Abstract
Background
Scant data describe exocrine pancreatic insufficiency (EPI) secondary to immune checkpoint inhibitor (ICI) use. The goal of this study is to describe the incidence, risk factors, and clinical characteristics of patients with ICI-related EPI.
Patients and Methods
A single center, retrospective case-control study was performed of all ICI-treated patients at Memorial Sloan Kettering Cancer Center between January 2011 and July 2020. ICI-related EPI patients had steatorrhea with or without abdominal discomfort or weight loss, started pancrelipase after initiation of ICI, and demonstrated symptomatic improvement with pancrelipase. Controls were matched 2:1 by age, race, sex, cancer type, and year of ICI start.
Results
Of 12 905 ICI-treated patients, 23 patients developed ICI-related EPI and were matched to 46 controls. The incidence rate of EPI was 1.18 cases per 1000 person-years and the median onset of EPI was 390 days after the first dose of ICI. All 23 (100%) EPI cases had steatorrhea that improved with pancrelipase, 12 (52.2%) had weight loss, and 9 (39.1%) had abdominal discomfort; none had changes of chronic pancreatitis on imaging. Nine (39%) EPI patients had episodes of clinical acute pancreatitis preceding the onset of EPI, compared to 1 (2%) control (OR 18.0 (2.5-789.0), P < .001). Finally, the EPI group exhibited higher proportions of new or worsening hyperglycemia after ICI exposure compared with the control group (9 (39.1%) vs. 3 (6.5%), P < .01).
Conclusion
ICI-related EPI is a rare but clinically significant event that should be considered in patients with late onset diarrhea after ICI treatment and often is associated with development of hyperglycemia and diabetes.
Keywords: checkpoint inhibitor, exocrine pancreatic insufficiency, clinical pancreatitis, diabetes
This article describes the incidence, risk factors for, and clinical characteristics of patients with immune checkpoint inhibitor-related exocrine pancreatic insufficiency.
Implications for Practice.
With the increased use of immune checkpoint inhibitors (ICI), rare ICI-associated pancreatic adverse events are being recognized. Here, we describe the clinical characteristics and risk factors for exocrine pancreatic insufficiency (EPI). The onset of EPI was 390 days from ICI initiation. ICI-induced EPI had an incidence rate of 1.18 cases per 1000 person-years. Clinical pancreatitis increases the risk of developing EPI by 18-fold. Compared to the controls, more patients with EPI developed new diabetes or had decompensation of their existing diabetes. EPI should be considered as a potential etiology of diarrhea late in the patient’s clinical course of ICI.
Background
Immune checkpoint inhibitors (ICIs), including cytotoxic T-cell lymphocyte-4 (CTLA-4) and programmed death-1 (PD-1)/ligand-1 (PD-L1) inhibitors, are effective in treating multiple advanced cancers and have widespread use in cancer treatment regimens. However, their use has led to a variety of immune-related adverse events (irAE), and common irAEs, such as those affecting dermatologic, gastrointestinal, hepatic, pulmonary, and endocrine organ systems, are well described. The increased use of ICIs is now bringing to light rarer irAEs, such as ICI-associated pancreatic adverse events, including pancreatitis, asymptomatic amylase/lipase elevations, endocrine pancreatic insufficiency, and exocrine pancreatic insufficiency. Of these, exocrine pancreatic insufficiency (EPI) has been least described in the literature with only rare reports to date.
Up to 35% of ICI-treated patients develop diarrhea, which is typically attributed to ICI-related enterocolitis; however, steatorrhea secondary to exocrine pancreatic insufficiency (EPI) has been described in small case reports.1-3 In a case-control study of 403 patients with melanoma, non-small cell lung cancer, and head and neck squamous cell cancer, 31 patients developed pancreatic atrophy on CT, of whom 4 patients developed EPI, which resolved with pancrelipase.4 In a retrospective review of the longitudinal cross-sectional imaging during immunotherapy of 25 patients who developed ICI-associated pancreatitis, 11 patients developed pancreatic atrophy, of whom 3 patients developed EPI.5 Despite the emerging recognition of this rare irAE, there are no large studies describing the incidence and clinical characteristics of these patients.
On the other hand, ICI-associated endocrine pancreatic insufficiency, defined as ICI-related glucose dysregulation secondary to altered functioning of the pancreatic islets of Langerhans, has been recognized in multiple case reports and case series, although larger studies are still limited. ICI-related diabetes mellitus (ICI-DM) has been primarily described as presenting with type 1 DM with diabetic ketoacidosis, although other phenotypes, such as the development of hyperglycemia without the need for insulin and the worsening of glycemic control in patients with type 2 DM, has been observed as well. Type 3c DM refers to pancreatogenic diabetes secondary to chronic pancreatitis; however, to our knowledge, worsening hyperglycemia in association with EPI from ICI therapies has not been described.
