Abstract
Objectives
To investigate the association between the neutrophil-to-lymphocyte ratio (NLR) and psoriasis.
Design
Cross-sectional study.
Setting
National Health and Nutrition Examination Survey 2011–2014.
Participants
A subsample of 8387 individuals aged 18 years and older were screened for inclusion, of whom 238 reported a diagnosis of psoriasis.
Primary and secondary outcome measures
Psoriasis and the severity of psoriasis were defined according to participants’ self-reports. Weighted logistic regression, subgroup and restricted cubic spline (RCS) analyses were conducted to estimate the potential relationship of the NLR with psoriasis.
Results
In the fully adjusted models, the fourth quartile of the NLR was significantly and positively associated with the presence of psoriasis using the first quartile as a reference (OR: 2.22, 95% CI: 1.27 to 3.87, p=0.01). Elevated NLR was associated with an increased odds of having more severe psoriasis for the highest quartile (vs the lowest quartile), with an OR of 2.43 (95% CI: 1.10 to 5.36, p=0.003). The association between the NLR and psoriasis differed across prespecified subgroups by age, sex, race, income and education. A non-linear correlation of the NLR with psoriasis was observed using univariable and multivariable RCS (all p for non-linearity <0.05).
Conclusions
The NLR was non-linearly and positively correlated with the presence of psoriasis, and our findings suggest a significant association between the NLR and the severity of psoriasis. The potential role and value in the clinical diagnosis and prognostic assessment of the NLR in psoriasis calls for further longitudinal studies.
Keywords: psoriasis, public health, epidemiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study included the use of a nationally representative sample and adopted strict methods of statistical adjustment to minimise potential confounding.
We have explored and identified for the first time the non-linear relationship between the neutrophil-to-lymphocyte ratio and psoriasis.
Causal inferences cannot be made, as this was a cross-sectional observational study.
The observed outcomes may be subject to recall bias because the diagnosis and severity of psoriasis were based on respondents’ self-reports.
Unknown and unmeasured confounders may have impacted our estimates.
Introduction
Psoriasis is a chronic and disfiguring skin disease affecting multiple systems and organs throughout the body that imposes tremendous physical and psychological burdens.1 Approximately 3% of the population and an estimated 7.5 million adults in the USA have received a diagnosis of psoriasis.2 It afflicts men and women at all ages in all countries.3 People living with psoriasis are at a higher risk of developing other severe systemic diseases than the general population, most commonly cardiovascular diseases (CVDs) and metabolic syndrome. Numerous studies have suggested associations between psoriasis and other comorbidities, such as gastrointestinal disease, kidney disease, malignancy and mood disorders.4 Psoriasis is an incurable disease that substantially impairs patients’ quality of life, and a large number of people suffer unnecessarily from psoriasis due to poor or delayed diagnosis, inadequate therapy, inappropriate care and social stigma.3 Therefore, the pressing need for increased awareness regarding psoriasis should be recognised.
Over the last two decades, the systemic inflammatory response induced by T lymphocytes has been considered to be predominant in the etiopathogenesis of psoriasis.5 The neutrophil-to-lymphocyte ratio (NLR) is an inexpensive and validated marker of systemic inflammation that can be readily calculated from existing datasets of routine laboratory tests.6 The NLR was first devised to offer a convenient and efficient measure to assess the intensity of systemic inflammation in critically ill patients following stressful events7 but later proved to exhibit prognostic value for clinical outcomes in various diseases.8–11 In recent years, this index has gained much attention owing to its wide availability and ease of access.12 13 A published study has reported that an increasing NLR is a risk factor for mortality related to heart disease, chronic lower respiratory disease and kidney disease.14 An increased NLR has been suggested as a predictor of poor survival in individuals with cancer.15 Moreover, there is a rapidly evolving body of literature indicating the presence of abnormal NLR in some psychiatric disorders.16–18
Since inflammation plays a pivotal role in the causative mechanisms of psoriasis, several researchers have sought to shed light on the involvement of the NLR in psoriasis. Emerging evidence indicates that the NLR and psoriasis are closely associated.19–22 However, previous studies were primarily limited by the relatively small enrolment of participants, and results on the relationship between the NLR and psoriasis severity remain inconclusive.23 24 Hence, we processed data from the National Health and Nutrition Examination Survey (NHANES) from 2011 through 2014 to carry out a large-scale study based on the US civilian population. Our purposes were to unravel the potential association of the NLR with psoriasis, and to clarify whether the NLR could be a valuable parameter indicating the extent of inflammation and disease in patients with psoriasis .
