Abstract
Background
Osteoarthritis (OA) is one of the most prevalent musculoskeletal diseases. There is currently no consensus on what is the best treatment to improve OA symptoms and slow disease progression. Diacerein is an anthraquinone synthesised in 1980 that interferes with interleukin‐1, an inflammatory mediator. It has been proposed that diacerein acts as a slow‐acting, symptom‐modifying and perhaps disease‐structure‐modifying drug for OA. This is an update of a Cochrane review first published in 2006.
Objectives
To assess the benefits and harms of diacerein for the treatment of adults with OA when compared with placebo and other pharmacologically active interventions (nonsteroidal anti‐inflammatory drugs (NSAIDs) and other symptom‐modifying, slow‐acting drugs) for OA.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library, Issue 10, 2013, MEDLINE (1966 to 2013), EMBASE (1980 to 2013), LILACS (1982 to 2013), and ACP Journal Club, and we handsearched reference lists of published articles. We also searched the World Health Organization International Clinical Trials Platform ( http://www.who.int/trialsearch/Default.aspx) to identify ongoing trials and screened reference lists of retrieved review articles and trials to identify potentially relevant studies. All searches were up to date as of March 2013. Pharmaceutical companies and authors of published articles were contacted. We searched the websites of the regulatory agencies using the keyword ‘diacerein’ in November 2013. No language restrictions were applied.
Selection criteria
Studies were included if they were randomised or quasi‐randomised controlled trials that compared diacerein with placebo or another active pharmacological intervention in participants with OA.
Data collection and analysis
Data abstraction and quality assessment were performed by two independent investigators, and their results were compared. The Cochrane risk of bias tool was used. The quality of evidence obtained was assessed using the GRADE approach.
Main results
We identified three new trials (141 participants), and this updated review now includes 10 trials, totalling 2,210 participants. The most frequent risk of bias was incomplete outcome data, identified in approximately 80% of the studies. Allocation concealment and random sequence generation were unclear in 90% and 40% of the studies, respectively, because of poor reporting.
Low‐quality evidence from six trials (1,283 participants) indicates that diacerein has a small beneficial effect on overall pain (measured on a 100 mm visual analogue scale) at three to 36 months (mean difference (MD) ‐8.65, 95% confidence interval (CI) ‐15.62 to ‐1.68), which is equivalent to a 9% pain reduction in the diacerein group (95% CI ‐16% to ‐2%) compared with the placebo group. This benefit may not be clinically significant.
No statistically significant differences in physical function (4 studies, 1006 participants) were noted between the diacerein and placebo groups (Lequesne impairment index, 0 to 24 points) (MD ‐0.29, 95% CI ‐0.87 to 0.28).
Low‐quality evidence from two trials (616 participants) on slowing of joint space narrowing (a decrease greater than 0.50 mm) in the knee or hip favoured diacerein over placebo (risk ratio (RR) 0.85, 95% CI 0.72 to 0.99), with an absolute risk difference of ‐6% (95% CI ‐15% to 2%) and a number needed to treat for an additional beneficial outcome (NNTB) of 14 (95% CI 8 to 203). Analysis of the knee joint alone (1 study, 170 participants) did not reach statistical significance (RR 0.94, 95% CI 0.51 to 1.74).
None of the trials of diacerein versus placebo measured quality of life. According to one trial (161 participants), which compared diacerein versus non‐steroidal anti‐inflammatory drugs (NSAIDs), the quality of life of participants in the two groups (as assessed by the Short Form (SF)‐36 health survey questionnaire (0 to 800 sum score)) did not differ significantly (MD ‐40.70, 95% CI ‐85.20 to 3.80).
Low‐quality evidence from seven trials showed significantly more adverse events in the diacerein group compared with the placebo group after two to 36 months, mainly diarrhoea (RR 3.52, 95% CI 2.42 to 5.11), with an absolute risk increase of 24% (95% CI 12% to 35%), and a number needed to treat for an additional harmful outcome (NNTH) of 4 (95% CI 3 to 7).
No statistically significant differences in participant withdrawal due to adverse events were seen at two to 36 months for diacerein compared with placebo (RR 1.29, 95% CI 0.83 to 2.01).
A search of regulatory websites found a recommendation from the European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) that the marketing authorization of diacerein should be suspended across Europe because of harms (particularly the risk of severe diarrhoea and potentially harmful effects on the liver) outweighing benefits. However, this guidance is not final as the PRAC recommendation will be re‐examined.
Authors' conclusions
In this update, the strength of evidence for effectiveness outcomes was low to moderate. We confirmed that symptomatic benefit provided by diacerein in terms of pain reduction is minimal. The small benefit derived in terms of joint space narrowing is of questionable clinical relevance and was observed only for OA of the hip. With respect to adverse effects of diacerein, diarrhoea was most frequent. Given the recent guidance issued by the EMA recommending suspension of diacerein in Europe, the EMA website should be consulted for further recommendations regarding the use of diacerein.
Plain language summary
Diacerein for osteoarthritis
What is osteoarthritis and what is diacerein?
Osteoarthritis (OA) is the most common form of arthritis. In OA, the cartilage that protects the ends of the bones breaks down, causing pain and swelling. OA can affect any joint, but the knees, hips and hands are the joints most often studied in clinical trials. In all, 10% of the world’s population aged 60 or older have pain or disability from OA.
Diacerein is a slow‐acting drug taken as a pill that may slow the breakdown of cartilage and relieve pain and swelling.
The review searched for studies up to March 2013 about primary osteoarthritis affecting men and women (18 years and older) of any disease severity.
The review shows that in people with osteoarthitis:
‐ Pain may improve slightly more in people taking diacerein.
‐ Improvement in physical function is about the same for people taking diacerein,‐ or a placebo (fake pill). This may have happened by chance.
‐ Diacerein may slow the process of joint space narrowing slightly of the hip but may have little or no difference on the knee joint as it is seen on an x‐ray.
‐ Diacerein may cause side effects in the lower digestive tract, such as diarrhoea.
Further research is very likely to have an important impact on our confidence in these findings and is likely to change the estimates.
Best estimate of what happens to people with osteoarthritis who take diacerein
Pain after three to 36 months
‐ People who took diacerein rated their pain to be 9 points lower on a scale of 0 (no pain) to 100 (extreme pain) after taking the medication for three to 36 months (9% absolute improvement).
‐ People who took diacerein rated their pain to be 34 on a scale of 0 to 100 after taking the medication compared to people who took a fake pill and rated their pain to be 43 points on a scale of 0 to 100.
Physical function after two to 36 months (lower score means worse function)
‐ People who took diacerein rated their physical function to be 0.30 points lower on a scale of 0 to 24 after taking the medication for two to 36 months (0% absolute improvement).
‐ People who took diacerein rated their physical function to be 9.3 on a scale of 0 to 24 after taking the medication compared to people who took a fake pill and rated their physical function to be 9 points on a scale of 0 to 24.
Radiographic progression ‐ how the joint looks on an x‐ray (reduction in joint space narrowing of at least 0.5 mm)
‐ Seven more people who took placebo had radiographic progression (absolute difference of 7%).
‐ 42 of every 100 people who took diacerein experienced reduction in joint space narrowing of at least 0.5 mm compared to 49 of every 100 people who took a fake pill.
Quality of life
‐ The review authors found no studies about quality of life of people who took diacerein compared with placebo.
‐ There was no difference in quality of life of people who took diacerein compared with non‐steroidal anti‐inflammatory drugs (NSAIDs). This may have happened by chance.
Side effects
‐ Twenty‐six more people who took diacerein experienced diarrhoea as a side effect (absolute difference of 26%).
‐ 36 of every 100 people who took diacerein experienced diarrhoea as a side effect compared to 10 of every 100 who took a fake pill.
Diarrhoea was the most common side effect and usually occurred during the first two weeks after the start of diacerein.
People who took diacerein were not more likely than people who took a placebo to stop taking the medication because of side effects.
In November 2013, the European Medicines Agency Pharmacovigilance Risk Assessment Committee (PRAC) recommended that the marketing authorisation of diacerein should be suspended across Europe because of harms outweighing benefits. However, this guidance is not final as the PRAC recommendation will be re‐examined.
Summary of findings
Summary of findings for the main comparison. Diacerein compared with placebo for osteoarthritis.
| Diacerein compared to placebo for osteoarthritis | ||||||
| Patient or population: participants with osteoarthritis Settings: outpatient Intervention: diacerein Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Diacerein | |||||
| Pain overall visual analogue scale for pain Scale from: 0 to 100 Follow‐up: three to 36 months | Mean pain overall visual analogue scale for pain in control groups was 43 mm | Mean pain overall visual analogue scale for pain in the intervention groups was 8.65 points lower (15.62 to 1.68 lower) | 1,283 (six studies) | ⊕⊕⊕⊝ low1,2 | NNTB 6 (4 to 30)5 Absolute risk difference ‐9% (‐16% to ‐2%) Relative percentage change ‐19% (‐34% to ‐4%) |
|
| Physical function—Lequesne Impairment Index Scale from: 0 to 24 Follow‐up: two to 36 months | Mean physical function—Lequesne Impairment Index in control groups was 9 points | Mean physical function—Lequesne Impairment Index in intervention groups was 0.29 points lower (0.87 lower to 0.28 higher) | 1,006 (four studies) | ⊕⊕⊕⊝ moderate2 | NNTB: non–statistically significant5 Absolute risk difference 0% (‐4% to 1%) Relative percentage change ‐4% (‐11% to 4%) |
|
|
Radiographic progression—minimum joint space width (decreased over 0.50 mm during the study period) Follow‐up: 12 to 36 months |
494 per 1000 | 420 per 1000 (355 to 489) | RR 0.85 (0.72 to 0.99) | 616 (two studies) | ⊕⊕⊝⊝ low2,3 | NNTB 14 (8 to 203)5 Absolute risk difference ‐6% (‐15% to 2%) Relative percentage change ‐15% (‐28% to ‐1%) |
| Quality of Life not measured | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured.6 |
| Adverse event: diarrhoea Follow‐up: two to 36 months | 102 per 1000 | 359 per 1000 (247 to 521) | RR 3.52 (2.42 to 5.11) | 1.462 (seven studies) | ⊕⊕⊕⊝ low2,4 | NNTH 4 (7 to 3)7 Absolute risk difference 24% (12% to 35%) Relative percentage change 252% (142% to 411%) |
| Withdrawal due to adverse events Follow‐up: two to 36 months | 118 per 1000 | 153 per 1000 (98 to 238) | RR 1.29 (0.83 to 2.01) | 1,476 (seven studies) | ⊕⊕⊝⊝ low3,4 | NNTH: non–statistically significant7 Absolute risk difference 0% (‐3% to 4%) Relative percentage change 0% (‐29% to 41%) |
| Total number of serious adverse events not measured | See comment | See comment | Not estimable | ‐ | See comment | This outcome was not measured in any of our studies8 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1High heterogeneity (I2 = 84%). Reduction of heterogeneity could be explained by post hoc sensitivity analysis between studies, with follow‐up lasting longer than six months.
2No allocation concealment in most studies. 3Total number of events is less than 300. 4Unexplained heterogeneity. 5NNTB: number needed to treat for an additional beneficial outcome.
6One study compared diacerein versus non‐steroidal anti‐inflammatory drugs (NSAIDs) and there was no difference in the quality of life of participants in the two groups (MD ‐40.70, 95% CI ‐85.20 to 3.80).
7NNTH: number needed to treat for an additional harmful outcome.
8Although diacerein is known to cause diarrhoea as a side effect, the PRAC (http://www.ema.europa.eu) on 8 November 2013, concluded that there was a high number of cases, particularly of severe diarrhoea, which sometimes led to complications. The Committee was also concerned about liver problems that had been reported in some patients taking the medicine.
Background
Description of the condition
Osteoarthritis (OA) is the most prevalent musculoskeletal disease (ACR 2000; Picavet 2003). The World Health Organization (WHO) Scientific Group on Rheumatic Diseases estimates that 10% of the world’s population aged 60 or older have significant clinical problems attributed to OA (Woolf 2003). As the incidence and prevalence of OA increase with age, the increase in life expectancy will result in an increase in OA in the future (Sun 2007; Woolf 2003), making this disease an ever growing public health problem. More than 10% of the US adult population had clinical OA in 2005, and in 2009, OA was the fourth most common cause of hospitalisation. OA is the leading indication for joint replacement surgery; 905,000 knee and hip replacements were performed in 2009 at a cost of $42.3 billion (Murphy 2012). Obesity is a strong risk factor for OA of the knee and hip (Murphy 2012).
OA remains an enigmatic disease. It is defined as a condition characterised by focal areas of loss of articular cartilage within the synovial joints, associated with hypertrophy of the bone (osteophytes and subchondral bone sclerosis) and thickening of the capsule (Lawrence 1998; Zhang 2001). Recently, OA has been relabeled as a whole organ disease because pathological abnormalities such as periarticular muscle weakness, lax ligaments, low‐grade synovitis, meniscal degeneration and neurosensory system alteration are often present in these patients (Bijlsma 2012).
Description of the intervention
Treatments for OA include pharmacological and non‐pharmacological therapies and surgical procedures. Pharmacological therapies consist of topical agents, oral (systemic) agents, adjunct therapies and nutraceuticals (Bellamy 2006; Towheed 2006; Towheed 2008).
Although some drugs and/or compounds have been available for several decades and are integrated as standard practice in many countries, their efficacy has been demonstrated only over the past decade. Revision of drug registries by health authorities in various European countries in the 1990s led to appropriate clinical trials for available drugs (such as avocado extract), as well as drugs in development at that time (such as diacerein). This action of health authorities greatly improved knowledge regarding the level of evidence and characteristic treatment effects of these drugs (onset of action, carry‐over effect) (Hochberg 2001).
Current therapies for OA, including non‐steroidal anti‐inflammatory drugs (NSAIDs), although effective against symptoms of the disease, are palliative and do not stop disease progression. However, promising agents and compounds have been shown to reduce the severity of the disease, as well as the symptoms. Among them is diacerein, an oral interleukin (IL)‐1beta inhibitor. Its active derivative, rhein, is an anthraquinone found in plants of the genus Cassia. It has moderate anti‐inflammatory and analgesic activities (Spencer 1997).
How the intervention might work
Although OA is considered a non‐inflammatory disease, numerous studies have shown that inflammatory cytokines provide essential biochemical signals that simulate chondrocytes to release cartilage‐degrading enzymes. In addition, cytokines can be produced by synovial tissue cells and subchondral osteoblasts. IL‐1beta and tumour necrosis factor (TNF)‐alfa are key cytokines in the catabolic process of cartilage (Berembaum 2010).
In vitro and in vivo studies have demonstrated that diacerein acts not only on cartilage but in all tissues involved in the pathogenesis of OA, including synoviocytes, the synovial membrane, subchondral bone and chondrocytes. Besides its inhibitory effects on IL‐1, diacerein reduces other important mediators such as metalloproteinases, nitric oxide, ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs)‐4 and ADAMTS‐5 (Pelletier 2010).
Why it is important to do this review
Currently, clinical management of OA typically entails a combination of treatment options to reduce pain and improve tolerance to functional activity. Existing pharmacological therapies for OA help to reduce symptoms but are only moderately effective and leave patients with substantial pain and functional burden (Hunter 2011).
