Abstract
Background
The nitrogen balance estimates a protein net difference. However, since it has a number of limitations, it is important to consider the trajectory of the nitrogen balance in the clinical course of critically ill patients.
Objectives
We herein exploratively classified the nitrogen balance trajectory using a machine learning method.
Method
This is a post hoc analysis of a single-center prospective study for the patients admitted to our Emergency and Critical Center ICU. The nitrogen balance was evaluated with 24-h urine collection from ICU days 1–10 with 9 points. K-means clustering was performed to classify the nitrogen balance trajectory. We also evaluated factors associated with uncovered clusters.
Results
Seventy-six eligible patients were included in the present study. After clustering, the nitrogen balance trajectory was classified into 4 classes. Class 1 was trajected as a negative balance over 10 days (24 patients). Class 2 had a positive conversion on day 3 or 4 (8 patients). Class 3 had a positive conversion on day 8 or 9 (28 patients). Class 4 initially had a positive balance and then converted to a negative balance (16 patients). Sepsis complication and steroid use were associated with negative nitrogen balance trajectory. Class 2 was associated with lower length of hospital stay and femoral muscle volume loss, however, frequently had frailty and sarcopenia on admission. Active nutrition therapy intention was not correlated with positive trajectory.
Conclusions
The nitrogen balance trajectory in critically ill patients may be classified into 4 classes for clinical practice. Among patients emergently admitted to the ICU, the positive conversion of the nitrogen balance might be delayed over 10 days.
Keywords: Nitrogen balance, Protein, Critical care, Machine learning, K-means
Introduction
The nitrogen balance (NB) is a measure of nitrogen intake with nutrition provision and nitrogen output mainly with urine and estimates the net difference between protein synthesis and breakdown [1, 2]. Although the usefulness of NB in the critical care field has not yet been established in detail [3], NB is now being reevaluated as a monitoring tool for nutrition therapy that may be measured every day in critical care [4]. Singer et al. [5] proposed a strategy in which optimal energy delivery is decided using indirect colorimetry and protein delivery with NB 1 day before. Furthermore, NB and other barometers equivalent to NB have been shown to correlate with and reflect muscle volume changes in critically ill patients [6, 7].
However, since NB has a number of limitations, it cannot be introduced into clinical practice. While nitrogen intake is easily and stably calculated in critically ill patients whose nutrition is completely managed by medical staff, nitrogen output is affected by various factors and, thus, cannot be accurately evaluated [8–10]. Moreover, due to large daily variations, we need to measure NB daily for average or cumulative NB evaluations [11]. However, the avoidance of 24-h urine collection is recommended because it may increase the risk of infection by resistant bacteria [12].
Under these limitations in actual NB evaluations, predictions of the trajectory of NB in intensive care unit (ICU) clinical courses may provide very important information for critical care nutrition. While some patients show the positive conversion of NB as an anabolic phase, NB may also be negative for a long time period. The patterns of the NB trajectory and their frequency have not yet been clarified in critical care. Therefore, a more detailed understanding of these patterns will contribute to the appropriate adoption of treatments, including nutrition therapy, and early mobilization.
We herein performed the exploratory clustering of the NB trajectory in critically ill patients with machine learning method. The aim of this study was to clarify how the NB shift with time course in critical care, how frequently NB positive conversion would be occurred in the first several days in the acute phase, and whether active nutrition and mobilization would contribute to it, especially in the emergently admitted patients. In order to generate these relevant hypotheses, this study was investigated with the previous study dataset, in which we compared the active nutrition and mobilization intervention with standard care in the Emergency and Critical Care Center, as a post hoc analysis. We analyzed the frequency, characteristics, and risk factors for each NB trajectory pattern.
Materials and Methods
Study Design and Setting
The present study is a post hoc analysis of the Intensive Goal-directed REhabilitation with Electrical muscle stimulation and Nutrition (IGREEN) study [13]. To address potential sources of bias, we declare that Kensuke Nakamura planned this study, physiotherapists evaluated physical outcomes, Hidehiko Nakano collected data, and Kentaro Ogura conducted the clustering and statistical analysis.
IGREEN was a single-center, prospective, and historical control study conducted at Hitachi General Hospital, a tertiary hospital which had the medical and surgical ICU for patients from the emergency department, for patients exhibiting in-hospital acute deterioration, and severe patients following surgery. This ICU includes an 8-bed medical and surgical ICU with a 2:1 patient-nurse ratio, and a 10-bed emergency ward with a 4:1 patient-nurse ratio. Ten intensivists were dedicated to this ICU. Patients admitted to the former 8-bed ICU between October 2019 and December 2020 were included. In the former period between October 2019 and February 2020, patients were treated with standard care as the control group. In the latter period between September 2020 and December 2020, patients received active rehabilitation and nutrition therapy with high protein provision, named IGREEN bundles, as the intervention group. Exclusion criteria were as follows: younger than 20 years old, possible pregnancy, anuria, a lower extremity event, such as infection, injury, or amputation, the use of extracorporeal membrane oxygenation, expected to be discharged from the ICU within 2 days, admission to the ICU for a second time during the same hospital stay, and the designation of “do not attempt resuscitation.” The IGREEN study was approved by our Ethics Committee (2020-38) and was registered at the University Hospital Medical Information Network-clinical trials registry (UMIN000040290). Details on rehabilitation and nutrition therapy in standard care and IGREEN care were described in a previous study [13].