Methods
Study design and participants
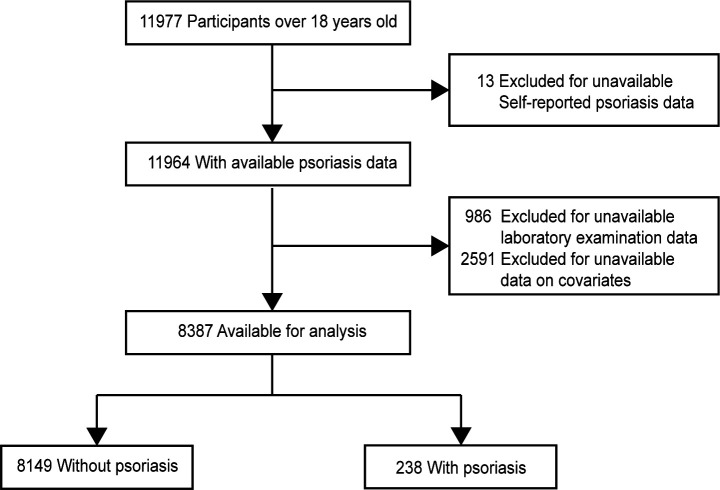
The NHANES is a biennial cross-sectional survey with the aim of tracking and evaluating the health and dietary nutrition status of community-dwelling US populations.25 The survey employs a complex, multistage cluster sampling method to ensure that it is representative of the nation as a whole.26 In this study, data from two NHANES cycles (2011–2012 and 2013–2014) were extracted for investigation, as these two cycles offer the most updated information on psoriasis.27 Our analyses were performed in conjunction with appropriate sampling weights to obtain unbiased estimates from the complicated NHANES sampling design.26 We included adult participants and excluded individuals who had missing or implausible data on self-reported psoriasis, neutrophil count or lymphocyte count and those with missing covariates. As a result, a total of 8387 individuals were ultimately included in the pool of eligible people. The flowchart of participant inclusion and exclusion is depicted in figure 1. NHANES was approved by the National Center for Health Statistics Institutional Review Board, and participants provided written informed consent.
Figure 1.
Flow diagram of participants screened from the National Health and Nutrition Examination Survey (NHANES) 2011 to 2014. Covariates included age, sex, race, income, education, body mass index, smoking, alcohol consumption, history of hypertension, diabetes and cardiovascular disease.
Diagnosis of psoriasis and measurement of the NLR
The outcome was a diagnosis of psoriasis based on a self-reported history of being told by a physician that they had psoriasis. To evaluate the severity levels of skin involvement, respondents were then asked to complete a set of questionnaires containing questions on the extent of psoriasis plaques on the body gauged by the number of palm-sized patches. Participants were needed to characterise their psoriasis into a category, including (1) little or no psoriasis, (2) only a few patches, (3) scattered patches and (4) extensive psoriasis. For the sake of avoiding an increase in sampling error, we merged (2), (3) and (4), which had smaller frequencies.
Our predictor variable of prime interest was the NLR, calculated by dividing the neutrophil count by the lymphocyte count, which can be derived from laboratory data. Blood specimen collection was undertaken at mobile examination centres. The Beckman Coulter methodology was applied to determine complete blood count parameters.28
Assessment of covariates
On the basis of published literature, we considered sociodemographic,29 lifestyle30 and comorbid factors31 32 that may affect both psoriasis and NLR as potential confounders, including age, sex, race, poverty income ratio (PIR), education, body mass index (BMI), smoking, alcohol consumption, and a history of hypertension, diabetes and CVD. We used the same terminology as NHANES to describe racial categories. The PIR was measured by dividing the household’s or individual’s income by a specific poverty guideline. We classified the PIR into three levels: low income (≤1.3), medium income (>1.3 to 3.5) and high income (>3.5). Educational attainment was grouped as high school or less, some college or associates (AA) degree and college graduate or above. Smokers were separated into the following categories: never smokers (those who have either never smoked or have smoked less than 100 cigarettes during their lifetime), former smokers (smoked at least 100 cigarettes but had quit currently) and current smokers. We defined alcohol drinkers as those who had consumed at least 12 drinks in any given year. Comorbid conditions were ascertained by respondent self-reports.
Statistical analysis
We used the Strengthening the Reporting of Observational Studies in Epidemiology cross-sectional checklist when writing our report.33 We first compared the baseline characteristics among individuals with and without psoriasis, using Student’s t-test for normally distributed quantitative variables, the non-parametric Kruskal-Wallis test for skewed quantitative variables, and the χ2 test for qualitative variables. Descriptive statistics are presented as the mean (SD) or median (IQR) for continuous variables and the number (percentage) of participants for categorical variables. Binary and multinomial logistic regression models were then fitted to estimate the relation of the NLR with psoriasis and psoriasis lesion severity, respectively. Three different models were developed. Model 1 was a basic unadjusted model. In Model 2, adjustments were made for age, sex, ethnicity, PIR and educational attainment. Model 3 included all variables in Model 2 plus BMI, smoking, alcohol consumption status and medical comorbidities (hypertension, diabetes and CVD). Linear trend tests were performed by treating the median concentration of each NLR quartile as a continuous variable. We entered the NLR into logistic regression analysis as a continuous variable and as a quartile categorical variable to explore the strength of risk association with psoriasis. Stratified analyses were conducted using multivariate logistic regression according to age (18–39, 40–59, 60–79 and ≥80 years), sex, race, PIR and education at baseline, incorporating a two-way interaction term between the NLR and subgroup status. We then used the restricted cubic spline (RCS) regressions with four knots to detect the possible non-linear relationship of the NLR with psoriasis. All analyses were performed using R software (V.4.1.3). P<0.05 (two-sided) was considered indicative of statistical significance.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct or reporting or dissemination plans of this research.