Starting in 1982 (Lingetti 1982), several trials tested diacerein for the treatment of OA, and since 1994, the drug has been marketed around the world, except in the United States of America. Based on the findings of several studies, it has been proposed that diacerein is a slow‐acting, symptom‐modifying and perhaps disease/structure‐modifying drug for OA. However, the importance of diacerein as an option for the treatment of OA needs to be clarified. Despite the long time elapsed since its discovery, published studies have not defined a clear place for the use of diacerein in the treatment of this disease as a symptom modifier or as a disease‐modifying agent that could retard the loss of cartilage.
We performed a review of these studies to gather up‐to‐date evidence to clarify the role of diacerein in the treatment of OA.
Objectives
To assess the benefits and harms of diacerein for the treatment of adults with OA when compared with placebo and other pharmacologically active interventions (nonsteroidal anti‐inflammatory drugs (NSAIDs) and other symptom‐modifying, slow‐acting drugs) for OA.
Methods
Criteria for considering studies for this review
Types of studies
Studies with the following characteristics were eligible for inclusion in the review.
Randomised controlled trials (RCTs) evaluating the benefits and harms of diacerein for OA.
Both placebo‐based and comparative studies were eligible.
Types of participants
All adults (age 18 years and older) with a diagnosis of primary OA at any site, including the axial and peripheral skeleton, who fulfilled the American College of Rheumatology (ACR) criteria (Altman 1986; Altman 1990) were eligible for inclusion. Primary OA is any OA for which a definite etiology (cause) is not found. Secondary OA is diagnosed when a specific cause for the disease can be identified, such as trauma or hypermobility; this type of OA was not included in this review.
Types of interventions
Studies evaluating benefits and/or harms of diacerein compared with:
placebo; and
other active treatments (non‐steroidal anti‐inflammatory drugs or other slow‐acting arthritis drugs).
Types of outcome measures
Seven important outcomes were selected for reporting.
For benefit, the outcomes were (1) pain, (2) physical function, (3) radiographic joint structure changes and (4) quality of life.
For safety, the outcomes were (5) number of participants experiencing any adverse event, (6) number of participants who withdrew because of adverse events and (7) number of participants experiencing any serious adverse event.
Benefits
1. Pain.
The measure of effectiveness was pain relief. To assess this outcome in accordance with the latest review of the OMERACT (international initiative to improve outcome measurement in rheumatology)‐3 (Bellamy 1997), the OMERACT‐6 (Pham 2003) recommends the use of standardised, validated instruments such as visual analogue scales (VASs) (Carlsson 1983), the Lequesne Functional Severity Index (Lequesne 1987) or the pain scales included in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (Bellamy 1988).
If data on more than one pain scale were provided for a trial, data were extracted on the pain scale that is highest in the following list, according to a previously described hierarchy of pain related outcomes.
Pain overall.
Pain on walking.
WOMAC pain subscale.
Pain on activities other than walking.
WOMAC global scale.
Lequesne Osteoarthritis Index global score.
Other algofunctional scale.
Patient’s global assessment.
Physician’s global assessment.
Other outcome.
No continuous outcome reported.
2. Physical function.
If data on more than one physical function scale were provided for a trial, data were extracted according to the hierarchy presented in the following list.
Global disability score.
Walking disability.
WOMAC disability subscore.
Composite disability scores other than WOMAC.
Disability other than walking.
WOMAC global scale.
Lequesne Osteoarthritis Index global score.
Other algofunctional scale.
3. Radiographic joint structure changes.
Radiographic progression of OA in studies lasting longer than one year include the following.
Minimum joint space width.
Median joint space width.
Semi‐quantitative measurement.
4. Quality of life.
Quality of life data were extracted from the following instruments.
Short Form (SF)‐12.
Short Form (SF)‐36.
Safety
The toxicity of diacerein was also considered a relevant outcome and was measured by the following.
5. Number of participants experiencing any adverse event.
6. Number of participants who withdrew because of adverse events.
7. Number of participants experiencing any serious adverse event.
Search methods for identification of studies
For identification of relevant studies, detailed search strategies were developed for each specific database to be searched. These strategies were based on the search strategy developed for MEDLINE (OVID) (Appendix 1) and revised appropriately for each database. The following databases were searched: Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 3, 2013) (Appendix 2), MEDLINE (1966 to March 2013), EMBASE (1980 to March 2013) (Appendix 3), ACP Journal Club (1991 to 2013) (Appendix 2), LILACS (1982 to March 2013) (Appendix 4) and International Clinical Trials Register (World Health Organization, March 2013) (Appendix 5).
The reference lists of all identified citations were manually searched. In addition, letters were sent to study authors and to content experts to ask for assistance in retrieving additional RCTs, especially those that were unpublished. The manufacturers of diacerein (Negma‐Lerads and TRB‐Pharma) were contacted for additional trials. For this version of the Cochrane Review, the search was updated to March 2013.
For safety assessments, we searched the websites of the regulatory agencies (US Food and Drug Administration‐MedWatch (http://www.fda.gov/Safety/MedWatch/default.htm) , European Medicines Evaluation Agency (http://www.ema.europa.eu), Australian Adverse Drug Reactions Bulletin (http://www.tga.gov.au/safety/ews‐monitoring.htm), and UK Medicines and Healthcare products Regulatory Agency (MHRA) pharmacovigilance and drug safety updates (http://www.mhra.gov.uk/Safetyinformation/index.htm) using the keyword ‘diacerein’ on 26 November 2013.
No language or date of publication restrictions were applied.
Data collection and analysis
Selection of studies
Two review authors (TSAF and CRM) independently reviewed the references identified through the search strategy and selected those that fulfilled the selection criteria. Differences regarding selection were solved by a third review author (VFMT).
Data extraction and management
Two review authors (TSFA and CRM) independently extracted data from eligible studies. Review authors were not masked to report authors, journals, dates of publication, sources of financial support or results. Any disagreements were resolved through discussion with or by seeking the opinion of a third review author (VFMT). Data extracted included study characteristics and outcome data. For studies with more than one publication, the main trial report was used as the reference, and additional details were derived from secondary papers.
Assessment of risk of bias in included studies
This updated version of the review assessed independently and in duplicate the risk of bias in included studies using the risk of bias tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of interventions, version 5.1.0 (Higgins 2011). The domains investigated included the following.
Sequence generation.
Allocation concealment.
Blinding of participants.
Blinding of outcome assessment.
Incomplete outcome data addressed.
Selective outcome reporting.
Each domain was classified as having 'low risk of bias', 'high risk of bias' or 'unclear risk of bias'.
Measures of treatment effect
For dichotomous outcomes, results were expressed as a risk ratio (RR), that is, the proportion of events in the treatment group in relation to the proportion of events in the control group, with 95% confidence intervals (95% CIs). When overall results were significant, the number needed to treat for an additional beneficial outcome (NNTB) was calculated. The NNTB is the number of participants who need to be treated with the intervention to prevent one event. The NNTB was also calculated for radiographic progression. Continuous outcomes were analysed according to standardised mean differences (SMDs), using an inverse variance with random approach.
Unit of analysis issues
The unit of analysis was the participant. For studies containing more than two intervention groups, to make multiple pair‐wise comparisons between all possible pairs of intervention groups, we included each group of participants only once in the meta‐analysis, in accordance with the procedure recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
As far as possible, data were analysed on an intention‐to‐treat (ITT) basis, and attempts were made to obtain missing data from the original trial lists. When some data were unavailable, only the available data were analysed.
For dichotomous outcomes that measured adverse events (e.g. number of withdrawals due to adverse events), the withdrawal rate was calculated using the number of participants who received treatment as the denominator (worst‐case analysis). For dichotomous outcomes that measured benefits, the worst‐case analysis was calculated using the number of randomly assigned participants as the denominator. For continuous outcomes (e.g. pain), we calculated mean difference (MD) or the SMD using the number of participants analysed at the time point. If the number of participants analysed was not presented for each time point, the number of randomly assigned participants in each group at baseline was used.
When possible, missing standard deviations were computed from other statistics such as standard errors. CIs or P values were calculated according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If it was not possible to calculate standard deviations, we imputed them, for example, from other studies in the meta‐analysis (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was measured by Tau2 and I2 and by calculating a Chi2 test with P < 0.10 considered significant. The I2 cutoff point for considering substantive heterogeneity is 50% (Higgins 2003).
Assessment of reporting biases
In future updates, if sufficient numbers of studies (more than 10) are eligible for inclusion, a funnel plot (Egger 1997) will be used to assess publication bias.
Data synthesis
For clinically homogeneous studies, we pooled outcomes in a meta‐analysis using the random‐effects model as a default.
Subgroup analysis and investigation of heterogeneity
When possible, subgroup analysis was done as follows: different doses of diacerein, hip OA versus knee OA versus spine OA versus hand OA and different functional classes of OA. Studies analysing knee and hip OA were separated to assess whether results changed in the meta‐analysis graphics (subgroup analyses). In this update, subgroup analyses for spine versus hand OA and for different functional classes of OA were not performed because RCTs included in this review did not provide these data.
Sensitivity analysis
Sensitivity analysis was carried out to explore heterogeneity. Studies of longer than six months' duration were pooled together to explore effect size differences and robustness of the results. Sensitivity analysis according to length of follow‐up was not included in the original protocol because it was decided post hoc that this would be performed.
In future updates, if sufficient studies are eligible, other sensitivity analyses could be carried out. Heterogeneity in the results of the meta‐analysis should be assessed both by inspecting graphical presentations (funnel plot) (Egger 1997) if more than 10 studies are included and by calculating a Chi2 test with P values < 0.1 considered as significant. In future updates of this review, differences in populations, interventions and assessments of outcomes could be explored in analyses of heterogeneity.
Grading of Evidence
The Cochrane Collaboration has adopted the principles of the GRADE approach for evaluating the quality of evidence for outcomes reported in systematic reviews (Grade 2008). The GRADE approach specifies four levels of quality. The highest quality rating is for randomised trial evidence. However, review authors can downgrade randomised trial evidence to moderate, low or even very low quality evidence, depending on the presence of the five factors. Usually, quality ratings will fall by one level for each factor, up to a maximum of three levels for all factors. If very severe problems are noted for any one factor (e.g. when assessing limitations in design and implementation, all studies were unconcealed, were unblinded and lost more than 50% of their participants to follow‐up), randomised trial evidence may fall by two levels because of that factor alone.
These five factors that constitute the GRADE approach include the following.
Limitation in the design or implementation of available studies, suggesting high level of bias.
Indirecteness of evidence (indirect population, intervention, control and outcomes).
Unexplained heterogeneity or inconsistency of results (including problems with subgroup analysis).
Imprecision of results (wide confidence intervals).
High probability of publication bias.
Summary of findings tables
The summary of findings tables present the main findings in this updated review. They provide key information concerning quality of the evidence, magnitude of effect of the interventions examined and the sum of available data on the main outcomes. Seven important outcomes were included in the summary of findings tables.
Pain.
Physical function.
Radiographic joint structure changes.
Quality of life.
Number of participants experiencing any adverse event.
Number of participants who withdrew because of adverse events.
Number of participants experiencing any serious adverse event.
Results
Description of studies
Results of the search
The search retrieved 324 (25 Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 10, 2013), 184 MEDLINE, 90 EMBASE, 24 LILACS, 1 ACP Journal) references, and nine additional reports were obtained through other sources (eight reference lists and one abstract from a conference meeting), which after de‐duplication resulted in 272 citations. After the titles and abstracts of these references were screened, 40 full‐text articles were selected; 30 of these studies were excluded, and 10 fulfilled the selection criteria. Three new studies were included in this updated version of the 2006 systematic review. See the study flow diagram for further details (Figure 1).
1.
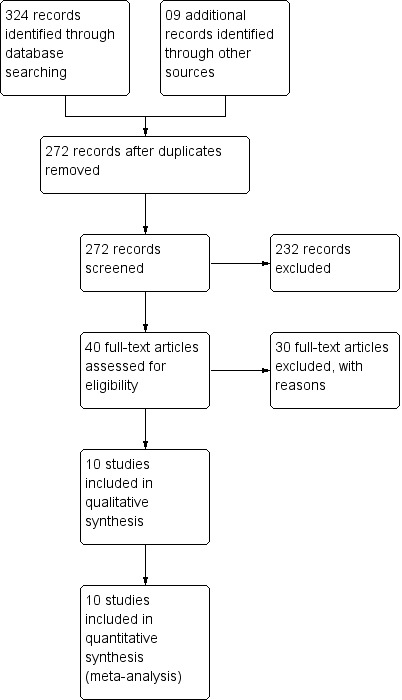
Study flow diagram.
Included studies
The 10 identified studies are listed in the Characteristics of included studies table. Years of publication ranged from 1994 to 2009. All were double‐blinded, randomised, parallel‐group trials and included a total of 2,210 adults with a mean age of 59.69 (± 8.90) years. A total of 996 participants were randomly assigned to treatment with diacerein, and 1,214 were randomly assigned to one of the comparator groups (NSAIDs or placebo or other symptom‐modifying, slow‐acting drugs for OA). These studies were performed in France (Chantre 2000; Dougados 2001; Lequesne 1998; Nguyen 1994; Pham 2004), the UK (Pham 2004), Canada (Pelletier 2000), Israel (Pelletier 2000), China (Zheng 2006), the Czech Republic (Pavelka 2007), Thailand (Louthrenoo 2007) and India (Brahmachari 2009). Seven of the ten studies compared diacerein with placebo (Brahmachari 2009; Dougados 2001; Lequesne 1998; Nguyen 1994; Pavelka 2007; Pelletier 2000; Pham 2004 ), two compared diacerein with other symptom‐modifying, slow‐acting drugs for OA—Harpadol and the hyaluronic acid compound NRD101 (Chantre 2000; Pham 2004) and three compared diacerein with NSAIDs: tenoxicam (Nguyen 1994), diclofenac (Zheng 2006) and piroxicam (Louthrenoo 2007).
Only knee or hip OA was evaluated in the 10 included studies. None of the studies evaluated OA in other segments such as hands or spine. In two studies, only the hip joint was evaluated (Dougados 2001; Nguyen 1994). The knee was evaluated in six RCTs (Brahmachari 2009; Louthrenoo 2007; Pavelka 2007; Pelletier 2000; Pham 2004; Zheng 2006), and two RCTs (Chantre 2000; Lequesne 1998) assessed both knee and hip joints.
Participants with primary OA were evaluated in all studies, and radiographs of the target joint were obtained in nine of the ten studies. In all included studies, the diagnosis of OA was based on valid clinical and radiographic findings in accordance with the ACR criteria; one study also included the Lequesne criteria (Lequesne 1998). The Kellgren and Lawrence radiographic gradation of OA was used to evaluate the radiographic diagnosis of OA (Kellgren 1957).
Duration of the studies ranged from two months to three years. Six studies (Brahmachari 2009; Chantre 2000; Dougados 2001; Louthrenoo 2007; Nguyen 1994; Pelletier 2000) mentioned the duration of disease, and the mean was 4.69 years. The number of participants randomly assigned ranged from 64 (Brahmachari 2009) to 521 (Dougados 2001), and the number of dropouts in the diacerein groups ranged from three of 86 (Louthrenoo 2007) to 65 of 262 (Dougados 2001).