Study Participants
Patients in the IGREEN study were included in the present study, while patients in whom renal replacement therapy was performed for 10 days and in whom deficits in NB of three points or more occurred over 10 days were excluded. Exclusion criteria in the IGREEN study were as follows: younger than 20 years old, possible pregnancy, anuria, a lower extremity event, such as infection, injury, or amputation, the use of extracorporeal membrane oxygenation, expected to be discharged from the ICU within 2 days, admission to the ICU for a second time during the same hospital stay, and the designation of “do not attempt resuscitation.”
Measurements and Outcomes
NB was prospectively evaluated with 24-h urine collection from ICU days 1–10 with 9 points. NB was calculated as follows: NB = protein delivery (g/day)/6.25 – total urine nitrogen (g/day) × 1.25 [14]. Total calorie and protein deliveries were calculated by a hospital nutritionist. The other outcomes were evaluated as follows: femoral muscle volume (mL) evaluated with plain computed tomography on days 1 and 10, the ICU mobility scale (IMS) each day, Medical Research Council (MRC) scores and functional status scores for the ICU (FSS-ICU) at ICU discharge, the Barthel Index and grip strength at hospital discharge, and N-titin/Cre on days 1, 3, 5, and 7. N-titin was measured using an enzyme-linked immunosorbent assay kit (#27900 Human Titin N-Fragment Assay Kit; Immuno-Biological Laboratories, Fujioka, Japan) [7], and we used the value of the spot urine N-titin level divided by the spot urine creatinine concentration and by 10 (N-titin/Cre) (pmol/mgCre). Femoral muscle volume was evaluated by a radiologist who was blinded for IGREEN/usual care, and IMS, MRC scores FSS-ICU, the Barthel Index, and grip strength were prospectively evaluated by physiotherapists who were also blinded for IGREEN/usual care.
Age, sex, body mass index, sequential organ failure assessment (SOFA) scores, acute physiology and chronic health evaluation (APACHE) II scores, the Charlson comorbidity index, the main diagnosis, sepsis complication, and laboratory data, including C-reactive protein (CRP), albumin, and the lymphocyte count over 10 days, were extracted from electronic medical record and confirmed by a physician. Sepsis was determined by Sepsis-3 criteria [15] including suspected cases. eGFR was calculated as 194 × (Cre−1.094 × Age−0.287) in males or as 194 × (Cre−1.094 × Age−0.287) × 0.739 in females.
The proportion of survival discharge, the lengths of ICU and hospital stays, and the use of adjunctive therapy, such as mechanical ventilation, renal replacement therapy, steroids, and continuous neuromuscular blocking agents, were also evaluated. Steroid doses were recorded as the total amount of a hydrocortisone equivalent within 10 days.
Clustering of the NB Trajectory
To elucidate the patterns of the NB trajectory, we performed a clustering analysis. Although variations in NB were significant for each patient, a decrease in these variations improves the accuracy of establishing whether similar patterns are in the same group. Since variations in NB are not clinically significant, we normalized the NB trajectory. The normalization method is based on dividing the 10-day NB value by the absolute maximum value in each patient. In addition, we performed linear interpolations for missing nitrogen values. The trajectory of NB on each day in each patient and normalized trajectory are shown in online supplementary Figure 1 (for all online suppl. material, see https://doi.org/10.1159/000532126). Using normalized data, we performed clustering with the K-means method.
K-means clustering was performed on normalized data. Because the sample sizes of 20 per each cluster [16] were aimed in the 76 eligible patients in this study, they were classified into four classes, which were confirmed to be a reasonable number of classes by the Elbow method (online suppl. Fig. 2). In each class, the mean value of NB on each day and the 95% confidential interval was calculated and described as the trajectory.