Results
Of 11 977 participants aged over 18 years from the 2011 to 2014 NHANES cycles, those with missing relevant data (n=3590) were excluded from the analyses, leaving 238 adults with psoriasis and 8149 adults without psoriasis for inclusion. A total of 4254 were females (50.7%), and 4133 were males (49.3%), with an average age of 48.7 years.
Table 1 illustrates the demographic, lifestyle and clinical features of the included subjects stratified by the presence and absence of psoriasis. In comparison to the group without psoriasis, individuals with psoriasis were older (51.3 vs 47.2, p<0.001) and more likely to be non-Hispanic whites (79.7% vs 68.4%, p=0.001) but less likely to be current smokers (10.9% vs 20.0%, p<0.001). The prevalence of hypertension (42.2% vs 32.7%, p=0.032), diabetes (16.7% vs 10.0%, p=0.006) and CVD (12.1% vs 8.2%, p=0.024) at baseline was higher among participants with psoriasis than among those without psoriasis. In addition, higher NLR levels were observed in patients who had psoriasis (2.4 vs 2.0, p<0.001). The psoriasis and non-psoriasis groups did not differ significantly with regard to sex, income, educational attainment, BMI or alcohol consumption.
Table 1.
Baseline characteristics of participants with and without psoriasis
| Characteristics | Without psoriasis (n=8149) | With psoriasis (n=238) | P value |
| Age, mean (SD), years | 47.2 (17.0) | 51.3 (15.4) | <0.001 |
| Sex | 0.408 | ||
| Female | 4128 (51.0) | 126 (53.8) | |
| Male | 4021 (49.0) | 112 (46.2) | |
| Race | 0.001 | ||
| Mexican American | 904 (7.9) | 15 (4.1) | |
| Other Hispanic | 759 (5.8) | 24 (5.1) | |
| Non-Hispanic white | 3467 (68.4) | 136 (79.7) | |
| Non-Hispanic black | 1842 (10.7) | 28 (4.8) | |
| Non-Hispanic Asian | 931 (4.6) | 26 (4.0) | |
| Other race | 246 (2.6) | 9 (2.3) | |
| Poverty income ratio | 0.81 | ||
| Low | 2793 (23.9) | 84 (22.7) | |
| Medium | 2800 (34.5) | 76 (33.8) | |
| High | 2556 (41.6) | 78 (43.5) | |
| Education | 0.405 | ||
| High school or less | 3444 (35.4) | 91 (30.4) | |
| Some college or AA degree | 2559 (32.8) | 79 (33.4) | |
| College graduate or above | 2146 (31.8) | 68 (36.2) | |
| BMI | 0.071 | ||
| Underweight (<18.5) | 133 (1.4) | 0 (0.0) | |
| Normal (18.5 to <25) | 2382 (28.8) | 52 (20.8) | |
| Overweight (25 to <30) | 2618 (33.2) | 89 (41.6) | |
| Obese (30 or greater) | 3016 (36.6) | 97 (37.6) | |
| Smoking status | <0.001 | ||
| Never smoker | 4594 (56.2) | 121 (49.4) | |
| Former smoker | 1888 (23.9) | 82 (39.7) | |
| Current smoker | 1667 (20.0) | 35 (10.9) | |
| Alcohol drinker | 0.899 | ||
| No | 2157 (20.6) | 65 (20.3) | |
| Yes | 5992 (79.4) | 173 (79.7) | |
| Hypertension | 0.032 | ||
| No | 5207 (67.3) | 129 (57.8) | |
| Yes | 2942 (32.7) | 109 (42.2) | |
| Diabetes | 0.006 | ||
| No | 7090 (90.0) | 190 (83.3) | |
| Yes | 1059 (10.0) | 48 (16.7) | |
| History of CVD | 0.024 | ||
| No | 7352 (91.8) | 197 (87.9) | |
| Yes | 797 (8.2) | 41 (12.1) | |
| NLR | 2.0 (1.5, 2.6) | 2.4 (1.8, 3.2) | <0.001 |
Median (SD) or median (IQR) for continuous; n (%) for categorical.
AA, associates; BMI, body mass index; CVD, cardiovascular disease; NLR, neutrophil-to-lymphocyte ratio.
The results of binary logistic regression are summarised in table 2. In univariate models, NLR as a continuous variable was associated with a 19% increased risk of psoriasis (OR: 1.19, 95% CI: 1.11 to 1.28, p<0.001), and the OR for quartile 4 was significantly higher than the OR for quartile 1 (Q4 vs Q1—OR: 2.62, 95% CI: 1.58 to 4.32, p<0.001). The association between the NLR and psoriasis persisted even after adjusting for sociodemographic variables (OR: 1.16, 95% CI: 1.08 to 1.24, p<0.001). In the fully adjusted models, those with the highest quartile of the NLR had more than two times greater odds of having psoriasis than those with the lowest quartile (Q4 vs Q1—OR: 2.22, 95% CI: 1.27 to 3.87, p=0.01).
Table 2.
