Abstract
The vaginal microbiome is dominated by Lactobacillus spp. and the depletion of these microorganisms have been associated with adverse conditions that can affect women’s health. Disturbance of the vaginal niche with a non-lactobacillary microbiota is associated with susceptibility to some diseases, such as obstetric alterations and infertility, resulting in failure in natural pregnancies and increased demand for assisted reproduction treatments. The present study sought to understand the influence of Lactobacillus spp. and fertility female. A systematic search was performed in the following databases: PubMed, MEDLINE, SciELO and LILACS, using the keywords: “Microbiome”; “Lactobacillus” and “Female Infertility”, published in the last five years. The search resulted in 92 articles; however, 38 articles were excluded due to duplicity, 23 articles were excluded in the selection title/abstract, leaving 31 articles for full reading. In the end, 18 articles were analyzed. The studies encompassed a total of 2,011 women, using 27 types of samples to verify the composition of the microbiome. The eighteen articles that reported the microbiome of fertile women were constituted by a dominance of Lactobacillus spp. who joined to positive predictive outcomes in reproduction, while infertile women showed a dysbiotic profile. Therefore, analyzing bacterial patterns would allow a personalized diagnosis, which could favor personalized therapy for prevention and treatment of certain diseases.
Keywords: microbiome, Lactobacillus, infertility, reproduction, dysbiosis
INTRODUCTION
The microbiota is the formation of communities of microorganisms that live inside or on the external surface of the human body and its genomic constitution is called microbiome. Under ideal circumstances, the vaginal microbiota is populated by over 200 bacterial species, which suffer genetic, environmental and behavioral influences, in addition to be influenced by the oral, rectal and penile microbiota (Auriemma et al., 2021; Martin, 2012; Mendling, 2016).
The healthy vaginal microbiota consists mainly of resident species of Lactobacillus, such as L. crispatus, L. iners, L. jensenii and L. gasseri. These microorganisms act as probiotics and inhibit the overgrowth of other bacterial species, for several direct and indirect antipathogenic mechanisms. Directly by producing active components such as lactic acid and hydrogen peroxide (H2O2), which kill or directly inhibit pathogens. In an indirect way, they form microcolonies that adhere to the epithelial cells and create a physical barrier against the adhesion of certain microorganisms, in addition to promoting the stimulation of host defense mechanisms against infections Sexually Transmitted Diseases (STIs) (Auriemma et al., 2021; Ceccarani et al., 2019; Franasiak & Scott, 2015; Jespers et al., 2012; Younes et al., 2018).
Throughout the menstrual cycle, hormonal fluctuations influence conditions environmental conditions of the vaginal lumen and, in turn, resident bacteria. The vaginal microbiota of mother serves as a source of colonization for the baby and around the first two to four weeks after birth, maternal estrogen promotes proliferation and thickening of the vaginal mucosa. Subsequently, the accumulation of lactic acid leads to a decrease in pH vaginal. This phase is short-lived, as maternal estrogen is metabolized, the epithelium vagina begins to thin and the glycogen levels decrease, thus raising the pH vaginal. In prepuberty, the microbiota is populated by a wide range of species of aerobic, strictly anaerobic and enteric bacteria, being compared to that of women adults with bacterial vaginosis. With menarche comes follicular development, leading to systemic production of estrogen, which causes the vaginal epithelium to begin to thicken and increase deposition of glycogen, mainly in the intermediate cells. The epithelial maturation ends up selecting microorganisms such as Lactobacillus, Atopobium, Leptotrichia, Leuconostoc, Megasphaera, Pediococcus, Streptococcus and Weissella. The fluctuations hormones throughout the menstrual cycle influence environmental conditions and transform inhabiting bacteria. Seen therefore an increase in the rate of Lactobacillus throughout the cycle menstrual cycle and, in contrast, the concentration of non-Lactobacillus species tend to be higher in menstruation. In post-menopause there are low concentrations of Lactobacillus and other bacteria, allowing the growth of a variety of other pathogenic species and enteric. As the epithelium becomes very thin as estrogen levels decrease, reduces the production and secretion of glycogen (Godha et al., 2018).
We understand, therefore, that the vaginal microbiota is mainly dominated by Lactobacillus spp. and depletion of these organisms is associated with several adverse conditions such as premature birth, pelvic inflammatory disease, increased risk of STIs such as Human Immunodeficiency Virus (HIV), Herpes Virus (HSV), Papillomavirus Human (HPV), chlamydia, trichomonas and multiple symptoms affecting quality of life female (Buchta, 2018; van Oostrum et al., 2013; Younes et al., 2018).
Clinically, the disturbance of the vaginal niche, with a non-lactobacillary microbiota, characterized as dysbiosis. Dysbiosis is defined as the imbalance of populations and/or microbiota functions and changes in microbiome diversity, being associated with the mostdifferent sites in the human body. In certain sites, dysbiosis can promote disease inflammatory bowel diseases, metabolic disorders, multiple sclerosis, allergies, asthma, autism and cancer (Requena & Velasco, 2021; Weiss & Hennet, 2017).
Recently, the imbalance of the vaginal microbiota has been pointed out as a possible interfere with female fertility. The World Health Organization (2020) classifies the infertility as the inability of a couple of reproductive age to conceive within a period of 12 months having sex without the use of contraceptives. Infertility can be caused by a series of factors: sexual diseases, obesity, smoking, sedentary lifestyle, illicit drugs, alcoholism, exposure to chemicals, radiation, stress, activities physical excess, non-recommended diets and age.
It is important to emphasize that the causes of infertility can be found in women or men, there are also the joint causes of male and females for couple infertility. Regarding infertile women, they have already been predisposing factors such as endometriosis, ovulatory problems, and age group (Félis et al., 2019; Starc et al., 2019).
Since the factors underlying infertility are complex and wide-ranging, approximately 40% of cases cannot be explained by anovulation or pathology tubal; these cases are defined as ‘unexplained infertility’ or ‘female infertility unspecified’. The “unexplained infertility” is much discussed since the diagnosis can be related to lack of a specific test, due to misdiagnosis or factors psychological (Félis et al., 2019; Hong et al., 2020).
