Abstract
Background
Patients with type 2 diabetes mellitus (T2DM) are commonly at high risk for developing cognitive dysfunction. Antidiabetic agents might be repurposed for targeting cognitive dysfunction in addition to modulation on glucose homeostasis. This study aimed to evaluate the impact of dipeptidyl peptidase-4 inhibitors (DPP-4i) on cognitive function in T2DM.
Methods
PubMed, Embase, Cochrane Library and Web of Science were systematically searched from inception to September 30, 2023. Weighted mean differences were calculated using the Mantel-Haenszel (M-H) fixed or random effects model based on the degree of heterogeneity among studies. Heterogeneity was evaluated using a Chi-squared test and quantified with Higgins I2. Sensitivity analysis was performed with the leave-one-out method, and publication bias was evaluated according to Begg’s and Egger’s tests.
Results
Six clinical trials involving 5,178 participants were included in the pooled analysis. Administration of DPP-4i generally correlated with an increase of Mini-Mental State Examination (MMSE) scores (1.09, 95% CI: 0.22 to 1.96). DPP-4i alleviated cognitive impairment in the copying skill subdomain of MMSE (0.26, 95% CI: 0.12 to 0.40). Treatment with DPP-4i also resulted in an increase of Instrumental Activities of Daily Living (IADL) scores (0.82, 95% CI: 0.30 to 1.34). However, DPP-4i produced no significant effects on Barthel Activities of Daily Living (BADL) scores (0.37, 95% CI: -1.26 to 1.99) or other test scores.
Conclusions
DPP-4i treatment favourably improved cognitive function in patients with T2DM. Further trials with larger samples should be performed to confirm these estimates and investigate the association of different DPP-4i with cognitive function among diabetic patients.
Trial registration in PROSPERO
CRD42023430873.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-023-01985-y.
Keywords: Dipeptidyl peptidase-4 inhibitors, Cognitive function, MMSE, IADL, Type 2 Diabetes Mellitus
Introduction
The number of patients with diabetes is increasing yearly worldwide. According to a report by the World Health Organization, the number of diabetic patients would reach 366 million in 2030 [1]. Among them, approximately 90% of patients were diagnosed with type 2 diabetes mellitus (T2DM) [2]. A previous study explored the association of social risk factors with diabetes, demonstrating that patients’ educational levels and ethnicity contributed more to the development of diabetes than other factors [3]. The social factor of stress is also linked with the onset of T2DM [4]. Both modifiable (for example, obesity, smoking, diet, insufficient physical activity, excessive alcohol consumption and even unhealthy sleep) and nonmodifiable risk factors (for example, age, sex and ethnicity) contribute to the development of T2DM [5]. Among them, aging is a key factor leading to an increasing number of diabetic individuals [6]. A similar increasing trend has also been observed in related neurodegenerative diseases. Cognitive dysfunction is one of the manifestations of diabetes. Diabetes has been identified as a risk factor for cognitive dysfunction, including cognitive decline, mild cognitive impairment (MCI) and dementia [7]. Diabetic patients are likely to develop neurodegenerative complications ranging from cognitive impairment to dementia [8]. Additionally, patients with neurological diseases are at a high risk for developing diabetes [9, 10]. Accumulating evidences have demonstrated a positive correlation between diabetes and cognitive dysfunction [11–14]. Furthermore, multiple cognitive domains, including execution and spatial skills, are affected during the development of cognitive dysfunction in diabetic state [15]. Although numerous efforts have been made to alleviate cognitive dysfunction, no effective agents could arrest or reverse the process underlying cognitive impairment.
An increased understanding of the common pathology between diabetes and cognitive disorders has led to a focus on the therapeutic potential of antidiabetic agents for treating cognitive dysfunction [16–20]. High levels of glycosylated haemoglobin (HbA1c) have been found to positively correlate with an increased risk of cognitive dysfunction [21]. Antidiabetic regimens are expected to be repurposed for treating cognitive impairment in addition to modulation of glucose homeostasis. In a large observational study involving 176,250 participants, antidiabetic agents, including metformin, dipeptidyl peptidase-4 inhibitor (DPP-4i), glucagon-like peptide-1 (GLP-1) analogues and sodium glucose cotransporter 2 inhibitors (SGLT2i), significantly reduced the risk of dementia in T2DM [22]. Among these antidiabetic regimens, DPP-4i have served as oral agents by interacting with DPP-4 since 2006 [23]. A previous study demonstrated that high concentrations of DPP-4 or enhanced DPP-4 activity might be positively linked with cognitive impairment in elderly participants with T2DM [24]. DPP-4 affects cognitive function in diabetic patients through multiple signalling pathways involving inflammatory reactions, oxidative stress and mitochondrial dysfunction [25]. Inhibition of DPP-4 potentially produced an effect on cognitive function in addition to the action on GLP-1 [26]. In fact, studies have indicated that DPP-4i presented pleiotropic effects in addition to their antihyperglycaemic actions. For example, sitagliptin significantly alleviated memory impairment by promoting neurogenesis and reducing oxidative stress [27]. An improvement of the Mini-Mental State Examination (MMSE) score has been observed in diabetic patients undergoing treatment with DPP-4i [28]. Compared to nonusers, DPP-4i users showed a slower cognitive decline based on MMSE scores [29]. Linagliptin was also demonstrated to slow the progression of premature aging by enhancing cerebral blood flow in gene-modified mouse models [30]. In contrast, other studies presented no beneficial effects on cognitive function during DPP-4i treatment. Furthermore, DPP-4i were even suggested to deteriorate cognitive function and produce neurodegenerative toxicity in diabetic patients. For example, sitagliptin increased tau phosphorylation in the hippocampus of diabetic rats, leading to a caution on the administration of sitagliptin in treating Alzheimer’s disease (AD) [31].
To date, no definite conclusion has been achieved regarding the potential effects of DPP-4i on improving cognitive dysfunction in T2DM [32]. This meta-analysis was designed to ascertain whether DPP-4i could improve or deteriorate cognitive function in T2DM.
Methods
Search strategy
This meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [33]. The protocol was registered in the PROSPERO system (CRD42023430873). PubMed, Embase, Cochrane Library and Web of Science were systematically searched from inception to September 30, 2023. Key words combined with free words were used to perform the search. The index words mainly included (dipeptidyl peptidase-4 inhibitors) AND (Diabetes Mellitus, Type 2) AND (cognitive function).
Study selection
Relevant studies were screened by two reviewers. Disagreements were resolved by consulting a third researcher until consensus was reached. Eligible trials evaluating the impact of DPP-4i on MMSE scores, Barthel activities of daily living (BADL) scores, instrumental activities of daily living (IADL) scores and scores on other tests were recorded. The inclusion criteria were as follows: (i) trials evaluating the effect of DPP-4i on cognitive function and related scores; (ii) presented sufficient information on the pre- and post-treatment MMSE, BADL, IADL scores and other assessment scores or provided a difference score; and (iii) enrolled patients with T2DM. Animal studies, letters, case reports, meeting abstracts and narrative reviews were excluded. Studies without sufficient information on pre- or posttreatment-related cognitive scores were also excluded. The inclusion and exclusion criteria were objectively evaluated by two reviewers.
