Abstract
Appendiceal cancer is a rare, orphan disease with no therapies currently approved by the FDA for its treatment. Given the limited data regarding drug efficacy, these tumors have historically been treated with chemotherapy designed for colon cancer. However, an overwhelming body of molecular data has demonstrated that appendiceal adenocarcinoma is a distinct entity with key molecular differences from colon cancer, notably rare APC mutation. Recognizing that APC loss-of-function is thought to contribute to taxane resistance and that taxanes are effective in the treatment of other gastrointestinal tumors, including gastric, esophageal, and small bowel adenocarcinoma, we completed a single-center retrospective study to assess efficacy. In a cohort of 13 patients with metastatic appendiceal adenocarcinoma, treated with taxane chemotherapy the median overall survival was 8.8 months. Of 10 evaluable patients, we observed 3 responses, 4 patients with stable disease, and 3 with progression (30% response rate, 70% disease control rate). The results of this study showing activity of taxane-based chemotherapy in appendiceal adenocarcinoma support further clinical investigation of taxane therapy in this orphan disease.
Keywords: appendiceal cancer, taxane chemotherapy
Appendiceal cancer is a rare, orphan disease with no currently approved therapies. This study assessed efficacy of taxane chemotherapy in a cohort of 13 patients with metastatic appendiceal adenocarcinoma, with promising results.
Introduction
Appendiceal tumors encompass a rare and diverse group of neoplasms, with appendiceal adenocarcinoma (AA) being the most common histologic subtype.1 Despite a unique natural history characterized by metastatic spread limited to the peritoneum, as well as growing evidence that appendiceal tumors are molecularly distinct from colorectal cancer (CRC),2 current National Comprehensive Cancer Network (NCCN) guidelines suggest that appendiceal tumors should be treated with the same chemotherapy as used for CRC. However, recent prospective and retrospective studies have called into question the dogma that appendiceal tumors respond to chemotherapy similarly to colon cancer.3,4 In particular, a prospective, randomized, crossover design trial showed that patients with low-grade mucinous AAs do not derive benefit from 5-FU-based chemotherapy,4 highlighting the need to drug development efforts specific to appendix cancer.
Taxanes have been shown to be ineffective in colorectal cancer but are active in small bowel adenocarcinoma (SBA).5 It has been reported that APC loss-of-function is a mechanism of taxane resistance.6 Unlike CRC, where APC is mutated in >70% of tumors, APC mutation is uncommon in subtypes of appendiceal cancer (9.0%).2 We, therefore, hypothesized that taxane chemotherapy would have activity in AA.
Materials and Methods
The MD Anderson adapted version of the Palantir-Foundry software system was used to perform an automated query of the MD Anderson GI Medical Oncology database to identify patients with AA treated with paclitaxel-based regimens between 2003 and 2022. Manual chart review was performed to confirm patients met eligibility criteria and to extract outcome variables. Eligible patients had pathologic diagnosis of AA, mucinous adenocarcinoma, signet ring cell adenocarcinoma, or goblet cell adenocarcinoma, and more than one dose of taxane-based therapy and were not enrolled in a clinical trial. Radiographic response was assessed retrospectively based upon the treating physician’s assessment and categorized as response, stable disease, progression; response could not be evaluated (NA) if the patient did not have imaging performed after starting taxane therapy. Biochemical response was evaluated based on the percent change in tumor marker before and after treatment. Patients with a greater than 20% decrease were categorized as response, less than 20% change as stable, and greater than 20% increase as progression. Those whose respective tumor markers were not elevated were indicated as such, and those whose tumor markers were not measured were classified as NA. In cases where biochemical response differed from radiographic response, radiographic response was used for final response determination. Median overall survival (OS) was determined using the Kaplan-Meier method.
Results
Thirteen patients with AA treated with paclitaxel-based therapy were identified and met inclusion criteria. Median age was 64 years (range 29-77) with roughly equal splits between male and female as well as well, moderate, and poorly differentiated tumors (Supplementary Table S1). All 13 patients had inoperable peritoneal disease at time of treatment, and 4 (30.1%) had prior surgical resection. The cohort was heavily pretreated with median of 3 prior lines of therapy (range 1-5), 10 of the 13 had prior treatment with either FOLFOX or CapeOx. Ten patients received taxane-based combinations (gemcitabine combination in 7, platinum combination in 2, and fluoropyrimidine combination in 1), 3 received paclitaxel monotherapy. Four of the combination-treated patients received nab-paclitaxel, the rest paclitaxel.
