Abstract
Introduction
Although frailty is commonly considered as a syndrome of old individuals, recent studies show that it can affect younger adults, too. Whether and how frailty differs in younger adults compared to old is however unknown. To this end, we analyzed the prevalence, characteristics, and risk factors of early-life (aged <65) and late-life (aged ≥65) frailty.
Methods
We analyzed individuals in the UK Biobank (N = 405,123) and Swedish Screening Across the Lifespan Twin (SALT; N = 43,641) study. Frailty index (FI) scores ≥0.21 were used to demarcate frailty. Characteristics of early-life versus late-life frailty were analyzed by collating the FI items (deficits) into domains and comparing the domain scores between younger and older frail individuals. Logistic regression was used to assess the risk factors of frailty.
Results
The pooled prevalence rates of frailty were 10.3% (95% confidence interval [CI]: 2.7–32.7), 14.4% (95% CI: 4.5–37.2), 19.2% (95% CI: 2.5–68.5) in individuals aged ≤55, 55–64, 65–74, respectively. Younger frail adults (aged <65) had higher scores in immunological, mental wellbeing, and pain-related domains, whereas older frail adults (aged ≥65) had higher scores in cardiometabolic, cancer, musculoskeletal, and sensory-related domains. Higher age, female sex, smoking, lower alcohol consumption, lower education, obesity, overweight, low income, and maternal smoking were similarly associated with the risk of early-life and late-life frailty.
Conclusion
Frailty is prevalent also in younger age groups (aged <65) but differs in some of its characteristics from the old. The risk factors of frailty are nevertheless largely similar for early-life and late-life frailty.
Keywords: Frailty, Younger adults, Characteristics, Frailty domain, Risk factors
Introduction
Frailty has become a public health priority with the rapidly growing aging populations [1]. Individuals with higher frailty scores are at a higher risk of adverse outcomes, such as falls, hospitalization, and mortality [2]. While there is currently no international standardization on how to measure frailty, the two dominant models are the Fried frailty phenotype (FP) and Rockwood frailty index (FI) [3, 4]. The FP measures frailty as a physical syndrome and categorizes individuals as robust, pre-frail, and frail based on reduced grip strength, slow gait speed, weight loss, low physical activity, and exhaustion. The FI defines frailty in broader terms to achieve a multidimensional definition [3]. It is a continuous score, calculated by counting the number of health deficits across medical, physiological, functional, and social domains, thereby providing good sensitivity and feasible assessment in younger adults as well [4].
Frailty has almost exclusively been analyzed in older populations. Younger individuals are also often excluded from the studies based on the assumption that early-life frailty is very different from late-life frailty. However, there is currently no evidence to support that assumption. Frailty is, however, known to be present among younger individuals. In community-dwelling adults aged 50–59, 60–69, and 70–79, the prevalence rates of frailty were 6%, 12%, and 18%, measured using the FP, and 23%, 23%, and 25%, measured using the FI, respectively [5]. A recent study found a trend that frailty is increasing in prevalence and severity in US men aged >20 and women aged >35, highlighting the need to more rigorously address the implications of frailty in younger adults [6]. To date, few studies have shown that frailty has an impact on adverse outcomes in younger individuals [7–9]. Our previous studies in Swedish and UK samples have shown that higher frailty scores confer a relatively greater risk of mortality at midlife than in old age [10–12], with others reporting similar results [8, 9]. Moreover, individuals having increasing frailty trajectories already at midlife are more likely to die before age 70 compared to those having lower trajectories [13]. Otherwise, studies into frailty in younger individuals are scarce. The need for better understanding of frailty in younger and middle-aged adults has nevertheless been recognized and stressed [14].
Risk factors of frailty in sociodemographic, clinical, lifestyle, and biological domains have been extensively assessed in older adults with relatively consistent findings across studies. Among the most robust findings are those on increasing age, female sex, and socioeconomic deprivation that have been shown to increase the risk of late-life frailty [15]. Early-life exposures, such as childhood stress have also been regarded as risk factors for late-life frailty [15–17]. Risk factors for early-life frailty are however unstudied; understanding on this matter is urgently needed to be able to tackle frailty early on. To respond to the quest for better understanding of frailty in younger adults, we used population-based data from two large samples including younger individuals – the UK Biobank and the Swedish Screening Across the Lifespan Twin Study (SALT) – and analyzed the prevalence, characteristics, and risk factors of early-life and late-life frailty. We additionally performed the analyses stratified by sex to assess potential sex differences.