Association between the NLR and the presence of psoriasis
| Variable | Model 1 | Model 2 | Model 3 | |||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| NLR (continuous) | 1.19 (1.11, 1.28) | <0.001 | 1.16 (1.08, 1.24) | <0.001 | 1.15 (1.05, 1.25) | 0.006 |
| NLR (quartile) | ||||||
| Q1 (≤1.47) | Reference | Reference | Reference | |||
| Q2 (1.47–1.96) | 1.14 (0.67, 1.94) | 0.613 | 1.05 (0.61, 1.82) | 0.849 | 1.05 (0.59, 1.89) | 0.848 |
| Q3 (1.96–2.63) | 1.48 (0.93, 2.36) | 0.095 | 1.34 (0.83, 2.18) | 0.218 | 1.31 (0.77, 2.24) | 0.282 |
| Q4 (>2.63) | 2.62 (1.58, 4.32) | <0.001 | 2.25 (1.35, 3.76) | 0.004 | 2.22 (1.27, 3.87) | 0.01 |
| P for trend | <0.001 | 0.001 | 0.004 | |||
Model 1: no covariates were adjusted. Model 2: adjusted for age, sex, race, income and education. Model 3: adjusted for age, sex, race, income, education, body mass index, smoking status, alcohol consumption, history of hypertension, diabetes and cardiovascular disease.
NLR, neutrophil-to-lymphocyte ratio.
Findings from the multinomial logistic regression are detailed in table 3. A pronounced correlation was found between the NLR and the severity of psoriasis, except for a slight non-significant relationship between the NLR and those with little or no psoriasis after adjusting for all variables, regardless of whether the NLR was used as a continuous (OR: 1.08, 95% CI: 1.00 to 1.17, p=0.06) or quartile variable (Q4 vs Q1—OR: 2.04, 95% CI: 1.00 to 4.17, p=0.052). In all models, the ORs of psoriasis severity increased as the quartile of the NLR increased. Compared with participants with an NLR ≤1.47 (Q1), those with an NLR >2.63 (Q4) had a significant increase in the odds of ‘few patches to extensive psoriasis’ (Q4 vs Q1—OR: 2.43, 95% CI: 1.10 to 5.36, p=0.003). High NLR values were associated with having more severe psoriasis.
Table 3.
Association between the NLR and the severity of psoriasis
| Model | Psoriasis | Continuous | P value | OR (95%CI) | P value | |||
| Severity | OR (95% CI) | Q1 | Q2 | Q3 | Q4 | |||
| Model 1 | Little or no psoriasis | 1.13 (1.06, 1.21) | <0.001 | Reference | 1.30 (0.59, 2.85) | 1.81 (0.97, 3.35) | 2.41 (1.30, 4.48) | 0.01 |
| Few patches to extensive psoriasis | 1.22 (1.11, 1.34) | <0.001 | Reference | 0.95 (0.37, 2.44) | 1.09 (0.47, 2.55) | 2.86 (1.34, 6.07) | <0.001 | |
| Model 2 | Little or no psoriasis | 1.10 (1.03, 1.18) | 0.008 | Reference | 1.18 (0.52, 2.72) | 1.62 (0.85, 3.09) | 2.07 (1.08, 3.96) | 0.034 |
| Few patches to extensive psoriasis | 1.20 (1.10, 1.31) | <0.001 | Reference | 0.89 (0.35, 2.29) | 1.00 (0.41, 2.44) | 2.49 (1.17, 5.29) | 0.003 | |
| Model 3 | Little or no psoriasis | 1.08 (1.00, 1.17) | 0.06 | Reference | 1.21 (0.49, 2.96) | 1.61 (0.80, 3.22) | 2.04 (1.00, 4.17) | 0.052 |
| Few patches to extensive psoriasis | 1.19 (1.07, 1.33) | 0.004 | Reference | 0.88 (0.33, 2.34) | 0.95 (0.36, 2.54) | 2.43 (1.10, 5.36) | 0.003 | |
In multinomial logistic regression models, the association between the NLR and psoriasis severity was tested with patients never diagnosed with psoriasis as the reference group. Q1, NLR ≤1.47; Q2, NLR 1.47–1.96; Q3, NLR 1.96–2.63; Q4, NLR >2.63.
NLR, neutrophil-to-lymphocyte ratio.
Stratified analyses were undertaken by dividing the participants into prespecified subgroups of sociodemographic position at baseline to assess the consistency of the relationship between the main predictors and outcome (online supplemental table S1). An increased NLR was a risk factor for the presence of psoriasis in participants aged 40–59 (OR: 1.28, 95% CI: 1.05 to 1.56, p=0.019) and 60–79 (OR: 1.24, 95% CI: 1.09 to 1.41, p=0.004) years. For each unit increase in the NLR, the adjusted OR for psoriasis risk was 1.22 in females (p=0.016), 1.13 in males (p=0.03), 1.17 in non-Hispanic white individuals (p=0.006), 2.50 in other races (p=0.023) and 1.21 in participants with a medium PIR (p=0.021). For the subgroup stratified by education level, the association of the NLR with psoriasis was non-significant only in the ‘Some College or AA degree’ stratification (OR: 0.96, 95% CI: 0.77 to 1.20, p=0.693). Moreover, there was no evidence of interaction effects between multiple stratification factors and the NLR (all p for interaction >0.1).
