Abstract
Femtosecond laser (FSL) applications in corneal surgery have increased since its inception. Corneal surgery has undergone a tremendous transformation thanks to the introduction of FSL technology. This laser makes precise, three-dimensional incisions while causing minimal damage to surrounding tissue. This review updates and summarizes current and upcoming FSL applications in corneal surgery, current commercially available FSL, and its respective applications. Refractive surgery applications include laser in-situ keratomileusis flaps, refractive corneal lenticule extraction such as small incision lenticule extraction, astigmatic keratotomy, intracorneal ring segments tunnels for keratoconus including corneal allogenic intrastromal ring segments, and presbyopia treatments with intrastromal pockets for corneal inlays and intrastromal incisions (INTRACOR). Keratoplasty applications include penetrating keratoplasty trephination; superficial and deep anterior lamellar keratoplasty trephination, lamellar dissection, and tunnel creation; posterior lamellar keratoplasty donor and recipient preparation; Bowman layer transplantation donor, and recipient preparation; and stromal keratophakia. Other applications include conjunctival graft preparation in pterygium surgery, and keratopigmentation (corneal tattooing). FSL is a surgical instrument widely used in corneal surgery because it improves reproducibility and safety in many procedures.
Keywords: Anterior lamellar keratoplasty, astigmatic keratotomy, bowman layer transplantation, cornea, corneal pockets, descemet stripping automated endothelial keratoplasty, femtosecond laser, intracorneal ring segments, laser in-situ keratomileusis, penetrating keratoplasty, refractive corneal lenticule extraction, small incision lenticule extraction, stromal keratophakia
Introduction
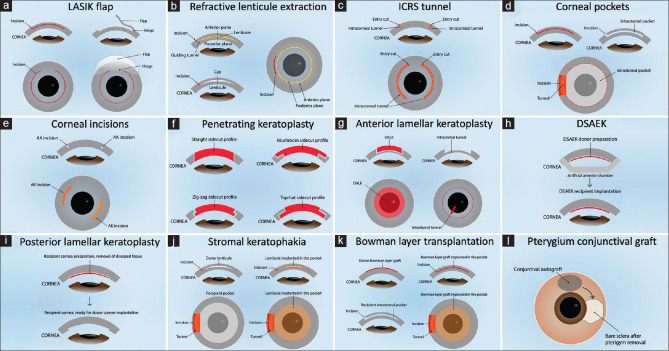
This is a narrative review of the current applications of femtosecond laser (FSL) in corneal surgery. Advantages of using an FSL over a manual blade to create corneal incisions include increased reproducibility, predictability, and safety.[1] Limitations include new complications, a learning curve, and higher costs. The first ophthalmic FSL application was the creation of flaps in laser in-situ keratomileusis (LASIK), approved in 2001.[2] Multiple applications and improvements have been developed to date: LASIK flaps, refractive corneal lenticule extraction (RCLE), intracorneal ring segments (ICRS) tunnels, astigmatic keratotomy (AK), intrastromal pockets, intrastromal incisions, penetrating and lamellar keratoplasties, stromal keratophakia, Bowman layer transplantation (BLT), pterygium and conjunctiva surgery, and others [Figure 1 and Table 1].[1-5]
Figure 1.
Femtosecond Laser Applications in Corneal Surgery. (a) Laser in-situ keratomileusis flap. (b) Refractive corneal lenticule extraction. (c) Intracorneal ring segments tunnel. (d) Intrastromal corneal pockets. (e) Corneal incisions, such as astigmatic keratotomy, intrastromal incisions, and clear cornea incisions. (f) Penetrating keratoplasty donor and recipient trephination. (g) Anterior lamellar keratoplasty, such as deep anterior lamellar keratoplasty and superficial anterior lamellar keratoplasty donor and recipient trephination and dissection. (h) Descemet’s stripping automated endothelial keratoplasty donor preparation and recipient implantation. (i) Posterior lamellar keratoplasty recipient preparation and implantation. (j) Bowman layer transplantation donor bowman graft and recipient pocket preparation. (k) Stromal keratophakia donor lenticule and recipient pocket preparation. (l) Pterygium conjunctival autograft preparation
Table 1.
Femtosecond laser applications in corneal surgery
| Surgical procedure | Use and advantages |
|---|---|
| Refractive surgery | |
| LASIK | Refractive procedure. Precise flap depth, side cut, diameter, and shape |
| RCLE | Refractive procedure. Precise refractive lenticule shape, thickness, and depth. Small incision, flap-free procedure less dry eye |
| AK | Astigmatic management. Incision of precise depth, curvature, and location. Reliable outcomes and fewer complications |
| ICRS | Keratoconus treatment. Precise tunnel depth, length, width, radius of curvature, and location. Fewer complications. Can be used with CAIRS |
| Intrastromal pocket for corneal inlay | Presbyopia management. An intrastromal pocket of precise diameter, and depth for corneal inlay implantation, for |
| INTRACOR | Presbyopia management. INTRACOR with intrastromal circular incisions to make the cornea multifocal |
| Keratoplasty | |
| PKP | Donor and host precise trephination cuts in multiple shapes (straight, zig-zag, hat, mushroom) and good donor-host apposition |
| ALK: DALK and SALK | Partial trephination, precise lamellar dissection Debulking, manual DALK, tunnel for big bubble DALK |
| PLK | DSAEK donor preparation PLK recipient preparation: Removes host posterior stroma and endothelium, followed by a DSAEK/DMEK donor cornea implantation |
| BLT | Keratoconus treatment. Donor of precise diameter and fewer tears, but slightly thicker. Recipient pocket is safe and reliable in depth and size |
| Stromal keratophakia | Keratoconus and hyperopia treatment. Precise and safe donor lenticule and recipient intrastromal pocket creation |
| Others | |
| Conjunctival graft for pterygium surgery | Ultrathin conjunctival autograft, more predictable size and depth |
| Other applications | Case reports and small series have demonstrated the feasibility of keratopigmentation (corneal tattooing), corneal biopsy, stromal drug delivery, Boston keratoplasty donor preparation, and intrastromal implantation of biopolymers and an artificial cornea |
LASIK=Laser in situ-keratomileusis, RCLE=Refractive corneal lenticule extraction, ICRS=Intracorneal ring segments, CAIRS=Corneal allogenic intrastromal ring segments, INTRACOR=Intrastromal correction of presbyopia, AK=Astigmatic Keratotomy, PKP=Penetrating keratoplasty, ALK=Anterior lamellar keratoplasty, DALK=Deep ALK, SALK=Superficial ALK, EK=Endothelial keratoplasty, DSAEK=Descemet stripping automated EK, PLK=Posterior lamellar keratoplasty, BLT=Bowman layer transplantation, DMEK=Descemet’s membrane EK
Femtosecond Laser Concepts
The FSL is a neodymium glass solid-state photo-disruptor infrared laser (wavelength: 1053 nm) that safely passes unaffected through the cornea. The FSL uses ultrafast pulses of short duration (200–600 fs), high repetition rate (150–20,000 kHz), and low energy (0.05–2.5 μJ). It produces small spots of a few microns that separate and cut the corneal tissue. It is sharply focused on the targeted tissue allowing tridimensional incisions of precise and reproducible depth, length, and shape with very little tissue damage and a smooth surface.[1-4] The FSL creates a laser-induced optical breakdown when the focused laser energy in a small spot area exceeds the electron-to-nucleus bonding energy in the tissue. This results in ionization, release of free electrons, and creation of plasma that vaporizes the focused tissue and creates cavitations of gas bubbles in the tissue. These cavitation bubbles separate and cut the tissue, and when they are in place, one after the other forms lines of cavitations that lead to incisions in the tissues [Figure 2].[1,4] An interface device is in contact with the cornea; this interface surface can be flat, curved, or noncontact liquid. The latter could help to improve cut geometry by avoiding the deformation of the cornea. Some platforms have real-time optic coherence tomography to improve visualization, precision, and incision customization. FSL specifications and availability of applications are shown in Table 2 and vary between model and brand.
Figure 2.
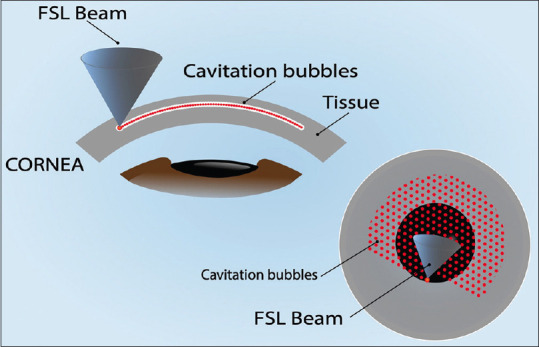
Illustration of Femtosecond Laser (FSL) Photo-disruption of Corneal Tissue. The FSL beam is sharply focused on the tissue and generates cavitation bubbles, arranged one next to the other to form lines of cavitations that separate and cut the tissue, forming incisions in the tissue
Table 2.
