Abstract
Background
In advanced urothelial cancers (UC), immune checkpoint inhibitors (ICI) show promise as a durable therapy. Immune-related adverse events (irAEs), a side effect of ICIs, may serve as an indicator of beneficial response. We investigated the relationship between irAEs and clinical outcomes in patients with advanced UC who received ICI.
Materials and Methods
In this retrospective study, we investigated 70 patients with advanced UC treated with ICIs at Winship Cancer Institute from 2015 to 2020. Data on patients were collected through chart review. Cox’s proportional hazard model and logistic regression were applied to estimate the association with overall survival (OS), progression-free survival (PFS), and clinical benefit (CB). The possible lead-time bias was handled in extended Cox regression models.
Results
The median age of the cohort was 68. Over one-third (35%) of patients experienced an irAE, with skin being the most frequent organ involved (12.9%). Patients that experienced at least one irAE had significantly enhanced OS (HR: 0.38, 95% CI, 0.18-0.79, P = .009), PFS (HR: 0.27, 95% CI, 0.14-0.53, P < .001), and CB (OR: 4.20, 95% CI, 1.35-13.06, P = .013). Patients who experienced dermatologic irAEs also had significantly greater OS, PFS, and CB.
Conclusion
Of patients with advanced UC that had undergone ICI therapy, those who had irAEs, especially dermatologic irAEs, had significantly greater OS, PFS, and CB. These results may suggest that irAE’s may serve as an important marker of durable response to ICI therapy in urothelial cancer. The findings of this study need to be validated with larger cohort studies in the future.
Keywords: immune-related adverse events, urothelial cancer, bladder cancer, checkpoint inhibitor, outcomes
This article reports on the relationships between immune-related adverse events and clinical outcomes in patients with advance urothelial cancer who received immune checkpoint inhibitor therapy.
Implications for Practice.
This study found that patients with advanced urothelial cancer undergoing ICI who experienced irAEs, especially those of the skin, had significantly improved clinical outcomes compared to those who did not experience an irAE. With the increasing use of ICIs in urothelial cancers, our results suggest the occurrence of an irAE, particularly in the skin, may act as a clinical marker of beneficial therapy response and improved outcome for that patient.
Introduction
Bladder cancer is a common and complex disease that requires multidisciplinary specialties for optimal treatment. There are approximately 81 000 new cases and 17 000 deaths in the US, and over 700 000 cases estimated worldwide.1,2 The most common form of bladder cancer, urothelial cancers (UC), is subdivided into the often less aggressive non-muscle invasive disease, and muscle-invasive disease, representing 75% and 25% of UC cases respectively. While non-muscle invasive UC has improved outcomes and prognosis compared to invasive muscular and metastatic UC, it still has recurrence rates of over 50% with up to 30% progression.2-4 For patients with metastatic disease, frontline therapy with platinum-based therapies and second-line chemotherapies provides a limited median overall survival.5-8
Over the past several years, agents targeting tumor-associated antigens and immune system evasion have fortified treatments against advanced urothelial cancers. For advanced UC, anti-PD-1/PD-L1 therapies have demonstrated improved survival for patients with advanced disease and are approved as second-line and maintenance therapies for those that have failed or are ineligible for platinum-based therapies.9,10 While these ICIs show significant promise, only a subset of patient tumors show response to ICIs.11 As a result, clinical biomarkers that provide prognostic clues of durable response to ICIs are being investigated. In urothelial cancer, sites of metastasis and scoring systems that combine serum markers and patient functional status have shown promise in forecasting response to ICIs, but investigation into other prognostic biomarkers in urothelial cancer remains limited.4,12 Considering the poor prognosis of advanced UC, and the infrequent but promising potential ICI response, it is essential to identify clinical biomarkers to better forecast which patients will benefit from ICIs.
There has been growing interest in the unique side effects of ICIs that some patients experience, known as Immune-related adverse events (irAEs). irAEs have variable onset, presentation, severity, and outcomes.13,14 A growing number of malignancies show improved survival when irAEs occur after ICI. In melanoma, non-small cell lung cancer, and metastatic renal cell carcinoma, irAEs were associated with improved outcomes.15-18 A similar finding of significant improvement in survival associated with irAEs was reported recently in one of the only studies of irAEs in UC.19 IrAE’s represent an important consideration for patients undergoing ICI therapy that remains understudied, particularly in genitourinary cancers.
