Summary
Background
Carriers of cancer predisposing variants (CPVs) are at an increased risk to develop subsequent malignant neoplasms (SMNs) among childhood cancer survivors. We aim to investigate whether CPVs contribute to the risk of SMN-related late-mortality.
Methods
CPVs affecting 60 genes associated with well-established autosomal-dominant cancer-predisposition syndromes were characterized through whole-genome or whole-exome sequencing for 12469 (6172 male and 6297 female) survivors, including 4402 from the St. Jude Lifetime Cohort (SJLIFE) and 8067 from the Childhood Cancer Survivor Study (CCSS). SMNs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE). Cause-specific late-mortality was based on linkage with the National Death Index and systematic cohort follow-up. Fine & Gray subdistribution hazard models estimated SMN-related late-mortality starting from the first biospecimen collection, where non-SMN-related mortality was treated as a competing risk.
Findings
Among all survivors, 641 (5.1%) carried CPVs, which were significantly associated with increased severity of SMNs (CTCAE-grade≥4 vs. CTCAE-grade<4: Odds Ratio=2.15, 95% CI=1.18–4.19, P=0.0085). A total of 263 (2.1%) SMN-related deaths, and 426 (3.4%) non-SMN-related deaths occurred. Cumulative SMN-related mortality at 10-years from the first biospecimen collection was higher in CPV carriers (SJLIFE: 3.7% [95%CI =1.2–8.5]; CCSS: 6.9% [4.1–10.7]) vs. non-carriers (SJLIFE: 1.5% [1.0–2.1]; CCSS: 2.1% [1.7–2.5]). After adjusting for genetic ancestry, sex, age at diagnosis and cancer treatment exposures, carrying a CPV was associated with an increased risk of SMN-related mortality (SJLIFE: subdistribution hazard ratio [95% CI] =3.40 [1.37–8.43], P=0.0082; CCSS: 3.58 [2.27–5.63], P<0.0001).
Interpretation
Childhood cancer survivors with a CPV are at a significantly increased risk of SMN-related mortality, further highlighting the importance of genetic counseling and clinical genetic testing for CPVs. Identifying survivors at higher risk of SMNs and implementing early personalized cancer surveillance and prevention strategies may reduce the substantial SMN-related mortality.
Keywords: cancer predisposing variants, childhood cancer survivors, subsequent malignant neoplasms, late-mortality, survivorship care
Introduction
Despite improvement in five-year survival for children and adolescents diagnosed with cancer1,2, this high-risk population still experiences a nine-times greater risk for deaths after surviving 5 years than the age-sex-matched general population3. Among all causes of death, recurrence or progression of the primary cancer accounts for the majority of early deaths (within five years from diagnosis)4, whereas subsequent-malignant-neoplasms (SMNs) represent the most prominent contributor to late-mortality (five or more years from diagnosis)4,5. Recent studies have investigated the influence of treatment modalities on mortality risk over time or across treatment eras6, however, there is a paucity of research addressing the host genetic factors associated with late-mortality, particularly those underlying the SMN-related late-mortality7,8.
Cancer treatments (e.g., radiotherapy (RT) and chemotherapy) play a significant role in the excessive risk of SMNs observed among long-term survivors of childhood cancer9,10. We have previously demonstrated that pathogenic/likely pathogenic cancer predisposing variants (CPVs) also contribute to the increased risk for SMN development11, where CPV carriers exhibit a 1.8-times higher rate of developing any subsequent neoplasm compared with non-carriers in the St. Jude Lifetime Cohort Study (SJLIFE). Here, we expand the sample size to more than 12,000 in total by adding additional survivors from the Childhood Cancer Survivor Study (CCSS)12–14 to investigate potential contribution of CPVs to SMN-related late-mortality among long-term childhood cancer survivors who may have received prior genotoxic exposures including RT and different chemotherapeutic agents.
Methods
Study Participants
Two retrospectively-constructed cohort studies with prospective follow-up of five-year childhood cancer survivors with corresponding germline whole-genome or whole-exome sequencing data were included in the current analysis, i.e., the SJLIFE and the CCSS. SJLIFE, which enrolls five-year survivors of childhood cancer diagnosed and treated at St. Jude Children’s Research Hospital (SJCRH) from 1962 to 2012, involves periodic comprehensive clinical assessments of survivors as previously described12. CCSS participants are five-year survivors of childhood cancer diagnosed between 1970 and 1986 (the original CCSS cohort) and between 1987 and 1999 (the expansion CCSS cohort) at one of the 31 institutions in North America who provide periodic self-reported outcomes. Participants from both St. Jude Lifetime Cohort (SJLIFE) and Childhood Cancer Survivor Study (CCSS) provided informed consent. If a survivor was enrolled in both studies, he/she is excluded from the CCSS study in the current analysis to avoid duplication of data. The SJLIFE study was approved by the St. Jude Institutional Review Board (IRB), and the CCSS was approved by the IRB of each participating center. In both studies, self- and/or proxy-report questionnaires were used to assess demographic characteristics. The age at childhood cancer diagnosis is younger than 21 years for CCSS and 25 years for SJLIFE. There is no limit on attained age for eligibility. Both studies validate SMN and deaths through review of medical records and National Death Index (NDI) search, respectively. Cumulative dose of chemotherapy exposures within five years from initial childhood cancer diagnosis, including alkylating agents, anthracyclines, and epipodophyllotoxins15, were abstracted from medical records. Body-region-specific RT exposures within five years from childhood cancer diagnosis were determined from radiation oncology treatment records16. Therapy for a relapse or a secondary tumor that occurred five years after the original childhood cancer diagnosis was not considered.