Statistical Analysis
Results are expressed as the mean ± standard deviation or a median (interquartile range). Differences were assessed by the χ2 test and a one-way analysis of variance for normally distributed parameters. The Kruskal-Wallis test was applied for non-normally distributed data. To identify which factors correlate with the classification by K-means clustering, we created a heatmaps to show data distributions. The explanatory variables were age, sex, SOFA, APACHE, CCI, BMI, sepsis complication, femoral muscle volume on admission, mechanical ventilation, total steroids, eGFR, CRP, albumin, lymphocyte count and N-titin/Cre on admission, and IGREEN care intention. Since all of the categorical variables; male sex, sepsis complication, mechanical ventilation, and IGREEN care intention were binary variables, we used them as 1 and the others as −1. Other than the categorical variables were normalized to uniform scales. The heatmap to show data of each patient in each class, that of average data in each class, and that of the coefficients of the explanatory variables for each class in multiclass classification logistic regression model were made.
All statistical analyses other than the multivariable analysis were performed using JMP 14 software (SAS Institute Inc., Cary, NC, USA). The multivariable analysis was conducted using Python 3.8.3. p values <0.05 were considered to be significant. Heatmaps were made with seaborn (ver. 0.12.1) which was one of Python packages.
Results
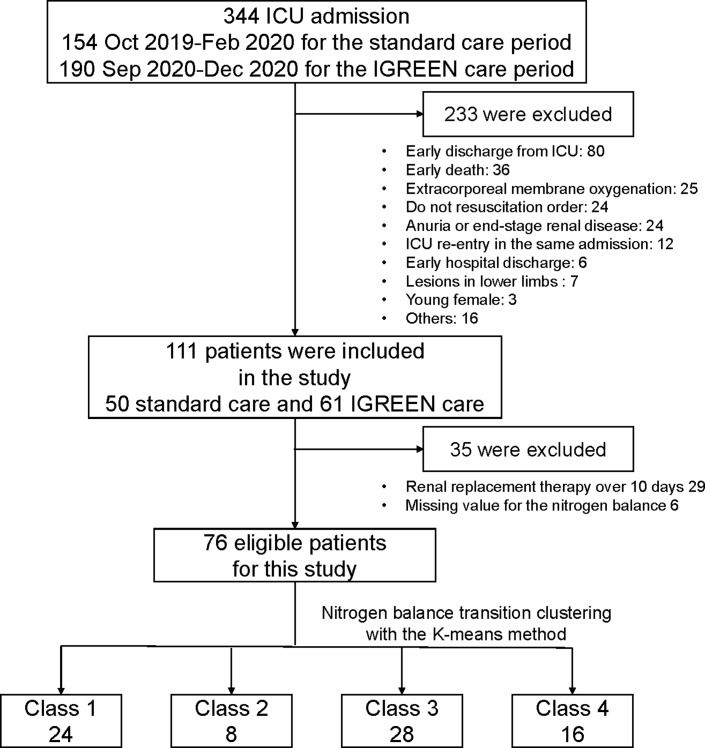
The study outline is shown in Figure 1. A total of 344 patients were admitted to our Emergency and Critical Care Center ICU. After the exclusion of 233 patients, 50 for standard care and 61 for IGREEN care were included and evaluated with each care intervention. Twenty-nine patients received renal replacement therapy, and 6 showed a deficit in NB of three points or more over 10 days. After the exclusion of these patients, 76 patients were eligible for inclusion in the present study.
Fig. 1.
Study outline.
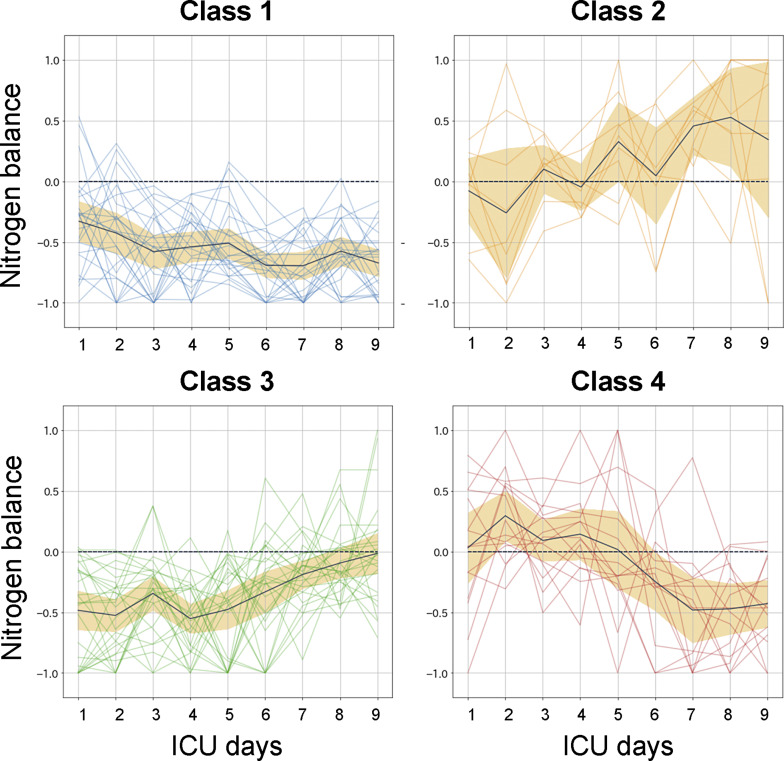
Four classes of the NB trajectory were finally classified (Fig. 2). The black line represents the trajectory of the mean value for NB, and the orange area shows the 95% confidential interval. Class 1 showed a negative balance over 10 days (24 patients). Class 2 had a positive conversion on day 3 or 4 (8 patients). Class 3 exhibited a positive conversion on day 8 or 9 (28 patients). Class 4 initially showed a positive balance and then converted to a negative balance (16 patients).
