Abstract
Introduction
The clinicopathological features of segmental membranous glomerulopathy (SMGN) have not been well characterized. The aim of this study was to investigate the prevalence and clinicopathological features of SMGN in adults.
Methods
Adult patients with biopsy-confirmed SMGN in the native kidney at our center between January 2017 to September 2020 were identified. The clinicopathological features of SMGN were collected. The glomerular deposition of IgG subclasses, M-type phospholipase A2 receptor 1 (PLA2R), thrombospondin type 1 domain-containing 7A (THSD7A), and neural epidermal growth factor-like 1 protein (NELL1) were tested. Clinical and pathologic features were comparable between NELL1-positive and NELL1-negative SMGN.
Results
A total of 167 patients with biopsy-proven SMGN were enrolled. During the same period, 32,640 (33.0%) out of 98,939 renal biopsies were diagnosed with membranous nephropathy (MN) in adults. SMGN accounted for 0.17% of total kidney biopsies and 0.51% of MN in adults. One hundred and fifty (89.8%) cases were isolated SMGN, and 17 (10.2%) cases were complicated with other kidney disease. Clinically, the median age of isolated SMGN patients was 41.5 years, with female (74%) predominance, and 33.1% had full nephrotic syndrome. Pathologically, IgG1 was the dominant subclass (92.5%), followed by IgG4 (45.0%). PLA2R and THSD7A staining were done in 142 and 136 isolated SMGN cases, respectively, in which, all the cases showed negative. NELL1 staining was done in 135 isolated SMGN cases; 58 cases (43.0%) showed positive. Fifty-eight patients (41.1%) had diffuse (≥90%) foot process effacement, and 119 patients (83.8%) had either stage I (38.0%) or stage II (45.8%) membranous alterations in patients with SMGN. Most patients with NELL1-positive SMGN were female. Patients with NELL1-positive SMGN were more likely with lower prevalence of full nephrotic syndrome than NELL1-negative SMGN.
Conclusions
SMGN is a relatively rare pathological type. Majority of patients with isolated SMGN were female, with a median age of 41.5 years, 33.1% had full nephrotic syndrome, absence of PLA2R and THSD7A, 43.0% with NELL1-positive, and mainly stage I or II MN (83.8%). NELL1 is the major target antigen of SMGN in adults.
Keywords: Clinicopathological features, Segmental membranous glomerulopathy, M-type phospholipase A2 receptor 1, Thrombospondin type 1 domain-containing 7A, Neural epidermal growth factor-like 1 protein
Introduction
Membranous nephropathy (MN), a common cause of the nephrotic syndrome in adults, is characterized by the accumulation of immune deposits along the glomerular capillary loops [1, 2]. Epidemiological data showed a remarkable rise of MN, and it has become the most common type of glomerulonephritis in patients aged 65 and above [3, 4]. The reported proportion of MN in primary glomerulonephritis range from 13% to 43% [4–7].
Segmental membranous glomerulopathy (SMGN), a variant of MN, is characterized by segmental staining of IgG in immunofluorescence (IF), segmental distribution of subepithelial deposits under light microscopy (LM), and electron microscopy (EM) [8–10]. Since the first report of the disease [11], so far only 200 cases have been reported [8–13]. Currently, there are few studies on the prevalence and clinicopathological characteristics of SMGN. M-type phospholipase A2 receptor 1 (PLA2R) and thrombospondin type 1 domain-containing 7A (THSD7A) antigen were the target antigens accounting for 70–80% and 1–5% of MN patients [14–17], respectively. Sethi et al. [18] recently detected neural epidermal growth factor-like 1 protein (NELL1) in the patients with PLA2R-negative MN. NELL1 is a cytoplasmic protein with a molecular weight of 90-kDa, which contains several highly conserved structural motifs including a secretory signal peptide, an N-terminal TSP-1-like (TSPN), a coiled-coil domain, 4 von Willebrand factor type C domains, and 6 epidermal growth factor-like domains [18]. PLA2R, THSD7A, and NELL1 were the target antigens of MN, and their expression in SMGN has been rarely studied. Therefore, in this report, we aimed to identify the clinicopathological characteristics of SMGN and analyze the presence of PLA2R, THSD7A, and NELL1 in SMGN patients.
Materials and Methods
Participants
In this study, SMGN was defined as subepithelial deposits less than 75% of the glomerular tuft [8] by LM, IF, and EM. SMGN secondary to hepatitis B virus, systemic lupus erythematosus, and Sjögren’s syndrome were excluded. Adult patients with biopsy-confirmed SMGN in the native kidney in Renal Pathology of King Medical Diagnostics Center between January 2017 to September 2020 were identified. The research was in compliance with the Declaration of Helsinki and approved by the Ethics Committee of King Medical Diagnostics Center, Guangzhou, China.
The diagnostic criteria for HBV-associated MN should meet the following conditions: (1) detection of the serologic manifestations of HBV infection and replicative virus in the blood; (2) detection of HBV-related protein antigens (HBsAg, HBeAg, and/or HBcAg) in the glomerular immune deposits; (3) excluding other secondary MN such as lupus nephritis [19, 20].
The diagnosis of Sjögren’s syndrome was based on the revised version of the diagnostic criteria of American-European Consensus Group [21]. Patients who meet the diagnostic criteria for Sjögren’s syndrome and were confirmed by renal biopsy as MN should be considered as MN associated with Sjögren’s syndrome.
Histopathologic Evaluation
The biopsy specimens were routinely analyzed by LM, IF, and EM. The number of glomeruli, glomerulosclerosis, segmental glomerulosclerosis, endocapillary hypercellularity, crescent formation, interstitial fibrosis and/or tubular atrophy (IF/TA) ≥25% was collected. Frozen sections were cut into 3–4 μm and then used for direct IF staining by using fluorescein isothiocyanate (FITC)-labeled antibodies to human IgG, IgA, IgM, C3, C1q (DAKO, Denmark) and FITC-labeled antibodies to human IgG1, IgG2, IgG3, IgG4 (Gene, China). Indirect IF was used to detect PLA2R (Sigma, USA), THSD7A (Sigma, USA), NELL1 (Sigma, USA), light chain kappa (DAKO, Denmark), and lambda (DAKO, Denmark). For patients with positive serum HBsAg, HBV biomarkers including HBsAg, HBcAg, and HBeAg (Gene, China) were regularly detected in the kidney by indirect IF. Semi-quantitative scoring of IF staining intensity from 0 to 3+ (0 negative, 1+ weak staining, 2+ moderate staining, 3+ strong staining). For EM examination of thin sections, a JEM-1400 PLUS electron microscope (Jeol, Tokyo, Japan) was used. Ehrenreich and Churg classification was used for the ultrastructural staging of MN [22].
