Abstract
Introduction
Vision loss is common in patients treated with radiotherapy for uveal melanoma. With proton beam irradiation (PBI), the prescribed dose is delivered to the tumor with a sharp dose reduction outside the target volume. However, radiation complications are likely to develop when tumors are located near the optic nerve or fovea. Treatment with light-activated AU-011 (belzupacap sarotalocan), an investigational drug which specifically targets tumor cells, may avoid these complications. We evaluated outcomes in a historical group of patients who fit eligibility criteria for AU-011 therapy and were treated with PBI.
Methods
A consecutive series of patients who received PBI for small choroidal melanoma at a single center between 1986 and 2016 were identified. Consistent with eligibility criteria in clinical trials of AU-011, patients were included when tumor dimensions did not exceed 2.5 mm in maximum thickness and 10.0 mm in largest basal diameter (LBD). Snellen visual acuities were converted to logMAR for analysis. Visual acuity outcomes were analyzed in patients with an initial acuity of logMAR 0.7 or better (equivalent to Snellen 20/100). Rates of visual acuity loss and mortality were calculated using the Kaplan-Meier method. Acuity loss by tumor location was compared using log-rank testing. Rates of tumor recurrence, neovascular glaucoma (NVG), and eye loss were also described.
Results
Two hundred and 22 patients were included in the study. The median age was 60.7 years (range 21.3–94.8 years). Median tumor thickness was 2.0 mm (range 1.2–2.5 mm), and median LBD was 8.0 mm (range 4.0–10.0 mm). Median follow-up was 6.9 years (range 1.0–30.2 years). In 204 patients with a baseline logMAR visual acuity of 0.7 or better, the mean baseline acuity was 0.15 (equivalent to Snellen 20/25), which decreased to 0.52 (approximately Snellen 20/70) by 5 years after PBI. Visual outcomes were significantly worse for patients with tumors located within 3 mm of the optic disc and/or fovea. Tumor recurrence (1.4%), NVG (4.5%), and eye loss (2.7%) were uncommon.
Discussion
Despite the advantageous dose distribution of protons, over half of patients with small choroidal melanomas located near the optic disc or fovea had a visual acuity equivalent to 20/80 or worse at 5 years after PBI. Treatment with AU-011 may allow better vision preservation in small tumors that carry a high risk of vision loss with radiotherapy.
Keywords: Choroidal melanoma, Radiation, Oncology
Introduction
Uveal melanoma is the most common primary intraocular malignancy. Successful local control of the majority of tumors can be achieved with radiotherapy, typically either external beam radiation therapy or plaque brachytherapy [1–3]. Unfortunately, visual outcomes are often poor following treatment. Risk factors for vision loss include poor initial acuity, greater tumor height and diameter, as well as proximity and radiation dose to the optic nerve and fovea [4–6].
The most frequently used form of external beam radiation is proton beam irradiation (PBI), which deploys charged-particle ionizing radiation. Because of the high-dose localization in proton Bragg peak, a relatively uniform radiation dose is delivered to the tumor with a sharp dose reduction outside the treatment area. Despite the theoretical advantages of PBI in sparing the critical structures for vision, many patients lose visual acuity in a dose- and time-dependent fashion following radiation treatment [6]. Patients with tumors near the optic nerve or fovea are at higher risk of vision loss compared to those with tumors located farther away from both structures. The most common reasons for vision loss were exudative retinal detachment, cataract, and radiation retinopathy [7].
Alternative therapies are desired to avoid radiation-related complications and preserve vision. AU-011 (belzupacap sarotalocan) is an investigational therapy consisting of viral-like particle bioconjugates that bind to heparan sulfate proteoglycans (HSPG) on the surface of tumor cells [8]. It is under investigation for use in small choroidal melanoma and small melanocytic lesions. The drug is delivered via intravitreal or suprachoroidal injection and is conjugated with a phthalocyanine photosensitizer, IRDye 700DX, which causes cellular necrosis when activated by ophthalmic laser at 689 nm wavelength. Theoretically, it may allow for better vision retention compared to radiotherapy by specifically targeting tumor cells.
Because of ethical and logistical concerns inherent to a randomized controlled trial for a rare malignancy, there is a lack of data on the relative efficacy and complication rates for AU-011 compared to existing therapy. We use historical data to examine outcomes for patients treated with PBI who met inclusion criteria for AU-011 clinical trials.