Methods
Study Design and Participants
In this population-based, cross-sectional study, we obtained data from the UK Biobank and SALT studies. Between 2006 and 2010, UK Biobank assessed 502,640 participants aged between 37 and 73 years. The SALT study, which is part of the Swedish Twin Registry, assessed 44,919 twin individuals aged between 41 and 97 years in 1998–2002 [18]. Both studies collected data on physical measurements, demographics, socioeconomics, lifestyle, environmental exposures, health factors, and medical history through a self-completed touch-screen questionnaire (UK Biobank) or a computer-assisted telephone interview (SALT) [19]. The UK Biobank study was approved by the North West Multi‐Centre Research Ethics Committee and the SALT study by the Swedish Ethical Review Authority.
FI Assessment
Construction of the FI has been done based on the Rockwood deficit accumulation model for both UK Biobank and SALT participants as previously described [4, 10, 11]. Altogether 49 items in the UK Biobank and 44 items in SALT were included. In both samples, the FI is based on self-reported deficits covering physiological and mental domains, general health status, signs and symptoms of disease, psychosocial health, and disabilities. The FI score is calculated by dividing the number of deficits by the total number of deficits considered. For example, an individual having 6 deficits of the 49 in the UK Biobank has an FI value of 0.12 (6/49). The items included in the FI and their coding are presented in online supplementary Tables 1 and 2 (for all online suppl. material, see https://doi.org/10.1159/000534131).
Stratification of the Samples into Younger and Older Individuals
To identify the age cut-off that best stratifies the sample into younger and older individuals, a principal component analysis of the FI items, followed by a linear model, was performed in the UK Biobank. The analysis is described in detail in online supplementary methods.
Risk Factors for Frailty
Baseline age, sex, body mass index, smoking status, alcohol consumption, education were analyzed for their associations with frailty in both UK Biobank and SALT. In addition, annual household income and maternal smoking were analyzed in UK and occupation in SALT. Assessment of these variables is presented in online supplementary methods.
Statistical Analysis
In the UK Biobank, 405,123 individuals with complete data on the 49 FI items were included in this study [11]. In SALT, to ensure maximal power, we first excluded individuals with missing data points in >20% and then performed multiple imputation for the 43,641 remaining individuals [10]. In the analysis of the characteristics, prevalence, and risk factors of frailty in younger (aged <65) and older (aged ≥65) adults, individuals with an FI value ≥0.21 were considered as frail, and the remaining individuals (FI <0.21) were considered as non-frail [20]. Differences in the study variables between younger (aged <65) and older (aged ≥65) adults and by sex were assessed using t test, χ2 test, and Kruskal-Wallis test as appropriate. The sample characteristics of individuals with and without sufficient FI items in UK Biobank and SALT were likewise assessed using t tests for continuous variables and χ2 tests for categorical variables. To gain insight into the burden of frailty across midlife and old age, the samples were stratified by 10-year age intervals (≤55, 55–64, 65–74, 75–85, and >85), and the prevalence of frailty was assessed in each age group. We derived the pooled prevalence estimates and 95% confidence intervals (CIs) using a generalized linear mixed model using a meta package in R in age groups of ≤55, 55–64, and 65–74 based on data from the UK Biobank and SALT [21].
For assessing the differences in the characteristics of younger (early-life frailty) and older (late-life frailty) frail individuals, the FI items were assigned into 11 and 12 domains in the UK Biobank and SALT, respectively. The domains are presented in online supplementary Tables 1 and 2. Domain scores were assessed by dividing the number of deficits one individual had in a given domain by the total number of deficits considered in this domain. For example, glaucoma, cataracts, and hearing difficulty were included in the sensory domain, and the sensory domain score of an individual with glaucoma and hearing difficulty, but no cataracts is (1 + 1 + 0)/3 = 0.67. Thus, a higher domain score indicates a worse health status in a given domain. To analyze differences in the domain scores between younger and older frail adults (frailty demarcated by FI ≥0.21), the means of the frailty domains scores were compared using linear regression. In SALT, the nonindependence of twin pairs was accounted for by using robust standard errors. In addition, we analyzed the prevalence of each individual frailty item between younger and older frail adults. In these analyses, we used the Benjamini-Hochberg false discovery rate method to adjust for multiple testing [22].
Multivariable logistic regression models were performed separately in younger (aged <65) and older (aged ≥65) individuals to assess the associations between the risk factors for early-life and late-life frailty. All predictor variables were included simultaneously to the model. FI level ≥0.21 was used as a dichotomous outcome variable in the models. We additionally performed the logistic regression models for the younger (aged <65) and older (aged ≥65) individuals stratified by sex. Nonindependence of the twin pairs in SALT was accounted for by using robust standard errors. All statistical analyses were performed using R 4.1.2.