With the development of state-of-the-art sequencing technology, high yield, the function of many bacteria considered normal in the vagina has been redefined. They developed concern not only about potentially pathogenic, but also in terms of changes in the entire structure of the vaginal microbiota. New molecular technologies may shed light on the role of bacteria in health gynecology, and also to elucidate how the change in the vaginal microbiota affects the susceptibility to diseases (Félis et al., 2019; Oliveira et al., 2019).
Female infertility brings serious psychosocial consequences, therefore, the prevention and management of female infertility are an integral component of services comprehensive sexual and reproductive health. Assisted reproduction has become an element comprehensive care for many women who have suffered from infertility over the past forty years (Esteves et al., 2019).
Assisted reproduction are the techniques used in the treatment of infertility, which manipulation of one or both gametes will take place. There are numerous techniques such as: intrauterine insemination (IUI), in vitro fertilization (IVF), intracytoplasmic injection of sperm (ICIS) (Souza & Alves, 2016).
In 2019, the Latin American Network of Assisted Reproduction announced that Brazil led the Latin American ranking of countries that performed the most breeding techniques assisted: 44,705 IVF cycles. In 25 years, 83,000 Brazilian babies were born through assisted reproduction treatments, demonstrating that assisted reproduction techniques have more evidence gained, since the decrease in fertility is an inevitable biological factor, combined with late motherhood (Foizer et al., 2014; Zegers-Hochschild et al., 2020).
Human reproduction can be considered inefficient, since the conception rate is 25-30% per cycle, of these, only 50% will pass by the 20th week of pregnancy; and of gestational losses, 75% are the result of implantation failure that are not recognized by the clinical point of view. About 5% of women will have at least two consecutive losses, while 75% will have at least one implantation failure (Borges Júnior et al., 2020).
With the growth of assisted reproduction techniques, it has been studied even more on the interference in their success. Some studies correlate that pathogens such as Mycoplasma tuberculosis, Chlamydia trachomatis and Neisseria gonorrhoeae. When present in the vaginal microbiome interfere with fertility and reproductive techniques assisted (Sirota et al., 2014).
Since the literature has discussed that some microorganisms when present in the microbiome of infertile women, can disrupt or decrease implantation rates in assisted reproduction treatments, this study was designed to establish which influence of Lactobacillus spp. in female fertility.
MATERIAL AND METHODS
This is a systematic literature review that addresses the influence of Lactobacillus spp. in female fertility. Studies that contributed to the hypothesis raised and, thus, to understand, scientifically, the relationship of changes in Lactobacillus spp. in the vaginal microbiota and in female fertility, highlighting the level of reliability and its clinical potential. It is noteworthy that it did not involve interventions in humans, therefore approval by an Ethics Committee was not required. We use the quote: “Preferred Reporting Items for Systematic Reviews and Meta-analysis” (PRISM) to report the results.
Search strategy
This systematic literature review started in August 2021 until April 2022. The following databases were used: PubMed, MEDLINE, SciELO and LILACS, using the keywords: “Microbiome”; “Lactobacillus” and “Female Infertility”, in English and Portuguese, in advanced search. Articles published in the last five years were used (from 2017 to April 2022) in order to provide the most up-to-date and recent data.
Article selection criteria
Full, original articles, in English, addressing infertility were included. female, microbiome, vaginal microbiota and assisted reproduction techniques. Were excluded, articles dealing with male infertility, review articles, case reports, animal studies, incomplete or unavailable.
Measurements
Articles were analyzed based on inclusion and exclusion criteria. After this choice, a complete reading was performed, where it was possible to identify the contribution to this job. The following were observed: the studied group (healthy women and infertile women), the assisted reproduction techniques portrayed in the study, the methodologies used in the studies, the main microorganisms correlated with the lactobaciliary change, the of success and failure in the use of assisted reproduction techniques in women with dysbiotic profile and other factors underlying changes in the vaginal microbiome.
RESULTS
Data collection and analysis
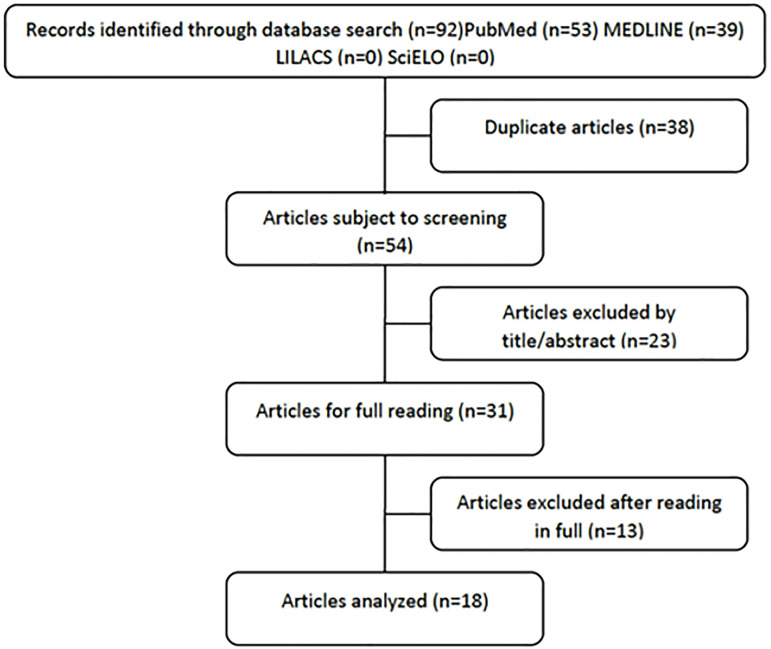
The records identified in the data search were: PubMed (n=53), MEDLINE (n=39), SciELO (n=0), LILACS (n=0), resulting in a total of 92 articles. after the search initial period, the duplicate articles were removed, which were 38 articles, leaving, therefore, 54 articles for screening. After reading the title and abstract, 23 articles that did not met the inclusion criteria, leaving 31 articles for full reading. After full reading, 18 articles were analyzed (Figure 1).
Figure 1.
Articles analyzed.
Description of included studies
The articles analyzed in the present study were published between 2017 and 2022 and met the inclusion and exclusion criteria. The studies encompassed a total of 2,011 women, being 512 (25.45%) fertile women and 1,499 (74.54%) infertile women.
