Abstract
Background
Preclinical models suggest synergy between anti-angiogenesis therapy, mammalian target of rapamycin (mTOR), and histone deacetylase inhibitors to promote anticancer activity.
Methods
This phase I study enrolled 47 patients between April 2012 and 2018 and determined safety, maximum tolerated dose (MTD), and dose-limiting toxicities (DLTs) when combining bevacizumab, temsirolimus, and valproic acid in patients with advanced cancer.
Results
Median age of enrolled patients was 56 years. Patients were heavily pretreated with a median of 4 lines of prior therapy. Forty-five patients (95.7%) experienced one or more treatment-related adverse events (TRAEs). Grade 3 TRAEs were lymphopenia (14.9%), thrombocytopenia (8.5%), and mucositis (6.4%). Grade 4 TRAEs included lymphopenia (2.1%) and CNS cerebrovascular ischemia (2.1%). Six patients developed DLTs across 10 dose levels with grade 3 infection, rash, mucositis, bowel perforation, elevated lipase, and grade 4 cerebrovascular ischemia. The MTD was dose level 9 (bevacizumab 5 mg/kg days 1 and 15 intravenously (IV) plus temsirolimus 25 mg days 1, 8, 15, and 22 IV and valproic acid 5 mg/kg on days 1-7 and 15-21 per orally (PO)). Objective response rate (ORR) was 7.9% with confirmed partial response (PRs) in 3 patients (one each in parotid gland, ovarian, and vaginal cancers). Stable disease (SD) ≥+6 months was seen in 5 patients (13.1%). Clinical benefit state (CBR: PR + SD ≥+6 months) was 21%.
Conclusion
Combination therapy with bevacizumab, temsirolimus, and valproic acid was feasible, but there were numerous toxicities, which will require careful management for future clinical development (ClinicalTrials.gov Identifier: NCT01552434).
Keywords: bevacizumab, temsirolimus, HIF-1 alpha, vascular endothelial growth factor, histone deacetylase inhibitors, valproic acid, clinical trial
This is the first study to evaluate the combination of bevacizumab, temsirolimus, and valproic acid in patients with advanced malignancies. This combination demonstrated modest efficacy among various solid tumors, but at the expense of toxicity.
Lessons Learned.
This trial highlights the importance of understanding the biological rationale with resistance to anticancer therapies and designing a trial where 3 well-known pathways promoting oncogenesis and resistance via VEGF, mTOR, and histone deacetylase was successfully implemented with astute therapy selection, dose escalation scheme, and manageable toxicities without overt overlap.
Objective responses were seen in high grade mucoepidermoid carcinoma of parotid, endometrioid ovarian carcinoma, and vaginal squamous cell carcinoma. Durable stable responses >6 months were seen in uveal melanoma, breast cancer, leiomyosarcoma, clear cell carcinoma of the kidney, and metastatic thymoma.
Prospective studies to elucidate this subset of patients with translational or biomarker-based studies are vital to harness the potential of this combination with bevacizumab and temsirolimus and valproic acid.
Discussion
This is the first study to evaluate the combination of bevacizumab, temsirolimus, and valproic acid in patients with advanced malignancies. This combination demonstrated modest efficacy among various solid tumors, but at the expense of toxicity. Treatment-related adverse events were seen in 45/47 (95.7%) of patients with 26/47 (55%) patients having grade 3/4 TRAEs. The maximum tolerated dose (MTD) was determined to be dose level 9 which was bevacizumab (5 mg/kg IV once every 14 days), temsirolimus (25 mg IV weekly), and valproic acid (5 mg/kg PO every other week). Except for temsirolimus, the other two drugs in this combination therapy were dosed well below their label indication including bevacizumab at 50% and valproic acid at 17% of the FDA approved doses (Table 1).
Table 1.
Dose-escalation schedule for bevacizumab/temsirolimus and valproic acid.
| Dose level | Dose and schedule (28-day cycle) | ||
|---|---|---|---|
| Temsirolimus* | Bevacizumab | Valproic acid | |
| Level -1 | 5 mg days 1, 8, 15, and 22 | 2.5 mg/kg day 1 and 15 | 5 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 0 | 5 mg days 1, 8, 15, and 22 | 5 mg/kg day 1 and 15 | 5 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 1 | 5 mg days 1, 8, 15, and 22 | 10 mg/kg day 1 and 15 | 5 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 2 | 12.5 mg days 1, 8, 15, and 22 | 2.5 mg/kg day 1 and 15 | 5 mg/kg rounded to nearest 250mg daily on days 1-7 and 15-21 |
| Level 3 | 12.5 mg days 1, 8, 15, and 22 | 7.5 mg/kg day 1 and 15 | 10 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 4 | 12.5 mg days 1, 8, 15, and 22 | 10 mg/kg day 1 and 15 | 10 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 5 | 20 mg days 1, 8, 15, and 22 | 2.5 mg/kg day 1 and 15 | 5 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 6 | 20 mg days 1, 8, 15, and 22 | 7.5 mg/kg day 1 and 15 | 10 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 7 | 20 mg days 1, 8, 15, and 22 | 10 mg/kg day 1 and 15 | 10 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 8 | 25 mg days 1, 8, 15, and 22 | 2.5 mg/kg day 1 and 15 | 5 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 9 | 25 mg days 1, 8, 15, and 22 | 5 mg/kg day 1 and 15 | 5 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
| Level 10 | 25 mg days 1, 8, 15, and 22 | 10 mg/kg day 1 and 15 | 10 mg/kg rounded to nearest 250 mg daily on days 1-7 and 15-21 |
In our study, objective responses were observed in three patients. The first patient had high-grade mucoepidermoid carcinoma of parotid with no actionable mutations and had confirmed PR (−62%) after 2 cycles of treatment with a 2.5-month duration of response. The second patient had endometrioid ovarian carcinoma with no actionable mutations and achieved confirmed PR (−50%) with a duration of response of 17 months. The third patient who achieved confirmed PR (−35%), had P16 positive vaginal squamous cell carcinoma with 8-month duration of response. In terms of prolonged stable disease (≥6 months), one patient with uveal melanoma with no actionable mutations had SD for 9 months. Another patient with PIK3CA and KRAS aberrated, hormone receptor positive (HR+), human epidermal growth factor receptor-2 (HER-2) negative breast cancer had stable disease for 11 months. A patient with PTEN and TP53 aberrated metastatic leiomyosarcoma had SD for 7 months while another patient with PTEN aberrated metastatic clear cell carcinoma of the kidney had SD for 8 months. The last patient had APC and NF1 aberrated metastatic thymoma had SD for 8 months.
