Abstract
Objectives:
To assess the frequency of olfactory dysfunction (OD) among individuals afflicted with coronavirus disease of 2019 (COVID-19).
Methods:
A comprehensive literature search was carried out across several bibliographical databases (PubMed, Scopus, Google Scholar, and Web of Science) to extract publications in the English language between January 2020 and December 2021 to report the incidence of OD alone or together with gustatory dysfunction (GD) among COVID-19 patients.
Results:
Based on eligibility criteria, 84 articles were included from 27 countries, comprising 36,903 patients, of whom 58.1% were females. The generality rates of olfactory impairment alone was 34.60% and in conjunction with GD was 11.36%. Patients with OD were subclassified into various categories, and the prevalence of anosmia was 20.85%, 5.04% for hyposmia, 8.88% for anosmia or hyposmia, 1.84% for parosmia, 0.78% for phantosmia, and 0.02% for hyperosmia, among COVID-19 patients.
Conclusion:
Clinical features associated with OD, either isolated or in combination with GD, are common in patients with COVID-19 and consider important signs of COVID-19 that may guide clinicians in the early phase of the disease.
PROSPERO Reg. No.: 417296
Keywords: anosmia, COVID-19, Hyposmia, olfactory dysfunction, SARS-CoV-2
The coronavirus of 2019 (COVID-19) pandemic has evolved into a worldwide emergency, posing a substantial public health challenge, with rapid dissemination and increased mortality. The global health crisis continues to affect the world today and is expected to do so in the future. Although, first observed in December 2019 in Hubei Province, China, it has spread rapidly worldwide. On 11 March 2020, COVID-19 was declared a ‘pandemic emergency’ by the World Health Organization (WHO). Currently, 274,628,461 confirmed cases and 5,358,978 deaths have been reported worldwide. 1
The COVID-19 is the result of an emerging beta-coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These are single-stranded RNA viruses that cause respiratory, hepatic, enteric, and neurological illnesses. The incubation period spans from 1-14 days, during which the most frequently encountered symptoms include fever, cough, shortness of breath, breathing difficulties, and fatigue. Furthermore, some individuals with COVID-19 have reported experiencing olfactory disorder and anosmia. 2,3 The intensity of these symptoms varies among individuals and is influenced by factors such as the duration of virus exposure, the patient’s age and gender, and the presence of underlying health conditions. 4
Healthcare professionals and researchers around the globe are endeavoring to gather a multitude of evidence aimed at comprehending the epidemiology, clinical characteristics, and predictive elements of COVID-19. The sinonasal tract plays a significant role in the pathogenesis of viral infections. 5 The relationship between loss of smell and COVID-19 was first proposed by Mao et al. 6 Since then, the number of studies explaining the relationship between olfactory dysfunction (OD) and other symptoms of COVID-19 has increased. 7,8 A recent systematic review carried out by Aziz et al 2 concluded that OD is a prevalent symptom in patients with COVID-19. On 26 March 2020 the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) reported that the COVID-19 anosmia reporting tool for clinicians which showed that anosmia was present in 73% of cases before the laboratory diagnosis of COVID-19 and was the main presenting symptom in 26.6% of the cases. 9,10 Due to the rising occurrence of olfactory symptoms in individuals with COVID-19, the Centers for Disease Control and Prevention have recently included ‘new loss of taste or smell’ in the roster of symptoms that can manifest 2-14 days following exposure to the virus. 11
Although OD is one of the most underreported symptoms of COVID-19, it is sometimes the only presenting symptom in these patients. 2 Therefore, a comprehensive comprehension of COVID-19 symptoms holds significant importance in early disease detection and transmission prevention. In light of this, this systematic review seeks to consolidate existing literature on OD in COVID-19, emphasizing the role of ear, nose, and throat (ENT) specialists in efforts to mitigate the impact of this severe pandemic.
Methods
The main objective of this study was to carry out a systematic assessment and description of documented instances of anosmia linked to infections caused by SARS-CoV-2. This structured review adhered to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. 12
Eligibility criteria
We systematically combed through clinical evidence, specifically seeking original peer-reviewed journal articles. These articles encompassed observational studies that explored the occurrence of OD in individuals afflicted with COVID-19. The range of publications published between January 2020 and December 2021 was limited. Case reports, case series, letters to the editor and replies, conference papers, book reviews, book chapters, newspaper and newsletter articles, expert opinions, theses and dissertations, and studies written in languages other than English were ruled out.
Data sources and search strategy
We carried out a thorough search of the scientific literature across various electronic bibliographic databases, including PubMed, Scopus, Google Scholar, and Web of Science. We collected all articles published between January 2020 and December 2021. The Scopus database was explored by S. S. S., Google Scholar database by H. S. S., and the Web of Science by N. M. M. Two investigators (A. F. T. and Z. A. Q.) independently examined all articles in a standardised manner to determine their eligibility and subsequently compared the eligible articles. A final review of the selected articles was carried out by all investigators (F. M. K., F. A. M., Amit F. W. H., and R.S.O.). The following search terms were used to screen the different databases: PUBMED (search until 29.12.2021): (anosmia) OR (loss of smell) OR (hyposmia) OR (olfactory dysfunction) AND (COVID 19) OR (coronavirus pandemic) OR (SARS-CoV-2); SCOPUS (search until 27.12.2021): (Anosmia OR hyposmia OR loss of smell OR olfactory dysfunction AND COVID-19 OR coronavirus); Google Scholar (search until 28.12.2021): Olfactory dysfunction or anosmia in COVID-19; Web of Science (search until 25.12.2021): ‘Olfactory dysfunction in COVID-19’ OR ‘Loss of smell in coronavirus pandemic’ OR ‘Anosmia/hyposmia in coronavirus pandemic’.
Data collection
The study followed a 2-phase approach. In Phase I, we commenced with an initial review of the study titles, followed by a subsequent assessment of their abstracts. This screening process adhered to predefined inclusion and exclusion criteria. Articles that met the eligibility criteria based on their titles and abstracts were then subject to a comprehensive evaluation for final eligibility. Any duplicate or irrelevant articles were systematically excluded from the review, and we procured the full texts of all studies with potential relevance.
Following the initial filtering phase, the chosen articles underwent a reference screening in Phase II to identify any new studies that might meet the eligibility criteria. Two independent reviewers carried out a thorough examination of the full-text articles and extracted pertinent data. Furthermore, the references cited in the selected articles were scrutinized for any relevant studies, and the Zotero software was employed to extract additional references. Additionally, we carried out a literature search by examining the reference lists of prior systematic reviews and meta-analyses. 2,13-19
All studies reporting anosmia (alone or in combination with gustatory dysfunction [GD]) in individuals with confirmed laboratory diagnoses of COVID-19 were incorporated. Studies involving patients with suspected, but unconfirmed, COVID-19 were not considered. To create a comprehensive overview, we assessed the included studies based on the following criteria: author, year of publication, country of study, the kind of study, patient information (age and gender), COVID-19 status, number of patients with olfactory impairment alone, number of patients with OD and GD, and data collection method (telephone survey, in-person interview, and elaborate questionnaire focused on olfactory ability), method of olfactory assessment, time of disease onset, duration of olfactory symptoms, time of recovery from olfactory symptoms, and treatment used for OD. In the end, a total of 84 articles met the criteria for inclusion in the systematic review. Figure 1 depicts a flowchart illustrating the article selection process.
Figure 1.
- Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart.
Outcome measures
The primary outcome was to estimate the prevalence of anosmia/hyposmia among patients with COVID-19. The secondary outcome was aimed to estimate the association between hypogeusia or ageusia and anosmia/hyposmia among patients with COVID-19.
Statistical analysis
All data obtained from the included studies were entered into a Microsoft Excel spreadsheet and analysed.
Results
Many studies and literature reviews have evaluated OD in COVID-19 positive individuals. We obtained 213 articles from the preliminary search, of which 84 were included in the final analysis, as shown in Figure 1.
A total of 36,903 patients were included in the 84 studies whose data we obtained. 9,20-102 The sample sizes for the different studies ranged from 8-8238. 22-79 In 2020, all articles (n=84) were published. 9,20-65 However, the majority of the publications (n=47) were published in 2020, 2021, and 2022. 66-102 Data from 25 different nations were included in the 84 papers (Table 1), whereas the majority of the 84 research (n=34) were cross-sectional (Figure 2). The age group most commonly represented in the studies (n=41) was 41-49 years. Among the 36,903 participants, 21,474 (58.1%) were women. The descriptive characteristics of the included studies (n=84) are presented in Table 1.
Table 1.
- Demographic characteristics of the included studies (n=84).