Among the methodologies used in studies to analyze the vaginal microbiome: 14 (77.77%) of the studies used amplification of the 16S ribosomal RNA (rRNA) gene, 2 (11.11%) studies used quantitative real-time PCR (Wee et al., 2018; Haahr et al., 2019) associated the use of these techniques, 1 (5.55%) study used culture aerobic, anaerobic and fungal routine, however Graspeuntner et al. (2018) and Azpiroz et al. (2021) used the three methodologies, 1 (5.55%) used the IS-pro technique. Graspeuntner et al. (2018) used complementary techniques, such as: ELISA and Immunblot.
The studies used 27 types of samples to verify the composition of the microbiome were: 14 (51.85%) vaginal swabs, 6 (22.22%) endometrial fluids and endometrial tissue, 3 (11.11%) cervical swabs, 1 (3.70%) rectal swabs, 1 (3.70%) urinary sample, 1 (3.70%) fecal sample, 1 (3.70%) cervix.
As for the association with assisted reproduction techniques: 12 (66.66%) studies addressed IVF, and Bernabeu et al. (2019), Koedooder et al. (2019) and Patel et al. (2022), jointly address ICSI, 1 (5.55%) study addressed patients submitted to assisted reproduction technologies without specifying the technique and 5 (27.77%) studies did not make the association between microbiota and assisted reproduction technique (Table 1).
Table 1. Studies.
| Study | Group of Study | Age | Methodology used | Techniques assisted by reproduction | Microbiome/ microbiota |
|---|---|---|---|---|---|
| Babu et al., 2017 | 200 women (84 healthy - Group and 116 infertile - group 2) | 18 years to 45 years | All swabs were subjected to culture aerobics, anaerobic and fungal from routine. | Does not portray | Group 1 - was dominated by
Lactobacillus followed by
Micrococcus, Enterococci and
Staphylococcus spp. negative
coagulase. Group 2 - Candida spp., Enterococcus followed by bacilli Gram negative, like Escherichia coli. |
| Wee et al., 2018 | 31 women (16 healthy 15 infertile) | 28 years to 49 years | Collection of samples vaginal, cervical and endometrial analyzed by amplification of RNA gene ribosomal 16S (rRNA) and expression endometrial from human genes selected by the reaction in chain of polymerase from transcription reverse quantitative. | Does not portray | It was observed a dominated microbiota By Lactobacillus. However, there were a trend of that women infertile had more often Ureaplasma in vagina and Gardnerella in the cervix. |
| Graspeuntner et al., 2018 | 210 women (26 women infertile, 21 women by having infertility infectious, 89 women fertile, 54 professionals of sex. | Does not portray | Were used swabs for culture bacterial conventional, swabs for PCR against C. trachomatis, N. gonorrhoeae, M. genitalium, M. hominis and U. urealitic, swabs for the sequencing of the 16S gene rRNA. | Does not portray | It was observed Lactobacillus as the most gender prominent, which declined among the women's groups infertile and professionals of sex. In contrast had an increase in genus Gardnerella and Prevotella us women's groups infertile and professionals of sex. |
| Bernabeu et al., 2019 | 31 women (in treatment of reproduction assisted) | 18 years to 50 years | Samples vaginal were harvested for analyze the V3 V4 region of the 16S rRNA and so if analyze the microbiome vaginal | Fertilization in in vitro (IVF) and Stimulation Ovarian controlled and injection intracytoplasmic the of sperm (ICSI). | Points a dominated microbiota by Lactobacillus (<90% Lactobacillus spp.) and one does not dominated by Lactobacillus, where the proportion of bacteria of the genus Gardnerella exceeds 10%. |
| Haahr et al., 2019 | 120 women (submitted to fertilization in vitro) | Age average of 40 years (Detour pattern 4.3) | Samples vaginal sequenced using the region V4 of the gene RNA ribosomal 16S with grouping of clades genomics of Gardnerella vaginalis. | Fertilization in vitro (IVF) | It was observed Lactobacillus as the dominant gender, however, the highest scores of Nugent were associated with greater diversity and included highs OTU loads corresponding to G. vaginalis, A. vaginae, Sneathia bloods and Prevotella spp. |
| Koedooder et al., 2019 | 192 women (67 women What got pregnant and 125 that don't got pregnant) | 20 years to 44 years | The composition of the microbiota vaginal was determined using the IS-pro technique. | Fertilization in vitro (IVF) and Stimulation ovarian controlled and injection intracytoplasmic the of sperm (ICSI). | Dominance of Lactobacillus crispatus was an important factor in prediction of pregnancy. |
| Fu et al., 2020 | 67 women (27 failed applicant of implantation and 40 group control) | 33.4±3.7 | Sequencing the 16S gene rRNA of microbiota vaginal | Fertilization in vitro (IVF) | It was observed a dominance of Lactobacillus in control group, in addition of that it was verified that the decrease of Lactobacillus, plays an important role in pathogenesis of failure applicant of implantation. |
| Zhao et al., 2020 | 122 women (30 women infertile and 92 women healthy) | 23 years to 40 years | Analysis of composition of microbiome vaginal using sequencing of the 16S gene rRNA. | Fertilization in vitro (IVF) | Infertile women present a decrease significant in diversity and richness of the microbiome in comparison with healthy women during the period of no ovulation (phase follicular), while the microbiome |
| Carosso et al., 2020 | 15 women (submitted to techniques of reproduction assisted) | Age ≤ 42 years old | A swab vaginal and the far end tip distal of the ET catheter were analyzed using the sequencing of the gene 16 SrRNA of last generation. | Fertilization in vitro (IVF) | The relative proportion of Lactobacillus vaginal and endometrial was decreased during the fertilization cycle in vitro, with a simultaneous increase of bacteria potentially pathogenic, such as Atopobium Escherichia-Shigella and Prevotella. |
| Karaer et al., 2021 | 223 women | 23 years to 39 years | The samples vaginal for identification of microbiota vaginal was carried out using sequencing last generation and categorized from according to region hypervariable V3-V4 in gene region 16S rRNA. | Fertilization in vitro (IVF) | presented dominance of
Lactobacillus in vaginal
microbiota. the abundance relative of Streptococcus and Gardnerella was increased in women who don’t got pregnant. |