Detection of Glomerular PLA2R, THSD7A, and NELL1 Expression By Indirect IF
Frozen kidney tissues were cut into 3–4 μm thick slices and fixed with acetone. Rabbit anti-human PLA2R antibody (Sigma, HPA012657), mouse anti-human THSD7A antibody (Sigma, AMAB91233), and rabbit anti-human NELL1 antibody (Sigma,HPA051535) were used as primary antibodies. FITC-labeled swine anti-rabbit immunoglobulins (Dako, F0205), FITC-labeled rabbit anti-mouse immunoglobulins (Dako, F0261), and FITC-labeled swine anti-rabbit immunoglobulins (Dako, F0205) were used as secondary antibodies, respectively. Paraffin sections were roasted, dewaxed, and digested with protease K. The subsequent steps for the paraffin sections were the same as for the frozen samples. Kidney biopsies from other patients such as minimal change disease and IgA nephropathy were also stained as negative controls.
Clinical and Laboratory Data
The data on age, gender, personal health history (hypertension, diabetes, tuberculosis, thyroid disease), edema, blood urea nitrogen (BUN), serum creatinine, hemoglobin, serum albumin, serum IgA, serum IgG, serum IgM, serum C3, microscopic hematuria (RBC >5/HP), 24-h urinary protein, and secondary etiology (autoantibodies, tumors, HBsAg, and medication usage) were obtained. Nephrotic range proteinuria was defined as proteinuria ≥3.5 g/d. Nephrotic syndrome was defined as nephrotic range proteinuria and hypoalbuminemia (<30 g/L). The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation [23]. Follow-up data included treatment, renal function, albumin, and urinalyses. Complete remission was defined as urine protein <0.3 g/d (uPCR <300 mg/g). Partial remission was defined as urine protein >0.3 but <3.5 g/d or at least 50% reduction at biopsy and <3.5 g/d, along with improvement in serum albumin and stabilization of serum creatinine [19, 24].
Statistical Analysis
All statistics were performed using SPSS (version 26.0). The measurement data were shown as mean ± standard deviation if they had a normal distribution. Median and interquartile range (25th to 75th percentiles) were used to show continuous variables having a skewed distribution. Student’s t test or Mann-Whitney U test was used for continuous variables, and the χ2 test was used for categorical variables. Two-tailed p values <0.05 were considered statistically significant.
Results
From January 2017 to September 2020, 32,640 (33.0%) out of 98,939 renal biopsies were diagnosed with MN in adults at our center. Among them, a total of 167 adult patients were identified as SMGN, from 127 hospitals across China. SMGN accounted for 0.17% of total kidney biopsies and 0.51% of MN in adults. One hundred and fifty (89.8%) of 167 patients with SMGN cases were isolated SMGN and 17 (10.2%) cases were complicated with other kidney disease, including 5 with hypertensive renal injury, 3 with IgA nephropathy, 3 with ANCA-related vasculitis, 3 with diabetic nephropathy, 1 with hyperuricemia nephropathy, 1 with ischemic kidney injury, and 1 with podocyte infolding glomerulopathy.
Baseline Characteristics of Patients with SMGN and Isolated SMGN
The median age of SMGN patients was 44 years, 31.7% were male, 17.1% had hypertension, 7.5% had diabetes, 45.6% had microscopic hematuria, 34.0% had full nephrotic syndrome, 74.2% had edema, 24.0% were with positive ANA, and 6.2% were with positive HBsAg.
As shown in Table 1, the median age of isolated SMGN patients was 41.5 years, and 26.0% were male. The median 24-h urine protein was 3.3 g/d, 48.2% had nephrotic range proteinuria, 58.0% had hypoalbuminemia, 44.0% had microscopic hematuria, and 33.1% had full nephrotic syndrome. Median serum creatinine and eGFR were 61.0 μmol/L and 107.1 mL/min/1.73 m2, respectively. Furthermore, 12.0% of them had eGFR less than 60 mL/min/1.73 m2. Serologic evaluation demonstrated positive ANA in 22.9% patients. HBsAg serologies were positive in 5.4% patients. Of the 150 patients with isolated SMGN, 101 did not have an identifiable secondary etiology of MN, 27 had abnormal autoimmune findings, but the patients did not satisfy the criteria for diagnosis of lupus and Sjögren’s syndrome (such as positive antinuclear antibody, anti-double stranded DNA, anti-SM, anti-SSA, 1 had undifferentiated connective tissue disease, 1 had rheumatoid arthritis, and 1 had ankylosing spondylitis), 7 had a history of HBV without deposition of HBV antigen in renal tissue, 5 had a history of tuberculosis, 9 had a history of hypothyroidism, 2 had plasma cell neoplasm, and one patient each had a history of esophageal cancer, colorectal carcinoma, thymic tumor, syphilis, and NSAID usage.
Table 1.
