Abstract
Valid and relevant models are critical for research to have biological relevance or to proceed in the right path. As well-established two-dimensional cell cultures lack niches and cues and rodent models differ in species, three-dimensional organoids emerged as a powerful platform for research. Cultured in vitro from stem cells, organoids are heterogeneous in cells and closely resemble the in vivo settings. Organoids also recapitulate the unique human features if cultured from a human source and are subjected to genetic modification. However, one type of organoid possesses only a limited selection of cells. In particular, the absence of vasculature and immune cells restricts the organoids from nutrition, cues, or critical interactions, undermining the validity of organoids as physiological or pathological models. To fill the current gap, there is an urgent need to provide organoids with vasculature and immune cells. In this paper, we review the methods to generate physiological and pathological organoid models and summarize ways to vascularize or immunize them. Our discussion continues with some advantages and disadvantages of each method and some emerging solutions to current problems.
Keywords: Organoids, Assembloids, Infectious diseases, Cancer
Introduction
What Are Organoids and Their Applications?
Organoids are self-organizing three-dimensional (3D) “in vitro miniature organs” that highly mimic the structure, function, and complexity of their in vivo counterpart organs including lung, liver, kidney, heart, brain, stomach, etc. They can be derived from stem cells such as human pluripotent stem cells (hPSCs), induced pluripotent stem cells (iPSCs), adult stem cells, or derived from surgically resected patient’s tumor biopsies, which recapitulate the physiological architecture of the patient’s tumor. Sato et al. have demonstrated that single Lgr5+ intestinal stem cells (ISCs) can self-aggregate and form crypt-villus organoids, resembling a normal gut. This study opens up new avenues for 3D culture models, shifting from simple 3D cell aggregates to organoids with complex structures. Making organoids out of hPSCs/iPSCs usually starts from embryoid body (EB) formations which are 3D aggregates formed by these cells in suspension culture, and EB could be developed into specific tissues derived from all three embryonic germ layers when cultured with tissue-specific induction medium. The differentiated EB are usually placed in the hydrogel, most commonly a Matrigel, in the presence of growth factors which could further allow the EB to form organoids with a complex structure. To date, different protocols using a cocktail of appropriate growth factors have been developed for directing the EB to a specific lineage. Organoids are particularly useful in studying human biology and diseases. Although two-dimensional (2D) cell culture systems and animal models have been the conventional method to study human biology and diseases, they have several limitations when extrapolating the results to a human context. 2D cell culture is an oversimplified model that fails to fully recapitulate in vivo environments. Although animal models can solve this problem, they are costly, time-consuming, and lack human physiological relevance due to species-specific differences [Chenchula et al., 2019]. Organoids are self-organizing 3D “in vitro miniature organs” that are either stem cell-derived or patient tumor-derived [Kim et al., 2020]. Embryonic stem cells (ESCs), iPSCs, and tissue-derived adult stem cells are the common source of stem cell-derived organoids, they could be assembled into complex structures when placed in the hydrogel, usually a Matrigel, in the presence of appropriate growth factors [Huch et al., 2015; Neal et al., 2018]. With their ability to replicate the architecture and physiology of human organs, the aforementioned limitations of existing models could be overcome. There has been a rise in its application in understanding developmental biology, human diseases, drug screening, and regenerative medicine.
Limitations of Existing Organoid Technology
Yet, organoids lack vasculature. Organs such as the kidney, brain, and heart, are highly vascularized tissues with a dynamic microenvironment, and a complete vasculature in their organoids is important to fully recapitulate their development and function in vivo. Moreover, vascularization is important for organoid development to prevent necrotic core caused by increased metabolic and nutritional demands that cannot be met by diffusion alone [Zhang et al., 2021].
Current techniques to vascularize organoids mainly depend on in vivo engraftment into an immune-deficient host such as NSG immunodeficient mice model, coculturing with endothelial cells (ECs) or mesodermal progenitor cells (MPCs) or gene editing to manipulate cells in organoids into adopting an endothelial fate [Birey et al., 2017; Mansour et al., 2018; Strobel et al., 2022]. Immune cells are also indispensable when modeling diseases such as cancer, viral infections, and autoimmune diseases. As a result, epithelial organoid-immune coculture systems have been established to study the epithelial-immune interactions during pathogenic events such as cancer progression and aberrant immune reactions, etc. [Bar-Ephraim et al., 2018]. Endowing organoids with immune cells is still a major challenge and lacks a standardized method. The most common technique is to coculture an organoid with specific immune cells that are either immortalized or autologous immune cells derived from patients. In this review, we will summarize the current strategies of supplying organoids with vasculature and immune cells, their pros, and cons, as well as propose the future directions of this new model system.
Vascularization and Immunization Strategies for Physiological Organoids
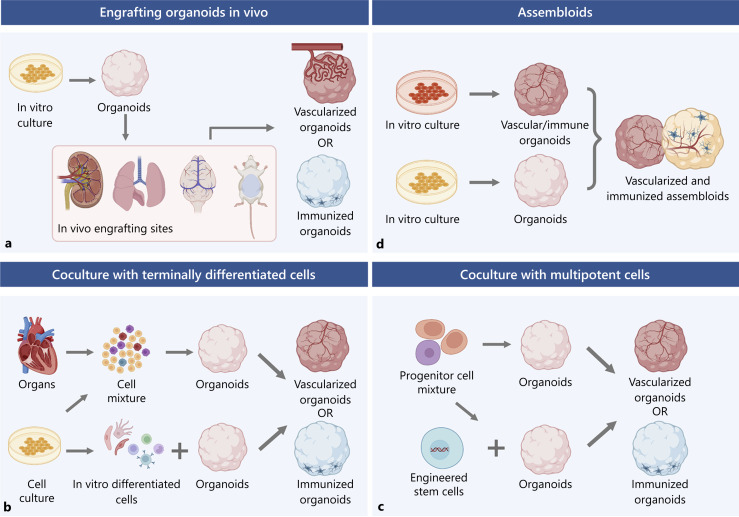
Engraft Organoids in vivo
Vascularization is necessary for valid models. For example, kidney culture models. Glomeruli, a patch of small vessels that filter wastes in the kidneys, do not form in an avascular environment. Some efforts well recapitulated the nephrogenesis using hPSCs, but including vascular progenitors for glomeruli genesis has been challenging [Morizane et al., 2015]. Only when a preferential selection for collecting duct progenitors was in place could renal endothelial undergo vascularization [Takasato et al., 2015]. Yet, in vivo engraftments offer another solution. Early in 2012, Xinaris et al. succeeded in generating renal organoids from E11.5 mice kidneys and subsequently implanting them below the kidney capsule of an athymic living rat host. In vivo transplantation resulted in glomeruli structures with fully differentiated capillary walls [Xinaris et al., 2012]. The success in utilizing vasculature-rich niches like kidneys in vivo was inspiring. Soon after, in 2014, another group implanted human intestine organoids (HIO) generated from human ESC or iPSC into mouse kidney capsules [Watson et al., 2014]. Their results showed a larger volume of HIO, similar to human intestine tissue, with more mature and differentiated cell types. Meanwhile, blood vessels of host origin were observed to be responsive to physiological cues within HIO. Through in vivo implantation, the HIO could also absorb selective molecules, indicating a better functional model [Watson et al., 2014]. Similarly, in vitro culture alone cannot fully capture the organ characteristics of the lung. In one study, multiple cell types and physiological structures were included in hPSC-derived human lung organoids (HLO), but immune cells or vasculature were absent [Dye et al., 2015]. The same group progressed to focus on bud tip-like organoids and followed the in vitro culture with in vivo engraftment into immunocompromised mouse lungs [Miller et al., 2018]. The engrafted organoids were able to persist and differentiate into lung-specific ECs, but no vasculature was reported [Miller et al., 2018]. Tan et al. also generated airway organoids using primary adult bronchial cells with original cell compositions [Tan et al., 2017]. During in vitro culture, fibroblasts and microvascular ECs were observed, yet CD31+ ECs were not sustainable. They then transplanted the organoids into NSG mice kidney capsules. Host vasculatures successfully invaded into organoids, although a regression was observed at a later time point. An improvement was shown in Chen et al.’s research where they used hESCs to generate HLO and again implanted the HLO into mouse kidney capsules. With 3D image reconstruction, they show tubular vasculatures within the organoids. They also achieved innervation from the host and were the first to generate alveolar structures by ectopic transplantation [Chen et al., 2018]. These results indicate in vivo vascularization is able to produce progressively mature tissue models. Besides the renal capsule, the peritoneal cavity can also serve as a vascularization niche, as demonstrated by Varzideh et al., in 2019. They cocultured cardiac progenitor cells with mesenchymal stem cells (MSCs) and ECs derived from hESC to generate cardiac organoids (COs). After in vitro self-organization, beating COs was formed, followed by implantation into the mouse peritoneal cavity. A significant increase in mature cardiomyocytes, vascularization, and normal blood flow was observed after implantation, demonstrating its advantage as a more physiologically relevant method to model cardiac functions [Varzideh et al., 2019].