Methods
This study was approved by the Institutional Review Board and was in accordance with the tenets of the Declaration of Helsinki. Retrospective chart review was performed of patients diagnosed with small choroidal melanoma who were treated with PBI at the Harvard Cyclotron or Francis H. Burr Proton Therapy Center at Massachusetts General Hospital between 1986 and 2016. Subjects were identified through the Uveal Melanoma Registry at the Massachusetts Eye and Ear (MEE), Boston, Massachusetts.
The selected inclusion parameters were consistent with eligibility criteria for the phase 2 trial of investigational AU-011 therapy; these included a clinical diagnosis of choroidal melanoma with a maximum tumor thickness greater than or equal to 0.5 mm and less than or equal to 2.5 mm on B-scan ultrasound, as well as largest basal diameter (LBD) less than or equal to 10.0 mm [9]. Total follow-up for ocular outcomes was calculated as the length of time between the first date of radiation therapy and the last visit. Subjects with less than 1 year of follow-up were excluded.
The protocol for PBI has been described previously [10]. Tumor localization surgery with the placement of fiducial markers was performed to aid in accurate targeting with the proton beam during radiation treatment. A computerized treatment planning system was used to create an individualized therapy plan for each patient. The radiation dose, corrected for the proton’s relative biological effectiveness (RBE), was either 50 or 70 Gy (RBE) delivered in 5 fractions [11]. Patients underwent subsequent surveillance examinations either at MEE or with their local ophthalmologist, typically at 6-month intervals during the first 5 years following PBI and annually thereafter. Ocular examination data were collected from MEE records or notes from patients’ local ophthalmologists. Mortality and cause of death were determined by periodic searches of the medical record, hard copy death certificates, and the National Death Index.
The predetermined study outcomes were visual acuity, tumor recurrence, neovascular glaucoma (NVG), loss of the eye, and all-cause and melanoma-related mortality. Snellen visual acuities were converted to logMAR, and visual outcomes were examined in patients with baseline visual acuity of logMAR 0.7 or better (equivalent to Snellen 20/100). Kaplan-Meier analyses were used to calculate the cumulative rates of visual acuity loss to logMAR ≥0.3 and ≥0.6, as well as mortality outcomes. Visual acuity outcomes were analyzed for patients who had tumors located within 3 mm of the optic disc and/or fovea, and therefore at high risk of vision loss, compared to those who had tumors located greater than 3 mm from both structures. The log-rank test was used to evaluate for statistically significant differences in rates of visual acuity loss by tumor location.
Results
A total of 222 patients met inclusion criteria (Table 1). Median patient age was 60.7 years (range 21.3–94.8 years), and 129 (58.1%) were female. Median tumor thickness was 2.0 mm (range 1.2–2.5 mm), and median LBD was 8.0 mm (range 4.0–10.0 mm). The median tumor distance from the optic disc was 2.25 mm (range 0–10.5 mm), and the median distance from the fovea was 1.95 mm (range 0–10.5 mm). The total radiation dose was 50 Gy (RBE) in 59 patients (26.6%) and 70 Gy (RBE) in 163 patients (73.4%). The median ocular follow-up time was 6.9 years (range 1.0–30.2 years). Thirteen patients (7.7%) received intravitreal injections following PBI; 12 received anti-VEGF injections, and 1 received steroid injections. The median number of injections was 11. The majority (160 subjects, 72.1%) were treated for choroidal melanoma prior to 2005 and thus did not receive anti-VEGF injections.
Table 1.