Clinical features of patients with isolated SMGN
| SMGN (N = 150) | |
|---|---|
| Age, years | 41.5 (32.8–51.3) |
| Male, n (%) | 39/150 (26.0) |
| BUN, mmol/L | 4.1 (3.3–6.0) |
| Serum creatinine, μmol/L | 61.0 (50.0–77.1) |
| Serum albumin, g/L | 26.0 (19.5–36.3) |
| eGFR, mL/min/1.73 m2 | 107.1 (87.3–120) |
| Hemoglobin, g/L | 135.0±20.8 |
| Serum IgA, g/L | 2.2 (1.6–2.8) |
| Serum IgG, g/L | 7.2 (5.1–10.7) |
| Serum IgM, g/L | 1.4 (0.9–2.0) |
| Serum C3, g/L | 1.2±0.3 |
| Urine protein, g/day | 3.3 (1.6–5.9) |
| No. of patients showing microscopic hematuria, % | 62/141 (44.0) |
| No. of patients showing full nephrotic syndrome, % | 42/127 (33.1) |
| No. of patients showing nephrotic range proteinuria, % | 54/112 (48.2) |
| No. of patients showing hypoalbuminemia, % | 83/143 (58.0) |
| Positive ANA, % | 25/109 (22.9) |
| Positive HBsAg, % | 7/130 (5.4) |
| No. of patients showing edema, % | 103/139 (74.1) |
| No. of patients with eGFR<60 mL/min/1.73 m2, % | 17/142 (12.0) |
LM Results in Patients with Isolated SMGN
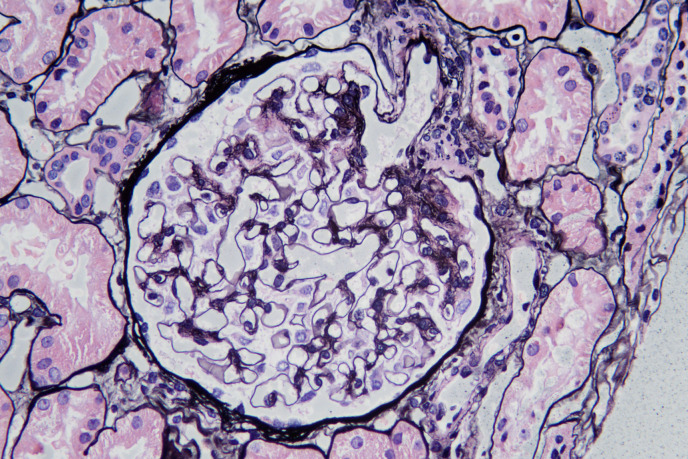
The GBMs of isolated SMGN had segmental “spikes” in 64 of 150 patients (shown in Fig. 1), with mild glomerular mesangial proliferation, 1 (0.7%) exhibited endocapillary hypercellularity, 1 (0.7%) exhibited crescent formation, 4 (2.7%) were with IF/TA ≥25%, and 13 (8.7%) were with segmental glomerulosclerosis. The median number of glomeruli and glomerulosclerosis in SMGN were 18 (13–23) and 0 (0–1), respectively.
Fig. 1.
Segmental “spikes” can be seen in SMGN on Jones methenamine silver (×200).
IF Results in Patients with Isolated SMGN
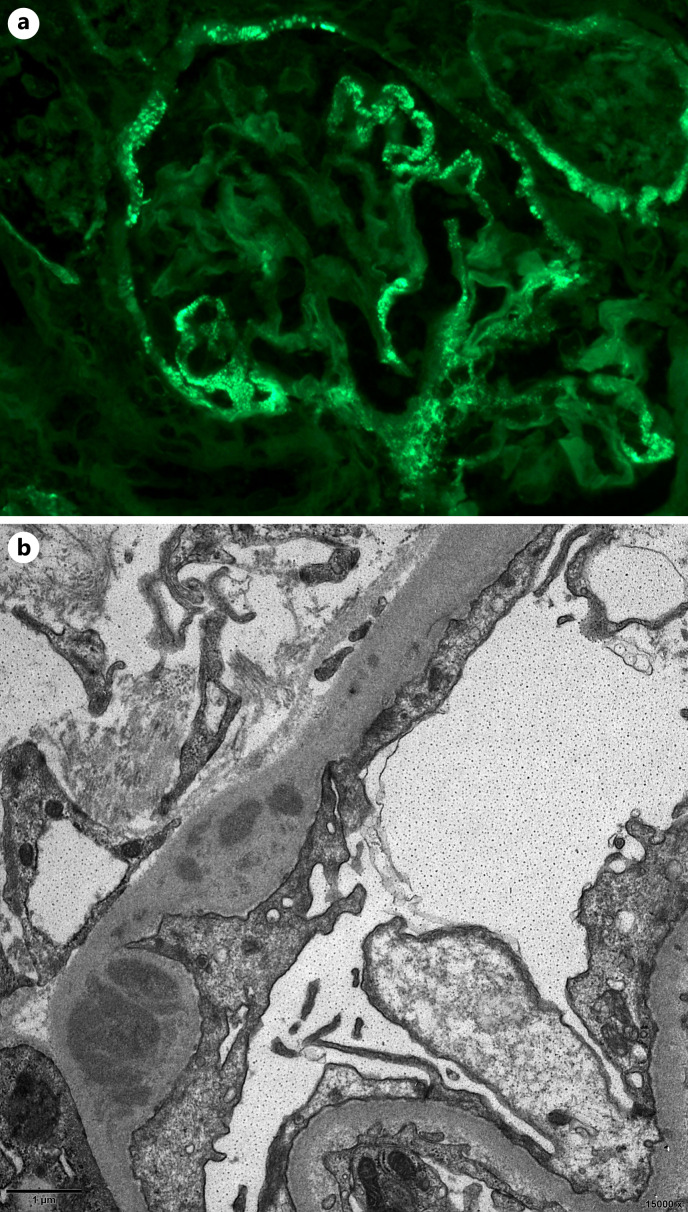
IF revealed IgG segmental deposition (shown in Fig. 2a) in 92.7% cases, frequently accompanied by IgM (69.3%). Fifteen patients (10.0%) showed segmental stain of IgG along Bowman capsule (shown in Fig. 3a). None of the cases had full-house IF and tubular basement membrane deposit. Light chain kappa and lambda were tested in 59 cases, and no light chain restriction was found.