Herein, we investigated the relationship between irAEs and clinical outcomes in patients with advanced urothelial cancer treated with ICI monotherapies. Because irAEs likely represent a robust and active innate response to therapy, we hypothesized that patients who experienced irAEs would have improved survival and clinical benefit compared to those who did not. In characterizing irAEs of advanced UC, we aim to broaden the understanding and utility of ICI in UC, their adverse events, and the utility of irAEs as a prognostic biomarker. Adding to the growing understanding of irAEs may develop new insights into the complex interplay tumor antigens, autoimmune disease, and organ homeostasis. Given the growing use of ICIs, we hope this study aids oncologists in discussing therapies and goals with their patients and their quality of life.
Materials and Methods
We retrospectively studied patients with advanced urothelial who were treated with PD-1 or PD-L1-based ICI-regimens at Winship Cancer Institute between 2015 and 2020. Patients in this study had histologic diagnosis of advanced UC and also having received at least one dose of the following ICIs: atezolizumab (anti PD-L1), pembrolizumab (anti PD-1), or nivolumab (anti PD-1) between 2015 and 2020. This study was approved by the institutional review board. Cohort data including age, sex, race, Eastern Cooperative Oncology Group (ECOG) performance status, histologic cancer grades, and ICI treatment course cohort were collected after review of the electronic medical record.
IrAEs were defined as adverse events with a probable immunologic basis that required surveillance and possible treatment with endocrine or immune suppressant therapy. IrAEs were subdivided into dermatologic, arthralgia, neuropathic, gastrointestinal (GI), or endocrine groupings. Dermatologic irAEs included pruritis, rash, and vitiligo-like pigmentation/depigmentation. irAE clinical data, including ICI duration and time to irAE, were collected from clinic notes, hospitalization notes, and radiology reports.
Clinical outcomes were measured using overall survival (OS), progression-free survival (PFS), and clinical benefit (CB). OS is defined as the time from first ICI dose to the date of death. PFS is defined as the time from first dose of ICI to radiographic/clinical progression or death. Patients with no record of death were censored at the date of last follow-up. CB was defined as best radiographic response of complete response, partial response, or stable disease for ≥ 6 months per RECIST v1.1. Radiographic responses to ICI were determined by reviewing clinic notes and radiology reports using RECIST v1.1. The senior author reviewed cases and determined responses in situations in which the response was not clearly documented.
Statistical Analysis
The univariate association between each covariate and study cohorts (irAE status yes vs. no) was assessed using Chi-square test or Fisher’s exact test for categorical covariates and ANOVA for numerical covariates. The univariate association of each covariate including study cohorts with OS and PFS was assessed using Cox proportional hazards models. Univariate analyses (UVA) were performed using logistic regression models to estimate odds ratios (ORs) for CB. Six patients were removed from CB analysis because of missing chart information. Multivariable analyses (MVA) were carried out by a backward variable selection approach with a removal criterion of 0.10. Kaplan-Meier curves were generated to compare the survival outcomes of the 2 cohorts. A supplementary analysis using an extended Cox model was performed to assess the association between time-dependent irAEs and OS,20,21 in which irAE was treated as a time-varying variable. Statistical analysis was conducted using SAS version 9.4 (SAS Institute, Cary, NC), and SAS macros developed by Biostatistics Shared Resource at Winship Cancer Institute.22
Results
Patient Demographics and Disease Entity
Demographics, baseline disease characteristics, and irAE incidences are presented in Table 1. A total of 70 patients were included in the study. The median age was 68.7 years (28.0–91.0) and 70% of participants were male (n = 49). Median follow-up was 20.07 months (95%CI, 12.47–30.79). Most patients were White (n = 50, 71.4%) and one-fifth were Black (n = 15). One-third of patients had ECOG PS greater than or equal to 2. Most patients (n = 64, 95%) had urothelial carcinoma, and most (n = 57, 81%) received at least 1 prior treatment before receiving an ICI agent.