Results
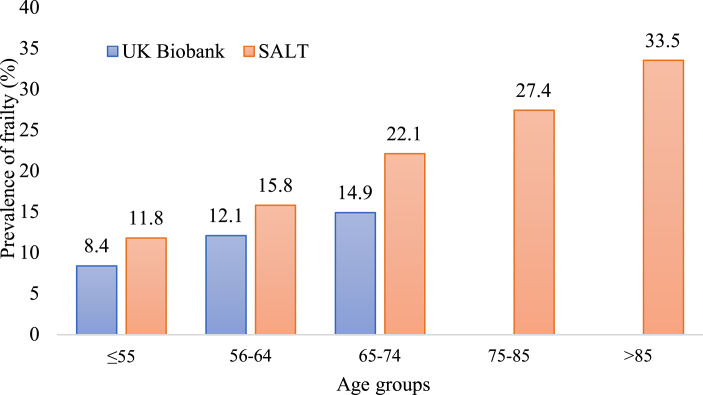
The characteristics of the study samples by age are presented in Tables 1 and 2 and by sex in online supplementary Table 3. In the UK Biobank and SALT, the mean baseline ages were 56.0 (SD 8.1) and 57.6 (SD 10.0) years, respectively. Significant differences were observed across all study variables between the younger (aged <65) and older (aged ≥65) adults (Tables 1, 2) and between men and women (online suppl. Table 3). The characteristics of individuals with and without sufficient items for creating the FI in UK Biobank and SALT are presented in online supplementary Table 4. Significant differences were found across the variables except for age in the UK Biobank and smoking status in SALT (online suppl. Table 4). The prevalence rates of frailty in the UK Biobank were 8.4%, 12.1%, and 14.9% in individuals aged ≤55, 55–64, and 65–74, respectively, whereas the prevalence rates in SALT were 11.8%, 15.8%, 22.1%, 27.5%, and 33.5% in individuals aged ≤55, 55–64, 65–74, 75–85, and >85, respectively (Fig. 1). The pooled prevalence rates of frailty were 10.3% (95% CI: 2.7–32.7), 14.4% (95% CI: 4.5–37.2), 19.2% (95% CI: 2.5–68.5%) in individuals aged ≤55, 55–64, 65–74 based on the UK Biobank and SALT data. The prevalence of frailty was significantly higher in younger women (aged <65) than in younger men (aged <65), the estimates being 11.7% versus 9.9% in the UK Biobank and 19.1% versus 11.9% in SALT (online suppl. Table 3). The age cut-off with the highest discrimination to stratify the sample into younger and older adults was found to be 65 (online suppl. Fig. 1).
Table 1.
Characteristics of the study population in the UK Biobank
| UK Biobank | ||||
|---|---|---|---|---|
| all (N = 307,568) | younger adults (<65 years) (N = 254,955) | older adults (≥65 years) (N = 52,613) | p value | |
| Frailty, N (%) | ||||
| Non-frail (FI <0.21) | 274,254 (89.2) | 229,341 (90.0) | 44,913 (85.4) | <0.01 |
| Frail (FI ≥0.21) | 33,314 (10.8) | 25,614 (10.0) | 7,700 (14.6) | |
| Age, mean (SD) | 56.0 (8.1) | 53.8 (7.0) | 66.9 (1.5) | <0.01 |
| Sex, N (%) | ||||
| Women | 161,295 (52.4) | 137,063 (53.8) | 24,232 (46.1) | <0.01 |
| Men | 146,273 (47.6) | 117,892 (46.2) | 28,381 (53.9) | |
| Tobacco use status, N (%) | ||||
| Never | 171,637 (55.8) | 145,799 (57.2) | 25,838 (49.1) | <0.01 |
| Previous | 106,004 (34.5) | 82,925 (32.5) | 23,079 (43.9) | |
| Current | 29,927 (9.7) | 26,231 (10.3) | 3,696 (7.0) | |
| Alcohol consumption, N (%) | ||||
| Less than weekly | 86,699 (28.2) | 71,127 (27.9) | 15,572 (29.6) | <0.01 |
| Weekly | 220,869 (71.8) | 183,828 (72.1) | 37,041 (70.4) | |
| Education, N (%) | ||||
| Low | 40,773 (13.3) | 26,387 (10.3) | 14,386 (27.3) | <0.01 |
| Intermediate | 152,288 (49.5) | 128,343 (50.3) | 23,945 (45.5) | |
| High | 114,507 (37.2) | 100,225 (39.3) | 14,282 (27.1) | |
| BMI, N (%) | ||||
| Underweight | 1,513 (0.5) | 1,283 (0.5) | 230 (0.4) | <0.01 |
| Normal weight | 102,752 (33.4) | 87,535 (34.3) | 15,217 (28.9) | |
| Overweight | 131,467 (42.7) | 106,721 (41.9) | 24,746 (47.0) | |
| Obese | 71,836 (23.4) | 59,416 (23.3) | 12,420 (23.6) | |
| Income, mean (SD) | 2.69 (1.20) | 2.83 (1.18) | 1.99 (1.00) | <0.01 |
| Maternal smoking, N (%) | ||||
| No | 215,722 (70.1) | 175,714 (68.9) | 40,008 (76.0) | <0.01 |
| Yes | 91,846 (29.9) | 79,241 (31.1) | 12,605 (24.0) | |
BMI, body mass index; FI, frailty index; IQR, interquartile range; SALT, Screening Across the Lifespan Twin Study; SD, standard deviation.