| Studies | Study design | Study location | Study duration | Total number of patients with COVID-19 | Age (years) | Male/female | COVID status |
|---|---|---|---|---|---|---|---|
| Kaye et al 9 | Pilot | US | Mar 2020 to Apr 2020 | 237 | 39.6±14.6 | M:46 | RT-PCR confirmed |
| F:54 | |||||||
| Klopfensteina et al 20 | Retrospective | France | Mar 2020 | 114 | 47±16 | M:33.0% | RT-PCR confirmed |
| F:670% | |||||||
| Agrawal et al 21 | Retrospective | US | Apr 2020 | 42 | 65.5 | M:75.0% | RT-PCR confirmed |
| F:250% | |||||||
| Gilania et al 22 | Retrospective | Iran | Mar 2020 to Apr 2020 | 8 | Range: 22-44 | M:25.0% | RT-PCR confirmed (05/08) |
| F:75.0% | |||||||
| Vaira et al 23 | Cohort | Italy | Mar 2020 to Apr 2020 | 72 | 49.2 | M:37.0% | RT-PCR confirmed |
| F:630% | |||||||
| Menni et al 24 | Cross-sectional | UK | Mar 2020 | 1702 | M:179 (+) | RT-PCR confirmed (n=579) | |
| 40.79 (+) | F:400 (+) | ||||||
| 41.22 (-) | M:297 (-) | ||||||
| F:826 (-) | |||||||
| Hopkin et al 25 | Observational cohort | UK | Mar 2020 | 382 | 40-49 | M:25.4% | RT-PCR confirmed (80%) |
| F:74.6% | |||||||
| Moein et al 26 | Case control | Iran | Mar 2020 | 120 (60 cases - 60 controls) | 46.55 | M:66.0% | RT-PCR confirmed (n=60) |
| F:340% | |||||||
| Speth et al 27 | Prospective | US | Mar 2020 to Apr 2020 | 103 | 46.8 | M:48.5% | RT-PCR confirmed |
| F:51.5% | |||||||
| Coelho et al 28 | Longitudinal (cohort) | US | Apr 2020 | 220 | 42.8 | M:21.8% | RT-PCR confirmed (n=93; 42.3%) |
| F:78.2% | |||||||
| Roland et al 29 | Cohort study | US | Mar 2020 to Apr 2020 | 620 | M:35.0% (+) | RT-PCR confirmed (n=145) | |
| 40 (+) | F:65.0% (+) | ||||||
| 38 (-) | M:22.0% (-) | ||||||
| F:78.0% (-) | |||||||
| Zayet et al 30 | Retrospective | France | Mar 2020 | 217 | 39.8 | M:16.8% | RT-PCR confirmed (n=95) |
| F:83.2% | |||||||
| Boscolo–Rizzo et al 31 | Cross-sectional | Italy | Mar 2020 to Apr 2020 | 214 | - | - | RT-PCR confirmed (n=54) |
| Lee et al 32 | Prospective cohort | Korea | Mar 2020 | 3191 | 46 | M:37.3% | RT-PCR confirmed |
| F:62.7% | |||||||
| Vaira et al 33 | Multicentre cohort | Italy | - | 345 | 48.5 | M:42.3% | RT-PCR confirmed |
| F:7.7% | |||||||
| Lechien et al 34 | Prospective (questionnaire based survey) | France | - | 417 | 36.9±11.4 | M:36.9% | RT-PCR confirmed |
| F:63.1% | |||||||
| Hopkin et al 35 | Online survey | UK | Apr 2020 | 2428 | 30-39 (median) | M:27.0% | RT-PCR confirmed (n=80) |
| F:73.0% | |||||||
| Jalessi et al 36 | Prospective descriptive | Iran | Feb 2020 to Mar 2020 | 100 | 52.94 | M:67.4% | RT-PCR confirmed |
| F:32.6% | |||||||
| Lechien et al 37 | Cross-sectional | Spain | - | 16 | 36.0±10.1 | M:50.0% | RT-PCR confirmed |
| F:50.0% | |||||||
| Valeria et al 38 | Cross-sectional | Italy | Mar 2020 | 355 | 50 (40-59.5) | M:54.0% | RT-PCR confirmed |
| F:46.0% | |||||||
| Villarreal et al 39 | Descriptive observational single-centre | Spain | Apr 2020 | 230 | 43 (18-62) (median) | M:15.0% | RT-PCR confirmed |
| F:85.0% | |||||||
| Qiu et al 40 | Cross-sectional | China Germany France | Mar 2020 to Apr 2020 | 394 | 39 | M:57.0% | RT-PCR confirmed |
| F:43.0% | |||||||
| Tham et al 41 | Retrospective and cross-sectional | Singapore | Mar 2020 to Apr 2020 | 1065 | 34 (median) | M:87.6% | RT-PCR confirmed |
| F:12.4% | |||||||
| Naeinia et al 42 | Cross-sectional | Iran | Apr 2020 to May 2020 | 49 | 45±12.2 | M:44.9% | RT-PCR confirmed (n=49) |
| F:55.1% | |||||||
| Otte et al 43 | Cross-sectional | Germany | - | 91 | 43.01±12.69 | M:50.5% | RT-PCR confirmed |
| F:49.5% | |||||||
| Al-Ani et al 44 | Retrospective | Qatar | May 2020 to June 2020 | 141 | 35.91±10.069 | M:50.3% | RT-PCR confirmed |
| F:49.6% | |||||||
| Altin et al 45 | Prospective | Istanbul | Mar 2020 to Apr 2020 | 81 | 54.16±16.98 | M:50.6% | RT-PCR confirmed |
| F:49.4% | |||||||
| D’Ascanio et al 46 | Prospective case-control | US | Feb 2020 to Apr 2020 | 43 | 58.1 | M:67.0% | RT-PCR confirmed |
| F:33.0% | |||||||
| Cazolla et al 47 | Prospective | US | Mar 2020 to May 2020 | 67 | 65±13.1 | M:67.2% | RT-PCR confirmed |
| F:32.8% | |||||||
| Chiesa-Estomba et al 48 | Prospective | Belgium | Mar 2020 | 751 | 41±13 | M:36.4% | RT-PCR confirmed |
| F:63.6% | |||||||
| Karimi-Galougahi et al 49 | Prospective cross-sectional | Iran | March 2020 | 76 | 38.5±10.6 | M:40.8% | RT-PCR confirmed |
| F:59.2% | |||||||
| La Torre et al 50 | Case control | Italy | March 2020 | 30 cases - 75 controls | 43.6 | M:30.7% | RT-PCR confirmed (n=30) |
| F:69.3% | |||||||
| Kosugi et al 51 | Cross-sectional | Brazil | Mar 2020 to Apr 2020 | 253 | 36 (median) | M:40.9% | RT-PCR confirmed (n=145) |
| F:59.1% | |||||||
| Gorzkowski et al 52 | Cross-sectional | France | March 2020 | 229 | 39.7±13.7 | M:35.8% | RT-PCR confirmed |
| F:64.2% | |||||||
| Lechien et al 53 | Cross-sectional | Australia | Mar 2020 to May 2020 | 88 | 42.6±11.2 | M:33.0% | RT-PCR confirmed |
| F:67.0% | |||||||
| Martin Sanz et al 54 | Case-control | Spain | Mar 2020 to Apr 2020 | Cases: 215 (60.6%) | 42.9±0.67 | M:9.2% | RT-PCR confirmed (n=215; 60.6%) |
| Controls: 140 (39.4%) | F:80.8% | ||||||
| Mazzatenta et al 55 | Cross-sectional | Italy | - | 100 | 63±15 | M:70.0% | RT-PCR confirmed |
| F:30.0% | |||||||
| Meini et al2020 56 | Cross-sectional | Italy | April 2020 | 100 | 65 | M:60.0% | RT-PCR confirmed |
| F:40.0% | |||||||
| Mishra et al 57 | Cross-sectional | India | - | 74 | 17.2 | M:43 | RT-PCR confirmed |
| F:31 | |||||||
| Moein et al 58 | Cohort study | Iran | Mar 2020 to May 2020 | 100 | 45.40 (11.80; 23-76) | M:67.0% | RT-PCR confirmed |
| F:33.0% | |||||||
| Mohamud et al 59 | Retrospective double centre | Somalia | Apr 2020 | 60 | 45.7 (13.5) | M:70.0% | RT-PCR confirmed |
| F:30.0% | |||||||
| Sayin et al 60 | Cross-sectional | Turkey | - | 128 (64 [+] and 64 [-]) | 38.63±10.08 | M:37.5% | RT-PCR confirmed |
| F:62.5% | |||||||
| Talavera et al 61 | Retrospective cohort | Spain | Mar 2020 to Apr 2020 | 576 | 67.2 | M:56.7% | RT-PCR confirmed |
| F:43.3% | |||||||
| Yan et al 62 | Retrospective | California | Mar 2020 to Apr 2020 | 169 | 53.5 (40-65) | M:34.6% | RT-PCR confirmed |
| F:65.4% | |||||||
| Lechien et al 63 | Cross-sectional | France | - | 86 | 41.7±11.8 | M:34.9% | RT-PCR confirmed |
| F:65.1% | |||||||
| Barillari et al 64 | Cross-sectional | Italy | Apr 2020 | 294 | 42.1±12.3 | M:50.0% | RT-PCR confirmed (n=179) |
| F:50.0% | |||||||
| Kim et al 65 | Cross-sectional | Korea | Mar 2020 | 172 | 26 (median) | M:38.4% | RT-PCR confirmed |
| F:61.6% | |||||||
| Leedman et al 66 | Cross-sectional | Australia | Nov 2020 to Dec 2020 | 56 | 55.34±16.81 | M:46.4% | RT-PCR confirmed |
| F:54.6% | |||||||
| Kusnik et al 67 | Cross-sectional | Germany | Mar 2020 to July 2020 | 43 (+) | 41.2±16.2 (+) | M:44.0% | RT-PCR confirmed (n=43) |
| 668 (-) | 40.9±14.5 (-) | F:66.0% | |||||
| Makaronidis et al 68 | Community based cohort | UK | Apr 2020 to May 2020 | 467 | 39.67±12.12 | M:28.8% | RT-PCR confirmed |
| F:70.9% | |||||||
| Poerbonegoro et al 69 | Cross-sectional | Indonesia | Nov 2020 to Dec 2020 | 51 | 30.04±1.39 | M:54.9% | RT-PCR confirmed |
| F:45.1% | |||||||
| Bayrak et al 70 | Cross-sectional | Turkey | - | 105 | 55.9±17.6 | M:50.5% | RT-PCR confirmed |
| F:49.5% | |||||||
| Abdelmaksoud et al 71 | Prospective | Egypt | May 2020 to Aug 2020 | 134 | 47.8±15.8 | M:58.2% | RT-PCR confirmed |
| F:42.8% | |||||||
| Goyal et al 72 | Prospective cohort | India | Sep 2020 to Jan 2021 | 574 | 46.60 | M:2.1% | RT-PCR confirmed |
| F:1.0% | |||||||
| Soh et al 73 | Cross-sectional | Singapore | May 2020 to July 2020 | 1983 | 25 (median) | - | RT-PCR confirmed |
| Cousyn et al 74 | Prospective cohort | France | Mar 2020 to Apr 2020 | 98 | 34.5 (27.9-47.9) | M:24.5% | Positive RT-PCR tests (n=96) or positive SARS-CoV-2 antibody tests (n=2) |
| F:75.5% | |||||||
| Bakhshaee et al 75 | Longitudinal | Iran | Mar 2020 to Apr 2020. | 502 | 46.8±18.5 | M:47.6% | RT-PCR confirmed |
| F:52.4% | |||||||
| Sayin et al 76 | Cross-sectional | Turkey | Mar 2020 to May 2020 | 52 | 61.32±12.53 | M:69.2% | RT-PCR confirmed |
| F:30.8% | |||||||
| Printza et al 77 | Cross-sectional | Greece | Mar 2020 to Apr 2020 | 140 | 51.6±6.8 | M:62.0% | RT-PCR confirmed |
| F:38.0% | |||||||
| Kumar et al 78 | Prospective | India | May 2020 to Aug 2020 | 141 | 15.2 | M:58.9% | RT-PCR confirmed |
| F:41.1% | |||||||
| Kant et al 79 | Retrospective | Turkey | Mar 2020 to Oct 2020 | 8238 | 51.3±18.5 | M:60.8% | RT-PCR confirmed |
| F:39.2% | |||||||
| Chaturvedi et al 80 | Retrospective | India | Mar 2021 | 277 | 51.47±14.15 | M:70.8% | RT-PCR confirmed |
| F:29.2% | |||||||
| Parente-Arias et al 81 | Observational cohort | Spain | Mar 2020 | 151 | 41±12.15 | M:35.1% | RT-PCR confirmed |
| F:64.9% | |||||||
| Mubaraki et al 82 | Retrospective | KSA | May 2020 to Jul 2020 | 1022 | 15-39 | M:60.9% | RT-PCR confirmed |
| F:39.1% | |||||||
| D Silva et al 83 | Cross-sectional | Brazil | Apr 2020 | 166 | 44.7±11.6 | M:65.0% | RT-PCR confirmed (n=85) |
| F:35.0% | |||||||
| Bhatta et al 84 | Multicentric prospective | India, Nepal, Maldives | Apr 2020 to Jan 2021 | 188 | 33.1±1.7 | M:54.2% | RT-PCR confirmed |
| F:45.8% | |||||||
| Hameed et al 85 | Descriptive observational cross-sectional | Iraq | Mar 2020 to Apr 2020 | 35 | 11-60 | - | RT-PCR confirmed |
| Savtale et al 86 | Cross-sectional | India | Oct 2020 | 180 | 37.8±12.5 | M:33.4% | RT-PCR confirmed |
| F:66.6% | |||||||
| Horvath et al 87 | Retrospective | Australia | Feb 2020 to Apr 2020 | 102 | 45 | M:40.0% | RT-PCR confirmed |
| F:60.0% | |||||||
| Shaikh et al 88 | Retrospective | India | Aug 2020 to Sep 2020 | 1070 | 50-59 | M: 1.8 | RT-PCR confirmed |
| F:1.0 | |||||||
| Khan et al 89 | Cross-sectional | India | Mar 2021 to Jun 2021 | 224 | 35.4±15.5 | M:54.9% | RT-PCR confirmed |
| F:46.1% | |||||||
| Lee et al 90 | Cross-sectional | Israel and Canada | Mar 2020 to Jun 2020 | 350 | 47.0 | M:42.6% | RT-PCR confirmed |
| F:56.9% | |||||||
| Others:0.6% | |||||||
| Koul et al 91 | Cross-sectional | India | May 2020 to Aug 2020 | 300 | 37 | M:74.0% | RT-PCR confirmed |
| F:26.0% | |||||||
| Kandemirli et al 92 | Prospective | Turkey | May 2020 to Jun 2020 | 23 | 29 (median) | M:39.1% | RT-PCR confirmed |
| F:60.9% | |||||||
| Altundag et al 93 | Cross-sectional | Turkey | Mar 2020 | 135 | 39.8±11.3 | M:54.8% | RT-PCR confirmed |
| F:46.2% | |||||||
| Dev et al 94 | Case control | India | May 2020 to Jun 2020 | Cases: 55 Controls: 55 | 36 | M:58.0% | RT-PCR confirmed |
| F:42.0% | |||||||
| Korkmaz et al 95 | Prospective | Germany | - | 116 | 57.24±14.32 | M:50.0% | RT-PCR confirmed |
| F:50.0% | |||||||
| Babaei et al 96 | Retrospective | Iran | Dec 2020 to Mar 2021 | 235 | 43.95±15.27 | - | RT-PCR confirmed |
| Nouchi et al 97 | Cross-sectional | France | Mar 2020 to Mar 2020 | 390 | 66 (median) | M:64.0% | RT-PCR confirmed |
| F:36.0% | |||||||
| Polat et al 98 | Cross-sectional | Istanbul | - | 217 | 41.74 | M:59.4% | RT-PCR confirmed |
| F:40.6% | |||||||
| Renaud et al 99 | Cohort | France | Apr 2020 | 97 | 38.8 | M:30.9% | RT-PCR confirmed |
| F:69.1% | |||||||
| Rizzo et al 100 | Prospective | UK | - | 202 | 57 (median) | M:45.4% | RT-PCR confirmed |
| F:54.6% | |||||||
| Thakur et al 101 | Prospective | India | Sep 2020 to Oct 2020 | 250 | 21-80 | M:57.6% | RT-PCR confirmed |
| F:42.4% | |||||||
| Teaima et al 102 | Prospective | Egypt | Aug 2020 to Oct 2020 | 1031 | 18-69 | M:31.8% | RT-PCR confirmed |
| F:68.2% |
COVID-19: coronavirus disease - 2019, US: the United States of America, UK: the United Kingdom, KSA: Kingdom of Saudi Arabia, M: male, F: female, RT-PCR: reverse transcription-polymerase chain reaction test, (+): positive COVID-19, (-): negative COVID-19
Figure 2.