bmjopen-2023-077596supp001.pdf (54.4KB, pdf)
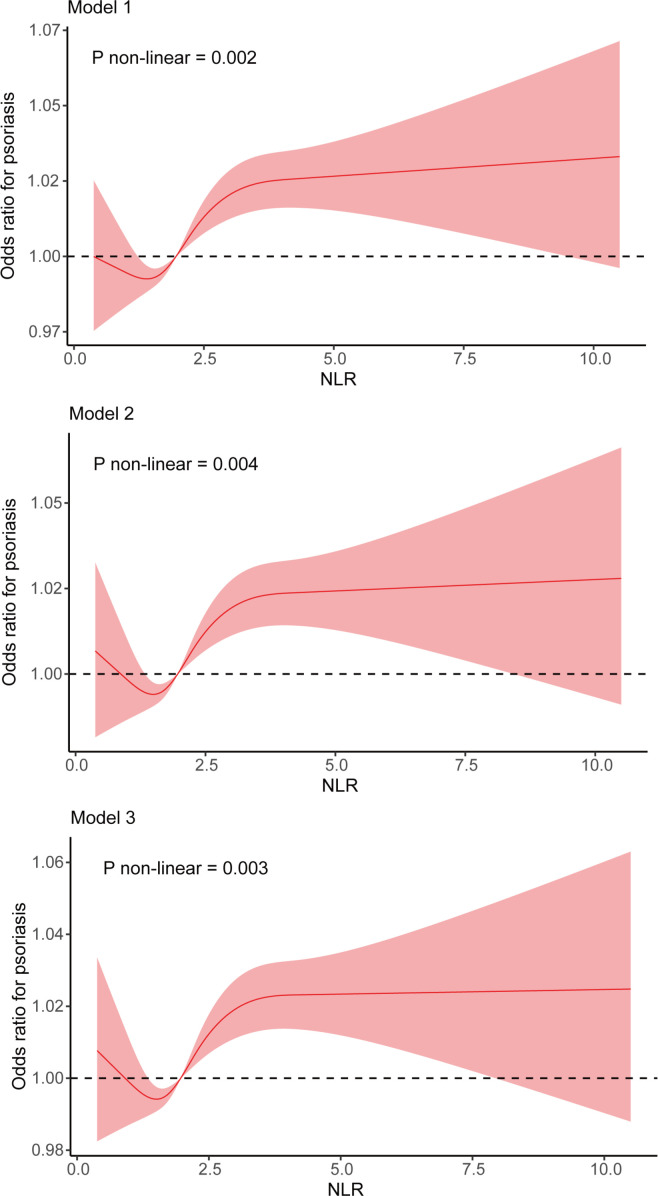
To flexibly model and visualise the relationship between the NLR and psoriasis, RCSs were used (figure 2). We observed a strong non-linear association between the NLR and psoriasis. In Model 1, the OR of psoriasis decreased continuously before the NLR reached 1.40, after which it started to increase and became relatively stable when the NLR reached 2.97 or higher (p for non-linearity=0.002). Similar results were observed in the multivariable-adjusted models. The curve plots of Model 2 and Model 3 showed that the OR values decreased within a lower range of NLR, reached the lowest point at an NLR of 1.50 and then started to increase. The RCS curves of Model 2 and Model 3 reached a plateau after the NLR reached 3.08 and 3.13, respectively (Model 2: p for non-linearity=0.004; Model 3: p for non-linearity=0.003). In the partially adjusted model (ie, Model 2), the OR value for psoriasis was 1 when the NLR was 0.84 and 2.01, while in the fully adjusted model (ie, Model 3), the OR value for psoriasis was 1 when the NLR was 0.89 and 2.01.
Figure 2.
Non-linear association between the NLR and psoriasis by restricted cubic spline regression. The fitted regression line is shown as a red solid line; black dashed lines indicate where the OR equals 1; 95% CI is represented by a shaded region. NLR, neutrophil-to-lymphocyte ratio.
Discussion
In this observational study, we analysed standardised data from a large cohort of participants in a US population sample. Our study identified that the NLR was increased in patients with psoriasis and positively correlated with the disease severity. Taking into account that an imbalance in the baseline characteristics of participants may modify the association between the NLR and psoriasis, adjustments were made for potential confounders in regression analysis; nevertheless, we still detected a significant association of the NLR with psoriasis, indicating that this association cannot be solely attributed to risk factors and that the NLR could independently predict either the presence of psoriasis or the severity of psoriatic skin lesions. In the multivariable-adjusted RCS analysis, the NLR showed a strong non-linear association with psoriasis risk, with the lowest risk at an NLR of 1.50. We also found that an NLR ranging between 0.89 and 2.01 was associated with a lower risk of psoriasis after accounting for all covariates, which meant that an NLR within an appropriate range might be a protective factor against psoriasis.