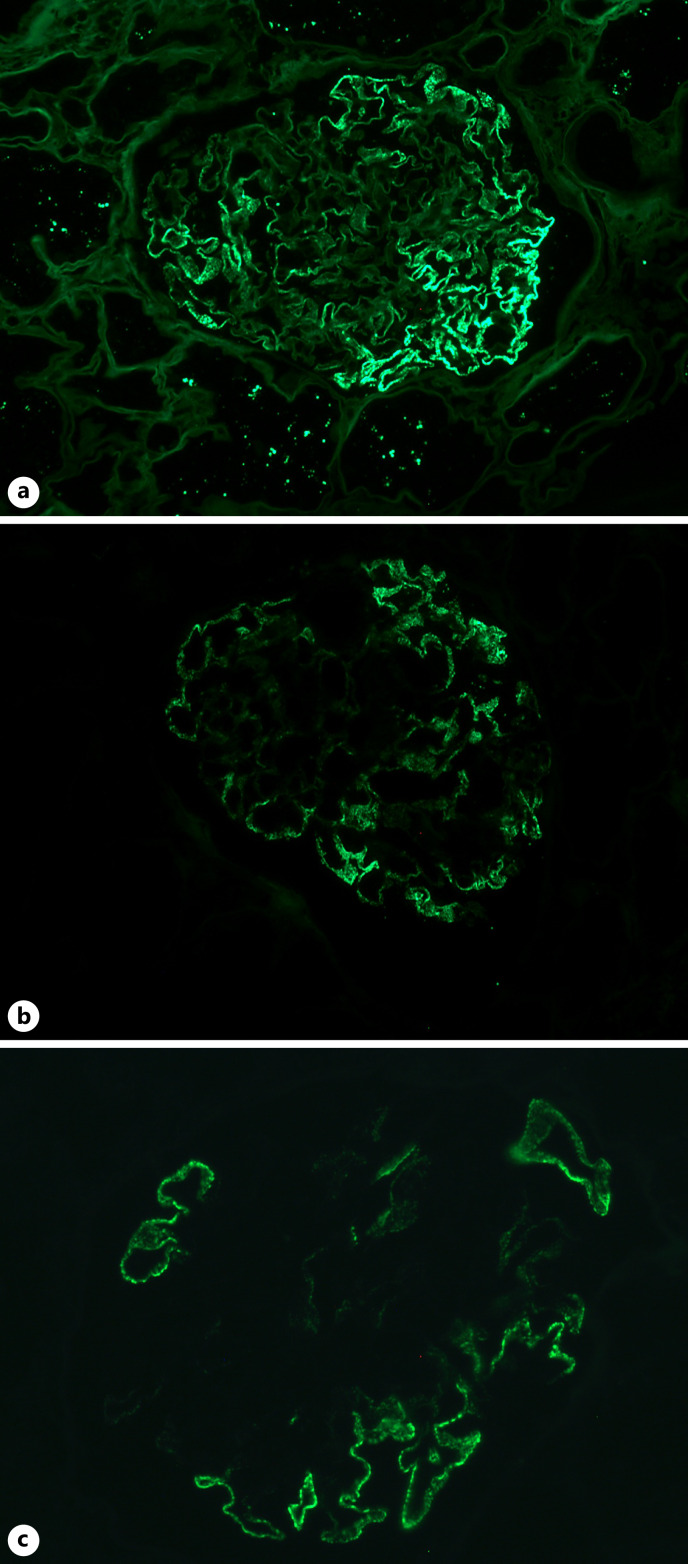
Fig. 2.
IF findings in SMGN. a Segmental granular staining of IgG (×200) along the capillary loops. b Segmental granular staining of IgG1 (×200) along the capillary loops. c Segmental granular staining of neural epidermal growth factor-like 1 protein (NELL1) (×400) along the capillary loops.
Fig. 3.
Deposits in glomerular Bowman capsule in SMGN patients. a Segmental deposition of IgG (×400) in glomerular Bowman capsule. b EDD can be seen in glomerular Bowman capsule by EM.
Forty cases had IgG subclass staining in isolated SMGN due to adequacy of samples. There were IgG1 staining in 37 cases (92.5%, shown in Fig. 2b), IgG4 in 18 biopsies (45.0%), IgG2 in 11 biopsies (27.5%), and IgG3 in 4 biopsies (10.0%).
PLA2R and THSD7A staining were done in 142 and 136 isolated SMGN cases, respectively, in which, all the cases showed negative. NELL1 staining was done in 135 isolated SMGN cases; 58 cases (43.0%) showed positive.
EM Results in Patients with Isolated SMGN
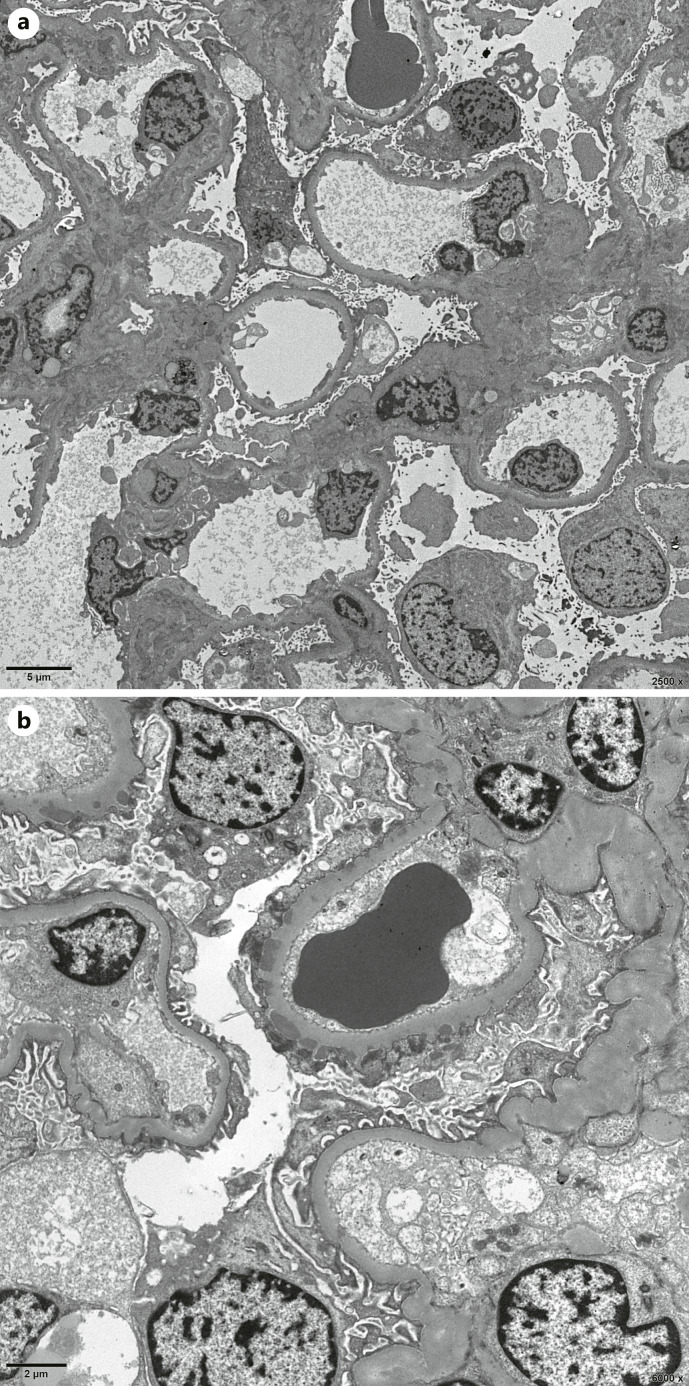
Ultrastructural examination was available for 142 (including 1 case was unable to distinguish foot processes) of 150 isolated SMGN cases. As shown in Table 2, 58 patients (41.1%) had diffuse (≥90%) foot process effacement (shown in Fig. 4a), and 119 patients (83.8%) had either stage I (38.0%) or stage II (45.8%, shown in Fig. 4b) membranous alterations in patients with SMGN. Seventeen patients (12.0%) with SMGN exhibited mesangial electron dense deposits (EDD), and 2 patients (1.4%) exhibited subendothelial EDD. None of the biopsies had endothelial tubuloreticular inclusions and substructure in the deposits.