The primary goal of this manuscript is to describe the largest study to date of patients who developed EPI secondary to ICI use to better understand their clinical characteristics and to identify risk factors of EPI. The primary outcomes are the incidence of EPI secondary to ICI use, the time of onset of EPI after starting ICI, and the clinical characteristics of patients with EPI. The secondary outcomes are the clinical risk factors of EPI and the correlation of ICI-induced exocrine and endocrine pancreatic insufficiency.
Methods
A single-center, retrospective case-control study was conducted of adult patients with metastatic cancer treated with an ICI at Memorial Sloan Kettering Cancer Center (MSKCC) between January 2011 and July 2020. The study was approved by the Institutional Review Board at MSKCC and was HIPAA complaint. Study reporting followed STROBE guidelines for reporting observational studies.6
Study Population
The study included patients who met all the following criteria: (1) age 18 years or older and (2) received ICI therapy for advanced malignancies. Patients were excluded if they had an ICD9/10 code for (1) pancreatic cancer or pancreatic metastasis (157.0-157.9/C25-C25.9) or (2) a history of pancreatic surgery (V88.11/Z90.41/Z90.410/Z90.411). The electronic data warehouse at MSK was queried for all patients with any of the following: (1) an ICD9/10 code for steatorrhea (579.4/K90.3), (2) ICD9 code for other specified diseases of the pancreas (577.8) or ICD10 code for exocrine pancreatic insufficiency (K86.81), or (3) a new prescription for pancrelipase that postdated initiation of ICI therapy. All patients identified were then manually chart-reviewed to confirm that they met inclusion and exclusion criteria and had a diagnosis of EPI. Criteria for diagnosis of EPI were patients who met all of the following criteria: (1) started pancrelipase after prior receipt of an ICI, (2) had clearly documented symptoms of EPI, specifically steatorrhea, with or without associated abdominal discomfort or weight loss, and (3) demonstrated symptomatic improvement of steatorrhea with pancrelipase. To identify risk factors of EPI, controls were then selected from all cancer patients treated with ICI therapy at MSK, who met exclusion criteria as above, and who survived >6 months from the start of ICI therapy to allow for the development of pancreatic toxicity. Two controls were selected for each EPI case by the statistician, matched by age (± 5 years), cancer type, gender, year of the first dose of ICI, and race. Fig. 1 shows a flowchart of case and control patient selection.
Figure 1.
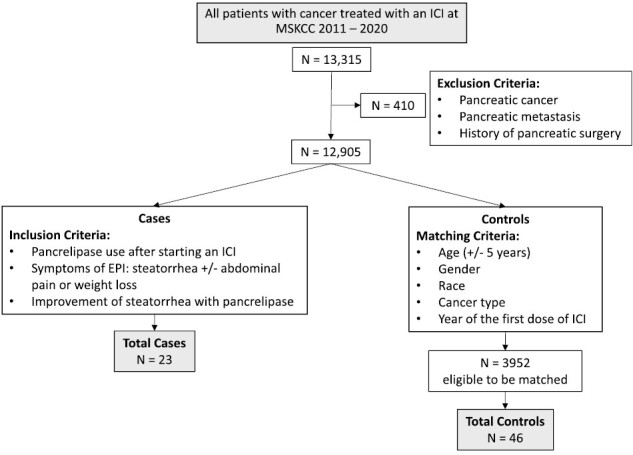
Flowchart of case and control patient selection.
Definitions and Outcomes of Interest
Data were collected through a combination of electronic data extraction and manual chart review and included patient demographics such as age, gender, race, and body mass index (BMI). Malignancies were categorized as brain, gastrointestinal/hepatobiliary, genitourinary, gynecologic, leukemia/lymphoma, lung/head and neck (H&N), melanoma, and sarcoma. ICI agents, including nivolumab, ipilimumab, pembrolizumab, atezolizumab, durvalumab, and tremelimumab, were categorized as anti-CTLA-4 monotherapy, anti-PD-(L)1 monotherapy, or combination anti-CTLA-4/anti-PD(L)-1 therapy. The median number of ICI doses and median duration of ICI therapy were recorded.
Clinical steatorrhea was ascertained by chart review when terminology including oily, yellow, floating, and greasy stools was documented by clinicians. Biochemical markers of pancreatic inflammation, including amylase and lipase, and of EPI, including fecal fat (positive Sudan stain or >20% on spot fecal fat) and elastase (<100 mcg/g), were recorded when available. The time of onset of EPI was defined from the first dose of ICI to the date of EPI symptom onset. Clinical acute pancreatitis was defined by the revised Atlanta classification as the presence of 2 of 3 criteria: symptomatic abdominal pain, imaging findings of pancreatic inflammation, or biochemical lipase or amylase elevation above 3 times upper limit of normal. If the lipase or amylase level was below 3 times the upper limit of normal, cross-sectional imaging confirmed pancreatitis. Phenotypes of hyperglycemia were defined as the decline in glycemic control after the first dose of ICI and were determined by a new HbA1c ≥6.5 after the start of ICI or by the initiation of new diabetes medications after ICI use. The time of onset of hyperglycemia was defined from the first dose of ICI to the date of first HbA1c ≥6.5 or date of the starting a new diabetes medication.