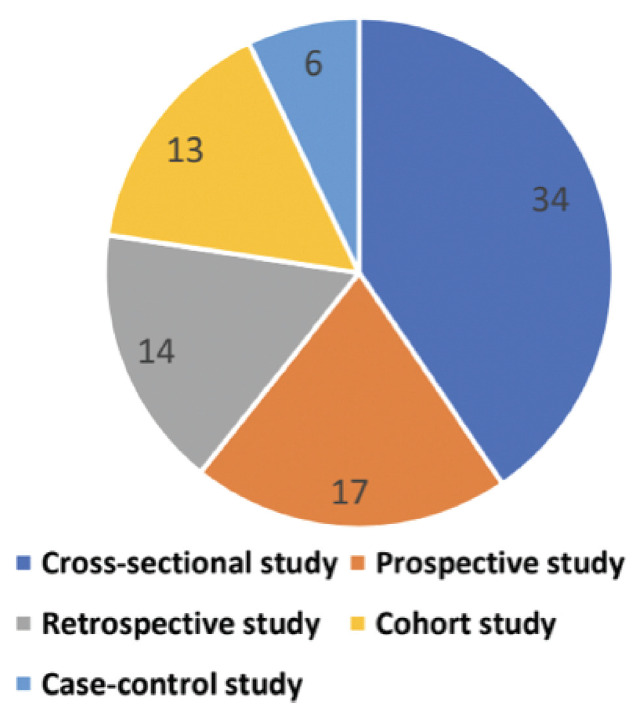
- Classification of the type of studies included in the systematic review (n=84).
The prevalence of OD is presented in Table 2. Among the 84 studies, 81 reported only OD, 40 reported the combination of OD and GD as a single entity, and 37 reported both the prevalence of OD alone and the combination of both. 9-102 A total of 33,231 patients were identified for the evaluation of OD, among them, 11,499 (34.60%) reported experiencing OD alone, whereas 3777 (11.36%) patients reported a combination of OD and GD. The number of patients with OD in the included studies ranged from 3-179635-60 with the estimated prevalence of OD ranging from 3.9-100%. 22,38,42,53,92 Similarly, the patients reporting both OD and GD ranged from 122-517102 with an estimated prevalence ranging from 3.9-90.9%. 41,43 The patients with OD were sub-classified into various categories. In our systematic review, among COVID-19 positive individuals, the prevalence rates of anosmia was 20.85%, 5.04% for hyposmia, 8.88% for anosmia or hyposmia, 1.84% for parosmia, 0.78% for phantosmia, and 0.02% for hyperosmia. A detailed description of this process is provided in Table 3.
Table 2.
- Details of olfactory dysfunction experienced by the coronavirus disease-19 positive individuals (n=84).
| Authors | Patients with OD | Patients with OD + GD | Mode of collecting data | Objective assessment of OD | Onset of OD (days) | Duration of OD (days) | Recovery time (days) | Treatment given |
|---|---|---|---|---|---|---|---|---|
| Kaye et al 9 | Anosmia: 173/237 (73%) | - | COVID-19 Anosmia reporting tool | - | Before: 73.0% Concomitant: 40.0% After: 27.0% | 7.2±3.1 Complete recovery: 85.0% (within 10) | - | |
| Klopfensteina et al 20 | Anosmia: 54/114 | 46/114 (with hypogeusia) | Medical records | - | 4.4 | 8.9 | 7-13 (35.0%) 4-6 (30.0%) 1-3 (16.0%) 14-20 (14.0%) 21-27 (5.0%) | - |
| Agrawal et al 21 | - | 03/42 | Medical records | - | - | - | - | |
| Gilania et al 22 | Anosmia: 8/8 (100%) | 1/8 (12.5%) (with ageusia) | Medical records | - | After 4: 1 Sudden onset: 2 After 2: 5 | - | - | - |
| Vaira et al 23 | Mild hyposmia (70-80): 22 (30.6%) Moderate hyposmia (50-60): 33 (45.8%) Severe hyposmia (20-40): 3 (4.2%) Anosmia (0-10): 2 (2.8%) | 30/72 (41.7%) | Telephone | CCCRC scoring system | - | - | Within 5: 35.8% After 5: 30.2% No recovery: 34% | - |
| Menni et al 24 | - | 342/1702 (59.0%) | COVID RADAR symptom tracker app | - | - | - | - | - |
| Hopkin et al 25 | Anosmia: 330/382 (74.4%) Very severe: 17.3% | - | - | 7 (60.0%) | 7-14 | 21 (71.0%) | - | |
| Moein et al 26 | Anosmia: 7/60 (12.0%) | 20/60 (17.0%) | Questionnaire | Mean UPSIT score: (34.10, p<0.001) Anosmia: 35/60 (58.0%) Severely microsmic: 20/60 (33.0%) Moderate microsmia: 16/60 (27.0%) Mild microsmia: 8/60 (13.0%) Normosmia: 1/60 (2.0%) | - | - | - | - |
| Speth et al 27 | 62/103 (61.2%) Anosmia:63, Hyposmia: 14 | - | Telephone | Mild VAS scores: 6.3% Moderate: 12.7%; severe: 81.0% | 1-8.7% Mean onset: 3.4 | 0-12 | - | - |
| Coelho et al 28 | 22/220 (26.5%), Anosmia: 116 (56.3%) | 54 (65.1%) | Web-based survey | - | - | - | - | - |
| Roland et al 29 | Anosmia/hyposmia: 137/145 COVID | - | Questionnaire | - | - | - | - | - |
| Zayet et al 30 | Anosmia in COVID-19: 137 (63.2%)negative: 217 (14.8%) | COVID-19 positive/negative-54.7%/9.0% | Medical records | - | - | - | - | - |
| Boscolo-Rizzo et al 31 | - | COVID-19: 63.0% Negative: 15.0% | Telephone | - | - | - | - | - |
| Lee et al 32 | Anosmia: 135/3191 (27.7%) | 254/3191 (52.0%) | Telephone | - | - | 7 | 21 | - |
| Vaira et al 33 | Anosmia: 22/345 (6.4%) | 203 (58.8%) | Telephone | UPSIT function scores Hyposmia: mild-76 (22.0%), moderate-59 (17.1%), severe-45 (13.0%); and ansomia: 61 (17.7%) | 14.8 | ≤7: 191 (74.6%) >7: 65 (25.4%) | Olfactory recovery: 70 (31.1%); normal: 21 (30%), mild hyposmia: 39 (55.7%), and moderate hyposmia: 10 (14.2%) | - |
OD: olfactory dysfunction, COVID: coronavirus disease-2019, GD: gustatory dysfunction, CCCRC: connecticut chemosensory clinical research center, UPSIT: University of Pennsylvania smell identification test, VAS: visual analog scale
Table 3.