Five trials analysed treatment carry‐over effect (Brahmachari 2009; Lequesne 1998; Louthrenoo 2007; Pavelka 2007; Zheng 2006) for up to two months after cessation of the intervention. The carry‐over effect refers to the remaining effect of the drug after its discontinuation.
Good overall agreement was reached between two investigators (TSAF and CRM) regarding data extracted from the 10 RCTs. Consensus was reached for all discrepancies.
Excluded studies
A total of 30 studies were excluded for the following reasons: inadequate study design for this review (Adami 1985; Bogliola 1991; Carrabba 1987; Delcambre 1994; Fagnani 1998; Kay 1980; Linguetti 1982; Mantia 1987; Marcolongo 1988; Mathieu 1999; Mazzaro 1989; Renapurkar 2010; Sharma 2008), duplicate publication (Delcambre 1996; Leblan 2000; Tang 2004; Valat 1997), incomplete data and unsuccessful personal contact with authors (Ascherl 1994; Fioravanti 1985; Mattara 1985; Mordini 1986; Pietrogrande 1985; Portioli 1987; Schulitz 1994; Seisenbayev 2012) and inappropriate inclusion criteria (Baliga 2010; Singh 2012). The studies Vignon 2002, Villani 1998 and Villermay 1994 are not clinical trials. Reasons for exclusion are listed in the Characteristics of excluded studies table.
One study (Shin 2013) is awaiting classification depending on the response of the study authors regarding information necessary to the process of inclusion.
Risk of bias in included studies
Pre randomisation inclusion and exclusion criteria were provided by all 10 RCTs. Study authors and pharmaceutical companies were contacted to provide data.
The most frequent risk of bias was incomplete outcome data, identified in approximately 80% of the studies, followed by lack of blinding of clinical outcome assessment in about 20% and selective reporting in 10% of the studies. Almost all (90%) studies did not provide details on allocation concealment (unclear).
Approximately 40% of the included studies were unclear about random sequence generation. See the risk of bias graph and the risk of bias summary for additional details (Figure 2; Figure 3).
2.
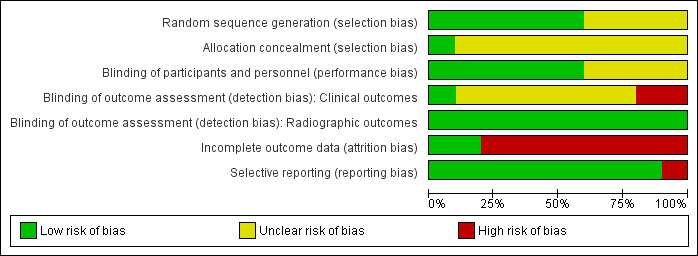
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
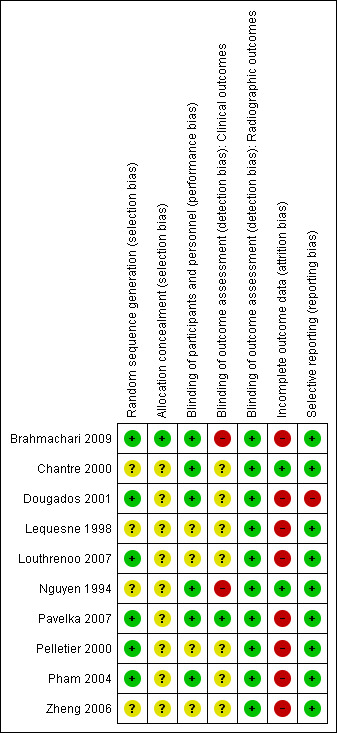
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation sequences were adequately described with low risk of bias in six studies (Brahmachari 2009; Dougados 2001; Louthrenoo 2007; Pavelka 2007; Pelletier 2000; Pham 2004). Three studies used computer‐generated number lists as their randomisation method (Brahmachari 2009; Louthrenoo 2007; Pavelka 2007), two studies used blocked randomisation (Dougados 2001; Pelletier 2000) and one study used central randomisation (Pham 2004).
Four studies (Chantre 2000; Lequesne 1998; Nguyen 1994; Zheng 2006) were classified as having unclear risk of bias for this domain.
Allocation concealment was not described (unclear risk of bias) in any of the studies, except one. The author of Brahmachari 2009 replied to our contact and informed that he had used sealed opaque envelopes.
Blinding
Six studies (Brahmachari 2009; Chantre 2000; Dougados 2001; Nguyen 1994; Pavelka 2007; Pham 2004) were classified as having low risk of bias for blinding of participants and personnel. Four of these studies (Dougados 2001; Chantre 2000; Pavelka 2007; Pham 2004) described adequate double‐blinding processes. Brahmachari 2009 blinded only participants.
Four studies provided no information on blinding of participants and personnel and therefore were categorised as having unclear risk of bias (Lequesne 1998; Louthrenoo 2007; Pelletier 2000; Zheng 2006). No studies were categorised as having high risk of bias for performance bias.
Seven studies provided no information on blinding of clinical outcome assessors and therefore were categorised as having unclear risk of bias (Chantre 2000; Dougados 2001; Lequesne 1998; Louthrenoo 2007; Pelletier 2000; Pham 2004; Zheng 2006). Two studies were categorised as having high risk of bias for detection bias (Brahmachari 2009; Nguyen 1994).
Two studies (Dougados 2001; Pham 2004) were classified as having low risk of bias for blinding of radiographic outcome assessment because they described adequate blinding processes for radiographic outcomes: The radiologists were unaware of the identity of the participants when they read their X‐rays to evaluate structural outcomes. The other eight studies, which did not evaluate radiographic outcomes, were classified as having low risk of bias.
Incomplete outcome data
Two studies (Chantre 2000; Nguyen 1994) were classified as having low risk of bias on this item because all randomly assigned participants were included in the ITT analyses.
Seven studies were classified as having high risk of bias (Dougados 2001; Lequesne 1998; Louthrenoo 2007; Pavelka 2007; Pelletier 2000; Pham 2004; Zheng 2006). In three of these studies (Brahmachari 2009; Dougados 2001; Pelletier 2000), ITT analysis was used for all participants who took at least one dose of the medication, and the last observation carried forward (LOCF) method was used for those with missing values.
Pham 2004 evaluated efficacy outcomes using ITT analysis (all randomly assigned participants); however, radiographic evaluation was done only for participants who had at least two different X‐rays to compare.
Two studies (Louthrenoo 2007; Pavelka 2007) reported different numbers of participants in the baseline and outcomes tables. These studies provided no information on the reasons for exclusion of these participants at the end of the study.
Lequesne 1998 evaluated pain using a 0 to 100‐mm VAS for all randomly assigned participants. The number of participants for the second effectiveness outcome in this study (Lequesne Impairment Index) was different, and the authors provided no explanation for this discrepancy.
Selective reporting
All proposed outcomes were evaluated in nine studies (Brahmachari 2009; Chantre 2000; Lequesne 1998; Louthrenoo 2007; Nguyen 1994; Pavelka 2007; Pelletier 2000; Pham 2004; Zheng 2006). One study (Dougados 2001) was classified as having high risk of bias on this domain because it did not provide data for the outcome "patient evaluation of the treatment" using a 0 to 5 Likert scale .
Effects of interventions
See: Table 1
Comparison 1: diacerein compared with placebo
Benefits
1. Pain.
Visual analog scale for pain (0 to 100 mm); 1,283 participants from six studies:Brahmachari 2009; Dougados 2001; Lequesne 1998; Nguyen 1994; Pelletier 2000; Pham 2004 (Analysis 1.1).
1.1. Analysis.
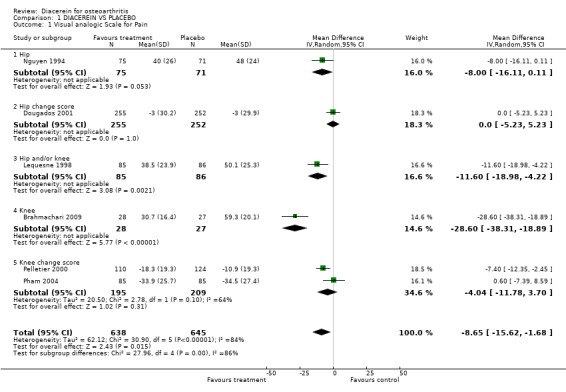
Comparison 1 DIACEREIN VS PLACEBO, Outcome 1 Visual analogic Scale for Pain.
The pooled summary MD (random effect) of these six studies was ‐8.65 (95% CI ‐15.62 to ‐1.68), with high heterogeneity (I2 = 84%). A negative MD in this case means that diacerein was superior to placebo in reducing pain. The absolute risk difference was ‐9% (95% CI ‐16% to ‐2%), and the relative percentage change was ‐19% (95% CI ‐34% to ‐4%).
The effect of diacerein was similar to that of placebo in the two studies that followed participants for longer than six months, according to the post hoc sensitivity analysis. The pooled MD of these two studies was 0.48 (95% CI ‐3.90 to 4.86) with no heterogeneity (I2 = 0%). See Analysis 4.1.
4.1. Analysis.
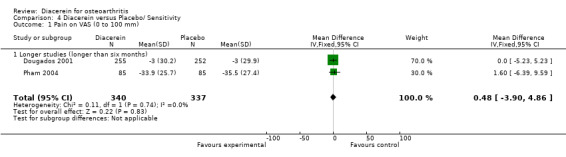
Comparison 4 Diacerein versus Placebo/ Sensitivity, Outcome 1 Pain on VAS (0 to 100 mm).
2. Physical function.
Lequesne Impairment Index (0 to 24 points); 1,006 participants from four studies:Dougados 2001; Lequesne 1998; Nguyen 1994; Pham 2004 (Analysis 1.2).
1.2. Analysis.
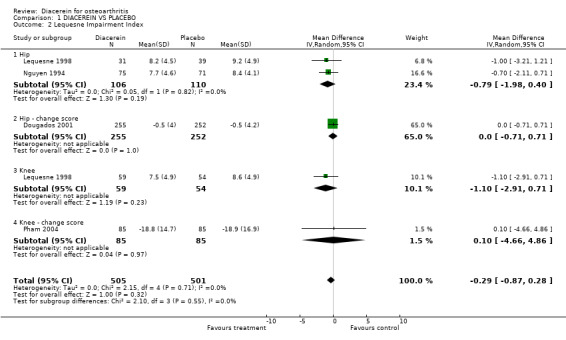
Comparison 1 DIACEREIN VS PLACEBO, Outcome 2 Lequesne Impairment Index.
The pooled summary MD (random effect) of these four studies was ‐0.29 (95% CI ‐0.87 to 0.28). A negative MD in this case means that diacerein was superior to placebo in terms of its ability to improve Lequesne Index scores, but this effect did not reach statistical significance. The absolute risk difference was 0% (95% CI ‐4% to 1%), and the relative percentage change was ‐4% (95% CI ‐11% to 4%).
3. Radiographic joint structure changes.
Minimum joint space width decreased over 0.50 mm during the study period; 616 participants from two studies:Dougados 2001; Pham 2004 (Analysis 1.7).
1.7. Analysis.
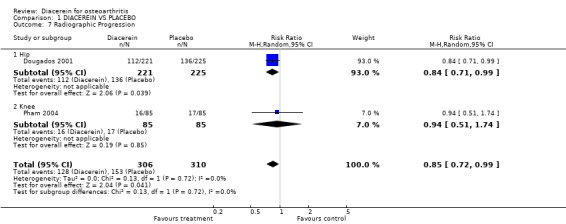
Comparison 1 DIACEREIN VS PLACEBO, Outcome 7 Radiographic Progression.
When diacerein was compared with placebo for changes in minimum joint space width for the knee or hip, the summary RR (random effect) was 0.85 (95% CI 0.72 to 0.99). Diacerein slowed the radiological progression for hip OA (the most representative study was Dougados 2001, with RR 0.84, 95% CI 0.71 to 0.99) but not for knee OA (RR 0.94, 95% CI 0.51 to 1.74). The number needed to treat for an additional beneficial outcome (NNTB) was 14 (95% CI 8 to 203). The absolute risk difference was ‐6% (95% CI ‐15% to 2%), and relative percentage change was ‐15% (95% CI ‐28% to ‐1%).
4. Quality of life.
This outcome was not reported by the studies included in this comparison.
Safety
5. Number of participants experiencing any adverse event.
The pooled RR (random effect) for diarrhoea was 3.52 (95% CI 2.42 to 5.11) in six studies (726 participants taking diacerein and 736 taking placebo). The RR for dyspepsia was 0.98 (95% CI 0.61 to 1.58) when 526 participants in the diacerein group were compared with 533 participants in the placebo group (four studies). See Analysis 1.8. The number needed to treat for an additional harmful outcome (NNTH) for diarrhoea was 4 (95% CI 3 to 7). The absolute risk difference was 24% (95% CI 12% to 35%), and the relative percentage change was 252% (95% CI 142% to 411%).
1.8. Analysis.
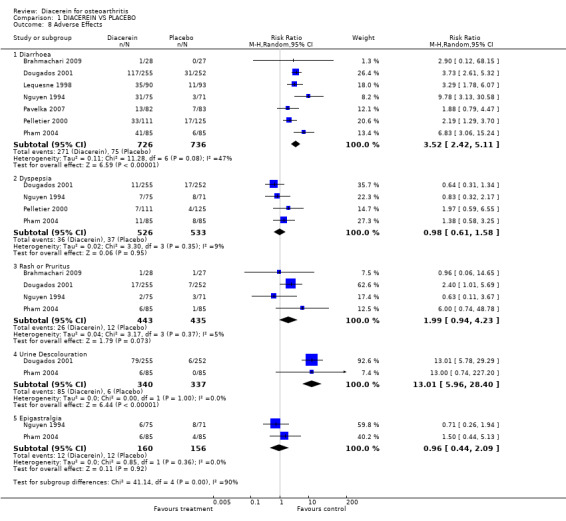
Comparison 1 DIACEREIN VS PLACEBO, Outcome 8 Adverse Effects.
6. Number of participants who withdrew because of adverse events.
The pooled RR (random effect) for withdrawals due to adverse effects was 1.29 (95% CI 0.83 to 2.01) in 733 participants taking diacerein versus 743 participants using placebo (seven studies). See Analysis 1.9. The NNTH was not calculated, as the result was not statistically significant. The absolute risk difference was 0% (95% CI ‐3% to 4%), and the relative percentage change was 0% (95% CI ‐29% to 41%).
1.9. Analysis.
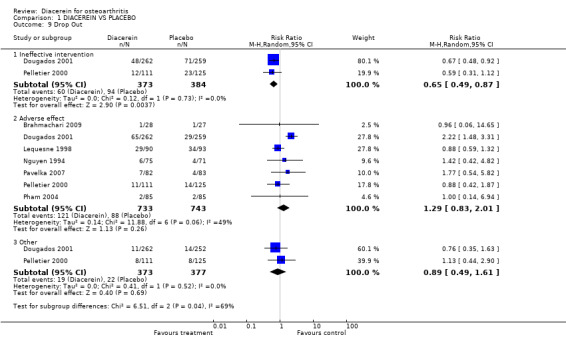
Comparison 1 DIACEREIN VS PLACEBO, Outcome 9 Drop Out.