Fig. 2.
Clustering of the nitrogen balance trajectory. After normalization, the trajectory of the nitrogen balance was classified into 4 classes using clustering with the K-means method. ICU, intensive care unit.
Basic characteristics in each class are shown in Table 1. No significant differences were observed in age, sex, disease severity, or main diagnosis. Sepsis complication was the most prevalent in class 3; 18 (64.3%). Intention of the active nutrition therapy protocol; IGREEN care was frequent in class 4, but not in class 2. Laboratory findings on admission were not different significantly.
Table 1.
Basic characteristics
| Class 1 | Class 2 | Class 3 | Class 4 | p value | |
|---|---|---|---|---|---|
| N | 24 | 8 | 28 | 16 | |
| Age, years | 66.6±16.0 | 70.4±26.3 | 73.1±12.8 | 73.8±12.7 | 0.41 |
| Male sex, n (%) | 15 (62.5) | 5 (62.5) | 21 (75.0) | 11 (68.8) | 0.78 |
| Body mass index, kg/m2 | 22.5±4.9 | 20.6±3,6 | 22.1±4.9 | 23.1±3.8 | 0.67 |
| Charlson comorbidity index | 1 (0, 2) | 2.5 (0.25, 3) | 1 (1, 2) | 2 (1, 2) | 0.063 |
| SOFA on admission | 6.5 (4, 9.5) | 7.5 (4.5, 10) | 6 (4, 8) | 7 (5.25, 9.5) | 0.81 |
| APACHE II on admission | 16 (12, 22.75) | 14.5 (12.25, 23) | 15.5 (9.5, 18.75) | 15 (13, 17) | 0.84 |
| Main diagnosis, n (%) | 0.40 | ||||
| Cardiopulmonary arrest | 2 (8.3) | 1 (12.5) | 4 (14.3) | 0 (0) | |
| Cardiovascular disease | 1 (4.2) | 0 (0) | 0 (0) | 0 (0) | |
| Heart failure | 4 (16.7) | 1 (12.5) | 0 (0) | 0 (0) | |
| Endocrine and metabolic disorders | 1 (4.2) | 0 (0) | 0 (0) | 1 (6.3) | |
| Gastrointestinal bleeding | 0 (0) | 0 (0) | 1 (3.6) | 0 (0) | |
| Post-surgery | 2 (8.3) | 0 (0) | 2 (7.1) | 2 (12.5) | |
| Respiratory failure | 2 (8.3) | 1 (12.5) | 2 (7.1) | 2 (12.5) | |
| Infection | 5 (20.8) | 3 (37.5) | 13 (46.4) | 11 (68.8) | |
| Stroke | 3 (12.5) | 1 (12.5) | 1 (3.6) | 0 (0) | |
| Trauma | 4 (16.7) | 1 (12.5) | 5 (17.9) | 0 (0) | |
| Sepsis complication, n (%) | 10 (41.7) | 3 (37.5) | 18 (64.3) | 6 (37.5) | 0.22 |
| Adjunctive treatments | |||||
| Mechanical ventilation, n (%) | 21 (87.5) | 5 (62.5) | 22 (78.6) | 9 (56.3) | 0.12 |
| Steroid dose, mg | 0 (0, 575) | 0 (0, 0) | 175 (0, 837.5) | 0 (0, 512.5) | 0.24 |
| Neuromuscular blocking agent, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 0.45 |
| IGREEN care, n (%) | 14 (58.3) | 3 (37.5) | 16 (57.1) | 13 (81.3) | 0.16 |
| Laboratory data on day 1 | |||||
| White blood cell count, /μL | 119.5 (97.25, 162.25) | 78.5 (54.5, 139.25) | 99 (64.5, 138.25) | 97 (86, 134) | 0.19 |
| Lymphocyte count, /μL | 840 (518.5, 989.25) | 691.5 (342.75, 1,135) | 717 (499.75, 1,757.5) | 860 (588, 1,072) | 0.90 |
| Albumin, g/dL | 3.2 (2.9, 3.4) | 2.6 (2.3, 3.2) | 3.05 (2.55, 3.475) | 2.8 (2.6, 3) | 0.15 |
| Creatinine, mg/dL | 0.89 (0.67, 1.18) | 0.85 (0.56, 1.10) | 1.01 (0.77, 1.43) | 1.05 (0.99, 1.44) | 0.25 |
| eGFR, mL/min | 62.3 (44.7, 80.5) | 73.1 (46.3, 80.5) | 56.6 (36.6, 74.5) | 44.3 (38.3, 58.7) | 0.21 |
| CRP, mg/dL | 3.5 (0.97, 5.99) | 3.08 (1.355, 13.1875) | 7.775 (2.5475, 12.175) | 5.89 (4.08, 14.75) | 0.11 |
SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation; IGREEN, Intensive Goal-directed REhabilitation with Electrical muscle stimulation and Nutrition; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein.