In conclusion, the combination of bevacizumab, temsirolimus, and valproic acid showed modest clinical efficacy across an array of advanced sold tumors; however, numerous toxicities were reported, which would require careful monitoring and management during future clinical development.
| Trial Information | |
|---|---|
| Disease | All solid tumors |
| Stage of disease/treatment | Metastatic/advanced |
| Prior therapy | Allowed/no limit |
| Type of study | Phase I |
| Primary endpoint | safety and tolerability and to determine the maximum tolerated dose (MTD) of the combinational treatment of bevacizumab, and temsirolimus with valproic acid |
| Secondary endpoints | Anti-Tumor Efficacy |
| Investigator’s analysis | Level of activity did not meet planned endpoint |
Additional Details of Endpoints or Study Design
This was a phase I open-label, dose-escalation study that enrolled adult patients with advanced malignancies and was conducted between April 2012 and April 2018. The primary endpoint of this study was to access safety and tolerability and to determine the MTD of the combinational treatment of bevacizumab, and temsirolimus with valproic acid.
Patients were recruited and treated at the University of Texas, MD Anderson Cancer Center (MDACC). The study was approved by the Institutional Review Board (IRB) in accordance with the Declaration of Helsinki, Good Clinical Practice, and all federal, state and local regulatory guidelines. Consent was obtained from all patients prior to study enrollment. Each cycle was 28 days. Bevacizumab was administered at 2.5, 5, 7.5, or 10 mg/kg by IV infusion on days 1 and 15. Temsirolimus was given at 5, 12.5, 20, or 25 mg by IV infusion on days 1, 8, 15, and 22. Valproic acid was administered PO daily at a dose of either 5 mg/kg or 10 mg/kg rounded to nearest 250 mg on days 1-7 and 15-21. Re-staging scans were performed every 8 weeks to evaluate patient responses. During the study period, no other investigational, commercial agents or therapies were allowed with the intent to treat the patient’s malignancy.
This protocol utilized a standard 3 + 3 dose escalation design.1 Ten dose levels were explored between bevacizumab (IV) 10 mg/kg Q2W + temsirolimus (IV) 5 mg QW + valproic acid (PO) 5 mg/kg (days 1-7 and 15-21 of a 28-day cycle) and bevacizumab (IV) 10 mg/kg Q2W + temsirolimus 25 mg IV QW + valproic acid (PO) 10 mg/kg IV QD (days 1-7 and 15-21 of a 28-day cycle). Initially, three patients were enrolled to one dose cohort and were evaluated for toxicity. If one of these three patients experienced a dose-limiting toxicity (DLT) during the first cycle, three additional patients were enrolled and treated at the same cohort. If, at any time, more than 33% of patients in that cohort experienced DLT, the cohort was closed, and dose escalation stopped. In this study, adverse events were evaluated and graded per Common Terminology Criteria for Adverse Events v3.0 (CTCAE v3.0). A DLT event was defined as a clinically significant adverse event that occurred during the first cycle and was possibly, probably, or definitely related to any of the study medications; including any grade 3 or 4 non-hematologic toxicity (except nausea and vomiting responsive to appropriate regimens, correctable electrolyte imbalances, or alopecia); any grade 4 hematologic toxicity lasting > 14 days despite supportive care; any grade 4 nausea of vomiting > 4 days despite maximum anti-nausea regiment; any other grade 3 non-hematologic toxicity including symptoms/signs of vascular leak or cytokine release syndrome; any severe or life-threatening complication or abnormality not defined in CTCAE v3.0 that was attributable to the therapy.
Key inclusion criteria were, advanced or metastatic cancer that was refractory to standard treatment, relapsed after standard treatment or had no standard treatment available; Eastern Cooperative Oncology Group (ECOG) performance ≤ 2; absolute neutrophil count ≥ 1 × 109/L; platelet count ≥ 50 × 109/L; creatinine ≤ 3 × the upper limit of normal (ULN); total bilirubin ≤ 3.0 mg/dL; aspartate transaminase (AST) and alanine transaminase (ALT) ≤ 5 × ULN; fasting level of total cholesterol ≤ 350 mg/dL; triglyceride level ≤ 400 mg/dL. Key exclusion criteria were clinically significant unexplained bleeding with 28 days prior to study entry; uncontrolled systemic vascular hypertension (systolic blood pressure > 140 mmHg, diastolic pressure > 90 mmHg on medication); clinically significant cardiovascular disease; history of hypersensitivity to bevacizumab, temsirolimus, or valproic acid; major surgery within 6 weeks of the study enrollment; pregnancy. Patients who had prior treatments with bevacizumab, temsirolimus, and/or valproic acid were allowed to participate.
Computed tomography or magnetic resonance imaging scans were performed at baseline and every two cycles (8 weeks) thereafter. Tumor measurements were performed according to Response Evaluation Criteria In Solid Tumors (RECIST) v1.0 to evaluate measurable target lesions for response.2 Prolonged stable disease (SD) was defined as lasting ≥+ 6 months.
Genetic analysis was performed to analyze phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), phosphatase and tensin homolog (PTEN), rapidly accelerated fibrosarcoma (RAF), rat sarcoma virus (RAS), and tumor protein p53 (TP53) aberrations for patients who had archival tissue samples available. Deoxyribonucleic acid (DNA) was extracted and purified from formalin-fixed, paraffin-embedded archival tumor tissue or blood samples. Polymerase chain reaction (PCR)-based primer extension assay, or next generation sequencing (NGS)-based analysis were used to screen for genetic mutations and copy number variations in target genes. All tests were performed in Clinical Laboratory Improvement Amendment (CLIA)-certified molecular diagnostics laboratories at MD Anderson Center and Foundation Medicine.