- Classification of the olfactory dysfunction (n=84).
| Olfactory dysfunction category | Number of studies | n (%) out of 33,231patients |
|---|---|---|
| Anosmia | 29 | 6929 (20.8) |
| Hyposmia | 4 | 1676 (5.0) |
| Anosmia or hyposmia | 17 | 2953 (8.9) |
| Parosmia | 9 | 613 (1.8) |
| Phantosmia | 4 | 262 (0.8) |
| Hyperosmia | 2 | 7 (0.02) |
Values are presented as numbers and precentages (%).
| Authors | Patients with OD | Patients with OD + GD | Mode of collecting data | Objective assessment of OD | Onset of OD (days) | Duration of OD (days) | Recovery time (days) | Treatment given |
|---|---|---|---|---|---|---|---|---|
| Lechien et al 34 | 357/417 (85.6%), anosmia: 284 (79.6%); hyposmia: 73 (20.4%); phantosmia: 12.6%; and parosmia: 32.4% | Anosmia-16 (37.2%) Hyposmia-4 (9.3%) | Questionnaire | - | 9.77±5.68, before: 11.8%, after: 65.4%, concomitant: 22.8% | - | Anosmia: 1-4 (33.0%), 5-8 (39.6%), 9-14 (24.2%), and >15 (3.3%) | Oral/nasal corticosteroids: 70.0/8.0%; nasal saline irrigation: 17.0%; other: 3.0% |
| Hopkin et al 35 | Anosmia: 1796/2428 (74.4%) | - | - | <7 (n=1487; 61.0%) Before-14.9%; concomitant-39.3%; after-45.8% | - | - | Nasal steroids; 20 patients; only 3-oral steroids | |
| Jalessi et al 36 | 22/100 (23.9%), anosmia:9 (40.9%); hyposmia: 13 (59.1%); hyperosmia: 2 | - | Questionnaire | - | First symptom-6.5% Time of onset-3.41±2.46 | 10.73±8.26 | 21 (95.4%) | - |
| Lechien et al 37 | Anosmia: 16/16 | - | Questionnaire | The mean SNOT-22 score - 28.8±18.0; mean Sniffin’ Stick score-4.6±1.7 | At presentation-100% | - | 19.8±12.8 | - |
| Valeria Dell’Era et al 38 | Anosmia: 14/355 (3.9%) | 249/355 (70.0%) | Medical records and interview | Baseline smell perception of 10 (range: 3-10) | First symptom-8.7% | - | 14.0-49.5% | - |
| Villarreal et al 39 | Anosmia: 157/230 (68.0%) | - | Questionnaire | Average OD-8.2 in the modified VAS (range: 2-10) | - | 11 | >28.0-26.0% | - |
| Qiu et al 40 | Anosmia: 61/394 (15.0%) | 93/394 (240.0%) | Medical records | Mild-54.0%; moderate-37.0%; severe-17.0% Mean VAS score-3.60±3.62 (IQR: 0-7) The mean scores of QOD-QoL 37.0%/23.0% | - | - | - | - |
| Tham et al 41 | Anosmia: 126/1065 (11.8%) | 41/1065 (3.9%) | Questionnaire | - | - | 14 | - | - |
| Naeinia et al 42 | 49/49, anosmia: 42 (85.7%); hyposmia: 7 (14.3%) | - | Questionnaire | - | Sudden onset-91.8% | - | - | - |
| Otte et al 43 | 41/91 (45.0%), normosmic:49, hyposmic: 41 | 80/91 (90.9%) | Questionnaire | Odour T: 6.31±0.25; odour D: 11.63±0.26; odour I: 12.92±0.21; TDI score: 30.87±0.5 | 57.94±1.40 | - | - | - |
| Al-Ani et al 44 | Anosmia: 7/141 (5.0%) | 12/141 (8.5%) | Medical records | - | - | 6.89±3.056 | 3-12 | - |
| Altin et al 45 | Anosmia: 29/81 (35.8%) | 20 (24.7%) | Questionnaire | - | - | - | - | - |
| D’Ascanio et al 46 | 26/43, partial hyposmia: 6 (23.0%); Total anosmia: 20 (77.0%) | - | Questionnaire | - | Concomitant-07; before-04 | 5 | 30 | - |
| Cazolla et al 47 | 44/67 (65.7%), anosmia: 10 (22.7%); hyposmia: 34/67 | 6 (8.9%) | Questionnaire | VAS scores: severe-38.6%; moderate-29.6%; mild-9.1% | - | 10±6 | 35 (52.2%)-14 | - |
OD: olfactory dysfunction, GD: gustatory dysfunction, SNOT: sinonasal outcome test, IQR: interquartile range, VAS: visual analog scale, T: threshold, D: discrimination, I: identification
| Authors | Patients with OD | Patients with OD + GD | Mode of collecting data | Objective assessment of OD | Onset of OD (days) | Duration of OD (days) | Recovery time (days) | Treatment given |
|---|---|---|---|---|---|---|---|---|
| Chiesa-Estomba et al 48 | Anosmia: 621/751 (83.0%), total loss: 621 (83.0%), partial loss:130 (17.0%) | - | Questionnaire | - | - | - | Complete recovery-367 (49.0%) | Nasal/oral corticosteroids-9.0%/8.0%; nasal saline irrigation-20.0% |
| Karimi-Galougahi et al 49 | Anosmia: 46 (60.5%), hyposmia: 30 (39.5%) | Questionnaire | Sudden onset-63.2%; before-24; concomitant-7; after-41 | Complete/partial recovery-30.3%/44.7% | ||||
| La Torre et al 50 | Isolated anosmia: 1/30 (3.3%), cases:14 (46.7%), controls: 5 (6.7%) | Cases/controls-12 (40.0%)/3 (4.0%) | Interview | - | - | - | - | - |
| Kosugi et al 51 | 145/253, anosmia: 126 (86.9%), hyposmia: 19 (13.1%) | - | Online questionnaire | - | - | 15 | Full recovery-72 (52.6%); partial-46 (33.6%); no-19 (13.9%) | - |
| Gorzkowski et al 52 | Anosmia: 5/229 (3.6%), permanent: 136 (97.1%), fluctuating: 4 (2.8%), parosmia: 21(15.0%), phantosmia: 17 (12.1%) | 140/229 (61.1%) | Telephone | Questionnaire-complete smell loss (0)-90 (64.3%); profound smell loss (1-3)-31 (22.1%); moderate smell loss (4-7)-19 (13.6%); mild smell loss (8-9)-0 | Concomitant-14.2%; before-77.8%; after-4.3% | - | 26 (95.7%) | - |
| Lechien et al 53 | 88/88, anosmia: 35 (40.0%), hyposmia: 31 (35.0%) | - | Questionnaire | SNOT-22: 33.6±18.2; sQOD-NS: 10.8±5.5 The mean Sniffin’-Sticks test-11.14±3.2 | Concomitant-29.7%; before-21.6%; after-44.6% | 14 (25.0%); 15-30 (10.2%); 31-45 (28.4%) | - | |
| Martin Sanz et al 54 | 138/215 (64.1%), hyposmia: 64.1% | - | Questionnaire | VAS score 0-2: 78 (56.5%); 3-5: 33 (23.9%); 6-8: 20 (14.4%); 7 (5.1%) | - | 10.66±0.44 | 14.0-85.4% | |
| Mazzatenta et al 55 | 61/100, hyposmic: 34.0%, Severe-hyposmic: 48.0%, anosmic: 13.0% | - | Interview | - | 7.65±5.18 | - | 14 | - |
| Meini et al 56 | Anosmia/hyposmia: 29/100 | 28/100 | Interview | - | - | 18 | F-26 M-14 | - |
| Mishra et al 57 | Anosmia: 11/74 (14.8%) | - | Questionnaire | - | - | 21 | - | |
| Moein et al 58 | Anosmia: 28/100 (28.0%) | 18/100 (18.0%) | Questionnaire | UPSIT function scores-Normosmia (31-40) 4.0%; mild microsmia (28-30) 13.0%; moderate microsmia (24-27) 24.0%; severe microsmia (17-23) 41.0%; anosmia (6-16) 18.0% | within 28 | - | ||
| Mohamud et al 59 | Anosmia: 24/60 (40.0%) | - | Medical records | - | Before-5.0%; concomitant-10.0%; after-18.3%; not remember-6.7% | - | <5: 25.0%; 5-10: 5.0%; unrecovered: 10.0% | - |
| Sayin et al 60 | 65/128 (51.6%), anosmia: 8 (12.5%), hyposmia: 33 (51.6%), parosmia: 11 (17.2%) | 34/64 (53.1%) | Online questionnaire | VAS score for COVID positive group-5.48±2.18 | Before/after diagnosis: 53.1%/18.8% | - |
OD: olfactory dysfunction, COVID: coronavirus disease-2019, GD: gustatory dysfunction, CCCRC: connecticut chemosensory clinical research center, UPSIT: University of Pennsylvania smell identification test, VAS: visual analog scale
| Authors | Patients with OD | Patients with OD + GD | Mode of collecting data | Objective assessment of OD | Onset of OD (days) | Duration of OD (days) | Recovery time (days) | Treatment given |
|---|---|---|---|---|---|---|---|---|
| Talavera et al 61 | Anosmia: 146/576 (25.3%) | - | Questionnaire | - | - | - | - | - |
| Yan et al 62 | Anosmia/hyposmia COVID-19 admitted: 7/169 (26.9%), COVID-19 positive ambulatory: 68/169 (66.7%) | - | Medical records | - | - | - | - | - |
| Lechien et al 63 | 32/86 (38.6%) Total anosmia: 61.4%, partial loss: 38.6% | - | Questionnaire | - | - | 17 | - | - |
| Barillari et al 64 | Anosmia/hyposmia: 207/294 | - | Online questionnaire | Mean SNOT score-2.39±1.61 of the 5 items (parosmia, hyposmia, anosmia, phantosmia, and GD) inserted | Before 11.6%; after 57.1%; concomitant 31.3% | - | Persistence of symptoms-31.4%; 1-4 (22.2%); 5-8 (15.4%); 9-15 (24.3%). | - |
| Kim et al 65 | Hyposmia: 68/172 (39.5%) | - | Questionnaire | - | - | - | - | - |
| Leedman et al 66 | Anosmia/hyposmia: 36/56 (64.3%) | - | Questionnaire | UPSIT function category score Normosmia-64.3%; Mild microsmia-14.3%; Moderate microsmia-14.3%; Severe microsmia-3.5%; Anosmia-3.5% | - | - | After 6 months of COVID-19: 11 (19.6%) | - |
| Kusnik et al 67 | Anosmia/hyposmia: 25/43 | - | Questionnaire | - | - | 6 | - | - |
| Makaronidis et al 68 | Anosmia: 38/467 (10.0%), partial loss: 358 (93.7%), complete loss: 92 (25.7%), parosmia: 113 (29.7%) | 83.7% (319/467) | Questionnaire | - | - | - | Full resolution-206 (57.7%); no/partial resolution-151 (42.3%) | - |
| Poerbonegoro et al 69 | Anosmia/hyposmia: 34/51 (66.7%) | 19/34 (55.9%) | Interview and questionnaire | VAS scores-Severe (7-10) 20 (68.9%); Moderate (4-6) 8 (27.7%); Mild (0-3) 1 (3.4%) | Before diagnosis-21/29 (72.4%); after-8/29 (27.5%) | - | - | - |
| Bayrak et al 70 | Anosmia/hyposmia 56/105 (53.3%) | - | Questionnaire | VAS score-1.64±2.56 (beginning of the study) and 6.19±3.12 at the end of the second month | - | - | 31 (55.0%)-one month; 16 (28.0%)-2 months; 28.8±21.0 days | - |
| Abdelmaksoud et al 71 | Total 105/134 (78.4%) Anosmia 80 (59.7%) Hyposmia 25 (18.6%) | Questionnaire | - | - | - | 7 days-zinc therapy 18 days-not received zinc therapy | Zinc therapy | |
| Goyal et al 72 | 200/574 (34.84%) Hyposmia/anosmia 73 (36.5%)/115 (57.5%) Parosmia 12 (6.0%) | 163/574 (28.4%) | Questionnaire | - | First symptom-49 (24.5%) Within 7 days-136 (68.0%); between 7-14 days-15 (7.5%) | After 1 week/2 weeks/1 month/2 months/no recovery-68 (34.0%)/74 (37.0%)/33 (16.5%)/18 (9.0%)/7 (3.5%) | - |
OD: olfactory dysfunction, GD: gustatory dysfunction, COVID: coronavirus disease, SNOT: sinonasal outcome test, UPSIT: University of Pennsylvania smell identification test, VAS: visual analog scale