7. Number of participants experiencing any serious adverse event.
This outcome was not described in the studies included in this comparison.
Comparison 2: diacerein compared with non‐steroidal anti‐inflammatory drugs (NSAIDs)
Benefits
Three RCTs (Louthrenoo 2007; Nguyen 1994; Zheng 2006) with 150, 184 and 161 participants, respectively, compared diacerein with NSAIDs. The Nguyen study evaluated hip OA, and the other two studies evaluated knee OA.
1. Pain.
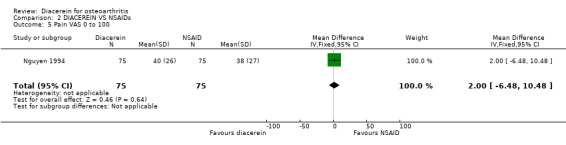
Visual analog scale for pain (0 to 100 mm); 150 participants from one study:Nguyen 1994.
No statistically significant differences were noted between the two interventions. Only one study (Nguyen 1994) evaluated pain reduction according to a 0 to 100‐mm VAS scale and reported no differences between the two interventions. The summary MD was 2.00 (95% CI ‐6.48 to 10.48).
WOMAC pain subscale; 184 participants from one study:Louthrenoo 2007.
One study evaluated this outcome over 16 weeks of treatment by comparing diacerein versus piroxicam; no statistically significant difference was observed between the two interventions. The summary MD was 14.00 (95% CI ‐10.15 to 38.15).
Pain on walking 20 m; 231 participants from one study:Zheng 2006.
One study did not show statistically significant differences between diacerein and diclofenac. The summary MD was 1.30 (95% CI ‐3.81 to 6.41).
2. Physical function.
WOMAC disability subscore; 345 participants from two studies:Louthrenoo 2007; Zheng 2006 (Analysis 2.1).
2.1. Analysis.
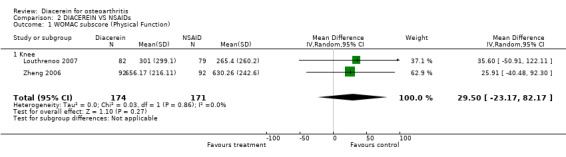
Comparison 2 DIACEREIN VS NSAIDs, Outcome 1 WOMAC subscore (Physical Function).
The summary MD was 29.50 (95% CI ‐23.17 to 82.17).
3. Radiographic joint structure changes.
No study assessed this outcome.
4. Quality of life.
SF‐36 (sum score 0‐800): 374 participants in two studies:Louthrenoo 2007; Zheng 2006.
Only one study provided data on SF‐36 results allowing analysis Louthrenoo 2007. Afer 16 weeks of active treatment, there were no statistically significant differences between the groups. At the end of the treatment, both groups had similar variations in the scores for each dimension of the SF‐36 health survey questionnaire. MD was ‐40.70 (95% confidence interval; ‐85.20 to 3.80) (Analysis 2.2).
2.2. Analysis.
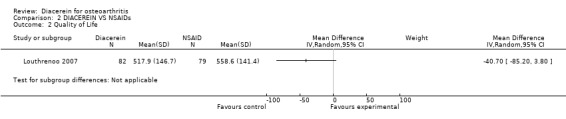
Comparison 2 DIACEREIN VS NSAIDs, Outcome 2 Quality of Life.
Zheng 2006 informed that there were no statistically significant differences between both intervention groups without reporting specific numerical data.
Safety
5. Number of participants experiencing any adverse event.
The pooled RR (random effect) for diarrhoea was 3.20 (95% CI 1.58 to 6.49) with 77 of 253 participants in the diacerein group versus 23 of 252 participants in the NSAIDs group (three studies). The RR for dyspepsia was 0.69 (95% CI 0.29 to 1.61) in three studies (Analysis 2.3).
2.3. Analysis.
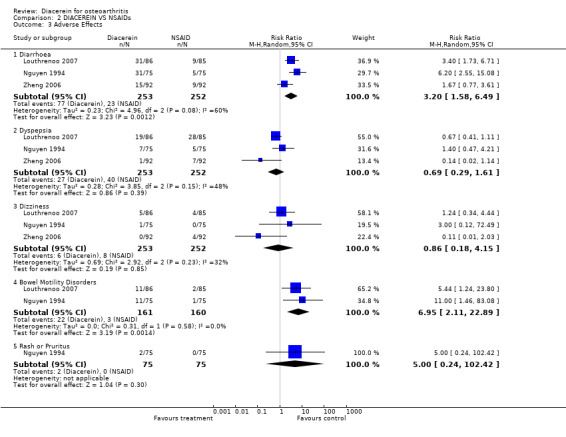
Comparison 2 DIACEREIN VS NSAIDs, Outcome 3 Adverse Effects.
6. Participants who withdrew because of adverse events.
The pooled RR (random effect) for withdrawals due to adverse events was 0.96 (95% CI 0.38 to 2.44) in three studies with 534 participants (Analysis 2.4).
2.4. Analysis.
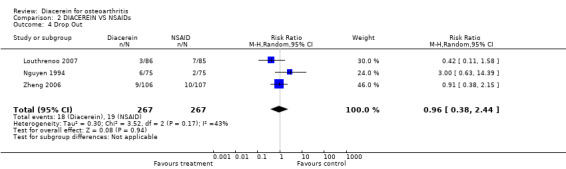
Comparison 2 DIACEREIN VS NSAIDs, Outcome 4 Drop Out.
7. Participants experiencing any serious adverse event.
None of the studies analysed this outcome.
Comparison 3: diacerein compared with other symptomatic slow‐acting drugs for osteoarthritis (SYSADOA)
Two RCTs (Chantre 2000; Pham 2004) consisting of 338 participants compared diacerein versus two SYSADOA drugs: intra‐articular NRD101 (a hyaluronic acid high‐molecular‐weight, ‐1.900 kDa polysaccharide) for 12 months versus Harpadol or devil's claw (a perennial South African herbaceous plant with anti‐inflammatory and analgesic effects attributed to its iridoid glycoside) for four months.
Benefits
1. Pain.
Visual analog scale for pain (0 to 100 mm); 338 participants from two studies:Chantre 2000; Pham 2004 (Analysis 3.1).
3.1. Analysis.
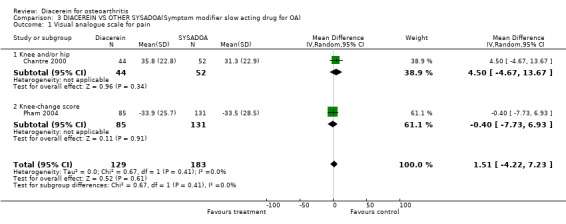
Comparison 3 DIACEREIN VS OTHER SYSADOA(Symptom modifier slow acting drug for OA), Outcome 1 Visual analogue scale for pain.
For pain assessed through a VAS scale, the comparison between diacerein and NRD101 resulted in a subgroup MD (random effect) of 4.50 (95% CI ‐4.67 to 0.13.67), and the comparison between diacerein and Harpadol resulted in a subgroup MD (random effect) of 0.40 (95% CI ‐7.73 to 6.93).
2. Physical function.
Lequesne Impairment Index (0 to 24 points); 338 participants from two studies:Chantre 2000; Pham 2004 (Analysis 3.2).
3.2. Analysis.
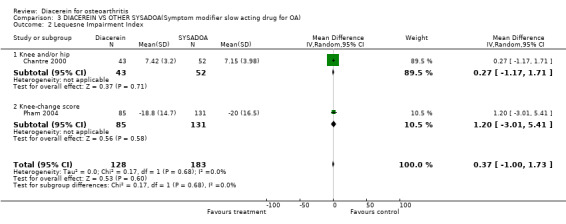
Comparison 3 DIACEREIN VS OTHER SYSADOA(Symptom modifier slow acting drug for OA), Outcome 2 Lequesne Impairment Index.
For the Lequesne Index, comparison between diacerein and NRD101 yielded a pooled MD (random effect) of 0.27 (95% CI ‐1.17 to 1.71), and for the comparison between diacerein and Harpadol, the pooled MD (random effect) was 1.20 (95% CI ‐3.01 to 5.41).
3. Radiographic joint structure changes.
MInimum joint space width decreased by more than 0.50 mm during the study period; 216 participants from one study:Pham 2004.
Radiographic progression was assessed in one study (Pham 2004), and no statistically significant difference was noted between diacerein and NRD101: RR (random effect) 1.07 (95% CI 0.60 to 1.91) after one year of observation.
4. Quality of life.
None of the studies assessed this outcome.
Safety
5. Number of participants experiencing any adverse event.
The most frequent adverse event was diarrhoea, with RR 4.26 (95% CI 2.54 to 7.16) (Analysis 3.4).
3.4. Analysis.
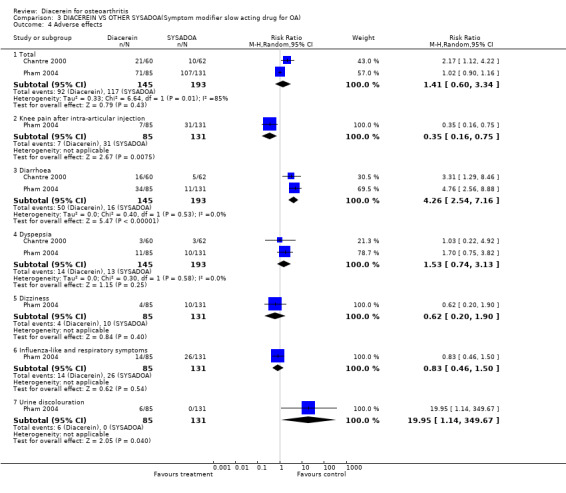
Comparison 3 DIACEREIN VS OTHER SYSADOA(Symptom modifier slow acting drug for OA), Outcome 4 Adverse effects.
6. Participants who withdrew because of adverse events.
The proportion of dropouts in the diacerein groups was similar to that in the SYSADOA group; RR was 1.42 (95% CI 0.78 to 2.58) (Analysis 3.4).
7. Participants experiencing any serious adverse event.
This outcome was not reported by the studies.
Subgroup analysis: carry‐over effect
Five studies (Brahmachari 2009; Lequesne 1998; Louthrenoo 2007; Pavelka 2007; Zheng 2006) analysed the carry‐over effect. The time for outcome measurement without the drug was four weeks (Brahmachari 2009), eight weeks (Lequesne 1998), four weeks (Louthrenoo 2007), 12 weeks (Pavelka 2007) and four weeks (Zheng 2006).
1. Pain.
Visual analog scale for pain (0 to 100 mm); 470 participants from three studies:Brahmachari 2009; Lequesne 1998; Zheng 2006 (Analysis 5.1).
5.1. Analysis.
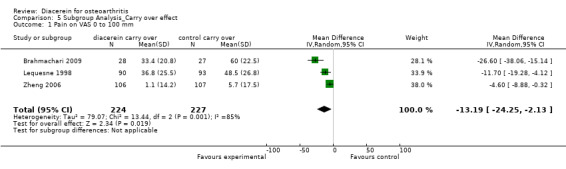
Comparison 5 Subgroup Analysis_Carry over effect, Outcome 1 Pain on VAS 0 to 100 mm.
The summary MD was ‐13.19 (95% CI ‐24.25 to ‐2.13).
WOMAC Index subscale pain; 339 participants from two studies:Louthrenoo 2007; Pavelka 2007 (Analysis 5.2).
5.2. Analysis.
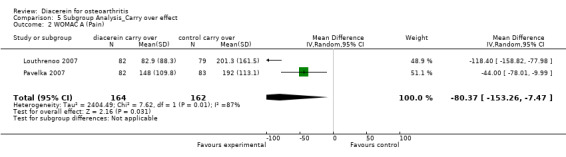
Comparison 5 Subgroup Analysis_Carry over effect, Outcome 2 WOMAC A (Pain).
The summary MD was ‐80.37 (95% CI ‐153.26 to ‐7.47).
2. Physical function.
WOMAC Index subscale physical function; 381 participants from three studies:Brahmachari 2009; Louthrenoo 2007; Pavelka 2007 (Analysis 5.4).
5.4. Analysis.
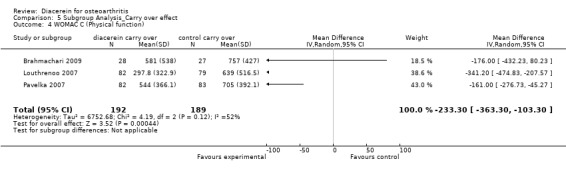
Comparison 5 Subgroup Analysis_Carry over effect, Outcome 4 WOMAC C (Physical function).
The summary MD was ‐233.30 (95% CI ‐363.30 to ‐103.30).
Only one study (Pelletier 2000) studied different doses of diacerein, and the only reported difference between groups was related to adverse events. Participants who received 50 mg/d had significantly fewer adverse effects than the group treated with 100 mg; participants treated with 150 mg/d had the highest overall rate of withdrawals (20% in the 150‐mg diacerein group vs 10 % in the placebo group). This is why we did not perform this subgroup analysis.
Results from search of regulatory websites:
The FDA MedWatch, Australian Adverse Drug Reactions Bulletin, and UK Medicines and Healthcare products Regulatory Agency, did not have any warnings regarding diacerein. However, a notice on the European Medicines Agency (EMA) website, dated November 8, 2013, from the Pharmacovigilance Risk Assessment Committee (PRAC) recommended that the marketing authorization of diacerein should be suspended across Europe because of harms (particularly the risk of severe diarrhoea and potentially harmful effects on the liver) outweighing benefits (PRAC 2013). This guidance is not final and the EMA website should be consulted for future guidance on this issue.
Liver adverse effects was not an outcome pre‐specified for this review. However, after becoming aware of the PRAC recommendation, we re‐assessed the included studies for this outcome. We did not find evidence of liver adverse effects in the studies included in this review. Blood samples were collected to evaluate liver function in all studies, except two (Chantre 2000; Lequesne 1998). Only one patient discontinued the diacerein treatment due to deterioration in hepatic function (ALT up to 97 U/L) in the Zheng study (Zheng 2006).
Discussion
Summary of main results
This updated systematic review identified 10 randomised controlled trials. We found that the symptomatic benefit of diacerein in participants with OA of the knee or hip was minimal or none when compared with placebo. Minimal benefit was noted in terms of joint space narrowing for hip OA, and was uncertain for knee OA. Adverse effects related to the gastrointestinal tract (diarrhoea) were frequent, and safety concerns could make use of this drug non‐beneficial.
This review included trials published between 1994 and 2009 that allocated and analysed 2,210 participants with knee or hip OA. The average age of participants was 60 years, and 63% of them were women. These participants were treated with diacerein (996 participants) compared with placebo or other active interventions (1,214 participants).