The cranial window is another site that has easy access to host vasculatures and became the primary transplantation site for organoid research by Takebe and colleagues [Dye et al., 2016; Daviaud et al., 2018; Revah et al., 2022]. In the beginning, the group focused on the generation of liver “organ buds,” a similar form of organoid, from mixing human iPSCs (hiPSCs), MSCs, and ECs. They found that, after in vitro generation of liver buds, in vivo transplantation into mouse cranial window vascularized the buds and stimulated more specific hepatic cell fate and functions [Daviaud et al., 2018]. Furthermore, rescuing liver failure mouse models proved the proper functions of the buds [Revah et al., 2022]. The same method to generate liver buds could also be in vivo vascularized under the renal capsule and peritoneum [Revah et al., 2022]. Later, Takebe et al. expanded the methods to generate organ buds from a mixture of primary cells and vascularize them in vivo. It is worth noticing that MSCs and ECs were important components of the cell mixture. While MSCs were indispensable for the self-assembly of organ buds, ECs provided vascularization potential in vivo [Dye et al., 2016]. The group’s effort also successfully covered various organ types, including kidney, lung, intestine, etc. [Dye et al., 2016].
Another fast-advancing field of organoid research is establishing brain organoid models. The lack of vessel structures has been a bottleneck for brain organoid research as it limits the size and maturation of the model and induces much apoptosis. Similar in vivo vascularization strategies were seen in the past few years. For example, Mansour et al. managed to implant unguided, hESC-derived human brain organoids into the retrosplenial cortex of an adult mouse brain. After 8 months of engraftment, this method led to progressive differentiation and maturation of organoids, including gliogenesis and integration of microglia. They observed functional neuronal networks and blood vessels in the grafts [Mansour et al., 2018]. Neurons within the organoids were able to grow into multiple regions of the host brain, which further exhibited neuronal activity, suggesting a connection of graft-to-host functional synapses [Mansour et al., 2018]. In the same vein, Daviaud et al. used hiPSC-derived organoids for in vivo implantation. They engrafted the organoids into a mouse frontoparietal cortex lesion [Deuse et al., 2011b]. Subsequently, vasculatures from the host were shown to infiltrate into the center of the graft. Neurons within organoids also showed proliferation and further differentiation after 2–4 weeks of in vivo growth, indicating healthy growth and adaptation. The group also did a parallel experiment engrafting neural progenitor cells (NPCs) instead, but NPC engraftment showed less vascularization and a progressive retraction after 4 weeks. This also provided insights that structured cellular arrangements such as organoids, serve as a better platform to facilitate graft-to-host adaptation.
Successful as the attempts of in vivo vascularization of brain organoids were, none of them reported the cognitive changes in the host after implantation, which is unique and critical for brain modeling. A recent report filled the gap by probing mice behaviors after transplantation [Deuse et al., 2011a]. They transplanted hESC-derived brain organoids into the somatosensory cortex of an athymic rat and verified rat-origin vasculatures and microglia. They then devised a rewarding experiment and found that the transplanted organoids were able to drive reward-seeking behaviors. Optogenetics further revealed circuit connections between the host and grafts. This latest progress demonstrated that in vivo brain organoids can not only achieve vascularization and immunization but also engage with behavior control, enabling circuit-level research [Deuse et al., 2011a].
Notably, some in vivo vascularization attempts were more successful with bio-engineering aids. For example, in 2016, Dye et al. used a microporous poly (lactide-co-glycolide) scaffold to hold HLOs before transplanting them into a mouse fat pad. In previous studies, no ECs of lung specificity were successfully derived from the host environment. Yet in this study, they not only observed robust vascularization but also the recovered lung ECs after 8 weeks with correct EC structures and differentiation [Deuse et al., 2019].
In summary, implanting organoids in vivo provides a robust approach to vascularizing and immunizing organoids by offering sources of vasculatures and suitable growing niches (Fig. 1a). The vascularized organoids show better maturation and adaptation in vivo, while some are even able to engage in a higher level of behavior modulation. Bio-engineering methods can also enhance certain models. However, it must be kept in mind that due to potential graft-versus-host disorders, animals used in such experiments must be immune-deficient, limiting their application in organoid immunization and modeling inflammatory diseases (Table 1). With this regard, engineering immunologically hPSCs could be a solution. Advances have been made in generating hypoimmunogenic stem cells [Abud et al., 2017; Han et al., 2019; Matheus et al., 2022] which can overcome much of the incompatibility.
Fig. 1.
Methods to vascularize or immunize physiological organoids. a An in vivo engrafting method that involves organoids cultured in vitro and an in vivo engrafting site. Often the engrafted animals are rodents, and the sites are either consistent with the organoid types or highly vascularized sites, including but not limited to the kidney capsule, lung, brain, and peritoneal cavity of rodents. These methods can achieve vascularization from a host origin and, in the case of the brain, some degree of microglia integration. b Coculture with terminally differentiated cells from different origins achieves vascularization or immunization. There are multiple resources of differentiated cells, including primary organs or tissues, established cell lines, or in vitro induction from stem cells. Usually, for vascularization or immunization, ECs or immune cells (T cell subsets or macrophages) are incorporated. The cells can either be cocultured with preformed organoids or assembled to form new organoids. c Coculture with multipotent cells involves progenitor or stem cells. Progenitor cells such as MPCs or macrophage progenitor cells are examples of generating vasculature or immune cells in organoids. Stem cells are pre-engineered to express certain genes for cell fate precondition. These multipotent cells can be either cocultured with preformed organoids or assembled to form new organoids. d The assembloid technique involves putting different organoids together to form a more complex fusion. Organoids can be separately generated in vitro before fusion and may be arranged in a position to improve the outcome (flanking). The assembloids can achieve vascularization and incorporate immune cells simultaneously.
Table 1.