Neutrophils and T lymphocytes play critical roles in the development and progression of psoriasis. Massive infiltration of neutrophils within the dermis and epidermis is one of the classic histological features of psoriasis.34 As the first line of defence against immune attack, neutrophils are actively recruited to the affected skin and are responsible for propagating inflammation.35 Respiratory burst, degranulation and neutrophil extracellular trap formation are the main anti-inflammatory mechanisms of neutrophils that contribute to the immunopathogenesis of psoriasis.36 T lymphocytes that produce high levels of interleukin-17 (IL-17) driven by IL-23 have been corroborated as pathogenic culprits in psoriasis.5 IL-17 mediates effects on keratinocytes, which facilitates the recruitment of more IL-17-producing lymphocytes and neutrophils into inflamed psoriatic lesions, establishing a self-amplifying feedback loop to maintain and exacerbate inflammatory events in psoriasis.5 37 The NLR, originating from the ratio of neutrophils to lymphocytes in peripheral blood, may have the ability to mirror the balance between innate and adaptive immune responses.32 Aberrant NLR values are representative of inflammatory conditions in the body, but as of now, there is no universally acceptable NLR cut-off value that defines its range of normalcy.38 The circulating levels of neutrophils and lymphocytes vary from person to person and fluctuate over the course of an individual’s disease. In addition, patients’ medication usage has an impact on peripheral leucocyte levels. Thus, it remains a challenge to ensure that the NLR becomes a reasonable and individually standardised predictor of health outcomes.
Recently, a number of investigators have examined the diagnostic and prognostic value of the NLR in psoriasis. In an observational study comprising 60 patients with psoriasis along with 50 healthy controls, increased NLR values were found in the patient group when compared with controls, which is in line with our results.39 Prior studies have shown that greater NLR values were associated with higher scores on the Psoriasis Area and Severity Index (PASI).40 41 Similar findings were mirrored in another study that reported a significant increase in the NLR in patients with PASI scores of 10 or more compared with patients with PASI scores of less than 10.20 Nevertheless, several studies failed to find a significant association between the NLR and the clinical severity of psoriasis.23 42 Moreover, the NLR was proposed to be a robust predictor for the emergence of psoriatic arthritis in patients who had psoriasis.20 43 44 It has been reported that the NLR is capable of predicting all-cause mortality and cardiovascular risk,45 and this index might be a novel biomarker to assess the risk of subclinical atherosclerosis in patients with psoriasis.22 46 The NLR is believed to have the potential to predict treatment response because a remarkable reduction in the NLR was observed in patients with psoriasis who underwent effective treatment.47 48 As mentioned above, the NLR was increased in patients with psoriasis, but it declined after treatment, whereas initial works investigating the relationship of the NLR with psoriasis severity produced inconsistent results. These studies were limited by sample size and bias, and the association between the NLR and psoriasis may be influenced by sociodemographic characteristics,29 personal health habits49 50 and individual medical history.51 52 Given that a too high or too low NLR might signal a pathological state, there is a strong likelihood of a nonlinear relationship between the NLR and psoriasis that has not yet been explored and identified in earlier studies.
Our study has some strengths and clinical implications. Foremost, we used a nationally representative sample from NHANES, which offered sufficient statistical power to draw a conclusion and made our findings likely generalisable to the entire US population. The NHANES database contain comprehensive information on sociodemographic status, lifestyle exposures, physical measurements and medical history, which enabled us to control for numerous confounding factors. Additionally, the NLR is a readily available index that may assist clinicians in identifying patients at high risk of severe psoriasis.
However, there were several limitations to this study that should be noted. First, as this was a cross-sectional observational study, inferences regarding whether this association is causal cannot be drawn. The true causality and possible mechanisms underlying the relationship between the NLR and psoriasis should be further examined. Second, because the definition of psoriasis and comorbidities (eg, hypertension, diabetes and CVD) relied on self-reports from the respondents instead of diagnoses made by two or more experienced dermatologists, the observed outcomes may be subject to recall bias. Additionally, the extent of psoriatic skin involvement was assessed by questionnaires instead of structured diagnostic scales, such as the Psoriasis Area Severity Index, which might affect the validity of the findings. NHANES categorises psoriasis as (1) little or no psoriasis, (2) only a few patches, (3) scattered patches and (4) extensive psoriasis, which cannot represent the severity of psoriasis in clinical practice. Finally, although we controlled for multiple potential confounders, unknown and unmeasured confounders may have impacted our estimates. For instance, the use of immunomodulatory drugs may influence the correlation between the NLR and psoriasis.53 54 However, NHANES did not collect any information on the use of immunomodulatory medication. Future rigorously designed and sufficiently powered studies with greater use of immunomodulatory drugs may offer valuable insights into the complex interplay between psoriasis and the NLR.
Conclusion
In summary, our study elucidated that the NLR was independently associated with psoriasis and that the association was non-linear rather than simply linear. We also found evidence in favour of a clear link between the NLR and psoriasis severity. However, further research is warranted to elaborate the detailed mechanism of the NLR in psoriasis.
Supplementary Material
Acknowledgments
We thank all the investigators of National Health and Nutrition Examination Survey who provided the original data.
Footnotes
Contributors: JH designed the research, conducted statistical analysis and wrote the manuscripts. ML and NL directed the study and revised the manuscripts. All authors contributed to the article and approved the submitted version. ML is responsible for the overall content as the guarantor.
Funding: This work was supported by grants from National Natural Science Foundation of China (82173432), CAMS Innovation Fund for Medical Sciences (2017-I2M-1-017, 2021-I2M-1-001).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Open access data are available on the NHANES website (www.cdc.gov/nchs/nhanes/).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
NHANES was approved by the National Center for Health Statistics Institutional Review Board, and participants provided written informed consent.