| Authors | Patients with OD | Patients with OD + GD | Mode of collecting data | Objective assessment of OD | Onset of OD (days) | Duration of OD (days) | Recovery time (days) | Treatment given |
|---|---|---|---|---|---|---|---|---|
| Soh et al 73 | Anosmia-59/1938 (3.0%) Symptomatic-34 (4.4%) Asymptomatic-25 (2.1%) | - | Questionnaire | - | - | - | - | - |
| Cousyn et al 74 | 95/98 (97%) Hyposmia-9/95 (96.9%); anosmia-86/95 (90.5%); parosmia-6/95 (6.3%); phantosmia-15/95 (15.8%) | Telephone | - | 2 days before COVID-19 diagnosis | - | 20 days | - | |
| Bakhshaee et al 75 | 173/502 (37.9%) Anosmia-108 (22.0%) Hyposmia-94 (19.1%) Parasmia-17 (3.7%) Hyperosmia-5 (1.1%) | Medical records | VAS scores-2.5±2.5; 8.3±2.1; and 9.4±1.6 at the first evaluation, in 2 weeks, and after 1 month of follow-up (p<0.001) | Sudden-71 (60.2%); gradual-47 (39.8%); concomitant-72 (51.1%) | - | After 2 weeks in 18 (25.3%) anosmic and 37 (46.8%) hyposmic | - | |
| Sayin et al 76 | 03/52 Hyposmia-18 (85.78%); anosmia-3 (14.28%) | 18/52 | Questionnaire | - | Before ICU stay-15 (68.2%) | - | - | - |
| Printza et al 77 | Anosmia/hyposmia-57/140 (41%) | 48/140 (34.0%) | Telephone | VAS scores-mild-3 (5.0%); moderate-12 (21.0%); severe-11 (19.0%); extremely severe (anosmia)-31 (54.0%) | First symptom-15 (26.0%) | 11.5±13.3 days | Recovery-50 (88.0%)-61 days Median recovery time-10 days | - |
| Kumar et al 78 | 12/141 Hyposmia-16/141 Anosmia-18/141 | 28/141(19.8%) | Questionnaire | - | First symptom-13.5% | 2-15 days | Within 7 days; After 15 days-3 patients | - |
| Kant et al 79 | Anosmia/hyposmia 1756/8238 (21.3%) | - | Questionnaire | - | 2.9±2.3 days after the onset of COVID-19 | 9.4±2.7 days | Improved 2-5 days-78.1% Within 14 days-16.2%; after 14 days-3.2% | - |
| Chaturvedi et al 80 | Anosmia/hyposmia 130/277 (47.7%) | 153/277 (55.0%) | Telephone | - | With other symptoms-58.2% | - | 5-10 days (64.1%); <5 days-34.8% >14 days-11.1% | - |
| Parente-Arias et al 81 | 8/151 (8.1%) Anosmia-75/151 (49.7%) Hyposmia-26 (17.2%) Isolated anosmia-2 (1.3%) | 99/151 (65.6%) | Telephone | - | Same day-19/75 (25.3%) | 4.4±0.6 days | First 2 months (85.3%) | - |
| Mubaraki et al 82 | 541/1022 (53.0%) Anosmia-32.7%; hyposmia-20.3% | - | Telephone | - | - | Anosmia/hyposmia-12.1±10.3/8.7±8.3 | - | - |
| D Silva et al 83 | 45/166 (53.0%) Hyposmia-45 (53.0%) | - | Online questionnaire | 8.3±4.7 days | ||||
| Bhatta et al 84 | 112/188 (60.6%) Hyposmia-36.1%; anosmia-20.2%; parosmia-4.2% | - | Questionnaire | - | - | Hyposmia/anosmia/parosmia-8/5/2 days | After 4 months Anosmia-97.4%; hyposmia-95.6%; parosmia-100% | - |
| Hameed et al 85 | 4/35 Anosmia-4 Anosmia and hypogeusia-2 | 2/35 | Questionnaire | - | - | 7-14 days | - | - |
| Savtale et al 86 | Anosmia/hyposmia-90/180 (55.5%) | - | Verbal survey | - | - | 20.5 days | - | - |
OD: olfactory dysfunction, GD: gustatory dysfunction, COVID: coronavirus disease, VAS: visual analog scale, ICU: intensive care unit
| Authors | Patients with OD | Patients with OD + GD | Mode of collecting data | Objective assessment of OD | Onset of OD (days) | Duration of OD (days) | Recovery time (days) | Treatment given |
|---|---|---|---|---|---|---|---|---|
| Horvath et al 87 | 66/102 (65.0%) Hyposmia-23.0%; anosmia42.0% | 75/102 (74.0%) | Online questionnaire | - | - | - | - | - |
| Shaikh et al 88 | 34/1070 Hyposmia-3.2% Anosmia-7.3% | 150 (14.0%) | Questionnaire | - | - | - | - | - |
| Khan et al 89 | Anosmia/hyposmia 19/224 (8.4%) | 64/224 (28.6%) | Questionnaire | UPSIT function scores normal-142 (63.4%); mild hyposmia 39 (17.4%); moderate hyposmia 18 (8.0%); severe hyposmia 13 (5.8%); anosmia 12 (5.4%) | Within 5 days-(58/102 (56.8%) First sign-10.1% Sudden in onset-7.1% | - | - | - |
| Lee et al 90 | Anosmia-89/350 (25.4%) Hyposmia-56/350 (16.0%) | - | Telephone | - | First symptom-10% | - | 4 weeks (90.4%) | - |
| Koul et al 91 | 83/231 (55.33%) Anosmia-57.3%; hyposmia-28.7% | 46/231 (30.7%) | Questionnaire | - | - | - | 1 month (78.0%) | - |
| Kandemirli et al 92 | Anosmia-23/23 | - | Questionnaire | Sniffin’ Sticks test Threshold-1 (1±2.25); discrimination-2 (0±3); identification-3 (0±4); TDI-4 (1±8.5) | Sudden onset-4/23; after-12 concomitant-07 | - | - | - |
| Altundag et al 93 | Anosmia/hyposmia-80/135 (59.3%) | - | Telephone | VAS scores-Group 1-8.4±1.9; Group 2-7.6±2; Group 3-6.2±2.6 | - | 7.8±3.1 (2-15) days | Group 1/2/3-28.6%/50.0%/66.7% | - |
| Dev et al 94 | Anosmia-53/55 (96.0%) | 39 (71.0%) | Medical records | Mean VAS scores 5.52±2.08 | 7 days | - | 30 days | |
| Korkmaz et al 95 | Anosmia/hyposmia-43/116 (37.9%) | - | Questionnaire | - | - | - | - | - |
| Babaei et al 96 | Anosmia-207/235 (88.5%)-4 weeks and 219 (93.2%)-8 weeks | - | Interview | - | First symptom-23 (9.8%); Onset (mean)-3.88 day | - | 19.42±8.81 days | - |
| Nouchi et al 97 | Hyposmia 129/390 (33.0%) | 106 (27.0%) | Telephone | - | - | - | Persistent hyposmia-34.0% | - |
| Polat et al 98 | Anosmia 72/217 (33.2%) | - | Interview | - | 3 (1-13) days | - | 13 (3-30) days | - |
| Renaud et al 99 | 43/51 (84.3%) Anosmia 23 (45.1%) Hyposmia 27 (52.9%) Parosmia 14 (27.5%) Phantosmia 13 (25.5%) | - | Questionnaire | CCCRC-QOD scores ranges 0-10/11-25/26-50/51-75/76-90/91-95/96-t-5 (9.8%)/3 (5.8%)/9 (17.7%)/9 (17.7%)/13 (25.5%)/5 (9.8%)/7 (13.7%) Identification test-5 (9.8%)/5 (9.8%)/6 (11.8%)/7 (13.7%)/9 (17.7%)/9 (17.7%)/10 (19.5%)/ | - | - | After 4 months-<15/16-30/30-60/60-90-11(47.8%)/5 (21.7%)/6 (26.1%)/1 (4.4%) | - |
| Rizzo et al 100 | 110/202 (60.1%) Normosmia 58 (28-34) Microsmia 77 (16-27) Anosmia-10 (5-15) | - | Telephone | CAUPSIT score-25.5; mildly microsmic-54(37.2%); moderately microsmic 16 (11.0%); severely microsmic-7 (4.8%); anosmic 10 (6.9%) | - | - | Complete resolution/partial/no improvement 85 (77.3%)/22 (20.0%)/3 (2.7%) | - |
OD: olfactory dysfunction, GD: gustatory dysfunction, UPSIT: University of Pennsylvania smell identification test, TDI: olfactory test, VAS: visual analog scale, CCCRC-QOD: connecticut chemosensory clinical research center - questionnaire of olfactory disorders, CAUPSIT: culturally adapted University of Pennsylvania smell identification test
| Authors | Patients with OD | Patients with OD + GD | Mode of collecting data | Objective assessment of OD | Onset of OD (days) | Duration of OD (days) | Recovery time (days) | Treatment given |
|---|---|---|---|---|---|---|---|---|
| Thakur et al 101 | Anosmia/hyposmia-179/250 (71.6%) | - | Oral questionnaire | - | Before-44(17.6%); after-77 (30.8%); concomitant-58(23.2%) | Recovery time-1-4/5-8/9-14/more than 15 days-17 (6.8%)/87 (34.8%)/103(41.2%)/43 (17.2%) | - | |
| Teaima et al 102 | Anosmia-67.9%; hyposmia-30.0%; phantosmia-18.0%; parosmia-28.4% | Anosmia & ageusia-50.2%; hyposmia & hypogeusia-23.3% | Questionnaire | - | After COVID symptoms-43.5% Sudden onset-80.4% | After 6 months-complete/partial/no recovery-66.0%/22.1%/11.9% | - |
OD: olfactory dysfunction, GD: gustatory dysfunction, COVID: coronavirus disease
The most common method used to evaluate OD was the questionnaire (n=43) followed by telephonic conversation (n=15), medical records (n=11), personal face-to-face interview of the patient (n=7), online questionnaire (n=5), and email (n=2), COVID RADAR symptom tracker app (n=1), and COVID-19 anosmia reporting tool (n=1).
In our systematic review, the loss of smell as the first and only symptom was described in 8 studies. 36,38,72,77,78,89,90,96 The occurrence of olfactory symptoms before the generalised symptoms of COVID-19 was reported by 14 studies. 9,34,35,46,49,52,53,59,60,64,69,74,76,101 The sudden onset of olfactory symptoms was reported by 7 studies. 22,42,49,75,89,92,102 Only 4 studies included patients who received treatment for OD. 34,35,48,71 Details of the onset time, duration, recovery time, and treatment of OD are shown in Table 2.
Discussion
Coronavirus (SARS-CoV-2) is a global threat, resulting in widespread infections and fatalities across the world. The disease remains an active pandemic and a serious threat to healthcare systems worldwide. At first, the primary classical symptoms of COVID-19 were believed to be fever, cough, fatigue, and shortness of breath. However, more recently, OD has emerged as a prominent symptom that can aid in the detection of asymptomatic carriers of COVID-19. 27
This systematic review uncovered a significant body of research documenting the loss of the sense of smell among COVID-19 patients across multiple continents. We included data from 27 countries, of which the studies published in India contributed to 11 (13.09%), 9 (10.71%) in France, 8 (9.52%) in Italy, 8 (9.52%) in Iran, and 7 (8.33%) in the United States of America (US), of the total studies included in this review. 9-101 In terms of the study population, India, France, Italy, Iran, and the US carried out substantial contribution to the sample size, accounting for 3388 (9.1%) in India, 2042 (5.53%) in France, 1585 (4.29%) in Italy, 1190 (3.22%) in Iran, and 132 (3.57%) in the US of the participants. A female predominance was observed in our systematic review (58.1%), similar to the results of a meta-analysis carried out by Saniasiaya et al 14 (61.4%) and a systematic review carried out by Aziz et al 2 (53.1%).