Procedures
Whole-genome sequencing (WGS, 30 folds) was performed for 4402 survivors in the SJLIFE cohort with DNA derived from peripheral blood mononuclear cells17, and 2839 survivors in the CCSS expansion cohort with DNA derived from buccal/saliva samples as previously described18. The entire collection of WGS data for the SJLIFE and CCSS expansion cohorts is accessible from St. Jude Cloud (https://stjude.cloud), and whole-exome sequencing (WES, 40 folds) data of 5451 survivors from the CCSS original cohort was downloaded from dbGaP (phs001327.v2). Because 218 survivors are participants in both SJLIFE and CCSS studies (confirmed by the genotype concordance rate >98% based on pairwise comparisons for 5451 × 4402 pairs), survivors from the CCSS cohort were excluded. Five additional CCSS survivors with no date of death were also excluded. Finally, a total of 12469 survivors (4402 in SJLIFE, 5228 in the CCSS original cohort and 2839 in the CCSS expansion cohort) with DNA sequencing data were included in the current analysis (Fig. 1). All participants provided written informed consent for biospecimen collection for research purposes. In SJLIFE, self-reported SMNs were verified by review of pathology reports and medical records. Grading procedures were previously outlined13 for the spectrum of benign and malignant subsequent neoplasms experienced by childhood cancer survivors and mapped using histology-based International Classification of Diseases for Oncology, Third Edition (ICD-O-3), in combination with lesion site and surgical International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. In CCSS, self-reported SMNs were also confirmed with pathology reports and the grading procedures were previously described19. Essentially, basal cell carcinoma was graded a 2, thyroid carcinoma a 3, and all other cancers a 4 if the person lived, and a 5 if the person died. The grading used in our analyses represents the maximum grade at the last follow-up. The NDI, which provides underlying causes of death for deceased patients according to the criteria of the International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10), served as the primary source for cause of death. The latest available NDI search cutoff dates were December 31st, 2016 for SJLIFE and December 31st, 2017 for CCSS. For CCSS, NDI was the only source of cause of death. For SJLIFE, additional death events were ascertained with systematic cohort follow-up continuing until December 31st, 2019, where medical records were reviewed and adjudicated to determine the specific cause of death, differentiating SMN-related death from death due to other causes, e.g., recurrence/progression of primary malignancy, cardiovascular or other health conditions. Our recently published SJLIFE study examined the NDI-based cause of death with and without medical record reviews and found no appreciable difference between the two7. CPVs in a set of well-established 60 cancer predisposition genes (denoted as SJCPG60) have been previously curated among 3006 SJLIFE survivors11. Sequencing variants for the remaining 1396 SJLIFE survivors and 8067 CCSS survivors were analyzed by following the same approach11,20. Germline data from the non-cancer cohort from the Genome Aggregation Database (gnomAD)21 and the ClinVar22 database were utilized to facilitate pathogenicity classification.
Fig. 1.
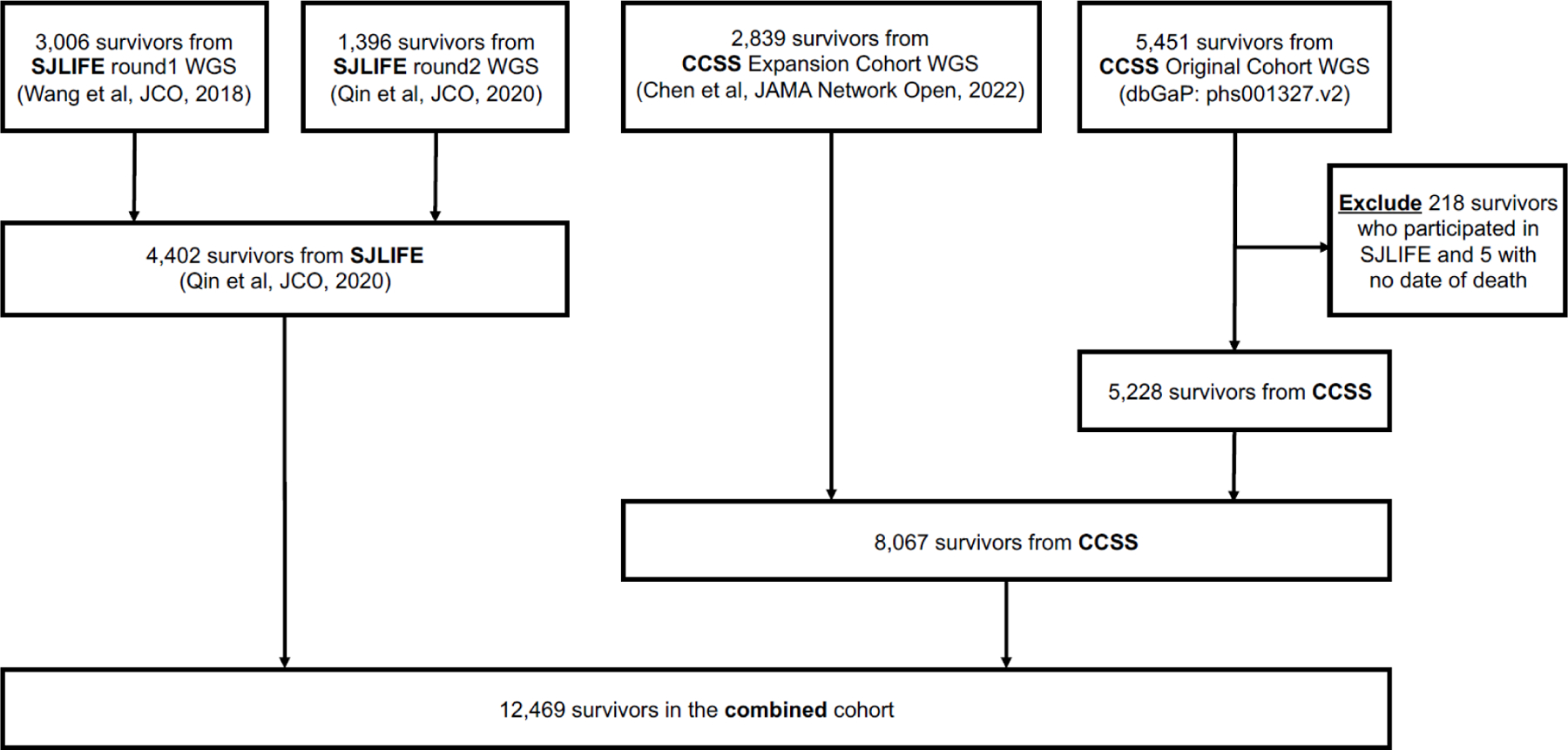
Flow-chart illustrates how the combined cohort was derived from multiple sources.
Statistical Analyses
Late-mortality was evaluated from time of the first biospecimen collection (blood for SJLIFE and saliva/buccal for CCSS survivors) until either death or December 31st, 2019 for SJLIFE and December 31st, 2017 for CCSS. Standardized mortality ratios (SMR) and absolute excess risk (AER) were calculated to quantify the difference of mortality risk, among all survivors, CPV carriers and non-carriers, in each cohort as compared with mortality rates in the U.S. population using National Center for Health Statistics data23, matched by age, calendar year, and sex. The Gray method24 was used to estimate the cumulative incidence of all-cause late-mortality as well as SMN-related mortality (with all other causes treated as competing risk events) for CPV carriers versus non-carriers, and assess the statistical significance of the differences in cumulative incidence by CPV status for SJLIFE and CCSS separately.