Baseline patient demographics and tumor characteristics, categorized by tumor location
| Characteristic | Within 3 mm of disc and/or fovea (n = 163) | >3 mm from disc and fovea (n = 59) | All patients (n = 222) |
|---|---|---|---|
| Age at time of PBI, years, median (range) | 60.4 (27.1–94.8) | 62.1 (21.3–81.4) | 60.7 (21.3–94.8) |
| Sex, female, n (%) | 96 (58.9) | 33 (55.9) | 129 (58.1) |
| Tumor thickness, mm, median (range) | 2.0 (1.2–2.5) | 2.0 (1.2–2.5) | 2.0 (1.2–2.5) |
| LBD, mm, median (range) | 8.0 (4.0–10.0) | 9.0 (6.0–10.0) | 8.0 (4.0–10.0) |
| Distance from optic disc, mm, median (range) | 1.35 (0–6.75)* | 6 (3.75–10.5)* | 2.25 (0–10.5) |
| Distance from fovea, mm, median (range) | 1.1 (0–7.5)** | 7.5 (3.5–10.5)** | 1.95 (0–10.5) |
| Baseline logMAR VA ≤0.7 (20/100), n (%) | 145 (88.9) | 59 (100) | 204 (91.9) |
| Follow-up time for ocular outcomes, years, median (range) | 6.1 (1.0–30.2) | 9.9 (1.9–30.1) | 6.9 (1.0–30.2) |
| Follow-up time for survival, years, median (range) | 13.1 (1.5–30.2) | 15.8 (2.2–30.1) | 13.7 (1.5–30.2) |
| Total radiation dose, 70 Gy (RBE), n (%) | 107 (65.6) | 56 (94.9) | 163 (73.4) |
PBI, proton beam irradiation; VA, visual acuity.
*1 missing; **2 missing.
Visual Acuity Outcomes
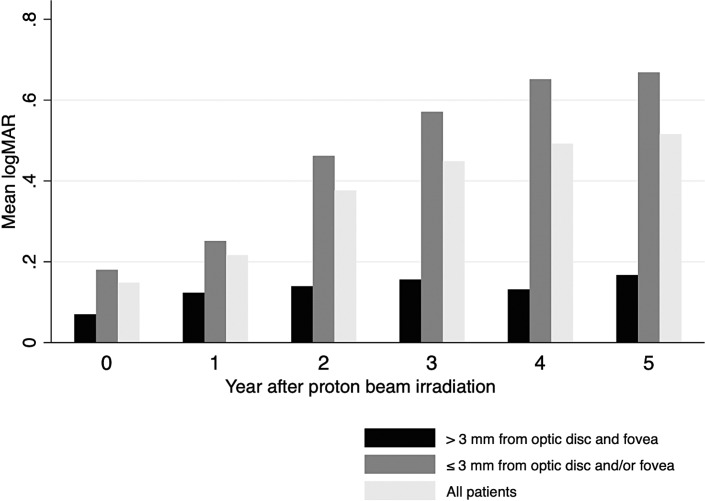
There were 204 patients with a baseline logMAR visual acuity of 0.7 or better (equivalent to Snellen 20/100). The mean baseline logMAR acuity was 0.15 (equivalent to Snellen 20/25). This declined to logMAR 0.22, 0.38, 0.45, 0.49, and 0.52 during years 1–5 after PBI, respectively (approximately Snellen 20/30, 20/50, 20/60, 20/60, and 20/70) (Fig. 1). Kaplan-Meier analyses showed that 53.3% of patients had vision loss to logMAR 0.3 or worse (Snellen 20/40), and 42.3% had logMAR 0.6 or worse (Snellen 20/80) by 5 years after PBI (Table 2).
Fig. 1.
Mean logMAR visual acuity at years 0–5 after proton beam therapy. Among patients starting with logMAR acuity of 0.7 or better (equivalent to Snellen 20/100), the mean baseline acuity was logMAR 0.15 (Snellen 20/25). The mean acuity progressively declined to logMAR 0.52 (approximately Snellen 20/70) by 5 years after PBI.
Table 2.