*The categories of income in the UK Biobank are as follows: 1 = GPB <18,000; 2 = GPB 18,000–GPB 30,999; 3 = GPB 31,000–GPB 51,999; 4 = GPB 52,000–GPB 100,000; 5 = GPB >100,000. p values for comparison between younger and older adults.
Table 2.
Characteristics of the study population in the SALT
| SALT | ||||
|---|---|---|---|---|
| all (N = 38,381) | younger adults (<65 years) (N = 29,164) | older adults (≥65 years) (N = 9,217) | p value | |
| Frailty, N (%) | ||||
| Non-frail (FI <0.21) | 32,400 (84.4) | 25,317 (86.8) | 7,083 (76.8) | <0.01 |
| Frail (FI ≥0.21) | 5,981 (15.6) | 3,847 (13.2) | 2,134 (23.2) | |
| Age, mean (SD) | 57.6 (10) | 53.0 (5.7) | 72.1 (5.8) | <0.01 |
| Sex, N (%) | ||||
| Women | 19,732 (51.4) | 15,229 (52.2) | 4,503 (48.9) | <0.01 |
| Men | 18,649 (48.6) | 13,935 (47.8) | 4,714 (51.1) | |
| Tobacco use status, N (%) | ||||
| Never | 14,446 (37.6) | 10,327 (35.4) | 4,119 (44.7) | <0.01 |
| Previous | 15,621 (40.7) | 11,747 (40.3) | 3,874 (42.0) | |
| Current | 8,314 (21.7) | 7,090 (24.3) | 1,224 (13.3) | |
| Alcohol consumption, N (%) | ||||
| Less than weekly | 12,406 (32.3) | 7,850 (26.9) | 4,556 (49.4) | <0.01 |
| Weekly | 25,975 (67.7) | 21,314 (73.1) | 4,661 (50.6) | |
| Education, N (%) | ||||
| Low | 9,985 (26.0) | 5,280 (18.1) | 4,705 (51.0) | <0.01 |
| Intermediate | 21,922 (57.1) | 18,279 (62.7) | 3,643 (39.5) | |
| High | 6,474 (16.9) | 5,605 (19.2) | 869 (9.4) | |
| BMI, N (%) | ||||
| Underweight | 468 (1.2) | 332 (1.1) | 136 (1.5) | <0.01 |
| Normal weight | 20,381 (53.1) | 15,712 (53.9) | 4,669 (50.7) | |
| Overweight | 14,496 (37.8) | 10,797 (37.0) | 3,699 (40.1) | |
| Obese | 3,036 (7.9) | 2,323 (8.0) | 713 (7.7) | |
| Occupation, N (%) | ||||
| Low | 10,292 (26.8) | 7,435 (25.5) | 2,857 (31.0) | <0.01 |
| Medium | 23,555 (61.4) | 17,926 (61.5) | 5,629 (61.1) | |
| High | 4,534 (11.8) | 3,803 (13.0) | 731 (7.9) | |
BMI, body mass index; FI, frailty index; IQR, interquartile range; SALT, Screening Across the Lifespan Twin Study; SD, standard deviation.
Fig. 1.
Prevalence of frailty across age categories in the UK Biobank and SALT. SALT, Screening Across the Lifespan Twin Study.