Six studies were pooled for analysis of pain reduction assessed through a 0 to 100‐mm VAS. When diacerein and placebo were compared, the MD was ‐8.65 (95% CI ‐15.62 to ‐1.68, P < 0.01), supporting mild efficacy of diacerein as opposed to placebo. This result is based on studies with large heterogeneity (I2 = 84%). No significant reduction in heterogeneity was observed when the previously described sensitivity analysis was performed. Diacerein was statistically non‐significant in reducing the Lequesne Index score according to five comparisons: MD was ‐0.29 (95% CI ‐0.87 to 0.28) without heterogeneity (I2 = 0%).
Radiographic progression of disease was less pronounced for hip OA (RR 0.84, 95% CI 0.71 to 0.99) than for knee OA (RR 0.94, 95% CI 0.51 to 1.74).
Diacerein compared with NSAIDs was statistically non‐significant for physical function measured by the WOMAC Index, with MD 29.50 (95% CI ‐23.17 to 82.17).
According to studies that analysed the carry‐over effect (Brahmachari 2009; Lequesne 1998; Louthrenoo 2007; Pavelka 2007; Zheng 2006), diacerein remains effective for at least two months after treatment interruption. Reasons for this were not explained. Pooled results of three of these studies (Brahmachari 2009; Lequesne 1998; Zheng 2006) for pain reduction produced MD of ‐13.19 (95% CI ‐24.25 to ‐2.13) on a 0 to 100‐mm VAS.
A statistically significant increase in the risk of adverse effects was noted for participants allocated to diacerein compared with those given placebo, mainly diarrhoea. The NNTH for diarrhoea was 4, but this did not lead to a statistically significant increase in withdrawals due to adverse events (RR 1.29, 95% CI 0.83 to 2.01).
No difference between diacerein and placebo was reported in terms of upper gastrointestinal symptoms. The second most prevalent adverse effect was urine discolouration (25% in the diacerein group vs 1.7% in the placebo group)—a clinically irrelevant effect. It should be noted that this effect is unrelated to renal function. Allergic events affecting the skin (pruritus, rash) were more frequent in the diacerein groups (Analysis 1.8).
In November 2013, the European Medicines Agency Pharmacovigilance Risk Assessment Committee (PRAC) recommended that the marketing authorization of diacerein should be suspended across Europe because of harms (particularly the risk of severe diarrhoea and potentially harmful effects on the liver) outweighing benefits (PRAC 2013). However, the website states that this recommendation will be re‐examined.
Overall completeness and applicability of evidence
This review has several limitations. First of all, most of the included studies were small and were too short in duration, given that the therapeutic effects of diacerein start after approximately six to eight weeks of use. Only three of the 10 studies lasted longer than 24 weeks. Second, all studies allowed participants to take analgesics and even NSAIDs during the trial, and this could have influenced the results related to pain and evaluation of adverse effects. Finally, in daily clinical practice, OA can affect other joints besides the knees and hips, but these other joints were not evaluated in the included studies.
Quality of the evidence
All studies included in this review had some type of risk of bias: selection bias or detection bias or attrition bias. Five randomised trials had lower risk of bias in most of the categories (Brahmachari 2009; Chantre 2000; Nguyen 1994; Pavelka 2007; Pham 2004), but their primary outcomes were different. Brahmachari 2009 evaluated the primary efficacy and safety of diacerein compared with placebo over a short time; Chantre 2000 evaluated the non‐inferiority of Harpagophytum procumbens compared with diacerein for pain and functional disability improvement; Pham 2004 evaluated the efficacy and safety of a hyaluronic acid intra‐articular compared with placebo and diacerein in a long‐term study; Nguyen 1994 evaluated the efficacy and safety of diacerein compared with non‐steroidal anti‐inflammatory drugs; and Pavelka 2007 analysed the carry‐over effect after three months of therapy. This diversity of bias can reduce the meaning of the results.
Only one (Pavelka 2007) of the 10 studies blinded assessors for clinical outcomes; all studies were classified as having low risk for radiographic outcomes. Radiological progression of OA is evaluated in long‐term studies, and this review retrieved two studies that included this analysis. Radiographic joint space width, measured in millimetres, is currently considered the preferred technique to evaluate the structural progression of OA, which is required by regulatory agencies (Hellio 2009). Unfortunately, the studies evaluated different joints. Radiological progression of knee OA was evaluated after one year (Pham 2004) with no statistically significant differences noted, and hip OA was evaluated after three years, with a small difference favouring diacerein over placebo (Dougados 2001).
Compliance gives an indication of drug tolerability and acceptability by participants. Although all studies described in their Methods section that compliance was assessed by pill counting, investigators did not analyse this parameter, thereby hindering estimations of drug tolerability. Included studies do not provide information on how often or what doses of analgesics or NSAIDs were used by randomly assigned participants. This information would be important in assessing the overall effectiveness and safety of the treatment.
Only one of the 10 studies (Brahmachari 2009) had adequate allocation concealment, and six (Brahmachari 2009; Dougados 2001; Louthrenoo 2007; Pavelka 2007; Pelletier 2000; Pham 2004) used an adequate method to generate a random sequence. Eight of ten studies had high risk of bias for incomplete outcome data. Only one study(Dougados 2001) was selective in its reporting of results and data. Consequently, evidence was downgraded to moderate for physical function/Lequesne impairment and to low for all other outcomes, using the GRADE assessment of quality (Table 1).
Potential biases in the review process
Strengths of this review include the detailed electronic search strategy; all important databases were included in the search. At least two independent investigators were involved in all steps of the review, from screening of retrieved references, to reading, abstraction and quality assessment of included studies. An additional investigator was consulted to solve discrepancies until consensus was reached.
Weaknesses of this review include a low response rate when attempts were made to contact authors of the included studies. In addition, the pharmaceutical companies contacted (TRB Chemedica, Negma Lerads) did not reply to our questions regarding missing data.
Agreements and disagreements with other studies or reviews
Eight studies retrieved from the reference list of another review (Rintelen 2006) were excluded from our review. They were unpublished reports (Mantia 1987; Portioli 1987) or presentations from the Italian Society of Rheumatology (Mattara 1985; Mordini 1986; Pietrogrande 1985), except one study that did not show data consistent with other studies (Mattara 1985) and two others that were already excluded in the first version of this review (2006) because they compared diacerein associated with other effective drugs versus standard treatment (Fagnani 1998; Marcolongo 1988). Over the past three years, we have repeatedly contacted authors of those eight studies and representatives of the pharmaceutical industry to ask for additional details and unpublished data, but we have received no reply. The pharmaceutical company that sponsored several of these studies did not send us the complete data for analyses. Bartels et al (Bartels 2010) also did not include these studies in their meta‐analyses.
Results of this updated review coincide with those reported by Bartels (Bartels 2010), who analysed six of these studies and reported a small beneficial effect of diacerein in the treatment of OA. As in our review, those authors found a small reduction in pain and lower efficacy in the studies of longest duration.
As mentioned in our Results, the EMA PRAC recommended that the marketing authorization of diacerein should be suspended because of harms related to the risk of severe diarrhoea and potentially harmful effects on the liver (PRAC 2013). While the results of this review found evidence of an increased risk of diarrhea, a post‐hoc assessment of the included studies for liver adverse effects was not found in these RCTs.
Authors' conclusions
Implications for practice.
There is low quality evidence that diacerein provides a small symptomatic benefit in pain improvement that may not be clinically significant. Another small benefit (of low quality, as assessed by GRADE) in terms of joint space narrowing was noted in hip OA while the result for knee OA was not statistically significant. There was low quality of evidence indicating that diacerein can cause adverse effects such as diarrhoea, which was described as the most frequent adverse event by all studies that documented and analysed this outcome.
The European Medicines Agency Pharmacovigilance Risk Assessment Committee (PRAC) recommended the suspension of diacerein‐containing medicines across Europe because of harms outweighing benefits. However, this guidance is not final as the PRAC recommendation will be re‐examined.
Implications for research.
This review provides the most recent evidence on (1) the clinical effectiveness of diacerein for pain reduction and physical function improvement in patients with OA and (2) the effect of this drug on the radiographic progression of hip and knee OA.
This evidence, however, is based on studies with methodological shortcomings, qualifying the evidence as low and moderate. These findings show that additional trials are needed to further assess the effectiveness of this drug for pain reduction and physical function. These outcomes are better measured by WOMAC scales and/or the Lequesne Index, as well as by patient global evaluation (Dworkin 2011).
Structural variables usually assess the rate and extent of cartilage breakdown revealed by radiographic space width or cartilage volume, as measured by magnetic resonance imaging. Such outcome variables are accurate, have high intrinsic validity and are usually considered as the primary outcome to be assessed in studies of disease‐modifying OA drugs (Dougados 2004).
It is very important that researchers design studies of good methodological quality, lasting longer than six months and providing blinding of outcome assessors, including radiographic progression and symptomatic improvement. In addition to the VAS pain scale, the quality of life index and global assessment by participants and investigators should be included in future studies.
What's new
| Date | Event | Description |
|---|---|---|
| 14 January 2014 | New search has been performed | With the addition of three new trials including 141 participants, the updated review now reports findings of a total of 10 studies including 2,210 participants. New tools used in the review downgraded the quality of the evidence. |
| 14 January 2014 | New citation required and conclusions have changed | Two new review authors, Cristiane Rufino Macedo and Lara Maxwell, were included in this update. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 2 April 2008 | Amended | CMSG ID C028‐R |
| 1 April 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank the Cochrane Musculoskeletal Editorial Team, mainly Ms Elizabeth Ghogomu, for their thoughtful comments and suggestions of ways to minimise bias in the review.
Appendices
Appendix 1. MEDLINE search strategy
1. exp osteoarthritis/ 2. osteoarthr$.tw. 3. (degenerative adj2 arthritis).tw. 4. arthrosis.tw. 5. or/1‐4 6. Diacetylrhein/ 7. Diacerein.tw. 8. Diacerhein.tw. 9. Rhein.tw. 10. Diacetylrhein.tw. 11. Anthraquinone Derivative/ 12. Anthraquinone$.tw. 13. or/6‐12 14. 5 and 13
Appendix 2. CENTRAL search strategy
CDSR, ACP, DARE, HTA
1. exp osteoarthritis/
2. osteoarthr$.tw.
3. (degenerative adj2 arthritis).tw.
4. arthrosis.tw.
5. or/1‐4
6. Diacetylrhein.tw.
7. Diacerein$.tw.
8. Diacerhein.tw.
9. Rhein.tw.
10. Anthraquinone Derivative.tw.
11. exp Anthraquinones/
12. Anthraquinone$.tw.
13. or/6‐12
14. 5 and 13
Appendix 3. EMBASE search strategy
1. 'osteoarthritis'/exp
2. osteoarthr*:ab,ti
3. (degenerative NEAR/2 arthritis):ab,ti
4. arthrosis:ab,ti
5. #1 OR #2 OR #3 OR #4
6. 'diacerein'/exp
7. diacerein*:ab,ti
8. diacerhein:ab,ti
9. diacetylrhein:ab,ti
10. 'anthraquinone derivative'/exp
11.'anthraquinone derivative':ab,ti
12. anthraquinon*:ab,ti
13. rhein:ab,ti
14. #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13
15. #6 AND #14
Appendix 4. LILACS search strategy
((TW:Osteoarthritis) OR (TW:Osteoartrite) OR (TW: 'Arthritis, Degenerative') OR (MH:C05.550.114.606$) OR (MH:C05.799.613$) OR (TW:osteoarthr$) OR (TW: 'degenerative arthritis') OR (TW: 'artrite degenerativa') OR (TW:arthrosis) OR (TW:artrose)) AND ((TW:Diacetylrhein) OR (TW:Diacerein$.) OR (TW:Diacerhein) OR (TW:rhein) OR (TW: 'Anthraquinone Derivative') OR (TW:Anthraquinone$) OR (TW:Antraquinonas) OR (TW:Antraquinonas) OR (TW:Anthracenediones) OR (MH:D02.455.426.559.847.117.159$) OR (MH:D02.806.100$) OR (MH:D04.615.117.159$))
Appendix 5. International Clinical Trials Register search strategy
Keywords CONTAINS: (Osteoarthritis) OR (degenerative arthritis OR (arthrosis)) AND ((Diacetylrhein) OR (Diacerhein) OR (rhein) OR (Anthraquinone Derivative) OR (Anthraquinone))
Data and analyses
Comparison 1. DIACEREIN VS PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Visual analogic Scale for Pain | 6 | 1283 | Mean Difference (IV, Random, 95% CI) | ‐8.65 [‐15.62, ‐1.68] |
| 1.1 Hip | 1 | 146 | Mean Difference (IV, Random, 95% CI) | ‐8.0 [‐16.11, 0.11] |
| 1.2 Hip change score | 1 | 507 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐5.23, 5.23] |
| 1.3 Hip and/or knee | 1 | 171 | Mean Difference (IV, Random, 95% CI) | ‐11.60 [‐18.98, ‐4.22] |
| 1.4 Knee | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐28.60 [‐38.31, ‐18.89] |
| 1.5 Knee change score | 2 | 404 | Mean Difference (IV, Random, 95% CI) | ‐4.04 [‐11.78, 3.70] |
| 2 Lequesne Impairment Index | 4 | 1006 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.87, 0.28] |
| 2.1 Hip | 2 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.98, 0.40] |
| 2.2 Hip ‐ change score | 1 | 507 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.71, 0.71] |
| 2.3 Knee | 1 | 113 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.91, 0.71] |
| 2.4 Knee ‐ change score | 1 | 170 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐4.66, 4.86] |
| 3 WOMAC total | 2 | 399 | Mean Difference (IV, Random, 95% CI) | ‐58.69 [‐173.90, 56.52] |
| 3.1 Knee | 1 | 165 | Mean Difference (IV, Random, 95% CI) | ‐148.0 [‐306.34, 10.34] |
| 3.2 Knee change score | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐20.00 [‐33.38, ‐6.62] |
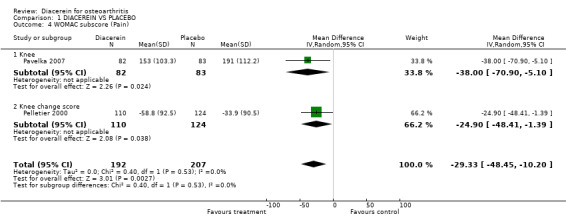
| 4 WOMAC subscore (Pain) | 2 | 399 | Mean Difference (IV, Random, 95% CI) | ‐29.33 [‐48.45, ‐10.20] |
| 4.1 Knee | 1 | 165 | Mean Difference (IV, Random, 95% CI) | ‐38.0 [‐70.90, ‐5.10] |
| 4.2 Knee change score | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐24.9 [‐48.41, ‐1.39] |
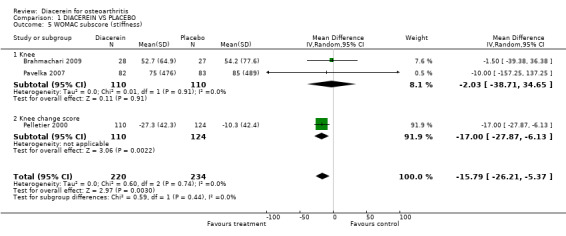
| 5 WOMAC subscore (stiffness) | 3 | 454 | Mean Difference (IV, Random, 95% CI) | ‐15.79 [‐26.21, ‐5.37] |
| 5.1 Knee | 2 | 220 | Mean Difference (IV, Random, 95% CI) | ‐2.03 [‐38.71, 34.65] |
| 5.2 Knee change score | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐17.0 [‐27.87, ‐6.13] |
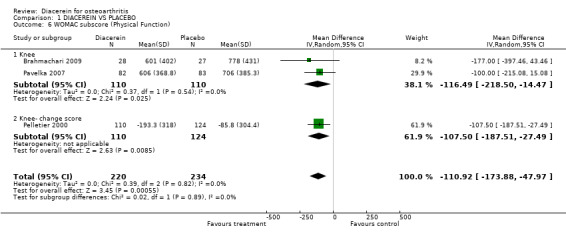
| 6 WOMAC subscore (Physical Function) | 3 | 454 | Mean Difference (IV, Random, 95% CI) | ‐110.92 [‐173.88, ‐47.97] |
| 6.1 Knee | 2 | 220 | Mean Difference (IV, Random, 95% CI) | ‐116.49 [‐218.50, ‐14.47] |
| 6.2 Knee‐ change score | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐107.50 [‐187.51, ‐27.49] |
| 7 Radiographic Progression | 2 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 0.99] |
| 7.1 Hip | 1 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 0.99] |
| 7.2 Knee | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.51, 1.74] |
| 8 Adverse Effects | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Diarrhoea | 7 | 1462 | Risk Ratio (M‐H, Random, 95% CI) | 3.52 [2.42, 5.11] |
| 8.2 Dyspepsia | 4 | 1059 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.61, 1.58] |
| 8.3 Rash or Pruritus | 4 | 878 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.94, 4.23] |
| 8.4 Urine Descolouration | 2 | 677 | Risk Ratio (M‐H, Random, 95% CI) | 13.01 [5.96, 28.40] |
| 8.5 Epigastralgia | 2 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.44, 2.09] |
| 9 Drop Out | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Ineffective intervention | 2 | 757 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.49, 0.87] |
| 9.2 Adverse effect | 7 | 1476 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.83, 2.01] |
| 9.3 Other | 2 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.49, 1.61] |