Current commercially available femtosecond lasers for cornea surgery*
| Model and (Brand) | Laser specifications | Corneal procedures | Cataract | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||||
| Wave length (nm) | Pulse repetition rate (kHz) | Pulse duration (fs) | Pulse energy (µJ) | Interface surface | Centration | Visuali-zation | Mobile | LASIK | RCLE | AK | ICRS | ISP | PKP | ALK | PLK | Other | ||
| Femto LDV Z8 and NEO (Ziemer, CH) | 1045 | 100–10,000 | 250 | 0.050–2.5 | Flat and curved applanation, and liquid | Semi automatic | OCT and virtual | Yes | • | CLEAR | • | • | • | • | • | • | FLAP, BLT, SKP | • |
| Femto LDV Z6 (Ziemer, CH) | 1045 | 100–10,000 | 250 | <1 | Flat applanation | Manual | Virtual | Yes | • | • | • | • | • | • | • | BLT, SKP | ||
| Femto LDV Z4 (Ziemer, CH) | 1045 | 100–10,000 | 250 | <1 | Flat applanation | Manual | Virtual | Yes | • | • | • | |||||||
| Visumax 800 (Carl Zeiss, DE) | 1043 | 2000 | 220–580 | 0.110–0.150 | Curved applanation | Semi automatic | Virtual and visual | No | • | SMILE | • | • | • | • | BLT, SKP | |||
| Visumax 500 (Carl Zeiss, DE) | 1043 | 500 | 220–580 | <1 | Curved applanation | Manual | Virtual and visual | No | • | SMILE | • | • | • | • | BLT, SKP | |||
| Wavelight FS200 (Alcon, USA) | 1030 | 200 | 350 | <2.4 | Flat applanation | Computer | Virtual and visual | No | • | • | • | • | • | |||||
| LenSx (Alcon, USA) | 1030 | 60 | 600–800 | >15 | Curved soft fit | Computer | OCT | No | • | • | • | • | • | |||||
| ELITA (J&J, USA) | 1053 | - | 150 | 0.06 | - | Computer | Virtual | No | • | SILK | • | |||||||
| iFS Intralase 150 (J&J, USA) | 1053 | 150 | 600–800 | 0.3–2.5 | Flat applanation | Computer | Virtual | No | • | • | • | • | • | • | ||||
| Catalys (J&J, USA) | 1030 | 120 | <600 | 3–10 | Liquid | Computer | OCT and virtual | No | • | • | ||||||||
| ATOS (Schwind, DE) | 1030 | Up to 4000 | <295 | 0.075–0.135 | Curved applanation | Semi automatic | Virtual | No | • | Smart-sight | ||||||||
| Victus (B&L, DE) | 1040 | 80–160 | 290–550 | 6–10 | Curved applanation | Computer | OCT and virtual | No | • | • | • | • | • | • | • | • | ||
| LensAR (LENSAR, USA) | 1030 | 80 | 500 | 7–15 | Fluid filled | Computer | OCT and virtual | No | • | • | • | • | • | |||||
| 520F (Technolas Perfect Vision, DE) | 1053 | - | - | - | Curved applanation | Manual | Visual | No | • | • | INTRACOR | |||||||
*Public information, according to the specifications and description by the manufacturer. CH: Switzerland, DE: Germany, LASIK: Flap creation, RCLE: SMILE, CLEAR, SILK and smart-sight, ICRS: Tunnel creation, ISP: Multiple uses such as presbyopia corneal inlay implantation, stromal keratophakia, bowman transplantation and drug delivery, PKP: Trephination using multiple shapes for donor and recipient, mushroom, zig-zag, Christmas tree, top hat, ALK: SALK and DALK. Donor and recipient trephination and stromal bed incisions; and big-bubble air needle tunnel creation, EK: DSAEK and PLK, AK: Partial thickness or intrastromal arcuate incisions, FLAPS: FLAPS; conjunctival autograft preparation, BLT: BLT; donor preparation and recipient pocket creation, SKP: SKP, donor lenticule and recipient stromal pocket creation. LASIK=Laser in situ-keratomileusis, RCLE=Refractive corneal lenticule extraction, SMILE=Small incision lenticule extraction, CLEAR=Cornea lenticule extraction for advanced refractive correction, SILK=Sutureless intrastromal lamellar keratoplasty, ICRS=Intracorneal ring segment, ISP=Intrastromal pocket, PKP=Penetrating keratoplasty, ALK=Anterior lamellar keratoplasty, SALK=Superficial ALK, DALK=Deep ALK, EK=Endothelial keratoplasty, DSAEK=Descemet stripping automated EK, PLK=Posterior lamellar keratoplasty, AK=Astigmatic keratotomy, FLAPS=Femtosecond laser-assisted pterygium surgery, BLT=Bowman layer transplantation, SKP=Stromal keratophakia, INTRACOR=Intrastromal correction of presbyopia, OCT=Optic coherence tomography, •=Corneal procedures that can be performed with this FSL model
Laser in-situ Keratomileusis
Over the years, LASIK has become an increasingly popular method to reduce or eliminate glasses dependency.[6] The most frequently performed FSL application in corneal surgery is the creation of the LASIK flap, known as FSL-assisted LASIK (FS-LASIK). Flap creation is a fundamental step during LASIK; the consistency and predictability of the flap thickness and diameter are crucial.[7] FSL has the advantage of creating consistent flaps with better thickness accuracy than mechanical microkeratome (MK).[7] In FS-LASIK, the flap thickness is constant across its center and periphery. In contrast, in MK-LASIK, the flap is thinnest at the center and gradually thicker toward the periphery, resulting in a meniscus shape.[8] The peripheral edge of the flap differs between MK and FSL. A study reported that the MK-LASIK flap edge had an oblique configuration, while the FS-LASIK flap edge was perpendicular to the corneal surface.[9] The FSL flap size, shape, depth, and side cut orientation can be adjusted by the surgeon by controlling its parameters. The flap is typically 8–10 mm in diameter and 90–120 μm thick [Figure 1a].[10]
Compared to MK-LASIK, the FS-LASIK has similar visual, refractive, and safety outcomes, such as mean refractive spherical equivalent (MRSE) and uncorrected visual acuity (UCVA).[11] According to a metanalysis in 2020, FS-LASIK is similar to MK-LASIK in the early and midterm follow-up and does not have a clear advantage in efficacy, accuracy, and safety profile.[12] A recent systematic review found no clear superiority of one technique regarding complications and safety between FS-LASIK and MK-LASIK.[7] However, FS-LASIK has greater flap customization (thickness, diameter, and side-cut angle) and increased predictability, accuracy, and precision of flap creation.[7,13]
A retrospective study found that the flap complication rate was similar in MK-LASIK and FS-LASIK (14.2% vs. 15.2%).[14] Two of the most reported unique intraoperative complications associated with FSL flap creation are forming an opaque bubble layer (OBL) and vertical gas breakthrough (VGB). OBL is the accumulation of gas bubbles temporarily detained in the intrastromal interface, creating transient corneal opacity. Excessive OBL may interfere with eye tracker pupil recognition and generate difficulties in flap lifting.[15] No severe complications have been reported after OBL.[15] VGB can occur when cavitation bubbles dissect superiorly toward Bowman’s layer and through the epithelium. Risk factors include a thin flap, corneal scar, previous radial keratotomy, and microscopic breaks in the Bowman layer (BL).[16] Intraoperative complications were more frequent with earlier FSL generations. Two unique postoperative complications of FS-LASIK are transient light sensitivity syndrome (TLSS) and rainbow glare. TLSS occurs 2–6 weeks after uneventful LASIK. It is characterized by sudden and severe episodes of light sensitivity and eye discomfort that occur spontaneously or in response to bright lights despite having good UCVA and minimal slit lamp findings. TLSS improves with topical steroids, which is believed to be related to the changes in the corneal nerves following surgery.[16] Rainbow glare is characterized by the appearance of colored rings or halos around point light sources (headlights or streetlamps), particularly in low-light conditions. It is a self-limiting condition, lasting up to several months after surgery. Even with the latest advances in FSL technology, rainbow glare remains a mild optical side effect, although it does not appear to interfere with visual acuity.[7]
Refractive Corneal Lenticule Extraction
RCLE is a minimally invasive new technique to correct refractive errors that require only the FSL. It creates a small incision in the cornea and a refractive lens-shaped lenticule of stromal tissue removed through the incision, eliminating the need for a corneal flap [Figures 1b and 3].[5] Small incision lenticule extraction (SMILE) was the first technique and has gained popularity among ophthalmologists worldwide. RCLE with FSL and flap (ReLEx FLEx) was the precursor to SMILE, which involves creating a flap in the cornea using an FSL to access and remove a lenticule. While ReLEx FLEx is still used in some centers, SMILE has become the preferred technique due to its advantages in visual recovery, patient comfort, and the potential for fewer complications.[5,17] One of the main advantages of SMILE is that it preserves more of the anterior corneal tissue and does not require a flap, preventing flap-related complications and quicker recovery of dry eye.[5,18] It is theoretically speculated that SMILE retains more corneal biomechanical strength; however, conflicting results are favoring either SMILE[19] and FS-LASIK.[20]
Figure 3.