Table 1.
Descriptive statistics of urothelial cancer patient cohort receiving immune-checkpoint inhibitors.
| Variable | Level | N (%) = 70 |
|---|---|---|
| Age | Median | 69.50 |
| (Range) | (28-91) | |
| Race | Asian | 5 (7.1) |
| Black | 15 (21.4) | |
| White | 50 (71.4) | |
| Gender | Female | 21 (30.0) |
| Male | 49 (70.0) | |
| Urothelial carcinoma | No a | 3 (4.5) |
| Yes | 64 (95.5) | |
| Missing | 3 | |
| ECOG PS | 0 | 32 (46.4) |
| 1 | 19 (27.5) | |
| ≥2 | 18 (26.1) | |
| Missing | 1 | |
| Prior lines | 0 | 13 (18.6) |
| 1 | 32 (45.7) | |
| ≥2 | 25 (35.7) | |
| irAE | No | 45 (64.3) |
| Yes | 25 (35.7) | |
| Follow-up time | Median mo | 20.07 |
| (Range) | (12.47-30.79) | |
| Time from C1D1 to irAE (weeks) | Median mo | 6.29 |
| (Range) | (0.29-71.43) |
aThe value “no” defined as variant histology.
Abbreviations: ECOG PS; Eastern Cooperative Oncology Group performance scale; irAE, immune related adverse event; C1D1, day 1 of cycle 1 of immune checkpoint inhibitor therapy.
Immune Checkpoint Inhibitor Toxicities
Of all patients receiving ICI, 35% experienced at least one irAE. The median time from ICI initiation to irAE was 6.29 weeks (Table 1). There were no significant differences in demographic and status characteristics between those that did and did not experience an irAE (Supplementary Table 1). The most common irAEs were dermatologic (12.9%) followed by only 3 reports of gastrointestinal, endocrine, and arthralgia irAEs each (Table 2).
Table 2.
Incidence of end organ immune related adverse events in urothelial cancer patient cohort receiving immune-checkpoint inhibitors.
| Variable | Level | N (%) = 70 |
|---|---|---|
| Dermatologic irAE | No | 61 (87.1) |
| Yes | 9 (12.9) | |
| Missing | 0 | |
| Neuropathy irAE | No | 14 (93.3) |
| Yes | 1 (6.7) | |
| Missing | 55 | |
| Arthralgia irAE | No | 12 (80.0) |
| Yes | 3 (20.0) | |
| Missing | 55 | |
| Gastrointestinal irAE | No | 14 (82.4) |
| Yes | 3 (17.6) | |
| Missing | 53 | |
| Endocrine irAE | No | 12 (80.0) |
| Yes | 3 (20.0) | |
| Missing | 55 |
Abbreviation: irAE, immune related adverse event.
Associations Between irAEs and Clinical Outcomes
UVA analysis of associations between irAEs and clinical outcomes are reported in Supplementary Table 2. irAEs and dermatologic irAEs were all associated with significantly improved OS, PFS, and CB (P-values < .04). Further investigation with MVA analysis of the association between irAEs and clinical outcomes are presented in Table 3; Fig. 1. Patients that experienced an irAE had significantly improved OS (HR: 0.38, 95%CI, 0.18-0.79, P = .009, Fig. 1), significantly longer PFS (HR: 0.27, 95% CI, 0.14-0.53, P < .001, Fig. 1), and significantly greater CB (OR: 4.20, 95% CI, 1.35-13.06, P = .013). Specifically, patients who experienced dermatologic irAEs had significantly greater OS, PFS, and CB (all P < .02) (Table 3; Fig. 1). CB rates of patients who experienced irAEs and those that did not were 52% and 23% respectively. CB rates of patients who experienced dermatologic irAEs and those who did not were 67% and 29% respectively. Associations between other irAEs and clinical outcomes did not reach significance (Table 1). MVA Clinical outcomes associated with time-dependent irAEs did not reach significance (Table 4). However, we still see a trend of a better OS among patients who experienced irAE (HR = 0.87). The extended Cox model treated irAE as a time-varying and handled immortal time bias appropriately.23 The non-significant findings may be improved with future sample size accumulation.