| Drug information—Drug 1 | |
|---|---|
| Generic/working name | Bevacizumab |
| Company name | Genentech |
| Drug type | Antibody |
| Drug class | Anti-angiogenic; Anti-VEGF-A |
| Dose | 5 |
| Unit | mg/kg |
| Route | IV |
| Schedule of administration | day 1 and 15 every 28 days |
| Drug Information—Drug 2 | |
|---|---|
| Generic/working name | Temsirolimus |
| Company name | Wyeth Pharmaceuticals, Inc. |
| Drug type | Small molecular inhibitor |
| Drug class | specific inhibitor of mTOR |
| Dose | 5 |
| Unit | mg |
| Route | IV |
| Schedule of administration | days 1, 8, 15, and 22 every 28 days |
| Drug Information—Drug 3 | |
|---|---|
| Generic/working name | Valproic acid |
| Company name | Various |
| Drug type | organic weak acid |
| Drug class | Anti-epileptic |
| Dose | 5 |
| Unit | mg/kg |
| Route | PO |
| Schedule of administration | Daily on days 1-7 and 15-21 every 28 days |
| Patient Characteristics | |
|---|---|
| Number of patients, male | 22 |
| Number of patients, female | 25 |
| Stage | IV |
| Age: median (range) | 56 (21-78) years |
| Number of prior systemic therapies: median (range) | 4 (0-9) |
| Performance status: ECOG | 0: 2 1: 43 2: 2 3: 0 4: 0 |
| Cancer Types or Histologic Subtypes | Number |
|---|---|
| Adrenocortical | 1 |
| Bartholin’s gland adenoid cystic carcinoma | 1 |
| Breast | 4 |
| Cervix | 2 |
| Colorectal | 6 |
| Endometrial | 2 |
| Esophageal | 2 |
| Glioblastoma | 4 |
| Kidney | 1 |
| Lung | 1 |
| Melanoma | 2 |
| Salivary gland | 1 |
| Pituitary carcinoma | 1 |
| Parotid | 1 |
| Ovarian | 4 |
| Sarcoma | 5 |
| Squamous cell carcinoma head and neck | 6 |
| Squamous cell carcinoma of skin | 1 |
| Thymoma | 1 |
| Vaginal | 1 |
| Primary Assessment Method | |
|---|---|
| Title | Objective response rate |
| Number of patients screened | 69 |
| Number of patients enrolled | 47 |
| Number of patients evaluable for toxicity | 47 |
| Number of patients evaluated for efficacy | 38 |
| Evaluation method | RECIST 1.0 |
| Response assessment, CR | 0 (0%) |
| Response assessment, PR | 3 (7.9%) |
| Response assessment, SD | 23 (60%) |
| Response assessment, PD | 12 (31.5%) |
Outcome Notes
Patient Demographic and Clinical Characteristics
Between April 2012 and April 2018, 47 patients with advanced, metastatic tumors were enrolled onto this study. Median age for enrolled patients was 56 years (range 21-78) and the majority were female (53%). The most frequent tumor types enrolled were Head and Neck Squamous Cell Carcinoma (HNSCC; 7 patients, 14.9%) and colorectal (6 patients, 12.8%), followed by breast (4 patients, 8.5%), glioblastoma (4 patients, 8.5%), and ovarian (4 patients, 8.5%). Patients were heavily pretreated with 28 patients (59.6%) having received at least four prior lines of therapy. Five patients (10.6%) had received prior mTOR inhibitors (1 patient received temsirolimus, 2 patients received everolimus, and 2 patients received MLN0128. Twenty patients (42.6%) had received prior bevacizumab therapy, 4 patients had prior pazopanib exposure, 3 patients had aflibercept previously, and one patient each had prior regorafenib, sorafenib, and axitinib therapy. No patients had received prior valproic acid-based therapies, but 2 patients had treatment with vorinostat and KA-2507 (histone deacetylase, HDAC6 inhibitor), respectively.
The median number of treatment cycles (cycle = 28 days) was 2 (range, 1-22). Twenty-one patients (44.7%) received at least 3 cycles of treatment. All patients have discontinued the treatment. The primary reasons for discontinuation were disease progression (22 patients, 46.8%), clinical progression (11 patients, 23.4%), toxicities (7 patients, 14.8%) death unrelated to therapy (3 patients, 6.3%), withdrew consent (2 patients; %), lost to follow up (1 patient; 2.3%%), and holding therapy for quality of life (1 patient; 2.3%).
Toxicity Assessment
Standard 3 + 3 dose-escalation design was followed to enroll patients in this study up to dose level 10. Table 2 summarizes numbers of patients treated and DLT events observed in each dose level. A total of six patients experienced DLTs. At dose level 2, one patient experienced a G3 elevated lipase. At dose level 5, one patient experienced G3 mucositis. At dose level 6, one patient with metastatic SCC of the tongue with no bowel issues experienced a G3 bowel perforation secondary to therapy. At dose level 10, two out of six patients experienced DLT events: one patient experienced a G3 infection, and the other patient experienced multiple adverse events (AEs) resulting in death (eg, 3 dizziness, G3 neuropathy, G3 weakness, G3 gait abnormality and G4 cerebrovascular ischemia). Per protocol, dose escalation was stopped at dose level 10 and 4 additional patients were enrolled to dose level 9. One of these 4 additional patients enrolled at dose level 9 experienced one G3 rash DLT event.
Table 2.
Dose level (28-day cycle), toxicities (G3/4),* and response.
| Dose level | N | Temsirolimus IV on days 1, 8, 15, 22 | Bevacizumab IV on days 1, 15 | Valproic acid on days 1-7, and 15-21 | SD≥6 months or PR/total treated | Grade (G) 3/4 Toxicity (N) * |
| 1 | 3 | 5 mg | 10 mg/kg | 5 mg/kg rounded to nearest 250 mg | 0/3 | G3 Hypophosphatemia (1) G3 Thrombocytopenia (1) |
| 2 | 6 | 12.5 mg | 2.5 mg/kg | 5 mg/kg rounded to nearest 250 mg | 1/6 |
G3 Elevated Lipase (1)
^
G3 Mucositis (1) |
| 3 | 4 | 12.5 mg | 7.5 mg/kg | 10 mg/kg rounded to nearest 250 mg | 1/4 | |
| 4 | 3 | 12.5 mg | 10 mg/kg | 10 mg/kg rounded to nearest 250 mg | 0/3 | G3 Hypertriglyceridemia (1) |
| 5 | 6 | 20 mg | 2.5 mg/kg | 5 mg/kg rounded to nearest 250 mg | 1/6 |
G3 Mucositis (1)
^
G3 Thrombocytopenia (1) |
| 6 | 6 | 20 mg | 7.5 mg/kg | 10 mg/kg rounded to nearest 250 mg | 2/6 | G3 Anemia (1) G3 Bowel Perforation (1)^ G3 Mucositis (1) G3 Lymphopenia (2) G3 Elevated Lipase (1) G3 Thrombocytopenia (1) G3 Neutropenia (1) |
| 7 | 3 | 20 mg | 10 mg/kg | 10 mg/kg rounded to nearest 250 mg | 0/3 | G3 Thrombocytopenia (1) G3 Lymphopenia (1) |
| 8 | 3 | 25 mg | 2.5 mg/kg | 5 mg/kg rounded to nearest 250 mg | 0/3 | G3 Lymphopenia (1) G3 Hypokalemia (1) G4 Lymphopenia (1) |
| 9 | 7 | 25 mg | 5 mg/kg | 5 mg/kg rounded to nearest 250 mg | 2/7 | G3 Lymphopenia (2) G3 Neutropenia (1) G3 Hypertriglyceridemia (1) G3 Rash (1)^ G3 Perirectal Abscess (1) G3 Pericoronitis (1) |
| 10 | 6 | 25 mg | 10 mg/kg | 10 mg/kg rounded to nearest 250 mg | 1/6 | G3 Hypophosphatemia (1) G3 Lymphopenia (1) G3 Intestinal Obstruction (1) G3 Bowel Perforation (1) G3 Hyperglycemia (1) G3 Infection (1)^ G3 Dizziness (1)^ G3 Neuropathy (1)^ G3 Weakness (1)^ G3 Gait-walking (1)^ G4 CNS Cerebrovascular Ischemia (1)^ |
*Adverse events deemed at least possibly related to treatment, graded based on Common Terminology Criteria for Adverse Events, version 3.0 (CTCAEv3.0).