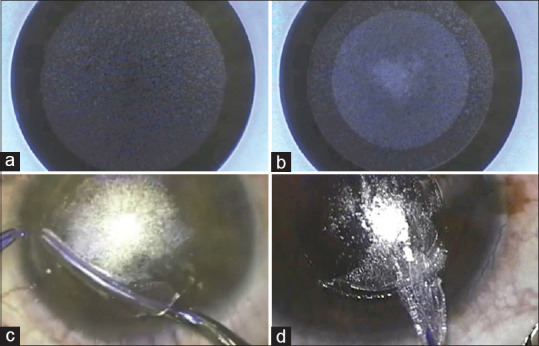
Small Incision Lenticule Extraction Procedure. (a) Posterior surface of lenticule cut, (b) Anterior surface of lenticule cut, (c) Lenticule dissection, (d) Lenticule extraction
SMILE treats myopia with and without astigmatism[21,22] and hyperopia with and without astigmatism.[23,24] Most of the SMILE publications have been on myopia. The excellent results of current corneal laser refractive surgery make it difficult to find clear superiority between LASIK, PRK, or SMILE, and the results are generally comparable. Myopic SMILE has achieved similar outcomes to FS-LASIK concerning visual acuity, refractive error, and proportion of cases with loss of lines of best-corrected visual acuity in many studies.[5,21,22,25] SMILE could induce less dry eye and less damage to the corneal nerves in myopia and high myopia.[22]
In a systematic review and meta-analysis of 18 studies that included 3,466 eyes, the postoperative MRSE was -0.02 D, and the postoperative UDVA was 20/20 or better in 94.5% of eyes.[22] A 2016 systematic review and meta-analysis found similar outcomes in safety and efficacy.[22] Another more recent systematic review and meta-analysis of 11 randomized clinical trials published in 2023 also found that SMILE and FS-LASIK were comparable concerning the safety and efficacy as shown as follows: residual MRSE (mean difference (MD)-0.04, P = 0.22), the proportion of eyes losing one or more lines of corrected distance visual acuity (CDVA) (risk ratio [RR]: 1.14; P = 0.70), the proportion of eyes with UCVA of 20/20 or better (RR: 0.99; 95% confidence interval [CI], 0.94–1.05; P = 0.71), postoperative UCVA (MD: 0.01; P = 0.13), residual refraction within ± 1.0 D (RR: 1.00; P = 0.60), postoperative astigmatism within ± 0.25, 0.5 and 1.0 D (RR: 0.80, 0.99, 1.00; P = 0.60, 0.86, 0.87), and postoperative higher order aberrations (RR: 0.00; P = 0.99). However, FS-LASIK might be superior in predictability with a higher proportion of eyes within 0.5D, and SMILE might have fewer spherical aberrations.[21] Studies have confirmed the safety of SMILE as similar or better than FS-LASIK,[21,22] including a lower risk of dry eye disease,[22] and less corneal nerve damage.[26] Potential complications include suction loss, cap rupture, epithelial ingrowth, lenticule remnants, interface inflammation, epithelial defects, and delayed epithelialization.[27]
In addition to SMILE, other new RCLE techniques use the same basic principles of SMILE to correct refractive errors. Cornea lenticule extraction for advanced refractive-correction (CLEAR) and SmartSight minimally invasive lenticule extraction. CLEAR uses a smaller incision (1.5 mm) than SMILE (2.5 mm) and can correct higher levels of myopia. SmartSight is a modified version of SMILE that leaves a portion of the lenticule in the cornea to improve corneal biomechanical stability.[5,28,29] Overall, RCLE is a promising advancement in refractive surgery.
Astigmatic Keratotomy
A variety of corneal incisions can be performed. AK or arcuate incision to reduce corneal astigmatism has been used for many years. Peripheral corneal or limbal relaxing incisions are like AK but placed more peripherally. Cutting the cornea with a paired or single AK into a specific depth reduces astigmatism by flattening the corneal curvature in one axis.[30] Manual AK (MAK) is performed with a fixed or adjustable depth diamond blade. A limitation is that the depth, arc length, and regularity of corneal incisions during surgery may be unpredictable, leading to a higher risk of complications such as inadvertent perforation, wound dehiscence, epithelial downgrowth, infection, irregular astigmatism, or astigmatism under-correction. FSL-assisted AK (FSAK) can create more controlled and symmetric arcuate incisions of precise depth, length arc, shape, and location [Figure 1e].[30-33] Thus, making FSAK safer, more effective, more predictable, and more reliable than MAK and with fewer complications.[30,31,34] FSAK can customize the incision characteristics, usually 75%–90% in-depth, but can also be intrastromal (IAK), avoiding the risk of infection. FSAK can correct naturally occurring or induced astigmatism after various types of surgeries, such as penetrating keratoplasty,[32,33,35] deep anterior lamellar keratoplasty (DALK),[35] cataract surgery,[36] and glaucoma surgery.[30,31] In addition, FSL-assisted wedge resection has been reported as an effective way to correct high astigmatism after PKP.[37] The amount of astigmatism that can be reduced with FSAK is significantly higher, up to 2.5–4.5D, compared to MAK, which typically reduces up to 1.5 D. FSAK can achieve more significant improvements than MAK in CDVA, UCVA, corneal and manifest astigmatism, success index, and correction index.[32,33,35,38]
Intracorneal Ring Segments
A valuable application of FSL is the creation of intrastromal tunnels to insert ICRS in managing ectatic corneal disorders such as keratoconus.[1,39] Corneal ectatic diseases are characterized by progressive thinning and bulging of the cornea, resulting in irregular astigmatism, visual distortion, and reduced visual acuity.[39] The insertion of ICRS has been in use since the year 2000; it is a well-established treatment for these disorders, as it can help to reshape the cornea and improve visual function.[39-41] The ICRS are medical devices made of polymethyl methacrylate with variable thickness, geometries, and diameters. ICRS induces flattening of the cornea depending on the ICRS thickness and distance from the center of the cornea.[41]
Traditional ICRS insertion methods involve manual cornea dissection to create an intrastromal tunnel. Then the ICRS is inserted into the pocket or tunnel, where it exerts pressure on the cornea to reshape it and improve visual function. Associated complications include corneal perforation, infection, and implant displacement. In FSL-assisted ICRS implantation, the laser creates an intrastromal corneal pocket or tunnel of a desired depth, diameter, angle, and shape.[Figures 1c and 4].[41] The advantages of FSL are the precision, consistency, and reliability in creating the intrastromal corneal pocket or tunnel. FSL reduces the risk of complications and improves the accuracy of ICRS placement.[41] FSL can create a customized pocket or tunnel that fits the size and shape of the ICRS. This could help optimize visual outcomes and reduce the risk of infection and implant displacement, as the pocket or tunnel is created without direct contact with the cornea.[42]
Figure 4.
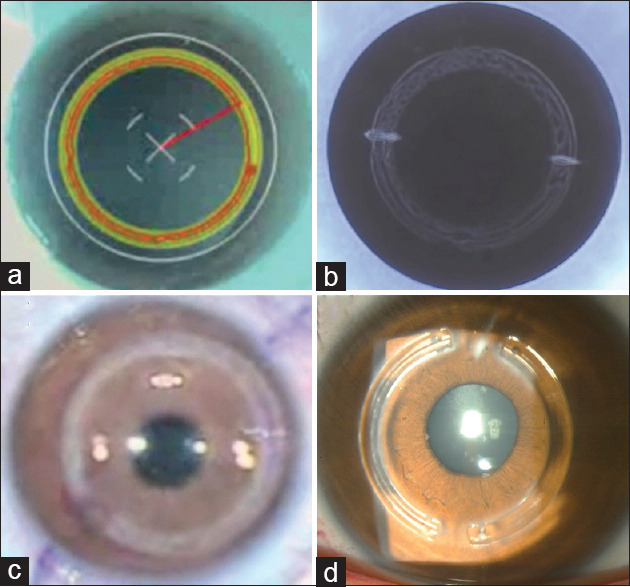
Intracorneal Ring Segments. (a) Femtosecond laser screen showing laser treatment plan, (b) Femtosecond laser pattern and incisions, (c) Femtosecond laser intrastromal tunnels, (d) Clinical picture after intrastromal ring segments implantation
Several studies have shown that FSL-assisted ICRS insertion is a safe and effective treatment for corneal ectatic disorders. There is a clear advantage of fewer intraoperative complications with FSL than traditional manual tunnel creation.[43,44] Complications include corneal perforation, infection, and implant displacement, although rare and significantly less frequent than mechanical techniques.[43,44] Regarding visual, refractive, and aberrometric outcomes, FSL and mechanical techniques offer similar outcomes.[40,41,43,44] A recent similar technique of FSL tunnel creation has been used in conjunction with corneal allogenic intrastromal ring segments as a potential treatment option for corneal ectasias.[45]
Presbyopia Management with Femtosecond Laser
There are two FSL corneal applications for presbyopia, the intrastromal correction of presbyopia (INTRACOR) and an intrastromal pocket for corneal inlay implantation. The INTRACOR creates five intrastromal circular incisions 2–4 mm in diameter placed around the pupil of the nondominant eye. This induces topographic and aberrometric changes that make a multifocal cornea with a central steepening of 1–2 D.[46] It can be performed with the Technolas® FSL (Technolas Perfect Vision®, Germany). Patients showed improvement in binocular uncorrected near vision, with moderate patient satisfaction.[46-48] However, concerns about the loss of UCVA and CDVA and the irreversibility of the procedure limited its use. Further evaluation is needed.[47,48]
Several models of intrastromal corneal inlays for the management of presbyopia have been developed. Currently, only two are commercially available, Kamra and Flexivue. Kamra increases the depth of focus with a pinhole effect; it is a small aperture inlay of 3.8 mm made of polyvinylidene difluoride and pigmented with carbon nanoparticles. Flexivue is a bifocal transparent refractive inlay of 3.2 mm made of a biocompatible hydrophilic acrylic material, with a central plano refraction and a peripheral plus correction from +1.5 to +3.5 D.[49] The manufacturers recommend the use of FSL to create the intrastromal pocket and to implant the corneal inlay in the nondominant eye.[49] The FSL can create intrastromal pockets of a reliable and precise depth (200–300 μm), diameter (9.2–9.5 mm), and centration [Figure 1d].[49] The inlay must be centered in the pupillary center and the first Purkinje image.[49] In principle, it is a reversible procedure. A systematic review showed that all improved near UCVA, and patients were moderately satisfied.[49,50] There are concerns about long-term complications since many will lose one or more lines of CDVA.[49,50] Other complications are refractive instability, decentration of the inlay, corneal haze with a potential loss of CDVA, infectious keratitis, and epithelial ingrowth.[49] Recently, allograft corneal implants made of SMILE lenticules have been used to correct presbyopia and hyperopia with promising outcomes.[51]
Penetrating Keratoplasty
Since the beginning of corneal transplantation in the year 1905, improvements have been made to achieve better outcomes. Lamellar keratoplasty is the preferred option for corneal transplantation. Full-thickness keratoplasty should be reserved for advanced-stage diseases that compromise all the layers of the cornea.[52] Conventional PKP with manual trephination achieves good results and vision improvement; however, induced astigmatism affects visual outcomes.[53,54] Induced astigmatism can be secondary to the sutures, scarring, and irregular corneal borders.