Table 3.
Associations between immune related adverse events and clinical outcomes in urothelial cancer patients using multivariable analysis.a
| Variable | OS | PFS | CB | ||||
|---|---|---|---|---|---|---|---|
| HR (CI) | P-value | HR (CI) | P-value | OR (CI) | P-value | ||
| irAE (n = 25) |
0.38 (0.18-0.79) |
0.009 b | 0.27 (0.14-0.53) | <0.001 b | 4.2 (1.35-13.06) | 0.013 b | |
| Median months (CI) | NR (9.9, NR) | 8.5 (3.3, NR) | |||||
| No irAE (n = 45) |
1 | 1 | 1c | ||||
| Median months (CI) | 7.1 (2.4, 11.7) | 2.2 (1.7, 3.2) | |||||
| Dermatologic irAE (n = 9) |
0.23 (0.07-0.78) |
0.019 b | 0.18 (0.06-0.53) | 0.002 b | 7.68 (1.51-38.93) |
0.014 b | |
| Median months (CI) | NR (5.5, NR) | 33.3 (2.8, NR) | |||||
| No dermatologic irAE (n = 61) |
1 | 1 | 1c | ||||
| Median months (CI) | 9.2 (5.8, 12.4) | 2.6 (2.2, 3.8) | |||||
Statistically significant P-values are bolded.
aMultivariable analysis was built using the backward selection method at alpha level of 0.1.
bStatistical significance at alpha < 0.05.
cSix patients were removed from CB analysis due to missing chart information.
Abbreviations: CB, clinical benefit; HR, hazard ratio; irAE, immune related adverse events; OS, overall survival; PFS, progression-free survival; OR, odds ratio
Figure 1.
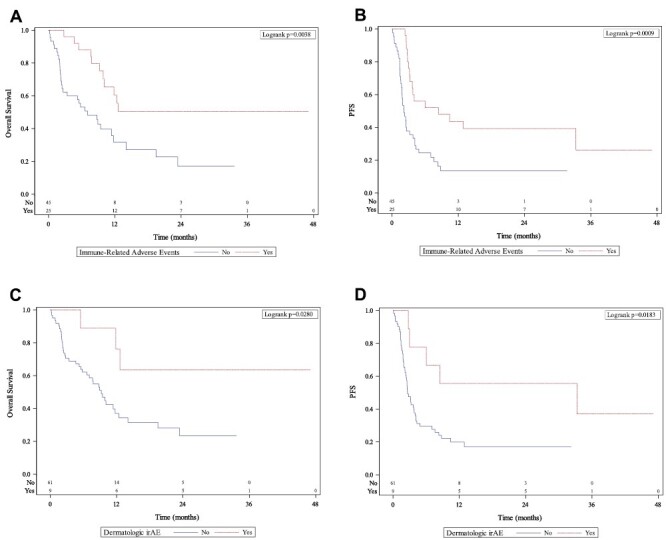
Kaplan-Meir curves with occurrence of immune-related adverse events (A, B), dermatologic immune-related adverse events (C, D) and their association to overall survival (left column) and progression-free survival (right column). Abbreviations: irAE, immune-related adverse event; PFS, progression-free survival.
Table 4.
Multivariable extended Cox proportional hazard model for overall survival time dependent immune-related adverse events in urothelial cancer patient cohort.
| Overall survival | ||||
|---|---|---|---|---|
| Covariate | Level | Hazard ratio (95% CI) | HR P-value | Overall P-value |
| irAE | Yes | 0.87 (0.40-0.1.9) | .734 | .734 |
| No | — | — | ||
| ECOG PS | 1 | 1.26 (0.54-2.91) | .595 | .009 |
| ≥2 | 3.49 (1.53-7.97) | .003 | ||
| 0 | — | — | ||
| Prior lines | 1 | 4.04 (1.31-12.48) | .015 | .035 |
| ≥2 | 4.62 (1.38-15.38) | .013 | ||
| 0 | — | — | ||
Statistically significant P-values are bolded.