Potential risk factors of EPI were hypothesized to be the type of ICI received, total doses of ICI, duration of ICI treatment, prior lipase or amylase elevations, and prior clinical pancreatitis. Known risk factors of EPI include smoking, alcohol use, and gallstone disease or prior cholecystectomy. They were considered possible confounders and recorded.
CT scan radiology reports for EPI cases were reviewed for evidence of prior pancreatic inflammation, including heterogeneity, edema, or stranding of pancreatic parenchyma, or atrophy before the development of EPI.
Statistical Analysis
Descriptive statistics were presented as counts and percentages for categorical variables; and mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables, as appropriate. Exact conditional logistic regression model was used for univariate analysis of risk factors of EPI. We used SAS software (version 9.4; SAS Institute Inc, Cary, NC) in all analyses. All statistical tests were 2-tailed, and P-values of <.05 were considered statistically significant.
Results
Of 13 315 patients treated with ICI therapy at MSKCC between 2011 and 2020, 12 905 patients met inclusion criteria, of whom 23 patients developed clinically confirmed EPI (Fig. 1). All 23 (100%) patients presented with steatorrhea, 12 (52.2%) with weight loss, and 9 (39.1%) with abdominal pain. Steatorrhea improved for all patients with the initiation of pancrelipase. 10 patients had fecal fat checked and 6 patients had fecal elastase checked; all 10 (100%) had an elevated fecal fat and all 6 (100%) had a low fecal elastase. Median time to onset of EPI was 390 days (IQR 252-578 days) (Fig. 2). The incidence rate of EPI was 1.18 cases per 1000 person-years in patients exposed to ICI therapy.
Figure 2.
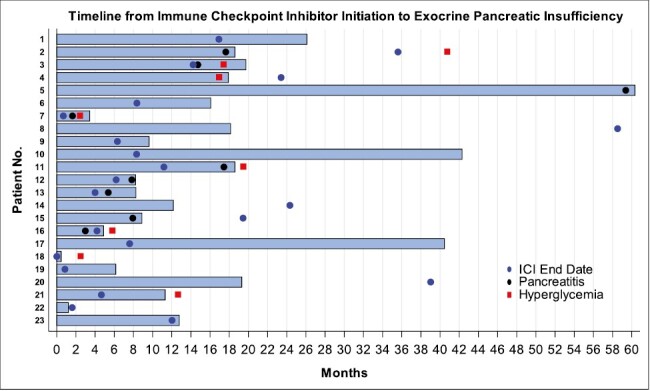
Clinical presentation of patients with ICI-related EPI. Legend: Blue bar—time from ICI initiation to the development of EPI, blue circle—ICI end date, black circle—pancreatitis, red square—hyperglycemia. Data is shown over 5 years from the start of ICI therapy. 15 patients completed ICI therapy prior to developing EPI; 8 patients continued ICI therapy after the development of EPI; 9 patients developed clinical pancreatitis, and 9 patients developed hyperglycemia. *Data limited by 5-year timeline: Patient 5 completed ICI therapy 6.2 years after starting ICI. Patient 8 developed hyperglycemia 8 years after the first dose of ICI.
Clinical characteristics of the 23 EPI cases and 46 matched controls are detailed in Table 1. Melanoma was the most frequent malignancy (35%), followed by genitourinary malignancies (21.7%) and lung or head and neck (17.4%). A similar number of EPI patients were treated with combination anti-CTLA-4/anti-PD(L)-1 therapy (48%) versus anti-PD(L)-1 therapy alone (52%). There was no significant difference in the type of ICI received between cases and controls (P = .30). The EPI group received a median of 16 doses of ICI (IQR 9-40) and had median exposure to ICI of 255 days (IQR 128-714), which was similar to the control group (P = .11 and P = .39, respectively). There was no statistical difference in the proportions of smoking, alcohol use, and gallstone disease or prior cholecystectomy between the two groups, which are known risk factors of chronic pancreatitis and EPI.
Table 1.