Table 2.
Pathologic features of patients with isolated SMGN
| SMGN (N = 150) | |
|---|---|
| Immunofluorescence | |
| IgG positivity, % | 139/150 (92.7) |
| IgA positivity, % | 6/150 (4.0) |
| IgM positivity, % | 104/150 (69.3) |
| C3 positivity, % | 25/150 (16.7) |
| C1q positivity, % | 2/150 (1.3) |
| Full-house IF, % | 0/150 (0.0) |
| IgG Bowman capsule deposits, % | 15/150 (10.0) |
| PLA2R, % | 0/142 (0.0) |
| THSD7A, % | 0/136 (0.0) |
| NELL1, % | 58/135 (43.0) |
| IgG subclass (N = 40) | |
| IgG1, % | 37/40 (92.5) |
| IgG2, % | 11/40 (27.5) |
| IgG3, % | 4/40 (10.0) |
| IgG4, % | 18/40 (45.0) |
| Electron microscopy (N = 142) | |
| No. diffuse (≥90%) foot process effacement, % | 58/141 (41.1) |
| Stage of MN | |
| Stage I, % | 54/142 (38.0) |
| Stage II, % | 65/142 (45.8) |
| Stage III, % | 20/142 (14.1) |
| Stage IV, % | 3/142 (2.1) |
| Mesangial EDD, % | 17/142 (12.0) |
| Subendothelial EDD, % | 2/142 (1.4) |
Fig. 4.
EM findings. a Electron micrograph reveals extensive effacement of the podocyte foot processes. b Normal capillary loop and capillary loop with segmental subepithelial EDD.
Clinical and Pathologic Characteristics of SMGN Patients with and without NELL1 Expression in Glomerular Immune Deposits
As shown in Table 3, among 58 patients with NELL1-positive SMGN, 13 men and 45 women (women-men ratio of 3.5), with a median age of 40 years, were analyzed. Median 24-h urine protein was 2.9 g/d, and 17.6% had full nephrotic syndrome. Serologic evaluation demonstrated positive ANA in 5 of 49 patients (10.2%) and low C3 in 2 of 39 patients (5.1%). Five patients had a history of tuberculosis. One patient had esophageal cancer, 1 patient had multiple myeloma, while the remaining 56 patients had no evidence of malignancy. NELL1 staining showed segmental bright granular GBM staining in all 58 patients. Patients with NELL1-positive SMGN were more likely with a higher serum albumin, lower urine protein, lower prevalence of full nephrotic syndrome, lower prevalence of ANA than NELL1-negative SMGN (p < 0.05). While age, sex, BUN, serum creatinine, eGFR, and the incidence of cancer did not differ between the two groups (p > 0.05).
Table 3.
Clinical characteristics of isolated SMGN patients with and without NELL1 expression in glomerular immune deposits
| NELL1-positive | NELL1-negative | p value | |
|---|---|---|---|
| Patients, n | 58 | 77 | |
| Age, years | 40.0 (32.8–50.3) | 44.0 (33.0–52.0) | 0.574 |
| Male, n (%) | 13/58 (22.4) | 24/77 (31.2) | 0.259 |
| BUN, mmol/L | 4.0 (3.2–5.4) | 4.4 (3.5–6.2) | 0.220 |
| Serum creatinine, μmol/L | 56.0 (47.9–71.4) | 62.5 (53.3–79.5) | 0.068 |
| Serum albumin, g/L | 32.4 (24.1–36.7) | 22.5 (17.9–35.6) | 0.004 |
| eGFR, mL/min/1.73 m2 | 110.5 (97.9–122.7) | 105.4 (86.1–117.7) | 0.161 |
| Urine protein, g/day | 2.9 (1.4–4.4) | 3.9 (2.6–7.5) | 0.003 |
| No. of patients showing microscopic hematuria, % | 23/56 (41.1) | 30/71 (42.3) | 0.893 |
| No. of patients showing full nephrotic syndrome, % | 9/51 (17.6) | 30/63 (47.6) | 0.001 |
| No. of patients showing nephrotic range proteinuria, % | 16/46 (34.8) | 34/55 (61.8) | 0.007 |
| No. of patients showing hypoalbuminemia, % | 23/55 (41.8) | 52/74 (70.3) | 0.001 |
| Positive ANA (%) | 5/49 (10.2) | 15/53 (28.3) | 0.021 |
| Positive HBsAg (%) | 3/51 (5.9) | 3/68 (4.4) | 1.000 |
| No. of patients showing edema, % | 37/52 (71.2) | 56/73 (76.7) | 0.483 |
| No. of patients with eGFR<60 mL/min/1.73 m2, % | 9/56 (16.1) | 6/72 (8.3) | 0.177 |
| Cancer, % | 2/58 (3.4) | 2/77 (2.6) | 1.000 |
Biopsies from NELL1-positive SMGN patients showed no significant difference in IgG positivity (98.3 vs. 89.6%, p = 0.077), IgA positivity (0.0 vs. 5.2%, p = 0.212), IgM positivity (65.5 vs. 71.4%, p = 0.463), C3 positivity (22.4 vs. 13.0%, p = 0.149), C1q positivity (0.0 vs. 1.3%, p = 1.000), compared to NELL1-negative SMGN. Twenty cases had IgG subclass staining in NELL1-positive SMGN due to adequacy of samples. There were IgG1 staining in 19 cases (95.0%), IgG4 in 12 biopsies (60.0%), IgG2 in 7 biopsies (35.0%), and IgG3 in 2 biopsies (10.0%).