Abbreviations: ECOG PS; Eastern Cooperative Oncology Group performance scale; HR, hazard ratio; irAE, immune related adverse event.
Discussion
In this retrospective study, we investigated associations of clinical outcomes with irAEs in UC patients treated with PD-1/PD-L1 checkpoint inhibitors. The incidence of patients experiencing at least one irAE in the cohort was 35%, and the highest rate of occurrence was in the skin. UC patients who experienced an irAE, and specifically a dermatologic irAE, had significantly improved OS, PFS, and CB compared to those who did not experience an irAE. These findings suggest that irAE’s are associated with improved survival measures, and could forecast better outcomes for a patient with advanced urothelial cancer undergoing ICI therapy. IrAEs represent a unique development to our understanding at the intersections of autoimmunity, cancer biology, and immunology, and their occurrence raises questions of costs and benefits to the patient. IrAEs likely represent collateral damage from a robust immune response and invasion of a quiescent system ignited by checkpoint blockade. Concurrent immune activation and anti-tumor response would lead to observed improvements for patients. Taken together, irAEs may even serve as a clinical indicator of beneficial response.
The results of this study generate important contributions to the limited investigation of irAEs in urothelial cancer. In a multicenter retrospective study of urothelial cancer patients, Kijima et al found that an irAE was significantly associated with improved OS and PFS.24 Another study of 40 patients found significantly increased OS associated with only grade 2+ irAEs in urothelial cancer patients.19 In addition to corroborating significant associations between irAEs and significantly improved OS and PFS, this is the first study to report significantly increased clinical benefit associated with irAEs in advanced urothelial cancer. Interestingly, Kijima and colleagues found that only endocrine irAEs were associated with improved OS. This association between endocrine irAEs has been reported in a large study of renal cancer irAEs.18,25 In our study, we found that dermatologic irAEs were significantly associated with OS, PFS, and CB. This is the first study to find a significant association between cutaneous irAEs and improved clinical outcomes in urothelial cancer. This significant improvement in clinical outcomes associated with dermatologic irAEs is consistent with large studies of irAEs in melanoma and non-small cell lung cancers on ICIs.15,16,26 While some irAEs can become life-threatening, dermatologic irAEs are frequently minor, only require topical steroids, and often do not require withdrawal of ICI.26 Thus, the occurrence of dermatologic irAEs may be a more specific and useful tool than a non-specific irAE in forecasting benefits for patients. The notion of skin irAEs as a more practical prognostic biomarker is underscored by the still clouded picture of benefits vs. risks of other end organ irAEs and severe irAEs.12,17 Investigation into other clinical biomarkers that may predict the type and severity of irAE’s and the impact of irAE treatment in urothelial cancer are critical in providing clarity to irAEs and their utility. Radiologic, genetic, and serum markers have shown promise in helping predict outcomes from irAEs in other cancers treated with ICIs and should be carried out for urothelial cancers as well.27-29 Further investigation into biomarkers that can predict irAEs may help clinicians assess risk in balancing risk and benefit when their patient presents with an irAE. The exact mechanism of how these irAEs arise remains difficult to study and unknown. Overlap between cancer types and irAE target organ raise more questions about the balance between autoimmunity and anti-tumor efficacy that require broad molecular and clinical research to address.
The mechanism of irAEs has been theorized to be due to a varied combination of antibody cross-reactivity, cytokine release, and T-cell infiltration.13,17,30 Moreover, they bring about questions surrounding the interplay of auto-immunity and cancer biology that are essential to answer as ICIs gain prevalence in treatment. Early studies found PD-1 knockout models demonstrated a lupus-like disease, but irAEs were not seen until human clinical trials began, establishing a multifactorial mechanism to irAE’s.31,32 The risk of dermatologic irAEs and their positive response are likely individual to the patient. Indeed, increased genetic risk of cutaneous autoimmune diseases like psoriasis has been shown to associate with dermatologic irAEs and improved ICI response in urothelial cancer.28 Accordingly, histologic studies of cutaneous irAEs remain limited with respect to the diverse skin changes reported with PD-1/PD-L1 blockade but have shown similar patterns to psoriasis and other th-17 axis diseases.26 Furthermore, histologic studies of irAEs remain critical in linking theorized mechanisms to pathology. Perhaps autoimmune susceptibility lends an eager immune system ready to respond to checkpoint blockade (and generate an irAE in the process).