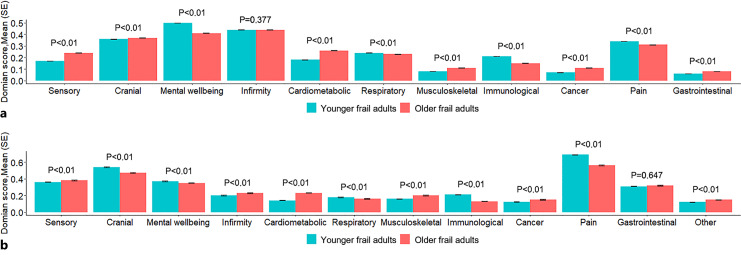
Comparing the mean frailty domain scores between younger (aged <65) and older (aged ≥65) frail adults (FI ≥0.21) revealed statistically significant differences across most of the domains, yet the effect sizes of the differences were generally small (Fig. 2). The greatest differences with consistent findings in both samples were seen in pain, immunological, and mental wellbeing domains, with younger frail adults having higher domain scores compared to older frail adults, whereas older frail adults had higher mean scores in sensory, cardiometabolic, musculoskeletal, and cancer domains (Fig. 2). The exact numeric mean scores of each domain are listed in online supplementary Table 5. The differences in the prevalence rates of the individual FI items largely mirrored the differences in the domain scores (online suppl. Fig. 2).
Fig. 2.
Mean of domain score of frailty in younger and older frail adults of UK Biobank (a) and SALT (b). Younger frail adults = individuals aged <65, old frail adults = individuals aged ≥65. The p values are for the comparison between younger and older frail individuals. The p value threshold level is corrected to 0.045 based on Benjamini-Hochberg false discovery rate method.
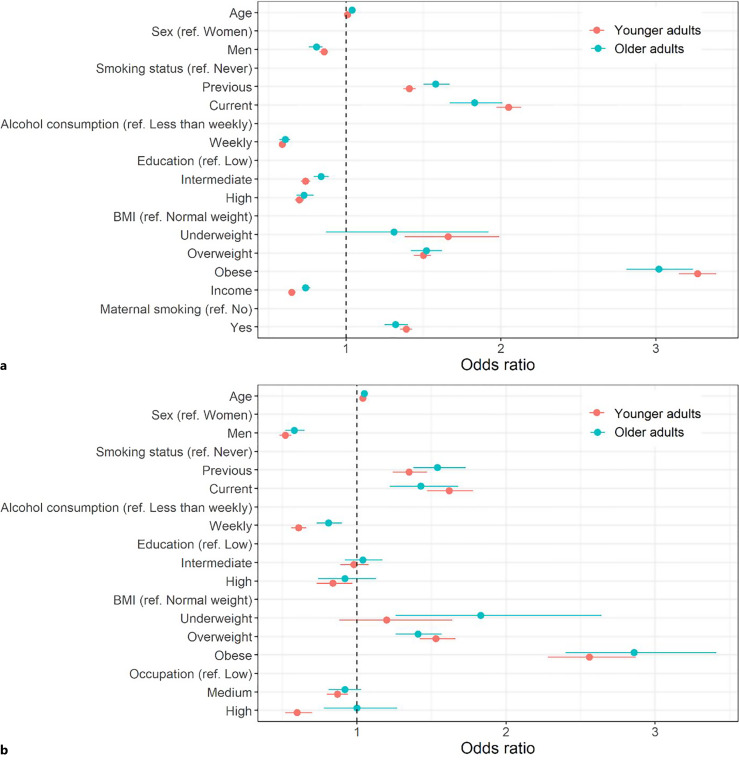
Higher age, female sex, being a previous or current smoker, consuming alcohol less than weekly, overweight, and obesity were similarly associated with early-life and late-life frailty in both samples (Fig. 3; the numeric point estimates are presented in online suppl. Table 6). Underweight was associated with early-life frailty in UK Biobank, and with late-life frailty in SALT. Low education, low income, and maternal smoking history were associated with both early-life and late-life frailty in the UK Biobank. In SALT, low education and low occupation level were associated with early-life frailty but not with late-life frailty. The results of the sex-specific logistic regression were largely similar to the full sample with no marked sex differences observed (online suppl. Table 7; online suppl. Fig. 3).
Fig. 3.
Associations of the common risk factors with frailty in younger (aged <65) and older (aged ≥65) adults in the UK Biobank (a) and SALT (b) in a multivariable logistic regression model. The whiskers present 95% confidence intervals for the estimates. BMI, body mass index.
Discussion
In two large population-based cohorts from the UK and Sweden, we found that the pooled prevalence rates of frailty were 10.3%, 14.4%, and 19.2% in individuals aged ≤55, 55–64, 65–75, respectively. We identified 65 as the cut-off age having the greatest discrimination in frailty between younger and older individuals and thus used this cut-off in the analysis on the characteristics and risk factors of early-life and late-life frailty. Early-life frailty was characterized by worse health in mental wellbeing, pain-related, and immunological domains, whereas late-life frailty was characterized by worse health in sensory, cardiometabolic, musculoskeletal, and cancer domains. Higher age, female sex, being a previous or current smoker, lower alcohol consumption, obesity, overweight, low income, and maternal smoking were similarly associated with the risk of early-life and late-life frailty. However, low occupational level was associated only with early-life but not with late-life frailty. No marked sex differences were found in the risk factor associations.