Data extraction
The following data were extracted by two reviewers: first author’s name, publication year, study location, gender ratio, number of participants in the DPP-4i and control groups, mean age, body mass index (BMI), treatment duration, diabetes duration, baseline levels of HbA1c, and baseline and post-treatment scores of cognitive tests. The longest therapy duration information was extracted if multiple follow-ups were reported in the same study. Parameters of cognitive function included MMSE, Verbal Fluency Test (VFT) and Trial Making Test (TMT). The comprehensive geriatric scales included the BADL, IADL, Center for Epidemiologic Studies Depression Scale (CES-D), Global Deterioration Scale (GDS) and Mini Nutritional Assessment (MNA). All the parameters were extracted as an absolute value pre- and post-treatment in T2DM. The ratio of TMT was recorded and pooled to analysis.
Quality assessment
The quality of randomized controlled trials (RCTs) was evaluated according to Cochrane Handbook for Systematic Reviews of Interventions as our previous study [34]. The following domains were assessed: random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessment; incomplete outcome data; selective outcome reporting and other potential sources of bias. According to the Cochrane criteria, a judgement of ‘yes’ indicates a low risk of bias, while ‘no’ indicates a high risk of bias. A judgement of ‘unclear’ indicated an unknown risk of bias. The Newcastle‒Ottawa Scale was used to assess the quality of non-RCTs included [35].
Quantitative data synthesis
Meta-analysis was performed to examine cognitive test scores. Weighted mean difference (WMD) and 95% confidence interval (CI) were calculated to examine test scores among T2DM participants. A fixed effects or random effects model was used based on the heterogeneity, which was quantified with the I2 index. Pooled analysis was considered to be statistically significant when P value was < 0.05. Sensitivity analysis was performed using the leave-one-out method to examine the influence of each individual study. Publication bias was also examined by Begg’s test and Egger’s test if there were at least five studies reporting an outcome. Statistical analysis was performed with Review Manager and STATA 12.0 software.
Results
Characteristics of the included studies
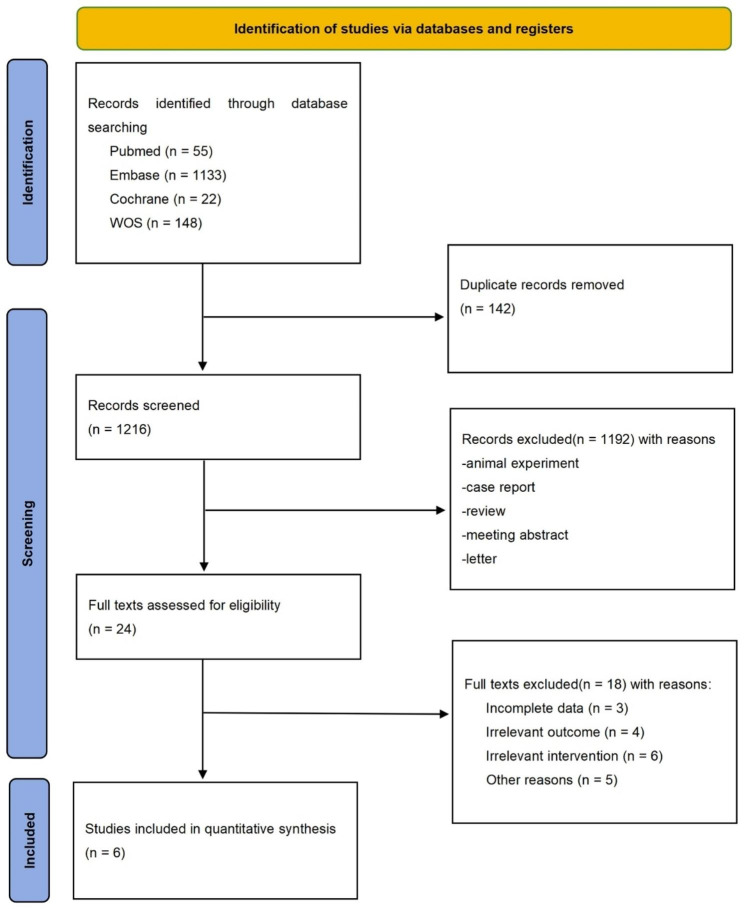
After a detailed literature search, six studies ultimately met the inclusion criteria [36–41]. The search process is demonstrated in Fig. 1. For the MMSE, 2,650 patients were placed in the DPP-4i group, and 2,528 patients were placed in the control arm. Two studies lasted less than 1 year (ranging from 1 to 6 months), and four studies lasted more than 1 year (ranging from 13 to 26 months). Two studies had a sample size larger than 1000. The baseline characteristics of included studies were shown in Table 1. Participants received DPP-4i treatment with linagliptin, vildagliptin and sitagliptin. Cognitive function was evaluated by the scores of MMSE, VFT and TMT. The comprehensive geriatric scales included tests of BADL, IADL, CES-D, GDS and MNA. Overall, the quality of the identified trials was moderate (see supplementary Tables 1 and supplementary Table 2).
Fig. 1.
PRISMA flow chart for study selection
Table 1.
Demographic characteristics of the studies included
| Study/year | Location | Treatment arm (n) |
Sex (male/female) |
Duration of follow-up (months) |
Age (years) |
Duration of diabetes (years) |
BMI (kg/m2) |
HbA1c (%) |
MMSE score (0[worst]-30[best]) |
|---|---|---|---|---|---|---|---|---|---|
| Isik,2017 [36] | Turkey |
sita: 43 met: 69 insu: 22 |
sita: 47/57 met + insu: 35/66 |
6 |
77.05 ± 8.50 77.12 ± 7.87 77.61 ± 9.40 |
13.38 ± 8.5 12.95 ± 4.99 15.66 ± 6.97 |
28.86 ± 4.21 29.78 ± 4.71 28.33 ± 5.24 |
7.29 ± 1.49 6.90 ± 1.29 7.50 ± 1.19 |
22.73 ± 5.64 23.55 ± 5.38 23.50 ± 4.03 |