To calculate the associations between CPV status and all-cause mortality, a multivariable Cox proportional hazard regression model was fit with the CPV carrier status as an independent variable and covariates including genetic ancestry, sex, age at primary childhood cancer diagnosis, and cancer treatment exposures. Survivors with missing covariate information were excluded from regression models. The time-scale was years since the first biospecimen collection. All survivors alive at the last follow-up were censored. Because of the high correlations among the body-region-specific radiotherapy variables (appendix 2 p 3), only chest-RT was included in the model. The adjusted hazard ratios (HRs) were estimated by the partial maximum likelihood method with 95% confidence intervals (CI) included. Two-sided p-values were calculated using the standard large-sample inference methods. To calculate the association of SMN-related mortality with CPV status, the Fine & Gray competing risk method25 was used to estimate the subdistribution hazard ratios (subHRs) by treating other-cause deaths as competing risks; survivors with unknown cause of deaths were excluded from this analysis. On the contrary, for the association of other-cause mortality, SMN-related deaths were treated as competing risks. Fixed-effects meta-analysis using the inverse variance-weighted average method (IVW) was performed to combine the estimates for the two cohorts. I2 and Phet based on the Cochran’s Q statistic were calculated for measurement of heterogeneity. As a post-hoc analysis, cause-specific hazard ratios (csHRs) were also estimated using Cox regression model by censoring the competing risk. As post-hoc sensitivity analyses, models adjusting for time from childhood cancer diagnosis to the first biospecimen collection or the overall effect of radiotherapy and chemotherapy were evaluated, and results were compared. Furthermore, the association between CPV status and SMN-related mortality by chest RT (doses ≥20Gy vs. not exposed and doses <20Gy) as well as the association between CPV status and SMN-related mortality by exposures to alkylating agents (doses in the 2nd and 3rd tertile vs. not exposed and doses in the 1st tertile) were evaluated using appropriate contrast statement in PROC PHREG in SAS 9.4. Associations between CPV status and severity grades of SMN were based on Fisher’s exact test. A two-sided p value of less than 0·05 was considered statistically significant.
Analyses were conducted using SAS software (SAS 9.4, Cary NC, USA) and R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria)26, weighted by inverse probability of sampling to account for the under-sampling of acute lymphoblastic leukemia survivors in the CCSS expansion cohort.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
A total of 12,469 survivors who underwent WGS or WES were derived from SJLIFE and CCSS cohorts (Fig. 1). Of these eligible survivors (4402 in SJLIFE and 8067 in CCSS) with genetic data (Table 1), childhood cancer was mostly diagnosed between 0 and 4 years old with 1881 (42.7%) and 3012 (37.3%) for SJLIFE and CCSS studies, respectively, followed by 5–9 years, 10–14 years and ≥15 years; the oldest age at childhood cancer diagnosis was 23.6 years for the SJLIFE study and 21.0 years for the CCSS study; 2090 (47.5%) among SJLIFE and 4207 (52.2%) among CCSS were females; 3463 (78.7%) in SJLIFE and 6874 (85.2%) in CCSS were non-Hispanic White; acute lymphoblastic leukemia was the most common childhood cancer in both SJLIFE (1259, 28.6%) and CCSS (1949, 24.2%), followed by central nervous system (CNS) tumors and Hodgkin lymphoma. 2123 (48.2%) survivors in SJLIFE and 4140 (51.3%) survivors in CCSS were treated with chest-RT; 2473 (56.2%) received alkylating agents in SJLIFE, and 3481 (43.2%) in CCSS; 2451 (55.7%) received anthracyclines in SJLIFE, and 3102 (38.5%) in CCSS; 1488 (33.8%) received epipodophyllotoxins in SJLIFE and 818 (10.1%) in CCSS. Detailed population characteristics were shown separately by the sub-cohorts (i.e., CCSS expansion cohort vs. the CCSS original cohort) (appendix 2 pp 4–5). Population characteristics for sequenced survivors and not-sequenced survivors were also provided in the appendix 2 pp 6–7.
Table 1.
Characteristics of the study populations
| Variable | Category | SJLIFE, N (%) |
CCSS, N (%) |
||||
|---|---|---|---|---|---|---|---|
| Overall N=4402 | CPV carriers N=294 (6.7) | CPV non-carriers N=4108 (93.3) | Overall N=8067 | CPV carriers N=347 (4.3) | CPV non-carriers N=7720 (95.7) | ||
| Race/Ethnicity | Missing | 2 (0.0) | 0 (0.0) | 2 (0.0) | |||
| Non-Hispanic White | 3463 (78.7) | 227 (77.2) | 3236 (78.8) | 6874 (85.2) | 290 (83.6) | 6584 (85.3) | |
| Non-Hispanic Black | 700 (15.9) | 46 (15.6) | 654 (15.9) | 224 (2.8) | 12 (3.5) | 212 (2.7) | |
| Other | 237 (5.4) | 21 (7.1) | 216 (5.3) | 969 (12.0) | 45 (13.0) | 924 (12.0) | |
| Sex | Female | 2090 (47.5) | 154 (52.4) | 1936 (47.1) | 4207 (52.2) | 184 (53.0) | 4023 (52.1) |
| Male | 2312 (52.5) | 140 (47.6) | 2172 (52.9) | 3860 (47.8) | 163 (47.0) | 3697 (47.9) | |
| Diagnosis | Acute lymphoblastic leukemia | 1259 (28.6) | 36 (12.2) | 1223 (29.8) | 1949 (24.2) | 69 (19.9) | 1880 (24.4) |
| Central nervous system (CNS) | 648 (14.7) | 63 (21.4) | 585 (14.2) | 1431 (17.7) | 99 (28.5) | 1332 (17.3) | |
| Hodgkin lymphoma | 467 (10.6) | 8 (2.7) | 459 (11.2) | 1037 (12.9) | 22 (6.3) | 1015 (13.1) | |
| Other malignancies* | 2028 (46.1) | 187 (63.6) | 1841 (44.8) | 3650 (45.2) | 157 (45.2) | 3493 (45.2) | |
| Chest RT | None | 2230 (50.7) | 143 (48.6) | 2087 (50.8) | 3401 (42.2) | 162 (46.7) | 3239 (42.0) |