A summary of strategies in organoid vascularization and immunization for both physiological organoids and pathological organoids and their advantages and limitations
| Methods | Features | Advantages | Limitations |
|---|---|---|---|
| Physiological organoids | |||
| Engrafting organoids in vivo | Put in vitro-produced organoids into immunodeficient animals; involving vasculatures or immune cells of host origins | Better adapted into host environment as structured organoids; more likely to be vascularized in vasculature-rich sites; able to achieve a higher level of maturation; incorporating functional vessels containing blood flow | Restricted on immunodeficient animals; limited application in immunizing organoids due to potential graft-versus-host diseases; organoids subject to regression and loss of cell type specificity |
| Coculture with terminally differentiated cells | Use terminally differentiated cells from primary sources, cell lines, stem cells, or induced pluripotent cells | Suitable for either vascularization or immunization; relatively easy to obtain in vitro or from primary sources with relative ease. Potentially able to model abnormal conditions with primary cells | Limited types of cells introduced in one go; subject to less organ specificity regarding terminally differentiated cells; more challenges involved in producing certain types of cells, like microglia |
| Coculture with multipotent cells | Use multipotent cells such as progenitors and stem cells | Able to induce organ-specific cells and cells like microglia with physiological cues rather than additional cytokines; capable of introducing several cell types in one go | Subject to low or uncertain conversion rate of multipotent cells; still without simultaneous immunization or vascularization |
| Assembloids | Fuse different types of organoids together | Able to introduce vasculatures and immune cells simultaneously; able to model more systematic processes like angiogenesis and migration | More challenges are involved in producing multiple organoids and fusing them well |
| Organoids for studying infectious diseases | |||
| Coculture-infected organoids with immune cells | Organoids such as brain organoids and intestinal organoids were infected with pathogens and cocultured with immune cells such as microglia, T lymphocytes, and DCs etc. | Useful for studying pathogen-induced inflammatory response that leads to tissue damage | Only one type of immune cell is incorporated into the organoids, and fails to mimic a complete host immune response against pathogens |
| Tumor organoids | |||
| De novo generation of tumor organoids | Induce oncogenic mutations in wild-type organoids through gene editing, e.g., CRISPR-Cas9 | Useful for oncogene screening, studying molecular mechanisms for tumor initiation and progression etc. | De novo-generated organoids do not contain other cellular components, e.g., immune cells in the TME which makes it hard to study the effect of other cells on tumor development |
| Tumor-endothelial-immune coculture model | Allow tumor spheroids, ECs, and immune cells to self-organize to form 3D tumor organoids with vasculature and immune cells | Useful for studying specific immune-tumor interactions | Only limited types of immune cells can be cocultured with tumor spheroids at once; does not guarantee physiologically relevant spatial distribution and organization in the TME |
| PTOs | Derived from patient’s tumor tissues that recapitulate the biological characteristics of the primary tumor, retain native vasculature, immune cells, and stromal components | Preserves diverse cell types including stromal fibroblasts, macrophages, NK cells, etc. enabling the study of complex interactions between tumors and their microenvironment | The short half-life of endogenous immune components |
While the in vivo vascularization and immunization strategies could be hindered by graft-versus-host incompatibility, some other commonly used approaches rely entirely on in vitro. Different approaches to coculture organoids will be discussed as follows.
Coculture with Terminally Differentiated Cells
Another common way to vascularize or immunize organoids is to incorporate cells of relevant identity. Hence, coculturing cells from multiple lineages to form organoids or coculturing preformed organoids with other critical cell populations are also among researchers’ choices.
To better recapitulate the tissue microenvironment, some researchers use primary cells with physiological compositions to assemble organoids. Caspi et al. first demonstrated that cardiac tissues can be vascularized in vitro by creating multicellular scaffolds composed of cardiomyocytes, ECs, and embryonic fibroblasts. The presence of ECs greatly improved cardiomyocytes’ cell survival and proliferation, and also enhanced their differentiation and maturation. Adding additional cells such as pericytes and smooth muscle cells is important to further enhance blood vessel integrity, altogether suggesting that vascularization is necessary to maintain the function of COs in vitro. Although vascularization of heart organoids was addressed in many studies, immune cells are not included so far. It was previously reported that heart resident immune cells play an unexpected role in heart homeostasis. For example, it was found that macrophages played a role in removing old mitochondria and electrical conduction. Moreover, heart failure may be caused by inflammatory cell subtypes such as macrophages, natural killer cells (NK), mast cells, T cells, and B cells, etc. Heart organoids incorporating these cells can allow us to understand the contribution of these immune cells to the inflammatory process that causes heart failure in an in vitro heart organoid model. Therefore, it would be useful in understanding immune cardiac diseases and recapitulating the physiological heart function of COs if more studies can focus on incorporating immune cells into these engineered heart tissues.
In one study focusing on COs, primary ventricular cardiac cells from neonatal rats were isolated and grown in hanging-drop cultures [Zhao et al., 2020]. Researchers found a microcapillary vascular network formed by cardiac ECs. The vascularized model was capable of modeling cardiac fibrosis with TGFβ1 treatment, providing a better pathogenesis investigation platform [Zhao et al., 2020]. This study proved that incorporating multiple cell types produces more physiologically relevant models. However, organoids from primary rat cells still deviate from human physiology. Considering the accessibility of primary human tissues, organoids based on human-origin cell lines or hESC may be more valuable and informative. Progress was made by Polonchuk et al., in 2017 using human coronary artery ECs, iPSC-derived cardiac fibroblasts, and cardiomyocytes of either primary or iPSC origin. The research demonstrated that the use of different cardiomyocyte origins could produce robust vascular networks within organoids. The models were further used to test for cellular toxicity and a potential mechanism of NO toxicity was discovered [Figtree et al., 2017]. Similarly, another group cocultured multiple cell types together to obtain blood-brain barrier (BBB) organoids [Polonchuk et al., 2017]. BBB is a highly specialized arrangement of the vasculature that regulates the selective molecular uptake into the brain. The researchers used a low-adhesion culture to assemble human brain vascular ECs, pericytes, and primary astrocytes together and BBB-like structures were observed under confocal microscopy [Polonchuk et al., 2017]. This research suggested that incorporating cell types like brain ECs can facilitate vascular structure acquisition and pave the way for vascularized brain organoids and assemblies.
In 2018, vascularized brain organoids were achieved by Pham et al. by coculturing guided brain organoids with the same origin ECs. They obtained robust vascularization at the organoids’ periphery, with some vessel structures invading a STEM121+, central nervous system marker region. The vascularization was enhanced after engrafting the organoids into mice. Particularly, the ECs in organoids were shown to self-assemble into tubular structures and penetrate toward the center [Bergmann et al., 2018], showing that terminally differentiated cells can be cued by organoids to have higher structures.
Coculturing organoids with terminally differentiated cells are also commonly used to immunize organoids. Immune response contributes to tissue maturation, regeneration, and some pathogenesis. Hence, in 2015, in order to study the immune modulation during intestinal injury healing, Lindemans et al. cocultured mice intestinal organoids with lamina propria lymphocytes. They found that the coculture increased the size of organoids, as well as ISC regeneration and proliferation via IL-22 [Pham et al., 2018]. In 2018, another group cocultured intestinal organoids with either CD4+ T helper cell subsets or cytokines and demonstrated that immune cells regulate the ISC status, organoid size, and niches [Lindemans et al., 2015]. They further elucidated the interaction mechanism between ISCs and T helper cells or their key cytokines and proposed three mechanisms of the MHCII presenting system within the intestines [Lindemans et al., 2015]. Regarding pathogenesis, Roh et al. [2019] cocultured human colon organoids with monocyte-derived macrophages and further insulted the system with E. coli to mimic infection. A more relevant structure was achieved with the help of a sponge scaffold. Macrophage infiltration was already observed in noninfected models. After infection, macrophage showed altered morphology, increased migration, and significant pro-inflammatory cytokine secretion, resembling a state of active inflammatory bowel disease [Biton et al., 2018]. However, the contribution of immune cells to tissue status may be more dynamic. In one study that cocultured intestinal organoids together with ISCs and T effector memory cells [Roh et al., 2019], it was shown that the effects of TNF-a and CD4+ T cells were spectrum-like. Both low doses of TNF-a and CD4+ T cells promoted organoid growth and maturation, while high doses led to inhibition [Roh et al., 2019].