| Borzi,2019 [37] | Italy |
vild + met: 30 met: 30 |
vild + met: 14/16 met:13/17 |
6 |
77.67 ± 8.23 75.46 ± 7.89 |
16.72 ± 5.81 15.49 ± 6.83 |
29.55 ± 5.92 28.48 ± 6.69 |
8.02 ± 1.27 7.09 ± 1.21 |
21.00 ± 1.41 20.77 ± 1.22 |
| Biessels,2019 [38] | Netherland |
lina: 800 pla: 745 |
lina: 503/297 pla: 501/244 |
30 |
67.8 ± 8.3 67.7 ± 8.0 |
14.8 ± 9.2 15.4 ± 9.4 |
32.5 ± 5.1 32.8 ± 5.3 |
7.8 ± 0.9 7.8 ± 0.9 |
28.3 ± 1.7 28.2 ± 1.8 |
| Xue, 2020 [39] | China |
dpp4i: 30 sulfo: 30 |
dpp4i: 17/13 sulfo: 14/16 |
6 |
68.5 ± 7.1 67.4 ± 5.9 |
8.17 ± 3.05 8.97 ± 2.65 |
25.71 ± 3.76 25.59 ± 4.28 |
8.56 ± 1.25 8.86 ± 3.17 |
25.42 ± 1.22 25.37 ± 1.16 |
| Bulut,2020 [40] | Turkey |
vild: 43 pla: 35 con: 52 |
vild: 18/25 pla + con: 20/32 |
6 |
74.4 ± 7.9 79.7 ± 4.8 74.2 ± 7.8 |
18.4 ± 9.9 11.3 ± 7.6 16.4 ± 10.6 |
29.3 ± 5.1 28.5 ± 4.2 29.1 ± 4.5 |
7.63 ± 1.37 6.01 ± 0.49 7.12 ± 1.57 |
21.81 ± 6.16 24.94 ± 4.31 26.04 ± 5.04 |
| Biessels,2021 [41] | Netherland |
lina: 1618 glim: 1545 |
lina: 1002/616 glim: 958/587 |
40 |
64.4 ± 9.1 64.4 ± 9.3 |
7.7 ± 6.2 7.4 ± 5.9 |
30.8 ± 5.0 30.7 ± 4.9 |
7.1 ± 0.5 7.1 ± 0.6 |
28.5 ± 1.7 28.5 ± 1.7 |
Values are expressed as the mean ± SD. Abbreviations: n: number of participants per group; HbA1c: glycated haemoglobin; sita: sitagliptin; vild: vildagliptin; pla: placebo; con: conventional treatment; met: metformin; insu: insulin; sulfo: sulfonylurea; lina: linagliptin; gli: glimepiride; MMSE: Mini-Mental State Examination
Effect of DPP-4i treatment on cognitive function
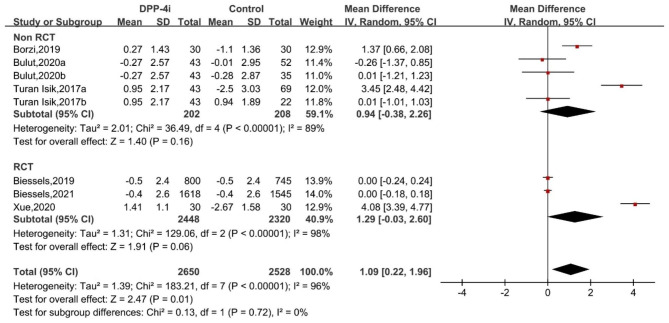
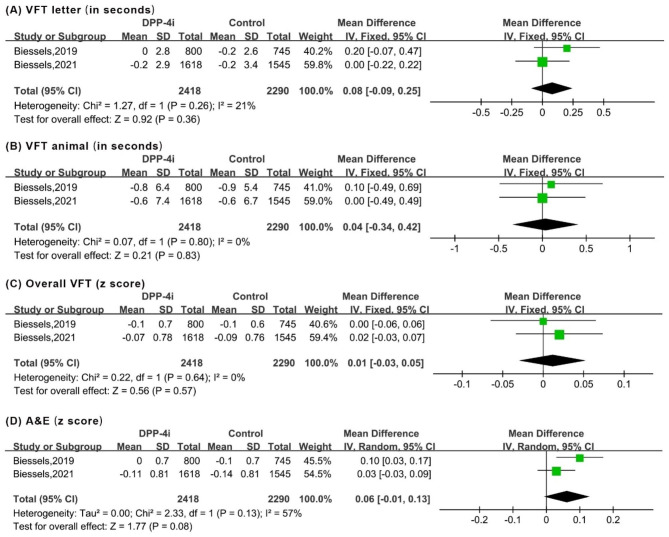
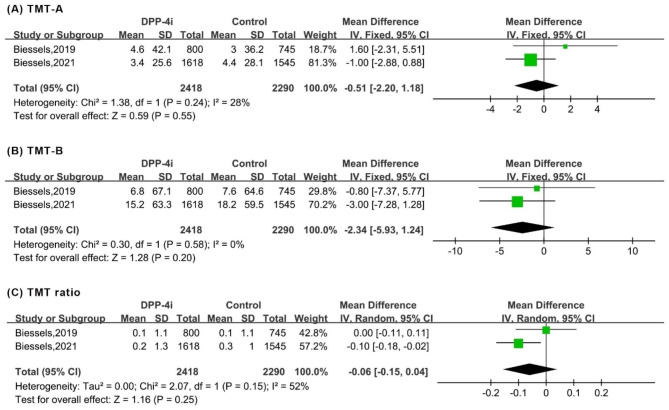
Meta-analysis suggested that DPP-4i produced an improvement of the overall MMSE score (WMD: 1.09, 95% CI: 0.22 to 1.96). Regarding the subdomains of MMSE, significant improvements were observed in copying skills (WMD: 0.26, 95% CI: 0.12 to 0.40). However, DPP-4i produced no significant effects on other subdomains, including temporal orientation, records, language, attention and recall (Fig. 2). Similarly, for VFT and TMT, DPP-4i treatment showed no significant improvements, as indicated by outcomes of VFT letter 60 (in seconds), A&E (z score), VFT animals 60 (in seconds), overall VFT 60 (z score), TMT-A (in seconds), TMT-B (in seconds) and TMT ratio (Figs. 3 and 4).
Fig. 2.
Forest plot for the analysis of the impact of DDP-4i on MMSE total and subdomain scores
Fig. 3.
Forest plot for the analysis of the impact of DDP-4i on the VFT score
Fig. 4.
Forest plot for the analysis of the impact of DDP-4i on the TMT score
Effect of DPP-4i treatment on comprehensive geriatric assessment
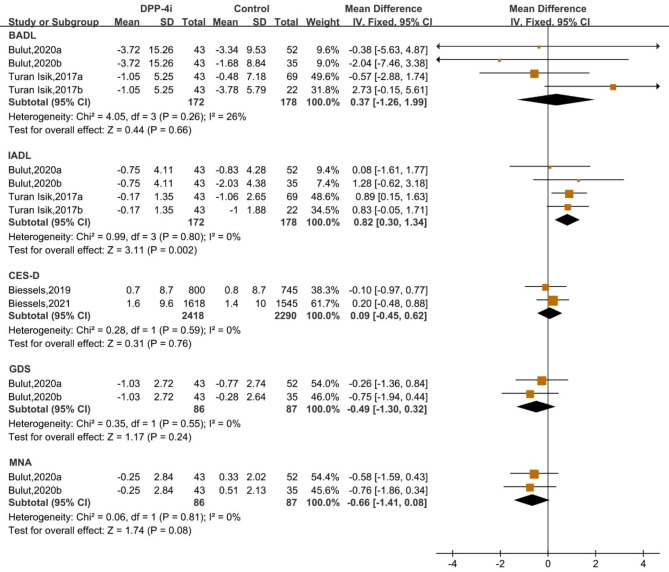
DPP-4i treatment was found to produce a favourable effect on comprehensive geriatric assessment, as shown by a significant change of IADL score (WMD: 0.82, 95% CI: 0.30 to 1.34). DPP-4i treatment produced no significant effect on BADL score (WMD: 0.37, 95% CI: -1.26 to 1.99). Similarly, CES-D, GDS and MNA scores were not modulated during DPP-4i treatment (Fig. 5).
Fig. 5.
Forest plot for the analysis of the impact of DDP-4i on the comprehensive geriatric assessment
Sensitivity test and publication bias evaluation
Sensitivity analysis revealed that the beneficial effect of DPP-4i on MMSE scores was stable (WMD: 1.09, 95% CI: 0.22 to 1.96, N = 8 studies, heterogeneity P = 0.014) (Supplementary Fig. 1). This finding indicated that a significant difference across studies is an overall effect from all studies included. Furthermore, no publication bias of cognitive function was detected by Begg’s test (P = 0.62) or Egger’s test (P = 0.15) (Supplementary Fig. 2).
Discussion
In the current meta-analysis, the pooled estimates of MMSE and IADL scores confirmed an improvement of cognitive function in diabetic patients receiving DPP-4i treatment. DPP-4i could be potentially used for diabetes-related cognitive disorders in clinical practice.