Clinical characteristics of the EPI cases and matched controls.
| Cases (n = 23) | Controls (n = 46) | Total (n = 69) | P value | |
|---|---|---|---|---|
| Age, mean (SD) | 61.5 (8.0) | 61.6 (8.1) | 61.6 (8.0) | .91 |
| Sex, n (%) | ||||
| Male | 16 (70) | 32 (70) | 48 (70) | |
| Female | 7 (30) | 14 (30) | 21 (30) | |
| BMI, mean (SD) | 29.7 (6.5) | 28.1 (5.4) | 28.6 (5.8) | .22 |
| Race, n (%) | ||||
| Caucasian | 22 (96) | 44 (96) | 66 (96) | |
| Non-Caucasian | 1 (4) | 2 (4) | 3 (4) | |
| Diabetes/hyperglycemia, n (%) | ||||
| DM prior to ICI | 2 (9) | 6 (13) | 8 (12) | .92 |
| New hyperglycemia after ICI | 9 (39) | 3 (7) | 12 (17) | .002 |
| None | 12 (52) | 37 (80) | 49 (71) | |
| Cancer type, n (%) | ||||
| Brain | 1 (4) | 2 (4) | 3 (4) | |
| GI/hepatobiliary | 2 (9) | 4 (9) | 6 (9) | |
| Gynecologic | 1 (4) | 2 (4) | 3 (4) | |
| Leukemia/lymphoma | 1 (4) | 2 (4) | 3 (4) | |
| Lung/H&N | 4 (17) | 8 (17) | 12 (17) | |
| Melanoma | 8 (35) | 16 (35) | 24 (35) | |
| Sarcoma | 1 (4) | 2 (4) | 3 (4) | |
| Genitourinary | 5 (22) | 10 (22) | 5 (22) | |
| ICI Type, n (%) | .30 | |||
| PD-1/PD-(L)1 | 12 (52) | 31 (67) | 43 (62) | |
| CTLA-4/PD-(L)1 | 11 (48) | 15 (33) | 26 (38) | |
| Total doses of ICI | ||||
| Median (IQR) | 16 (9-40) | 13.5 (6-26) | 14 (7-30) | .11 |
| Days on ICI | ||||
| Median (IQR) | 255 (128-714) | 245 (111-582) | 255 (122-590) | .39 |
| Prior smoking history, n (%) | ||||
| Current | 3 (13) | 7 (15) | 10 (14) | 1.00 |
| Previous | 8 (35) | 17 (37) | 25 (36) | .87 |
| Never | 12 (52) | 19 (41) | 31 (45) | |
| Missing | 0 (0) | 3 (7) | 3 (43) | |
| Alcohol use history, n (%) | ||||
| Heavy | 3 (13) | 6 (13) | 9 (13) | 1.00 |
| Social | 10 (43) | 20 (43) | 30 (43) | .93 |
| None | 8 (35) | 17 (37) | 25 (36) | |
| Missing | 2 (9) | 3 (7) | 5 (7) | |
| Prior gallstone disease, n (%) | ||||
| Yes | 3 (13) | 7 (15) | 10 (14) | 1.00 |
| No | 20 (87) | 39 (85) | 59 (86) | |
| Prior cholecystectomy, n (%) | ||||
| Yes | 5 (22) | 5 (11) | 10 (14) | .4 |
| No | 18 (78) | 41 (89) | 59 (86) | |
| Prior history of clinical pancreatitis, n (%) | ||||
| Yes | 9 (39) | 1 (2) | 10 (14) | .0007 |
| No | 14 (61) | 45 (98) | 59 (86) | |
| Maximum lipase post ICI | ||||
| Median (IQR) | 202 (90-573) | 35.5 (26-79) | 79 (28-379) | .02 |
| Maximum amylase post ICI | ||||
| Median (IQR) | 116 (69-258) | 107 (69-170) | 110.5 (69-190) | .71 |
| Abnormal lipase post ICI (Lipase>78), n (%) | ||||
| Yes | 15 (65) | 6 (13) | 21 (30) | |
| No | 4 (17) | 16 (35) | 20 (29) | |
| Missing | 4 (17) | 24 (52) | 28 (41) | |
| Abnormal amylase post ICI (amylase>100), n (%) | ||||
| Yes | 9 (39) | 13 (28) | 22 (32) | .79 |
| No | 8 (35) | 10 (22) | 18 (26) | |
| Missing | 6 (26) | 23 (50) | 29 (42) | |
Data were matched by age (± 5 years), gender, race, cancer type, and year of the first dose of ICI. P values were calculated by exact conditional logistic regression.
Abbreviatons: DM, diabetes; EPI, exocrine pancreatic insufficiency; ICI, immune checkpoint inhibitor; IQR, interquartile range; SD, standard deviation..