^A dose-limiting toxicity.
Abbreviation: N, number of patients experiencing toxicity.
Forty-five patients (95.7%) experienced one or more adverse events that were at least possibly related to the treatment. Most AEs were grades 1 and 2 and were reversible. Table 3 summarizes treatment-related AEs (TRAEs) at all dose levels. The most common grades 1 and 2 TRAEs reported in more than 20% of patients were hypercholesterolemia (57.4%), hypertriglyceridemia (53.2%), thrombocytopenia (53.2%), hyperglycemia (38.3%), mucositis (38.3%), anorexia (36.2%), elevated AST (34%), leukopenia (34%), anemia (31.9), fatigue (29.8%), nausea (29.8%), rash (29.8%), headache (27.7%), diarrhea (23.4%), and proteinuria (23.4%). Grade 3 TRAEs included lymphopenia (14.9%, DL6, 7, 9, 10), thrombocytopenia (8.5%; DL1, 5, 6, 7), mucositis (6.4%, DL2, 5, 6), hypophosphatemia (4.3%; DL1 and 10), elevated lipase (4.3%, DL 2 and 6), hypertriglyceridemia (.3%, DL4 and 9), bowel perforation (4.3%, DL6 and 10), neutropenia (4.3%, DL6 and 9), anemia (2.1%, DL6), hypokalemia (2.1%, DL8), rash (2.1%, DL9), perirectal abscess (2.1%s, DL9), pericoronitis (2.1%, DL9), intestinal obstruction (2.1%, DL10), hyperglycemia (2.1%, DL10), infection (2.1%, DL10), dizziness (2.1%, DL10), neuropathy (2.1%, DL10), weakness (2.1%, DL10), and gait abnormality 2.1%, DL10). Grade 4 treatment-related AEs include lymphopenia (2.1%, DL8), and cerebrovascular ischemia (2.1%, DL10). At dose level 10, one patient diagnosed with GBM experienced 5 DLT events during the first cycle of therapy (G3 dizziness, G3 neuropathy, G3 weakness, G3 gait-walking and G4 CNS cerebrovascular ischemia). She did not finish the first cycle and was taken off study due to toxicities.
Table 3.
Adverse events at all dose levels.
| Adverse event of all grades | Dose level, temsirolimus (mg) and bevacizumab (mg/kg) and valproic acid (mg/kg) |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose level | 1, (5/10/5) (n = 3) |
2, (12.5/2.5/5) (n = 6) |
3, (12.5/7.5/10) (n = 4) |
4, (12.5/10/10) (n = 3) |
5, (20/2.5/5) (n = 6) |
6, (20/7.5/10) (n = 6) |
7, (20/10/10) (n = 3) |
8, (25/2.5/5) (n = 3) |
9, (25/5/5) (n = 7) |
10, (25/10/10) (n = 6) |
Total (n = 47) |
|||||||||||
| Grade | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 |
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 |
| Acne | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Anemia | 1 | 0 | 3 | 0 | 3 | 0 | 1 | 0 | 3 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 15 | 1 |
| Anorexia | 0 | 0 | 4 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 17 | 0 |
| Basophilia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Bilateral lower extremity edema | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Bleeding (vaginal) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Bowel perforation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Chest pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Cough | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| CNS cerebrovascular ischemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Confusion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 |
| Constipation | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| Cognitive disturbance | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Decreased PT/INR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Dermatitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| Diarrhea | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 11 | 0 |
| Diffuse gallbladder wall edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Dizziness | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 1 |
| Dry eye | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Dysgeusia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 |
| Dyspnea | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 6 | 0 |
| Dysuria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 0 |
| Elevated ALT | 1 | 0 | 4 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 9 | 0 |
| Elevated ALP | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 8 | 0 |
| Elevated amylase | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 0 | |
| Elevated AST | 3 | 0 | 3 | 0 | 3 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 16 | 0 |
| Elevated bilirubin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Elevated BUN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 0 |
| Elevated Creatinine | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 8 | 0 |
| Elevated D-dimer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Elevated LDH | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 9 | 0 |
| Elevated Lipase | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 4 | 2 |
| Epistaxis | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 8 | 0 |
| Facial numbness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Fall | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Fatigue | 0 | 0 | 4 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 4 | 0 | 14 | 0 |
| Fever | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Fistula | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Gait-walking | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Gallstone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Glycosuria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 |
| Hand and foot syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Headache | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 13 | 0 |
| Hematuria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 |
| Hemoglobinuria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Hypercalcemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Hypercholesterolemia | 3 | 0 | 4 | 0 | 3 | 0 | 2 | 0 | 3 | 0 | 4 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 4 | 0 | 27 | 0 |
| Hyperglycemia | 2 | 0 | 4 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 3 | 1 | 18 | 1 |
| Hyperkalemia | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| Hypertension | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 6 | 0 | |
| Hypernatremia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 5 | 0 | |
| Hypertriglyceridemia | 3 | 0 | 5 | 0 | 2 | 0 | 2 | 1 | 2 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 3 | 0 | 25 | 2 |
| Hyperphosphatemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Hypoalbuminemia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 | 0 |
| Hypocalcemia | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| Hypokalemia | 1 | 0 | 2 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 8 | 1 |
| Hypomagnesemia | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| Hyponatremia | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 5 | 0 |
| Hypophosphatemia | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 |
| Hypothermia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Indigestion | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 |
| Insomnia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 5 | 0 |
| Intestinal obstruction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Jaw pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Ketonuria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 7 | 0 |
| Leukopenia | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 16 | 0 |
| Lip infection (fever blister) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Lymphopenia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 1 | 2 | 2 | 0 | 2 | 4 | 1 | 9 | 8 |
| Malaise | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Mucositis | 0 | 0 | 1 | 1 | 1 | 0 | 3 | 0 | 3 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | 3 | 0 | 3 | 0 | 18 | 3 |
| Myositis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Myalgia | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 6 | 0 |
| Nausea | 1 | 0 | 3 | 0 | 4 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 14 | 0 |
| Neuropathy | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 8 | 1 |
| Neutropenia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 2 | 0 | 2 | 1 | 0 | 0 | 8 | 2 |
| Neutrophilia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 0 |
| Pericoronitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| Perirectal abscess | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Pneumonitis | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Positive urine bacteria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Positive urine bilirubin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 |
| Positive urine casts | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Positive urine glucose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 |
| Positive urine leuk. est. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 3 | 0 |
| Positive urine mucous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 |
| Positive urine RBCs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Positive urine uric acid crystals | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Positive urine WBCs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 |
| Positive urobilinogen | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Presyncope | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Proteinuria | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 11 | 0 |
| Pruritic scalp rash | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Pruritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Radiation recall wound | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Rash | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 1 | 1 | 0 | 14 | 1 |
| Sinusitis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Tachycardia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Thinning of nails | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Thrombocytopenia | 0 | 1 | 3 | 0 | 3 | 0 | 0 | 0 | 4 | 1 | 5 | 1 | 2 | 1 | 3 | 0 | 2 | 0 | 3 | 0 | 25 | 4 |
| Upper back pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| UTI | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Vision flashing lights, floater | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Vomiting | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 9 | 0 |
| Wound (right foot) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Weakness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 1 |
| Weight loss | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 0 |
| Xerostomia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Antitumor Activity
All 47 patients had disease that was measurable per RECIST v1.0 on baseline scans. However, 9 patients were taken off the study before completing cycle 2 and did not reach restaging assessment. Three patients developed clinical progression before reaching the first restaging scans, one patient withdrew consent, and another was lost to follow up. Four patients came off secondary to toxicity related to therapy (one patient each with G2 pneumonitis, G3 bowel micro-perforation, G3 perineal skin and soft tissue infection, and G4 cerebrovascular ischemia and gait abnormality). For the 38 patients who had at least one post-baseline restaging scan, the best RECIST response for each patient is depicted in Figure 1. In this waterfall plot, 7 patients were assigned a value of +21% for clinical progression or new lesions upon restaging. objective response rate (ORR) was 7.9% as confirmed partial response (PR) was observed in 3 patients. Stable disease (SD) ≥+ 6 months were observed in 5 patients (13.1%). Clinical Benefit Rate (CBR: CR+ PR + SD ≥+ 6 months) was 21% (8/38). Table 4 provides detailed information of patients with PR or SD ≥+ 6 months.