| Hao et al., 2021 | 100 women (51 subjected to IVF, where 25 got pregnant, 49 submitted transfers of embryo, where 27 They were clinically pregnant) | 20 years to 40 years old age | The regions variables 3 and 4 (V3-V4) of 16S rRNA gene were amplified and sequenced. | Fertilization in vitro (IVF) | a minor proportion of Lactobacillus for other bacteria in cervical microbiota was associated with decrease in chances of pregnancy clinic. |
| Sun et al., 2021 | 184 women | Age Reproductive | Samples vaginas, from cervix and of the cavity uterine for sequencing of 16S rRNA. | Does not portray | It was observed that the microbiota
cervico vaginal was dominated by
Lactobacillus. At the general, the composition of the microbiota in uterine cavity was more diverse than that in the vagina and cervix of the uterus. |
| Koedooder et al., 2021 | 85 women (in treatment of reproduction assisted) | 18 years to 43 years old | The composition microbial was Determined by the reaction in chain of polymerase of V1-V3 regions of the gene bacterial 16S rRNA | Fertilization in vitro (IVF) | A decrease significant in plenty of species of Lactobacillus as well like an increase significant in the species of Staphylococcus was observed in women who got pregnant after treatment of IVF/IVF-ICSI. |
| Azpiroz et al., 2021 | 307 women (287 infertile and 20 group control) | 21 years to 39 years old. | Samples of swab were collected from vagina and the straight. The composition microbial by NGS and the expression of miRNA by PCR in time real. | Fertilization in vitro (IVF) | Infertile patients showed lower bacterial richness and increase in ratio Firmicutes / Bacteroidetes in rectal level and augmentation of reason Lactobacillusbrevis / Lactobacillusiners in samples vaginal in relation to the fertile group. |
| Lüll et al., 2022 | 25 women (with infertility) | 28 years to 42 years old | Analysis of sample of fabrics and fluids for sequencing of 16S rRNA. | Fertilization in vitro (IVF) | It was observed that the dominance of Lactobacillus genus is a factor important that influences the composition microbial. |
| Sezer et al., 2022 | 52 women (26 infertile and 26 fertile) | 20 years to 45 years old | Samples vaginal and endometrial were analyzed by PCR quantitative in real time. | Does not portray | It was found that the microbiota in vaginal samples from patients with infertility, it was harmed as much to the number of Lactobacillus. In addition addition, it was portrayed that the women with microbiota lactobaciliary impaired had increased risk for infertility. |
| Villani et al., 2022 | 90 women (with diagnosis in infertility, 2 patients excluded, remaining 88, which 39 got pregnant and 49 negative) | 24 years to 40 years old | Vaginal swabs which after the extraction of DNA microbial, the regions variables V3-V4 of the gene 16S rRNA were amplified and sequenced | Assisted reproduction’s Technologies | It was found that the plenty of Lactobacillus was favorable for positive results. In addition plenty of Lactobacillus crispatus and iners, respectively increased and decreased in the group favorable in comparison to unfavorable group. |
| Patel et al., 2022 | 31 women (11 fertile and 20 infertile, being 10 with failure appellant and 10 with infertility unexplained) | Does not portray | The samples faecal and vaginal, the regions variables V2-V3 of the gene 16S rRNA were amplified and sequenced. | Fertilization In vitro (IVF) and Injection intracytoplasmic the of sperm (ICSI) | The vaginal microbiota was dominated
by genre Lactobacillus, with
Lactobacillus iners being the most species
abundant among the groups. Compared with the infertile cohort, the growth excessive of bacteria anaerobic, associated with dysbiosis vaginal, like Leptotrichia and Snethia, occurred in the controls. |
The composition of the vaginal microbiome of fertile and infertile women
The studies compare the vaginal microbiome of fertile and infertile women. Since 512 (25.45%) of fertile women showed dominance of Lactobacillus in the vaginal microbiota, while 1,499 (74.54%) infertile women had a higher microbial diversity and decrease in the number of Lactobacillus.
Gardnerella spp. was present in the microbiome of infertile women in 66.6%, followed by Atopobium spp. and Prevotella spp. in 38.8%, Escherichia coli in 27.7%, Streptococcus spp., Sneathia and Staphylococcus in 22.2%, Enterococcus spp. by 16.6%. Other microorganisms cited in infertile women were: Candida spp. (Babu et al., 2017), Ureaplasma spp. (Wee et al., 2018), Chlamydia trachomatis (Graspeuntner et al., 2018), Mycoplasma hominis (Sezer et al., 2022).
Some studies have associated the presence of Lactobacillus crispatus as an important predictor of pregnancy (Graspeuntner et al., 2018; Bernabeu et al., 2019; Haahr et al., 2019; Koedooder et al., 2019; Villani et al., 2022). On the other hand, studies of Graspeuntner et al. (2018), Haahr et al. (2019) and Villani et al. (2022) associated the presence of Lactobacillus iners with a more varied microbiota.
The studies also address changes in the vaginal microbiome during use of assisted reproduction techniques. Carosso et al. (2020) noted that despite the dominance of Lactobacillus in the vaginal microbiome is permanent after the IVF cycle, there was a decrease in abundance. The same was seen by Koedooder et al. (2021) and Villani et al. (2022).
DISCUSSION
In 25.45% of the articles analyzed in the present study, it was observed that the microbiota of fertile women is constituted by a dominance of Lactobacillus spp. Lactobacillus spp. were first described in 1901, being a genus of aerobic bacteria in the form of rod and immobile, Gram-positive, non-spore-forming, acid-tolerant and capable of produce lactic acid by fermentation of carbohydrates from the phylum Firmicutes (Chee et al., 2020; Dempsey & Corr, 2022; Zhang et al., 2019).
Members of the Lactobacillus genus are abundant and predominant in the vaginal niche of healthy women of reproductive age, reaching a concentration of 10 7 cfu/mL of sample vaginal and 80% of all microbial content. Including: Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners and Lactobacillus jensenii (Chee et al., 2020; Parolin et al., 2021).
It is widely demonstrated that vaginal lactobacilli are involved in maintenance of the state of vaginal eubiosis and one of the main functions of lactobacilli is to activate the glycogen metabolism. Glycogen produced by vaginal epithelial cells is transformed into lactic acid, inducing a low vaginal pH (3.8-4.4). This creates an environment unfavorable for the growth of pathogenic bacteria and sexually transmitted infections (Di Simone et al., 2020; Parolin et al., 2021).