As shown in Table 4, ultrastructural examination was available for 54 of 58 NELL1-positive SMGN and 75 of 77 NELL1-negative SMGN. Eleven of NELL1-positive SMGN cases showed diffuse (≥90%) foot process effacement, 4 exhibited mesangial EDD, and none of the biopsies had subendothelial EDD. Patients with NELL1-positive SMGN were more likely with a lower prevalence of diffuse (≥90%) foot process effacement. There was a significant difference in the composition ratio of stage II and III MN between NELL1-positive and negative patients (p < 0.05).
Table 4.
Pathologic features in isolated SMGN patients with and without NELL1 expression in glomerular immune deposits
| NELL1-positive | NELL1-negative | p value | |
|---|---|---|---|
| Immunofluorescence | |||
| IgG positivity, % | 57/58 (98.3) | 69/77 (89.6) | 0.077 |
| IgA positivity, % | 0/58 (0.0) | 4/77 (5.2) | 0.212 |
| IgM positivity, % | 38/58 (65.5) | 55/77 (71.4) | 0.463 |
| C3 positivity, % | 13/58 (22.4) | 10/77 (13.0) | 0.149 |
| C1q positivity, % | 0/58 (0.0) | 1/77 (1.3) | 1.000 |
| IgG Bowman capsule deposits, % | 5/58 (8.6) | 7/77 (9.1) | 0.924 |
| IgG subclass | |||
| IgG1, % | 19/20 (95.0) | 16/17 (94.1) | 1.000 |
| IgG2, % | 7/20 (35.0) | 4/17 (23.5) | 0.447 |
| IgG3, % | 2/20 (10.0) | 0/17 (0.0) | 0.489 |
| IgG4, % | 12/20 (60.0) | 12/17 (70.6) | 0.501 |
| Electron microscopy | |||
| No. diffuse (≥90%) foot process effacement, % | 11/53 (20.8) | 41/75 (54.7) | <0.001 |
| Stage of MN | |||
| Stage I, % | 20/54 (37.0) | 31/75 (41.3) | 0.622 |
| Stage II, % | 30/54 (55.6) | 26/75 (34.7) | 0.018 |
| Stage III, % | 2/54 (3.7) | 18/75 (24.0) | 0.002 |
| Stage IV, % | 2/54 (3.7) | 0/75 (0.0) | 0.173 |
| Mesangial EDD, % | 4/54 (7.4) | 11/75 (14.7) | 0.204 |
| Subendothelial EDD, % | 0/54 (0.0) | 2/74 (2.7) | 0.508 |
Follow-Up
Follow-up data were obtained for 35 of 150 patients with isolated SMGN. Of the 35 patients, 20 received immunosuppression (6 with glucocorticoids and calcineurin inhibitors, 6 with glucocorticoids only, 3 with glucocorticoids and cyclophosphamide, 2 with glucocorticoids and Tripterygium wilfordii, 1 with glucocorticoids and hydroxychloroquine, 1 with Tripterygium wilfordii only, and 1 with calcineurin inhibitors only), 13 received non-immunosuppressive therapy (12 received renin angiotensin aldosterone system inhibition, and 1 received traditional Chinese medicine), 1 died of tumor, and 1 underwent maintenance hemodialysis. Among the 33 patients, the mean follow-up was 12.1 ± 10.5 months. The median proteinuria at follow-up was 0.28 (0.08–1.76) g/d. The average eGFR at follow-up was 107.8 ± 15.2 mL/min/1.73 m2, and serum albumin was 40.6 ± 7.5 g/L. Among the 35 patients mentioned above, 31 underwent NELL1 staining, of which 14 were NELL1-positive and 17 were NELL1-negative (including 1 died of tumor). At the end of the follow-up period, 7 patients (50.0%) had complete remission of proteinuria, 3 (21.4%) had partial remission, 4 (28.6%) had no remission in patients with NELL1-positive. One patient with NELL1-positive was diagnosed with ovarian cancer. Ten patients (62.5%) had complete remission of proteinuria, 3 (18.8%) had partial remission, 3 (18.8%) had no remission in patients with NELL1-negative. There were no significant differences observed in the treatment response between two groups (p > 0.05).
Discussion
To the best of our knowledge, our current study is the first and the largest multicenter cohort of SMGN. We found that SMGN is a relatively rare pathological type, accounting for 0.17% of total kidney biopsies and 0.51% of MN in adults. The frequency of SMGN in patients with MN is unclear and varies greatly in the reported literature. It represents 2.5% of MN in adults [8], however, up to 29% in children [10]. The data in the above articles were all higher than ours. The demographics and diagnostic criteria may affect the frequency of SMGN. There are no definite diagnostic criteria for SMGN. Obana et al. [10] defined SMGN as partial glomerular tuft involvement but did not give the percentage of glomerular clusters. Segawa et al. [25] identified SMGN as lesions comprising <50% of glomerular clusters. Kudose et al. [8] proposed a definition that requires the involvement of glomerular tuft at least 25% and no more than 75%. However, in our data, we have 28 cases (18.7%) with isolated SMGN less than 25% of the glomerular tuft by IF or EM. These cases do not meet the diagnostic criteria of minimal change disease. Thus, we included these patients in the study and defined SMGN as no more than 75% of the total glomerular capillaries.
So far, the clinicopathological features of SMGN have not been well characterized [8–10, 12, 13]. In pediatric patients, Obana et al. [10] found that SMGN had a higher frequency of C1q staining and mesangial EDD, but their clinical manifestations and staging of MN were similar to those of MN. Segawa et al. [25] showed that IgG1 and IgG3 were the dominant subclasses. In adult patients, Kudose et al. [8] showed that SMGN had an elderly age, about one-third had nephrotic syndrome, IgG1 predominance, and predominantly early-stage MN. In our current study, the median age of isolated SMGN patients was 41.5 years, with female predominance, 12.0% with mesangial EDD, and the majority of patients absent of C1q staining. Our data confirmed that IgG1 was the dominant subclass, followed by IgG4, and rarely accompanied IgG3. The above results suggest that the clinicopathological and pathogenesis of SMGN in childhood may be different from that in adults. In addition, we also found IgG deposition in the Bowman capsule in 10% of SMGN patients, which we currently cannot explain.