Among the 23 EPI patients, 9 patients (39.1%) developed clinical pancreatitis prior to developing EPI vs. only 1 patient (2.2%) who had clinical pancreatitis in the control group. All patients with clinical pancreatitis responded to conservative management; none progressed to necrotizing pancreatitis. The median time to onset of EPI from the date of clinical pancreatitis was 35 days (IQR 29-58). Development of clinical pancreatitis conferred an 18-fold (95% CI 2.5-789.0, P = .0007) risk of developing EPI in the context of ICI exposure. The median maximum lipase after starting ICI was significantly higher among the EPI patients (202, IQR 90-573) compared to the controls (35.5, IQR 26-79; P = .02), with 15 (65%) EPI patients having a documented lipase elevation above the upper limit of normal versus 6 (13%) control patients. There was no statistical difference in median maximal amylase after starting ICI or in amylase elevation above the upper limit of normal between cases and controls. Blood lipase and amylase are not routinely measured in patients on ICI at our institution; thus, there was a higher proportion of control patients in whom lipases were not checked (52.2% controls vs. 17.4% cases) and amylases were not checked (50% controls vs 26.1% cases). On review of CT scans prior to EPI development, 7 of the 23 cases had evidence of pancreas inflammation; however, none of the cases had atrophy or chronic pancreatitis noted on imaging. In the 23 EPI cases, 16 patients (69.6%) had a concomitant irAE. Colitis and hepatitis were the most common concomitant irAEs (Table 3).
Table 3.
Concomitant immune-related adverse events among EPI cases.
| History of prior or concomitant immune-related adverse events | Number of cases |
|---|---|
| Colitis | 6 |
| Hepatitis | 5 |
| Thyroiditis | 3 |
| Pneumonitis | 2 |
| Hypophysitis | 1 |
| Nephritis | 1 |
| Adrenal insufficiency | 1 |
| Arthritis | 1 |
| Skin | 1 |
The proportions of pre-existing diabetes prior to ICI use were similar between the case and control groups (2 (8.7%) vs. 6 (13.0%) respectively, P = .92). However, the EPI group exhibited higher proportions of new hyperglycemia after exposure to ICIs, compared with the control group (9 (39.1%) vs. 3 s (6.5%), P < .01). BMI was similar between the 2 groups (29.7 vs. 28.1 respectively, P = .22), suggesting similar metabolic profiles prior to ICI exposure.
The specific characteristics of the EPI patients with hyperglycemia are summarized in Table 2. The median time to onset of hyperglycemia after ICI exposure was 518 days (IQR 178-595 days). Of the 2 patients who had pre-existing type 2 DM, both progressed from requiring only oral antidiabetic medications prior to ICI to requiring insulin after ICI treatment. Two patients exhibited new-onset type 1 DM with autoantibodies. Three patients were exposed to steroids for other irAEs immediately preceding their presentation of acute hyperglycemia. One of the 3 was able to discontinue antidiabetic agents by 3 months after presentation suggesting steroid-induced hyperglycemia, but the other 2 required ongoing medication use for DM. One patient had transient hyperglycemia with HbA1c 6.5 but was able to control this with lifestyle modification and did not require antidiabetic medications. Notably, 7 of the 9 patients (77%) developed EPI and hyperglycemia within 10 weeks of the other (Fig. 2).
Table 2.
Characteristics of EPI patients with hyperglycemia.
| Patient | Cancer type | ICI | DM prior to ICI | Time to onset of exocrine insufficiency (weeks) | Time to onset of hyperglycemia (weeks) | Presenting features | Recent steroid use | DM agent | Type of hyperglycemia | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Presenting glucose (mmol/L) | Presenting HbA1c (%) | Presenting fructosamine (mcmol/L) | C peptide | Autoantibodies | |||||||||
| 55M | Melanoma | CTLA-4/ PD-1 | No | 81 | 178 | DKA | 307 | 8.9 | NR | 0.1 | IA-2 | No | Insulin | Type 1 DM |
| 73F | Lymphoma | PD-1 | No | 86 | 76 | Asymptomatic | 129 | 6.5 | 288 | NR | NR | No | none | Unclear |
| 62M | Genitourinary | PD-1 | No | 78 | 74 | Asymptomatic | 172 | 6.4 | NR | NR | NR | No | PO | Type 2 DM |
| 59M | GI/HPB | PD-L1 | Yes | 70 | NA | NA | NA | NA | NA | 0.41 | NR | No | Insulin | Type 2 DM |
| 67M | Melanoma | CTLA-4/ PD-1 | No | 15 | 11 | Acute Hyperglycemia | 404 | 9.3 | 346 | 2.94 | NR | Yes | PO | Type 2 DM |
| 61F | Melanoma | CTLA-4/ PD-1 | No | 79 | 413 | Asymptomatic | NR | NR | NR | NR | NR | No | PO | Type 2 DM |
| 56F | Genitourinary | CTLA-4/ PD-1 | No | 81 | 85 | Asymptomatic | 123 | 7.1 | 274 | 2.11 | NR | No | none | Type 2 DM |
| 50F | Lung | CTLA-4/ PD-1 | Yes | 39 | NA | NA | NA | NA | NA | 3.06 | NR | No | Insulin + PO | Type 2 DM |
| 57M | Lung | PD-1 | No | 21 | 25 | Acute Hyperglycemia | 594 | 9.8 | NR | 1.19 | None | Yes | PO | Steroid-induced hyperglycemia |
| 47M | Melanoma | CTLA-4/ PD-1 | No | 2 | 11 | Acute Hyperglycemia | 470 | 8 | NR | <0.2 | GAD | Yes | Insulin | Type 1 DM |
| 68M | Sarcoma | CTLA-4/ PD-1 | No | 49 | 55 | Asymptomatic | 131 | 6.5 | NR | NR | NR | No | PO | Type 2 DM |
Abbreviations: DKA, diabetic ketoacidosis; DM, diabetes; EPI, exocrine pancreatic insufficiency; GI, gastrointestinal; HPB, Hepato-Pancreato-Biliary; ICI, immune checkpoint inhibitor; NA, not applicable due to presence of diabetes before ICI administration; NR, not reported; PO, oral.