Figure 1.
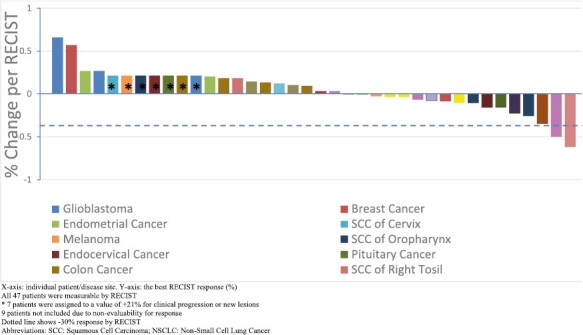
Waterfall plot depicting best RECIST response by patient. X-axis: individual patient/disease site. Y-axis: the best RECIST response (%). All 47 patients were measurable by RECIST * 7 patients were assigned to a value of +21% for clinical progression or new lesions. 9 patients not included due to non-evaluability for response dotted line shows −30% response by RECIST. Abbreviations: SCC: squamous cell carcinoma; NSCLC: non-small cell lung cancer.
Table 4.
Stable disease ≥ + 6 months or partial response (PR) by RECIST and characterization by patient.
| Patient ID | Cancer type | Dose level | Best response by RECIST v1.0 | Number of prior systemic regimens | Duration of treatment (weeks) | PTEN mut | PIK3CA mut | RAS mut | RAF mut | P53 mut |
| 41 | Ocular melanoma | 2 | SD (−3%) | 4 | 36 | ND | N | ND | ND | ND |
| 50 | HR+, HER2- Breast | 3 | SD (−9%) | 9 | 43 | N | E542K | KRAS G12S | N | N |
| 73 | Mucoepidermoid Parotid Cancer | 5 | PR (--62%) | 1 | 19 | N | N | N | N | N |
| 101 | Endometroid ovarian carcinoma | 6 | PR (−50%) | 6 | 97 | N | N | N | N | N |
| 107 | Leiomyosarcoma | 6 | SD (−4%) | 7 | 29 | deletion | N | N | N | V147fs*23 |
| 120 | Vaginal Squamous Cell carcinoma | 9 | PR (−35%) | 8 | 50 | N | E545K, I391M | N | N | N |
| 138 | Clear cell carcinoma of kidney | 9 | SD (−16%) | 5 | 31 | I150fs*4 | N | N | N | N |
| 127 | Thymoma | 10 | SD (−16%) | 6 | 31 | N | N | N | N | N |
Abbreviations: mut, mutation; ND, not done; N, no; HR, hormone receptor; HER-2, human epidermal growth factor receptor 2.
One patient with high grade mucoepidermoid carcinoma of parotid, treated at dose level 5 achieved PR (-62%). This patient had a 2.5-month duration of response. A second patient with endometrioid ovarian carcinoma, treated at dose level achieved PR (-50%) with a duration of response of 17 months. The third patient who achieved PR (-35%) had P16 positive vaginal squamous cell carcinoma and was treated at dose level 9. She had a duration of response of 8 months. This patient had PIK3CA E545K and I391M mutations.
The six patients who had SD ≥+ 6 months included one with ocular melanoma treated at dose level 2, one with breast cancer treated at dose level 3 who had PIK3CA E452K and KRAS G12S mutations, one with leiomyosarcoma treated at dose level 6 who had PTEN deletion, and TP53 V147fs*23 mutations, one with clear cell kidney cancer treated at dose level 9 who had PTEN I150fs*4 mutation, and one patient with thymoma treated at dose level 10. Please see Table 4 for additional details.
Molecular Analysis and Association with Response
For patients who had archival tissue samples available, genetic testing was performed to analyze PIK3CA, PTEN, RAF, RAS, and TP53 mutations. Table 5 is a summary of genetic analysis of patients tested with detailed molecular aberration, cancer type, and best response information.
Table 5.