Similar efforts were also made regarding brain organoids. Microglia plays an essential role in neurological diseases such as Alzheimer’s Disease. However, primary human microglia is notoriously difficult and rare to obtain and culture. In order to overcome this obstacle, Abud et al. utilized hiPSC to generate induced hematopoietic progenitors and then obtained human microglial-like cells. Coculturing with hiPSC-derived brain organoids allowed the modeling of various human microglial-like cell functions such as invasion and response to injury, which also indicated successful immunization [Schreurs et al., 2019] (Fig. 1b; Table 1).
Coculture with Multipotent Cells
Other than coculturing organoids with various terminally differentiated cells, using progenitors or stem cells may offer a more physiologically relevant model with the potential to vascularize and immunize organoids in one go. Wörsdörfer et al. cocultured brain organoids with hiPSC-derived MPCs to vascularize the structure. MPC-derived formed blood vessels displayed a hierarchical organization and plasticity. The endothelial network was expanding along with the growth of organoids and could respond to both anti- or pro-angiogenesis drugs. Additionally, pericyte markers and Iba1 signals were detected, indicating the potential incorporation of pericytes and prevascular macrophages [Worsdorfer et al., 2019]. Progenitor cell coculture can result in multiple cell types, consistent with their physiological conditions. Regarding immunization, while Abud et al. committed to in vitro differentiation of microglia, another group proved that primitive macrophage progenitors gave rise to microglia under physiological cues [Xu et al., 2021]. The researchers cocultured hPSC-derived NPCs together with macrophage progenitors to generate brain organoids and around 8% of the cells were identified as microglia. Functionally, the microglia were able to phagocytize neural progenitors and remove apoptotic cells within organoids and respond to ZIKA invasion. Meanwhile, the immunized organoids increased in size with more mature molecular signatures. A ventricle-like zone was formed, and good responses toward stimulation were recorded in the patch clamp, indicating better neuronal functions [Xu et al., 2021]. This study bypassed the difficult process of microglia induction and instead utilized the progenitors and environmental cues, providing a more straightforward method of immunizing the organoids.
On the other hand, others incorporate multipotent cells into organoids by genetically engineering stem cells to overexpress genes determining cell fate, resulting in more mature organoids. Cakir et al. induced ETS variant transcription factor 2 overexpression in hESCs and cocultured the engineered hESCs with nonengineered ones. With guided brain organoid induction, the coculture resulted in a comprehensive vascular network structure composed of more mature neurons than previous methods. For example, some neurons responded to continuous stimulation with adaptation-like multiple-action potential patterns. Some BBB-like characteristics were also observed. Additionally, compared to conventional brain organoids, vascularized organoids via genetic engineering had better angiogenic results and were better perfused after in vivo transplantation [Cakir et al., 2019]. The same group established a similar approach to immunizing brain organoids as well [Cakir et al., 2022]. Myeloid-specific transcription factor PU.1 was induced into an optimal ratio of hESCs, followed by brain organoid differentiation. The organoids possessed not only functional microglia but also its complement system and chemokine system as well. The established immune system was able to clear amyloid beta deposition and reduce associated neuronal death. These models demonstrated that by incorporating multipotent cells, organoids could gain multiple cell lineages and functions that further promote maturation and provide more physiologically relevant models (Fig. 1c; Table 1).
Assembloids
Coculturing organoids with different cell types opened possibilities like vascularization and immunization. However, a simple mixture of cells does not guarantee physiologically relevant spatial distribution and organization [Vogt, 2021]. Assembloids, a more advanced approach fusing organoids with specific identities together, may well provide a better solution. Such an approach has been employed in brain organoids research. A recent example flanked the brain organoids with two vessel organoids on each side to achieve a high level of vascularization [Sun et al., 2022b]. Not only did the vessels invade the brain organoids and form a comprehensive vasculature network, but they also converted to a brain-specific type. For the brain organoids, the number of neural progenitors increased, and functional microglia were found within the model. The organoids also displayed functional BBB features [Sun et al., 2022b] (Fig. 1d). As a result, this study showed that assembloids could achieve vascularization and immunization simultaneously, elevating the level of maturation of especially complicated organoids such as the brain (Table 1).
Vascularizing and Immunizing Pathological Organoids for Disease Modeling
Organoid Model for Studying Infectious Diseases
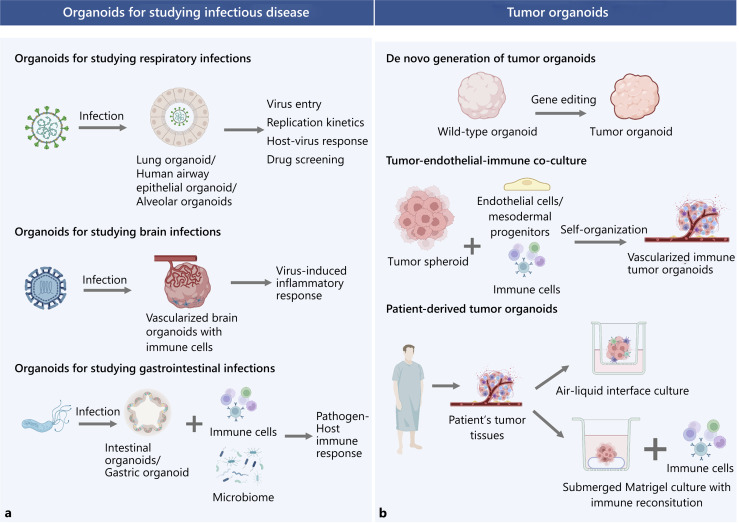
Organoids have been utilized to study respiratory, gastrointestinal, and neuronal infections caused by viruses, bacteria, parasites, fungi, etc. [Blutt and Estes, 2022]. The COVID-19 pandemic has enhanced the development of organoid technology to elucidate SARS-CoV-2 pathogenesis. Since SARS-CoV2 enters through the upper respiratory tract and mainly affects the lungs, various organoids such as human airway epithelium organoids [Milewska et al., 2020], hPSC-derived lung organoids [Duan et al., 2020], alveolar organoids [Lamers et al., 2021], have been developed to study the mechanism of virus entry, replication kinetics, host responses, and for drug screening. Although these organoids contain various cell types including epithelial cells, goblet, ciliated and alveolar cells, vasculature, and immune cells are not incorporated into the system. Cytokine storm syndrome, a hyperactive immune response, is a major cause of SARS-CoV-2 death [Thepmankorn et al., 2021]. Recent studies showed that SARS-Cov-2 not only affects specific organs but also damages the airway endothelium [Schimmel et al., 2021]. However, coculturing SARS-Cov-2 with ECs was shown to only support virus entry and replication, indicating that the effect of SARS-Cov-2 on vasculature may be organ and tissue dependent [Flaumenhaft et al., 2022]. A cardiomyocyte-macrophage coculture model by another study group also revealed that cardiomyocyte recruits macrophages upon infection, leading to macrophage-mediated hyperinflammation and apoptosis, explaining cardiac complications in some COVID-19 patients [Yang et al., 2021]. These findings highlight the importance of using organoids with vasculature and immune cells to comprehensively understand COVID-19 pathogenesis. Moreover, many pathogens including HIV, SARS-Cov-2, and ZIKA virus etc. can invade the central nervous system, leading to neuroinflammation [Dando et al., 2014] that is caused by the invasion of microglia, CD4+, and CD8+ T cells [Ní Chasaide and Lynch, 2020; Pellegrini et al., 2020]. In light of this, cerebral organoids have been developed to model the process of pathogens’ neuro-invasion [Qian et al., 2017; Depla et al., 2022]. For example, HIV-1 neuropathogenesis was studied using 3D human brain organoids composed of neurons and astrocytes cocultured with HIV-infected microglia. They found that HIV-infected microglia release inflammatory molecules such as TNFα and IL-1β that correlates with the increase in viral replication and leads to neuronal damage [Dos Reis et al., 2020]. This tri-culture model is superior to using conventional brain organoids as it demonstrated key pathological features associated with HIV-induced inflammatory response.