In the study by Koedooder et al. (2019) pay attention to the dominance of Lactobacillus crispatus and reported to be an important factor in predicting pregnancy (<60%). In literature we found that L. crispatus produces lactic acid and other compounds that are potent inhibitors of associated bacterial species, mainly bacterial vaginosis. Therefore, L. crispatus seems to be a promising species because it is associated with vaginal health and negatively associated with bacterial vaginosis and preterm birth (Abdelmaksoud et al., 2016).
Infertile women (74.54%) had a greater diversity of microorganisms and a decrease in the proportion of Lactobacillus in the vaginal microbiota when compared to fertile women. The literature has portrayed that alterations in the dominance of lactobacilli and a microbiota with high bacterial diversity, are associated with an increased risk of infections, spontaneous preterm birth, and pelvic inflammatory disease (Di Simone et al., 2020).
In the study by Babu et al. (2017), it was observed that women with infertility had a low percentage of Lactobacillus and had vaginosis asymptomatic. In the literature we found that women with bacterial vaginosis, the microbiome of lactobacilli, which produce hydrogen peroxide, are responsible for maintaining from an acidic environment, which ends up being replaced by invasive pathogens, such as Gardnerella vaginalis, Prevotella spp. and Mobiluncus spp.. This substitution promotes a pH that sets the environment for bacterial vaginosis, in addition, G. vaginalis produces a biofilm that provides a matrix for the adhesion of other pathogenic bacteria, in addition to hinder the penetration of antibiotic therapy and eradication of the infection (Bagnall & Rizzolo, 2017).
In 66.6% of the articles analyzed in the present study, the presence of Gardnerella spp. in the vaginal microbiome of infertile women. In 1955, Gardnerella was known as the main organism involved in bacterial vaginosis, being a Gram-facultative anaerobic variable, its infection results in higher vaginal pH, thin discharge, fishy odor and presence of epithelial cells covered with bacteria. Sometimes the infection can is asymptomatic, even so it can be accompanied by serious consequences for the health conditions, such as premature birth and pelvic inflammatory disease, and may facilitate the acquisition of sexually transmitted infections (Morrill et al., 2020; Wong et al., 2018).
Bacterial vaginosis is a dysbiosis, as it causes a condition in which there is a decrease in of lactobacilli levels and overgrowth of several bacteria from other taxonomic groups (Gardnerella, Atopobium, Mobiluncus, Prevotella, Bacteroides, Anaerococcus, Peptostreptococcus, Sneathia, Leptotrichia and members of the Clostridia class, among others). Proposes that vaginal dysbiosis is linked to inflammatory states and is associated with adverse obstetrics. Bacterial vaginosis has been linked to infertility, although the cause that leads patients to be infertile has not yet been elucidated, it is known that the association between microbiota of a patient with bacterial vaginosis and subsequent inflammation can lead to reduced fertility (Di Simone et al., 2020; Morrill et al., 2020; Reiter & Kellogg Spadt, 2019).
It was observed in 38.8% of the articles analyzed in the present study the presence of microorganism Atopobium spp. in the microbiota of infertile women. Atopobium spp. was described in 1999, has a variable morphology from elongated cocci to bacilli, with Grampositive, and may be present singly, in pairs or in small chains. although already its presence in the microbiota of healthy women has been verified, it has been demonstrated that Atopobium is more frequently found in the vaginal microbiota of patients with bacterial vaginosis, as it is an important component in the formation of biofilms (Rodriguez Jovita et al., 1999; Zhou et al., 2004).
The microorganism Prevotella spp. was also found in the microbiota of infertile women in 38.8% of the analyzed articles. Prevotella spp. was named after the French microbiologist A.R. Prevot, a pioneer in anaerobic microbiology, is a Gram-anaerobic negative, which stains weakly by Gram, of the phylum Bacteroidetes, which also includes the clinically important genera Bacteroides and Porphyromonas. Are classically considered commensal bacteria due to their extensive presence in the healthy human body and its rare involvement in infections. Only a few strains have been reported to give rise to endogenous opportunistic infections, including chronic infections, abscesses and anaerobic pneumonia. However, it has been associated that the interaction between Prevotella and the immune system, can promote inflammatory disease and its abundance has been seen increases with the severity of bacterial vaginosis, in addition to being inversely correlated with the presence of Lactobacillus (Larsen, 2017; Murray et al., 2020).
As for assisted reproduction techniques, it was observed that patients undergoing IVF or ICSI showed a decrease in Lactobacillus levels and an increase in bacteria such as: Staphylococcus, Atopobium, Escherichia-Shigella and Prevotella. The studies also pointed out that women with recurrent implantation failures had a greater microbial diversity, in addition to showing a decrease in the number of Lactobacillus, reporting that this decrease plays an important role in the pathogenesis of recurring deployment. The literature points out that genital dysbiosis (for example, vaginal or endometrial tissue) was associated with lower odds of live births in reproductive Technologies (ART), by decreasing pregnancy rates and increasing the risk of miscarriages (Mauries et al., 2021).
Moreno et al. (2016) report that the existence of an endometrial microbiota highly stable during the acquisition of endometrial receptivity is a predictive factor positive for successful implementation. However, the pathological modification of your profile is associated with poor reproductive outcomes for in vitro fertilization (IVF) patients. He was demonstrated that the presence of a microbiota not dominated by Lactobacillus in a receptive endometrium was associated with significant decreases at implantation (60.7% vs. 23.1%; P=0.02).
Moreno et al. (2022) analyzed the endometrial microbiome of 342 infertile women. clinics in Europe, America and Asia. In their results, they observed that women with presence of microorganisms such as Atopobium, Bifidobacterium, Chryseobacterium, Gardnerella, Haemophilus, Klebsiella, Neisseria, Staphylococcus and Streptococcus do not were successful in the in vitro fertilization (IVF) technique, but women whose microbiome showed dominance of Lactobacillus were successful in the procedure. Therefore, the analysis of the composition of the endometrial microbiota before the transfer of the embryo is a useful biomarker for predicting reproductive outcome, offering an opportunity to further improve diagnostic and treatment strategies.