Manual trephination can cause irregular borders that affect the graft-host junction. An eccentric trephination is generated when the trephine is placed decentered, and the graft and host curvatures will not match. When the trephine is tilted, an oval opening can happen. Manual trephination is performed in the host cornea from the epithelial and donor cornea from the endothelial sides. Due to this, the posterior cornea of the recipient tends to have a larger diameter than the anterior cornea, while the donor cornea experiences the opposite effect; this is known as vertical tilt. A horizontal torsion occurs when the two first cardinal sutures are not placed symmetrically.[55] For these reasons, new technologies and instrumentation have been developed to make more precise cuts with better geometry.[56]
FSL-assisted PKP (femto-PKP) was first used in the year 2005 to trephine the host and donor corneas accurately.[57] FSL allows trephination to the donor and host corneas, improving incision geometry with more accurate angles and avoiding vertical tilt.[56] Different trephination shapes and patterns have been explored to enhance donor-host union adherence, such as straight cut, zig-zag, mushroom, top hat, and Christmas tree [Figure 1f].[58] These configurations increase the contact area between the graft and host, improving wound scarring and enhancing the strength of the donor-host union.[59] It is hypothesized that this reduces the risk of traumatic wound dehiscence, but no evidence supports it. This allows for earlier removal of stitches,[60] reducing the risks of suture-related infections and speeding up visual rehabilitation. An artificial anterior chamber is needed to perform FSL donor PKP trephination. It is performed from the epithelial side and prevents vertical tilt. In addition, FSL can create orientation marks in the donor and recipient cornea to enhance the placement of the cardinal sutures and reduce the risk of horizontal torsion.[61]
Femto-PKP achieves good visual outcomes with low degrees of postoperative astigmatism. However, two meta-analyses failed to show significant differences in induced astigmatism between femto-PKP and manual PKP.[62,63] This might be because postoperative astigmatism is multifactorial, depending on suturing and scarring process after PKP. An advantage of femto-PKP was founded in a meta-analysis that showed significantly lower endothelial cell loss.[62] Different platforms are used to perform femto-PKP, and there are variations in their interface with the cornea, which can be flat, curved, or noncontact liquid. The noncontact could help to improve cut geometry by avoiding the deformation of the cornea.[56] FSL with real-time ASOCT can have more customizable incisions.
Deep anterior Lamellar Keratoplasty
It is indicated in corneal diseases affecting the anterior stroma with a healthy endothelium, such as keratoconus, stromal dystrophies, and scars.[52] DALK has some advantages compared to PKP, for example, less risk of rejection, less risk of glaucoma, and avoiding the risk of open sky surgery.[64] DALK is performed using techniques such as the manual, big bubble,[65] and viscoelastic dissection.[66] All are complex techniques, time-consuming, with a steep learning curve, and sometimes with unexpected outcomes.[67] FSL technology has allowed for more precise and predictable dissection during DALK.[68-70]
A primary advantage of FSL-assisted DALK (FSL DALK) is its ability to remove damaged stroma by creating a precise and reproducible predescemetic dissection at the recipient cornea. This allows for a better interface junction with the stromal donor cornea.[68,69,71] In addition, like PKP, the laser can be used to customize the shape and size of the corneal graft, resulting in a more accurate tight fit.[69] It can create different cutting patterns, including zig-zag, mushroom, and inverted mushroom. These interfaces reduce the risk of irregular astigmatism and allow early sutural removal.[58] Another advantage of FSL DALK is the reduced risk of intraoperative complications such as perforation.[71] The laser allows for a more controlled and less traumatic dissection of the corneal tissue. This reduces the risk of damage to the surrounding structures and creates a smooth stromal bed in the recipient cornea. Evidence has demonstrated that FSL DALK exhibits a lower incidence of corneal perforation and a reduced need for conversion to PKP compared to manual DALK.[71]
The FSL can be used to create an intrastromal guiding tunnel for the big bubble technique. Studies have described tunnel placement at a distance ranging from 50 to 130 μ away from Descemet’s membrane (DM).[68,72,73] FSL improves the precision and predictability of the tunnel, reducing the risk of perforation or incomplete big-bubble formation [Figure 1g].[73] FSL DALK has shown promise in improving the success of big-bubble formation.
Multiple studies comparing the visual outcomes of FSL and manual DALK have demonstrated similar results.[69,70,74-76] Except for one study that found better visual outcomes at 1 year with FSL DALK using FSL only to trephine the cornea, the lamellar dissection was manually performed with a diamond knife.[77] One[77] study reported better CDVA 3 months after FSL DALK compared to manual DALK but similar outcomes after 1 year.[75] FSL DALK may contribute to faster visual recovery, leading to better CDVA in the early postoperative period. There[74] are no significant differences in the refractive outcomes between manual and FSL DALK.[69,75,76] The safety of FSL DALK has been supported by studies showing that the endothelial cell density remains stable from 1 month to 2 years after surgery.[76,77] Further research is needed to enhance the technique, maximize its benefits and ensure consistent outcomes.
Posterior Lamellar Keratoplasty
Posterior lamellar keratoplasty (PLK) is an advanced surgical technique used to treat endothelial dysfunction of the cornea.[52] This procedure has gained popularity due to its numerous advantages over traditional PKP.[78] PLK offers improved visual and refractive outcomes, reduces the risk of rejection, and has become the preferred choice when the corneal stroma remains healthy.[78] The two main variants of PLK, Descemet’s stripping automated endothelial keratoplasty (DSAEK), and DM endothelial keratoplasty (DMEK), have revolutionized the treatment of endothelial dysfunction, ensuring better patient outcomes and faster visual recovery.[79] FSL facilitates the precise formation of the descemetorhexis on the receptor cornea during DSAEK and DMEK procedures.[80,81] In addition, it is utilized to prepare the donor graft for DSAEK surgery, ensuring optimal fit and enhancing surgical outcomes.[82]
Traditional methods of preparing donor grafts for DSAEK involve using an MK, which carries a high risk of perforation and lacks predictability in graft thickness.[83] To address these limitations, FSL technology has been studied as an alternative approach. FSL allows for donor graft preparation using an artificial anterior chamber, starting the procedure from the epithelial side [Figure 1h].[84] After the creation of the lenticule with FSL, it is dissected, trephined, and prepared to be used with the conventional DSAEK technique. Conventional FSL preparation can result in a rough graft surface due to scattered laser energy in the posterior stroma or because of the posterior stromal collagen arrangement.[85,86] Studies have explored the feasibility of endothelial-side preparation using a viscoelastic coating for protection.[87] These studies have shown promising outcomes, including smoother graft surfaces and minimal loss of endothelial cells. One study has documented the utilization of FSL for DMEK graft preparation. In this study, FSL-assisted trephination was conducted before the peeling of the DM to mitigate the potential occurrence of peripheral tears caused by the manual trephination process and subsequent embedding of the DM into the stromal tissue.[88]
Recipient preparation during PLK consists of a circular peeling of DM, usually called descemetorhexis, commonly performed using inverse hooks. However, the diameter of the descemetorhexis tends to be unpredictable, and small fragments of DM remain attached to the stromal tissue. Such residual fragments can compromise the adherence of the PLK graft, increase the rates of rebubbling, and compromise the overall success of the graft.[89] FSL technology has been utilized in recipient preparation for descemetorhexis, enabling the creation of a posterior lenticule with a thickness ranging from 100 to 150 microns. The lenticule has the same in which endothelium, DM, and a thin layer of the posterior stroma are removed. The lenticule has a diameter like the graft.[80] This lenticule is removed to expose the stroma that will receive the graft [Figure 1i]. Comparative studies demonstrated that manual descemetorhexis had a higher incidence of graft detachment on the first postoperative day than FSL-assisted descemetorhexis. However, it is important to note that these studies reported similar visual outcomes and similar rates of endothelial cell loss across both groups.[80,90] In addition, the utilization of FSL for descemetorhexis enables the removal of the DM and endothelium and aids in removing posterior stromal scarring. Removing scar tissue is crucial as it can impact the visual outcomes of PLK procedures. The integration of FSL technology in PLK procedures has facilitated the precise formation of the descemetorhexis during DSAEK and DMEK, ensuring optimal graft fit and enhancing surgical outcomes. FSL-assisted graft preparation for DSAEK has shown reproducible lenticule creation avoiding de MK complications. Future research should focus on refining FSL-assisted techniques and exploring additional benefits in PLK procedures.