The pathogenesis of SMGN is still incompletely elucidated [8–10]. One hypothesis is that SMGN is an early stage of idiopathic MN [10]. Repeated renal biopsy was performed on one child in our center. Two years later, after the 1st biopsy, the patient showed persistent SMGN same as the first biopsy. Similar cases were also found in the report of Kudose et al. [8] and Obana et al. [10]. These repeat biopsy results contradict the above hypothesis. Another hypothesis is that SMGN may be a secondary MN [25]. In our study, some patients with SMGN had autoimmune abnormalities, tumors, HBV, and thyroid diseases, indicating that some SMGN patients may be secondary MN. Last but not least, the target antigens are involved in the pathogenesis of SMGN. In this study, PLA2R and THSD7A staining were both negative, which is consistent with Kudose et al. [8]. The reported proportion of NELL1 staining in SMGN was 29.4% [8]. In this study, 43.0% of isolated SMGN patients were NELL1-positive. The prevalence of NELL1-positive SMGN was higher in our study than previously reported. NELL1, rather than PLA2R or THSD7A, is the target antigen of SMGN in adults [8, 18]. Therefore, the remaining nearly 50% of the target antigens of SMGN have remained elusive. With the application of mass spectrometry [26, 27], we believe that novel antigens of SMGN will be discovered soon.
NELL1, as a newly recognized target antigen for MN, has not yet been fully recognized. In this study, the majority of NELL1-positive SMGN were female, with a median age of 40 years, unlike in the study by Sethi et al. [18]. We compared the clinicopathological features between NELL1-positive and negative SMGN, and the prevalence of full nephrotic syndrome of NELL1-positive SMGN was significantly lower than that of NELL1-negative SMGN. However, during follow-up, we found that there was no statistically significant difference in treatment response between the two groups. As this study was a multicenter retrospective study and treatment regimens were not uniform, some patients were using steroid monotherapy, which is currently not recommended by KDIGO guidelines [19]. The prevalence of malignancy in NELL1-positive MN is variable [28]. Caza et al. [29] reported that NELL1-associated MN was the most common type of MN with a malignancy association. However, studies from China and Japan with NELL1-associated MN have not identified tumors [24, 28, 30]. In our study, 2 of the 58 patients with NELL1-positive SMGN had tumors at the time of diagnosis. However, there was no statistically significant difference in the incidence of cancer between the NELL1-positive and negative groups. The relationship between NELL1-positive MN and tumors may be geographically influenced. In addition, some studies have shown that the NELL1-positive MN were associated with drugs, infections, autoimmune disease, and sarcoidosis [28, 30, 31]. Therefore, the role of NELL1 should be examined in further studies.
There were some limitations in this study. First, we did not detect anti-NELL1 antibodies in serum. Second, PLA2R, THSD7A, NELL1, and IgG subclass staining were not available in all cases. Third, follow-up was not available for all the patients, and the treatment regimens were not uniform. Finally, the clinical data of a few patients were incomplete.
Conclusion
SMGN is a relatively rare pathological type, accounting for 0.17% of total kidney biopsies and 0.51% of MN in adults. Majority of patients with isolated SMGN were female, with a median age of 41.5 years, 33.1% had full nephrotic syndrome, IgG1 subclass predominance, 43.0% with NELL1-positive, and mainly stage I or II MN (83.8%). NELL1 is the target antigen of SMGN in adults.
Acknowledgments
The authors thank 34 nephrologists for their contributions to this study, including Lixia Xu, Ming Li, Xueping Wu, Yang He, Shuangyan Bu, Xiaonan Qiu, Fan Zhang, Wen Zhao, Baojuan Jia, Zhiping Zhang, Tingting Feng, Haofei Hu, Zaiyou Dai, Shanshan Sun, Hutang Zhang, Hongxia Zheng, Cheng Li, Lin Ning, Huoguang Zhu, Runying Zhao, Li Xing, Min Liu, Ling Xu, Chao Xu, Min Liu, Fei Tan, Meilan Liu, Ying Liu, Li Zhang, Li Xu, Feng Du, Hongjun Wang, Qiankun Zhang, and Shaoyong Gong.
Statement of Ethics
This study protocol was reviewed and approved by the Ethics Committee of King Medical Diagnostics Center, Guangzhou, China (approval number: 2021029). The patient informed consent was waived in view of the retrospective nature of the study. This study was performed fulfilling the principles of the Helsinki Declaration.
Conflict of Interest Statement
No potential conflict of interest relevant to this article was reported.
Funding Sources
This work was supported by the National Science Fund for Distinguished Young Scholars (82025024).
Author Contributions
All authors have reviewed and contributed to this manuscript. Shuangshuang Zhu was involved in study design, diagnosis of SMGN, data collection, follow-up, statistical analysis, drafting and revising the manuscript. Xiaotao Hou and Bei Luo were involved in electron microscopy filmmaking and evaluation. Shuling Yue and Lin Wang were involved in the diagnosis of SMGN. Xiaoting Liu, Kongshan Li, and Qiming Liang were involved in performing LM and IF. Xiaomeng Xu and Zhen Song were involved in revising the manuscript. Zheya Zhou was involved in data collection. Lei Zheng and Wenfang Chen were involved in designing the study and revising the manuscript.
Funding Statement
This work was supported by the National Science Fund for Distinguished Young Scholars (82025024).
Data Availability Statement
Data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author.