Tumor molecular analysis.
| Gene | N/total tested (%) | Molecular aberration | Patient ID | Cancer rype | Best response comments |
|---|---|---|---|---|---|
| PIK3CA | 43/47 | E542K | 50 | Breast | 9% decrease |
| E542K | 114 | Endocervical | 3% increase with new lesion | ||
| E545K | 66 | Breast | NE, off study for toxicities after completing cycle 1 | ||
| E545K | 111 | Esophageal | 10% increase | ||
| E545K, I391M | 120 | Vaginal SCC | 35% decrease | ||
| I391M | 95 | Leiomyosarcoma | NE, patient withdrew consent before restaging | ||
| I391M | 131 | Colon | 9% increase | ||
| M1043T | 96 | Endometrial | 27% increase | ||
| H1047R | 104 | SCC of tongue | NE, patient did not finish cycle 1, patient was taken off study for G3 bowel perforation, | ||
| K111_I112delinsN | 122 | Colon | Clinical progression | ||
| PTEN | 40/47 | Q110* | 95 | Leiomyosarcoma | NE, patient withdrew consent before restaging |
| Deletion | 99 | SCC of tonsil | 18% increase | ||
| Deletion | 107 | Leiomyosarcoma | 4% decrease | ||
| L325P | 108 | GBM | 27% increase | ||
| R335* | 112 | GBM | 66% increase | ||
| Deletion | 124 | Leiomyosarcoma | 4% decrease | ||
| I50fs*4 | 138 | Kidney | 16% decrease | ||
| K267N | 139 | GBM | Clinical progression | ||
| S338fs*6 | 131 | Colon | 9% increase | ||
| Rearrangement intron 4 | 132 | SCC of oropharynx | 26% decrease | ||
| splice site 254-2A>G | 133 | Colon | NE, patient was taken off study due to clinical progression after 1 cycle of treatment | ||
| R130* | 134 | GBM | NE, patient was taken off study due to toxicities, patient did not finish cycle 1 treatment | ||
| HRAS | 39/47 | G13R | 113 | SCC of Oropharynx | 13% increase with new lesion |
| KRAS | 43/47 | G13D | 7 | Colon | 13% increase |
| KRAS AMP | 14 | Breast | 1% decrease | ||
| G12V | 34 | Colon | 18% increase | ||
| G12V | 131 | Colon | 9% increase | ||
| G12V | 133 | Colon | NE, patient was taken off study due to clinical progression after 1 cycle of treatment | ||
| G12S | 50 | Breast | 9% decrease | ||
| Q61L | 52 | Colon | NE, patient was taken off study due to clinical progression, patient did not finish cycle 1 treatment | ||
| G12D | 111 | Esophageal | 10% increase | ||
| G12S | 122 | Colon | Clinical progression | ||
| NRAS | 41/47 | Q61R | 64 | Melanoma | 7% increase with new lesion |
| G61K | 113 | SCC of Oropharynx | 13% increase with new lesion | ||
| TP53 | 40/47 | R175H | 14 | Breast | 1% decrease |
| R175H | 96 | Endometrial | 27% increase | ||
| R248W | 33 | SCC of Larynx | 23% decrease | ||
| F270C | 34 | Colon | 18% increase | ||
| R196* | 66 | Breast | NE, off study for toxicities after completing cycle 1 | ||
| G245D | 67 | Ovarian | 7% decrease | ||
| E11Q, R342* | 72 | SCC of Esophagus | 14% increase | ||
| E258K | 104 | SCC of tongue | NE, patient did not finish cycle 1, patient was taken off study for G3 bowel perforation, | ||
| V147fs*23 | 107 | Leiomyosarcoma | 4% decrease | ||
| L111P | 109 | Ovarian | 3% increase | ||
| R240W | 112 | GBM | 66% increase | ||
| H179Y | 116 | SCC of skin | 11% decrease | ||
| R248W | 122 | Colon | Clinical progression | ||
| S121fs*11 | 147 | Ovarian | NE, patient was taken off study due to newly discovered brain metastases, patient did not finish cycle 1 treatment | ||
| I232_N235del | 133 | Colon | NE, patient was taken off study due to clinical progression after 1 cycle of treatment | ||
| V274fs | 151 | Endometrium | 20% increase | ||
| R248Q | 131 | Colon | 9% increase |
Abbreviations: NE, no response evaluation; GBM, Glioblastoma Multiforme; SCC, squamous cell carcinoma; AMP, amplification.
Forty-three patients were tested for PIK3CA aberrations, and 10 patients were positive (10/43, 23.3%). The most commonly detected PIK3CA mutations were E545K (3/43, 7%) and I391M (3/43, 7%). Of the 3 patients detected with PIK3CA E545K mutation, one with vaginal cancer (#120, also had PIK3CA I391M mutation) had PR with 35% decrease and was treated for a total of 11 cycles. One patient with esophageal cancer (#111, also had KRAS G12D mutation) had SD for 6 cycles. One patient with breast cancer (#66, also had TP53 R196* mutation) was taken off study due to toxicities after 1 cycle of treatment.
Forty patients were tested for PTEN gene aberrations and 12 patients had molecular alterations (12/40, 30%) of which PTEN deletion (3/12, 25%) was the predominant alteration. For 3 patients with PTEN deletion, one patient with HNSCC (#99) was treated for 3 cycles with stable disease before withdrawing consent. One leiomyosarcoma patient (#107, also had TP53 V147fs*23 mutation) had SD for 7 cycles of treatment. Another leiomyosarcoma patient (#124) had SD for 4 cycles of treatment.
KRAS testing was performed on tumor samples from 43 patients, and 9 were positive for a KRAS aberration. The most commonly detected KRAS mutation was G12V (3/9, 33%) and G12S (2/9, 22.2%). All three patients with KRAS G12V mutated tumor had colorectal cancer. One patient (#34) had SD for 4 cycles of treatment. One patient (#131, also had concurrent PIK3CA I391M, PTEN S338fs*6, and TP53 R248Q mutations) had SD for 4 cycles of treatment. One patient (#133, also had PTEN splice site 254-2A>G and TP53 I232_N235del mutations) had clinical progression before finishing cycle 1 treatment. Of the two patients with KRAS G12S mutation, one breast cancer patient (#50, with concurrent PIK3CA E542K mutation) had SD > + 6 months. One patient with colorectal cancer (#122, also had TP53 R248W mutation) had clinical progression before finishing cycle 1 treatment. HRAS mutation was tested in 39 patients and 1 patient was positive (1/39, 2.6%). NRAS mutation was tested in 41 patients and 2 patients were positive (2/41, 4.9%).
| Assessment, Analysis, and Discussion | |
|---|---|
| Completion | Study completed |
| Investigator’s Assessment | Level of activity did not meet planned endpoint |
In this study, the most common non-hematologic adverse events (observed in ≥ 20% of patients) were anorexia, diarrhea, elevated AST, fatigue, headache, hypercholesterolemia, hyperglycemia, hypertriglyceridemia, mucositis, nausea, proteinuria, and cutaneous rash. Hyperglycemia and hyperlipidemia have been reported as common adverse events after temsirolimus treatment, affecting 17-26% and 6-27% treated patients, respectively.3 In our study, hyperglycemia, hypercholesterolemia and hypertriglyceridemia were noted in 67%, 57% and 53% of patients, respectively. Dermatitis occurs in 47-75% of patients treated with temsirolimus in prior studies.4 We observed dermatitis in 37% of our patients, which is lower than the reported incidence for single agent temsirolimus. Although, a phase II temsirolimus trial reported fatigue in 71% of their patients, in our study, fatigue was observed in only 30% of patients. Mucositis was also observed at a lower incidence (38%) compared to the 46% incidence rate reported in a phase 3 trial of single agent temsirolimus in renal cell carcinoma.5 Proteinuria has been reported to occur in 32% of patients with ovarian cancer treated with single agent bevacizumab.6 In our study, proteinuria was observed at a lower rate in our patients at 23% likely secondary to the lower dose of bevacizumab at 50% of FDA approved dosing.