Stromal Keratophakia
Stromal keratophakia is an additive refractive surgery; no tissue is removed from the cornea. Manual stromal keratophakia was almost abandoned because of the difficulties and limitations of shaping a donor stromal lenticule and creating a smooth and regular host stromal pocket to implant the donor lenticule.[91] FSL-assisted stromal keratophakia revolutionized the procedure by creating reliable lenticules and pockets. It can create intrastromal pockets with smooth and uniform surfaces, reliable depth, and diameter. It can shape donor stromal lenticules of specific thickness, diameter, and curvature to be further implanted into the host stromal pocket [Figure 1j].[91]
The donor lenticule can be obtained either from SMILE lenticules or donor corneas.[91] SMILE lenticules (~6–7 mm in diameter) can be frozen, stored, and decellularized. They can be reshaped to a precise thickness, diameter, and radius of curvature. These lenticules can be either convex or concave, plus, or minus lens-shaped. The intrastromal pocket is usually at 100–250 μm depth.[4,91,92] This procedure is particularly promising as an alternative treatment to avoid keratoplasty in keratoconus and can be combined with cross-linking. A recent meta-analysis found it safe, with no reported adverse events of persistent haze, perforation, or rejection. Meanwhile, significantly improving UCVA (2 lines) and CDVA (1.7 lines), reducing MRSE (-2.3D), flattening the cornea (-3D of Kmax), increasing corneal thickness, and improving corneal sphericity or Q-value.[92] Three options of SMILE lenticule have been studied in keratoconus treatment, doughnut-shaped (a lenticule with a central trephination), hyperopic, and myopic SMILE lenticules.[92] Stromal keratophakia has also been reported as a feasible option to treat high hyperopia,[93] aphakia, and presbyopia.[91] A variant of stromal keratophakia is lenticule addition keratoplasty,[91] where stored SMILE lenticules could be used in various procedures such as tectonic keratoplasty,[94-96] corneal or scleral thinning after pterygium,[97] or limbal dermoid excision.[49,98]
Bowman’s Layer Transplantation
Bowman’s layer transplantation (BLT) is a new keratoconus treatment aimed to delay or avoid keratoplasty in corneas too thin or too advanced, not candidates for corneal cross-linking or ICRS.[99] This is an additive procedure because no tissue is removed from the host. The donor BL is implanted into an intrastromal host pocket in the mid-stroma. The limitations of manually peeling BL are less predictability, graft tearing, irregular surface, and irregular borders. Manual intrastromal pocket dissection limitations include the risk of perforation on the anterior or posterior corneal surface, unpredictable depth, and uneven surface.[100] FSL-assisted BLT is a newer and more promising procedure. The advantage is that it makes BL grafts more predictable and reproducible with no tears, regular borders, and even surfaces, although slightly thicker than manual dissection.[101] FSL-assisted mid-stromal pockets have more predictable depth and no perforations [Figure 1k].[101-103] A recent paper reported 97 and 100% successful BL grafting and pocket creation, respectively.[103] The FSL could be programmed using the anterior lamellar keratoplasty software to obtain BL graft and the intrastromal pocket standard software. The outcomes of BLT are encouraging for flattening the cornea, stabilizing the keratoconus, and stabilising the keratoconus while maintaining CDVA.[99,100,103]
Pterygium Surgery
FSL-assisted pterygium surgery has been recently proposed as an alternative to achieve ultrathin conjunctival autograft (CAG) of more predictable size and thickness than the gold standard manual preparation [Figure 1l].[104,105] The CAG was prepared using the lamellar keratoplasty module to achieve a 7 mm × 10 mm diameter ellipsoidal shape and 60 μm depth.[105] One comparative study found that FSL-assisted CAG achieved thinner grafts and less thickness variability. No significant differences in CDVA, recurrence rate, astigmatism, or discomfort were noted.[105] Cosmetic outcomes were graded good-to-excellent in 93% of cases.[106]
Other Procedures
Some studies have reported the feasibility of using the existing software to expand the FSL applications in corneal surgery. A case series on FSL-assisted keratopigmentation (corneal tattooing) uses the ICRS software to create intrastromal channels and corneal incisions of precise depth to safely place the pigments into the corneal stroma.[107] A long-term study reported keratopigmentation safety and efficacy, with all patients improving symptoms and cosmetics and 95% improving CDVA.[108] A case report shows how to make a corneal biopsy using the anterior lamellar keratoplasty or the flap software.[109] A case report on Boston type 1 keratoprosthesis donor cornea preparation uses FSL to have precise centration of the peripheral and the inner 3-mm central trephination.[110] A case report used partial thickness corneal incisions with ICRS software as an alternative method for drug delivery in nonresponding intrastromal infectious keratitis.[111] Other case reports use FSL-assisted intrastromal pockets to implant intrastromal KeraKlear artificial cornea in congenital aniridia[112] or insert silicon oil as palliative management of bullous keratopathy in blind eyes.[113]
The Future
Two nonablative laser procedures under development are worth mentioning,[4,29] the laser-induced refractive index change (LIRIC) and the nonlinear optical corneal cross-linking (NLO CXL). The LIRIC is a tissue-sparing procedure that uses low-pulse energy, below the damage threshold, to induce intrastromal changes, increase the refractive index and modify the aberrations of the cornea. LIRIC may be a promising alternative for those not candidates for LASIK or RCLE.[93,114] The NLO CXL is a promising alternative to cross-linking to halt keratoconus progression by creating intrastromal stiffening and flattening through low-energy intrastromal laser pulses and epithelial channels creation that improve riboflavin penetration into the stroma.[115,116] Finally, SMILE-derived stromal lenticules can be used as a scaffold for regenerative therapy.[117]
Conclusions
The FSL has earned its place in contemporary corneal surgery as a versatile and valuable tool that enables the creation of three-dimensional corneal incisions of great precision, reproducibility, and safety. Its utility and advantages have been proven in corneal refractive surgery, keratoplasty, and other corneal and ocular surface diseases.
Declaration of patient consent and Ethical approval
The institutional review board of hospital approved this study (Number: CE-2018-0032). Informed consent was waived by the IRB.
Data availability statements
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this pape.
Acknowledgments
The development of the illustrations owes a great deal to the significant contributions made by Hector Leos Olvera and Carlos Augusto Lopez Acevo.
References
- 1.Han SB, Liu YC, Mohamed-Noriega K, Mehta JS. Application of femtosecond laser in anterior segment surgery. J Ophthalmol. 2020;2020:8263408. doi: 10.1155/2020/8263408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callou TP, Garcia R, Mukai A, Giacomin NT, de Souza RG, Bechara SJ. Advances in femtosecond laser technology. Clin Ophthalmol. 2016;10:697–703. doi: 10.2147/OPTH.S99741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sioufi K, Zheleznyak L, MacRae S, Rocha KM. Femtosecond lasers in cornea and refractive surgery. Exp Eye Res. 2021;205:108477. doi: 10.1016/j.exer.2021.108477. [DOI] [PubMed] [Google Scholar]
- 4.Latz C, Asshauer T, Rathjen C, Mirshahi A. Femtosecond-laser assisted surgery of the eye: Overview and impact of the low-energy concept. Micromachines (Basel) 2021;12:122. doi: 10.3390/mi12020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuest M, Mehta JS. Advances in refractive corneal lenticule extraction. Taiwan J Ophthalmol. 2021;11:113–21. doi: 10.4103/tjo.tjo_12_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkler von Mohrenfels C, Khoramnia R, Salgado J, Wüllner C, Donitzky C, Maier M, et al. First clinical results with a new 200 kHz femtosecond laser system. Br J Ophthalmol. 2012;96:788–92. doi: 10.1136/bjophthalmol-2011-300073. [DOI] [PubMed] [Google Scholar]
- 7.Kanclerz P, Khoramnia R. Flap thickness and the risk of complications in mechanical microkeratome and femtosecond laser in situ keratomileusis: A literature review and statistical analysis. Diagnostics (Basel) 2021;11:1588. doi: 10.3390/diagnostics11091588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn H, Kim JK, Kim CK, Han GH, Seo KY, Kim EK, et al. Comparison of laser in situ keratomileusis flaps created by 3 femtosecond lasers and a microkeratome. J Cataract Refract Surg. 2011;37:349–57. doi: 10.1016/j.jcrs.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Shetty R, Malhotra C, D’Souza S, Wadia K. WaveLight FS200 versus hansatome LASIK: Intraoperative determination of flap characteristics and predictability by hand-held bioptigen spectral domain ophthalmic imaging system. J Refract Surg. 2012;28:S815–20. doi: 10.3928/1081597X-20121005-01. [DOI] [PubMed] [Google Scholar]