The outcomes in each class are shown in Table 2. Mortality, the length of the ICU stay, the duration of mechanical ventilation, and N-titin/Cre as a muscle injury biomarker were similar among classes. The length of the hospital stay was significantly longer for classes 4, 1, 3, and 2, in that order (p = 0.037). Regarding physical outcomes, class 2 had the lowest femoral muscle volume loss rate; however, they had the lowest muscle volume on day 1 and BMI. Other physical outcomes, such as the Barthel Index, MRC score, FSS-ICU, and grip strength was the lowest in class 2.
Table 2.
Outcomes
| Class 1 | Class 2 | Class 3 | Class 4 | p value | |
|---|---|---|---|---|---|
| n | 24 | 8 | 28 | 16 | |
| In-hospital mortality, n (%) | 5 (20.8) | 3 (37.5) | 4 (14.3) | 2 (12.5) | 0.49 |
| Length of hospital stay, days | 26.5 (18.25, 50.25) | 12 (11, 17.5) | 26 (15.5, 42) | 39 (15, 60) | 0.037 |
| Length of ICU stay, days | 5.5 (4, 9.75) | 5 (3.25, 7.25) | 6.5 (4.25, 10.75) | 6.5 (4, 9) | 0.52 |
| Duration of mechanical ventilation, days | 3 (1, 6) | 3.5 (0.25, 4.75) | 3 (1, 4.75) | 1.5 (0, 4.75) | 0.72 |
| Average nitrogen intake days 1–2, g/day | 5.4 (2.3, 8.9) | 6.3 (1.8, 8.8) | 4.5 (3.0, 9.5) | 8.4 (5.9, 10.6) | 0.097 |
| Average nitrogen intake days 3–6, g/day | 8.9 (5.6, 14.1) | 11.5 (6.7, 15.4) | 11.5 (5.6, 13.7) | 15.9 (14.1, 18.5) | 0.0019 |
| Average nitrogen intake days 7–10, g/day | 6.8 (5.3, 12.2) | 11.1 (5.7, 16.2) | 12.3 (7.4, 15.1) | 12.4 (8.9, 15.6) | 0.043 |
| Average nitrogen output days 1–2, g/day | 11.4 (8.4, 14.9) | 7.7 (5.2, 8.4) | 11.2 (7.7, 15.3) | 9.3 (7.1, 11.6) | 0.033 |
| Average nitrogen output days 3–6, g/day | 12.9 (8.7, 19.2) | 8.4 (5.4, 12.1) | 13.2 (9.0, 16.8) | 11.6 (9.1, 15.2) | 0.079 |
| Average nitrogen output days 7–10, g/day | 16.2 (10.8, 19.3) | 7.0 (3.5, 8.3) | 11.1 (8.0, 14.4) | 14.0 (11.6, 18.0) | 0.0010 |
| Mean N-titin/Cre, pmol/mgCre | 60.8 (34.3, 191.4) | 94.6 (13.2, 257.3) | 87.6 (26.2, 161.7) | 54.5 (23.3, 86.8) | 0.73 |
| Day 1 N-titin/Cre, pmol/mgCre | 86.7 (30.3, 290.8) | 29.8 (12.2, 135.9) | 77.2 (33.8, 206.6) | 42.9 (27.2, 160.2) | 0.79 |
| Day 3 N-titin/Cre, pmol/mgCre | 32.4 (15.7, 141.8) | 101.3 (21.0, 357.2) | 85.9 (16.7, 177.2) | 61.8 (24.1, 108.9) | 0.74 |
| Day 5 N-titin/Cre, pmol/mgCre | 34.3 (21.8, 111.7) | 93.1 (23.8, 171.8) | 69.5 (17.0, 138.9) | 35.8 (14.3, 68.8) | 0.37 |
| Day 7 N-titin/Cre, pmol/mgCre | 50.3 (20.3, 163.6) | 49.8 (12.6, 166.2) | 48.1 (21.2, 103.0) | 28.8 (13.7, 65.4) | 0.49 |
| Physical outcomes | |||||
| Femoral muscle volume | |||||
| Day 1, mL | 3,945 (3,041, 4,935) | 2,850 (1,332.5, 3,665) | 3,280 (2,422.5, 4,132.5) | 3,445 (2,087.5, 4,612.5) | 0.22 |
| Day 10, mL | 3,290 (2,310, 4,337.5) | 2,365 (1,297.5, 3,402.5) | 2,845 (2,022.5, 3,617.5) | 2,915 (1,978, 4,112.5) | 0.39 |
| Loss from days 1 to 10, % | 14.5 (9.87, 17.2) | 6.1 (2.68, 12.6) | 12.3 (8.84, 18.4) | 10.0 (6.35, 14.7) | 0.050 |
| Barthel Index before admission | 100 (100, 100) | 52.5 (5, 100) | 100 (100, 100) | 100 (61.25, 100) | 0.11 |
| MRC score at ICU discharge | 46 (3, 56) | 44 (21, 58.75) | 55 (28.75, 59) | 56 (44, 60) | 0.081 |
| FSS-ICU at ICU discharge | 8 (1, 17) | 5.5 (1.5, 17.5) | 9 (3, 20.5) | 15 (6, 21) | 0.38 |
| Grip strength at hospital discharge, kg | 12.6 (0, 21.8) | 4 (0, 26.8) | 16.75 (9.2, 26.5) | 16.4 (9.2, 26.5) | 0.34 |
| Barthel Index at hospital discharge | 50 (2.5, 100) | 25 (0, 75) | 70 (2.5, 100) | 45 (5, 95) | 0.66 |
MRC, Medical Research Council scores; FSS-ICU, functional status scores for the intensive care unit.
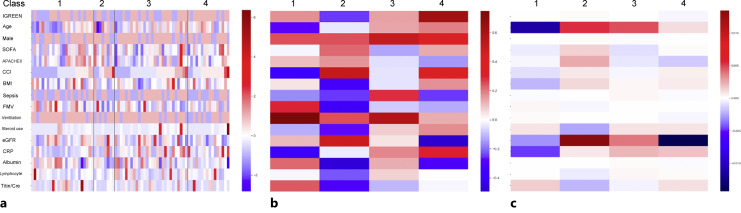