The sense of smell is one of the various special sensations. Olfactory dysfunction is subclassified into complete loss of smell (anosmia), partial loss of smell (hyposmia), distorted sense of smell (parosmia), olfactory hallucinations (phantosmia), and a heightened sense of smell (hyperosmia). Regarding the aetiology of OD in general, nearly 200 causes exist, but the most commonly observed eare related to age, congenital, head trauma, post-viral, toxins (smoking or work-related), drugs (local anaesthesia, nifedipine, antimicrobials, antidepressants, and immunosuppressants), and diseases related to the sinonasal tract (allergic and non-allergic rhinitis, septal deviation, and chronic rhinosinusitis with nasal polyposis). 103
In our comprehensive review, all 84 studies consistently demonstrated a robust link between the loss of smell and SARS-CoV-2 infection. Within this set, 81 studies specifically highlighted the occurrence of isolated OD, 40 studies reported a concurrent presentation of OD and GD as a unified symptom, and 37 studies reported the prevalence of both isolated OD and the combined presence of both dysfunctions. The estimated prevalence of loss of smell among 33,231 individuals with COVID-19 included in this review was 34.60% (range of prevalence from 3.9-100%). 22,38,42,53,92 Our estimated prevalence was slightly lower than the global pooled prevalence found in systematic reviews carried out by Aziz et al 2 (52.0%) with 51 included articles, da Costa et al 15 (60.7%) with 6 included articles, Hannum et al 17 (50.2%) with 34 included articles, and Agyeman et al 18 (41%) with 24 included articles, where the sample size was small, whereas, our systematic review included 84 studies. In a meta-analysis carried out by Saniasiaya et al, 14 it was determined that the prevalence of OD among COVID-19 patients stood at 47.85% (95% confidence interval [CI]: [41.20-54.50]). 14 Tong et al 13 found an overall prevalence of 52.73% (range of prevalence 5.14-98.33%) among 1,627 patients in 10 studies. Ibekwe et al 16 reported a global pooled prevalence of 48.47% (ranging from 4.23-98.33%) among 19,424 patients with COVID-19 included in 27 studies. Owing to the increased prevalence of loss of smell among patients with COVID-19, the ENT Society of the United Kingdom stated that individuals complaining of anosmia while not exhibiting other clinical features might be hidden carriers of COVID-19 and are responsible for the rapid spread of COVID-19. Such individuals should self-isolate for 14 days to stop the chain of infection. 104
The combined loss of smell and taste was less frequently reported in our systematic review, with only 40 studies including data from 3,777 individuals with COVID-19, resulting in a prevalence of 11.36% (generality ranging from 3.9-90.9%. 41,43 A meta-analysis carried out by Tong et al 13 revealed that the generality of both dysfunctions ranged from 5.61-92.65% among 626 patients in 9 studies. Ibekwe et al 16 demonstrated an estimated pooled generality of 35.04% (range of prevalence from 7.96-75.74%) in 13 studies involving 5,977 patients with COVID-19. A multicentric European study included in the review reported the commonness of OD to be 85.6% and GD to be 88.8%. 34 The data regarding the combined prevalence of OD and GD are limited as most systematic reviews have only reported the commonness of either OD or GD.
Pathophysiology
The precise pathophysiological mechanisms underlying the loss of smell in individuals with COVID-19 remain incompletely comprehended, but there are a few hypotheses that have already been presented in the literature. Zhou et al 105 unveiled a new SARS-CoV-2 infection on February 3, 2020. Their study elucidated the invasion of human lower respiratory system cells by SARS-CoV-2 through the utilization of ACE2 and transmembrane protease serine 2 receptors. Among these receptors, ACE2 is predominantly located on cells in various tissues, including the lungs, liver, kidneys, gastrointestinal (GI) tract, and even the nasal epithelium. 106 Respiratory epithelial cells and supporting olfactory cells act as the chief reservoir site and the second most susceptible site for the replication of this deadly virus, as they harbour the highest concentration of the 2 above-mentioned genes (abACE2 and TMPRSS2) responsible for smell loss. 107,108 Based on this hypothesis, 3 mechanisms have been postulated for the loss of smell. First, infection of the nasal mucosa by SARS-CoV-2 triggers the inflammatory process of the respiratory and olfactory mucosa, creating a barrier to the odour of the aromatic particles present in the air between the olfactory neurones and mucosa, leading to disruption of the process of odour detection. 109 The second mechanism is the direct attack of the virus to the olfactory mucosa causing inhibition of the transmission of olfactory signals, leading to temporary or permanent dysfunction of the olfactory mucosa. 110,111 The final mechanism involves the virus infiltrating the cribriform plate, thereby infecting the olfactory bulb. This allows the virus to follow the olfactory pathway, ultimately reaching the brain and impacting the olfactory cortex in the temporal lobe, leading to a loss of the sense of smell. 112 Hence, the involvement of any one or all of these mechanisms is responsible for the temporary or permanent loss of smell caused in COVID-19 positive individuals.
Symptoms
To better understand the prevalence of OD, clinical symptoms, and the correlation between these symptoms and disease progression in individuals with COVID-19, the AAO-HNS has provided a COVID-19 anosmia reporting tool. 10 Similarly, in our review, the objective assessment of olfactory symptoms was carried out in 14 studies using the University of Pennsylvania smell identification (UPSIT, n=6), odour threshold Sniffin’ Sticks (n=5), sinonasal outcome (SNOT, n=2), and connecticut chemosensory clinical research center (CCCRC, n=2) tests were used. 23,26,33,37,43,53,58,64,66,89,92,99,100 In the meta-analyses carried out by Saniasiaya et al 14 of 4 studies and Aziz et al 2 of 8 studies (out of 51), utilised objective assessments. Saniasiaya et al 14 found a higher prevalence of OD using an objective evaluation (72.10%) rather than a subjective one (44.53%). In another systematic review carried out by Hannum et al, 17 6 studies (out of 34) used the objective assessment method, and the prevalence of OD was found to be high using objective methods (77% vs. 44%). A meta-analysis carried out by Tong et al 13 reported a higher prevalence of OD using the UPSIT compared to other instruments. Each method has advantages and disadvantages. Objective methods quantify smell loss better because they are standardised, whereas subjective methods, such as questionnaires and interviews, have more flexibility and variability, are easy to use, and are cost-efficient. However, they lack standardisation and are subject to recall bias.
Smell loss is one of the most underreported symptoms in patients with COVID-19, and sometimes it can be the only complaint of the patient. In our systematic review, the occurrence of loss of smell as the first and only symptom was described in 8 studies 36,38,72,77,78,89,90,96 and the sudden onset of olfactory symptoms was reported in 7 studies. 22,42,49,75,89,92,102 The AAO-HNS found that anosmia was the first symptom in 26.6% of patients. 10 The occurrence of olfactory symptoms before the generalised symptoms of COVID-19 was reported in 14 studies. 9-101 Giorli et al 19 in their meta-analysis reported the early appearance of olfactory symptoms as compared to other ones in 11.8% of patients. While developing the COVID-19 anosmia reporting tool for clinicians, the AAO-HNS reported in their study that the occurrence of anosmia before the diagnosis of SARS-CoV-2 was found in 73% of patients. 10 The AAO-HNS also suggested that the possibility of COVID-19 should be considered among individuals with a sudden loss of anosmia or ageusia in the absence of other respiratory symptoms. 113
Imaging
Imaging modalities are not routinely required in patients with OD because in most cases, they are negative and of no use. As per the consensus guidelines by the British Rhinological Society (BRS), when a patient exhibits a loss of smell alongside other nasal symptoms persisting for 4-6 weeks (irrespective of COVID-19 status), it is recommended to carry out nasal endoscopy prior to resorting to imaging procedures. 114 The BRS states that if patients present with a loss of smell for more than 4-6 weeks along with the presence of neurological manifestations, brain MRI should be carried out regardless of COVID-19 status. 114 In the present review, imaging modalities were used in only 3 studies. 37,42,92 The utility of these modalities has not yet been proven and they are only reserved for patients with persistent OD.
Prognosis
The treatment of OD depends on the aetiology of smell loss; however, it is required only in cases where OD does not improve spontaneously or persists even after 2 weeks. Generally, the management of OD involves addressing its root cause, employing medical interventions such as oral and topical steroids, and considering surgical options like septoplasty, turbinoplasty, and endoscopic sinus surgery. 103 As for the treatment of OD in COVID-19 patients, the BRS has established a set of consensus guidelines. These guidelines encompass various approaches, including olfactory training and support (for patients experiencing a loss of smell lasting more than 2 weeks), the use of intranasal corticosteroid sprays, intranasal corticosteroid drops (recommended for patients with both a loss of smell and nasal symptoms lasting more than 2 weeks), oral corticosteroids (suitable for patients with a loss of smell and other nasal symptoms for 2 weeks, provided they have resolved their COVID-19 symptoms), and the consideration of alpha-lipoic acid or omega-3 supplements (particularly for individuals with isolated loss of smell lasting more than 2 weeks). 114 In the present review, 4 studies mentioned specific treatments for smell loss. 34,35,48,71 In a systematic review carried out by Saniasiaya et al, 14 there was no mention of a particular treatment protocol for addressing olfactory impairment. Similarly, most of the studies included in our review did not employ a specific treatment approach for OD. This choice is influenced by the uncertainty surrounding the effectiveness of oral steroids, as well as concerns regarding their potential to promote upper respiratory tract infections.
The prognosis of OD depends on the underlying cause; however, in most cases, patients recover within 30 days without treatment, suggesting a good prognosis. In our review, the outcome/recovery of olfactory symptoms was mentioned in 48 studies. Of these 48 studies, the persistence of olfactory symptoms after one month was observed in 13. 23-102 Hopkin et al 25 in their study concluded that an improvement in the loss of smell within a week of onset was observed in 80% of patients. A study carried out by Mendonca et al 115 stated that the presence of OD among patients with COVID-19 can be a sign of a good prognosis.
Study strength & limitations
The strength of this systematic review lies in its sample size, as we attempted to include studies from multiple continents. In addition, we depicted the prevalence of OD alone and in combination with GD that has not been previously reported by many studies. Although we carried out an extensive literature search, our systematic review had certain limitations. Since we only included studies published in bibliographic databases and in the English language, excluding unpublished and grey literature, certain biases such as language bias and publication bias are present in the systematic review. Second, we did not consider the role of pre-existing diseases in patients with COVID-19, as they can exaggerate the COVID-19 disease and its symptoms. In addition, objective evaluations were carried out in only a small number of studies. Furthermore, owing to the controversial association between COVID-19 and OD, loss of smell has been underreported in many studies, leading to an underestimation of the overall rampancy of these symptoms. Hence, more studies and systematic reviews should be carried out to overcome these drawbacks.
In conclusion, the rampancy of OD alone was 34.60% and in combination with GD was it was 11.36%, in COVID-19 positive individuals. After classifying OD, variations were observed in the prevalence of anosmia (20.85%), hyposmia (5.04%), anosmia or hyposmia (8.88%), parosmia (1.84%), phantosmia (0.78%), and hyperosmia (0.02%) in patients with COVID-19.