The median OS from the start of taxane therapy was 8.77 months (range 0.7-31.0 months, Fig. 1A) with 3 of 13 patients still alive at time of analysis. Four patients stopped therapy after 3 or fewer taxane treatments due to either small bowel obstruction (SBO) or deteriorating performance status, median progression-free survival (PFS) for the remaining 9 patients was 7.4 months (range 0.8-15.5 months, Supplementary Table S1). Median time on treatment was 2.5 months (range 0.2-13.1 months, Fig. 1B) with 3 patients remaining on treatment at time of analysis. Of the 10 patients that could be assessed for radiographic response, 3 showed response, 4 with stable disease, and 3 with progression for a response rate of 30% and a disease control rate of 70% (Fig. 1C). Of the 9 patients with elevated CEA, biochemical response was seen in 3 (33%), stable disease in 3 (33%), and progression in 3 (33%). Of the 6 patients with elevated CA 19-9, biochemical response was seen in 2 (33%), stable disease in 2 (33%), and progression in 2 (33%). Two patients received first-line carboplatin and paclitaxel, both had response; the third responding patient was treated with gemcitabine and nab-paclitaxel. Presumably, the addition of a second cytotoxic agent contributed to response, however, the activity of carboplatin and gemcitabine in appendiceal cancer is unknown. One patient was initially diagnosed with a primary ovarian tumor and changed to 5-FU and bevacizumab after 3 cycles once expert pathology review made diagnosis of AA. The second patient had concurrent diagnoses of stage III squamous cell carcinoma of the lung and goblet cell adenocarcinoma of the appendix and was treated initially with weekly carboplatin and paclitaxel with concurrent radiation and then carboplatin and paclitaxel plus pembrolizumab per NSCLC guidelines. Treatment with carboplatin and paclitaxel is ongoing, with marked radiographic improvement in peritoneal carcinomatosis and complete response of NSCLC based on imaging and endobronchial biopsies. There was not an associated between grade and radiographic response. Three of four GNAS mutant tumors did not respond, consistent with prior reports suggesting intrinsic resistance to therapy in the case of mutant GNAS.3
Figure 1.
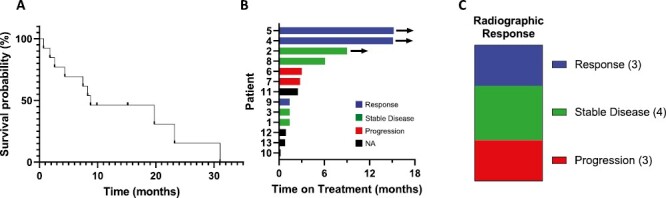
Outcomes for patients with AA treated with taxane-based therapy. (A) Overall survival from the start of treatment. (B) Time on treatment. (C) Radiographic response.
Discussion
Complete cytoreductive surgery (CRS) with heated intraperitoneal chemotherapy (HIPEC) remains the treatment of choice for patients with metastatic AA;7 however, for many patients the extent of peritoneal metastatic disease precludes this treatment option. There is unfortunately very little prospective data to guide chemotherapy choice for these nonoperative patients. To our knowledge, this is the largest cohort of patients with appendiceal cancer treated with taxane-based therapy reported in the literature. Although we recognize the inherent limitations of this small, retrospective study design, the favorable disease control rate in a heavily pretreated cohort indicates that taxane-based chemotherapy is active in AA and should be further studied in a prospective fashion. These findings are consistent with the activity of taxanes in SBA5,8 and are also consistent with the activity of intraperitoneal injection of paclitaxel in orthotopic PDX models of appendiceal cancer.9,10
Supplementary Material
Contributor Information
Julia Dansby, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Aditya More, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Mohammad Zeineddine, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Abdelrahman Yousef, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Alisha Bent, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Farshid Dayyani, Division of Hematology/Oncology, UC Irvine Health, Irvine, CA, USA.