Yet, studies suggest a much more complex process. For one, irAE may clinically manifest similarly to known autoimmune disease, but the histology does not always match.13,33 For example, cancer patients with pre-existing autoimmune disease receiving PD-1/PD-L1 blockade have similar incidence and severity of irAEs to patients without autoimmune disease while maintaining a similar improvement to survival after the event.32,34 More likely, irAEs of treated urothelial cancer patients may represent a shared antigen-antibody response between urothelial cancer and irAE organ cells. Studies of irAEs in melanoma and non-small cell lung cancer found shared antigens and subsequent auto-antibody formation between the skin and respective cancer cells.35,36 Given that our findings align with the incidence, and positive outcomes seen between dermatologic irAEs and these cancers, their mechanism may be quite similar. While the characterization of irAEs continues, more effort in documenting specifics of the irAE are necessary. Indeed, this was an important limitation in our own study that limited further analysis regarding associations between severity and cutaneous manifestation. There are many cutaneous features that fall under dermatologic irAEs that may represent entirely different mechanisms and prognostic capabilities. Improved detailing of these events and their characteristics are critical for future retrospective and multi-center studies with thoroughly matched controls if every facet of irAEs is to be revealed.
Our study found irAEs, and specifically dermatologic irAEs, associated with increased OS, PFS, and CB. To cover possible lead-time bias, extended Cox model analysis was also performed and did not produce significant results. The extended Cox model treated irAE as a time-varying and handled immortal time-bias23 appropriately. The non-significant findings may be improved with future sample size accumulation mainly because a small number of cases and short follow-up time lead to non-significance in the extended Cox model. Further investigation into potential re-challenge is warranted given these results and recent implications if ICI rechallenge in UC.37 Taken together, these results substantiate the notion that irAEs may indeed serve as prognostic markers of beneficial response and outcomes in urothelial cancer. Additionally, the specific association of dermatologic irAEs with improved outcomes aligns with trends seen in other thoroughly studied cancers treated with ICIs. These findings add to a limited number of studies on irAEs in urothelial cancer, and hopefully generate continued investigation into their mechanism and ultimately help oncologists better discuss therapy and goals with their patients.
While we found significant results implicating irAEs with positive outcomes in urothelial cancer, there were limitations in our study. There exists the implicit possibility of selection bias because this was a retrospective study with a relatively small sample size. To reduce sampling, we included urothelial cancer subtypes in the present cohort of PD-1/PD-L1 treated patients, regardless of urothelial cancer location or prior therapies received. Patients were only followed for 6 years after initial therapy, so long-term outcomes of irAEs could not be reasonably assessed. The small sample size likely led to an underestimation of all irAE events and non-significant differences in outcomes after extended Cox analysis. Larger retrospective cohort studies will be needed to more thoroughly investigate irAEs in urothelial cancer. Because of variability in medical record documentation, characterization of skin reactions was broad, and determining severity of irAEs was very limited. Improved characterization of skin reactions, locations, and features will be important in linking mechanisms to clinical manifestation. Along with variability in irAE documentation, details regarding steroid use and severity were limited and are an important and understudied element of irAEs in urothelial cancers. Larger and longer retrospective studies across multiple medical centers are essential to better match patients and investigate specific irAEs subtype outcomes, associations, and mechanisms. Continuing to investigate irAE’s will be critical not only to illuminate our understanding of the balance between auto-immunity and cancer but also will help guide clinicians in predicting and providing the best therapy for their patients.