Using FI ≥0.21 to demarcate frailty, our findings on the prevalence of frailty – 8.4% and 11.8% in individuals aged ≤55 and 12.1% and 15.8% in individuals aged 55–64 in the UK Biobank and SALT, respectively – align with previous results in similar age groups [5]. Previous studies have also analyzed the prevalence of frailty in even younger individuals. The Canadian Health Measures Study reported that the prevalence of frailty is 1.8% in the 18–34 age group, 4.3% in the 35–49 age group, and 11.6% in the 50–64 age group using the FI to measure frailty [7]. A Chinese Kadoorie Biobank study, likewise using the FI, reported somewhat lower prevalence estimates: 0.8% in individuals aged <50 years and 3.5% in individuals aged 50–64 years [8]. Overall, previous prevalence estimates of frailty in individuals aged 18–65 have ranged widely from 3.9% to 63%, the variation arising from different inclusion criteria and scales to measure frailty [14]. Indeed, our current prevalence estimate of 12.1% using the FI in individuals aged 55–65 in the UK Biobank is higher than a previous estimate of 3% using the FP in the same age group in the UK Biobank [9]. The higher estimate is probably due to the FI being a multidimensional and more sensitive measure than the FP, especially in younger individuals. The presence of frailty in younger adults suggests that frailty screening could be extended to younger age groups to facilitate early identification of at-risk individuals.
In the analysis of the characteristics of frailty, we used a novel approach of collating the individual FI items into domains and analyzed the domain scores in younger (aged <65) and older (aged ≥65) frail adults, denoting early-life and late-life frailty, respectively. The most notable findings were younger frail adults having higher scores, indicative of worse health in mental wellbeing, pain-related, and immunological domains, and older frail adults having higher scores in cardiometabolic, cancer, musculoskeletal, and sensory-related domains. The fact that mental wellbeing and pain-related health problems stood out as being strongly characteristic of early-life frailty suggests that younger individuals presenting with clustering of such health issues should receive close attention in healthcare. Overall, our findings indicate that while frailty is indeed different in younger and older adults in some of its characteristics, for most of the domains the differences were very small, albeit statistically significant. Our results would thus be in support of a hypothesis that early-life and late-life frailty are not totally different entities. More studies are nevertheless needed as systematic analyses into the characteristics of early-life and late-life frailty are lacking.
To the best of our knowledge, only one previous study has looked at the prevalence of the individual FI items in frail individuals across age. The Canadian Health Measures Study assessed the FI item prevalence in age groups 18–49, 50–64, and >65 years and found a higher prevalence of diabetes, cancer, and arthritis in older individuals, and higher prevalence of persistent cough and asthma in younger individuals [7]. They found no differences in the prevalence of liver disease, kidney dysfunction, thyroid problems, overall health, and sleeping problems. These results are similar to our findings on the prevalence of the individual FI items between younger and older frail adults, suggesting that some aspects of frailty differ with age, while others do not.
Increasing age, female sex, smoking, underweight, overweight, obesity, low wealth, and lower education have all been previously identified as common risk factors for frailty in older adults [17, 23–25]. Here, we found that higher age, female sex, smoking, consuming alcohol less than weekly (in reference to a weekly use), overweight, obesity, lower income, and maternal smoking were similarly associated with increased risk of early-life and late-life frailty. Interestingly, high occupation level was associated with a lower risk of early-life frailty but not with late-life frailty. As occupation level was available only in SALT, more research is needed to confirm the association. Some cohort differences were also found. Underweight was associated with early-life frailty in the UK Biobank and late-life frailty in SALT. The reason for the difference is unknown; however, in our previous longitudinal study [17], we found that underweight was associated with higher FI scores from approximately age 60 onwards with an increasing effect size toward older age, suggesting that underweight might be a greater risk factor in later life. The protective associations of the socioeconomic variables – education and income level in the UK Biobank and education and occupation level in SALT were also, overall, stronger in the UK Biobank than in SALT. The finding on lower alcohol consumption being associated with a higher risk of early-life and late-life frailty in both samples is consistent with previous findings [26, 27]. The reasons for heavier alcohol consumption being associated with lower levels of frailty across studies are not totally clear, but possible explanations include social bonding as alcohol is often consumed socially when it can reinforce social networks and prevent social isolation, a “sick quitter” effect, and survival bias [27]. When analyzed longitudinally, heavier alcohol consumption has nevertheless not been found to protect against incident frailty [26]. Overall, the risk factor associations were similar in younger and older adults to a degree that allows us to hypothesize that early-life and late-life frailty may have shared etiologies, even though the physiological manifestations may differ.