Discussion
This is the largest study describing new onset exocrine pancreatic insufficiency (EPI) in cancer patients treated with anti-CTLA-4 and/or anti-PD(L)-1 therapy. We observed that EPI is a rare pancreatic toxicity, with only 23 (0.17%) patients with clinically confirmed EPI found among 12 905 ICI-treated cancer patients without prior pancreatic injury (cancer, surgery, or metastasis), with an incidence rate of 1.18 cases of ICI-induced EPI per 1000 person-years.
Clinically, all of the EPI patients in our study presented with steatorrhea, with fewer presenting with weight loss and abdominal pain. ICI-related diarrhea is typically attributed to ICI-colitis and is often treated empirically with steroids, but our study demonstrates that other etiologies of diarrhea should be considered, especially when the presentation is atypical for colitis. The onset of EPI from initiation of ICI was a median of 13 months, which contrasts with ICI-colitis that typically occurs sooner, a median of 2 months from the initiation of ICI.7 This observation of a long latency to development of EPI is consistent with a prior case-control study in which 4 patients with EPI manifested at a median of 9 months whereas the 4 controls for ICI colitis manifested at a median of 2 months from the initiation of ICI.4 Thus, we recommend that ICI-related EPI be considered in the differential diagnosis in particular for patients who develop diarrhea late after initial ICI exposure. Our study also shows that patients with prior ICI colitis (6 (26%) patients) can present with diarrhea secondary to ICI-related EPI. The high concordance rate with other irAEs (16 (70%) patients) should also raise diagnostic suspicion of EPI in patients presenting with late onset diarrhea.
Immune checkpoint inhibitor (ICI)-induced pancreatic injury (ICIPI) is characterized by inflammation in the pancreas, yet its clinical features and significance remain uncertain. In one study, pancreas enzyme elevation was observed in 4% of patients treated with ICI.8 The severity of ICIPI can range from isolated, asymptomatic lipase/amylase elevation to clinical pancreatitis or from mild hyperglycemia to medical emergencies such as diabetic ketoacidosis. Additionally, pancreatic atrophy and chronic pancreatitis have been seen on imaging. In a meta-analysis by George et al., the incidence of asymptomatic elevation of lipase or amylase after ICI treatment is 2.7%,9 and the incidence of grade 2 pancreatitis (asymptomatic pancreatic enzyme elevation and radiographic findings of pancreatitis) is 1.9%.9
Clinical pancreatitis was observed to be a clinically significant risk factor for the development of EPI, increasing the risk of EPI by 18-fold in our study. This is a novel finding, but it has been supported by existing observations in literature. In a retrospective study of longitudinal cross-sectional imaging of 25 patients who developed ICI-associated pancreatitis, 11 patients developed pancreatic atrophy, of whom 3 patients developed EPI.5 Additionally, a recent study from MD Anderson found chronic pancreatitis on imaging for one of 32 patients with typical symptoms of pancreatitis and 2 of 50 patients with isolated lipase elevation.8 However, unlike these studies, chronic pancreatitis or atrophy on imaging was not noted in our patients prior to or at the time of EPI development.