- 10.Farjo AA, Sugar A, Schallhorn SC, Majmudar PA, Tanzer DJ, Trattler WB, et al. Femtosecond lasers for LASIK flap creation: A report by the American Academy of Ophthalmology. Ophthalmology. 2013;120:e5–20. doi: 10.1016/j.ophtha.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Eldaly ZH, Abdelsalam MA, Hussein MS, Nassr MA. Comparison of laser in situ keratomileusis flap morphology and predictability by WaveLight FS200 femtosecond laser and moria microkeratome: An anterior segment optical coherence tomography study. Korean J Ophthalmol. 2019;33:113–21. doi: 10.3341/kjo.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahuam-López N, Navas A, Castillo-Salgado C, Graue-Hernandez EO, Jimenez-Corona A, Ibarra A. Laser-assisted in-situ keratomileusis (LASIK) with a mechanical microkeratome compared to LASIK with a femtosecond laser for LASIK in adults with myopia or myopic astigmatism. Cochrane Database Syst Rev. 2020;4:CD012946. doi: 10.1002/14651858.CD012946.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZH, Jin HY, Suo Y, Patel SV, Montés-Micó R, Manche EE, et al. Femtosecond laser versus mechanical microkeratome laser in situ keratomileusis for myopia: Metaanalysis of randomized controlled trials. J Cataract Refract Surg. 2011;37:2151–9. doi: 10.1016/j.jcrs.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 14.Duffey RJ. Thin flap laser in situ keratomileusis: Flap dimensions with the moria LSK-One manual microkeratome using the 100-microm head. J Cataract Refract Surg. 2005;31:1159–62. doi: 10.1016/j.jcrs.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 15.Courtin R, Saad A, Guilbert E, Grise-Dulac A, Gatinel D. Opaque bubble layer risk factors in femtosecond laser-assisted LASIK. J Refract Surg. 2015;31:608–12. doi: 10.3928/1081597X-20150820-06. [DOI] [PubMed] [Google Scholar]
- 16.Sahay P, Bafna RK, Reddy JC, Vajpayee RB, Sharma N. Complications of laser-assisted in situ keratomileusis. Indian J Ophthalmol. 2021;69:1658–69. doi: 10.4103/ijo.IJO_1872_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamiya K, Kobashi H, Shimizu K, Igarashi A. Clinical outcomes of penetrating keratoplasty performed with the VisuMax femtosecond laser system and comparison with conventional penetrating keratoplasty. PLoS One. 2014;9:e105464. doi: 10.1371/journal.pone.0105464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah R, Shah S, Sengupta S. Results of small incision lenticule extraction: All-in-one femtosecond laser refractive surgery. J Cataract Refract Surg. 2011;37:127–37. doi: 10.1016/j.jcrs.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 19.Guo H, Hosseini-Moghaddam SM, Hodge W. Corneal biomechanical properties after SMILE versus FLEX, LASIK, LASEK, or PRK: A systematic review and meta-analysis. BMC Ophthalmol. 2019;19:167. doi: 10.1186/s12886-019-1165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashemi H, Roberts CJ, Elsheikh A, Mehravaran S, Panahi P, Asgari S. Corneal biomechanics after SMILE, femtosecond-assisted LASIK, and photorefractive keratectomy: A matched comparison study. Transl Vis Sci Technol. 2023;12:12. doi: 10.1167/tvst.12.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao L, Zhang M, Wang D, Zhao Q, Wang S, Bai H. Small incision lenticule extraction (SMILE) and laser in situ keratomileusis (LASIK) Used to treat myopia and myopic astigmatism: A systematic review and meta-analysis of randomized clinical trials. Semin Ophthalmol. 2023;38:283–93. doi: 10.1080/08820538.2022.2107399. [DOI] [PubMed] [Google Scholar]
- 22.Shen Z, Shi K, Yu Y, Yu X, Lin Y, Yao K. Small Incision lenticule extraction (SMILE) versus femtosecond laser-assisted in situ keratomileusis (FS-LASIK) for myopia: A systematic review and meta-analysis. PLoS One. 2016;11:e0158176. doi: 10.1371/journal.pone.0158176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moshirfar M, Bruner CD, Skanchy DF, Shah T. Hyperopic small-incision lenticule extraction. Curr Opin Ophthalmol. 2019;30:229–35. doi: 10.1097/ICU.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 24.Reinstein DZ, Sekundo W, Archer TJ, Stodulka P, Ganesh S, Cochener B, et al. SMILE for hyperopia with and without astigmatism: Results of a prospective multicenter 12-month study. J Refract Surg. 2022;38:760–9. doi: 10.3928/1081597X-20221102-02. [DOI] [PubMed] [Google Scholar]
- 25.Han T, Xu Y, Han X, Zeng L, Shang J, Chen X, et al. Three-year outcomes of small incision lenticule extraction (SMILE) and femtosecond laser-assisted laser in situ keratomileusis (FS-LASIK) for myopia and myopic astigmatism. Br J Ophthalmol. 2019;103:565–8. doi: 10.1136/bjophthalmol-2018-312140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed-Noriega K, Riau AK, Lwin NC, Chaurasia SS, Tan DT, Mehta JS. Early corneal nerve damage and recovery following small incision lenticule extraction (SMILE) and laser in situ keratomileusis (LASIK) Invest Ophthalmol Vis Sci. 2014;55:1823–34. doi: 10.1167/iovs.13-13324. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez-Miranda A, Ramirez-Luquin T, Navas A, Graue-Hernandez EO. Refractive lenticule extraction complications. Cornea. 2015;34(Suppl 10):S65–7. doi: 10.1097/ICO.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 28.Pradhan KR, Arba-Mosquera S. Three-month outcomes of myopic astigmatism correction with small incision guided human cornea treatment. J Refract Surg. 2021;37:304–11. doi: 10.3928/1081597X-20210210-02. [DOI] [PubMed] [Google Scholar]
- 29.Knox WH. Inventing a new way to see clearly: Non-invasive vision correction with femtosecond lasers. Technol Innov. 2019;20:385–98. [Google Scholar]
- 30.Vickers LA, Gupta PK. Femtosecond laser-assisted keratotomy. Curr Opin Ophthalmol. 2016;27:277–84. doi: 10.1097/ICU.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 31.Chang JS. Femtosecond laser-assisted astigmatic keratotomy: A review. Eye Vis (Lond) 2018;5:6. doi: 10.1186/s40662-018-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffart L, Proust H, Matonti F, Conrath J, Ridings B. Correction of postkeratoplasty astigmatism by femtosecond laser compared with mechanized astigmatic keratotomy. Am J Ophthalmol. 2009;147:779–87. doi: 10.1016/j.ajo.2008.12.017. e1. [DOI] [PubMed] [Google Scholar]
- 33.Sorkin N, Mimouni M, Santaella G, Kreimei M, Trinh T, Yang Y, et al. Comparison of manual and femtosecond astigmatic keratotomy in the treatment of postkeratoplasty astigmatism. Acta Ophthalmol. 2021;99:e747–52. doi: 10.1111/aos.14653. [DOI] [PubMed] [Google Scholar]
- 34.Yan Q, Han B, Ma ZC. Femtosecond laser-assisted ophthalmic surgery: From laser fundamentals to clinical applications. Micromachines (Basel) 2022;13:1653. doi: 10.3390/mi13101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.anNakhli F, Khattak A. Vector analysis of femtosecond laser-assisted astigmatic keratotomy after deep anterior lamellar keratoplasty and penetrating keratoplasty. Int Ophthalmol. 2019;39:189–98. doi: 10.1007/s10792-017-0803-0. [DOI] [PubMed] [Google Scholar]
- 36.Visco DM, Bedi R, Packer M. Femtosecond laser-assisted arcuate keratotomy at the time of cataract surgery for the management of preexisting astigmatism. J Cataract Refract Surg. 2019;45:1762–9. doi: 10.1016/j.jcrs.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Ghanem RC, Azar DT. Femtosecond-laser arcuate wedge-shaped resection to correct high residual astigmatism after penetrating keratoplasty. J Cataract Refract Surg. 2006;32:1415–9. doi: 10.1016/j.jcrs.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 38.Fadlallah A, Mehanna C, Saragoussi JJ, Chelala E, Amari B, Legeais JM. Safety and efficacy of femtosecond laser-assisted arcuate keratotomy to treat irregular astigmatism after penetrating keratoplasty. J Cataract Refract Surg. 2015;41:1168–75. doi: 10.1016/j.jcrs.2014.08.046. [DOI] [PubMed] [Google Scholar]
- 39.Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R, Jr, Guell JL, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34:359–69. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 40.Benoist d'Azy C, Pereira B, Chiambaretta F, Dutheil F. Efficacy of different procedures of intra-corneal ring segment implantation in keratoconus: A systematic review and meta-analysis. Transl Vis Sci Technol. 2019;8:38. doi: 10.1167/tvst.8.3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakellaris D, Balidis M, Gorou O, Szentmary N, Alexoudis A, Grieshaber MC, et al. Intracorneal ring segment implantation in the management of keratoconus: An evidence-based approach. Ophthalmol Ther. 2019;8:5–14. doi: 10.1007/s40123-019-00211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izquierdo L, Rodríguez AM, Sarquis RA, Altamirano D, Henriquez MA. Intracorneal circular ring implant with femtosecond laser: Pocket versus tunnel. Eur J Ophthalmol. 2022;32:176–82. doi: 10.1177/1120672121994729. [DOI] [PubMed] [Google Scholar]
- 43.Monteiro T, Alfonso JF, Franqueira N, Faria-Correira F, Ambrósio R, Jr, Madrid-Costa D. Comparison of clinical outcomes between manual and femtosecond laser techniques for intrastromal corneal ring segment implantation. Eur J Ophthalmol. 2020;30:1246–55. doi: 10.1177/1120672119872367. [DOI] [PubMed] [Google Scholar]
- 44.Struckmeier AK, Hamon L, Flockerzi E, Munteanu C, Seitz B, Daas L. Femtosecond laser and mechanical dissection for ICRS and myoring implantation: A meta-analysis. Cornea. 2022;41:518–37. doi: 10.1097/ICO.0000000000002937. [DOI] [PubMed] [Google Scholar]