Cumulative rates (95% CI) of visual acuity loss to logMAR ≥0.3 and logMAR ≥0.6 at years 1–5 after PBI, stratified by tumor location
| Year after PBI | Acuity loss to logMAR ≥0.3 | Acuity loss to logMAR ≥0.6 | ||||
|---|---|---|---|---|---|---|
| within 3 mm of disc and/or fovea | >3 mm from disc and fovea | all patients | within 3 mm of disc and/or fovea | >3 mm from disc and fovea | all patients | |
| Year 1 | 13.1 (8.6–19.8) | 10.2 (4.7–21.2) | 12.3 (8.5–17.6) | 4.8 (2.3–9.9) | 3.4 (8.6–12.9) | 4.4 (2.3–8.3) |
| Year 2 | 34.9 (27.6–43.4) | 11.9 (5.8–23.3) | 28.1 (22.4–34.9) | 19.3 (13.7–26.9) | 5.2 (1.7–15.2) | 15.2 (10.9–21.0) |
| Year 3 | 48.9 (40.7–57.8) | 13.8 (7.1–25.7) | 38.5 (31.9–45.8) | 33.0 (25.7–41.8) | 5.2 (1.7–15.2) | 24.8 (19.3–31.7) |
| Year 4 | 60.5 (52.0–69.1) | 18.0 (10.1–31.1) | 47.9 (40.8–55.5) | 44.3 (36.1–53.5) | 5.2 (1.7–15.2) | 32.8 (26.5–40.3) |
| Year 5 | 67.2 (58.7–75.5) | 20.4 (11.7–34.0) | 53.3 (46.1–61.0) | 57.6 (48.8–66.7) | 5.2 (1.7–15.2) | 42.3 (35.2–50.2) |
| Log-rank p < 0.001 | Log-rank p < 0.001 | |||||
Visual outcomes in patients with tumors located within 3 mm of the optic disc and/or fovea were markedly worse than those observed in patients with tumors located more than 3 mm from both structures. Five-year cumulative rates of acuity loss to logMAR 0.3 or worse were 67.2% (95% CI: 58.7–75.5) in patients with tumors located near one or both structures, compared to 20.4% (95% CI: 11.7–34.0) in patients with tumors located farther away (log-rank p < 0.001). The differences were even more dramatic for acuity loss to logMAR 0.6 or worse. Five-year cumulative rates were 57.6% (95% CI: 48.8–66.7) and 5.2% (95% CI: 1.7–15.2) in patients with tumors located within 3 mm of the optic disc and/or fovea versus those more than 3 mm from both structures, respectively (log-rank p < 0.001).
Tumor Recurrence, NVG, and Eye Loss
Tumor recurrence was diagnosed in 3 patients (1.4%) at a median time of 41.2 months (range 30.6–62.6 months). NVG was present in 10 patients (4.5%) at a median time of 53.9 months (range 24.1–129.9 months). Enucleation was performed in 6 patients (2.7%) at a median time of 59.1 months (range 43.25–73.6 months). The reasons for enucleation were tumor recurrence in 1 patient, NVG in 3 patients, and blind painful eye in 2 patients. There were 209 patients (94.1%) with no local ocular complications.
Mortality
The Kaplan-Meier all-cause mortality rate was 5.8% at 5 years (95% CI: 3.3–9.9) and 18.4% at 10 years (95% CI: 13.6–24.6) after PBI. The melanoma-related mortality rate was 0.5% at 5 years (95% CI: 0.1–3.6) and 4.1% at 10 years (95% CI: 2.0–8.4) after PBI.
Discussion
In this historical group of patients with small choroidal melanoma who met inclusion criteria for investigational AU-011 therapy, we found that there was initial visual acuity retention following PBI. However, the proportion of subjects maintaining good acuity declined over years 2–5 following radiation therapy, particularly those with tumors located in close proximity to the critical structures for vision.
Patients with tumors located near the optic nerve or fovea are known to have a higher rate of vision loss after PBI [7, 12]. Vascular risk factors such as diabetes may also increase the risk of radiation retinopathy [12, 13]. Small choroidal melanomas have a high frequency of being located near the optic nerve or fovea, as seen in the Collaborative Ocular Melanoma Study [14]. We found that about 75% of small melanomas treated at our institution were located within 3 mm of either or both structures.
In this series of patients with small choroidal melanoma no more than 2.5 mm in thickness and 10 mm in LBD located near the optic disc and/or fovea, over half had visual acuity worse than 20/80 at 5 years after PBI. Similarly, high rates of vision loss after plaque brachytherapy have also been reported. Among patients with small choroidal melanoma no more than 3 mm in thickness, with any location, about 40% had visual acuity worse than 20/200 at 5 years after plaque treatment [15]. The all-cause and melanoma-associated mortality rates in our series were also similar to those reported previously for small choroidal melanoma [14, 15].
A recent paper examining lower-dose PBI for small-medium choroidal melanoma near the optic nerve and/or fovea found similar tumor control rates for those treated with 50 Gy (RBE) rather than the standard 70 Gy (RBE) dose [11]. Visual acuity of at least 20/200 was more likely to be retained in the 50 Gy (RBE) group in the first 5 years after treatment, but the acuity difference between the groups narrowed after 10 years of follow-up.