1.3. Analysis.
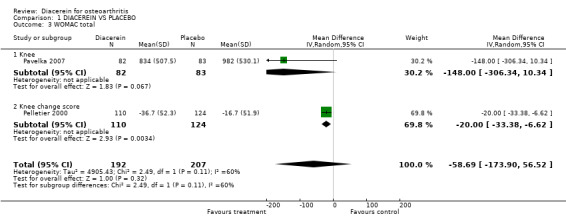
Comparison 1 DIACEREIN VS PLACEBO, Outcome 3 WOMAC total.
1.4. Analysis.
Comparison 1 DIACEREIN VS PLACEBO, Outcome 4 WOMAC subscore (Pain).
1.5. Analysis.
Comparison 1 DIACEREIN VS PLACEBO, Outcome 5 WOMAC subscore (stiffness).
1.6. Analysis.
Comparison 1 DIACEREIN VS PLACEBO, Outcome 6 WOMAC subscore (Physical Function).
Comparison 2. DIACEREIN VS NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 WOMAC subscore (Physical Function) | 2 | 345 | Mean Difference (IV, Random, 95% CI) | 29.50 [‐23.17, 82.17] |
| 1.1 Knee | 2 | 345 | Mean Difference (IV, Random, 95% CI) | 29.50 [‐23.17, 82.17] |
| 2 Quality of Life | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Adverse Effects | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Diarrhoea | 3 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 3.20 [1.58, 6.49] |
| 3.2 Dyspepsia | 3 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.29, 1.61] |
| 3.3 Dizziness | 3 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.18, 4.15] |
| 3.4 Bowel Motility Disorders | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 6.95 [2.11, 22.89] |
| 3.5 Rash or Pruritus | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.24, 102.42] |
| 4 Drop Out | 3 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.38, 2.44] |
| 5 Pain VAS 0 to 100 | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐6.48, 10.48] |
| 6 Womac Pain | 1 | 161 | Mean Difference (IV, Fixed, 95% CI) | 14.0 [‐10.15, 38.15] |
| 7 Pain on walking 20 m | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐3.81, 6.41] |
2.5. Analysis.
Comparison 2 DIACEREIN VS NSAIDs, Outcome 5 Pain VAS 0 to 100.
2.6. Analysis.
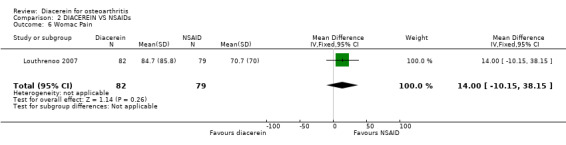
Comparison 2 DIACEREIN VS NSAIDs, Outcome 6 Womac Pain.
2.7. Analysis.
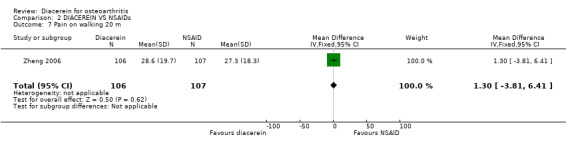
Comparison 2 DIACEREIN VS NSAIDs, Outcome 7 Pain on walking 20 m.
Comparison 3. DIACEREIN VS OTHER SYSADOA(Symptom modifier slow acting drug for OA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Visual analogue scale for pain | 2 | 312 | Mean Difference (IV, Random, 95% CI) | 1.51 [‐4.22, 7.23] |
| 1.1 Knee and/or hip | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 4.50 [‐4.67, 13.67] |
| 1.2 Knee‐change score | 1 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐7.73, 6.93] |
| 2 Lequesne Impairment Index | 2 | 311 | Mean Difference (IV, Random, 95% CI) | 0.37 [1.00, 1.73] |
| 2.1 Knee and/or hip | 1 | 95 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐1.17, 1.71] |
| 2.2 Knee‐change score | 1 | 216 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐3.01, 5.41] |
| 3 Radiographic progression | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.60, 1.91] |
| 4 Adverse effects | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Total | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.60, 3.34] |
| 4.2 Knee pain after intra‐articular injection | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.16, 0.75] |
| 4.3 Diarrhoea | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 4.26 [2.54, 7.16] |
| 4.4 Dyspepsia | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.74, 3.13] |
| 4.5 Dizziness | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.20, 1.90] |
| 4.6 Influenza‐like and respiratory symptoms | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.50] |
| 4.7 Urine discolouration | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 19.95 [1.14, 349.67] |
| 5 Dropout | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.78, 2.58] |
3.3. Analysis.
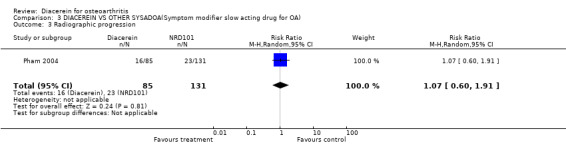
Comparison 3 DIACEREIN VS OTHER SYSADOA(Symptom modifier slow acting drug for OA), Outcome 3 Radiographic progression.
3.5. Analysis.
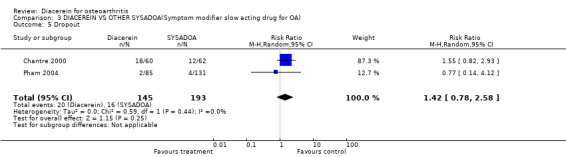
Comparison 3 DIACEREIN VS OTHER SYSADOA(Symptom modifier slow acting drug for OA), Outcome 5 Dropout.
Comparison 4. Diacerein versus Placebo/ Sensitivity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain on VAS (0 to 100 mm) | 2 | 677 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐3.90, 4.86] |
| 1.1 Longer studies (longer than six months) | 2 | 677 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐3.90, 4.86] |
Comparison 5. Subgroup Analysis_Carry over effect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain on VAS 0 to 100 mm | 3 | 451 | Mean Difference (IV, Random, 95% CI) | ‐13.19 [‐24.25, ‐2.13] |
| 2 WOMAC A (Pain) | 2 | 326 | Mean Difference (IV, Random, 95% CI) | ‐80.37 [‐153.26, ‐7.47] |
| 3 WOMAC B (Stiffness) | 3 | 381 | Mean Difference (IV, Random, 95% CI) | ‐20.42 [‐30.52, ‐10.31] |
| 4 WOMAC C (Physical function) | 3 | 381 | Mean Difference (IV, Random, 95% CI) | ‐233.30 [‐363.30, ‐103.30] |
5.3. Analysis.
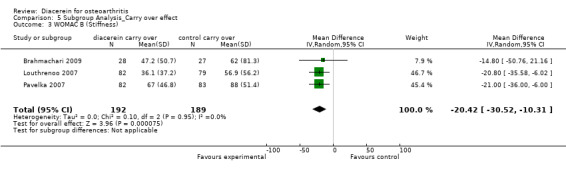
Comparison 5 Subgroup Analysis_Carry over effect, Outcome 3 WOMAC B (Stiffness).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brahmachari 2009.
| Methods | Randomised controlled trial Single‐blind (participants were blinded) Placebo‐controlled |
|
| Participants | Individuals between 35 and 70 years of age, with primary, symptomatic, tibiofemoral OA fulfilling ACR criteria and Kellgren‐Lawrence grades II and III with pain score ≥ 35 mm on a 100‐mm VAS scale | |
| Interventions | Diacerein 50‐mg capsules twice daily or placebo capsules twice daily Duration eight weeks with evaluation and after 12 weeks without treatment |
|
| Outcomes | Pain on movement in 0 to 100 mm VAS WOMAC stiffness and physical function Clinical global impression by investigator on a 5‐point Likert scale Use of rescue medication |
|
| Notes | The study drug was provided by Macleods Pharmaceuticals Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Diacerein and placebo arms were randomly assigned using a computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding only for the participants |
| Blinding of outcome assessment (detection bias) Clinical outcomes | High risk | Single blind. Each participant was followed up at monthly intervals for 12 weeks. Outcomes assessed were pain, WOMAC subscores for stiffness and physical function, clinical global assessment by the physician and use of rescue medication |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis used a modified intention‐to‐treat method, including all participants who attended at least one postbaseline visit. Missing values were dealt with by the last observation carried forward method |
| Selective reporting (reporting bias) | Low risk | Appropriate methods to compare results were used and well described |
Chantre 2000.
| Methods | Randomised controlled trial Double‐blind Multi‐centre (30 centres) Parallel‐group | |
| Participants | Outpatients with OA of hip or knee Country: France N = 122 Mean age, years: 61.5 Female 63%; male 37% |
|
| Interventions | Diacerein 50 mg BID + six capsules of placebo per day versus six capsules of Harpadol(R) + two capsules of placebo per day. Duration: 16 weeks |
|
| Outcomes | Pain (0 to 100 VAS scale) Lequesne Functional Index Use of NSAIDs | |
| Notes | Arkopharma Laboratories is represented as the first author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation in blocks of four to ensure uniform distribution to the treatment groups at each study centre. Trial samples were numbered consecutively and were handed out to participants in the order of inclusion. Treatment groups were matched with respect to age, gender, weight and duration of arthrosis |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind. Medications were packaged individually and labelled with the study number, the participant number and the randomisation number |
| Blinding of outcome assessment (detection bias) Clinical outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT participants and per‐protocol participants were described without disparities |
| Selective reporting (reporting bias) | Low risk | All data were properly reported |
Dougados 2001.
| Methods | Randomised controlled trial Double‐blind Multi‐centre (26 centres) Parallel‐group |
|
| Participants | Outpatients with hip OA Country: France N = 507 Mean age, years: 62 ± 7 Female 60%; male 40% |
|
| Interventions | Diacerein 50 mg BID versus placebo BID Duration: three years of treatment |
|
| Outcomes | Radiography once a year (JWS measurement) Pain (100‐mm VAS) Functional Lequesne Index Analgesic use Need for signal hip joint replacement |
|
| Notes | Supported in part by a grant from Negma Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The centralised allocation schedule was prepared using a blocked randomisation technique (blocking factor of 4) |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Participants were randomly assigned to receive one indistinguishable capsule of placebo or diacerein |
| Blinding of outcome assessment (detection bias) Clinical outcomes | Unclear risk | No reference was made to assessors' blinding in evaluating clinical outcomes |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | A central reader expert radiologist was unaware of participants' identity, study group, signal hip and sequence of radiographs |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 252 participants were randomly assigned to placebo and 255 to diacerein. ITT analysis in 247 placebo and 246 diacerein participants. PP analysis in 138 placebo and 131 diacerein |
| Selective reporting (reporting bias) | High risk | Outcomes were analysed at baseline and at the end of the study. One evaluation described in the baseline table was not reported at the end of the study: participant assessment on the Likert scale |
Lequesne 1998.
| Methods | Randomised controlled trial Double‐blind Parallel‐group Multi‐centre (35 centres) |
|
| Participants | Outpatients with knee or hip OA Country: France N = 183 Mean age, years: 61.5 ± 10.9 |
|
| Interventions | Zero to two months: diacerein 50 mg BID + diclofenac 50 mg BID versus placebo + diclofenac 50 mg BID Two to six months: diacerein 50 mg BID versus placebo Six to eight months: without treatment (carry‐over effect evaluation) |
|
| Outcomes | Pain (0 to 100‐mm VAS scale) Functional Lequesne Impairment Index Global efficacy evaluation for participant and physician (participant scale 0 to 7 and physician scale 0 to 5) Safety scale 0 to 4 at the end of the study Analgesic and NSAID use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Clinical outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 93 participants in the placebo group and 90 in the diacerein group. ITT analysis was done for only some outcomes |
| Selective reporting (reporting bias) | Low risk | All data were properly reported |
Louthrenoo 2007.
| Methods | Randomised controlled trial Multi‐centre (five medical schools in Thailand) Double‐blind Piroxicam‐controlled Parallel‐group |
|
| Participants | Participants with tibiofemoral X‐ray confirmed Kellgren‐Lawrence grade II or III OA according to ACR criteria between 40 and 65 years of age, with knee pain of at least 40 mm on at least two items of the WOMAC A, on a 0 to 100‐mm VAS scale, for at least 15 days | |
| Interventions | Diacerein 100 mg/ d (N = 86) versus piroxicam 20 mg/d (N = 85). 16 weeks | |
| Outcomes | WOMAC A, B, C SF‐36 Paracetamol consumption |
|
| Notes | Supported by a grant from TRB Chemedica International | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant was randomly assigned to a treatment group using a randomisation table generated by a validated computer software |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Clinical outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A good description of withdrawals and the reasons for them were provided. 171 were randomly assigned (diacerein 86 and control 85); however, ITT analysis was performed in only 161 (82 diacerein, 79 control). 10 participants (four diacerein, six control) were excluded from ITT because they did not take any dose of medication. 150 participants completed the study |
| Selective reporting (reporting bias) | Low risk | All data were properly reported |
Nguyen 1994.