Six studies were identified to evaluate the impact of DPP-4i on cognitive function in T2DM. In the study by Isik et al., elderly diabetic patients with or without cognitive dysfunction were administered with sitagliptin. During a follow-up of 6 months, sitagliptin increased MMSE scores in patients with AD compared to metformin treatment [36]. In addition, concomitant administration of vildagliptin with conventional antidiabetic treatment also improved cognitive function by targeting copying subdomain in older diabetic patients [40]. This improving effect on cognition might come from an increase of brain GLP-1 levels. Further study demonstrated that vildagliptin prevented the reduction of MMSE in elderly diabetic patients on the basis of metformin treatment [37]. Two studies by Biessels et al. evaluated the impact of linagliptin on cognition in diabetic patients, and no decline of cognitive performance were observed when compared to control group [38, 41]. It is interesting to find that fewer patients in linagliptin treatment group developed depressive manifestations. DPP-4i also improved cognitive ability in elderly diabetic patients with post-stroke MCI, which might be associated with a reduction of Aβ expression and inflammatory response [39].
The exact mechanism through which DPP-4i modulated cognitive function remains unclear. Numerous studies have revealed that multiple common pathologies underlie diabetes and cognitive decline, involving insulin resistance (IR), mitochondrial disorders, oxidative stress, glucose fluctuation and inflammatory response [27, 42–45]. DPP-4i might modulate cognitive function by targeting one or more of the signalling pathways above in a direct or indirect manner. IR is commonly present in diabetic patients and serves as an important risk factor for developing cognitive dysfunction. Additionally, neuroimaging results revealed subtle cerebral abnormalities in the insulin-resistant brain [46]. Upregulation of DPP-4 expression in the liver was usually accompanied by IR [47], while insulin sensitivity could be significantly improved when DPP-4 expression was downregulated [48]. Furthermore, DPP-4i users demonstrated an improved insulin sensitivity and secreted more insulin from beta-cells, a phenomenon partially correlated with a reduction of DPP-4 activity [49]. Additionally, vildagliptin treatment effectively improved neuronal insulin sensitivity and prevented brain mitochondrial dysfunction, thus contributing to the alleviation of cognitive impairment induced by a high-fat diet [50].
In a previous study, DPP-4 induced mitochondrial dysfunction and reduced cognitive function in T2DM, a process mediated by the protease-activated receptor 2 (PAR2)-dependent signalling pathway in the hippocampus. A reversal of mitochondrial dysfunction was achieved when DPP-4 gene was silenced, followed by an improvement of cognitive dysfunction in db/db mice [51]. An upregulation of DPP-4 expression had been suggested to be related to cognitive impairment via an overload of mitochondrial calcium, while cognitive impairment was alleviated after downregulation of DPP-4 expression [52]. The improvement in mitochondrial function might be linked to an alleviation of oxidative stress and inflammatory response in DPP-4i-treated subjects. Furthermore, enhanced DPP-4 activity increased oxidative stress and activated inflammatory response, partially contributing to cognitive impairment in T2DM [53]. Linagliptin favourably alleviated cognitive dysfunction through downregulating NADPH expression in streptozotocin-induced diabetic models, a process mediated by a reduction of oxidative stress [54]. Sitagliptin presented an anti-inflammatory effect through activating NF-E2-related factor 2 (Nrf2), thus leading to an alleviation of excessive autophagy in lipopolysaccharide (LPS)-induced inflammatory models [55]. Additionally, DPP-4i restricted diabetes-associated cognitive deficits by modulating neuroinflammatory indicators of caveolin 1 (Cav 1) and brain-derived neurotrophic factor (BDNF), thus reducing inflammatory response in the brain [56]. A previous study also explored the association of glucose fluctuation with cognitive function. Patients with glucose fluctuation commonly demonstrated an impaired performance of TMT-B and VFT [45].
The efficacy of DPP-4i on cognitive function was also found to be indirectly associated with a reduction of DPP-4 activity [44]. Plasma levels of DPP-4 substrates, including GLP-1, stromal derived factor 1α (SDF-1α) and brain natriuretic peptide (BNP), were increased during DPP-4i treatment. These substrates were either activated or inactivated by DPP-4 to partially modulate related signalling pathways involving inflammation or oxidative stress, thus facilitating cognitive impairment in diabetic conditions. Furthermore, substrates of SDF-1 and GLP-1 have also been suggested to provide neuroprotection in AD models [57]. DPP-4i prolonged the enzymatic degradation mediated by DPP-4, thus enhancing the neuroprotective effect of those substrates [58]. Additionally, DPP-4i have been shown to improve cognitive function by increasing concentrations of adiponectin receptor 1 and hypothalamic acetylcholine in T2DM [59]. In a recent study, DPP-4i increased the recruitment of circulating brain stem cells towards lesion sites, thus resulting in an increase of synaptic plasticity and neurogenesis. This suggested that DPP-4i might improve cognitive function by promoting hippocampal neurogenesis in AD [60].
In the current pooled analysis, DPP-4i were shown to improve MMSE scores in T2DM patients. The pooled estimate was consistent with a study performed by Rizzo et al., in which MMSE and composite cognitive function scores were also significantly improved in elderly diabetic patients with MCI during DPP-4i treatment [61]. Similarly, vildagliptin was shown to alleviate diabetes-associated cognitive impairment by reducing apoptosis-related protein levels in the hippocampus [62]. A beneficial effect of DPP-4i on cognitive function was also found in an insulin-resistant context. For example, cognitive decline was restored after vildagliptin administration in insulin-resistant obese subjects [63]. Further studies should be performed to fully explore the effect of DPP-4i on cognitive function in T2DM. In addition to an improvement of MMSE scores, DPP-4i restored neuroplasticity in patients with T2DM, while no favourable effects on odour detection and olfactory memory were observed [64]. Notably, the improved neurological function might not correlate with glycaemic control from DPP-4i treatment. Indeed, the effect of DPP-4i on cognitive function might be common in multiple neurodegenerative diseases. DPP-4i have also been shown to reverse amyloid deposition in AD patients with cognitive impairment [65]. The protective effect might potentially correlate with an enhanced glucose uptake, an action mediated by GLP-1 [66]. An animal study also showed that linagliptin ameliorated cognitive decline in a tauopathy mouse model [67]. In contrast, other studies showed that DPP-4i exerted no significant effect on modulating cognitive function in T2DM. For example, saxagliptin provided no neuroprotection or improvement of cognitive or motor function in 6-hydroxydopamine-induced Parkinson’s disease (PD) models [68]. Inconsistent results above might be attributed to the heterogeneity of models and contexts.
Sensitivity analysis revealed some heterogeneity in the outcomes of the identified studies. The heterogeneous result might come from a physiological or pathological effect or a lack of significant neurological changes during DPP-4i treatment [69]. Different patient ages and combined drug use also produced an effect on cognitive function during DPP-4i treatment. These multiple results on cognition might also result from different test measures used at baseline among these studies. Meta-regression was not conducted owing to the limited number of included studies. The correlation between DPP-4i treatment and cognitive function should be further explored considering variables including races or disease-related complications at baseline. DPP-4i improved pancreatic β-cell function in both fasting and postprandial states in T2DM. The reversal of hippocampal IR mediated by alogliptin had been reported to improve cognitive function in an AD model [70]. Further studies needed to be performed to analyse the association of insulin secretion with cognitive function in DPP-4i users.