Furthermore, intestinal organoids [García-Rodríguez et al., 2020] or gastric organoids [Pompaiah and Bartfeld, 2017] have also been developed to study gastrointestinal infections such as H. pylori infection. Holokai L et al. cocultured H. pylori-infected gastric organoids with cytotoxic T lymphocytes (CTLs) sampled from patients and discovered that upregulation of PD-L1 on gastric epithelium inhibits CTL proliferation and prevents T cell-mediated killing of H. pylori-infected cells, which contributes to gastric cancer progression [Holokai et al., 2019]. Moreover, several models incorporating dendritic cells (DCs) discovered that there is increased recruitment of DCs into gastric organoids by chemokines, enhancing phagocytosis against H. pylori infection [Kao et al., 2010; Sebrell et al., 2019]. Since gut microbiota also affects the host’s immune response against pathogens, microbes are often injected into the lumen intestinal organoids to understand how the immune system and gut microbiome work in tandem to fight off pathogens [Cheng et al., 2019]. A human gut-on-a-chip model has been developed to incorporate common intestinal microbes to mimic the gut microenvironment, enhancing drug development [Kim et al., 2012] (Fig. 2a)
Fig. 2.
a, b A summary of methods to generate pathological organoids for disease modeling. Organoids have been used to study respiratory, brain, and gastrointestinal infections. Coculturing infected organoids with immune cells allow the precise modeling of the interactions between pathogen and host immune responses. Tumor organoids can be generated by inducing oncogenic mutations in wild-type organoids, coculturing with endothelial and immune cells, and derived from a patient’s tumor tissues. ALI culture can retain endogenous vasculature and immune cells from patients, while submerged Matrigel culture requires immune reconstitution by coculturing with autologous immune cells.
Tumor Organoids
For tumor organoids, the necrotic core is not a problem since it is also present in solid tumors caused by inadequate vascularization and other metabolic stress. However, it is still important to include vasculature in tumor organoids because tumor vasculature could promote tumor metastasis which is often the predominant cause of cancer mortality. Innate immune cells such as macrophages and monocytes can be recruited to the endothelium and enhance vascular inflammation and tumor attachment that contributes to cancer metastasis. Therefore, including the vasculature and immune cells in tumor organoid models could further enhance our understanding of the molecular mechanisms of metastasis and thus accelerate drug development to inhibit metastasis. Moreover, the tumor microenvironment (TME) is composed of a heterogeneous mixture of cells, e.g., stromal cells, blood vessels, and immune cells, as well as noncellular components, e.g., ECM, cytokines, chemokines, and growth factors. Although cancer organoids with only epithelial components are routinely used for drug screening, immune compartments are indispensable for immune-oncology or immunotherapy research [Kim et al., 2020]. The recent advances in 3D tumor organoids with vasculature and immune components provide a cost-effective and representative approach to studying certain pathogenic processes in cancer, such as angiogenesis and metastasis, and intratumoral heterogeneity. This enhances the physiological relevance of drug screening and aids the development of precision medicine. 3D tumor organoids can also serve as a platform to re-educate and expand tumor-specific T cells for adoptive cell therapy. Here, we summarized three major methods to construct 3D tumor organoids: (1) de novo generation of tumor organoids, (2) tumor spheroid-immune-endothelial coculture, (3) patient-derived tumor organoids (PTOs) (Fig. 2b).
De novo Generation of Tumor Organoids
Tumor organoids can be generated using a bottom-up approach, where PSC-derived wild-type organoids were genetically manipulated to initiate tumorigenesis [Lo et al., 2020]. Colorectal cancer models (CRC) have been developed by knocking out tumor suppressor genes, e.g., Apc, Smad4, Tp53, or knocking in oncogenes using CRISPR-Cas9 genome editing to mimic different CRC patient groups [Matano et al., 2015]. Tumor suppressor gene knockout in breast organoids can also generate breast tumor organoids which respond to chemotherapy [Dekkers et al., 2020]. Moreover, Shan Bian et al. have been able to recapitulate brain tumor formation by introducing oncogenic mutations in cerebral organoids [Bian et al., 2018]. Tumor organoids generated from gene editing enhance our understanding of the molecular events associated with tumor initiation. However, tumor progression is a result of a complex interplay between the tumor and the TME, which cannot be studied in tumor organoids generated from this method alone.
Tumor Spheroid-Immune-Endothelial Coculture
Another method to generate vascularized tumor organoids is by using a self-organizing approach. Tumor spheroids are self-assembling aggregates of tumor cells without a scaffold and can be made from homogenous cancer cell lines [Ho and Msallam, 2021]. Tumor spheroids can be cocultured with immune cells/ECs, mimicking the TME. Wörsdörfer et al. cocultured MPCs with breast cancer spheroids, in which they successfully generated a functional vasculature along with fibroblasts. These vascularized organoids are connected to the host vascular system through blood perfusion in a chick embryo model [Worsdorfer et al., 2019]. Another study cocultured human colon tumor-derived spheroids with allogenic T cells and NK cells to assess their infiltration, activation, and function in the CRC TME. Additionally, they also investigated the therapeutic potential of antibodies targeting inhibitory receptors on immune cells [Courau et al., 2019]. Another study group also cocultured ECs, fibroblasts, and native and immortalized immune cell lines to renal cell carcinoma cell lines to generate a 3D heterotypic spheroid, which was subsequently used to evaluate the efficacy of drug combination [Rausch et al., 2021]. Tri-coculture of nonsmall cell lung carcinoma spheroids with cancer-associated fibroblasts (CAFs) and monocytes recreates an immunosuppressive TME, allowing the study of macrophage polarization [Rebelo et al., 2018].
The major advantage of this coculture model is that it is easier to establish compared to a complex tumor organoid model that recapitulates the 3D architecture of TME, yet exhibits more resemblance compared to 2D coculture, therefore, it is particularly useful when studying specific immune-tumor interactions. However, the low complexity of this system may not fully recapitulate the events in the TME. For example, Gheed Al-Hity et al. developed a 3D coculture model by coculturing breast tumor spheroid and splenocytes isolated from mice that contain B cells, T cells, and DCs, which was used to study the effect of stress hormones on immune infiltration and tumor growth [Al-Hity et al., 2021]. However, this model did not incorporate important barriers in the TME that limits tumor infiltration such as stroma, vasculature, regulatory T cells, etc. [Labani-Motlagh et al., 2020]. Therefore, this oversimplified in vitro model may not accurately predict in vivo immune infiltration.
Patient-Derived Tumor Organoids
Patient-derived xenograft (PDX) is a cancer model where a patient’s tumor tissues are engrafted into immunocompromised mice and have been widely used for preclinical cancer research [Murphy, 2016]. However, PDX lacks an intact immune system which makes it hard to study immuno-oncology. Although PDX can be reconstituted with a human immune system which allows immunotherapeutic drug development, it is costly, time-consuming, and may suffer from xenograft-versus-host diseases and incomplete immune reconstitution [De La Rochere et al., 2018]. PTOs, which are generated from patients’ tumor biopsies preserve endogenous TME immune components and serve as a better alternative. PTOs can be expanded long-term in vitro which can be used for high-throughput drug screening, which is a more cost-effective personalized cancer model compared to PDX [Yang et al., 2018].