| Methods | Randomised controlled trial
Allocation concealment Double‐blind Placebo‐controlled Parallel‐group Multi‐centre |
|
| Participants | Outpatients with hip OA Country: France N = 288; included in this review: 221 Mean age, years: 60 ± 16 Female 57%; male 43% |
|
| Interventions | One capsule (50‐mg capsule of diacerein or matching placebo capsules) twice daily and one tablet (20‐mg tablet of tenoxicam or matching placebo tablets) Duration: eight weeks |
|
| Outcomes | Pain (100‐mm VAS scale) Functional Lequesne Impairment Index Analgesic consumption Participant overall assessment (0 to 4 scale) |
|
| Notes | Supported in part by Negma Pharma Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Pills were unidentifiable and all participants were allowed to take analgesics |
| Blinding of outcome assessment (detection bias) Clinical outcomes | High risk | The same investigator did the clinical evaluation and applied questionnaires |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawn participants were well described, and all randomly assigned participants were evaluated |
| Selective reporting (reporting bias) | Low risk | All data were properly reported |
Pavelka 2007.
| Methods | Randomised controlled trial Double‐blind Placebo‐controlled Multi‐centre |
|
| Participants | Outpatients with tibiofemoral OA (knee) according to ACR criteria | |
| Interventions | Diacerein 50 mg twice a day (N = 82) versus placebo (N = 83) | |
| Outcomes | WOMAC for pain (A), WOMAC B, WOMAC C and total WOMAC Tenderness of target knee on palpation 100 mm VAS Acetaminophen intake, tablets/d |
|
| Notes | Supported by a joint grant from TRB Chemedica International and Glynn Brothers Chemicals AG | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind for participants and personnel |
| Blinding of outcome assessment (detection bias) Clinical outcomes | Low risk | Blinding for assessors |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 168 participants were randomly assigned; however, 165 participants were included in the ITT analysis |
| Selective reporting (reporting bias) | Low risk | All data were properly reported |
Pelletier 2000.
| Methods | Randomised controlled trial
Double‐blind Parallel‐group four‐arm trial Multi‐centre (25 centres) |
|
| Participants | Outpatients with knee OA
Country: Canada and Israel, N = 484; included in this review: 236
Mean age, years: 63.5 ± 8.9
Female 79.6%; male 20.4% |
|
| Interventions | Diacerein 25 mg twice daily versus diacerein 50 mg twice daily versus diacerein 75 mg twice daily versus placebo (one capsule twice daily) Duration 16 weeks |
|
| Outcomes | Pain (0 to 100‐mm VAS scale)
WOMAC Index
Handicap (0 to 100‐mm VAS)
Participant and physician overall assessment on a 0 to 100‐mm VAS at the end of the study
Knee joint swelling (0 to 3)
Duration of morning stiffness in minutes
Joint mobility assessed with a goniometer Safety evaluation |
|
| Notes | Supported by a grant from Les Laboratoires Negma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The centralised allocation schedule was prepared by using a blocked randomisation technique (blocking factor eight). Treatments were divided between the two countries (treatments 1 to 500 in Israel and 600 to 1000 in Canada) and were allocated to each centre |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Clinical outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three populations were evaluated: one for safety and two for efficacy Safety population received the medications at least once. An intention‐to‐treat population was evaluated with at least one postbaseline visit. A per‐protocol population with all postbaseline visits completed was also evaluated. Four participants were excluded from ITT analysis |
| Selective reporting (reporting bias) | Low risk | All data were properly reported |
Pham 2004.
| Methods | Randomised controlled trial
Double‐blind Three arms Multi‐centre (46 centres) |
|
| Participants | Outpatients with knee OA
Country: France and UK
N = 301 Mean age, years: 65 Female 70%; male 30% |
|
| Interventions | Diacerein 50 mg BID + 3 × 3 intra‐articular injections of saline solution versus 3 × 3 HA intra‐articular injections (NRD101) and placebo capsules versus 3 × 3 intra‐articular injections of saline solution and daily placebo capsules Duration: one year |
|
| Outcomes | Pain (0 to 100‐mm VAS)
Lequesne Impairment Index
Participants' global assessment (0 to 100 VAS)
Percentage of painful days (0 to 100 VAS)
Assessment of treatment efficacy by participant and investigator (0 to 5 scale) at the end of the study Radiography after one year (JWS measurement) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomised allocation schedule was centralised (Cassene Laboratories) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) Clinical outcomes | Unclear risk | No reference about assessors' blinding to evaluate clinical outcomes |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | Two observers who were unaware of participants' identity, study group, signal knee and sequence of radiographs |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis was done for efficacy outcomes. Structural evaluation was analysed only in participants with X‐rays in the baseline table. Nine participants were excluded from the analysis |
| Selective reporting (reporting bias) | Low risk | All data were properly reported |
Zheng 2006.
| Methods | Randomised controlled trial Double‐blind Parallel‐group Multi‐centre |
|
| Participants | Outpatients with knee OA
Country: China N = 184 Mean age, years: 58.5 82% female; 18% male |
|
| Interventions | Diacerein 2 × 50 mg capsules and three tablets of placebo versus diclofenac 3 × 25 mg tablets and two tablets of placebo Duration: three months with a follow‐up period of one month | |
| Outcomes | Pain on walking 20 m on a 100‐mm VAS WOMAC Index Effusion or swelling of soft tissue/tenderness of target joint Efficacy judgements by participants and investigators | |
| Notes | Diacerein and diclofenac were provided by Kunming Jida Pharmaceutical Co | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The same packaging boxes were used for both groups with the medications |
| Blinding of outcome assessment (detection bias) Clinical outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Radiographic outcomes | Low risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not performed |
| Selective reporting (reporting bias) | Low risk | All data were properly reported |
BID: twice a day. HA: hyaluronic acid.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adami 1985 | Not a randomised controlled trial |
| Ascherl 1994 | Unpublished study. Diacerein versus placebo for knee OA. Unsuccessful TRB Chemedica contact |
| Baliga 2010 | Inappropriate inclusion criteria |
| Bogliola 1991 | Not a randomised controlled trial The study describes diacerein 50 mg/d every 60 days versus a 30‐day interval compared with falgo balneo therapy for one year |
| Carrabba 1987 | Not a randomised controlled trial. Study analysed use of diacerein 100 mg/d in a group of 31 participants with OA without defined joint for four weeks, in another group of 20 OA participants using diacerein 100 mg/d in a cross‐over arm with naproxen 500 mg/d and in a third group of 20 fibromyalgia participants using diacerein 100 mg/d five days/wk for 12 weeks. The three cohorts were analysed for outcomes on a 0 to 3 scale |
| Delcambre 1994 | Not a randomised controlled trial. Study evaluated 1,221 participants with radiological OA of hip, knee or cervical or lumbar spine using diacerein 100 mg/d for three months as isolated therapy or associated with analgesic or NSAIDs. Outcome measurements inadequate for analysis (VAS 0 to 100 in five grades) |
| Delcambre 1996 | Duplication of the Delcambre 1994 publication |
| Fagnani 1998 | Randomised non blinded study using diacerein and standard therapy for OA including other slow acting anti osteoarthritic drugs plus several procedures that could cause confusion on improvement of end point measurements |
| Fioravanti 1985 | Report of diacerein effectiveness. Symposium presentation not published. Unsuccessfull TRB Chemedica contact |
| Kay 1980 | Not a randomised controlled trial |
| Leblan 2000 | Duplication of the publication of Chantre 2000 |
| Linguetti 1982 | Not a randomised controlled trial |
| Mantia 1987 | Clinical study report. Diacerein versus diclofenac for knee and hip OA. Data not available. Unsuccessfull TRB Chemedica contact |
| Marcolongo 1988 | Review on diacerein treatment. No specific data are available for analysis |
| Mathieu 1999 | Case series |
| Mattara 1985 | Data not available. Unsuccessful TRB Chemedica contact |
| Mazzaro 1989 | Not a trial |
| Mordini 1986 | Clinical study. Data not available. Unsuccessful TRB Chemedica contact |
| Pietrogrande 1985 | Data not available. Unsuccessful TRB Chemedica contact |
| Portioli 1987 | Data not available. Unsuccessful TRB Chemedica contact |
| Renapurkar 2010 | Not a randomised controlled trial |
| Schulitz 1994 | Data not available. Unsuccessful TRB Chemedica contact |
| Seisenbayev 2012 | Data not available. Unsuccessful personal contact |
| Sharma 2008 | Not a randomised controlled trial |
| Singh 2012 | Inappropriate inclusion criteria |
| Tang 2004 | Congress abstract later published by Zheng (Zheng 2006) with additional data |
| Valat 1997 | Duplication of the Fagnani study (Fagnani 1998) |
| Vignon 2002 | Comments about ECHODIAH study |
| Villani 1998 | Comment about placebo effects in trials using slow‐acting drugs for OA |
| Villermay 1994 | Comment about the large trial involving 1,221 participants described by Delcambre |
Characteristics of studies awaiting assessment [ordered by study ID]
Shin 2013.
| Methods | Double‐blind, randomised, controlled study; allocation concealment |
| Participants | 84 participants > 40 years of age, had at least one tender joint and had a joint pain visual analogue scale of 30 mm |
| Interventions | Participants received diacerein (50 mg) or placebo BID for 12 weeks |
| Outcomes | The primary end point was the Australian/Canadian Osteoarthritis Hand Index (AUSCAN) pain score at four weeks. Secondary end points were AUSCAN pain score at 12 weeks and AUSCAN physical function and stiffness score, participant and physician global assessments, functional index of hand OA scores and multi‐dimensional health assessment questionnaire results at four weeks and 12 weeks |
| Notes | The following additional information is pending:
|
Differences between protocol and review
No differences were noted between the objectives and methods described in the protocol and those included in the first published review. In this updated version, the review authors adhered to the current recommendations of the Cochrane Handbook for Assessment of Methodological Quality of Studies and included post hoc sensitivity analyses of studies with follow‐ups lasting longer than six months. Therefore, the Jadad scores described in the protocol were not used. As well, the search included a search of four main regulatory agency websites as per the current CMSG guidance.
Contributions of authors
Tania Sales de Alencar Fidelix (TSAF), Cristiane Rufino Macedo (CRM), Lara Maxwell (LM) and Virginia Fernandes Moça Trevisani (VFMT) contributed to the updated version of this review.
Sources of support
Internal sources
UNIFESP Escola Paulista de Medicina, Brazil.
Brazilian Cochrane Centre, Brazil.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Brahmachari 2009 {published data only}
- Brahmachari B, Chatterjee S, Ghosh A. Efficacy and safety of diacerein in early knee osteoarthritis: a randomized placebo‐controlled trial. Clinical Rheumatology 2009;28:1193‐1198. [DOI] [PubMed] [Google Scholar]
Chantre 2000 {published data only}
- Chantre P, Cappelaere A, Leblan D, Guedon D, Vandermander J, Fournie B. Efficacy and Tolerance of Harpagophytum Procumbens versus Diacerhein in Treatment of Osteoarthritis. Phytomedicine 2000;7:177‐183. [DOI] [PubMed] [Google Scholar]
Dougados 2001 {published data only}
- Dougados M, Nguyen M, Berdah L, Maziéres B, Vignon E, Lequesne M. Evaluation of Structure‐Modifying Effects of Diacerein in Hip Osteoarthritis. Arthritis & Rheumatism 2001;44(11):2539‐2547. [DOI] [PubMed] [Google Scholar]
Lequesne 1998 {published data only}
- Lequesne M, Berdah L, Gérentes I. Efficacy and Safety of Diacerein for the treatment of Knee and Hip Osteoarthritis [Efficacité et tolérance de la diacerhéine dans le traitment de la gonarthrose et de la coxarthrose]. La Revue du Praticien 1998;48:S31‐S35. [PubMed] [Google Scholar]
Louthrenoo 2007 {published data only}
- Louthrenoo W, Nilganuwong S, Aksaranugraha S, Asavatanabodee P, Saengnipanthkul S. The efficacy, safety and carry‐over effect of diacerein in the treatment of painful knee osteoarthitis: a randomised, double‐blind, NSAID‐controlled study. Osteoarthritis and Cartilage 2007;15:605‐614. [DOI] [PubMed] [Google Scholar]
Nguyen 1994 {published data only}
- Ngyen M, Dougados M, Berdah L, Amor B. Diacerhein in The Treatment of Osteoarthritis of The Hip. Arthritis & Rheumatism 1994;37(4):529‐36. [DOI] [PubMed] [Google Scholar]
Pavelka 2007 {published data only}
- Pavelka K, Trc T, Karpas K, Vítek P, Sedlacková M, Vlasáková V, Böhmová J, Rovenský J. The Efficacy and Safety of Diacerein in the Treatment of Painful Osteoarthritis of the Knee. Arthritis & Rheumatism 2007;56:4055‐4064. [DOI] [PubMed] [Google Scholar]
Pelletier 2000 {published data only}
- Pelletier JP, Yaron M, Haraoui B, Cohen P, Nahir M A, Choquette D, et al. Efficacy and Safety of Diacerein in Osteoarthritis of the Knee. Arthritis and Rheumatism 2000;43(10):2339‐2348. [DOI] [PubMed] [Google Scholar]
Pham 2004 {published data only}
- Pham T, Henanff AL, Ravaud P, Dieppe P, et al. Evaluation of symptomatic and structural efficacy of a new hyaluronic acid (HA) compound, (NRD101), when compared to diacerein and placebo in one‐year randomized controlled study in symptomatic knee osteoarthritis. Annals of Rheumatic Diseases 2004;63(12):161‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2006 {published data only}
- Zheng Wen‐Jie, Tang Fu‐Lin, Li Jun, Zhang Feng‐Chun, Li Zhan‐Guo, Su Yin, Wu Dong‐Hai, Ma Li, Zhou Hui‐Qiong, Huang Feng, Zhang Jiang‐Lin, Liang Dong‐Feng, Zhou Yi‐Xiong, Xu Hui. Efficacy and safety of diacerein in osteoarthritis of the knee: a randomized, multicenter, double‐dummy, diclofenac‐controlled trial in China. APLAR Journal of Rheumatology. 2006; Vol. 9:64‐69.
References to studies excluded from this review
Adami 1985 {published data only}
- Adami S, Bortolotti R, Guarrera G, Marini G, Rosini S, Zampieri A, Lo Cascio V. Diacerein in the Treatment of Degenerative Arthropathy [La Diacetilreina nel Trattamento delle Artropatie Degenerative]. La Clinica Terapeutica 1985;112:439‐443. [PubMed] [Google Scholar]
Ascherl 1994 {published and unpublished data}
- Ascherl R. Double blind, placebo‐controlled multicentre, phase III study of the efficacy and tolerability of diacerein (DA39) in patients with osteoarthritis of the knee (University of Lubeck). Köln, Germany:Madaus AG;. unpublished final clinical study report October 10, 1994. Madaus Report DA39KO.13.