Currently, there are genomic diagnostic tools for the receptivity endometrial tissue based on transcriptomic signature, composed of a microarray and a bioinformatic predictor for endometrial dating and to detect pathology of endometrial origin, the ERA - Endometrial Receptivity ARRAY. Díaz-Gimeno et al. (2011) performed a clinical trial with healthy women (88) with implantation failure (5) or hydrosalpinx (2) and exposed the ERA with a diagnostic tool that can be used clinically in reproductive medicine and gynecology to assess receptivity endometrial. Garrido-Gómez et al. (2013) corroborates by pointing out the possibility of ERA taking a new clinical concept of personalized embryo transfer by verifying the optimal day of endometrial receptivity, identified individually on a case-by-case basis.
It is important to report that there are tests that perform the metagenomic analysis of the endometrial microbiome to allow a better reproductive prognosis. A endometrial biopsy, which provides proportion of healthy bacteria, including Lactobacillus spp., in addition to classifying as normal, abnormal and dysbiotic microbiota or very low. These tests are based on Next Generation Sequencing (NGS) technology. to provide information on the endometrial microbiome, based on the DNA extraction and 16S ribosomal RNA gene sequencing from bacteria. Therefore, provides a microbiological view of the endometrium with the aim of improving management patients’ clinic.
A diagnostic method that also shows promising results is the real-time polymerase chain (RT-PCR) that can identify bacterial DNA with 75% sensitivity and 100% specificity, allowing the identification of bacteria cultivable or not, even without signs of infection (Borges Júnior et al., 2020).
It is known that a limiting factor for the use of such methodologies is need expensive machinery, inputs that are sometimes lacking in the market and of specialized work, since the professionals to conduct certain technologies of diagnosis require a high degree of specialization, with well-in-depth knowledge of molecular diagnostics and bioinformatics analysis, being an important impact factor on the quality of reactions that are introduced and offered to customers.
Today such tests are used in patients who had implantation failure, However, since the probability of conception rate is around 25%-30% in the face of a cycle and that 75% of implantation failures do not have clinical knowledge (Borges Júnior et al., 2020). It is suggested that such methodologies should be used as a factor to prevent all women who will be assisted by reproductive techniques.
CONCLUSION
The vaginal microbiome plays an important role in reproductive health. Therefore, analyzing bacterial patterns would allow a personalized diagnosis based on microbiota, which could favor personalized therapy for the prevention and treatment of certain diseases. We observed that the vaginal microbiome of fertile women showed dominance of Lactobacillus, while infertile women showed a decrease in Lactobacillus and increase in the variety of microorganisms. It was also seen that the Lactobacillus dominance is associated with positive predictive outcomes in reproduction and that vaginal dysbiosis is associated with unfavorable outcomes. As far as we know in Brazil it is not necessary to evaluate the vaginal microbiome for the use of assisted reproduction. But, we suggest that perhaps assessing the vaginal microenvironment would be a approach of interest, mainly for a favorable embryo implantation and a positive pregnancy outcome. Currently, diagnostic tools capable of to identify pathogenic bacteria in the female reproductive tract, these tests should be used in order to provide an opportunity to improve the clinical management of infertile patients.
REFERENCES
- Abdelmaksoud AA, Koparde VN, Sheth NU, Serrano MG, Glascock AL, Fettweis JM, Strauss JF, Buck GA, Jefferson KK. Comparison of Lactobacillus crispatus isolates from Lactobacillus-dominated vaginal microbiomes with isolates from microbiomes containing bacterial vaginosis-associated bacteria. Microbiology (Reading) 2016;162:466–475. doi: 10.1099/mic.0.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriemma RS, Scairati R, Del Vecchio G, Liccardi A, Verde N, Pirchio R, Pivonello R, Ercolini D, Colao A. The Vaginal Microbiome: A Long Urogenital Colonization Throughout Woman Life. Front Cell Infect Microbiol. 2021;11:686167. doi: 10.3389/fcimb.2021.686167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz MA, Orguilia L, Palacio MI, Malpartida A, Mayol S, Mor G, Gutiérrez G. Potential biomarkers of infertility associated with microbiome imbalances. Am J Reprod Immunol. 2021;86:e13438. doi: 10.1111/aji.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora and Incidence of Asymptomatic Vaginosis among Healthy Women and in Women with Infertility Problems of Reproductive Age. J Clin Diagn Res. 2017;11:DC18–22. doi: 10.7860/JCDR/2017/28296.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall P, Rizzolo D. Bacterial vaginosis: A practical review. JAAPA. 2017;30:15–21. doi: 10.1097/01.JAA.0000526770.60197.fa. [DOI] [PubMed] [Google Scholar]
- Bernabeu A, Lledo B, Díaz MC, Lozano FM, Ruiz V, Fuentes A, Lopez-Pineda A, Moliner B, Castillo JC, Ortiz JA, Ten J, Llacer J, Carratala-Munuera C, Orozco-Beltran D, Quesada JA, Bernabeu R. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J Assist Reprod Genet. 2019;36:2111–2119. doi: 10.1007/s10815-019-01564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges Júnior E, Braga DPAF, Setti AS. Reprodução Humana Assistida - Associação Instituto Sapientiae. 2ª. São Paulo: Atheneu; 2020. In Portuguese. [Google Scholar]
- Buchta V. Vaginal microbiome. Ceska Gynekol. 2018;83:371–379. [PubMed] [Google Scholar]