The study has some limitations. Most of the variables used in deriving the FI and the covariates were from self-reported data, which could lead to potential misclassification. Compared to the UK Biobank FI, the SALT FI has an overrepresentation of comorbidities to functional items. The results on the FI domain score analysis in younger and older frail individuals were nevertheless largely similar in both samples, suggesting that despite the different items included in the FIs, the domain score approach is a viable method to assess the characteristic of frailty in different samples. As the data were cross-sectional, we were unable to determine at which age exactly the individuals had become frail. Our previous longitudinal analysis nevertheless suggests that individuals who have high frailty scores already at or before midlife are more likely to die before age 70, whereas those who live up to ages 80+ only accrue significant increases in frailty after age ∼70 [13]. This would suggest that our older frail individuals are less likely to include those who were frail already before age 65, and more likely to include individuals who only became frail later in life. The strength of this study is the use of large population-based data that cover both younger and older individuals. In addition, as our samples included partially nonoverlapping birth cohorts, the largely consistent findings in the samples indicate that the results are robust against cohort effects and secular trends.
In summary, our study demonstrates that frailty is present in younger adults to a significant degree. Early-life frailty nevertheless differs from late-life frailty in some of its characteristics, yet the overall differences were not sizeable enough to consider early-life and late-life frailty as different entities. The risk factors for early-life and late-life frailty were also largely comparable, suggesting that similar interventions may be applicable in younger and older adults.
Statement of Ethics
This study has been reviewed and approved by The Swedish Ethical Review Authority (Dnr 97-051, Dnr 00-132, Dnr 2016/1888-31/1). In the UK Biobank, a written informed consent was obtained from all participants. In SALT, an informed consent was obtained from all participants as follows, the way of obtaining the consent being approved by the respective ethic authority. Participants were informed in writing prior to the computer-assisted telephone interview, with information that their participation was voluntary and that they could interrupt their participation at any time. At the end of the interview, participants were asked whether they could be contacted again for complementary questions and whether the Twin Registry could contact their physician to obtain medical records. After the interview, a letter was sent out to each participant confirming that they had been informed, had participated, and whether they agreed to recontact and/or contact with their physician. The present study was conducted following the guidelines of the Declaration of Helsinki.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the Academy of Finland (349335) to J.J.; the Instrumentarium Science Foundation to J.J.; the Swedish Research Council (2015-03255, 2018-02077 to J.J., 2019-01272 to S.H.); the Loo & Hans Osterman Foundation to S.H. and J.J.; the Foundation for Geriatric Diseases to S.H.; the Magnus Bergwall Foundation to S.H.; the Strategic Research Program in Epidemiology at Karolinska Institutet to S.H. and J.J.; and the China Scholarship Council (201806240009) to G.B.
Author Contributions
J.J. conceptualized and designed the study. G.B. performed the statistical analysis under the supervision of Y.W. G.B. and J.J. wrote the manuscript. S.H. contributed to the supervision of the work. J.K.L.M. and M.E. contributed to the acquisition of the study variables. All authors reviewed and approved the final manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding Statement
This work was supported by the Academy of Finland (349335) to J.J.; the Instrumentarium Science Foundation to J.J.; the Swedish Research Council (2015-03255, 2018-02077 to J.J., 2019-01272 to S.H.); the Loo & Hans Osterman Foundation to S.H. and J.J.; the Foundation for Geriatric Diseases to S.H.; the Magnus Bergwall Foundation to S.H.; the Strategic Research Program in Epidemiology at Karolinska Institutet to S.H. and J.J.; and the China Scholarship Council (201806240009) to G.B.
Data Availability Statement
Data used in the current study are not publicly available due to ethical reasons. However, applications for access to UK Biobank data can be made online. Further inquiries regarding access to SALT data can be directed to the Swedish Twin Registry (The Swedish Twin Registry steering committee; http://ki.se/en/research/the-swedish-twin-registry-1; contact: tvillingregistret@ki.se).