| >0 to <20 | 1418 (32.2) | 128 (43.5) | 1290 (31.4) | 2696 (33.4) | 118 (34.0) | 2578 (33.4) | |
| >=20 | 705 (16.0) | 17 (5.8) | 688 (16.7) | 1444 (17.9) | 41 (11.8) | 1403 (18.2) | |
| Unknown | 49 (1.1) | 6 (2.0) | 43 (1.0) | 526 (6.5) | 26 (7.5) | 500 (6.5) | |
| Alkylating agents | No | 1917 (43.5) | 175 (59.5) | 1742 (42.4) | 3780 (46.9) | 183 (52.7) | 3597 (46.6) |
| Yes | 2473 (56.2) | 118 (40.1) | 2355 (57.3) | 3481 (43.2) | 128 (36.9) | 3353 (43.4) | |
| Unknown | 12 (0.3) | 1 (0.3) | 11 (0.3) | 806 (10.0) | 36 (10.4) | 770 (10.0) | |
| Anthracyclines | No | 1947 (44.2) | 201 (68.4) | 1746 (42.5) | 4368 (54.1) | 205 (59.1) | 4163 (53.9) |
| Yes | 2451 (55.7) | 93 (31.6) | 2358 (57.4) | 3102 (38.5) | 115 (33.1) | 2987 (38.7) | |
| Unknown | 4 (0.1) | 0 (0.0) | 4 (0.1) | 597 (7.4) | 27 (7.8) | 570 (7.4) | |
| Epipodophyllotoxins | No | 2906 (66.0) | 219 (74.5) | 2687 (65.4) | 6810 (84.4) | 286 (82.4) | 6524 (84.5) |
| Yes | 1488 (33.8) | 73 (24.8) | 1415 (34.4) | 818 (10.1) | 38 (11.0) | 780 (10.1) | |
| Unknown | 8 (0.2) | 2 (0.7) | 6 (0.1) | 439 (5.4) | 23 (6.6) | 416 (5.4) | |
| Age at diagnosis | Overall (median, range) | 6.3 (0.0–23.6) | 2.3 (0.0–20.4) | 6.6 (0.0–23.6) | 7.4 (0.0–21.0) | 5.5 (0.0–20.8) | 7.5 (0.0–21.0) |
| 0–4 | 1881 (42.7) | 197 (67.0) | 1684 (41.0) | 3012 (37.3) | 162 (46.7) | 2850 (36.9) | |
| 5–9 | 958 (21.8) | 35 (11.9) | 923 (22.5) | 1852 (23.0) | 74 (21.3) | 1778 (23.0) | |
| 10–14 | 905 (20.6) | 41 (13.9) | 864 (21.0) | 1769 (21.9) | 61 (17.6) | 1708 (22.1) | |
| >=15 | 658 (14.9) | 21 (7.1) | 637 (15.5) | 1434 (17.8) | 50 (14.4) | 1384 (17.9) | |
| Age at first biospecimen, median (IQR) | 26.3 (20.4–33.9) | 22.0 (15.3–29.3) | 26.5 (20.6–34.1) | 30.6 (25.2,36.3) | 28.8 (23.2–34.5) | 30.7 (25.2–36.3) | |
| Time from diagnosis to the first biospecimen, median (IQR) | 17.8 (12.3–26.1) | 16.5 (11.4–22.3) | 17.9 (12.4–26.3) | 22.2 (18.4–26.1) | 22.0 (17.8–25.6) | 22.2 (18.4–26.1) | |
| Follow up time since the first biospecimen, median (IQR) | Censoring | 7.5 (3.1–9.5) | 6.0 (2.6–9.1) | 7.5 (3.2–9.5) | 13.5 (2.2–16.9) | 8.1 (2.1–16.6) | 13.5 (2.2–16.9) |
| Death | 4.9 (2.7–7.3) | 4.2 (3.2–5.5) | 5.1 (2.6–7.3) | 8.9 (4.6–13.0) | 6.9 (3.7–13.0) | 8.9 (4.7–13.0) | |
| Attained age, median (IQR) | Censoring | 33.2 (24.5–42.1) | 28.2 (18.3–36.9) | 33.6 (25.0–42.4) | 40.3 (34.3–47.6) | 38.0 (31.8–45.8) | 40.4 (34.4–47.7) |
| Death | 44.9 (34.8–52.5) | 40.7 (29.6–49.4) | 45.9 (35.4–52.9) | 43.9 (36.7–51.1) | 40.7 (34.6–45.7) | 44.3 (37.1–51.4) | |
| SMN | Yes | 298 (6.8) | 26 (8.8) | 272 (6.6) | 859 (10.6) | 57 (16.4) | 802 (10.4) |
| Late-Mortality | SMN-related | 44 (1.0) | 6 (2.0) | 38 (0.9) | 219 (2.7) | 22 (6.3) | 197 (2.6) |
| Unknown cause | 27 (0.6) | 3 (1.0) | 24 (0.6) | 49 (0.6) | 3 (0.9) | 46 (0.6) | |
| Other cause | 103 (2.3) | 7 (2.4) | 96 (2.3) | 323 (4.0) | 20 (5.8) | 303 (3.9) | |
Abbreviations: CPV, cancer predisposing variant; RT, radiotherapy; SJLIFE, St. Jude Lifetime Cohort Study; CCSS, Childhood Cancer Survivor Study; IQR, Inter-Quartile Range; SMN, subsequent malignant neoplasm.
Other malignancies included acute myeloid leukemia, germ cell tumor, neuroblastoma, non-Hodgkin lymphoma, retinoblastoma, sarcoma, Wilms tumor and other rare types.
Among all survivors, 641 (5.1%, 95% CI = 4.8%−5.5%) were CPV carriers, with 294 (6.7%) in SJLIFE and 347 (4.3%) in CCSS (Table 1; appendix 2 pp 8–29). The distribution of primary cancer diagnoses among survivors with the top 17 genes most frequently harboring CPVs in the combined cohort is provided in Fig. 1a, with RB1 (n=107), NF1 (n=104), BRCA2 (n=58), BRCA1 (n=41) and TP53 (n=34) ranked at the top. Prevalence of CPV carriers by childhood cancer diagnosis among survivors in the combined cohort is shown in Fig. 1b. The childhood cancer diagnosis with the highest prevalence of CPV carriers was retinoblastoma (RB) (110, 42.5%), followed by CNS tumors (161, 7.7%) and various solid tumors such as soft tissue sarcoma (STS) (64, 6.9%).
By following up survivors in CCSS from its inception in 1994 until December 31 2017 and from its initiation on April 27, 2007 in SJLIFE until December 31 2019, a total of 1,157 survivors developed SMNs (298 in SJLIFE and 859 in CCSS) with 83 carrying a CPV (Table 1). CPV status was significantly associated with increased severity of SMNs (CTCAE-grade≥4 vs. CTCAE-grade<4: Odds Ratio [OR] =2.15, 95% CI =1.18–4.19, P=0.0085) in the combined cohort and the association was consistent between the two cohorts (SJLIFE: OR =2.54, 95% CI =0.83–10.44, P=0.12; CCSS: OR =2.01, 95% CI =0.98–4.53, P=0.050) (Table 2). The most common SMNs diagnosed were breast cancer (250, 21.5%) and thyroid cancer (224, 19.3%) (appendix 2 p 30). The distribution of SMN types differed by CPV status in SJLIFE and CCSS (appendix 2 pp 31–32). A total of 263 SMN-related deaths occurred, including 44 in SJLIFE and 219 in CCSS. There was substantial heterogeneity in SMN types with the most common type of SMN resulting in death being CNS tumors (47, 17.9%) (appendix 2 p 33). If considering 426 other-cause deaths (103 in SJLIFE and 323 in CCSS) and 76 unknown-cause deaths (27 in SJLIFE and 49 in CCSS), a total of 174 died in SJLIFE (attained age at death: median [interquartile range, IQR] = 44.9 [34.8–52.5] years) and a total of 591 in CCSS (43.9 [36.7–51.1] years) based on the current data, where the duration of follow-up in SJLIFE is shorter and survivors are younger in SJLIFE (attained age at censoring: 33.2 [24.5–42.2] years) than in CCSS (40.3 [34.3–47.6] years) (Table 1).
Table 2.