The heatmaps of data in each class and that of the coefficients for each class in multiclass classification logistic regression model are shown in Figure 3. Sepsis complication and steroid use tended to be associated with negative NB trajectory (Fig. 3a, b). The other factors could not be found as risks for positive or negative trajectory. In the multivariable logistic regression analysis, these tendencies were also confirmed (Fig. 3c). The IGREEN care intention was not correlated with positive trajectory at all.
Fig. 3.
Heatmaps of patients’ data in each class and coefficients of the explanatory variables for each class in multivariable logistic regression analysis. a The data of each patient in each class. b The average data in each class. c The coefficients of each variable for each class in multiclass classification logistic regression model. Red boxes show positive association with that classification, and blue ones show negative association. BMI, body mass index; CCI, Charlson comorbidity index, eGFR, estimated glomerular filtration rate; IGREEN, Intensive Goal-directed REhabilitation with Electrical muscle stimulation and Nutrition; SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation; FMV, femoral muscle volume; MV, mechanical ventilation; CRP, C-reactive protein.
Discussion
The NB trajectory in critically ill patients was clustered into the following 4 classes: class 1, a trajected negative balance; class 2, conversion from a negative to positive balance on day 3 or 4; class 3, conversion from a negative to positive balance on day 8 or 9; and class 4, conversion from a positive to negative balance. Class 3 was the most frequent among critically ill patients in the Emergency and Critical Care Center, followed by class 1. Sepsis complication was more frequent in classes 3 and 1. Class 2 was associated with lower length of hospital stay and femoral muscle volume loss, however, frequently had frailty and sarcopenia on admission. The active nutrition therapy intention was not correlated with positive NB trajectory.
The daily NB shift in critically ill patients has been discussed in a number of studies. However, difficulties have been associated with the classification of patients into specific classes because of large daily variations in NB measurements. One of the greatest contributions of this study was considered that we could present the NB trajectory as discriminative 4 classes with compiling the daily variation using K-means method. In consideration of the transition of catabolism and metabolism in critically ill patients, these 4 classifications appear to be very reasonable.
Class 2, in which NB was converted from a negative to positive balance, appears to be the most favorable recovery process. Actually, this study showed the lowest length of hospital stay in class 2. The NB trajectory was considered to be this pattern when the main stress was from surgical or traumatic injury and no complications occurred in the clinical course [17]. In these patients, active full feeding is recommended from day 3 or 4, which is described as the transition into the late period of the acute phase in the guidelines of the European Society for Clinical Nutrition and Metabolism [18]. Unfortunately, the number of class 2 patients was the smallest in the present study, which may have been because subjects were ICU patients in the Emergency and Critical Care Center. The active nutrition therapy intention was not associated with class 2. The positive conversion of NB may be difficult with an intervention of nutrition therapy or rehabilitation alone.