Conclusion
We investigated associations of clinical outcomes with irAEs in patients with urothelial cancer treated with PD-1/PD-L1 checkpoint inhibitors. We found slightly more than one-third of the patients in the study experienced an irAE, and dermatologic irAEs had the greatest incidence. Our study found that patients with advanced UC who experienced irAEs, especially dermatologic irAEs, had significantly improved clinical outcomes while undergoing ICI therapy. Specifically, patients who experienced irAEs had significantly improved OS, PFS, and CB compared to those who did not experience irAEs. These results add important contributions to the investigation ICI therapy and their irAEs for UC, begging further inquiry into their subtypes, mechanism, and use as a clinical indicator. Larger multi-center studies are needed to validate these results, and further inquiry into irAEs may lead to better forecasting and outcomes for patients with advanced urothelial cancers.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Gregory E Sanda, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Julie M Shabto, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Subir Goyal, Departments of Biostatistics and Bioinformatics, Emory University, Atlanta, GA, USA.
Yuan Liu, Departments of Biostatistics and Bioinformatics, Emory University, Atlanta, GA, USA.
Dylan J Martini, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Bassel Nazha, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Jacqueline T Brown, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Lauren B Yantorni, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Greta Anne Russler, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Sarah Caulfield, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Department of Pharmaceutical Services, Emory University School of Medicine, Atlanta, GA, USA.
Shreyas S Joshi, Department of Urology, Emory University School of Medicine, Atlanta, GA, USA.
Vikram M Narayan, Department of Urology, Emory University School of Medicine, Atlanta, GA, USA.
Haydn Kissick, Winship Cancer Institute of Emory University, Atlanta, GA, USA; Department of Urology, Emory University School of Medicine, Atlanta, GA, USA.
Kenneth Ogan, Winship Cancer Institute of Emory University, Atlanta, GA, USA; Department of Urology, Emory University School of Medicine, Atlanta, GA, USA.
Viraj A Master, Department of Urology, Emory University School of Medicine, Atlanta, GA, USA.
Bradley C Carthon, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Omer Kucuk, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Mehmet Asim Bilen, Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, USA; Winship Cancer Institute of Emory University, Atlanta, GA, USA.
Conflict of Interest
Bassel Nazha serves on the scientific advisory board for Exelixis. Bradley C. Carthon reported consulting/advisory relationships with Astellas Medivation, Pfizer, and Blue Earth Diagnostics; and travel expenses from Bristol-Myers Squibb. Mehmet Asim Bilen reported consulting/advisory relationships with Exelixis, Bayer, Bristol-Myers Squibb, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, and Sanofi; and research funding (institutional) from Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, and Pfizer. The other authors indicated no financial relationships.
Author Contributions
Conception/design: G.E.S., J.M.S., S.G., Y.L., M.A.B. Provision of study material or patients: B.N., J.T.B., L.B.Y., G.A.R., S.C., S.S.J., V.M.N., H.K., K.O., V.A.M., B.C.C., O.K. Collection and/or assembly of data: G.E.S., J.M.S., S.G., Y.L., D.J.M., M.A.B. Data analysis and interpretation: G.E.S., J.M.S., S.G., Y.L., M.A.B. Manuscript writing: G.E.S., J.M.S., S.G., Y.L., M.A.B. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2. Kamat AM, et al. Bladder cancer. The Lancet .2016;388(10061):2796-2810. [DOI] [PubMed] [Google Scholar]
- 3. Moch, H., et al. , Tumours of the urinary tract. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed. Lyon, France: IARC, 2016. [Google Scholar]
- 4. Alhalabi O, Rafei H, Bilen MA, Shah AY.. Current landscape of immunotherapy in genitourinary malignancies. Adv Exp Med Biol. 2020;1244:107-147. 10.1007/978-3-030-41008-7_6. [DOI] [PubMed] [Google Scholar]
- 5. Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66(1):42-54. 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 6. Sternberg CN, Skoneczna I, Kerst JM, et al. ; European Organisation for Research and Treatment of Cancer Genito-Urinary Cancers Group. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3–pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(1):76-86. 10.1016/S1470-2045(14)71160-X. [DOI] [PubMed] [Google Scholar]
- 7. Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32(18):1889-1894. 10.1200/jco.2013.52.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477-1488. 10.1056/nejmoa1106106. [DOI] [PubMed] [Google Scholar]