There are two major approaches to culture PTOs, coculturing PTOs with autologous immune cells or PTO that contains endogenous immune cells without reconstitution. However, when PTO is cultured using a submerged Matrigel method, in which dissociated tumor cells are embedded in a dome-shaped 3D Matrigel submerged in a culture medium, only endogenous epithelial components of tumor cells are retained [Sun et al., 2022a]. Many reconstitution approaches have been developed for incorporating immune cells. In one study, they isolated bone marrow-derived DCs and activated them with tumor-conditioned medium containing tumor-associated antigens. The conditioned DCs were then cocultured with autologous CTLs and mouse or human gastric cancer organoids. Using this model, they demonstrated that inhibition of PD-L1 on tumors leads to cytotoxic T cell-induced tumor apoptosis [Chakrabarti et al., 2018]. To study the interactions between pancreatic ductal adenocarcinoma (PDAC) and CAF, one of the contributing factors of chemotherapy resistance, Giulia Biffi et al. cocultured CAFs with PDAC organoids. Using this model, they discovered that TGF-β and IL-1 secreted by PDAC contribute to CAF heterogeneity, hence resulting in ineffective targeting by chemotherapy [Biffi et al., 2019]. Moreover, Ethan Shelkey developed an immune-reactive tumor organoid model by coculturing CTLs with breast cancer in a hydrogel mixture, allowing the study of the effect of the microbiome on immune cells and the efficacy of immune checkpoint blockade in the TME [Shelkey et al., 2022].
PTOs can also be cocultured with peripheral blood mononuclear cells that lead to the reconstitution of multiple immune cell types. For instance, Krijn K Dijkstra et al. analyzed tumor-specific T cell responses by coculturing autologous epithelial tumor organoids with peripheral blood mononuclear cells, and this model clearly documented the process of tumor antigen-mediated CD8+ T cell proliferation [Dijkstra et al., 2018].
Nonetheless, PTOs can also be used to evaluate the cytotoxicity of CAR immune cells. Coculture CAR-NK92 cells targeting colon PDO and CAR-NK92 cells were shown to only kill tumor organoids carrying a solid tumor-specific antigen epidermal growth factor receptor variant III, whilst sparing normal organoids. This demonstrated that PDO can act as an ideal in vitro platform to study patient-specific responses to CAR immune cell therapy [Schnalzger et al., 2019].
Using air-liquid interface culture (ALI) allows PTOs to preserve their endogenous stroma and immune cells, giving insight into the syngeneic interactions between tumors and infiltrating immune cells without reconstitution [Yang et al., 2018]. Dissociated tumor fragments are embedded in a collagen gel layer in an inner Transwell dish, and culture medium is added to the outer Transwell such that the top half of the organoid is exposed to air whilst the bottom half is submerged in the medium, increasing oxygen supply to cells compared to the aforementioned submerged Matrigel method and therefore recapitulate the TME better. PTO from primary and metastatic tumors cultured using the ALI method was shown to retain stromal myofibroblast, CD3+ T cells with TCR repertoires that match the original tumor biopsies, macrophages, NK, NKT, etc. CD8+ T cells were activated upon PD-1/PD-L1 blockade, showing the potential application of ALI-cultured organoids for testing the efficacy of immune checkpoint blockade treatments. However, a key limitation of this approach is that the immune and stromal cells can only be maintained for a short time. Despite the addition of IL2 or anti-CD3/anti-CD28 to prolong the half-life of TILs and TAMs, they normally cannot survive in the culture for more than 2 months [Neal et al., 2018].
Discussion
In the development of organoids vascularization and immunization, the in vivo engraftment method contributed to much early progress. While the vascular-rich and more complex engraft destination endowed the organoids with a better chance to mature and develop higher level functions, graft-versus-host incompatibility still poses concerns. Fortunately, the advancement of hypoimmunogenic stem cells comes into aid. Using stem cells that are immunologically compatible could be a solution, which has been applied by regenerative medicine researchers. For instance, the generation of hypoimmunogenic hiPSCs solicited T-cell and macrophage responses [Votanopoulos et al., 2020]. They used RNA interference and antibody to block the expression of HLA class I, which resulted in alleviated immune rejection and engraftment survival in both xenogeneic and allogeneic settings [Bergdorf et al., 2020; Votanopoulos et al., 2020]. More recent advances utilized genetic engineering to create hypoimmunogenic stem cells. Knocking out partial or entire HLA class I gene and HLA class II genes in hPSCs successfully blunted adaptive immune rejection [Abud et al., 2017; Matheus et al., 2022]. Researchers also tried to simultaneously knock in CD47 [Matheus et al., 2022], PD-1, and HLA-G [Abud et al., 2017; Schreurs et al., 2019] to further suppress T-cell response and innate immune rejection. A similar approach was seen in Parent et al.’s paper, which deleted the majority of HLAs while retaining HLA-A2 and achieved similar results without concern about the lack of immune surveillance. Although promising progress has been made, hypoimmunogenic stem cells may still have limited applications. One apparent shortcoming is their limited ability in generating hematopoietic progenies with ablated immune modules [Ohta et al., 2019]. Plus, multiple rounds of genetic manipulation could have unwanted off-target effects [Ohta et al., 2019]. Therefore, the hypoimmunogenic stem cell strategy still awaits to be improved.
Current methods which rely on coculturing organoids with different cell types have indeed opened possibilities like vascularization and immunization within model systems. However, mixing cells with different identities does not guarantee physiologically relevant spatial distribution and organization. For instance, coculturing tumor organoids with specific immune cells can only be used to study specific immune-tumor interactions but cannot fully recapitulate the 3D architecture of the TME. Moreover, incorporating more immune cell types in a coculture system is increasingly difficult since the culture conditions are compromised between different cell types. Current protocols are not standardized and reproducible, which makes large-scale drug screening infeasible. For example, the source and ratio of immune cell type added to the coculture system are variables between research groups. Although using the ALI method for PTOs could retain a broader immune population, the short half-life of immune cells remains a challenge. Recently, immune-enhanced PTOs derived from melanoma patients have been developed by including their peripheral blood and lymph nodes. This retains 80% of the patient’s immune system, including an abundance of antigen-presenting cells, suggesting the potential use of lymph node tissues in reconstituting immune components [Votanopoulos et al., 2020]. A gentler extraction technique can be also developed to enhance the half-life of immune cells in PTOs. Bergdorf et al. developed a fine-needle aspiration method to extract PTOs, and results showed enhanced immune cell count, viability, and survival compared to conventional digestion methods [Bergdorf et al., 2020]. Assembloid models mostly focused on brain research, and limited studies have applied this technique to other contexts such as cancer. Previously, we have established a vascularized immune organoid (VIO) system from hPSCs which are composed of diverse immune cell types including monocytes, macrophages, NK, erythroblasts, and ECs [Ohta et al., 2019]. Generating assembloids by fusing VIO with other physiological organoids could model organ-specific hyperinflammation and cytokine storms caused by pathogens. When fusing VIO with cancer cells, we are anticipating creating tumor assembloids that highly resemble in vivo TME. Combining cutting-edge technologies such as spatial transcriptomics, single-cell profiling, and live imaging, we can screen for the deterministic factors in disease progression and use them as a platform to test for immunotherapeutic drugs. However, future work has to be done to optimize the culture conditions and the fusion ratio of the organoids to achieve a cellular composition that accurately mimics the in vivo environment.