Associations between CPV status and severity grades of SMN.
| |
CPV carriers, n (%) |
CPV non-carriers, n (%) |
Statistical Test |
||||
|---|---|---|---|---|---|---|---|
| Cohort | CTCAE-grade >=4 | CTCAE-grade <4 | CTCAE-grade >=4 | CTCAE-grade <4 | Odds Ratio | 95% CI | P# |
| SJLIFE | 22 (84.6) | 4 (15.4) | 186 (68.4) | 86 (31.6) | 2.54 | 0.83–10.44 | 0.12 |
| CCSS * | 47 (82.5) | 10 (17.5) | 562 (70.1) | 240 (29.9) | 2.01 | 0.98–4.53 | 0.050 |
| Combined | 69 (83.1) | 14 (16.9) | 748 (69.6) | 326 (30.4) | 2.15 | 1.18–4.19 | 0.0085 |
Abbreviations: CPV, cancer predisposing variant; SJLIFE, St. Jude Lifetime Cohort Study; CCSS, Childhood Cancer Survivor Study CTCAE, Common Terminology Criteria for Adverse Events.
Five with missing CTCAE-grade were not included: one was CPV carrier and four were non-carriers
Fisher Exact test.
The SMR for death due to SMNs was 4.7 (95% CI, 3.4–6.4) in SJLIFE and 6.3 (95% CI, 5.5–7.2) in CCSS (appendix 2 p 34), with CPV carriers having a more than three-times SMR compared to non-carriers (16.5 vs. 4.3 in SJLIFE; 18.2 vs. 5.9 in CCSS). In contrast, the SMR for other-cause mortality was only modestly higher among CPV carriers (3.4 vs. 2.3 in SJLIFE; 3.9 vs. 2.3 in CCSS). In addition, an overall AER for SMN-related mortality (SJLIFE: 1.2, 95% CI =0.8–1.7; CCSS: 2.2, 95% CI =1.9–2.6) was observed per 1,000 person years of follow-up and it was much higher among CPV carriers (3.3 in SJLIFE and 6.3 in CCSS) than non-carriers (1.1 in SJLIFE and 2.0 in CCSS).
The cumulative incidence of SMN-related mortality among CPV carriers were significantly higher as compared to non-carriers in SJLIFE (Fig. 2a) and CCSS (Fig. 2b). At 10 years from the first biospecimen collection, the cumulative incidence of SMN-related mortality was more than two-times higher among CPV carriers (3.7%, 95% CI =1.2–8.5) as compared to non-carriers (1.5%, 95% CI =1.0–2.1) in SJLIFE (appendix 2 p 35), and it was more than three-times higher among CPV carriers (6.9%, 95% CI =4.1–10.7) as compared to non-carriers (2.1%, 95% CI =1.7–2.5) in CCSS (appendix 2 p 36). In addition, the cumulative incidence of all-cause mortality was higher for CPV carriers (appendix 1 p 1; appendix 2 pp 35–36).
Fig. 2.
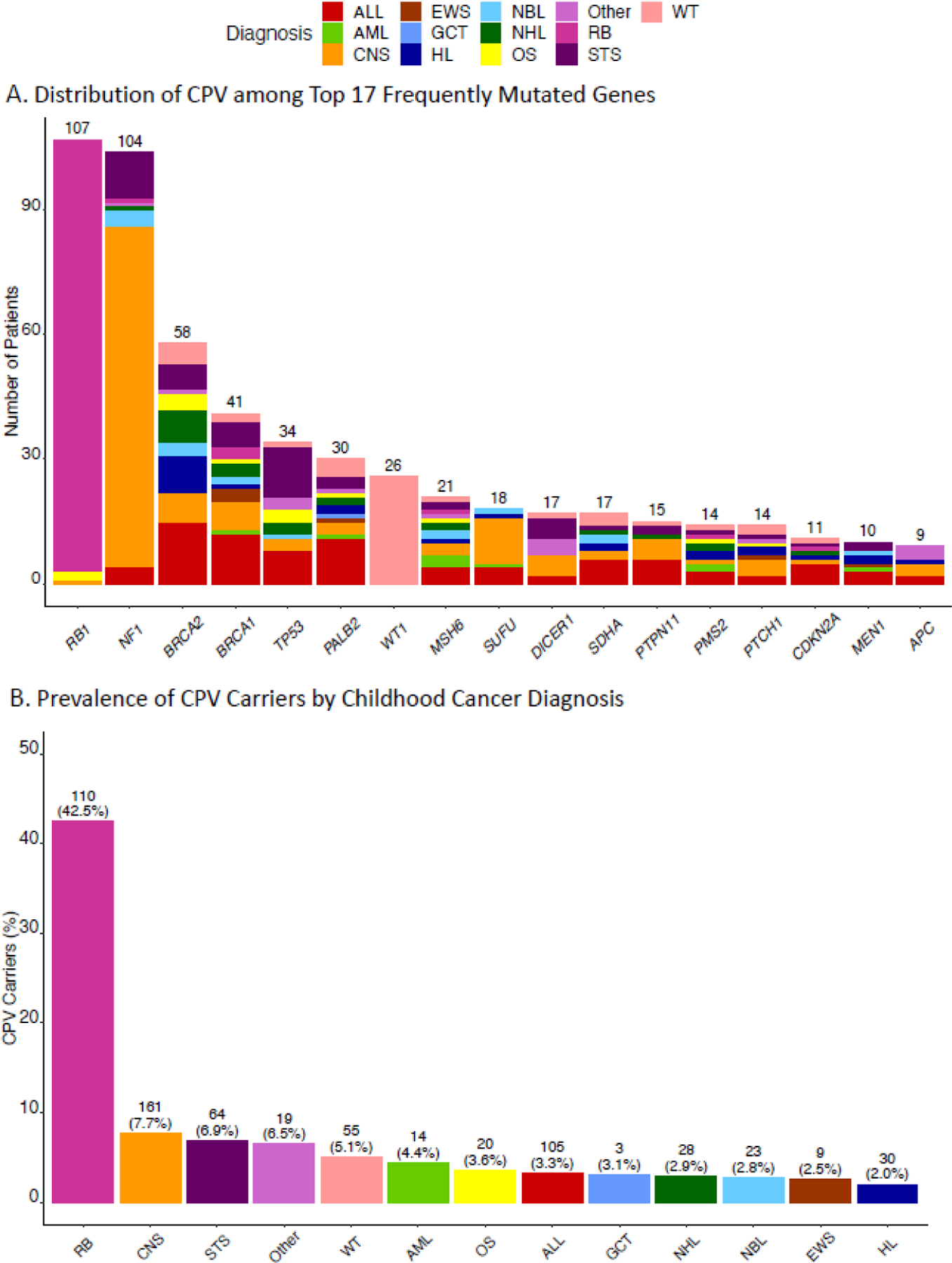
(a) Distribution of primary cancer diagnoses among 17 genes harboring CPV most frequently in the combined cohort; (b) Prevalence of CPV carriers by childhood cancer diagnosis.
In a multivariable regression analysis of SMN-related mortality combining the two cohorts, statistically significantly increased risk was observed for older age at childhood cancer diagnosis, exposure to chest-RT, and moderate to high exposure (2nd or 3rd tertile) to alkylating agents. Lower risk was observed for survivors who received low to moderate (1st or 2nd tertile) exposure to anthracyclines (appendix 2 pp 37–38). Similarly, significantly increased rates of all-cause mortality were observed among survivors treated with chest-RT and alkylating agents, but decreased risk of all-cause mortality were observed among survivors treated with low to moderate anthracyclines. In addition, moderate to high dose (2nd or 3rd tertile) of epipodophyllotoxins was also associated with decreased risk of all-cause mortality (appendix 2 pp 37–38).