- 45.Jacob S, Patel SR, Agarwal A, Ramalingam A, Saijimol AI, Raj JM. Corneal allogenic intrastromal ring segments (CAIRS) combined with corneal cross-linking for keratoconus. J Refract Surg. 2018;34:296–303. doi: 10.3928/1081597X-20180223-01. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz LA, Cepeda LM, Fuentes VC. Intrastromal correction of presbyopia using a femtosecond laser system. J Refract Surg. 2009;25:847–54. doi: 10.3928/1081597X-20090917-05. [DOI] [PubMed] [Google Scholar]
- 47.Khoramnia R, Fitting A, Rabsilber TM, Thomas BC, Auffarth GU, Holzer MP. Intrastromal femtosecond laser surgical compensation of presbyopia with six intrastromal ring cuts:3-year results. Br J Ophthalmol. 2015;99:170–6. doi: 10.1136/bjophthalmol-2014-305642. [DOI] [PubMed] [Google Scholar]
- 48.Holzer MP, Knorz MC, Tomalla M, Neuhann TM, Auffarth GU. Intrastromal femtosecond laser presbyopia correction:1-year results of a multicenter study. J Refract Surg. 2012;28:182–8. doi: 10.3928/1081597X-20120203-01. [DOI] [PubMed] [Google Scholar]
- 49.Fenner BJ, Moriyama AS, Mehta JS. Inlays and the cornea. Exp Eye Res. 2021;205:108474. doi: 10.1016/j.exer.2021.108474. [DOI] [PubMed] [Google Scholar]
- 50.Pluma-Jaramago I, Rocha-de-Lossada C, Rachwani-Anil R, Sánchez-González JM. Small-aperture intracorneal inlay implantation in emmetropic presbyopic patients: A systematic review. Eye (Lond) 2022;36:1747–53. doi: 10.1038/s41433-022-02032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moshirfar M, Henrie MK, Payne CJ, Ply BK, Ronquillo YC, Linn SH, et al. Review of presbyopia treatment with corneal inlays and new developments. Clin Ophthalmol. 2022;16:2781–95. doi: 10.2147/OPTH.S375577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–61. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 53.Perlman EM. An analysis and interpretation of refractive errors after penetrating keratoplasty. Ophthalmology. 1981;88:39–45. doi: 10.1016/s0161-6420(81)35086-6. [DOI] [PubMed] [Google Scholar]
- 54.Wiffen SJ, Maguire LJ, Bourne WM. Keratometric results of penetrating keratoplasty with the Hessburg-Barron and Hanna trephine systems using a standard double-running suture technique. Cornea. 1997;16:306–13. [PubMed] [Google Scholar]
- 55.Naumann GO. The Bowman lecture. Eye (Lond) 1995;9((Pt 4)):395–421. doi: 10.1038/eye.1995.98. [DOI] [PubMed] [Google Scholar]
- 56.Liu YC, Morales-Wong F, Patil M, Han SB, Lwin NC, Teo EP, et al. Femtosecond laser-assisted corneal transplantation with a low-energy, liquid-interface system. Sci Rep. 2022;12:6959. doi: 10.1038/s41598-022-11461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price FW, Jr, Price MO. Femtosecond laser shaped penetrating keratoplasty: One-year results utilizing a top-hat configuration. Am J Ophthalmol. 2008;145:210–4. doi: 10.1016/j.ajo.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 58.Bahar I, Kaiserman I, McAllum P, Rootman D. Femtosecond laser-assisted penetrating keratoplasty: Stability evaluation of different wound configurations. Cornea. 2008;27:209–11. doi: 10.1097/ICO.0b013e31815b7d50. [DOI] [PubMed] [Google Scholar]
- 59.Farid M, Steinert RF, Gaster RN, Chamberlain W, Lin A. Comparison of penetrating keratoplasty performed with a femtosecond laser zig-zag incision versus conventional blade trephination. Ophthalmology. 2009;116:1638–43. doi: 10.1016/j.ophtha.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Bahar I, Kaiserman I, Lange AP, Levinger E, Sansanayudh W, Singal N, et al. Femtosecond laser versus manual dissection for top hat penetrating keratoplasty. Br J Ophthalmol. 2009;93:73–8. doi: 10.1136/bjo.2008.148346. [DOI] [PubMed] [Google Scholar]
- 61.Mastropasqua L, Nubile M, Lanzini M, Calienno R, Trubiani O. Orientation teeth in nonmechanical femtosecond laser corneal trephination for penetrating keratoplasty. Am J Ophthalmol. 2008;146:46–9. doi: 10.1016/j.ajo.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Li X, Li W, Jiu X, Tian M. Systematic review and meta-analysis of femtosecond laser-enabled keratoplasty versus conventional penetrating keratoplasty. Eur J Ophthalmol. 2021;31:976–87. doi: 10.1177/1120672120914488. [DOI] [PubMed] [Google Scholar]
- 63.Peng WY, Tang ZM, Lian XF, Zhou SY. Comparing the efficacy and safety of femtosecond laser-assisted versus conventional penetrating keratoplasty: A meta-analysis of comparative studies. Int Ophthalmol. 2021;41:2913–23. doi: 10.1007/s10792-021-01826-w. [DOI] [PubMed] [Google Scholar]
- 64.Borderie VM, Sandali O, Bullet J, Gaujoux T, Touzeau O, Laroche L. Long-term results of deep anterior lamellar versus penetrating keratoplasty. Ophthalmology. 2012;119:249–55. doi: 10.1016/j.ophtha.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 65.Anwar M, Teichmann KD. Deep lamellar keratoplasty: Surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet's membrane. Cornea. 2002;21:374–83. doi: 10.1097/00003226-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Scorcia V, De Luca V, Lucisano A, Carnevali A, Carnovale Scalzo G, Bovone C, et al. Results of viscobubble deep anterior lamellar keratoplasty after failure of pneumatic dissection. Br J Ophthalmol. 2018;102:1288–92. doi: 10.1136/bjophthalmol-2017-311419. [DOI] [PubMed] [Google Scholar]
- 67.Unal M, Bilgin B, Yucel I, Akar Y, Apaydin C. Conversion to deep anterior lamellar keratoplasty (DALK): Learning curve with big-bubble technique. Ophthalmic Surg Lasers Imaging. 2010;41:642–50. doi: 10.3928/15428877-20100929-09. [DOI] [PubMed] [Google Scholar]
- 68.Liu YC, Wittwer VV, Yusoff NZ, Lwin CN, Seah XY, Mehta JS, et al. Intraoperative optical coherence tomography-guided femtosecond laser-assisted deep anterior lamellar keratoplasty. Cornea. 2019;38:648–53. doi: 10.1097/ICO.0000000000001851. [DOI] [PubMed] [Google Scholar]
- 69.Alio JL, Abdelghany AA, Barraquer R, Hammouda LM, Sabry AM. Femtosecond laser assisted deep anterior lamellar keratoplasty outcomes and healing patterns compared to manual technique. Biomed Res Int. 2015;2015:397891. doi: 10.1155/2015/397891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salouti R, Zamani M, Ghoreyshi M, Dapena I, Melles GR, Nowroozzadeh MH. Comparison between manual trephination versus femtosecond laser-assisted deep anterior lamellar keratoplasty for keratoconus. Br J Ophthalmol. 2019;103:1716–23. doi: 10.1136/bjophthalmol-2018-313365. [DOI] [PubMed] [Google Scholar]
- 71.Gadhvi KA, Romano V, Fernández-Vega Cueto L, Aiello F, Day AC, Gore DM, et al. Femtosecond laser-assisted deep anterior lamellar keratoplasty for keratoconus: Multi-surgeon results. Am J Ophthalmol. 2020;220:191–202. doi: 10.1016/j.ajo.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 72.Buzzonetti L, Petrocelli G, Valente P. Femtosecond laser and big-bubble deep anterior lamellar keratoplasty: A new chance. J Ophthalmol. 2012;2012:264590. doi: 10.1155/2012/264590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh B, Sharma S, Bharti N, Bharti S. A novel method of tunnel creation using intraoperative optical coherence tomography-guided deep anterior lamellar keratoplasty. Indian J Ophthalmol. 2021;69:3743–4. doi: 10.4103/ijo.IJO_531_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shehadeh-Mashor R, Chan CC, Bahar I, Lichtinger A, Yeung SN, Rootman DS. Comparison between femtosecond laser mushroom configuration and manual trephine straight-edge configuration deep anterior lamellar keratoplasty. Br J Ophthalmol. 2014;98:35–9. doi: 10.1136/bjophthalmol-2013-303737. [DOI] [PubMed] [Google Scholar]
- 75.Blériot A, Martin E, Lebranchu P, Zimmerman K, Libeau L, Weber M, et al. Comparison of 12-month anatomic and functional results between Z6 femtosecond laser-assisted and manual trephination in deep anterior lamellar keratoplasty for advanced keratoconus. J Fr Ophtalmol. 2017;40:e193–200. doi: 10.1016/j.jfo.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 76.Li H, Chen M, Dong YL, Zhang J, Du XL, Cheng J, et al. Comparison of long-term results after manual and femtosecond assisted corneal trephination in deep anterior lamellar keratoplasty for keratoconus. Int J Ophthalmol. 2020;13:567–73. doi: 10.18240/ijo.2020.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S, Wang T, Bian J, Wang F, Han S, Shi W. Precisely controlled side cut in femtosecond laser-assisted deep lamellar keratoplasty for advanced keratoconus. Cornea. 2016;35:1289–94. doi: 10.1097/ICO.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 78.Woo JH, Ang M, Htoon HM, Tan D. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2019;207:288–303. doi: 10.1016/j.ajo.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 79.Ong HS, Ang M, Mehta JS. Evolution of therapies for the corneal endothelium: Past, present and future approaches. Br J Ophthalmol. 2021;105:454–67. doi: 10.1136/bjophthalmol-2020-316149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Einan-Lifshitz A, Sorkin N, Boutin T, Showail M, Borovik A, Alobthani M, et al. Comparison of femtosecond laser-enabled descemetorhexis and manual descemetorhexis in descemet membrane endothelial keratoplasty. Cornea. 2017;36:767–70. doi: 10.1097/ICO.0000000000001217. [DOI] [PubMed] [Google Scholar]
- 81.Pilger D, von Sonnleithner C, Bertelmann E, Joussen AM, Torun N. Femtosecond laser-assisted descemetorhexis: A novel technique in descemet membrane endothelial keratoplasty. Cornea. 2016;35:1274–8. doi: 10.1097/ICO.0000000000000987. [DOI] [PubMed] [Google Scholar]