- 9. Galsky MD, Mortazavi A, Milowsky MI, et al. Randomized double-blind phase II study of maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer. J Clin Oncol. 2020;38(16):1797-1806. 10.1200/jco.19.03091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellmunt J, de Wit R, Vaughn DJ, et al. ; KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bilen MA, Shabto JM, Martini DJ, et al. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer. 2019;19(1):857. 10.1186/s12885-019-6073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morad G, Helmink BA, Sharma P, Wargo JA.. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309-5337. 10.1016/j.cell.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH.. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol. 2019;40(6):511-523. 10.1016/j.it.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886-894. 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378. 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—a systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. 10.1016/j.ctrv.2020.102134. [DOI] [PubMed] [Google Scholar]
- 18. Martini DJ, Goyal S, Liu Y, et al. Immune-Related adverse events as clinical biomarkers in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Oncologist. 2021;26(10):e1742-e1750. 10.1002/onco.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kobayashi K, Suzuki K, Hiraide M, et al. Association of immune-related adverse events with pembrolizumab efficacy in the treatment of advanced urothelial carcinoma. Oncology (Huntingt). 2020;98(4):237-242. 10.1159/000505340. [DOI] [PubMed] [Google Scholar]
- 20. Therneau, T.M., et al. , Modeling Survival Data: Extending the Cox Model. 2000: Springer. [Google Scholar]
- 21. Fisher LD, Lin DY.. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145-157. 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Nickleach Dana C, Zhang C, Switchenko JM, Kowalski J.. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS (®) macros. F1000Res. 2018;7:1955. 10.12688/f1000research.16866.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2007;167(4):492-499. [DOI] [PubMed] [Google Scholar]
- 24. Kijima T, Fukushima H, Kusuhara S, et al. Association between the occurrence and spectrum of immune-related adverse events and efficacy of pembrolizumab in Asian patients with advanced urothelial cancer: multicenter retrospective analyses and systematic literature review. Clin Genitourin Cancer. 2021;19(3):208-216.e1. 10.1016/j.clgc.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 25. Siddiqui BA, Gheeya JS, Goswamy R, et al. Durable responses in patients with genitourinary cancers following immune checkpoint therapy rechallenge after moderate-to-severe immune-related adverse events. J ImmunoTher Cancer. 2021;9(7):e002850. 10.1136/jitc-2021-002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83(5):1255-1268. 10.1016/j.jaad.2020.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nobashi T, Baratto L, Reddy SA, et al. Predicting response to immunotherapy by evaluating tumors, lymphoid cell-rich organs, and immune-related adverse events using FDG-PET/CT. Clin Nucl Med. 2019;44(4):e272-e279. 10.1097/RLU.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 28. Khan Z, Di Nucci F, Kwan A, et al. Polygenic risk for skin autoimmunity impacts immune checkpoint blockade in bladder cancer. Proc Natl Acad Sci USA. 2020;117(22):12288-12294. 10.1073/pnas.1922867117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tumeh PC, Harview Christina L, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568-571. 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Postow MA, Sidlow R, Hellmann MD.. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168. 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 31. Nishimura H, Nose M, Hiai H, Minato N, Honjo T.. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141-151. 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 32. Boland P, Pavlick Anna C, Weber J, Sandigursky S., Immunotherapy to treat malignancy in patients with pre-existing autoimmunity. J ImmunoTher Cancer. 2020;8(1):e000356. 10.1136/jitc-2019-000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. June CH, Warshauer JT, Bluestone JA.. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med. 2017;23(5):540-547. 10.1038/nm.4321. [DOI] [PubMed] [Google Scholar]
- 34. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME.. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018;168(2):121-130. 10.7326/M17-2073. [DOI] [PubMed] [Google Scholar]
- 35. Berner F, Bomze D, Diem S, et al. Association of checkpoint inhibitor–induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5(7):1043-1047. 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Moel EC, Rozeman EA, Kapiteijn EH, et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol Res. 2019;7(1):6-11. 10.1158/2326-6066.CIR-18-0245. [DOI] [PubMed] [Google Scholar]
- 37. Makrakis D, et al. Treatment rechallenge with immune checkpoint inhibitors in advanced urothelial carcinoma. Clin Genitourin Cancer. 2023;21(2):286-294. 10.1016/j.clgc.2022.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