After adjusting for genetic ancestry, sex, age at diagnosis, cancer treatment exposures, CPV carriers had a higher risk for SMN-related and all-cause mortality compared to non-carriers (Table 3). In the meta-analysis to combine both cohorts, the estimated subHR for SMN-related mortality was 3.54 (95% CI =2.36–5.32, P <0.0001), 1.61 for other-cause mortality (95% CI =1.07–2.42, P=0.021) and the estimated HR for all-cause mortality was 2.31 (95% CI =1.76–3.02, P <0.0001), which was consistent in both the SJLIFE cohort (SMN-related mortality: subHR =3.40, 95% CI =1.37–8.43, P=0.0082; all-cause mortality: HR =1.89, 95% CI =1.08–3.30, P=0.025; and other-cause mortality: subHR =1.18, 95% CI =0.50–2.80, P=0.70) and the CCSS cohort (SMN-related mortality: subHR =3.58, 95% CI =2.27–5.63, P<0.0001; all-cause mortality: HR =2.45, 95% CI =1.80–3.33, P<0.0001; and other-cause mortality: subHR =1.76, 95% CI = 1.11–2.78, P=0.016). To rule out of potential problem of delayed entry, we extended the model by adjusting for time from childhood cancer diagnosis to the first biospecimen collection as a covariate (appendix 2 p 39–43), and the association between CPV status and SMN-related mortality were similar in the SJLIFE cohort (subHR =3.80, 95% CI =1.47–9.82, P =0.0058) and the CCSS cohort (subHR =3.73, 95% CI =2.37–5.88, P<0.0001). To calculate cause-specific HR, we analyzed Cox regression models by censoring the competing events in the SJLIFE cohort (SMN-related mortality: csHR =3.36, 95% CI =1.36–8.32, P=0.0087; other-cause mortality: csHR =1.19, 95% CI =0.50–2.85, P=0.69) and the CCSS cohort (SMN-related mortality: csHR =3.71, 95% CI =2.34–5.86, P<0.0001; other-cause mortality: csHR =1.85, 95% CI =1.16–2.92, P=0.0091) (appendix 2 pp 44–47). Another alternative modeling adjusting for the overall effect of radiotherapy and chemotherapy was analyzed and the association between CPV status and late-mortality was similar (appendix 2 pp 48–49).
Table 3.
Associations between CPV Status and Late-Mortality.
| Cohort | SMN-related mortality |
Other-cause mortality |
All-cause mortality |
||||||
|---|---|---|---|---|---|---|---|---|---|
| sub HR |
95% CI | P-value | Sub HR |
95% CI | P-value | HR | 95% CI | P-value | |
| SJLIFE | 3.40 | 1.37–8.43 | 0.0082 | 1.18 | 0.50–2.82 | 0.70 | 1.89 | 1.08–3.30 | 0.025 |
| CCSS | 3.58 | 2.27–5.63 | <.0001 | 1.76 | 1.11–2.78 | 0.016 | 2.45 | 1.80–3.33 | <.0001 |
| Combined * | 3.54 | 2.36–5.32 | <.0001 | 1.61 | 1.07–2.42 | 0.021 | 2.31 | 1.76–3.02 | <.0001 |
Abbreviations: SJLIFE, St. Jude Lifetime Cohort Study; CCSS, Childhood Cancer Survivor Study; HR, hazard ratio; subHR, subdistribution hazard ratio; SMN, Subsequent Malignant Neoplasm.
Combined via fixed-effects meta-analysis based on two study specific models adjusting for genetic ancestry, sex, age at childhood cancer diagnosis, cancer treatment and exposures (chest-RT, alkylating agents, anthracyclines and epipodophyllotoxins). The follow-up started from the first biospecimen collection.
The SMN-related late-mortality occurred among 28 CPV carriers (6 in SJLIFE and 22 in CCSS) with substantial heterogeneity in types of SMNs (>10, including 9 CNS tumors among others), types of childhood cancer diagnoses (n=9, including 5 CNS and 5 Hodgkin lymphoma survivors among others), and cancer predisposition genes (n=17, including 8 NF1 and 6 TP53 among others) (appendix 2 p 50): the small counts prevented us from carrying out stratified analyses by SMN types, specific childhood cancer diagnosis or cancer predisposition genes.
In addition, the association between CPV status and SMN-related mortality was statistically significant (subHR =3.67, 95% CI =2.23–6.05, P <0.0001) among those who received none and less than 20 Gy of chest-RT but not in those who received ≥ 20 Gy of chest-RT (subHR =1.52, 95% CI =0.45–5.10, P=0.50), with moderate heterogeneity (Phet =0.17 and I2=47.1%) between the two subgroups (appendix 1 p 2; appendix 2 p 51). SubHR was elevated for CPV carriers who received moderate to high dose of alkylating agents (2nd and 3rd tertiles) (subHR =3.85, 95% CI =1.94–7.63, P=0.00011) compared to none or low dose (1st tertile) (subHR =2.83, 95% CI =1.50–5.34, P=0.0014), but the effect sizes were not statistically significantly different (Phet =0.49) (appendix 1 p 2; appendix 2 p 51).
Discussion
By combing the two largest cohorts of childhood cancer survivors with germline genome/exome sequencing data, in this study, we comprehensively examined the prevalence and spectrum of CPVs by specific cancer diagnosis and cancer predisposition gene. The prevalence of 6.7% for CPV carriers among 4,402 SJLIFE survivors is slightly higher than 5.8% (95% CI, 5.0% to 6.7%) that was previously reported based on a subset of 3,006 SJLIFE survivors11, and is much higher than the 4.3% for CCSS, primarily due to the different composition of childhood cancers in these two survivor cohorts. For instance, the highest prevalence of CPV carriers was found in survivors of childhood retinoblastoma (RB) (42.5%), a tumor type not included in the CCSS cohort. The difference of cancer diagnosis, along with the facts that SJCRH conducted clinical trials in treating childhood ALL with epipodophyllotoxins and the SJLIFE study enrolled patients treated more routinely with epipodophyllotoxins, leads to a much higher percentage of survivors treated with epipodophyllotoxins in SJLIFE than CCSS (Table 1).