The clinical characteristics linked to OD, whether in isolation or coupled with gustatory impairment, frequently manifest in COVID-19 patients. These manifestations serve as crucial indicators that can facilitate the early detection of the disease. Heightening awareness of these symptoms plays a pivotal role in ensuring the timely diagnosis and treatment of this serious COVID-19 condition.
Acknowledgment
The authors gratefully acknowledge Editage (www.editage.com) for their English language editing.
Footnotes
References
- 1. Coronavirus WHO. (COVID-19) dashboard. [Updated 2023; accessed 2021 Dec 28]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2. Aziz M, Goyal H, Haghbin H, Lee-Smith WM, Gajendran M, Perisetti A.. The association of “loss of smell” to COVID-19: a systematic review and meta-analysis. Am J Med Sci 2021; 361: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aziz M, Perisetti A, Lee-Smith WM, Gajendran M, Bansal P, Goyal H.. Taste changes (dysgeusia) in COVID-19: a systematic review and meta-analysis. Gastroenterology 2020; 159: 1132–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gengler I, Wang JC, Speth MM, Sedaghat AR.. Sinonasal pathophysiology of SARS-CoV-2 and COVID-19: a systematic review of the current evidence. Laryngoscope Investig Otolaryngol 2020; 5: 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, et al. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg 2020; 146: 674–675. [DOI] [PubMed] [Google Scholar]
- 8. Gane SB, Kelly C, Hopkins C.. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology 2020; 58: 299–301. [DOI] [PubMed] [Google Scholar]
- 9. Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC 3rd.. COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg 2020; 163: 132–134. [DOI] [PubMed] [Google Scholar]
- 10. American Academy of Otolaryngology-Head and Neck Surgery. COVID-19 anosmia reporting tool. [Updated 2021; accessed 2021 Dec 21]. Available from: https://www.entnet.org/content/reporting-tool-patients-anosmia-related-covid-19
- 11. Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020; 133: 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T.. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2020; 163: 3–11. [DOI] [PubMed] [Google Scholar]
- 14. Saniasiaya J, Islam MA, Abdullah B.. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID-19): a meta-analysis of 27,492 patients. Laryngoscope 2021; 131: 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Costa KVTD, Carnaúba ATL, Rocha KW, Andrade KCL, Ferreira SMS, Menezes PL.. Olfactory and taste disorders in COVID-19: a systematic review. Braz J Otorhinolaryngol 2020; 86: 781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ibekwe TS, Fasunla AJ, Orimadegun AE.. Systematic review and meta-analysis of smell and taste disorders in COVID-19. OTO Open 2020; 4: 2473974X20957975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hannum ME, Ramirez VA, Lipson SJ, Herriman RD, Toskala AK, Lin C, et al. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19-positive patients compared to subjective methods: a systematic review and meta-analysis. [Updated 2020; 2020 Feb]. Available from: https://www.medrxiv.org/content/10.1101/2020.07.04.20145870v1.full.pdf+html [DOI] [PMC free article] [PubMed]
- 18. Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R.. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc 2020; 95: 1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giorli A, Ferretti F, Biagini C, Salerni L, Bindi I, Dasgupta S, et al. A literature systematic review with meta-analysis of symptoms pevalence in covid-19: the relevance of olfactory symptoms in infection not requiring hospitalization. Curr Treat Options Neurol 2020; 22: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V, et al. Features of anosmia in COVID-19. Med Mal Infect 2020; 50: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM.. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berl) 2020; 7: 91–96. [DOI] [PubMed] [Google Scholar]
- 22. Gilani S, Roditi R, Naraghi M.. COVID-19 and anosmia in Tehran, Iran. Med Hypotheses 2020; 141: 109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck 2020; 42: 1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med 2020; 26: 1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hopkins C, Surda P, Whitehead E, Kumar BN.. Early recovery following new onset anosmia during the COVID-19 pandemic - an observational cohort study. J Otolaryngol Head Neck Surg 2020; 49: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL.. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol 2020; 10: 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR.. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg 2020; 163: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coelho DH, Kons ZA, Costanzo RM, Reiter ER.. Subjective changes in smell and taste during the COVID-19 pandemic: a national survey-preliminary results. Otolaryngol Head Neck Surg 2020; 163: 302–306. [DOI] [PubMed] [Google Scholar]
- 29. Roland LT, Gurrola JG 2nd, Loftus PA, Cheung SW, Chang JL.. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int Forum Allergy Rhinol 2020; 10: 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zayet S, Klopfenstein T, Mercier J, Kadiane-Oussou NJ, Lan Cheong Wah L, Royer PY, et al. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection 2021; 49: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boscolo-Rizzo P, Borsetto D, Spinato G, Fabbris C, Menegaldo A, Gaudioso P, Nicolai P, et al. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur Arch Otorhinolaryngol 2020; 277: 2637–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee Y, Min P, Lee S, Kim SW.. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci 2020; 35: e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020; 42: 1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 277: 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hopkins C, Surda P, Kumar N.. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology 2020; 58: 295–298. [DOI] [PubMed] [Google Scholar]
- 36. Jalessi M, Barati M, Rohani M, Amini E, Ourang A, Azad Z, et al. Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19. Neurol Sci 2020; 41: 2331–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lechien JR, Michel J, Radulesco T, Chiesa-Estomba CM, Vaira LA, De Riu G, et al. Clinical and radiological evaluations of COVID-19 patients with anosmia: preliminary report. Laryngoscope 2020; 130: 2526–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dell’Era V, Farri F, Garzaro G, Gatto M, Aluffi Valletti P, Garzaro M.. Smell and taste disorders during COVID-19 outbreak: cross-sectional study on 355 patients. Head Neck 2020; 42: 1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villarreal IM, Morato M, Martínez-RuizCoello M, Navarro A, Garcia-Chillerón R, Ruiz Á, et al. Olfactory and taste disorders in healthcare workers with COVID-19 infection. Eur Arch Otorhinolaryngol 2021; 278: 2123–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qiu C, Cui C, Hautefort C, Haehner A, Zhao J, Yao Q, et al. Olfactory and gustatory dysfunction as an early identifier of COVID-19 in adults and children: an international multicenter study. Otolaryngol Head Neck Surg 2020; 163: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tham AC, Thein TL, Lee CS, Tan GSE, Manauis CM, Siow JK, et al. Olfactory taste disorder as a presenting symptom of COVID-19: a large single-center Singapore study. Eur Arch Otorhinolaryngol 2021; 278: 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naeini AS, Karimi-Galougahi M, Raad N, Ghorbani J, Taraghi A, Haseli S, et al. Paranasal sinuses computed tomography findings in anosmia of COVID-19. Am J Otolaryngol 2020; 41: 102636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Otte MS, Eckel HNC, Poluschkin L, Klussmann JP, Luers JC.. Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol 2020; 140: 1032–1035. [DOI] [PubMed] [Google Scholar]
- 44. Al-Ani RM, Acharya D.. Prevalence of anosmia and ageusia in patients with COVID-19 at a primary health center, Doha, Qatar. Indian J Otolaryngol Head Neck Surg 2022; 74: 2703–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Altin F, Cingi C, Uzun T, Bal C.. Olfactory and gustatory abnormalities in COVID-19 cases. Eur Arch Otorhinolaryngol 2020; 277: 2775–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. D’Ascanio L, Pandolfini M, Cingolani C, Latini G, Gradoni P, Capalbo M, et al. Olfactory dysfunction in COVID-19 patients: prevalence and prognosis for recovering sense of smell. Otolaryngol Head Neck Surg 2021; 164: 82–86. [DOI] [PubMed] [Google Scholar]
- 47. Cazzolla AP, Lovero R, Lo Muzio L, Testa NF, Schirinzi A, Palmieri G, et al. Taste and smell disorders in COVID-19 patients: role of interleukin-6. ACS Chem Neurosci 2020; 11: 2774–2781. [DOI] [PubMed] [Google Scholar]
- 48. Chiesa-Estomba CM, Lechien JR, Radulesco T, Michel J, Sowerby LJ, Hopkins C, et al. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur J Neurol 2020; 27: 2318–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karimi-Galougahi M, Safavi Naini A, Ghorbani J, Raad N, Raygani N.. Emergence and evolution of olfactory and gustatory symptoms in patients with COVID-19 in the outpatient setting. Indian J Otolaryngol Head Neck Surg 2022; 74: 2743–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. La Torre G, Massetti AP, Antonelli G, Fimiani C, Fantini M, Marte M, et al. Anosmia and ageusia as predictive signs of COVID-19 in healthcare workers in Italy: a prospective case-control study. J Clin Med 2020; 9: 2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kosugi EM, Lavinsky J, Romano FR, Fornazieri MA, Luz-Matsumoto GR, Lessa MM, et al. Incomplete and late recovery of sudden olfactory dysfunction in COVID-19. Braz J Otorhinolaryngol 2020; 86: 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gorzkowski V, Bevilacqua S, Charmillon A, Jankowski R, Gallet P, Rumeau C, et al. Evolution of olfactory disorders in COVID-19 patients. Laryngoscope 2020; 130: 2667–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lechien JR, Journe F, Hans S, Chiesa-Estomba CM, Mustin V, Beckers E, et al. Severity of anosmia as an early symptom of COVID-19 infection may predict lasting loss of smell. Front Med (Lausanne) 2020; 7: 582802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martin-Sanz E, Riestra J, Yebra L, Larran A, Mancino F, Yanes-Diaz J, et al. Prospective study in 355 patients with suspected COVID-19 infection: value of cough, subjective hyposmia, and hypogeusia. Laryngoscope 2020; 130: 2674–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mazzatenta A, Neri G, D’Ardes D, De Luca C, Marinari S, Porreca E, et al. Smell and taste in severe COVID-19: self-reported vs. testing. Front Med (Lausanne) 2020; 7: 589409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meini S, Suardi LR, Busoni M, Roberts AT, Fortini A.. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: gender differences and recovery time in real-life. Eur Arch Otorhinolaryngol 2020; 277: 3519–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mishra P, Gowda V, Dixit S, Kaushik M.. Prevalence of new onset anosmia in COVID-19 patients: is the trend different between European and Indian population? Indian J Otolaryngol Head Neck Surg 2020; 72: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moein ST, Hashemian SM, Tabarsi P, Doty RL.. Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID-19 patients. Int Forum Allergy Rhinol 2020; 10: 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Farah Yusuf Mohamud M, Garad Mohamed Y, Mohamed Ali A, Ali Adam B.. Loss of taste and smell are common clinical characteristics of patients with COVID-19 in Somalia: a retrospective double centre study. Infect Drug Resist 2020; 13: 2631–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sayin İ, Yaşar KK, Yazici ZM.. Taste and smell impairment in COVID-19: an AAO-HNS anosmia reporting tool-based comparative study. Otolaryngol Head Neck Surg 2020; 163: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Talavera B, García-Azorín D, Martínez-Pías E, Trigo J, Hernández-Pérez I, Valle-Peñacoba G, et al. Anosmia is associated with lower in-hospital mortality in COVID-19. J Neurol Sci 2020; 419: 117163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS.. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol 2020; 10: 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M, Hans S, Calvo-Henriquez C, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck 2020; 42: 1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barillari MR, Bastiani L, Lechien JR, Mannelli G, Molteni G, Cantarella G, et al. A structural equation model to examine the clinical features of mild-to-moderate COVID-19: a multicenter Italian study. J Med Virol 2021; 93: 983–994. [DOI] [PubMed] [Google Scholar]