The current meta-analysis also revealed that DPP-4i had a significant effect on improving IADL scores. Although IADL is mainly an evaluation tool of daily living ability, this tool is also suggested to reflect cognitive function indirectly [36, 40]. Due to the limited studies identified, a solid conclusion could not be drawn regarding the impact of DPP-4i on other comprehensive geriatric assessments, including BADL, CES-D, GDS, and MNA. Further studies are also needed to illustrate this link in DPP-4i users. In fact, cognitive function consists of multiple test scores, which demonstrate an effect of cognition from different aspects. A preclinical study also showed that DPP-4i treatment inhibited oxidative stress and had a beneficial effect on diabetes-related dementia, a process independent of glucose reduction [54]. This finding helps to further elucidate the impact of DPP-4i on cognitive function, which might be somewhat dependent on related neurological proteins or related signalling pathways. Indeed, vildagliptin had been reported to restore cognitive function in neurological disease models by activating BDNF signalling cascades [71].
In the current study, DPP-4i produced no stronger effect on cognitive function than other antidiabetic agents. Numerous studies have been performed to evaluate the impact of antidiabetic agents on cognitive function. For metformin, controversy still exists regarding its impact on cognitive function. In a meta-analysis involving 19 studies, no significant improvement of cognitive function or dementia was found in T2DM patients during metformin treatment [72]. However, in another prospective observational study, elderly diabetic participants receiving metformin showed a slower cognitive decline and lower dementia risk compared to nonusers [73]. Acarbose serves as an alpha-glucosidase inhibitor and is poorly absorbed after administration. This agent exerted a glucose-lowering effect only in the intestine, and its effect on cognitive function was speculated to depend on glucose control [74]. In contrast, insulin-treated participants demonstrated an improvement in the attention test, which might be associated with an increase of plasma concentrations of beta-amyloid peptide in a fasting state [75]. Thiazolidinedione (TZD) treatment with pioglitazone has also improved cognitive impairment in T2DM by activating peroxisome proliferator-activated receptor gamma (PPAR-γ) [76]. A similar improvement in the memory test was also found in patients undergoing treatment with sulfonylureas, which was linked with a reduction of glucose levels, rather than an altered insulin condition [77]. In a recent promising study evaluating the effect of antidiabetic drugs on AD, GLP-1 receptor agonists delayed neurodegenerative processes associated with T2DM [78]. For AD patients, incretin-based therapies significantly improved learning and memory ability, an action correlating with a reduction of inflammatory response and tau hyperphosphorylation [79]. SGLT2i, a novel class of antidiabetic agents, also exerted an effect on cognitive function in T2DM. According to a retrospective cohort study, SGLT2i users showed a lower incidence of dementia than DPP-4i users [16]. This effect might be attributed to a favourable lipid-soluble nature of SGLT2i, allowing them to cross the blood‒brain barrier (BBB) and exert effects on brain cognition. The noninferiority effect observed between DPP-4i and other antidiabetic agents might also result from a balance of varied results from different antidiabetic classes. Detailed head-to-head trials should be designed to strictly evaluate comparative effects of DPP-4i with other agents on cognition.
Oral DPP-4i are well tolerated and safe to use in patients with T2DM. The neuroprotective effects reported in the above studies might indirectly derive from targeting their substrates or related signalling pathways. However, available DPP-4i in the current market could not cross the BBB, which inevitably limited their maximal effects on cognitive function. Omarigliptin, a novel DPP-4i designed to solve the issues of crossing BBB, is being used to treat diabetes-related neurodegenerative diseases [80]. Further clinical trials are also needed to deeply explore the mechanism of DPP-4i treatment in modulating cognitive function.
This current meta-analysis had some strengths. Available evidence was combined to evaluate cognitive function including executive and spatial skills during DPP-4i treatment in T2DM. Compared with individual studies, this meta-analysis could provide more evidence on the effect of DPP-4i on MMSE scores. Furthermore, the impact of DPP-4i on comprehensive geriatric assessment was also demonstrated in the current study.
Nevertheless, this meta-analysis had some limitations. First, the number of identified trials was small, and no subgroup analysis on age or sex were performed. Second, identified trials had differences in size, subject characteristics, cognitive scores at baseline and DPP-4i dosage. Diabetic patients were accompanied with other diseases including stroke were pooled, which inevitably confounded the impact of DPP-4i on cognition ability. Third, this meta-analysis covered sitagliptin, vildagliptin and linagliptin, while no further analysis on other DPP-4i were compared on MMSE scores or other scores due to the limited number of studies. Although more evidence from clinical studies were included, additional data from basic research should also be recorded to complete the conclusion. This could make the reference structure more stable. Finally, analysis on side effects should also be objectively performed when cognitive function was assessed in diabetic patients during DPP-4i treatment.
Conclusions
Available data from clinical trials suggested that DPP-4i produced an off-target favourable effect on cognitive function in patients with T2DM. Whether these significant improvements could translate into clinical value remains to be explored with further studies involving real world data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Relevant advice was provided by Professor Yanping Bao and Yanxue Xue of the National Institute on Drug Dependence and Beijing Key Laboratory of Drug Dependence, Peking University.
Abbreviations
- DPP-4i
dipeptidyl peptidase-4 inhibitor
- T2DM
type 2 diabetes mellitus
- MMSE
Mini-Mental State Examination
- BADL
Barthel Activities of Daily Living
- BDNF
brain-derived neurotrophic factor
- IADL
Instrumental Activities of Daily Living
- GDS
Global Deterioration Scale
- SGLT2i
sodium glucose cotransporter 2 inhibitors
- HbA1c
glycosylated haemoglobin
- RCT
randomized controlled trial
- IR
insulin resistance
- MNA
Mini Nutritional Assessment
- TMT
Trial Making Test
- VFT
Verbal Fluency Test
- A&E
Attention and executive functioning
- CES-D
Center for Epidemiologic Studies Depression Scale
- GLP-1
glucagon-like peptide 1
- LPS
lipopolysaccharide
- MCI
mild cognitive impairment
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- SDF-1α
stromal derived factor 1α
- NPY
neuropeptide Y
- Nrf2
NF-E2-related factor 2
- BNP
brain natriuretic peptide
- TZDs
thiazolidinediones
- PAR2
protease-activated receptor 2
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- Cav 1
caveolin 1
- BMI
body mass index
- BBB
blood‒brain barrier
- WMD:
weighted mean difference
- CI:
confidence interval
- PRISMA:
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- M-H
Mantel-Haenszel
- WOS
Web of Science
- NADPH
Nicotinamide adenine dinucleotide phosphate
Author contributions
All the authors helped to design, conduct and report this meta-analysis. J. M., R. Y. and C. Z. searched the literature, extracted the data and evaluated the risk of bias. J. M., and R. Y. wrote the main manuscript. J. M., R. Y. and C. Z. prepared Figs. 1, 2, 3, 4 and 5. X. B., X.Y. and Y.Y prepared the supplementary files. T. F. and X. L. had full access to all the data in the study and take full responsibility for the integrity of the data analysis. All the authors have read and approved the final manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of China (82071422) and Beijing Municipal Natural Science Foundation (7212031).
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jie Meng and Rui Yan These authors have contributed equally to this work and share first authorship.
Contributor Information
Tao Feng, Email: happyft@sina.com.