Conclusion
Human organoids are powerful tools that overcome the limitations of existing in vitro 2D monolayer cultures and in vivo animal models in disease modeling and drug screening. However, organoids lacking vasculature and immune cells hinder their application in preclinical and clinical immune-oncology research. Although strategies have been developed to vascularize and immunize organoids through coculturing with endothelial and immune cells, the model may not 100% recapitulate in vivo settings due to its low cellular complexity and physiologically irrelevant spatial distribution of tissues. Assembloid technology brings hope to vascularizing and immunizing organoids simultaneously by fusing immune organoids or blood vessel organoids with other physiological organoids and tumor tissues. Vascularized organoids with immune cells can benefit preclinical drug screening and the development of precision medicine for immunotherapy.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
S.Y. and N.W. wrote the manuscript. R.S. guided the manuscript.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 2017;94(2):278–93.e9. 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hity G, Yang F, Campillo-Funollet E, Greenstein AE, Hunt H, Mampay M, et al. An integrated framework for quantifying immune-tumour interactions in a 3D co-culture model. Commun Biol. 2021;4(1):781–12. 10.1038/s42003-021-02296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Ephraim YE, Kretzschmar K, Asra P, de Jongh E, Boonekamp KE, Drost J, et al. Modelling cancer immunomodulation using epithelial organoid cultures. bioRxiv. 2018:377655. [Google Scholar]
- Bergdorf K, Phifer C, Bharti V, Westover D, Bauer J, Vilgelm A, et al. High-throughput drug screening of fine-needle aspiration-derived cancer organoids. STAR Protoc. 2020;1(3):100212. 10.1016/j.xpro.2020.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann S, Lawler SE, Qu Y, Fadzen CM, Wolfe JM, Regan MS, et al. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc. 2018;13(12):2827–43. 10.1038/s41596-018-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, et al. Genetically engineered cerebral organoids model brain tumor formation. Nat Methods. 2018;15(8):631–9. 10.1038/s41592-018-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, et al. IL1-Induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9(2):282–301. 10.1158/2159-8290.CD-18-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545(7652):54–9. 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell. 2018;175(5):1307–20.e22. 10.1016/j.cell.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutt SE, Estes MK. Organoid models for infectious disease. Annu Rev Med. 2022;73:167–82. 10.1146/annurev-med-042320-023055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B, Tanaka Y, Kiral FR, Xiang Y, Dagliyan O, Wang J, et al. Expression of the transcription factor PU.1 induces the generation of microglia-like cells in human cortical organoids. Nat Commun. 2022;13(1):430. 10.1038/s41467-022-28043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019;16(11):1169–75. 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti J, Holokai L, Syu L, Steele NG, Chang J, Wang J, et al. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget. 2018;9(100):37439–57. 10.18632/oncotarget.26473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenchula S, Kumar S, Babu S. Comparitive efficacy of 3dimensional (3D) cell culture organoids vs. 2dimensional (2D) cell cultures vs. experimental animal models in disease modeling, drug development, and drug toxicity testing. Int J Cur Res Rev. 2019. [Google Scholar]
- Chen Y, Feng J, Zhao S, Han L, Yang H, Lin Y, et al. Long-term engraftment promotes differentiation of alveolar epithelial cells from human embryonic stem cell derived lung organoids. Stem Cells Dev. 2018;27(19):1339–49. 10.1089/scd.2018.0042. [DOI] [PubMed] [Google Scholar]
- Cheng H-Y, Ning M-X, Chen D-K, Ma W-T. Interactions between the gut microbiota and the host innate immune response against pathogens. Front Immunol. 2019;10:607. 10.3389/fimmu.2019.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courau T, Bonnereau J, Chicoteau J, Bottois H, Remark R, Assante Miranda L, et al. Cocultures of human colorectal tumor spheroids with immune cells reveal the therapeutic potential of MICA/B and NKG2A targeting for cancer treatment. J Immunother Cancer. 2019;7(1):74–14. 10.1186/s40425-019-0553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando SJ, Mackay-Sim A, Norton R, Currie BJ, St John JA, Ekberg JAK, et al. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin Microbiol Rev. 2014;27(4):691–726. 10.1128/CMR.00118-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviaud N, Friedel RH, Zou H. Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. eNeuro. 2018;5(6):ENEURO.0219–18.2018. 10.1523/ENEURO.0219-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers JF, Whittle JR, Vaillant F, Chen H-R, Dawson C, Liu K, et al. Modeling breast cancer using CRISPR-Cas9: mediated engineering of human breast organoids. J Natl Cancer Inst. 2020;112(5):540–4. 10.1093/jnci/djz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Rochere P, Guil-Luna S, Decaudin D, Azar G, Sidhu SS, Piaggio E. Humanized mice for the study of immuno-oncology. Trends Immunol. 2018;39(9):748–63. 10.1016/j.it.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Depla JA, Mulder LA, de Sá RV, Wartel M, Sridhar A, Evers MM, et al. Human brain organoids as models for central nervous system viral infection. Viruses. 2022;14(3):634. 10.3390/v14030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37(3):252–8. 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuse T, Seifert M, Phillips N, Fire A, Tyan D, Kay M, et al. Human leukocyte antigen I knockdown human embryonic stem cells induce host ignorance and achieve prolonged xenogeneic survival. Circulation. 2011a;124(11 Suppl):S3–9. 10.1161/CIRCULATIONAHA.111.020727. [DOI] [PubMed] [Google Scholar]
- Deuse T, Seifert M, Tyan D, Tsao PS, Hua X, Velden J, et al. Immunobiology of naïve and genetically modified HLA-class-I-knockdown human embryonic stem cells. J Cell Sci. 2011b;124(Pt 17):3029–37. 10.1242/jcs.087718. [DOI] [PubMed] [Google Scholar]
- Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174(6):1586–98.e12. 10.1016/j.cell.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Reis RS, Sant S, Keeney H, Wagner MCE, Ayyavoo V. Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Sci Rep. 2020;10(1):15209–17. 10.1038/s41598-020-72214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Han Y, Yang L, Duan F, Nilsson-Payant BE, Yaron TM, et al. Identification of candidate COVID-19 therapeutics using hPSC-derived lung organoids. bioRxiv. 2020:2020.05.05.079095. 10.1101/2020.05.05.079095. [DOI] [Google Scholar]
- Dye BR, Dedhia PH, Miller AJ, Nagy MS, White ES, Shea LD, et al. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife. 2016;5:e19732. 10.7554/eLife.19732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BR, Hill DR, Ferguson MAH, Tsai YH, Nagy MS, Dyal R, et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4:e05098. 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figtree GA, Bubb KJ, Tang O, Kizana E, Gentile C. Vascularized cardiac spheroids as novel 3D in vitro models to study cardiac fibrosis. Cells Tissues Organs. 2017;204(3–4):191–8. 10.1159/000477436. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R, Enjyoji K, Schmaier AA. Platelets: out of shape and misbehaving. Blood. 2022;140(21):2188–90. 10.1182/blood.2022018189. [DOI] [PubMed] [Google Scholar]
- García-Rodríguez I, Sridhar A, Pajkrt D, Wolthers KC. Put some guts into it: intestinal organoid models to study viral infection. Viruses. 2020;12(11):1288. 10.3390/v12111288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Wang M, Duan S, Franco PJ, Kenty JH-R, Hedrick P, et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc Natl Acad Sci U S A. 2019;116(21):10441–6. 10.1073/pnas.1902566116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T, Msallam R. Tissues and tumor microenvironment (TME) in 3D: models to shed light on immunosuppression in cancer. Cells. 2021;10(4):831. 10.3390/cells10040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holokai L, Chakrabarti J, Broda T, Chang J, Hawkins JA, Sundaram N, et al. Increased programmed death-ligand 1 is an early epithelial cell response to Helicobacter pylori infection. PLoS Pathog. 2019;15(1):e1007468. 10.1371/journal.ppat.1007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Gehart H, Van Boxtel R, Hamer K, Blokzijl F, Verstegen MMA, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160(1–2):299–312. 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, et al. Helicobacter pylori immune escape is mediated by dendritic cell: induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138(3):1046–54. 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12(12):2165–74. 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- Kim J, Koo B-K, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21(10):571–84. 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. 