynn E, Redshaw M, Heron J, Stein A, Quigley MA, Evans J, ... & Gray R. (2015). Mechanisms of resilience in children of mothers who self-report with depressive symptoms in the first postnatal year. PLoS One, 10(11), e0142898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawiak SJ, Shiba Y, Oikonomidis L, Windle CP, Santangelo AM, Grydeland H, ... & Roberts AC. (2018). Trajectories and milestones of cortical and subcortical development of the marmoset brain from infancy to adulthood. Cerebral Cortex, 28(12), 4440–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper FY, Majdandžić M, van de Ven PM, Jansen L, Doreleijers TA, Schuengel C, & de Vries AL (2017). Temperament traits and psychopathology in young clinically referred children compared to a general population sample. Child Psychiatry & Human Development, 48(6), 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe NN, Wen DJ, Poh JS, Chong YS, Broekman BF, Chen H, ... & Qiu A. (2018). Perinatal maternal depressive symptoms alter amygdala functional connectivity in girls. Human brain mapping, 39(2), 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983). State-trait anxiety inventory for adults. [Google Scholar]
- Spry EA, Aarsman SR, Youssef GJ, Patton GC, Macdonald JA, Sanson A, ... & Olsson CA. (2020). Maternal and paternal depression and anxiety and offspring infant negative affectivity: A systematic review and meta-analysis. Developmental Review, 58, 100934. [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, ... & Pariante CM. (2014). Effects of perinatal mental disorders on the fetus and child. The Lancet, 384(9956), 1800–1819. [DOI] [PubMed] [Google Scholar]
- Sun X, Shi L, Luo Y, Yang W, Li H, Liang P, ... & Wang D. (2015). Histogram-based normalization technique on human brain magnetic resonance images from different acquisitions. Biomedical engineering online, 14(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JC, Letourneau N, Campbell TS, Tomfohr-Madsen L, & Giesbrecht GF (2017). Developmental origins of infant emotion regulation: Mediation by temperamental negativity and moderation by maternal sensitivity. Developmental Psychology, 53(4), 611. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, ... & Casey B. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental science, 13(1), 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, & Sheridan MA (2010). A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in human neuroscience, 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas EA, Boes AD, Wemmie JA, Tranel D, & Nopoulos P (2010). Amygdala volume correlates positively with fearfulness in normal healthy girls. Social cognitive and affective neuroscience, 5(4), 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MK, Tronick EZ, Cohn JF, & Olson KL (1999). Gender differences in emotional expressivity and self-regulation during early infancy. Developmental psychology, 35(1), 175. [DOI] [PubMed] [Google Scholar]
- Wen DJ, Poh JS, Ni SN, Chong YS, Chen H, Kwek K, ... & Qiu, A. (2017). Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Translational psychiatry, 7(4), e1103–e1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer-Gembeck MJ, & Skinner EA (2016). The development of coping: Implications for psychopathology and resilience. Developmental psychopathology, 1–61. [Google Scholar]
- Zöllei L, Iglesias JE, Ou Y, Grant PE, & Fischl B (2020). Infant FreeSurfer: An automated segmentation and surface extraction pipeline for T1-weighted neuroimaging data of infants 0–2 years. Neuroimage, 218, 116946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou R, Tiemeier H, van der Ende J, Verhulst FC, Muetzel RL, White T, ... & El Marroun H. (2019). Exposure to maternal depressive symptoms in fetal life or childhood and offspring brain development: a population-based imaging study. American Journal of Psychiatry, 176(9), 702710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