Xin Liu, Email: ttyydrliu@126.com.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of Diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Petersmann A, Müller-Wieland D, Müller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, Schleicher E. Definition, classification and diagnosis of Diabetes Mellitus. Exp Clin Endocrinol Diabetes. 2019;127:1–s7. doi: 10.1055/a-1018-9078. [DOI] [PubMed] [Google Scholar]
- 3.Echouffo-Tcheugui JB, Caleyachetty R, Muennig PA, Narayan KM, Golden SH. Cumulative social risk and type 2 Diabetes in US adults: the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Eur J Prev Cardiol. 2016;23:1282–8. doi: 10.1177/2047487315627036. [DOI] [PubMed] [Google Scholar]
- 4.Hackett RA, Steptoe A. Type 2 Diabetes Mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol. 2017;13:547–60. doi: 10.1038/nrendo.2017.64. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 Diabetes Mellitus–present and future perspectives. Nat Rev Endocrinol. 2011;8:228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 6.Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF. Global, regional, and national burden and trend of Diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biessels GJ, Despa F. Cognitive decline and Dementia in Diabetes Mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14:591–604. doi: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with Diabetes Mellitus: guidance for daily care. Lancet Neurol. 2015;14:329–40. doi: 10.1016/S1474-4422(14)70249-2. [DOI] [PubMed] [Google Scholar]
- 9.Diniz Pereira J, Gomes Fraga V, Morais Santos AL, Carvalho MDG, Caramelli P, Braga Gomes K. Alzheimer’s Disease and type 2 Diabetes Mellitus: a systematic review of proteomic studies. J Neurochem. 2021;156:753–76. doi: 10.1111/jnc.15166. [DOI] [PubMed] [Google Scholar]
- 10.Carranza-Naval MJ, Vargas-Soria M, Hierro-Bujalance C, Baena-Nieto G, Garcia-Alloza M, Infante-Garcia C, Del Marco A. Alzheimer’s Disease and Diabetes: Role of Diet, Microbiota and Inflammation in Preclinical Models. Biomolecules 2021, 11. [DOI] [PMC free article] [PubMed]
- 11.Lyu F, Wu D, Wei C, Wu A. Vascular cognitive impairment and Dementia in type 2 Diabetes Mellitus: an overview. Life Sci. 2020;254:117771. doi: 10.1016/j.lfs.2020.117771. [DOI] [PubMed] [Google Scholar]
- 12.Dao L, Choi S, Freeby M. Type 2 Diabetes Mellitus and cognitive function: understanding the connections. Curr Opin Endocrinol Diabetes Obes. 2023;30:7–13. doi: 10.1097/MED.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 13.Moheet A, Mangia S, Seaquist ER. Impact of Diabetes on cognitive function and brain structure. Ann N Y Acad Sci. 2015;1353:60–71. doi: 10.1111/nyas.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun M, Wang L, Guo Y, Yan S, Li J, Wang X, Li X, Li B. The Association among Inflammatory Diet, Glycohemoglobin, and cognitive function impairment in the Elderly: based on the NHANES 2011–2014. J Alzheimers Dis. 2022;87:1713–23. doi: 10.3233/JAD-215688. [DOI] [PubMed] [Google Scholar]
- 15.Benedict RHB, Amato MP, DeLuca J, Geurts JJG. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19:860–71. doi: 10.1016/S1474-4422(20)30277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mui JV, Zhou J, Lee S, Leung KSK, Lee TTL, Chou OHI, Tsang SL, Wai AKC, Liu T, Wong WT, et al. Sodium-glucose cotransporter 2 (SGLT2) inhibitors vs. Dipeptidyl Peptidase-4 (DPP4) inhibitors for New-Onset Dementia: a propensity score-matched Population-based study with competing risk analysis. Front Cardiovasc Med. 2021;8:747620. doi: 10.3389/fcvm.2021.747620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaribeygi H, Rashidy-Pour A, Atkin SL, Jamialahmadi T, Sahebkar A. GLP-1 mimetics and cognition. Life Sci. 2021;264:118645. doi: 10.1016/j.lfs.2020.118645. [DOI] [PubMed] [Google Scholar]
- 18.Monney M, Jornayvaz FR, Gariani K. GLP-1 receptor agonists effect on cognitive function in patients with and without type 2 Diabetes. Diabetes Metab. 2023;49:101470. doi: 10.1016/j.diabet.2023.101470. [DOI] [PubMed] [Google Scholar]
- 19.Mone P, Lombardi A, Gambardella J, Pansini A, Macina G, Morgante M, Frullone S, Santulli G. Empagliflozin improves cognitive impairment in Frail older adults with type 2 Diabetes and Heart Failure with preserved ejection fraction. Diabetes Care. 2022;45:1247–51. doi: 10.2337/dc21-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawlos A, Broncel M, Woźniak E, Gorzelak-Pabiś P. Neuroprotective effect of SGLT2 inhibitors. Molecules 2021, 26. [DOI] [PMC free article] [PubMed]
- 21.Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, Yu JT. Diabetes Mellitus and risks of cognitive impairment and Dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. 2019;55:100944. doi: 10.1016/j.arr.2019.100944. [DOI] [PubMed] [Google Scholar]
- 22.Wium-Andersen IK, Osler M, Jørgensen MB, Rungby J, Wium-Andersen MK. Antidiabetic medication and risk of Dementia in patients with type 2 Diabetes: a nested case-control study. Eur J Endocrinol. 2019;181:499–507. doi: 10.1530/EJE-19-0259. [DOI] [PubMed] [Google Scholar]
- 23.Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 Diabetes Mellitus. Nat Rev Endocrinol. 2020;16:642–53. doi: 10.1038/s41574-020-0399-8. [DOI] [PubMed] [Google Scholar]
- 24.Sana SR, Li EY, Deng XJ, Guo L. Association between plasma dipeptidyl peptidase-4 levels and cognitive function in perinatal pregnant women with gestational Diabetes Mellitus. World J Clin Cases. 2021;9:10161–71. doi: 10.12998/wjcc.v9.i33.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Rawaf HA, Alghadir AH, Gabr SA. Molecular changes in circulating microRNAs’ expression and oxidative stress in adults with mild cognitive impairment: a biochemical and molecular study. Clin Interv Aging. 2021;16:57–70. doi: 10.2147/CIA.S285689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda Y, Nagase N, Tsuji A, Kitagishi Y, Matsuda S. Neuroprotection by dipeptidyl-peptidase-4 inhibitors and glucagon-like peptide-1 analogs via the modulation of AKT-signaling pathway in Alzheimer’s Disease. World J Biol Chem. 2021;12:104–13. doi: 10.4331/wjbc.v12.i6.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gault VA, Lennox R, Flatt PR. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes Metab. 2015;17:403–13. doi: 10.1111/dom.12432. [DOI] [PubMed] [Google Scholar]
- 28.Andersen ES, Deacon CF, Holst JJ. Do we know the true mechanism of action of the DPP-4 inhibitors? Diabetes Obes Metab. 2018;20:34–41. doi: 10.1111/dom.13018. [DOI] [PubMed] [Google Scholar]