Supplementary Material
References
- 1. Cesari M, Prince M, Thiyagarajan JA, De Carvalho IA, Bernabei R, Chan P, et al. Frailty: an emerging public health priority. J Am Med Dir Assoc. 2016;17(3):188–92. 10.1016/j.jamda.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Caoimh R, Sezgin D, O’Donovan MR, Molloy DW, Clegg A, Rockwood K, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50(1):96–104. 10.1093/ageing/afaa219. [DOI] [PubMed] [Google Scholar]
- 6. Blodgett JM, Rockwood K, Theou O. Changes in the severity and lethality of age-related health deficit accumulation in the USA between 1999 and 2018: a population-based cohort study. Lancet Healthy Longev. 2021;2(2):e96–104. 10.1016/S2666-7568(20)30059-3. [DOI] [PubMed] [Google Scholar]
- 7. Kehler DS, Ferguson T, Stammers AN, Bohm C, Arora RC, Duhamel TA, et al. Prevalence of frailty in Canadians 18-79 years old in the Canadian health measures survey. BMC Geriatr. 2017;17(1):28. 10.1186/s12877-017-0423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan J, Yu C, Guo Y, Bian Z, Sun Z, Yang L, et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. 2020;5(12):e650–60. 10.1016/S2468-2667(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3(7):e323–32. 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Ploner A, Karlsson IK, Liu X, Magnusson PKE, Pedersen NL, et al. The frailty index is a predictor of cause-specific mortality independent of familial effects from midlife onwards: a large cohort study. BMC Med. 2019;17(1):94. 10.1186/s12916-019-1331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams DM, Jylhävä J, Pedersen NL, Hägg S. A frailty index for UK biobank participants. J Gerontol A Biol Sci Med Sci. 2019;74(4):582–7. 10.1093/gerona/gly094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang M, Foebel AD, Kuja-Halkola R, Karlsson I, Pedersen NL, Hägg S, et al. Frailty index as a predictor of all-cause and cause-specific mortality in a Swedish population-based cohort. Aging. 2017;9(12):2629–46. 10.18632/aging.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bai G, Szwajda A, Wang Y, Li X, Bower H, Karlsson IK, et al. Frailty trajectories in three longitudinal studies of aging: is the level or the rate of change more predictive of mortality? Age Ageing. 2021;50(6):2174–82. 10.1093/ageing/afab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loecker C, Schmaderer M, Zimmerman L. Frailty in young and middle-aged adults: an integrative review. J Frailty Aging. 2021;10(4):327–33. 10.14283/jfa.2021.14. [DOI] [PubMed] [Google Scholar]
- 15. Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: a systematic review of longitudinal studies. PLoS One. 2017;12(6):e0178383. 10.1371/journal.pone.0178383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Xue QL, Odden MC, Chen X, Wu C. Linking early life risk factors to frailty in old age: evidence from the China Health and Retirement Longitudinal Study. Age Ageing. 2020;49(2):208–17. 10.1093/ageing/afz160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raymond E, Reynolds CA, Dahl Aslan AK, Finkel D, Ericsson M, Hägg S, et al. Drivers of frailty from adulthood into old age: results from a 27-year longitudinal population-based study in Sweden. J Gerontol A Biol Sci Med Sci. 2020;75(10):1943–50. 10.1093/gerona/glaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zagai U, Lichtenstein P, Pedersen NL, Magnusson PKE. The Swedish twin registry: content and management as a research infrastructure. Twin Res Hum Genet. 2019;22(6):672–80. 10.1017/thg.2019.99. [DOI] [PubMed] [Google Scholar]
- 19. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Romero-Ortuno R. An alternative method for Frailty Index cut-off points to define frailty categories. Eur Geriatr Med. 2013;4(5):299–303. 10.1016/j.eurger.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60. 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57(1):289–300. 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 23. Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65(4):377–81. 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 24. Ding YY, Kuha J, Murphy M. Multidimensional predictors of physical frailty in older people: identifying how and for whom they exert their effects. Biogerontology. 2017;18(2):237–52. 10.1007/s10522-017-9677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398. 10.1001/jamanetworkopen.2019.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kojima G, Jivraj S, Iliffe S, Falcaro M, Liljas A, Walters K. Alcohol consumption and risk of incident frailty: the English longitudinal study of aging. J Am Med Dir Assoc. 2019;20(6):725–9. 10.1016/j.jamda.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 27. Kojima G, Liljas A, Iliffe S, Jivraj S, Walters K. A systematic review and meta-analysis of prospective associations between alcohol consumption and incident frailty. Age Ageing. 2018;47(1):26–34. 10.1093/ageing/afx086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the current study are not publicly available due to ethical reasons. However, applications for access to UK Biobank data can be made online. Further inquiries regarding access to SALT data can be directed to the Swedish Twin Registry (The Swedish Twin Registry steering committee; http://ki.se/en/research/the-swedish-twin-registry-1; contact: tvillingregistret@ki.se).