- Carosso A, Revelli A, Gennarelli G, Canosa S, Cosma S, Borella F, Tancredi A, Paschero C, Boatti L, Zanotto E, Sidoti F, Bottino P, Costa C, Cavallo R, Benedetto C. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: a pilot study. J Assist Reprod Genet. 2020;37:2315–2326. doi: 10.1007/s10815-020-01878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarani C, Foschi C, Parolin C, D’Antuono A, Gaspari V, Consolandi C, Laghi L, Camboni T, Vitali B, Severgnini M, Marangoni A. Diversity of vaginal microbiome and metabolome during genital infections. Sci Rep. 2019;9:14095. doi: 10.1038/s41598-019-50410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee WJY, Chew SY, Than LTL. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact. 2020;19:203. doi: 10.1186/s12934-020-01464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey E, Corr SC. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front Immunol. 2022;13:840245. doi: 10.3389/fimmu.2022.840245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simone N, Santamaria Ortiz A, Specchia M, Tersigni C, Villa P, Gasbarrini A, Scambia G, D’Ippolito S. Recent Insights on the Maternal Microbiota: Impact on Pregnancy Outcomes. Front Immunol. 2020;11:528202. doi: 10.3389/fimmu.2020.528202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alamá P, Pellicer A, Simón C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95:50–60. doi: 10.1016/j.fertnstert.2010.04.063. 60.e1-15. [DOI] [PubMed] [Google Scholar]
- Esteves SC, Humaidan P, Roque M, Agarwal A. Female infertility and assisted reproductive technology. Panminerva Med. 2019;61:1–2. doi: 10.23736/S0031-0808.18.03553-X. [DOI] [PubMed] [Google Scholar]
- Félis KC, Campos AA, Silva AM, Carvalho IG, Pargeon JP, Almeida RJ. Repercussões psicossociais da infertilidade inexplicada em mulheres. Nursing (São Paulo) 2019;22:2818–2924. doi: 10.36489/nursing.2019v22i253p2818-2924. In Portuguese. [DOI] [Google Scholar]
- Foizer BRR, Silva KR, Vieira JDG, Amaral WN. Microbiological contamination in the laboratory of human reproduction and its implications for the success of assisted reproduction. Reprod Clim. 2014;29:66–70. doi: 10.1016/j.recli.2014.08.005. In Portuguese. [DOI] [Google Scholar]
- Franasiak JM, Scott RT Jr. Reproductive tract microbiome in assisted reproductive technologies. Fertil Steril. 2015;104:1364–1371. doi: 10.1016/j.fertnstert.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Fu M, Zhang X, Liang Y, Lin S, Qian W, Fan S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. mBio. 2020;11:e03242–19. doi: 10.1128/mBio.03242-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Gómez T, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simón C. Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril. 2013;99:1078–1085. doi: 10.1016/j.fertnstert.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Godha K, Tucker KM, Biehl C, Archer DF, Mirkin S. Human vaginal pH and microbiota: an update. Gynecol Endocrinol. 2018;34:451–455. doi: 10.1080/09513590.2017.1407753. [DOI] [PubMed] [Google Scholar]
- Graspeuntner S, Bohlmann MK, Gillmann K, Speer R, Kuenzel S, Mark H, Hoellen F, Lettau R, Griesinger G, König IR, Baines JF, Rupp J. Microbiota-based analysis reveals specific bacterial traits and a novel strategy for the diagnosis of infectious infertility. PLoS One. 2018;13:e0191047. doi: 10.1371/journal.pone.0191047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahr T, Humaidan P, Elbaek HO, Alsbjerg B, Laursen RJ, Rygaard K, Johannesen TB, Andersen PS, Ng KL, Jensen JS. Vaginal Microbiota and In Vitro Fertilization Outcomes: Development of a Simple Diagnostic Tool to Predict Patients at Risk of a Poor Reproductive Outcome. J Infect Dis. 2019;219:1809–1817. doi: 10.1093/infdis/jiy744. [DOI] [PubMed] [Google Scholar]
- Hao X, Li P, Wu S, Tan J. Association of the Cervical Microbiota With Pregnancy Outcome in a Subfertile Population Undergoing In Vitro Fertilization: A Case-Control Study. Front Cell Infect Microbiol. 2021;11:654202. doi: 10.3389/fcimb.2021.654202. 0.3389/fcimb.2021.654202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Ma J, Yin J, Fang S, Geng J, Zhao H, Zhu M, Ye M, Zhu X, Xuan Y, Wang B. The association between vaginal microbiota and female infertility: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020;302:569–578. doi: 10.1007/s00404-020-05675-3. [DOI] [PubMed] [Google Scholar]
- Jespers V, Menten J, Smet H, Poradosú S, Abdellati S, Verhelst R, Hardy L, Buvé A, Crucitti T. Quantification of bacterial species of the vaginal microbiome in different groups of women, using nucleic acid amplification tests. BMC Microbiol. 2012;12:83. doi: 10.1186/1471-2180-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaer A, Doğan B, Günal S, Tuncay G, Arda Düz S, Ünver T, Tecellioğlu N. The vaginal microbiota composition of women undergoing assisted reproduction: a prospective cohort study. BJOG. 2021;128:2101–2109. doi: 10.1111/1471-0528.16782. [DOI] [PubMed] [Google Scholar]
- Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, de Jonge JD, Poort L, Cuypers WJSS, Beckers NGM, Broekmans FJM, Cohlen BJ, den Hartog JE, Fleischer K, Lambalk CB, Smeenk JMJS, Budding AE, Laven JSE. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod. 2019;34:1042–1054. doi: 10.1093/humrep/dez065. [DOI] [PubMed] [Google Scholar]
- Koedooder R, Maghdid DM, Beckers NGM, Schoenmakers S, Kok DJ, Laven JSE. Dynamics of the urinary microbiome in pregnancy and the coincidental predictive value of the microbiota for IVF/IVF-ICSI outcome. Reprod Biomed Online. 2021;43:871–879. doi: 10.1016/j.rbmo.2021.07.018. [DOI] [PubMed] [Google Scholar]
- Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüll K, Saare M, Peters M, Kakhiani E, Zhdanova A, Salumets A, Boyarsky K, Org E. Differences in microbial profile of endometrial fluid and tissue samples in women with in vitro fertilization failure are driven by Lactobacillus abundance. Acta Obstet Gynecol Scand. 2022;101:212–220. doi: 10.1111/aogs.14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DH. The microbiota of the vagina and its influence on women’s health and disease. Am J Med Sci. 2012;343:2–9. doi: 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauries C, Ranisavljevic N, Gallet R, Fournier A, Gala A, Ferrières-Hoa A, Brouillet S, Hamamah S. Assessment of genital microbiota: An emerging approach in assisted reproductive techniques. Gynecol Obstet Fertil Senol. 2021;49:185–192. doi: 10.1016/j.gofs.2020.07.005. [DOI] [PubMed] [Google Scholar]
- Mendling W. In: Microbiota of the Human Body. Implications in Health and Disease. Schwiertz A, editor. Springer; Cham: 2016. Vaginal Microbiota; pp. 83–93. [PubMed] [Google Scholar]