- 29.Secnik J, Xu H, Schwertner E, Hammar N, Alvarsson M, Winblad B, Eriksdotter M, Garcia-Ptacek S, Religa D. The association of antidiabetic medications and Mini-mental State Examination scores in patients with Diabetes and Dementia. Alzheimers Res Ther. 2021;13:197. doi: 10.1186/s13195-021-00934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasegawa Y, Hayashi K, Takemoto Y, Cheng C, Takane K, Lin B, Komohara Y, Kim-Mitsuyama S. DPP-4 inhibition with linagliptin ameliorates the progression of premature aging in klotho-/- mice. Cardiovasc Diabetol. 2017;16:154. doi: 10.1186/s12933-017-0639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DH, Huh JW, Jang M, Suh JH, Kim TW, Park JS, Yoon SY. Sitagliptin increases tau phosphorylation in the hippocampus of rats with type 2 Diabetes and in primary neuron cultures. Neurobiol Dis. 2012;46:52–8. doi: 10.1016/j.nbd.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 Diabetes Mellitus. Nat Rev Endocrinol. 2021;17:484–95. doi: 10.1038/s41574-021-00507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Men P, Wang Y, Zhai S, Liu G. Impact of dipeptidyl peptidase-4 inhibitors on serum adiponectin: a meta-analysis. Lipids Health Dis. 2016;15:204. doi: 10.1186/s12944-016-0372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Jongh FW, Schaeffers A, Kooreman ZE, Ingels K, van Heerbeek N, Beurskens C, Monstrey SJ, Pouwels S. Botulinum toxin A treatment in facial palsy synkinesis: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2023;280:1581–92. doi: 10.1007/s00405-022-07796-8. [DOI] [PubMed] [Google Scholar]
- 36.Isik AT, Soysal P, Yay A, Usarel C. The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s Disease. Diabetes Res Clin Pract. 2017;123:192–8. doi: 10.1016/j.diabres.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Borzì AM, Condorelli G, Biondi A, Basile F, Vicari ESD, Buscemi C, Luca S, Vacante M. Effects of vildagliptin, a DPP-4 inhibitor, in elderly diabetic patients with mild cognitive impairment. Arch Gerontol Geriatr. 2019;84:103896. doi: 10.1016/j.archger.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Biessels GJ, Verhagen C, Janssen J, van den Berg E, Zinman B, Rosenstock J, George JT, Passera A, Schnaidt S, Johansen OE. Effect of Linagliptin on Cognitive performance in patients with type 2 Diabetes and Cardiorenal comorbidities: the CARMELINA Randomized Trial. Diabetes Care. 2019;42:1930–8. doi: 10.2337/dc19-0783. [DOI] [PubMed] [Google Scholar]
- 39.Xue J, Wang C, Pan C, Xing H, Xu L, Chen X, Wang X, Wang N. Effect of DPP-4 inhibitor on elderly patients with T2DM combined with MCI. Exp Ther Med. 2020;19:1356–62. doi: 10.3892/etm.2019.8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ates Bulut E, Sahin Alak ZY, Dokuzlar O, Kocyigit SE, Soysal P, Smith L, Isik AT. Cognitive and metabolic outcomes of vildagliptin addition to the therapy in patients with type 2 Diabetes Mellitus: 26 week follow-up study. Arch Gerontol Geriatr. 2020;88:104013. doi: 10.1016/j.archger.2020.104013. [DOI] [PubMed] [Google Scholar]
- 41.Biessels GJ, Verhagen C, Janssen J, van den Berg E, Wallenstein G, Zinman B, Espeland MA, Johansen OE. Effects of linagliptin vs glimepiride on cognitive performance in type 2 Diabetes: results of the randomised double-blind, active-controlled CAROLINA-COGNITION study. Diabetologia. 2021;64:1235–45. doi: 10.1007/s00125-021-05393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pintana H, Apaijai N, Chattipakorn N, Chattipakorn SC. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J Endocrinol. 2013;218:1–11. doi: 10.1530/JOE-12-0521. [DOI] [PubMed] [Google Scholar]
- 43.Kosaraju J, Murthy V, Khatwal RB, Dubala A, Chinni S, Muthureddy Nataraj SK, Basavan D. Vildagliptin: an anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s Disease. J Pharm Pharmacol. 2013;65:1773–84. doi: 10.1111/jphp.12148. [DOI] [PubMed] [Google Scholar]
- 44.Cheng Q, Cheng J, Cordato D, Gao J. Can dipeptidyl peptidase-4 inhibitors treat cognitive disorders? Pharmacol Ther. 2020;212:107559. doi: 10.1016/j.pharmthera.2020.107559. [DOI] [PubMed] [Google Scholar]
- 45.Xia W, Luo Y, Chen YC, Chen H, Ma J, Yin X. Glucose fluctuations are linked to disrupted Brain Functional Architecture and Cognitive Impairment. J Alzheimers Dis. 2020;74:603–13. doi: 10.3233/JAD-191217. [DOI] [PubMed] [Google Scholar]
- 46.Sim AY, Barua S, Kim JY, Lee YH, Lee JE. Role of DPP-4 and SGLT2 inhibitors connected to Alzheimer Disease in type 2 Diabetes Mellitus. Front Neurosci. 2021;15:708547. doi: 10.3389/fnins.2021.708547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baumeier C, Schlüter L, Saussenthaler S, Laeger T, Rödiger M, Alaze SA, Fritsche L, Häring HU, Stefan N, Fritsche A, et al. Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty Liver Disease. Mol Metab. 2017;6:1254–63. doi: 10.1016/j.molmet.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghorpade DS, Ozcan L, Zheng Z, Nicoloro SM, Shen Y, Chen E, Blüher M, Czech MP, Tabas I. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555:673–7. doi: 10.1038/nature26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bae J, Kim G, Lee YH, Lee BW, Kang ES, Cha BS. Differential effects of thiazolidinediones and Dipeptidyl Peptidase-4 inhibitors on insulin resistance and β-Cell function in type 2 Diabetes Mellitus: a propensity score-matched analysis. Diabetes Ther. 2019;10:149–58. doi: 10.1007/s13300-018-0541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013;37:839–49. doi: 10.1111/ejn.12088. [DOI] [PubMed] [Google Scholar]
- 51.Sun C, Xiao Y, Li J, Ge B, Chen X, Liu H, Zheng T. Nonenzymatic function of DPP4 in diabetes-associated mitochondrial dysfunction and cognitive impairment. Alzheimers Dement. 2022;18:966–87. doi: 10.1002/alz.12437. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Hui Y, Xu Z, Tan J, Yin K, Kuang L, Tang Y, Wei J, Zhong Q, Zheng T. Non-canonical function of DPP4 promotes cognitive impairment through ERp29-associated mitochondrial calcium overload in Diabetes. iScience. 2023;26:106271. doi: 10.1016/j.isci.2023.106271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muriach M, Flores-Bellver M, Romero FJ, Barcia JM. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev. 2014;2014:102158. doi: 10.1155/2014/102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ide M, Sonoda N, Inoue T, Kimura S, Minami Y, Makimura H, Hayashida E, Hyodo F, Yamato M, Takayanagi R, Inoguchi T. The dipeptidyl peptidase-4 inhibitor, linagliptin, improves cognitive impairment in streptozotocin-induced diabetic mice by inhibiting oxidative stress and microglial activation. PLoS ONE. 2020;15:e0228750. doi: 10.1371/journal.pone.0228750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong L, Deng J, Zhou X, Cai B, Zhang B, Chen X, Chen Z, Wang W. Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis. 2021;12:928. doi: 10.1038/s41419-021-04227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piątkowska-Chmiel I, Gawrońska-Grzywacz M, Popiołek Ł, Herbet M, Dudka J. The novel adamantane derivatives as potential mediators of inflammation and neural plasticity in Diabetes mice with cognitive impairment. Sci Rep. 2022;12:6708. doi: 10.1038/s41598-022-10187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chalichem NSS, Gonugunta C, Krishnamurthy PT, Duraiswamy B. DPP4 inhibitors can be a drug of choice for type 3 Diabetes: a Mini Review. Am J Alzheimers Dis Other Demen. 2017;32:444–51. doi: 10.1177/1533317517722005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yossef RR, Al-Yamany MF, Saad MA, El-Sahar AE. Neuroprotective effects of vildagliptin on drug induced Alzheimer’s Disease in rats with metabolic syndrome: role of hippocampal klotho and AKT signaling pathways. Eur J Pharmacol. 2020;889:173612. doi: 10.1016/j.ejphar.2020.173612. [DOI] [PubMed] [Google Scholar]