Baliga 2010 {published data only}
- Baliga VP, Bolmall CS, Jagiasi JD, Kumar MSA, Sankaralingam K, Veerappan V. Efficacy, safety and tolerability of diacerein MR 100 mg versus diacerein 50 mg in adult patients with osteoarthritis of the knee. Osteoarthritis and Cartilage 2010;18 SUPPL 2:S252. [Google Scholar]
Bogliola 1991 {published data only}
- Bogliolo A, Loi A, Perpignano G. "Fangobalneotherapy" and Diacerein in the Treatment of Hip and Knee Osteaoarthrosis [Fangobalneoterapia e Diacereina nel Trattamento dell'Osteoartrosi dell'Anca e del Ginocchio]. La Clinica Terapeutica 1991;137:3‐8. [PubMed] [Google Scholar]
Carrabba 1987 {published data only}
- Carrabba M, Mele G, Chevallard M, Angelini M. Diacereine, an "original"approach to the treatment of degenerative and/or extra‐articular rheumatism. [Diacereina: un approccio "originale" nel trattamento dei reumatismi degenerativi e/o extra‐articolari]. Minerva Medica 1987;78:179‐185. [PubMed] [Google Scholar]
Delcambre 1994 {published data only}
- Delcambre B, Taccoen A. ART 50 Study in the Update Rheumatologic Practice [Étude D'ART 50 en Pratique Rhumatologique Courante]. Revue Du Rhumatisme 1994;61:142S‐146S. [PubMed] [Google Scholar]
Delcambre 1996 {published data only}
- Delchambre B, Taccoen A. Study of ART 50 in the rheumatologic daily practice [Étude d'ART 50 en pratique rhumatologique quotidienne]. Le Revue du Praticien 1996;46:S49‐S52. [PubMed] [Google Scholar]
Fagnani 1998 {published data only}
- Fagnani F, Bouvenot G, Valat J‐P, Bardin T, Berdah L, Lafuma A, Bono I, Escchwege E, Dreiser R‐L. Medico‐Economic Analysis of Diacerein With or Without Standard Therapy in the Treatment of Osteoarthritis. Pharmacoeconomics 1998;13:135‐146. [DOI] [PubMed] [Google Scholar]
Fioravanti 1985 {published data only}
- Fioravanti A, Marcolongo R. Therapeutic effectiveness of diacerhein (DAR) in arthrosis of the knee and hip. Report presented at: The Toscana Medicina Symposuim on Diacereina. October 1, 1985.
Kay 1980 {published data only}
- Kay AGL, Griffiths LG, Volans GN, Grahame R. Prelimirary experience with diacetylrhein in the treatment of osteoarthritis. Current Medical Research and Opinion 1980;6(8):548‐551. [DOI] [PubMed] [Google Scholar]
Leblan 2000 {published data only}
- Leblan D, Chantre P, Fournié B. Harpagophytum procumbens in the treatment of knee and hip osteoarthritis. Four‐month results of a prospective, multicenter, double‐blind trial versus diacerhein. Join Bone Spine 2000;67:462‐7. [PubMed] [Google Scholar]
Linguetti 1982 {published data only}
- Linguetti M, D'Ambrosio PL, Grezia F, Sorrentino P, Lingetti E. A Controlled Study in the Treatment of Osteoarthritis with Diacetylrhein (ARTRODAR). Current Therapeutic Research 1982;31(3):408‐412. [Google Scholar]
Mantia 1987 {published and unpublished data}
- Mantia C. A controlled study of the efficacy and tolerability of diacetylrhein in the functional manifestations of osteoarthritis of the hip and the knee: a double‐blind study versus diclofenac. Palermo Hospital, Palermo, Italy; 1987.
Marcolongo 1988 {published data only}
- Marcolongo R, Fioravanti A, Adami S, Tozzi E, Mian M, Zampieri A. Efficacy and Tolerability of Diacerhein in the Treatment of Osteoarthrosis. Current Therapeutic Research 1988;43(5):878‐887. [Google Scholar]
Mathieu 1999 {published data only}
- Mathieu P. Interleucine 1 ‐ Results of a pilot study with diacerein (ART 50) for Knee Osteoarthrosis [L'interleukine 1 ‐ Résultats d'une étude "pilote" avec la diacerhéine (ART 50) dans la gonarthrose]. La Revue du Praticien 1999;49:S15‐S18. [PubMed] [Google Scholar]
Mattara 1985 {published data only}
- Mattara L. [DAR: indagini "controllate" nel trattamento della osteoartosi ]. Relazione presentata al LXXXVI Congresso Naz. Società Ital. Medicina Interna ‐ Sorrento. 1985; Vol. S:59.
Mazzaro 1989 {published data only}
- Mazzaro C, Bocchieri E, Tesolin FG, Ventre L, Romagnoli A. Clinical Evaluation of Diacereine in Osteoarthrosis Treatment [Valutazione clinica della Diacereina nel Trattamento dell'osteoartrosi]. Minerva Medica 1989;80(9):1025‐1027. [PubMed] [Google Scholar]
Mordini 1986 {published and unpublished data}
- Mordini M, Nencioni C, Lavagni A, Camarri E. Diacerhein vs naproxen in coxo‐gonarthrosis: double‐blind randomized study. Abstract presented at: The 27th Congress of the Italian Society of Rheumatology. October 30‐November 2, 1986.
Pietrogrande 1985 {published data only}
- Pietrogrande V, Leonardi M, Pacchioni C. Results of a clinical trial with a new drug, diacerhein in arthrosic patiens.. Report presented at: The LXXXVI Congress of the Italian National Society of Internal Medicine;. September 24, 1985.
Portioli 1987 {published and unpublished data}
- Portioli IA. Naproxen‐controlled study on efficacy and tolerability of diacetylrhein in the functional manifestations of osteoarthritis of the knee and hip: a double blind study versus naproxen. unpublished clinical study report; Santa Maria Nuova Hospital, Reggio Emilia, Italy 1987.
Renapurkar 2010 {published data only}
- Renapurkar DK. Mathur S, Rao KLJ. Evaluation of efficacy and safety of diacerein in osteoarthritis of knee joint. International Journal of Pharma and Bio Sciences 2010;1(3):1‐11. [Google Scholar]
Schulitz 1994 {published and unpublished data}
- Schulitz KP. Clinical investigation of the efficacy and tolerance of diacetylrhein (DAR) in the treatment of osteoarthritis of the knee. Köln, Germany: Madaus AG. Unpublished final clinical study report. Madaus Report RDA139. October 27 1994.
Seisenbayev 2012 {published data only}
- Seisenbayev A, Togizbaev. Three component treatment of osteoarthiitis. Rheumatology 2012;51 SUPPL 1:i29‐i30. [Google Scholar]
Sharma 2008 {published data only}
- Sharma A, Rathod R, Baliga VP. An Open Prospective Study on Postmarketing Evaluation of the Efficacy and Tolerability of Diacerein in Osteo‐arthritis of the knee (DOK). Journal of Indian Medical Association 2008;106:31‐34. [PubMed] [Google Scholar]
Singh 2012 {published data only}
- Singh K, Sharma R, Rai J. Diacerein as adjuvant to diclofenac sodium in osteoarthritis knee. International journal of rheumatic diseases 2012;15(1):69‐77. [PUBMED: 22324949] [DOI] [PubMed] [Google Scholar]
Tang 2004 {published data only}
- Tang FL, Wu DH, Lu ZG, Huang F, Zhou YX. The efficacy and safety of diacerein in treatmnet of painful osteoarthritis of the knee. 11º Asia Pacific League of Associations for Rheumatology Congress, Jeju Island, Korea. 2004:35.
Valat 1997 {published data only}
- Valat‐JP. Diacerein Pragmatic Study: Clinical, Life quality and socio‐ economic analysis [Étude pragmatique de la diacerheine: résultats sur les signes cliniques, la qualité de vie et les coûts médico‐économiques]. La Revue du Praticien 1997;47:S39‐S45. [PubMed] [Google Scholar]
Vignon 2002 {published data only}
- Vignon E. Results of ECHODIAH study in hip osteoarthritis [Résultats de l'essai thérapeutique ECHODIAH dans l"arthrose de hanche]. La Presse Médicale 2002;31(1):7‐9. [PubMed] [Google Scholar]
Villani 1998 {published data only}
- Villani P, Bouvenot G. Assessment of the placebo effect of symptomatic slow‐acting drugs given for osteoarthritis [Approche de l'intensité de l'effet placebo dans l'évaluation des anti‐arthrosiques symptomatiques d'action lente]. La Presse Médicale 1998;27(5):211‐214. [PubMed] [Google Scholar]
Villermay 1994 {published data only}
- Villermay D. A new medicine for Arthrosis [Un Nouveau Médicament Pour L'Arthrose]. revue de L'Infirmière 1995;3:10‐11. [PubMed] [Google Scholar]
References to studies awaiting assessment
Shin 2013 {published data only (unpublished sought but not used)}
- Kichul Shin, Joon Wan Kim, Ki won Moon, Ji Ae Yang, Eun Yeong Lee, MD, Yeong Wook Song, MD, Eun Bong Lee. The Efficacy of Diacerein in Hand Osteoarthritis:A Double‐Blind, Randomized, Placebo‐Controlled Study. Clinical Therapeutics 2013;35:431‐439. [DOI] [PubMed] [Google Scholar]
Additional references
ACR 2000
- American College of Rheumatology Siubcommittee on Osteoarthritis Guidelines. ACR recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update.. Arthritis & Rheumatism 2000;43(9):1905‐15. [DOI] [PubMed] [Google Scholar]
Altman 1986
- Altman R, Asch E, Bloch D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the knee. Arthritis and Rheumatism 1986;29:1039‐49. [DOI] [PubMed] [Google Scholar]
Altman 1990
- Altman R, Alarcon G, Appelrough D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis and Rheumatism 1990;33:1601‐10. [DOI] [PubMed] [Google Scholar]
Bartels 2010
- Bartels EM, Bliddal H, Schondorff PK, Altman RD, Zhang W, Christensen R. Symptomatic efficacy and safety of diacerein in the treatment of osteoarthritis: meta‐analysis of randomized placebo‐controlled trials. Osteoarthritis and Cartilage 2010;18:289‐296. [DOI] [PubMed] [Google Scholar]
Bellamy 1988
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Journal of Rheumatology 1988;15:1833‐40. [PubMed] [Google Scholar]
Bellamy 1997
- Bellamy N, Kirwan J, Boers M. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip and hand osteoarthritis. Consensus development at OMERACT III. Journal of Rheumatology 1997;24:700‐802. [PubMed] [Google Scholar]
Bellamy 2006
- Bellamy N, Campbell J, Welch V, Gee TL, Bourne R, Wells GA. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005321.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berembaum 2010
- Berembaum F, Sellam J. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature Reviews Rheumatology 2010;11:625‐35. [DOI] [PubMed] [Google Scholar]
Bijlsma 2012
- Bijlsma JWJ. Textbook on Rheumatic Diseases. First Edition. London: BMJ Group, 2012. [Google Scholar]
Carlsson 1983
- Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983;16:87‐101. [DOI] [PubMed] [Google Scholar]
Dougados 2004
- Dougados M. Outcome Measures for Clinical Trials of Disease Modifying Osteoarthritis Drugs in Patients with Hip Osteoarthritis.. The Journal of Rheumatology 2004;70:66‐9. [PubMed] [Google Scholar]
Dworkin 2011
- Dworkin RH, Pierce‐Sandner S, Turk DC, McDermott MP, Gibofsky A, Simon LS, Farrar JT, Katz NP. Outcome measures in placebo‐controlled trials of osteoarthritis: responsiveness to treatment effects in the REPORT database.. Osteoarthritis Cartilage 2011;19:483‐92. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Zellweger‐Zahner T, Scheider M, Junker C, Lengeler Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350:326‐9. [DOI] [PubMed] [Google Scholar]
Grade 2008
- Grading Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2008;336:924‐1051. [Google Scholar]
Hellio 2009
- Hellio Le Graverand‐Gastineau MP. OA clinical trials: current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed?. Osteoarthritis Cartilage 2009;17:1393‐401. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). www.cochrane‐handbook.org. UK: The Cochrane Collaboration, 2011. [Google Scholar]
Hochberg 2001
- Hochberg M C, Dougados M. Pharmacological therapy of osteoarthritis. Best Practice & Research Clinical Rheumatology 2001;15(4):583‐93. [DOI] [PubMed] [Google Scholar]
Hunter 2011
- Hunter DJ. Pharmacologic therapy for osteoarthritis ‐ the era of disease modification.. Nature Reviews Rheumatology 2011;7(1):13‐22. [DOI] [PubMed] [Google Scholar]
Kellgren 1957
- Kellgren JH, Lawrence JJ. Radiological assessment of osteoarthritis. Annals of Rheumatic Diseases 1957;16:494‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrence 1998
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis & Rheumatism 1998;41(4):778‐99. [DOI] [PubMed] [Google Scholar]
Lequesne 1987
- Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee. Validation ‐ value in comparison with other assessment tests.. Scandinavian Journal of Rheumatology 1987;(Suppl 65):85‐9. [DOI] [PubMed] [Google Scholar]
Lingetti 1982
- Lingetti M, D'Ambrosio PL, Grezia F, et al. A controlled study in the treatment of osteoarthritis with diacetylrhein (arthrodar). Current Therapeutic Research 1982;31(3):408‐12. [Google Scholar]
Murphy 2012
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population‐health perspective. American Journal of Nursing 2012;112(Suppl 1):S13‐9. [DOI] [PubMed] [Google Scholar]
Pelletier 2010
- Pelletier JM, Pelletier JP. Effects of diacerein at the molecular level in the osteoarthritis disease process. Therapeutical Advances in Muskuloskeletal Disease 2010;2(2):95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pham 2003
- Pham T, Heijde DV, Lassere M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome Variables for Osteoarthritis Clinical Trials: The OMERACT‐OARSI Set of Responder Criteria. Journal of Rheumatology 2003;30:1648‐54. [PubMed] [Google Scholar]
Picavet 2003
- Picavet HS, Hazes JM. Prevalence of self reported musculoskeletal diseases is high. Annals of the Rheumatic Diseases 2003;62(7):644‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
PRAC 2013
- The European Medicines Agency Pharmacovigilance Risk Assessment Committee (PRAC). PRAC recommends suspension of diacerein‐containing medicines. Available from http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Diacerein‐containing_medicines_for_oral_administration/human_referral_prac_000010.jsp&mid=WC0b01ac05805c516f 8 November 2013.
Rintelen 2006
- Rintelen B, Neumann K, Leeb BF. A Meta‐analysis of Controlled Clinical Studies With Diacerein in the Treatment of Osteoarthritis. Archives of Internal Medicine 2006;166:1899‐1902. [DOI] [PubMed] [Google Scholar]
Spencer 1997
- Spencer CM, Wilde MI. Diacerein. Drugs 1997;53(1):98‐106. [DOI] [PubMed] [Google Scholar]
Sun 2007
- Sun BH, Wu CW, Kalunian KC. New developments in osteoarthritis.. Rheumatic Diseases Clinic of North America 2007;33(1):135‐48. [DOI] [PubMed] [Google Scholar]
Towheed 2008
- Towheed T, Maxwell L, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC, Wells GA. Glucosamine therapy for treating osteoarthritis. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002946.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Towheed 2006
- Towheed TE, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004257.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolf 2003
- Woolf A, Pfleger B. Burden of major musculoskeletal conditions. Bulletin of the World Health Organization 2003;81(9):646‐656. [PMC free article] [PubMed] [Google Scholar]
Zhang 2001
- Zhang Y, Xu L, Nevitt MC, Aliabadi P, Yu W, Qin M, et al. Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: the Beijing Osteoarthritis Study. Arthritis & Rheumatism 2001;44(9):2065‐71. [DOI] [PubMed] [Google Scholar]