The most common hematologic toxicities were leukopenia and neutropenia which occurred in 26 (55%) patients however most of these AEs were of lower grade (G1/G2). Only two patients had G3/G4 neutropenia which was reversible with dose hold. We also observed thrombocytopenia, lymphopenia, and anemia in 25 (53%), 17 (36%) and 16 (34%) patients, respectively. In another trial examining combination therapy with temsirolimus, bevacizumab and cetuximab, the incidence of thrombocytopenia was 24% which was lower than observed in our study. This is likely attributable to the addition of valproic acid to bevacizumab and temsirolimus as valproic acid has an incidence of thrombocytopenia between 1%-27%.7 No patients developed thromboembolic complications.
Although there are no clinical data on the combination of bevacizumab, temsirolimus and valproic acid, preclinical studies have supported the rationale for combining these drugs. Brugarulos et al. found that Tuberous Sclerosis Complex-2 (TSC2) tumor suppressor protein regulates VEGF through mTOR-dependent and independent pathways.8 TSC2 loss leads to increase in HIF-1α and upregulates HIF-responsive genes like VEGF. The authors were able to demonstrate that rapamycin homogenized HIF levels in TSC2−/− cells, indicating that TSC2 regulates HIF by inhibiting mTOR. VEGF overproduction by TSC2−/− cells was suppressed by the HDAC inhibitor agent, Trichostatin A indicating its anti-VEGF properties. This study demonstrated that a synergistic interplay and crosstalk occurred in inactivated TSC2 between rapamycin and trichostatin regulating the mTOR, HIF, VEGF and HDAC pathways.8 However, it should be noted that there are emerging studies that demonstrated AKT/mTOR activation in tumor endothelial cells can contribute to antiangiogenic resistance by increasing the activity of mitogen-activated protein kinase (MAPK) and expression of the serine/threonine-protein kinase PIM-1which counteracts the anti-angiogenic efficacy of mTOR inhibitors.9-11 Further characterization of the complex interplay between the AKT/mTOR and VEGF pathway is needed.
Clinically, the safety of bevacizumab and everolimus in combination has been demonstrated in prior clinical trials with anti-tumor activity in refractory colorectal cancer, melanoma and renal cell carcinoma.12-14 Strickler et al. performed a phase I study of bevacizumab, everolimus, and panobinostat (LBH-589) in advanced solid tumors where patients received 10 mg of panobinostat three times weekly, 5 or 10 mg of everolimus daily, and bevacizumab at 10 mg/kg every 2 weeks.15 However, the combination regimen did not have an acceptable safety and tolerability profile with 2 DLTs in DL1 (Grade 3 oral mucositis; G2 esophagitis) and 2 DLTs in DL-1 (G2 ventricular arrythmias; G2 refractory skin rash). Regarding responses seen in this study, one patient (1/9, 11%) with advanced breast cancer had PR for 2 months. In contrast, in our study, we were able to establish an MTD and objective responses were observed in three patients.
Acknowledgments
We would like to thank all patients and families and staff who participated in this study. This is an investigator-initiated study with drugs obtained from commercial supply
Contributor Information
Blessie Elizabeth Nelson, Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Apostolia M Tsimberidou, Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Xueyao Fu, Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Siqing Fu, Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Vivek Subbiah, Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Anil K Sood, Department of Gynecologic Oncology and Reproductive Medicine, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Jordi Rodon, Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Daniel D Karp, Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
George Blumenschein, Department of Thoracic and Head and Neck Medical Oncology, University of Texas, MD Anderson Cancer Center, Houston, TX, USA.
Scott Kopetz, Department of Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Shubham Pant, Department of Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Sarina A Piha-Paul, Department of Investigational Cancer Therapeutics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Funding
NCI/NIH; P30CA016672 – Core Grant (CCSG Shared Resources)
Ethics Approval
The authors obtained MD Anderson Cancer Center Institutional Review Board approval and have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. For investigations involving human subjects, informed consent has been obtained from the participants involved.
Conflict of Interest
Apostolia M. Tsimberidou reports clinical trial research funding (received through the institution) from OBI Pharma USA Inc., IMMATICS, Parker Institute for Cancer Immunotherapy, Agenus, Tempus, Tvardi, Boston Biomedical, and Karus Therapeutics; and consulting or advisory role with Vincerx, Diaccurate, BrYet, Nex-I, Macrogenics, and BioEclipse. Siqing Fu reports research funding from AstraZeneca, Abbisko, Anaeropharma Science, Arrien Pharmaceuticals, BeiGene, BioAtla, LLC, Boehringer Ingelheim, Eli Lilly & Co., Hookipa Biotech, Huya Bioscience International, IMV, Inc., Innovent Biologics, Co., Ltd., Lyvgen Biopharm, Co., Ltd., MacroGenics, Medivir AB, Millennium Pharmaceuticals, Inc., Nerviano Medical Sciences, NeuPharma, Inc., Novartis, OncoMed Pharmaceuticals, Parexel International, LLC, Sellas Life Sciences Group, Soricimed Biopharma, Inc., Tolero Pharmaceuticals, NovoCure, Turnstone Biologics, Taiho Oncology, and Abbisko. Vivek Subbiah reports grants from Eli Lilly/LOXO Oncology, Blueprint Medicines Corporation, Turning Point Therapeutics, Boston Pharmaceuticals, and Helsinn Pharmaceuticals; grant and service in an advisory board/consultant position for Eli Lilly/Loxo Oncology; research grants from Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP, the University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, Pharmamar, and Medimmune; served on an advisory board and/or as a consultant for Helsinn, Incyte, QED Pharma, Daiichi-Sankyo, Signant Health, Novartis, Relay Therapeutics, Roche, and Medimmune; has received travel funds from Pharmamar, Incyte, ASCO, and ESMO; and has received other support from Medscape. Anil K. Sood reports consulting roles for Merck, GSK, ImmunoGen, Onxeo, Iylon, Kiyatec; he is also a share holder for BioPath and holds IP for EGFL6 antibody licensed to Top Alliance. Jordi Rodon has received personal fees from Novartis, Eli Lilly, Orion Pharmaceuticals, Servier Pharmaceuticals, Peptomyc, Merck Sharp & Dohme, Kelun Pharmaceuticals/Klus Pharma, Spectrum