- 59.Sakr HF. Effect of sitagliptin on the working memory and reference memory in type 2 diabetic Sprague-Dawley rats: possible role of adiponectin receptors 1. J Physiol Pharmacol. 2013;64:613–23. [PubMed] [Google Scholar]
- 60.Chalichem NSS, Sai Kiran PSS, Basavan D. Possible role of DPP4 inhibitors to promote hippocampal neurogenesis in Alzheimer’s Disease. J Drug Target. 2018;26:670–5. doi: 10.1080/1061186X.2018.1433682. [DOI] [PubMed] [Google Scholar]
- 61.Rizzo MR, Barbieri M, Boccardi V, Angellotti E, Marfella R, Paolisso G. Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. J Gerontol A Biol Sci Med Sci. 2014;69:1122–31. doi: 10.1093/gerona/glu032. [DOI] [PubMed] [Google Scholar]
- 62.Zhang DD, Shi N, Fang H, Ma L, Wu WP, Zhang YZ, Tian JL, Tian LB, Kang K, Chen S. Vildagliptin, a DPP4 inhibitor, alleviates diabetes-associated cognitive deficits by decreasing the levels of apoptosis-related proteins in the rat hippocampus. Exp Ther Med. 2018;15:5100–6. doi: 10.3892/etm.2018.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sa-Nguanmoo P, Tanajak P, Kerdphoo S, Jaiwongkam T, Wang X, Liang G, Li X, Jiang C, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. FGF21 and DPP-4 inhibitor equally prevents cognitive decline in obese rats. Biomed Pharmacother. 2018;97:1663–72. doi: 10.1016/j.biopha.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 64.Lietzau G, Davidsson W, Östenson CG, Chiazza F, Nathanson D, Pintana H, Skogsberg J, Klein T, Nyström T, Darsalia V, Patrone C. Type 2 Diabetes impairs odour detection, olfactory memory and olfactory neuroplasticity; effects partly reversed by the DPP-4 inhibitor Linagliptin. Acta Neuropathol Commun. 2018;6:14. doi: 10.1186/s40478-018-0517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel B, Sheth D, Vyas A, Shah S, Parmar S, Patel C, Patel S, Beladiya J, Pande S, Modi K. Amelioration of intracerebroventricular streptozotocin-induced cognitive dysfunction by Ocimum sanctum L. through the modulation of inflammation and GLP-1 levels. Metab Brain Dis 2022. [DOI] [PubMed]
- 66.Li Z, Zhang Y, Meng X, Li M, Cao W, Yang J, Xu X, Liu W, Li W, Cai Q, et al. A novel DPP-4 inhibitor gramcyclin a attenuates cognitive deficits in APP/PS1/tau triple transgenic mice via enhancing brain GLP-1-dependent glucose uptake. Phytother Res. 2022;36:1297–309. doi: 10.1002/ptr.7387. [DOI] [PubMed] [Google Scholar]
- 67.Nakaoku Y, Saito S, Yamamoto Y, Maki T, Takahashi R, Ihara M. The Dipeptidyl Peptidase-4 inhibitor linagliptin ameliorates high-fat Induced Cognitive decline in Tauopathy Model mice. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed]
- 68.Turnes JM, Bassani TB, Souza LC, Vital M. Ineffectiveness of saxagliptin as a neuroprotective drug in 6-OHDA-lesioned rats. J Pharm Pharmacol. 2018;70:1059–68. doi: 10.1111/jphp.12936. [DOI] [PubMed] [Google Scholar]
- 69.Gul O, Gul S. Mild cognitive decline in type 2 Diabetes Mellitus patients - risk factors and pathogenesis: role of DPP4 activity and future possible therapeutic targets. Neuropsychiatr Dis Treat. 2019;15:403–4. doi: 10.2147/NDT.S200663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rahman SO, Kaundal M, Salman M, Shrivastava A, Parvez S, Panda BP, Akhter M, Akhtar M, Najmi AK. Alogliptin reversed hippocampal insulin resistance in an amyloid-beta fibrils induced animal model of Alzheimer’s Disease. Eur J Pharmacol. 2020;889:173522. doi: 10.1016/j.ejphar.2020.173522. [DOI] [PubMed] [Google Scholar]
- 71.Mahmoud AMA, Mantawy EM, Wahdan SA, Ammar RM, El-Demerdash E. Vildagliptin restores cognitive function and mitigates hippocampal neuronal apoptosis in cisplatin-induced chemo-brain: imperative roles of AMPK/Akt/CREB/ BDNF signaling cascades. Biomed Pharmacother. 2023;159:114238. doi: 10.1016/j.biopha.2023.114238. [DOI] [PubMed] [Google Scholar]
- 72.Tabatabaei Malazy O, Bandarian F, Qorbani M, Mohseni S, Mirsadeghi S, Peimani M, Larijani B. The effect of metformin on cognitive function: a systematic review and meta-analysis. J Psychopharmacol. 2022;36:666–79. doi: 10.1177/02698811211057304. [DOI] [PubMed] [Google Scholar]
- 73.Samaras K, Makkar S, Crawford JD, Kochan NA, Wen W, Draper B, Trollor JN, Brodaty H, Sachdev PS. Metformin Use is Associated with slowed cognitive decline and reduced Incident Dementia in older adults with type 2 Diabetes: the Sydney Memory and Ageing Study. Diabetes Care. 2020;43:2691–701. doi: 10.2337/dc20-0892. [DOI] [PubMed] [Google Scholar]
- 74.Ogura J, Yamaguchi H. The effectiveness of antidiabetic Drugs in treating Dementia: a Peek into Pharmacological and Pharmacokinetic properties. Int J Mol Sci 2022, 23. [DOI] [PMC free article] [PubMed]
- 75.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–8. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 76.Alhowail A, Alsikhan R, Alsaud M, Aldubayan M, Rabbani SI. Protective effects of Pioglitazone on Cognitive Impairment and the underlying mechanisms: a review of literature. Drug Des Devel Ther. 2022;16:2919–31. doi: 10.2147/DDDT.S367229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strachan MW. Insulin and cognitive function in humans: experimental data and therapeutic considerations. Biochem Soc Trans. 2005;33:1037–40. doi: 10.1042/BST0331037. [DOI] [PubMed] [Google Scholar]
- 78.Colin IM, Szczepanski LW, Gérard AC, Elosegi JA. Emerging evidence for the use of Antidiabetic Drugs, glucagon-like peptide 1 receptor agonists, for the treatment of Alzheimer’s Disease. touchREV Endocrinol. 2023;19:16–24. doi: 10.17925/EE.2023.19.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferrari F, Moretti A, Villa RF. Incretin-based Drugs as potential therapy for neurodegenerative Diseases: current status and perspectives. Pharmacol Ther. 2022;239:108277. doi: 10.1016/j.pharmthera.2022.108277. [DOI] [PubMed] [Google Scholar]
- 80.Li X, Yin Y, Li W, Li S, Zhang D, Liu Z. Omarigliptin alleviates cognitive dysfunction in streptozotocin-induced diabetic mouse. Bioengineered. 2022;13:9387–96. doi: 10.1080/21655979.2022.2055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].