Robert Wolff, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Michael Overman, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
John Paul Shen, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Funding
This work was supported by the Col. Daniel Connelly Memorial Fund, the National Cancer Institute (K22 CA234406) to J.P.S., and the Cancer Center Support Grant (P30 CA016672), the Cancer Prevention & Research Institute of Texas (RR180035 to J.P.S., a CPRIT Scholar in Cancer Research), and a Conquer Cancer Career Development Award (CDA-7604125121 to J.P.S). to J.P.S. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or Conquer Cancer.
Conflict of Interests
Farshid Dayyani reported consulting/advisory relationships Eisai, Exelixis, Ipsen, and AstraZeneca; honoraria from Sirtex, Ipsen, and Servier; and research funding to institution from Amgen, AstraZeneca, BMS, Eisai, Exelixis, Roche, Ipsen, Natera, and Taiho. Robert Wolff reported royalty payments from McGraw-Hill, Coeditor of MD Anderson Manual of Medical Oncology. Michael Overman serves on the scientific advisory board for ACPMP. John Paul Shen reported consulting/advisory relationships with Engine Biosciences and NaDeNo Nanoscience; research funding from the NIH, ASCO, and CPRIT; medical advisory board for ACPMP. The other authors indicated no financial relationships.
Author Contributions
Conception/design: M.O., J.P.S. Provision of study material or patients: A.B., M.O., F.D., R.W., J.P.S
Collection and/or assembly of data: J.D., A.M., M.Z., A.Y., J.P.S. Data analysis and interpretation: J.D., J.P.S. Manuscript writing: J.D., J.P.S. Final approval of manuscript: All authors.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Raghav K, Shen JP, Jácome AA, et al. Integrated clinico-molecular profiling of appendiceal adenocarcinoma reveals a unique grade-driven entity distinct from colorectal cancer. Br J Cancer. 2020;123(8):1262-1270. 10.1038/s41416-020-1015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ang CS-P, Shen JP, Hardy-Abeloos CJ, et al. Genomic landscape of appendiceal neoplasms. JCO Precis Oncol. 2018;2(2):1-18. 10.1200/po.17.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foote MB, Walch H, Chatila W, et al. Molecular classification of appendiceal adenocarcinoma. J Clin Oncol. 2022;41(8):1553-1564. 10.1200/jco.22.01392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen JP, Yousef AM, Zeineddine FA, et al. Efficacy of systemic chemotherapy in patients with low-grade mucinous appendiceal adenocarcinoma: a randomized crossover trial. JAMA Network Open. 2023;6(6):e2316161-e2316161. 10.1001/jamanetworkopen.2023.16161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Overman MJ, Adam L, Raghav K, et al. Phase II study of nab-paclitaxel in refractory small bowel adenocarcinoma and CpG island methylator phenotype (CIMP)-high colorectal cancer. Ann Oncol. 2018;29(1):139-144. 10.1093/annonc/mdx688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Astarita EM, Maloney SM, Hoover CA, et al. Adenomatous polyposis coli loss controls cell cycle regulators and response to paclitaxel in MDA-MB-157 metaplastic breast cancer cells. PLoS One. 2021;16(8):e0255738. 10.1371/journal.pone.0255738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chicago Consensus Working Group. The Chicago Consensus on peritoneal surface malignancies: management of appendiceal neoplasms. Cancer. 2020;126(11):2525-2533. [DOI] [PubMed] [Google Scholar]
- 8. Aldrich JD, Raghav KPS, Varadhachary GR, Wolff RA, Overman MJ.. Retrospective analysis of taxane-based therapy in small bowel adenocarcinoma. Oncologist. 2019;24(6):e384-e386. 10.1634/theoncologist.2018-0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sugarbaker PH. Intraperitoneal paclitaxel: pharmacology, clinical results and future prospects. J Gastrointest Oncol. 2021;12(Suppl 1):S231-S239. 10.21037/jgo-2020-03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito I, Yousef AMG, Chowdhury S, et al. Intraperitoneal paclitaxel is a safe and effective therapeutic strategy for treating mucinous appendiceal adenocarcinoma. Cancer Res. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