To extend our previous finding that childhood cancer survivors who are CPV carriers have an increased risk to develop SMNs11, we assessed the contribution of CPVs to SMN-related late-mortality independent of genetic ancestry, sex, clinical and treatment risk factors based on time to event analysis, and found that the hazard of SMN-related mortality was significantly higher among CPV carriers compared to non-carriers. Notably, the overall strong association was primarily driven by survivors who received none or <20Gy chest-RT and the association was null among those who received ≥20Gy chest-RT. It is possible that high-dose of RT may obfuscate the CPV effect on risk of developing SMNs and hence SMN-related late-mortality, although the estimated subHR was not significantly different between those with high-dose of RT and those with no or low-dose RT. The findings suggest the potential importance of simultaneously considering both germline susceptibility and treatment exposures in determining the mortality risk with better precision among survivors of childhood cancer. Importantly, we found that CPV carriers tend to develop SMNs with CTCAE-graded ≥4 vs. those with CTCAE-graded <4 as compared to non-carriers, which more likely lead to SMN-related mortality. Based on this, we postulate that increasing surveillance and screening for carriers of CPV (especially, in genes with higher SMN penetrance and syndromes for which there is documented efficacy of screening27,28) may facilitate detecting SMNs at earlier stages, so the survivors of childhood cancer might fare better after SMNs. A personalized approach such as the one outlined for early detection and prevention of breast cancer including intensified screening for certain risk groups and prophylactic treatment for those deemed at the highest risk29. However, substantial heterogeneity in SMN types among those who died from SMNs suggested that the genetic contribution to SMN-related mortality is complex and the observed increased risk for SMN-related mortality among CPV carriers was not driven by one or a few specific SMNs.
Our study has the following limitations. First, WGS/WES data were available only for study participants who were alive at the first biospecimen collection, which can be variable with respect to follow-up length after cohort entry (5 years from childhood cancer diagnosis), may not represent all five-year survivors and potentially imposes certain bias, and CPVs associated with increased risk of early-mortality (e.g., relapse of a childhood cancer or subsequent acute myeloid leukemia) may be underrepresented. It has also been previously suggested that primary childhood cancer can be coded as cause of death, which may lead to lower estimates of SMN-related mortality30. Thus, the prevalence of CPV carriers and the association between CPV status and late-mortality risk are likely underestimated in our analysis, compared to the analysis involving all survivors. Second, while our specific treatment stratified analysis suggested different genetic effect effects within different treatment groups (e.g., none or chest-RT <20Gy vs. ≥20Gy), the substantial genetic heterogeneity and limited sample size prevented us from conducting more stratified analyses (e.g., by primary diagnosis groups or specific genes) and dissecting the contributions of CPV by considering gene-treatment interactions. Third, CPV carriers in the CCSS cohort were at a higher risk for SMN-related mortality than carriers in the SJLIFE cohort (rate of cumulative incidence of SMN-related mortality: 6.90% vs. 3.65% after 10 years since the first biospecimen collection), which could be partially due to survivors being older in CCSS (attained age: 40’s vs. 30’s in SJLIFE). Nevertheless, the adjusted genetic effect (CPV status) on risk of SMN-related mortality appeared to be very similar in SJLIFE and in CCSS (subHR: 3.40 vs. 3.58). Future studies are warranted to explore other factors underlying the difference between the lifetime data among these two childhood cancer cohorts as well as different treatment modalities for the SMNs. Finally, because survivors are relatively young (median age at censoring and median length of follow-up since the first biospecimen collection were 33.2 years and 7.4 years in SJLIFE, and 40.3 years and 12.6 years in CCSS, respectively), mortality risk associations with CPV carrier status may change with increasing age and extended follow-up.
Collectively, our findings provide compelling evidence of increased SMN-related late-mortality among childhood cancer survivors who are CPV carriers. Thus, these results have critical implications surrounding the provision of genetic counselling and testing and informing future clinical recommendations for precision medicine for this growing population. Identifying survivors at the highest risk for the development of SMN and implementing early personalized cancer surveillance and prevention strategies may reduce the substantial SMN-related late-mortality among long-term childhood cancer survivors.
Supplementary Material
Fig. 3. Cumulative incidence of SMN-related late-mortality among CPV carriers vs. non-carriers in SJLIFE (a); and CCSS (b).
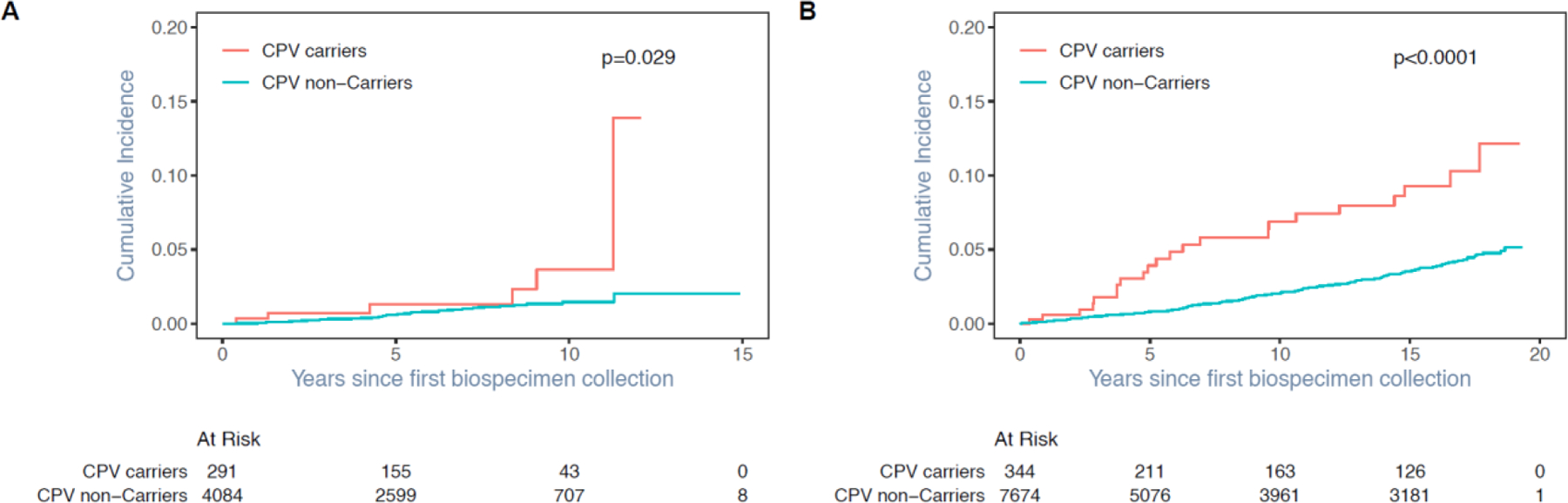
Note: There were 27 SJLIFE survivors and 49 CCSS survivors who died with unknown causes were excluded from the calculation of cumulative incidence of SMN-related mortality where other-cause deaths were treated as a competing risk.
Research in context.
Evidence before this study
We previously characterized the prevalence and spectrum of pathogenic/likely pathogenic (P/LP) variants in 60 genes associated with well-established autosomal-dominant cancer-predisposition syndromes, and demonstrated that carriers of germline cancer predisposing variants (CPVs) had an increased risk of developing subsequent malignant neoplasms (SMNs) independent of cancer treatment effects among survivors of childhood cancer. Given that SMNs represent the most prominent contributor to late-mortality (five or more years from diagnosis), it remained to be determined whether CPVs contribute to the risk of SMNs-related late-mortality. We systematically searched PubMed from database inception to Jan 4, 2022 with the terms “[childhood cancer or pediatric cancer] and [germline mutation or germline variant] and [cancer predisposition gene] and [sequencing] and [subsequent neoplasm or subsequent cancer or subsequent malignancy] and [mortality]” for research articles without language restriction in which the association of CPVs with SMN-related late-mortality was assessed in survivors of childhood cancer through next-generation sequencing. No published studies were found on this topic.