- Moreno I, Codoñer FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazán J, Alonso R, Alamá P, Remohí J, Pellicer A, Ramon D, Simon C. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215:684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- Moreno I, Garcia-Grau I, Perez-Villaroya D, Gonzalez-Monfort M, Bahçeci M, Barrionuevo MJ, Taguchi S, Puente E, Dimattina M, Lim MW, Meneghini G, Aubuchon M, Leondires M, Izquierdo A, Perez-Olgiati M, Chavez A, Seethram K, Bau D, Gomez C, Valbuena D, et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome. 2022;10:1. doi: 10.1186/s40168-021-01184-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill S, Gilbert NM, Lewis AL. Gardnerella vaginalis as a Cause of Bacterial Vaginosis: Appraisal of the Evidence From in vivo Models. Front Cell Infect Microbiol. 2020;10:168. doi: 10.3389/fcimb.2020.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology. Rio de Janeiro: Guanabara Koogan; 2020. [Google Scholar]
- Oliveira MS, Medeiros FC, Eleutério Junior J. Infertility and the vaginal microbiome: review study. J Bras Doenças Sex Transm. 2019;31:24–29. [Google Scholar]
- Parolin C, Croatti V, Laghi L, Giordani B, Tondi MR, De Gregorio PR, Foschi C, Vitali B. Lactobacillus Biofilms Influence Anti-Candida Activity. Front Microbiol. 2021;12:750368. doi: 10.3389/fmicb.2021.750368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Patel N, Pal S, Nathani N, Pandit R, Patel M, Patel N, Joshi C, Parekh B. Distinct gut and vaginal microbiota profile in women with recurrent implantation failure and unexplained infertility. BMC Womens Health. 2022;22:113. doi: 10.1186/s12905-022-01681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter S, Kellogg Spadt S. Bacterial vaginosis: a primer for clinicians. Postgrad Med. 2019;131:8–18. doi: 10.1080/00325481.2019.1546534. [DOI] [PubMed] [Google Scholar]
- Requena T, Velasco M. The human microbiome in sickness and in health. Rev Clin Esp. 2021;221:233–240. doi: 10.1016/j.rce.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Rodriguez Jovita M, Collins MD, Sjödén B, Falsen E. Characterization of a novel Atopobium isolate from the human vagina: description of Atopobium vaginae sp. nov. Int J Syst Bacteriol. 1999;49:1573–1576. doi: 10.1099/00207713-49-4-1573. [DOI] [PubMed] [Google Scholar]
- Sezer O, Soyer Çalışkan C, Celik S, Kilic SS, Kuruoglu T, Unluguzel Ustun G, Yurtcu N. Assessment of vaginal and endometrial microbiota by real-time PCR in women with unexplained infertility. J Obstet Gynaecol Res. 2022;48:129–139. doi: 10.1111/jog.15060. [DOI] [PubMed] [Google Scholar]
- Sirota I, Zarek SM, Segars JH. Potential influence of the microbiome on infertility and assisted reproductive technology. Semin Reprod Med. 2014;32:35–42. doi: 10.1055/s-0033-1361821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza KKPC, Alves OF. As principais técnicas de reprodução humana assistida. Saúde Ciênc Ação. 2016;2:26–37. In Portuguese. Available at: http://www.revistas.unifan.edu.br/index.php/RevistaICS/article/view/182 . [Google Scholar]
- Starc A, Trampuš M, Pavan Jukić D, Rotim C, Jukić T, Polona Mivšek A. Infertility and sexual dysfunctions: a systematic literature review. Acta Clin Croat. 2019;58:508–515. doi: 10.20471/acc.2019.58.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Ding H, Yu H, Ji Y, Xifang X, Pang W, Wang X, Zhang Q, Li W. Comprehensive Characterization of Microbial Community in the Female Genital Tract of Reproductive-Aged Women in China. Front Cell Infect Microbiol. 2021;11:649067. doi: 10.3389/fcimb.2021.649067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod. 2013;28:1809–1815. doi: 10.1093/humrep/det096. [DOI] [PubMed] [Google Scholar]
- Villani A, Fontana A, Barone S, de Stefani S, Primiterra M, Copetti M, Panebianco C, Parri C, Sciannamè N, Quitadamo PA, Tiezzi A, Santana L, Maglione A, D’Amato F, Perri F, Palini S, Pazienza V. Identifying Predictive Bacterial Markers from Cervical Swab Microbiota on Pregnancy Outcome in Woman Undergoing Assisted Reproductive Technologies. J Clin Med. 2022;11:680. doi: 10.3390/jcm11030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee BA, Thomas M, Sweeney EL, Frentiu FD, Samios M, Ravel J, Gajer P, Myers G, Timms P, Allan JA, Huston WM. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust N Z J Obstet Gynaecol. 2018;58:341–348. doi: 10.1111/ajo.12754. [DOI] [PubMed] [Google Scholar]
- Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74:2959–2977. doi: 10.1007/s00018-017-2509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YP, Tan GC, Wong KK, Anushia S, Cheah FC. Gardnerella vaginalis in perinatology: An overview of the clinicopathological correlation. Malays J Pathol. 2018;40:267–286. [PubMed] [Google Scholar]
- World Health Organization (WHO) Infertility. Geneva: WHO; 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/infertility . [Google Scholar]
- Younes JA, Lievens E, Hummelen R, van der Westen R, Reid G, Petrova MI. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018;26:16–32. doi: 10.1016/j.tim.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Crosby JA, Musri C, Souza MDCB, Martinez AG, Silva AA, Mojarra JM, Masoli D, Posada N. Assisted reproductive techniques in Latin America: The Latin American Registry, 2017. JBRA Assist Reprod. 2020;24:362–378. doi: 10.5935/1518-0557.20200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hou Q, Wang Y, Li W, Zhao H, Sun Z, Guo Z. Lactobacillus zhachilii sp. nov., a lactic acid bacterium isolated from Zha-Chili. Int J Syst Evol Microbiol. 2019;69:2196–2201. doi: 10.1099/ijsem.0.003362. [DOI] [PubMed] [Google Scholar]
- Zhao C, Wei Z, Yang J, Zhang J, Yu C, Yang A, Zhang M, Zhang L, Wang Y, Mu X, Heng X, Yang H, Gai Z, Wang X, Zhang L. Characterization of the Vaginal Microbiome in Women with Infertility and Its Potential Correlation with Hormone Stimulation during In Vitro Fertilization Surgery. mSystems. 2020;5:e00450–20. doi: 10.1128/mSystems.00450-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology (Reading) 2004;150:2565–2573. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]