- 82.Hosny MH, Marrie A, Karim Sidky M, GamalEldin S, Salem M. Results of femtosecond laser-assisted descemet stripping automated endothelial keratoplasty. J Ophthalmol. 2017;2017:8984367. doi: 10.1155/2017/8984367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Busin M, Madi S, Santorum P, Scorcia V, Beltz J. Ultrathin descemet's stripping automated endothelial keratoplasty with the microkeratome double-pass technique: Two-year outcomes. Ophthalmology. 2013;120:1186–94. doi: 10.1016/j.ophtha.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 84.AlióDel Barrio JL, Vargas V. Femtosecond laser-assisted deep lamellar descemet membrane endothelial keratoplasty for the treatment of endothelial dysfunction associated with posterior stromal scarring. Cornea. 2019;38:388–91. doi: 10.1097/ICO.0000000000001829. [DOI] [PubMed] [Google Scholar]
- 85.Kimakura M, Sakai O, Nakagawa S, Yoshida J, Shirakawa R, Toyono T, et al. Stromal bed quality and endothelial damage after femtosecond laser cuts into the deep corneal stroma. Br J Ophthalmol. 2013;97:1404–9. doi: 10.1136/bjophthalmol-2013-303328. [DOI] [PubMed] [Google Scholar]
- 86.Soong HK, Mian S, Abbasi O, Juhasz T. Femtosecond laser-assisted posterior lamellar keratoplasty: Initial studies of surgical technique in eye bank eyes. Ophthalmology. 2005;112:44–9. doi: 10.1016/j.ophtha.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 87.Liu YC, Teo EP, Adnan KB, Yam GH, Peh GS, Tan DT, et al. Endothelial approach ultrathin corneal grafts prepared by femtosecond laser for descemet stripping endothelial keratoplasty. Invest Ophthalmol Vis Sci. 2014;55:8393–401. doi: 10.1167/iovs.14-15080. [DOI] [PubMed] [Google Scholar]
- 88.Mckee HD, Jhanji V. Femtosecond laser-assisted graft preparation for descemet membrane endothelial keratoplasty. Cornea. 2018;37:1342–4. doi: 10.1097/ICO.0000000000001633. [DOI] [PubMed] [Google Scholar]
- 89.Tourtas T, Schlomberg J, Wessel JM, Bachmann BO, Schlötzer-Schrehardt U, Kruse FE. Graft adhesion in descemet membrane endothelial keratoplasty dependent on size of removal of host's descemet membrane. JAMA Ophthalmol. 2014;132:155–61. doi: 10.1001/jamaophthalmol.2013.6222. [DOI] [PubMed] [Google Scholar]
- 90.Sorkin N, Mednick Z, Einan-Lifshitz A, Trinh T, Santaella G, Telli A, et al. Three-year outcome comparison between femtosecond laser-assisted and manual descemet membrane endothelial keratoplasty. Cornea. 2019;38:812–6. doi: 10.1097/ICO.0000000000001956. [DOI] [PubMed] [Google Scholar]
- 91.Riau AK, Liu YC, Yam GH, Mehta JS. Stromal keratophakia: Corneal inlay implantation. Prog Retin Eye Res. 2020;75:100780. doi: 10.1016/j.preteyeres.2019.100780. [DOI] [PubMed] [Google Scholar]
- 92.Riau AK, Htoon HM, AlióDel Barrio JL, Nubile M, El Zarif M, Mastropasqua L, et al. Femtosecond laser-assisted stromal keratophakia for keratoconus: A systemic review and meta-analysis. Int Ophthalmol. 2021;41:1965–79. doi: 10.1007/s10792-021-01745-w. [DOI] [PubMed] [Google Scholar]
- 93.Brar S, Ganesh S, Sriganesh SS, Bhavsar H. Femtosecond intrastromal lenticule implantation (FILI) for management of moderate to high hyperopia:5-year outcomes. J Refract Surg. 2022;38:348–54. doi: 10.3928/1081597X-20220503-01. [DOI] [PubMed] [Google Scholar]
- 94.Pant OP, Hao JL, Zhou DD, Lu CW. Tectonic keratoplasty using femtosecond laser lenticule in pediatric patients with corneal perforation secondary to blepharokeratoconjunctivitis: A case report and literature review. J Int Med Res. 2019;47:2312–20. doi: 10.1177/0300060519841163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang H, Zhou Y, Zhao H, Xue J, Jiang Q. Application of the SMILE-derived lenticule in therapeutic keratoplasty. Int Ophthalmol. 2020;40:689–95. doi: 10.1007/s10792-019-01229-y. [DOI] [PubMed] [Google Scholar]
- 96.Pant OP, Hao JL, Zhou DD, Pant M, Lu CW. Tectonic keratoplasty using small incision lenticule extraction-extracted intrastromal lenticule for corneal lesions. J Int Med Res. 2020;48:300060519897668. doi: 10.1177/0300060519897668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pant OP, Hao JL, Zhou DD, Wang F, Lu CW. A novel case using femtosecond laser-acquired lenticule for recurrent pterygium: Case report and literature review. J Int Med Res. 2018;46:2474–80. doi: 10.1177/0300060518765303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wan Q, Tang J, Han Y, Ye H. Surgical treatment of corneal dermoid by using intrastromal lenticule obtained from small-incision lenticule extraction. Int Ophthalmol. 2020;40:43–9. doi: 10.1007/s10792-019-01201-w. [DOI] [PubMed] [Google Scholar]
- 99.van Dijk K, Parker JS, Baydoun L, Ilyas A, Dapena I, Groeneveld-van Beek EA, et al. Bowman layer transplantation: 5-year results. Graefes Arch Clin Exp Ophthalmol. 2018;256:1151–8. doi: 10.1007/s00417-018-3927-7. [DOI] [PubMed] [Google Scholar]
- 100.Tong CM, van Dijk K, Melles GRJ. Update on Bowman layer transplantation. Curr Opin Ophthalmol. 2019;30:249–55. doi: 10.1097/ICU.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 101.Parker JS, Huls F, Cooper E, Graves P, Groeneveld-van Beek EA, Lie J, et al. Technical feasibility of isolated Bowman layer graft preparation by femtosecond laser: A pilot study. Eur J Ophthalmol. 2017;27:675–7. doi: 10.5301/ejo.5000990. [DOI] [PubMed] [Google Scholar]
- 102.García de Oteyza G, González Dibildox LA, Vázquez-Romo KA, Tapia Vázquez A, Dávila Alquisiras JH, Martínez-Báez BE, et al. Bowman layer transplantation using a femtosecond laser. J Cataract Refract Surg. 2019;45:261–6. doi: 10.1016/j.jcrs.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 103.Barbosa Gonçalves T, Forseto AD, Martins AL, Pereira NC. Femtosecond laser-assisted Bowman layer transplantation for advanced keratoconus. Eur J Ophthalmol. 2022 doi: 10.1177/11206721221143163. 11206721221143163. [DOI] [PubMed] [Google Scholar]
- 104.Fuest M, Liu YC, Coroneo MT, Mehta JS. Femtosecond laser assisted pterygium surgery. Cornea. 2017;36:889–92. doi: 10.1097/ICO.0000000000001230. [DOI] [PubMed] [Google Scholar]
- 105.Liu YC, Ji AJ, Tan TE, Fuest M, Mehta JS. Femtosecond laser-assisted preparation of conjunctival autograft for pterygium surgery. Sci Rep. 2020;10:2674. doi: 10.1038/s41598-020-59586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ting DS, Liu YC, Lee YF, Ji AJ, Tan TE, Htoon HM, et al. Cosmetic outcome of femtosecond laser-assisted pterygium surgery. Eye Vis (Lond) 2021;8:7. doi: 10.1186/s40662-021-00230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alió JL, Rodriguez AE, Toffaha BT, Piñero DP, Moreno LJ. Femtosecond-assisted keratopigmentation for functional and cosmetic restoration in essential iris atrophy. J Cataract Refract Surg. 2011;37:1744–7. doi: 10.1016/j.jcrs.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Yin HY, Walter KA. Long-term outcome of femtosecond laser-assisted keratopigmentation: Using intacs channels for precise pigment deposition within the cornea. Cornea. 2021;40:1330–5. doi: 10.1097/ICO.0000000000002667. [DOI] [PubMed] [Google Scholar]
- 109.Yoo SH, Kymionis GD, O’Brien TP, Ide T, Culbertson W, Alfonso EC. Femtosecond-assisted diagnostic corneal biopsy (FAB) in keratitis. Graefes Arch Clin Exp Ophthalmol. 2008;246:759–62. doi: 10.1007/s00417-008-0785-8. [DOI] [PubMed] [Google Scholar]
- 110.Moshirfar M, Neuffer MC, Kinard K, Lependu MT, Sikder S. Femtosecond-assisted preparation of donor tissue for Boston type 1 keratoprosthesis. Clin Ophthalmol. 2011;5:1017–20. doi: 10.2147/OPTH.S22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pallikaris IG, Kymionis GD, Plaka AD, Binder PS, Kontadakis GA, Tsoulnaras KI. Femtosecond laser-assisted intra-corneal drug delivery. Semin Ophthalmol. 2015;30:457–61. doi: 10.3109/08820538.2013.874482. [DOI] [PubMed] [Google Scholar]
- 112.de Farias CC, Bezerra FM, Lamazales LL, Nosé W, Pereira Gomes JÁ. KeraKlear artificial cornea implantation assisted by femtosecond laser in eyes with aniridia. Cornea. 2022;41:635–9. doi: 10.1097/ICO.0000000000002998. [DOI] [PubMed] [Google Scholar]
- 113.Kymionis GD, Diakonis VF, Kankariya VP, Plaka AD, Panagopoulou SI, Kontadakis GA, et al. Femtosecond laser-assisted intracorneal biopolymer insertion for the symptomatic treatment of bullous keratopathy. Cornea. 2014;33:540–3. doi: 10.1097/ICO.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 114.Zheleznyak L, Butler SC, Cox IG, Huxlin KR, Ellis JD, Knox W, et al. First-in-human laser-induced refractive index change (LIRIC) treatment of the cornea. Invest. Ophthalmol. Vis. Sci. 2019;60(9):5079. [Google Scholar]
- 115.Bradford S, Mikula E, Juhasz T, Brown DJ, Jester JV. Nonlinear optical crosslinking (NLO CXL) for correcting refractive errors. Exp Eye Res. 2020;199:108199. doi: 10.1016/j.exer.2020.108199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bradford S, Mikula E, Kim SW, Xie Y, Juhasz T, Brown DJ, et al. Nonlinear optical corneal crosslinking, mechanical stiffening, and corneal flattening using amplified femtosecond pulses. Transl Vis Sci Technol. 2019;8:35. doi: 10.1167/tvst.8.6.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santra M, Liu YC, Jhanji V, Yam GH. Human SMILE-derived stromal lenticule scaffold for regenerative therapy: Review and perspectives. Int J Mol Sci. 2022;23:7967. doi: 10.3390/ijms23147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.