Alternatives to radiotherapy for choroidal melanoma have been described. Laser photocoagulation and transpupillary thermotherapy aimed to achieve local tumor control while minimizing damage to the optic nerve and fovea; however, they were found to have significant complications as well as higher rates of tumor recurrence compared to radiation therapy [16–18].
Investigational AU-011 therapy offers a potentially vision-sparing treatment for choroidal melanoma by specifically targeting cancer cells and avoiding damage to surrounding tissues. It consists of a recombinant virus-like particle conjugated with a photosensitizer. The drug binds to HSPG expressed on the surface of tumor cells and is activated by ophthalmic laser at 689 nm, causing cellular necrosis. Initial results from in vitro and animal models have been promising [8]. A phase 3 trial is currently planned. Because the mechanism of AU-011 requires tissue penetration by the drug and laser, it is more likely to be effective for thin choroidal lesions with a relatively small basal diameter.
This study demonstrates that patients with small choroidal melanomas located near the disc or fovea might be the most appropriate candidates for AU-011 therapy, as they are more likely to experience the benefit of a relatively vision-sparing treatment. In addition, some tumors located further from the disc and fovea may be too peripheral to treat using the slit lamp laser that is necessary to activate the drug.
Limitations of this study include its retrospective nature, non-standardized visual acuity measurement, and variations in management of radiation retinopathy. The best obtained visual acuity at each clinic visit was recorded, including the use of pinhole when applicable, but manifest refraction was not performed. The presence of visually significant cataract was not specifically accounted for but may be a source of reversible vision loss in some eyes. The use of intravitreal injections for prophylaxis or treatment of radiation retinopathy was left up to the discretion of the treating physician; however, the relatively small percentage (7.7%) of patients receiving injections is unlikely to have made a significant difference in the overall visual outcomes following PBI.
In AU-011 eligible patients with small choroidal melanoma treated with PBI, visual acuity deteriorated most significantly in those with tumors located near the optic disc and/or fovea over the first 5 years after radiotherapy. Excellent rates of local tumor control and a low rate of melanoma-related mortality were seen. The data presented here will serve as an important reference for physicians and patients considering treatment alternatives for small choroidal melanoma.
Acknowledgments
The authors would like to thank Ashley Go and Caleb Hartley for their assistance.
Statement of Ethics
This study was performed in line with the principles of the Declaration of Helsinki. The study was deemed exempt from requiring written informed consent by the Massachusetts General Brigham Institutional Review Board, approval number 2019P000606.
Conflict of Interest Statement
Dr. Kim receives research support as a clinical trial investigator for Aura Biosciences. Dr. Gragoudas is a consultant for Aura Biosciences.
Funding Sources
The authors did not receive support from any organization for the submitted work.
Author Contributions
I.K.K. and E.S.G. participated in the conception and design of the study. A.M.L. and F.W. performed data acquisition and analysis. A.T. and H.A.S. contributed to analysis. F.W. prepared the manuscript. All authors contributed to editing the manuscript.
Funding Statement
The authors did not receive support from any organization for the submitted work.
Data Availability Statement
The data that support the findings of this study are not publicly available as they contain information that could compromise the privacy of research participants. They are available from FW (frances_wu@meei.harvard.edu) upon reasonable request.