References
- 1.Boehncke W-H, Schön MP. Psoriasis. Lancet 2015;386:983–94. 10.1016/S0140-6736(14)61909-7 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol 2021;157:940–6. 10.1001/jamadermatol.2021.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Global report on psoriasis. Geneva: World Health Organization, 2016. Available: https://apps.who.int/iris/handle/10665/204417 [Google Scholar]
- 4.Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol 2017;76:377–90. 10.1016/j.jaad.2016.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol 2017;140:645–53. 10.1016/j.jaci.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferro D, Matias M, Neto J, et al. Neutrophil-to-lymphocyte ratio predicts cerebral edema and clinical worsening early after reperfusion therapy in stroke. Stroke 2021;52:859–67. 10.1161/STROKEAHA.120.032130 [DOI] [PubMed] [Google Scholar]
- 7.Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5–14. [PubMed] [Google Scholar]
- 8.Bhindi B, Hermanns T, Wei Y, et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. Br J Cancer 2016;114:207–12. 10.1038/bjc.2015.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Zheng H, Zhu X, et al. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res Clin Pract 2017;130:90–7. 10.1016/j.diabres.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 10.Boulos D, Proudman SM, Metcalf RG, et al. The neutrophil-lymphocyte ratio in early rheumatoid arthritis and its ability to predict subsequent failure of triple therapy. Semin Arthritis Rheum 2019;49:373–6. 10.1016/j.semarthrit.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 2020;81:e6–12. 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paliogiannis P, Fois AG, Sotgia S, et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev 2018;27:170113. 10.1183/16000617.0113-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupp MA, Cariolou M, Tzoulaki I, et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med 2020;18:360. 10.1186/s12916-020-01817-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song M, Graubard BI, Rabkin CS, et al. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep 2021;11:464. 10.1038/s41598-020-79431-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 16.Bhikram T, Sandor P. Neutrophil-lymphocyte ratios as inflammatory biomarkers in psychiatric patients. Brain Behav Immun 2022;105:237–46. 10.1016/j.bbi.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 17.Zulfic Z, Weickert CS, Weickert TW, et al. Neutrophil-lymphocyte ratio - a simple, accessible measure of inflammation, morbidity and prognosis in psychiatric disorders Australas Psychiatry 2020;28:454–8. 10.1177/1039856220908172 [DOI] [PubMed] [Google Scholar]
- 18.Mazza MG, Lucchi S, Tringali AGM, et al. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2018;84:229–36. 10.1016/j.pnpbp.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Minakawa S, Kaneko T, Matsuzaki Y, et al. Psoriasis area and severity index is closely related to serum C-reactive protein level and neutrophil to lymphocyte ratio in Japanese patients. J Dermatol 2017;44:e236–7. 10.1111/1346-8138.13942 [DOI] [PubMed] [Google Scholar]
- 20.Kim DS, Shin D, Lee MS, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis Vulgaris and Psoriatic arthritis. J Dermatol 2016;43:305–10. 10.1111/1346-8138.13061 [DOI] [PubMed] [Google Scholar]
- 21.Solak B, Dikicier BS, Erdem T. Impact of elevated serum uric acid levels on systemic inflammation in patients with psoriasis. Angiology 2017;68:266–70. 10.1177/0003319716657980 [DOI] [PubMed] [Google Scholar]
- 22.Kvist-Hansen A, Kaiser H, Krakauer M, et al. Neutrophil-to-lymphocyte ratio and the systemic immune-inflammation index as potential biomarkers of effective treatment and Subclinical Atherosclerotic cardiovascular disease in patients with psoriasis. J Eur Acad Dermatol Venereol 2023;37:e586–9. 10.1111/jdv.18860 [DOI] [PubMed] [Google Scholar]
- 23.Paliogiannis P, Satta R, Deligia G, et al. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: a systematic review and meta-analysis. Clin Exp Med 2019;19:37–45. 10.1007/s10238-018-0538-x [DOI] [PubMed] [Google Scholar]
- 24.Dey AK, Teague HL, Adamstein NH, et al. Association of neutrophil-to-lymphocyte ratio with non-calcified coronary artery burden in psoriasis: findings from an observational cohort study. J Cardiovasc Comput Tomogr 2021;15:372–9. 10.1016/j.jcct.2020.12.006 [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) . National health and nutrition examination survey (NHANES). About NHANES, Available: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- 26.Centers for Disease Control and Prevention (CDC) . National health and nutrition examination survey (NHANES). Sample Design, Available: https://wwwn.cdc.gov/nchs/nhanes/tutorials/SampleDesign.aspx
- 27.Centers for Disease Control and Prevention (CDC), National health and nutrition examination survey (NHANES) . NHANES Questionnaires, Datasets, and Related Documentation Available: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx [Google Scholar]
- 28.National Health and Nutrition Examination Survey (NHANES) . Laboratory Procedure Manual Available: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/cbc_g_met_he.pdf [Google Scholar]