- 65. Kim GU, Kim MJ, Ra SH, Lee J, Bae S, Jung J, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect 2020; 26: 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Leedman SR, Sheeraz M, Sanfilippo PG, Edgar DW, D’Aulerio GV, Robb DM, et al. Olfactory dysfunction at 6 months after coronavirus disease 2019 infection. J Laryngol Otol 2021; 135: 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kusnik A, Weiss C, Neubauer M, Huber B, Gerigk M, Miethke T, et al. Presence of gustatory and olfactory dysfunction in the time of the COVID-19 pandemic. BMC Infect Dis 2021; 21: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Makaronidis J, Firman C, Magee CG, Mok J, Balogun N, Lechner M, et al. Distorted chemosensory perception and female gender associate with persistent smell or taste loss in people with SARS-CoV-2 antibodies: a community based cohort study investigating clinical course and resolution of acute smell or taste loss in people with and without SARS-CoV-2 antibodies in London, UK. BMC Infect Dis 2021; 21: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Poerbonegoro NL, Reksodiputro MH, Sari DP, Mufida T, Rahman MA, Reksodiputro LA, Audindra S, et al. Cross-sectional study on the proportion of smell and taste disturbances in hospitalized COVID-19 patients. Ann Med Surg (Lond) 2021; 71: 102909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bayrak AF, Karaca B, Özkul Y.. Could smell and taste dysfunction in COVID-19 patients be a sign of the clinical course of the disease? Egypt J Otolaryngol 2021; 37: 106. [Google Scholar]
- 71. Abdelmaksoud AA, Ghweil AA, Hassan MH, Rashad A, Khodeary A, Aref ZF, et al. Olfactory disturbances as presenting manifestation among Egyptian patients with COVID-19: possible role of zinc. Biol Trace Elem Res 2021; 199: 4101–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goyal R, Kapoor A, Goyal MK, Singh R.. Alteration of smell and taste sensations in COVID-19 positive patients: a prospective cohort study in Western India. Indian J Otolaryngol Head Neck Surg 2021; 73: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Soh SHL, See A, Teo NWY, Tan HK, Palaniappan G, Lim MLA, et al. Prevalence of olfactory and taste dysfunction in COVID-19 patients: a community care facility study. Eur Arch Otorhinolaryngol 2021; 278: 3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cousyn L, Sellem B, Palich R, Bendetowicz D, Agher R, Delorme C, et al. Olfactory and gustatory dysfunctions in COVID-19 outpatients: a prospective cohort study. Infect Dis Now 2021; 51: 440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bakhshaee M, Barzegar-Amini M, Motedayen Z, Khojasteh-Taheri R, Rafiee M, Amini M, et al. Olfactory dysfunction in patients infected with 2019 novel coronavirus. Iran J Otorhinolaryngol 2021; 33: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sayın P, Altınay M, Cınar AS, Ozdemir HM.. Taste and smell impairment in critically ill patients with COVID-19: an intensive care unit study. Ear Nose Throat J 2021; 100: 174S–179S. [DOI] [PubMed] [Google Scholar]
- 77. Printza A, Katotomichelakis M, Valsamidis K, Metallidis S, Panagopoulos P, Panopoulou M, et al. Smell and taste loss recovery time in COVID-19 patients and disease severity. J Clin Med 2021; 10: 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kumar L, Kahlon N, Jain A, Kaur J, Singh M, Pandey AK.. Loss of smell and taste in COVID-19 infection in adolescents. Int J Pediatr Otorhinolaryngol 2021; 142: 110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kant A, Öztürk S, Arslan M, et al. Severity and reversibility of smell-taste dysfunction in Covid-19 patients. Acta Med Mediterr 2021; 37: 2559. [Google Scholar]
- 80. Chaturvedi HT, Patel VP, Vasava RR, Chaturvedi C.. Importance and correlation of sudden onset, presence and recovery of olfactory and gustatory dysfunctions in COVID-19 patients: a cross-sectional study. J Oral Maxillofac Pathol 2021; 25: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parente-Arias P, Barreira-Fernandez P, Quintana-Sanjuas A, Patiño-Castiñeira B.. Recovery rate and factors associated with smell and taste disruption in patients with coronavirus disease 2019. Am J Otolaryngol 2021; 42: 102648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mubaraki AA, Alrbaiai GT, Sibyani AK, Alhulayfi RM, Alzaidi RS, Almalki HS.. Prevalence of anosmia among COVID-19 patients in Taif city, Kingdom of Saudi Arabia. Saudi Med J 2021; 42: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Silva FTD, Sperandio M, Suzuki SS, Silva HPV, de Oliveira DG, Stefenon L, et al. Self-reported taste and smell impairment among patients diagnosed with COVID-19 in Brazil. Oral Dis 2022; 28: 2559–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bhatta S, Sharma D, Sharma S, Maharjan L, Bhattachan S, Shah MK, et al. Smell and taste disturbance in COVID-19 patients: a prospective multicenteric review. Indian J Otolaryngol Head Neck Surg 2022; 74: 2978–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hameed HM, Rashid FT, Al Qaisi T.. Olfactory and taste disorders (OTDs) in COVID-19 patients in Wasit Provence. Wasit J Sci Med 2020; 13: 18–23. [Google Scholar]
- 86. Savtale S, Hippargekar P, Bhise S, Kothule S.. Prevalence of otorhinolaryngological symptoms in COVID-19 patients. Indian J Otolaryngol Head Neck Surg 2022; 74: 3378–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Horvath L, Lim JWJ, Taylor JW, Saief T, Stuart R, Rimmer J, et al. Smell and taste loss in COVID-19 patients: assessment outcomes in a Victorian population. Acta Otolaryngol 2021; 141: 299–302. [DOI] [PubMed] [Google Scholar]
- 88. Shaik A, Raju K R, Priya S.. Profile of ENT manifestations among COVID-19 patients. IP J Orl Allied Sci 2021; 4: 1–5. [Google Scholar]
- 89. Khan I, Gupta V, Shukla SK.. Objective evaluation of olfactory and taste dysfunction among COVID-19 patients: a cross sectional study from Tribal India. Indian J Otolaryngol Head Neck Surg 2022; 74: 3193–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lee DJ, Daliyot D, Wang R, Lockwood J, Das P, Zimlichman E, et al. Comparative study of chemosensory dysfunction in COVID-19 in 2 geographically distinct regions. Ear Nose Throat J 2023; 102: 323–328. [DOI] [PubMed] [Google Scholar]
- 91. Koul D, Begh RA, Kalsotra P.. Olfactory and gustatory alterations in COVID-19 patients: a tertiary care COVID-19 centre inpatient experience. Indian J Otolaryngol Head Neck Surg 2022; 74: 2857–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kandemirli SG, Altundag A, Yildirim D, Tekcan Sanli DE, Saatci O.. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad Radiol 2021; 28: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Altundag A, Saatci O, Sanli DET, Duz OA, Sanli AN, Olmuscelik O, et al. The temporal course of COVID-19 anosmia and relation to other clinical symptoms. Eur Arch Otorhinolaryngol 2021; 278: 1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dev N, Sankar J, Gupta N, Meena RC, Singh C, Gupta DK, et al. COVID-19 with and without anosmia or dysgeusia: a case-control study. J Med Virol 2021; 93: 2499–2504. [DOI] [PubMed] [Google Scholar]
- 95. Özçelik Korkmaz M, Eğilmez OK, Özçelik MA, Güven M.. Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Eur Arch Otorhinolaryngol 2021; 278: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Babaei A, Iravani K, Malekpour B, Golkhar B, Soltaniesmaeili A, Hosseinialhashemi M.. Factors associated with anosmia recovery rate in COVID-19 patients. Laryngoscope Investig Otolaryngol 2021; 6: 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nouchi A, Chastang J, Miyara M, Lejeune J, Soares A, Ibanez G, et al. Prevalence of hyposmia and hypogeusia in 390 COVID-19 hospitalized patients and outpatients: a cross-sectional study. Eur J Clin Microbiol Infect Dis 2021; 40: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Polat B, Yilmaz NH, Altin G, Atakcan Z, Mert A.. Olfactory and gustatory dysfunctions in COVID-19 patients: from a different perspective. J Craniofac Surg 2021; 32: 2119–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Renaud M, Thibault C, Le Normand F, Mcdonald EG, Gallix B, Debry C, et al. Clinical outcomes for patients with anosmia one year after COVID-19 diagnosis. JAMA Netw Open 2021; 4: e2115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Boscolo-Rizzo P, Menegaldo A, Fabbris C, Spinato G, Borsetto D, Vaira LA, et al. Six-month psychophysical evaluation of olfactory dysfunction in patients with COVID-19. Chem Senses 2021; 46: bjab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Thakur K, Sagayaraj A, Prasad KC, Gupta A.. Olfactory dysfunction in COVID-19 patients: findings from a tertiary rural centre. Indian J Otolaryngol Head Neck Surg 2022; 74: 2840–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Teaima AA, Salem OM, Teama MAEM, Mansour OI, Taha MS, Badr FM, et al. Patterns and clinical outcomes of olfactory and gustatory disorders in 6 months: prospective study of 1031 COVID-19 patients. Am J Otolaryngol 2022; 43: 103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cho SH. Clinical diagnosis and treatment of olfactory dysfunction. Hanyang Rev 2014; 34: 107–115. [Google Scholar]
- 104. Hopkins C., Kumar N. Loss of sense of smell as a marker of COVID-19 infection. [accessed 2021 Dec 22]. Available from: https://www.entuk.org/sites/default/files/files/Loss%20of%20sense%20of%20smell%20as%20marker%20of%20COVID.pdf
- 105. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kipshidze N, Dangas G, White CJ, Kipshidze N, Siddiqui F, Lattimer CR, et al. Viral coagulopathy in patients with COVID-19: treatment and care. Clin Appl Thromb Hemost 2020; 26: 1076029620936776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv 2020; 6: eabc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Soler ZM, Yoo F, Schlosser RJ, Mulligan J, Ramakrishnan VR, Beswick DM, et al. Correlation of mucus inflammatory proteins and olfaction in chronic rhinosinusitis. Int Forum Allergy Rhinol 2020; 10: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Keyhan SO, Fallahi HR, Cheshmi B.. Dysosmia and dysgeusia due to the 2019 novel coronavirus; a hypothesis that needs further investigation. Maxillofac Plast Reconstr Surg 2020; 42: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Perlman S, Evans G, Afifi A.. Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J Exp Med 1990; 172: 1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 2020; 87: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. AAO-HN. Anosmia, hyposmia, and dysgeusia symptoms of coronavirus disease. [Updated 2020; accessed 2021 Dec 21]. Available from: https://www.entnet.org/content/aao-hns-anosmia-hyposmia-and-dysgeusia-symptoms-coronavirus-disease
- 114. Hopkins C, Alanin M, Philpott C, Harries P, Whitcroft K, Qureishi A, et al. Management of new onset loss of sense of smell during the COVID-19 pandemic - BRS consensus guidelines. Clin Otolaryngol 2021; 46: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mendonça CV, Mendes Neto JA, Suzuki FA, Orth MS, Machado Neto H, Nacif SR.. Olfactory dysfunction in COVID-19: a marker of good prognosis? Braz J Otorhinolaryngol 2022; 88: 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]