References
- 1. Gragoudas ES, Seddon JM, Egan K, Glynn R, Munzenrider J, Austin-Seymour M, et al. Long-term results of proton beam irradiated uveal melanomas. Ophthalmology. 1987 Apr;94(4):349–53. [DOI] [PubMed] [Google Scholar]
- 2. Reichstein DA, Brock AL. Radiation therapy for uveal melanoma: a review of treatment methods available in 2021. Curr Opin Ophthalmol. 2021 May 1;32(3):183–90. [DOI] [PubMed] [Google Scholar]
- 3. Jampol LM, Moy CS, Murray TG, Reynolds SM, Albert DM, Schachat AP, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report No. 19. Ophthalmology. 2020 Apr;127(4S):S148–57. [DOI] [PubMed] [Google Scholar]
- 4. Aziz HA, Singh N, Bena J, Wilkinson A, Singh AD. Vision loss following episcleral brachytherapy for uveal melanoma: development of a vision prognostication tool. JAMA Ophthalmol. 2016 Jun 1;134(6):615–20. [DOI] [PubMed] [Google Scholar]
- 5. Finger PT, Chin KJ, Yu GP; Palladium-103 for Choroidal Melanoma Study Group . Risk factors for radiation maculopathy after ophthalmic plaque radiation for choroidal melanoma. Am J Ophthalmol. 2010 Apr;149(4):608–15. [DOI] [PubMed] [Google Scholar]
- 6. Seddon JM, Gragoudas ES, Polivogianis L, Hsieh CC, Egan KM, Goitein M, et al. Visual outcome after proton beam irradiation of uveal melanoma. Ophthalmology. 1986 May;93(5):666–74. [DOI] [PubMed] [Google Scholar]
- 7. Seddon JM, Gragoudas ES, Egan KM, Glynn RJ, Munzenrider JE, Austin-Seymour M, et al. Uveal melanomas near the optic disc or fovea. Visual results after proton beam irradiation. Ophthalmology. 1987 Apr;94(4):354–61. [DOI] [PubMed] [Google Scholar]
- 8. Kines RC, Varsavsky I, Choudhary S, Bhattacharya D, Spring S, McLaughlin R, et al. An infrared dye-conjugated virus-like particle for the treatment of primary uveal melanoma. Mol Cancer Ther. 2018 Feb;17(2):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim IK; On Behalf of the AU-011 Investigator Group . A phase 2 trial of belzupacap sarotalocan (AU-011) A first-in-class targeted therapy for choroidal melanoma via suprachoroidal administration. Aura Biosciences. Investor Day; 2022 Oct 3. https://ir.aurabiosciences.com/static-files/c2ab5de2-53b2-469e-be4b-557abf1cac68. [Google Scholar]
- 10. Goitein M, Miller T. Planning proton therapy of the eye. Med Phys. 1983 Jun;10(3):275–83. [DOI] [PubMed] [Google Scholar]
- 11. Lane AM, Oxenreiter MM, Hashmi M, Aronow ME, Trofimov AV, Shih HA, et al. A comparison of treatment outcomes after standard dose (70 Gy) versus reduced dose (50 Gy) proton radiation in patients with uveal melanoma. Ophthalmol Retina. 2022 May 16;6(11):1089–97. [DOI] [PubMed] [Google Scholar]
- 12. Gragoudas ES, Li W, Lane AM, Munzenrider J, Egan KM. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology. 1999 Aug;106(8):1571–7; discussion 1577-1578. [DOI] [PubMed] [Google Scholar]
- 13. Melia BM, Abramson DH, Albert DM, Boldt HC, Earle JD, Hanson WF, et al. Collaborative Ocular Melanoma Study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology. 2001 Feb;108(2):348–66. [DOI] [PubMed] [Google Scholar]
- 14. Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997 Jul;115(7):886–93. [PubMed] [Google Scholar]
- 15. Shields CL, Sioufi K, Srinivasan A, Di Nicola M, Masoomian B, Barna LE, et al. Visual outcome and millimeter incremental risk of metastasis in 1780 patients with small choroidal melanoma managed by plaque radiotherapy. JAMA Ophthalmol. 2018 Dec 1;136(12):1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aaberg TM, Bergstrom CS, Hickner ZJ, Lynn MJ. Long-term results of primary transpupillary thermal therapy for the treatment of choroidal malignant melanoma. Br J Ophthalmol. 2008 Jun;92(6):741–6. [DOI] [PubMed] [Google Scholar]
- 17. Shields JA, Glazer LC, Mieler WF, Shields CL, Gottlieb MS. Comparison of xenon arc and argon laser photocoagulation in the treatment of choroidal melanomas. Am J Ophthalmol. 1990 Jun 15;109(6):647–55. [DOI] [PubMed] [Google Scholar]
- 18. Win PH, Robertson DM, Buettner H, McCannel CA, Bennett SR. Extended follow-up of small melanocytic choroidal tumors treated with transpupillary thermotherapy. Arch Ophthalmol. 2006 Apr;124(4):503–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available as they contain information that could compromise the privacy of research participants. They are available from FW (frances_wu@meei.harvard.edu) upon reasonable request.