References
- 1. Ronco P, Debiec H. Membranous nephropathy: current understanding of various causes in light of new target antigens. Curr Opin Nephrol Hypertens. 2021 May 1;30(3):287–93. 10.1097/MNH.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ravindran A, Madden B, Charlesworth MC, Sharma R, Sethi A, Debiec H, et al. Proteomic analysis of complement proteins in membranous nephropathy. Kidney Int Rep. 2020 May;5(5):618–26. 10.1016/j.ekir.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiong M, Wang L, Liu X, Yue S, Dong J, Li Y, et al. Kidney biopsies in elderly Chinese patients: a nationwide survey. Am J Kidney Dis. 2020 Aug;76(2):295–7. 10.1053/j.ajkd.2020.02.438. [DOI] [PubMed] [Google Scholar]
- 4. Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, et al. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol. 2016 Dec;27(12):3739–46. 10.1681/ASN.2016010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang L, Yao J, Kong X, Sun Q, Wang Z, Zhang Y, et al. Increasing prevalence of membranous nephropathy in patients with primary glomerular diseases: a cross-sectional study in China. Nephrology. 2017 Feb;22(2):168–73. 10.1111/nep.12739. [DOI] [PubMed] [Google Scholar]
- 6. Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, et al. Japan Renal Biopsy Registry: the first nationwide, web-based, and prospective registry system of renal biopsies in Japan. Clin Exp Nephrol. 2011 Aug;15(4):493–503. 10.1007/s10157-011-0430-4. [DOI] [PubMed] [Google Scholar]
- 7. Zhou FD, Zhao MH, Zou WZ, Liu G, Wang H. The changing spectrum of primary glomerular diseases within 15 years: a survey of 3,331 patients in a single Chinese centre. Nephrol Dial Transplant. 2009 Mar;24(3):870–6. 10.1093/ndt/gfn554. [DOI] [PubMed] [Google Scholar]
- 8. Kudose S, Santoriello D, Debiec H, Canetta PA, Bomback AS, Stokes MB, et al. The clinicopathologic spectrum of segmental membranous glomerulopathy. Kidney Int. 2021 Jan;99(1):247–55. 10.1016/j.kint.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 9. Choung HYG, Goldman B. Segmental membranous nephropathy. Clin Exp Nephrol. 2021;25(7):700–7. 10.1007/s10157-021-02056-1. [DOI] [PubMed] [Google Scholar]
- 10. Obana M, Nakanishi K, Sako M, Yata N, Nozu K, Tanaka R, et al. Segmental membranous glomerulonephritis in children: comparison with global membranous glomerulonephritis. Clin J Am Soc Nephrol. 2006 Jul;1(4):723–9. 10.2215/CJN.01211005. [DOI] [PubMed] [Google Scholar]
- 11. Ducrot H, Tsomi C, Jungers P, de Montera H, Hinglais N, Giromini M. Anatomoclinical study of extramembraneous glomerulonephritis. J Urol Nephrol. 1969 Sep;75(9):636–42. [PubMed] [Google Scholar]
- 12. Fujigaki Y, Tamura Y, Shibata S, Kondo F, Iwakura T, Kojima K, et al. A rare adult case with diffuse segmental membranous glomerulonephritis. Intern Med. 2017;56(13):1691–5. 10.2169/internalmedicine.56.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kondo A, Hashimura Y, Uchiyama T, Yoshikawa N, Minami H. Segmental membranous nephropathy with severe IgG3 deposition. Pediatr Int. 2018 Jun;60(6):597–8. 10.1111/ped.13560. [DOI] [PubMed] [Google Scholar]
- 14. Tomas NM, Huber TB, Hoxha E. Perspectives in membranous nephropathy. Cell Tissue Res. 2021 Aug;385(2):405–22. 10.1007/s00441-021-03429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beck LH Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009 Jul 2;361(1):11–21. 10.1056/nejmoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hofstra JM, Beck LH Jr., Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011 Jun;6(6):1286–91. 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomas NM, Beck LH Jr., Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014 Dec 11;371(24):2277–87. 10.1056/nejmoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020 Jan;97(1):163–74. 10.1016/j.kint.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 19. Kidney Disease Improving Global Outcomes KDIGO Glomerular Diseases Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021 Oct;100(4s):S1–s276. 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 20. Wang R, Wu Y, Zheng B, Zhang X, An D, Guo N, et al. Clinicopathological characteristics and prognosis of hepatitis B associated membranous nephropathy and idiopathic membranous nephropathy complicated with hepatitis B virus infection. Sci Rep. 2021 Sep 15;11(1):18407. 10.1038/s41598-021-98010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002 Jun;61(6):554–8. 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ehrenreich T, Churg J. Pathology of membranous nephropathy. Pathol Annu. 1968;3:389–433. [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang G, Sun L, Dong H, Wang Y, Xu X, Zhao Z, et al. Neural epidermal growth factor-like 1 protein-positive membranous nephropathy in Chinese patients. Clin J Am Soc Nephrol. 2021 May 8;16(5):727–35. 10.2215/CJN.11860720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Segawa Y, Hisano S, Matsushita M, Fujita T, Hirose S, Takeshita M, et al. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr Nephrol. 2010 Jun;25(6):1091–9. 10.1007/s00467-009-1439-8. [DOI] [PubMed] [Google Scholar]
- 26. Ahmad SB, Appel GB. Antigens, antibodies, and membranous nephropathy: a decade of progress. Kidney Int. 2020 Jan;97(1):29–31. 10.1016/j.kint.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 27. Alsharhan L, Beck LH Jr. Membranous nephropathy: core curriculum 2021. Am J Kidney Dis. 2021 Mar;77(3):440–53. 10.1053/j.ajkd.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 28. Sethi S. The many faces of NELL1 MN. Clin Kidney J. 2023 Mar;16(3):442–6. 10.1093/ckj/sfac237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caza TN, Hassen SI, Dvanajscak Z, Kuperman M, Edmondson R, Herzog C, et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 2021 Apr;99(4):967–76. 10.1016/j.kint.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwakura T, Ema C, Isobe S, Fujikura T, Ohashi N, Kato A, et al. Prevalence of neural epidermal growth factor-like 1- and exostosin 1/exostosin 2-associated membranous nephropathy: a single-center retrospective study in Japan. Sci Rep. 2022 Feb 22;12(1):2967. 10.1038/s41598-022-07037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spain RI, Andeen NK, Gibson PC, Samuels MH, Morris CD, Solomon AJ, et al. Lipoic acid supplementation associated with neural epidermal growth factor-like 1 (NELL1)-associated membranous nephropathy. Kidney Int. 2021 Dec;100(6):1208–13. 10.1016/j.kint.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author.