In the present study, there were many patients in whom NB was negative over 10 days or was converted to a positive balance on day 8 or 9, and these patients were classified as class 1 or 3. We need to consider a conversion strategy to full feeding at least until ICU day 7–10; however, overfeeding may occur because endogenous energy delivery may be continued to this period in these classes [19]. Since classes 1 and 3 were the most frequent in this study population of the Emergency and Critical Care Center, we should take care in nutrition progression, occasionally with continuing permissive underfeeding until ICU day 7–10, in the patients with emergency admission.
Unfortunately, it was difficult to predict completely which class the NB trajectory would be based only on basic characteristics on admission. Especially, the active nutrition therapy intention was not associated with class 2. NB trajectory would be strongly determined by the other characteristics or treatment courses than the nutrition therapy. Although the positive conversion of NB was difficult with the nutrition or rehabilitation intervention, high protein provision might be needed both in negative NB phase for protein sparing and in positive NB phase for recovery with protein synthesis, in the view of protein balance.
Among the characteristics, sepsis complication was the most frequent in class 3, followed by class 1, in which classes the negative NB prolonged. Sepsis is one of the most hyper-inflammatory and catabolic conditions [20]. We should consider such a NB trajectory in septic patients, and take care in the gradual increase of nutrition therapy. Furthermore, steroid use was identified as a risk factor for a negative NB trajectory. Steroid use had been discussed whether it was one of the risks for ICU acquired weakness or not [21]. Our results supported that the positive conversion of NB might be achieved by avoiding steroid use, although it is occasionally necessary for resuscitation.
Muscle volume was initially the lowest in class 2. This was reasonable because muscle is a source of amino acids under emergency conditions [21]. Actually, class 2 was associated with lower BMI, higher Charlson comorbidity index, and the worst physical outcomes, suggesting the frailty and sarcopenia. However, it should be originally resolved that patients with a large muscle mass may lose more muscle volume in critical care. The combination of active nutrition therapy and early mobilization may be one of the applicable countermeasures [13]. Anabolic enhancements may be another strategy, such as β-hydroxy-β-methylbutyrate [22] and oxandrolone [23].
There are the other several limitations that need to be addressed. Since this was a single-center observational study, selection bias cannot be excluded. Most of the patients examined were emergency admissions, while only a few were scheduled for surgery. Furthermore, the number of patients included in the present study was too small to conduct full machine learning with the preparation of a test set. In these points, this study was within an exploration, and generalizability was limited. Found knowledge and implications might be applied only in other emergency centers in the countries where a lot of elderly patients were admitted to these hospitals. Another limitation is that the NB trajectory and measurements after day 10 were not assessed. We did not assess the fecal nitrogen excretion in this study. Finally, there were already established factors which influenced the NB, such as types of diagnoses and steroid use. The population of this study was heterogenous. We should repeat similar studies with more well-designed/controlled variables.
Conclusions
In this explorative analysis, the NB trajectory in critically ill patients could be classified into 4 classes. Most of patients were the classes in which negative NB prolonged until ICU day 7–10. We might have to take care in nutrition progression, occasionally with continuing permissive underfeeding until this period, in the patients emergently admitted to the ICU including sepsis. Further investigation should be performed confirm the findings of this study.
Acknowledgments
The authors thank all the nursing specialists and physical therapists for their support for the study during their work.
Statement of Ethics
This study protocol was reviewed and approved by Hitachi General Hospital Ethics Review Board, approval number 2020-38. This study was registered at the University Hospital Medical Information Network-clinical trials registry (UMIN000040290). Written informed consent was obtained from each study participant.
Conflict of Interest Statement
Kentaro Ogura and Tadahiro Goto were employed by TXP Medical Co., Ltd.
Funding Sources
This study was funded by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research 21H03030.
Author Contributions
Kensuke Nakamura: conception of the study, interpretation, and drafting of the manuscript. Hidehiko Nakano, Daisuke Ikechi, Masaki Mochizuki, Yuji Takahashi: conduction of the study. Kentaro Ogura: data analysis and contribution to the manuscript. Tadahiro Goto: revision of the manuscript and supervision of the study. All authors have read and approved the manuscript.
Funding Statement
This study was funded by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research 21H03030.