Pharmaceuticals, Pfizer, Roche Pharmaceuticals, Ellipses Pharma, Certera, Bayer, Molecular Partners, NovellusDX, and IONCTURA SA; has received grants from Bayer, Novartis, Blueprint Pharmaceuticals, Spectrum Pharmaceuticals, Tocagen, Symphogen, BioAlta, Pfizer, GenMab, CytomX, Kelun-Biotech, Takeda-Millenium, GSK, and Ipsen; has received travel reimbursement from ESMO, the Department of Defense, Merck Sharp & Dohme, Louisiana State University, Kelun Pharmaceuticals/Klus Pharma, Huntsman Cancer Institute, Cancer Core Europe, Karolinska Cancer Institute, King Abdullah International Medical Research Center, Bayer, WIN Consortium, Jansen, and Molecular Partners; and has received other compensation from the European Journal of Cancer, VHIO/Ministero De Empleo Y Seguridad Social, Chinese University of Hong Kong, SOLTI, Elsevier, and GSK. Daniel D. Karp has received research funding through MD Anderson from Phosplatin Therapeutics, Pfizer, Arcus, Arqule, Bristol Myers Squibb, Eli Lilly, Five Prime Therapeutics, GSK, Genmab, Holy Stone Healthcare, Ipsen, Mirati Therapeutics, Novartis, Onco Response, Red Hill Biopharma, Rgenix, Sanofi-Aventis, Xencor, Astellas, Janssen, Affigen, Black Beret Life Sciences, and an NIH Clinical Translational Science Award. George Blumenschein receives personal fees and research funding from Amgen, Bayer, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Genentech, MedImmune, Merck, Roche, and Xcovery; research funding from Adaptimmune, Exelixis, GlaxoSmithKline, Immatics, Immunocore, Incyte, Kite Pharma, Macrogenics, Torque, AstraZeneca, Tmunity, Regeneron, Beigene, Novartis, and Repertoire Immune Medicines; and personal fees from Abbvie, Adicet, Amgen, Araid, Clovis Oncology, AstraZeneca, Bristol Myer Squibb, Celgene, Genentech, Gilead, Merck, Novartis, Roche, Virogin Biotech, Johnson & Johnson/Janssen, and Maverick Therapeutics. Scott Kopetz. has received consulting or advisory fees from Genentech, EMD Serono, Merck, Holy Stone, Novartis, Lilly, Boehringer Ingelheim, Boston Biomedical, AstraZeneca/MedImmune, Bayer Health, Pierre Fabre, Redx Pharma, Ipsen, Daiichi Sankyo, Natera, HalioDx, Lutris, Jacobio, Pfizer, Repare Therapeutics, Inivata, GlaxoSmithKline, Jazz Pharmaceuticals, Iylon, Xilis, Abbvie, Amal Therapeutics, Gilead Sciences, Mirati Therapeutics, Flame Biosciences, Servier, Carina Biotechnology, Bicara Therapeutics, Endeavor BioMedicines, Numab Pharma, and Johnson & Johnson/Janssen. Shubham Pant declares clinical trials research funding (paid to institution) from Arcus, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Holy Stone Healthcare Co., Tyme, Ipsen, Mirati Therapeutics, Inc., Novartis, Xencor, Astellas, and Janssen; and advisory board/consultant for 4D Pharma, Xencor, Ipsen, Zymeworks, Amal Therapeutics, and Novartis. Sarina A. Piha-Paul has received research and grant funding through MD Anderson from AbbVie, ABM Therapeutics, Acepodia, Alkermes, Aminex Therapeutics, Amphivena Therapeutics, BioMarin Pharmaceutical, Boehringer Ingelheim, Bristol Myers Squibb, Cerulean Pharma, Chugai Pharmaceutical, Curis, Daiichi Sankyo, Eli Lilly, ENB Therapeutics, Five Prime Therapeutics, Gene Quantum. Genmab A/S, GSK, Helix BioPharma, Incyte, Jacobio Pharmaceuticals, Medimmune, Medivation, Merck Sharp & Dohme, Novartis Pharmaceuticals, Pieris Pharmaceuticals, Pfizer, Principia Biopharma, Puma Biotechnology, Rapt Therapeutics, Seattle Genetics, Silverback Therapeutics, Taiho Oncology, Tesaro, TransThera Bio, and the NCI/NIH under award number P30CA016672.
Blessie Elizabeth Nelson and Xueyao Fu indicated no financial relationships.
Data Availability
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
- 1. Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45(3):925-937. [PubMed] [Google Scholar]
- 2. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 3. Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of cci-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22(5):909-918. 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 4. Raymond E, Alexandre J, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of cci-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22(12):2336-2347. 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 5. Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271-2281. 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 6. Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI.. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a gynecologic oncology group study. J Clin Oncol. 2007;25(33):5165-5171. 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 7. Liu X, Kambrick S, Fu S, et al. Advanced malignancies treated with a combination of the VEGF inhibitor bevacizumab, anti-EGFR antibody cetuximab, and the mTOR inhibitor temsirolimus. Oncotarget 2016;7(17):23227-23238. 10.18632/oncotarget.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG.. Tsc2 regulates VEGF through mTOR -dependent and -independent pathways. Cancer Cell 2003;4(2):147-158. 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 9. Dormond-Meuwly A, Roulin D, Dufour M, et al. The inhibition of MAPK potentiates the anti-angiogenic efficacy of mTOR inhibitors. Biochem Biophys Res Commun. 2011;407(4):714-719. 10.1016/j.bbrc.2011.03.086. [DOI] [PubMed] [Google Scholar]
- 10. Walpen T, Kalus I, Schwaller J, et al. Nuclear pim1 confers resistance to rapamycin-impaired endothelial proliferation. Biochem Biophys Res Commun. 2012;429(1-2):24-30. 10.1016/j.bbrc.2012.10.106. [DOI] [PubMed] [Google Scholar]
- 11. Faes S, Santoro T, Demartines N, et al. Evolving significance and future relevance of anti-angiogenic activity of mTOR inhibitors in cancer therapy. Cancers (Basel) 2017;9(11):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hainsworth JD, Infante JR, Spigel DR, et al. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma. Cancer. 2010;116(17):4122-4129. 10.1002/cncr.25320. [DOI] [PubMed] [Google Scholar]
- 13. Altomare I, Bendell JC, Bullock KE, et al. A phase II trial of bevacizumab plus everolimus for patients with refractory metastatic colorectal cancer. Oncologist 2011;16(8):1131-1137. 10.1634/theoncologist.2011-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hainsworth JD, Spigel DR, Burris HA III, et al. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol. 2010;28(13):2131-2136. [DOI] [PubMed] [Google Scholar]
- 15. Strickler JH, Starodub AN, Jia J, et al. Phase I study of bevacizumab, everolimus, and panobinostat (lbh-589) in advanced solid tumors. Cancer Chemother Pharmacol. 2012;70(2):251-258. 10.1007/s00280-012-1911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.