2020;11:940. 10.3389/fimmu.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers MM, van der Vaart J, Knoops K, Riesebosch S, Breugem TI, Mykytyn AZ, et al. An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells. EMBO J. 2021;40(5):e105912. 10.15252/embj.2020105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528(7583):560–4. 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y-H, Karlsson K, Kuo CJ. Applications of organoids for cancer biology and precision medicine. Nat Cancer. 2020;1(8):761–73. 10.1038/s43018-020-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36(5):432–41. 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, et al. Modeling colorectal cancer using CRISPR-Cas9: mediated engineering of human intestinal organoids. Nat Med. 2015;21(3):256–62. 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- Matheus F, Raveh T, Oro AE, Wernig M, Drukker M. Is hypoimmunogenic stem cell therapy safe in times of pandemics? Stem Cell Rep. 2022;17(4):711–4. 10.1016/j.stemcr.2022.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewska A, Kula-Pacurar A, Wadas J, Suder A, Szczepanski A, Dabrowska A, et al. Replication of severe acute respiratory syndrome coronavirus 2 in human respiratory epithelium. J Virol. 2020;94(15):e00957–20. 10.1128/JVI.00957-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Hill DR, Nagy MS, Aoki Y, Dye BR, Chin AM, et al. In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Rep. 2018;10(1):101–19. 10.1016/j.stemcr.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33(11):1193–200. 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JF. Patient-derived xenograft (PDX) models: an emerging platform for cancer drug development and translational research. MOJ Immunol. 2016;3(4):00094. 10.15406/moji.2016.03.00094. [DOI] [Google Scholar]
- Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175(7):1972–88.e16. 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ní Chasaide C, Lynch MA. The role of the immune system in driving neuroinflammation. Brain Neurosci Adv. 2020;4:2398212819901082. 10.1177/2398212819901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta R, Sugimura R, Niwa A, Saito MK. Hemogenic endothelium differentiation from human pluripotent stem cells in a feeder-and xeno-free defined condition. J Vis Exp. 2019;(148):e59823. 10.3791/59823-v. [DOI] [PubMed] [Google Scholar]
- Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, et al. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27(6):951–61.e5. 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, et al. Generation of human vascularized brain organoids. Neuroreport. 2018;29(7):588–93. 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonchuk L, Chabria M, Badi L, Hoflack JC, Figtree G, Davies MJ, et al. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci Rep. 2017;7(1):7005. 10.1038/s41598-017-06385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompaiah M, Bartfeld S. Gastric organoids: an emerging model system to study Helicobacter pylori pathogenesis. Curr Top Microbiol Immunol. 2017;400:149–68. 10.1007/978-3-319-50520-6_7. [DOI] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Jacob F, Song H, Ming G-L. Using brain organoids to understand Zika virus-induced microcephaly. Development. 2017;144(6):952–7. 10.1242/dev.140707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch M, Blanc L, De Souza Silva O, Dormond O, Griffioen AW, Nowak-Sliwinska P. Characterization of renal cell carcinoma heterotypic 3D co-cultures with immune cell subsets. Cancers. 2021;13(11):2551. 10.3390/cancers13112551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo SP, Pinto C, Martins TR, Harrer N, Estrada MF, Loza-Alvarez P, et al. 3D-3-culture: a tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials. 2018;163:185–97. 10.1016/j.biomaterials.2018.02.030. [DOI] [PubMed] [Google Scholar]
- Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, et al. Maturation and circuit integration of transplanted human cortical organoids. Nature. 2022;610(7931):319–26. 10.1038/s41586-022-05277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh TT, Chen Y, Paul HT, Guo C, Kaplan DL. 3D bioengineered tissue model of the large intestine to study inflammatory bowel disease. Biomaterials. 2019;225:119517. 10.1016/j.biomaterials.2019.119517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel L, Chew KY, Stocks CJ, Yordanov TE, Essebier P, Kulasinghe A, et al. Endothelial cells are not productively infected by SARS-CoV-2. Clin Transl Immunol. 2021;10(10):e1350. 10.1002/cti2.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnalzger TE, de Groot MH, Zhang C, Mosa MH, Michels BE, Röder J, et al. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019;38(12):e100928. 10.15252/embj.2018100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs RRCE, Baumdick ME, Sagebiel AF, Kaufmann M, Mokry M, Klarenbeek PL, et al. Human fetal TNF-alpha-cytokine-producing CD4(+) effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity. 2019;50(2):462–76.e8. 10.1016/j.immuni.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Sebrell TA, Hashimi M, Sidar B, Wilkinson RA, Kirpotina L, Quinn MT, et al. A novel gastric spheroid co-culture model reveals chemokine-dependent recruitment of human dendritic cells to the gastric epithelium. Cell Mol Gastroenterol Hepatol. 2019;8(1):157–71.e3. 10.1016/j.jcmgh.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelkey E, Oommen D, Stirling ER, Soto-Pantoja DR, Cook KL, Lu Y, et al. Immuno-reactive cancer organoid model to assess effects of the microbiome on cancer immunotherapy. Sci Rep. 2022;12(1):9983–13. 10.1038/s41598-022-13930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel HA, Moss SM, Hoying JB. Methods for vascularization and perfusion of tissue organoids. Mamm Genome. 2022;33(3):437–50. 10.1007/s00335-022-09951-2. [DOI] [PubMed] [Google Scholar]
- Sun C-P, Lan H-R, Fang X-L, Yang X-Y, Jin K-T. Organoid models for precision cancer immunotherapy. Front Immunol. 2022a;13:770465. 10.3389/fimmu.2022.770465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XY, Ju XC, Li Y, Zeng PM, Wu J, Zhou YY, et al. Generation of vascularized brain organoids to study neurovascular interactions. Elife. 2022b;11:e76707. 10.7554/eLife.76707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526(7574):564–8. 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- Tan Q, Choi KM, Sicard D, Tschumperlin DJ. Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials. 2017;113:118–32. 10.1016/j.biomaterials.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepmankorn P, Bach J, Lasfar A, Zhao X, Souayah S, Chong ZZ, et al. Cytokine storm induced by SARS-CoV-2 infection: the spectrum of its neurological manifestations. Cytokine. 2021;138:155404. 10.1016/j.cyto.2020.155404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varzideh F, Pahlavan S, Ansari H, Halvaei M, Kostin S, Feiz MS, et al. Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials. 2019;192:537–50. 10.1016/j.biomaterials.2018.11.033. [DOI] [PubMed] [Google Scholar]
- Vogt N. Assembloids. Nat Methods. 2021;18(1):27. 10.1038/s41592-020-01026-x. [DOI] [PubMed] [Google Scholar]
- Votanopoulos KI, Forsythe S, Sivakumar H, Mazzocchi A, Aleman J, Miller L, et al. Model of patient-specific immune-enhanced organoids for immunotherapy screening: feasibility study. Ann Surg Oncol. 2020;27(6):1956–67. 10.1245/s10434-019-08143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CL, Mahe MM, Munera J, Howell JC, Sundaram N, Poling HM, et al. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20(11):1310–4. 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsdorfer P, Dalda N, Kern A, Kruger S, Wagner N, Kwok CK, et al. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci Rep. 2019;9(1):15663. 10.1038/s41598-019-52204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, et al. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol. 2012;23(11):1857–68. 10.1681/ASN.2012050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Boreland AJ, Li X, Erickson C, Jin M, Atkins C, et al. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Rep. 2021;16(8):1923–37. 10.1016/j.stemcr.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Sun L, Liu M, Mao Y. Patient-derived organoids: a promising model for personalized cancer treatment. Oxford University Press; 2018. p. 243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Han Y, Jaffré F, Nilsson-Payant BE, Bram Y, Wang P, et al. An immuno-cardiac model for macrophage-mediated inflammation in COVID-19 hearts. Circ Res. 2021;129(1):33–46. 10.1161/CIRCRESAHA.121.319060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wan Z, Kamm RD. Vascularized organoids on a chip: strategies for engineering organoids with functional vasculature. Lab Chip. 2021;21(3):473–88. 10.1039/d0lc01186j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Lei A, Tian L, Wang X, Correia C, Weiskittel T, et al. Strategies for genetically engineering hypoimmunogenic universal pluripotent stem cells. iScience. 2020;23(6):101162. 10.1016/j.isci.2020.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]