Added value of this study
To our knowledge, this is the first time to comprehensively evaluate the contribution of CPVs to risk of SMN-related late-mortality among survivors of childhood cancer by combining the two largest cohorts of childhood cancer survivors with existing genome/exome sequencing data. We found that childhood cancer survivors who are CPV carriers have a significantly increased risk of SMN-related late-mortality. Accordingly, our study provides important new insights into the host genetic factors associated with the risk of SMN-related mortality, a significant adverse late effect following childhood cancer diagnosis.
Implications of all the available evidence
Our findings add substantial new information regarding the contribution of CPVs to the risk of SMN-related late-mortality among long-term survivors of childhood cancer, highlighting the importance of clinical germline testing for the presence of CPVs. Identifying survivors at the highest risk for SMNs and implementing early personalized cancer surveillance and prevention strategies may significantly reduce the burden of SMN-related late-mortality.
Acknowledgements
This research was supported by funding from the American Lebanese Syrian Associated Charities and by grants (CA195547 [K K Ness, M M Hudson], CA55727 [G T Armstrong], CA021765) from the National Institutes of Health to St. Jude Children’s Research Hospital. No authors are employed by NIH. The authors thank all the individuals who participated in this study.
Funding
American Lebanese Syrian Associated Charities; National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
We declare no competing interests.
Data sharing
The entire collection of whole-genome sequencing (WGS) data for the St. Jude Lifetime Cohort (SJLIFE, n=4,402) and Childhood Cancer Survivor Study (CCSS expansion, n=2,839) cohorts is now accessible from the St. Jude Cloud (https://stjude.cloud) under the accession number SJC-DS-1002. Additional phenotypic data including demographics, treatment and mortality about the study participants in the SJLIFE and CCSS expansion cohorts can be accessed via the survivorship portal (http://survivorship.stjude.cloud/). To protect patients’ privacy and confidentiality (in compliance with the IRB approval and the data sharing plan), WGS data download requires an application providing a purpose and project (medical or scientific research use only), completing a data access agreement, and a subsequent approval by the data access committee. Detailed instructions can be provided by contacting support@stjude.cloud. In addition, whole-exome sequencing data and the associated clinical data for 5,451 survivors from the CCSS original cohort is accessible from dbGaP (https://www.ncbi.nlm.nih.gov/gap/) with the accession: phs001327.v2.
Code availability
Analyses were conducted using SAS software (SAS 9.4, Cary NC, USA) and R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Custom code calling into the standard functions in SAS or R can be made available upon request.
Ethics
The St Jude Lifetime Cohort Study (SJLIFE) was approved by the St Jude institutional review board (IRB), and the Childhood Cancer Survivor Study (CCSS) was approved by the IRB of each participating center in North America. This current study is covered by the IRB approval for the 2 cohort studies.
References
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014; 14(1): 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014; 64(2): 83–103. [DOI] [PubMed] [Google Scholar]
- 3.Fidler MM, Reulen RC, Winter DL, et al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ 2016; 354: i4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2008; 100(19): 1368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA 2010; 304(2): 172–9. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med 2016; 374(9): 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrhardt MJ, Liu Q, Dixon SB, et al. Association of Modifiable Health Conditions and Social Determinants of Health With Late Mortality in Survivors of Childhood Cancer. JAMA Netw Open 2023; 6(2): e2255395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon SB LQ, Chow EJ, Oeffinger KC, Nathan PC, Howell RM, Leisenring WM, Ehrhardt MJ, Ness KK, Krull KR, Mertens AC, Hudson MM, Robison LL, Yasui Y, Armstrong GT. Specific causes of excess late mortality and association with modifiable risk factors among survivors of childhood cancer: A report from the Childhood Cancer Survivor Study (CCSS) cohort. Lancet 2023. [DOI] [PMC free article] [PubMed]
- 9.Turcotte LM, Liu Q, Yasui Y, et al. Chemotherapy and Risk of Subsequent Malignant Neoplasms in the Childhood Cancer Survivor Study Cohort. J Clin Oncol 2019; 37(34): 3310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017; 390(10112): 2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Wilson CL, Easton J, et al. Genetic Risk for Subsequent Neoplasms Among Long-Term Survivors of Childhood Cancer. J Clin Oncol 2018; 36(20): 2078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howell CR, Bjornard KL, Ness KK, et al. Cohort Profile: The St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. Int J Epidemiol 2021; 50(1): 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev 2017; 26(5): 666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009; 27(14): 2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2014; 61(1): 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stovall M, Donaldson SS, Weathers RE, et al. Genetic effects of radiotherapy for childhood cancer: gonadal dose reconstruction. Int J Radiat Oncol Biol Phys 2004; 60(2): 542–52. [DOI] [PubMed] [Google Scholar]
- 17.Qin N, Wang Z, Liu Q, et al. Pathogenic Germline Mutations in DNA Repair Genes in Combination With Cancer Treatment Exposures and Risk of Subsequent Neoplasms Among Long-Term Survivors of Childhood Cancer. J Clin Oncol 2020; 38(24): 2728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Song N, Dong Q, et al. Association of Single-Nucleotide Variants in the Human Leukocyte Antigen and Other Loci With Childhood Hodgkin Lymphoma. JAMA Netw Open 2022; 5(8): e2225647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006; 355(15): 1572–82. [DOI] [PubMed] [Google Scholar]
- 20.Edmonson MN, Patel AN, Hedges DJ, et al. Pediatric Cancer Variant Pathogenicity Information Exchange (PeCanPIE): a cloud-based platform for curating and classifying germline variants. Genome Res 2019; 29(9): 1555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016; 536(7616): 285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landrum MJ, Lee JM, Riley GR, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res 2014; 42(Database issue): D980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United States Department of Health and Human Services Centers for Disease Control and Prevention NCfHS. Compressed mortality file on CDC Wonder Online Database
- 24.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics 1988; 16(3): 1141–54, 14. [Google Scholar]
- 25.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999; 94(446): 496–509. [Google Scholar]
- 26.RCore Team R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing 2020.
- 27.Villani A, Shore A, Wasserman JD, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol 2016; 17(9): 1295–305. [DOI] [PubMed] [Google Scholar]
- 28.Villani A, Tabori U, Schiffman J, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: a prospective observational study. Lancet Oncol 2011; 12(6): 559–67. [DOI] [PubMed] [Google Scholar]
- 29.Pashayan N, Antoniou AC, Ivanus U, et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol 2020; 17(11): 687–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol 2001; 19(13): 3173–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