Data Availability Statement
Data are not publicly available due to ethical reasons. The datasets generated and/or analyzed during the present study are available from the corresponding author upon reasonable request. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. Millward, DJ. Identifying recommended dietary allowances for protein and amino acids: a critique of the 2007 WHO/FAO/UNU report. Br J Nutr. 2012;108(Suppl 2):S3–21. [DOI] [PubMed] [Google Scholar]
- 2. Hoffer LJ. Human protein and amino acid requirements. JPEN J Parenter Enteral Nutr. 2016;40(4):460–74. [DOI] [PubMed] [Google Scholar]
- 3. Weijs PJ, Wischmeyer PE. Optimizing energy and protein balance in the ICU. Curr Opin Clin Nutr Metab Care. 2013;16(2):194–201. [DOI] [PubMed] [Google Scholar]
- 4. Stoppe C, Wendt S, Mehta NM, Compher C, Preiser JC, Heyland DK, et al. Biomarkers in critical care nutrition. Crit Care. 2020;24(1):499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singer P, Bendavid I, BenArie I, Stadlander L, Kagan I. Feasibility of achieving different protein targets using a hypocaloric high-protein enteral formula in critically ill patients. Crit Care. 2021;25(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haines RW, Zolfaghari P, Wan Y, Pearse RM, Puthucheary Z, Prowle JR. Elevated urea-to-creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensive Care Med. 2019;45(12):1718–31. [DOI] [PubMed] [Google Scholar]
- 7. Nakano H, Hashimoto H, Mochizuki M, Naraba H, Takahashi Y, Sonoo T, et al. Urine titin N-fragment as a biomarker of muscle injury for critical illness myopathy. Am J Respir Crit Care Med. 2021;203(4):515–8. [DOI] [PubMed] [Google Scholar]
- 8. Calloway DH, Odell AC, Margen S. Sweat and miscellaneous nitrogen losses in human balance studies. J Nutr. 1971;101(6):775–86. [DOI] [PubMed] [Google Scholar]
- 9. Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr. 2003;77(1):109–27. [DOI] [PubMed] [Google Scholar]
- 10. Cheatham ML, Safcsak K, Brzezinski SJ, Lube MW. Nitrogen balance, protein loss, and the open abdomen. Crit Care Med. 2007;35(1):127–31. [DOI] [PubMed] [Google Scholar]
- 11. Allingstrup MJ, Esmailzadeh N, Wilkens Knudsen A, Espersen K, Hartvig Jensen T, Wiis J, et al. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr. 2012;31(4):462–8. [DOI] [PubMed] [Google Scholar]
- 12. Sekiguchi J, Teruya K, Horii K, Kuroda E, Konosaki H, Mizuguchi Y, et al. Molecular epidemiology of outbreaks and containment of drug-resistant Pseudomonas aeruginosa in a Tokyo hospital. J Infect Chemother. 2007;13(6):418–22. [DOI] [PubMed] [Google Scholar]
- 13. Nakano H, Naraba H, Hashimoto H, Mochizuki M, Takahashi Y, Sonoo T, et al. Novel protocol combining physical and nutrition therapies, Intensive Goal-directed REhabilitation with Electrical muscle stimulation and Nutrition (IGREEN) care bundle. Crit Care. 2021;25(1):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Konstantinides FN, Konstantinides NN, Li JC, Myaya ME, Cerra FB. Urinary urea nitrogen: too insensitive for calculating nitrogen balance studies in surgical clinical nutrition. JPEN J Parenter Enteral Nutr. 1991;15(2):189–93. [DOI] [PubMed] [Google Scholar]
- 15. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalmaijer ES, Nord CL, Astle DE. Statistical power for cluster analysis. BMC Bioinformatics. 2022;23(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sobotka L, Soeters PB. Basics in clinical nutrition: metabolic response to injury and sepsis. e-SPEN, Eur e-J Clin Nutr Metab. 2009;4:3. [Google Scholar]
- 18. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. [DOI] [PubMed] [Google Scholar]
- 19. Berger MM, Pichard C. Feeding should be individualized in the critically ill patients. Curr Opin Crit Care. 2019;25(4):307–13. [DOI] [PubMed] [Google Scholar]
- 20. Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2010;1(2):147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakamura K, Kihata A, Naraba H, Kanda N, Takahashi Y, Sonoo T, et al. β-Hydroxy-β-methylbutyrate, arginine, and glutamine complex on muscle volume loss in critically ill patients: a randomized control trial. JPEN J Parenter Enteral Nutr. 2020 Feb;44(2):205–12. [DOI] [PubMed] [Google Scholar]
- 23. Demling RH, DeSanti L. Oxandrolone induced lean mass gain during recovery from severe burns is maintained after discontinuation of the anabolic steroid. Burns. 2003;29(8):793–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not publicly available due to ethical reasons. The datasets generated and/or analyzed during the present study are available from the corresponding author upon reasonable request. Further inquiries can be directed to the corresponding author.