In our study, a similar number of EPI patients were treated with combination anti-CTLA-4/anti-PD(L)-1 therapy (48%) versus anti-PD(L)-1 therapy alone (52%); none of the cases or controls received anti-CTLA-4 monotherapy. On univariate analysis, the type of ICI, duration of total ICI use, and total doses of ICI administered were not found to be risk factors for EPI. To our knowledge, no prior studies have evaluated these as risk factors for EPI given the rarity of this diagnosis. However other ICI-associated pancreatic adverse events, such as ICI-pancreatitis and ICI-DM, have been associated with certain ICI therapies. In a meta-analysis of clinical trial data, there was a significantly higher incidence of ICI-pancreatitis in patients treated with anti-CTLA-4 compared to anti-PD-1 therapy and combination anti-CTLA-4/anti-PD(L)-1 therapy had a significantly higher incidence of pancreatitis compared to monotherapy.9 In contrast, the majority of ICI-DM cases are secondary to anti-PD(L)-1 therapy either alone or combination therapy, with very few cases with anti-CTLA monotherapy.10 Larger studies are needed to elucidate a possible association between the type of ICI and EPI.
ICI-related diabetes (ICI-DM) has been described as presenting as diabetic ketoacidosis or new-onset type 1 DM and is estimated to occur in 0.2%-1.0% of patients.11,12 In a case series of 283 patients with ICI-DM, the onset of DM ranged from 5 to 790 days after the first dose of ICI (median 116 days, IQR 58-207.5, n = 91) and PD-1 monotherapy was implicated in 76% of cases followed by combination PD-1/CTLA-4 therapies in 17%.10 Hyperglycemia and worsening of glycemic control in patients with type 2 DM have been described as well. Kotwal et al. identified 21 cases of DM in patients treated with ICI, 12 of whom developed new-onset insulin-dependent DM and 9 patients with type 2 DM who had worsening glycemic control with ICI therapy. These 9 patients’ HbA1c increased to a median of 10% in 6 months.13
This is the first case-control study to demonstrate an association between EPI and worsening of glycemic control. The most commonly accepted phenotype of ICI-DM has been type 1 DM, however, other phenotypes can manifest as mild to moderated hyperglycemia. In our study, we found that while 2 patients developed overt type 1 DM, 7 patients developed new hyperglycemia, and 2 patients had decompensation of their pre-existing DM. Additionally, the majority of patients developed the deterioration of glycemic control within weeks of exhibiting symptoms of EPI. Thus, despite the multiple phenotypes of hyperglycemia, this observation is highly suggestive of a loss of endocrine pancreatic function alongside the loss of exocrine pancreatic function. Thereby, a similar mechanism of pancreatic damage may account for both of these toxicities. However, studies regarding the mechanism of pancreatic toxicity are limited and conflicting. Zhang et al. found that of 11 patients who developed ICI-DM, only 2 (18%) patients had an abnormal lipase on the day of DM diagnosis and so they suggest that ICI therapy likely caused selective islet cell toxicity rather than broader pancreatic damage.14 In contrast, a German multicenter study showed that of 22 patients with ICI-DM, 12 (55%) had a lipase elevation around the time of DM diagnosis.15
The proposed biochemical mechanism of ICIPI involves ICIs blocking the inhibitory signals of T cells and thus promoting activation and expansion of T lymphocytes. CD3+ T lymphocytes infiltrate the pancreatic islets, disproportionally activating CD8+ T cells, rather than CD4+ T cells. This dense infiltration may trigger pancreatitis. The increased CD8+ T cells may damage the pancreatic exocrine acinar cells as well as endocrine beta cells, thereby predisposing to both exocrine and endocrine insufficiency.16
Strengths of our study include the identification of the largest number of cases to develop EPI to date as well as rigorous case-control matching to identify risk factors for this rare toxicity. The diagnosis of EPI was confirmed by detailed chart review and used strict clinical criteria, ie, prescription of pancrelipase for symptoms suspicious for EPI as well as documentation of clear symptomatic benefit after treatment with pancrelipase. Despite this being the largest presentation of cases of ICI-related EPI, there are some limitations to this study. First, this was a retrospective study and thus there were missing data as noted in Table 1. Second, the sample size of EPI cases is low despite our large ICI treatment population. Clinical EPI is a rare pancreatic toxicity that is newly being recognized and has only been reported in a few case series to date.1-5 Our case-control design helped study this rare disease and identify risk factors. Third, the date of onset of hyperglycemia is difficult to ascertain with certainty in retrospective review as hyperglycemia may have been present prior to the HbA1c being checked or to a new diabetic medication being prescribed. Fourth, as EPI has not been a well-recognized irAE, diagnostic suspicion bias may lead to underdiagnosis of milder cases and the true incidence of ICI-related EPI may be significantly higher. Similarly, milder DM/hyperglycemia phenotypes may be undiagnosed. Lastly, pancreatic injury is complex and factors other than ICI could have contributed to predisposing patients to EPI such as other treatments for their complex cancer care. Nonetheless, we excluded patients with other causes of overt pancreatic injury, including pancreatic cancer, pancreatic surgery, or pancreatic metastases to limit these confounders.