- 29.Howard R, Scheiner A, Kanetsky PA, et al. Sociodemographic and lifestyle factors associated with the neutrophil-to-lymphocyte ratio. Ann Epidemiol 2019;38:11–21. 10.1016/j.annepidem.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai Y-X, Wang S-C, Chou Y-J, et al. Smoking, but not alcohol, is associated with risk of psoriasis in a Taiwanese population-based cohort study. J Am Acad Dermatol 2019;80:727–34. 10.1016/j.jaad.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 31.Bu J, Ding R, Zhou L, et al. Epidemiology of psoriasis and comorbid diseases: A narrative review. Front Immunol 2022;13:880201. 10.3389/fimmu.2022.880201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Eliot M, Koestler DC, et al. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson heart study and modification by the Duffy antigen variant. JAMA Cardiol 2018;3:455–62. 10.1001/jamacardio.2018.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schön MP, Broekaert SMC, Erpenbeck L. Sexy again: the Renaissance of neutrophils in psoriasis. Exp Dermatol 2017;26:305–11. 10.1111/exd.13067 [DOI] [PubMed] [Google Scholar]
- 35.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 2014;32:227–55. 10.1146/annurev-immunol-032713-120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang C-C, Cheng W-J, Korinek M, et al. Neutrophils in psoriasis. Front Immunol 2019;10:2376. 10.3389/fimmu.2019.02376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiricozzi A, Romanelli P, Volpe E, et al. Scanning the Immunopathogenesis of psoriasis. Int J Mol Sci 2018;19:179. 10.3390/ijms19010179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buonacera A, Stancanelli B, Colaci M, et al. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci 2022;23:3636. 10.3390/ijms23073636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sirin MC, Korkmaz S, Erturan I, et al. Evaluation of monocyte to HDL cholesterol ratio and other inflammatory markers in patients with psoriasis. An Bras Dermatol 2020;95:575–82. 10.1016/j.abd.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polat M, Bugdayci G, Kaya H, et al. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in Turkish patients with chronic plaque psoriasis. Acta Dermatovenerol Alp Pannonica Adriat 2017;26:97–100. 10.15570/actaapa.2017.28 [DOI] [PubMed] [Google Scholar]
- 41.Sen BB, Rifaioglu EN, Ekiz O, et al. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis. Cutan Ocul Toxicol 2014;33:223–7. 10.3109/15569527.2013.834498 [DOI] [PubMed] [Google Scholar]
- 42.Ataseven A, Bilgin AU, Kurtipek GS. The importance of neutrophil lymphocyte ratio in patients with psoriasis. Mater Sociomed 2014;26:231–3. 10.5455/msm.2014.231-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen HT, Vo LDH, Pham NN. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as inflammatory markers in psoriasis: a case-control study. Dermatol Reports 2023;15:9516. 10.4081/dr.2022.9516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duran TI, Pamukcu M. Relationship between disease impact scores and C-reactive protein/albumin ratio in patients with Psoriatic arthritis. Croat Med J 2022;63:141–7. 10.3325/cmj.2022.63.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adamstein NH, MacFadyen JG, Rose LM, et al. The neutrophil-lymphocyte ratio and incident Atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J 2021;42:896–903. 10.1093/eurheartj/ehaa1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaiser H, Wang X, Kvist-Hansen A, et al. Biomarkers of Subclinical Atherosclerosis in patients with psoriasis. Sci Rep 2021;11:21438. 10.1038/s41598-021-00999-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Wiles C, Martinez LR, et al. Neutrophil-to-lymphocyte ratio decreases after treatment of psoriasis with therapeutic antibodies. J Eur Acad Dermatol Venereol 2017;31:e491–2. 10.1111/jdv.14334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersen CSB, Kvist-Hansen A, Siewertsen M, et al. Blood cell biomarkers of inflammation and cytokine levels as predictors of response to Biologics in patients with psoriasis. Int J Mol Sci 2023;24:6111. 10.3390/ijms24076111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budu-Aggrey A, Brumpton B, Tyrrell J, et al. Evidence of a causal relationship between body mass index and psoriasis: A Mendelian randomization study. PLoS Med 2019;16:e1002739. 10.1371/journal.pmed.1002739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei J, Zhu J, Xu H, et al. Alcohol consumption and smoking in relation to psoriasis: a Mendelian randomization study. Br J Dermatol 2022;187:684–91. 10.1111/bjd.21718 [DOI] [PubMed] [Google Scholar]
- 51.Miller IM, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol 2013;69:1014–24. 10.1016/j.jaad.2013.06.053 [DOI] [PubMed] [Google Scholar]
- 52.Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy 2021;122:474–88. 10.4149/BLL_2021_078 [DOI] [PubMed] [Google Scholar]
- 53.Blayney DW, Mohanlal R, Adamchuk H, et al. Efficacy of Plinabulin vs Pegfilgrastim for prevention of Docetaxel-induced neutropenia in patients with solid tumors: A randomized clinical trial. JAMA Netw Open 2022;5:e2145446. 10.1001/jamanetworkopen.2021.45446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loos AM, Liu S, Segel C, et al. Comparative effectiveness of targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: A systematic review and network meta-analysis. J Am Acad Dermatol 2018;79:135–44. 10.1016/j.jaad.2018.02.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-077596supp001.pdf (54.4KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Open access data are available on the NHANES website (www.cdc.gov/nchs/nhanes/).