Conclusion
Our study findings suggested that EPI is a rare, but clinically significant irAE and is often associated with endocrine pancreatic insufficiency. We identified clinical pancreatitis as a risk factor for developing ICI-related EPI. As the use of ICIs continues to increase and patient survival improves, clinicians may encounter side effects with a longer latency more frequently. Our study raises awareness of a likely underrecognized irAE that should be considered in the differential diagnosis of late onset diarrhea after treatment with ICI. Early recognition and initiation of pancrelipase can improve symptoms and quality of life for patients.
Acknowledgments
We would like to thank the MSKCC Information Systems’ data delivery group (DataLine), especially Joseph Schmeltz, for assistance with data acquisition.
Contributor Information
Deepika Satish, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
I-Hsin Lin, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
James Flory, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Hans Gerdes, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Michael A Postow, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Department of Medicine, Weill Cornell Medical College, New York, NY, USA.
David M Faleck, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Ethical Approval
This retrospective, case-control, observational, single-center study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center (MSKCC). Patient consent was not applicable given the retrospective nature of this study.
Conflict of Interest
Michael A. Postow has contracts/grants from RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, and AstraZeneca, and consults for BMS, Merck, Array BioPharma, Novartis, Eisai, and Pfizer. David M. Faleck has received consulting fees for Kaleido Biosciences, AzurRx, Mallinckrodt Pharmaceuticals, and Equillium. The other authors indicated no financial relationships.
Author Contributions
Conception/design: H.G., M.A.P., D.M.F. Provision of study material or patients: I.-H.L., D.M.F. Collection and/or assembly of data: D.S., I.-H.L., J.F. Data analysis and interpretation: D.S., I.-H.L., D.M.F. Manuscript writing: D.S., D.M.F. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hoadley A, Sandanayake N, Long GV.. Atrophic exocrine pancreatic insufficiency associated with anti-PD1 therapy. Ann Oncol. 2017;28(2):434-435. 10.1093/annonc/mdw626. [DOI] [PubMed] [Google Scholar]
- 2. Prasanna T, McNeil CM, Nielsen T, Parkin D.. Isolated immune-related pancreatic exocrine insufficiency associated with pembrolizumab therapy. Immunotherapy. 2018;10(3):171-175. 10.2217/imt-2017-0126. [DOI] [PubMed] [Google Scholar]
- 3. Koldenhof JJ, Suijkerbuijk KPM.. Diarrhoea during checkpoint blockade, not always colitis. Eur J Cancer. 2017;87:216-218. 10.1016/j.ejca.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 4. Eshet Y, Baruch EN, Shapira-Frommer R, et al. Clinical significance of pancreatic atrophy induced by immune-checkpoint inhibitors: a case–control study. Cancer Immunol Res. 2018;6(12):1453-1458. 10.1158/2326-6066.CIR-17-0659. [DOI] [PubMed] [Google Scholar]
- 5. Das JP, Postow MA, Friedman CF, Do RK, Halpenny DF.. Imaging findings of immune checkpoint inhibitor associated pancreatitis. Eur J Radiol. 2020;131:109250. 10.1016/j.ejrad.2020.109250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung VTF, Gupta T, Olsson-Brown A, et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: can IBD scoring point the way? Br J Cancer. 2020;123(2):207-215. 10.1038/s41416-020-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abu-Sbeih H, Tang T, Lu Y, et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J ImmunoTher Cancer. 2019;7(1):31. 10.1186/s40425-019-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. George J, Bajaj D, Sankaramangalam K, et al. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: a systematic review and meta-analysis. Pancreatology. 2019;19(4):587-594. 10.1016/j.pan.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 10. Wright JJ, Salem J-E, Johnson DB, et al. Increased reporting of immune checkpoint inhibitor–associated diabetes. Diabetes Care. 2018;41(12):e150-e151. 10.2337/dc18-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471-1480. 10.2337/dbi18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens. JAMA Oncol. 2018;4(2):173-182. 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotwal A, Haddox C, Block M, Kudva Yogish C.. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care. 2019;7(1):e000591. 10.1136/bmjdrc-2018-000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang AL, Wang F, Chang L-S, et al. Coexistence of immune checkpoint inhibitor-induced autoimmune diabetes and pancreatitis. Front Endocrinol (Lausanne). 2021;12:620522-620522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grimmelmann I, Momma M, Zimmer L, et al. Lipase elevation and type 1 diabetes mellitus related to immune checkpoint inhibitor therapy—a multicentre study of 90 patients from the German Dermatooncology Group. Eur J Cancer. 2021;149:1-10. 10.1016/j.ejca.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 16. Liu Y, Zhang H, Zhou L, et al. Immunotherapy-associated pancreatic adverse events: current understanding of their mechanism, diagnosis, and management. Front Oncol. 2021;11:627612-627612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


