Abstract
Background
More than 850 million people worldwide suffer from acute and chronic kidney diseases (CKD) which are tremendous socioeconomic burdens for society. Currently, the treatment choices for CKD are limited. There is a great need to understand the underlying mechanisms of the development of CKD in order to develop potential therapeutic strategies.
Summary
The alteration in cellular metabolism has emerged as an important common pathological mechanism in different kidney diseases. Metabolic intervening and reprogramming will yield new insights to prevent and slow the progression of kidney disease. As one essential component of cellular metabolisms in fuel-source preferences (glucose, fatty acids, or ketones), the polyamine compound metabolism comprising the metabolites (spermine, spermidine, and putrescine) and their biosynthetic and catabolic enzymes are an endogenous pathophysiological regulator that is arising as a potential therapeutic object for many diseases.
Key Messages
This article aimed to review current knowledge on polyamine metabolism and physiological processes, and its potential regulatory and beneficial roles in immunoregulation, mitochondrial homeostasis, autophagy, DNA damage, and kidney diseases, and thus provide a novel therapeutic opportunity for kidney diseases.
Keywords: Polyamines, Spermine, Spermidine, Kidney disease, Therapeutic potential
Introduction
There are more than 850 million people in the world suffering from acute and chronic renal diseases; especially, the incidence of chronic kidney disease (CKD) has been gradually increasing, affecting 8–16% of the global population [1, 2]. The cause of kidney diseases is multifactorial; the growing prevalence of diabetes, hypertension, and aging are the main contributors to the global rise in CKD [3]. In addition, CKD becomes one of leading causes of morbidity and mortality worldwide, but many patients with CKD have limited safe and effective treatment options and continue to progress to kidney failure, eventually requiring dialysis or kidney transplantation [4]. A thorough understanding of the pathogenesis of CKD is essential for the development of new therapeutic interventions. Specifically, there is an accumulating body of data supporting the major role of metabolic disorders in the development of CKD. Defective fatty acid oxidation, increased glycolysis, and abnormal amino acid metabolism have been observed in the kidney cells of CKD, all of which are critical contributors to the disease [5–7]. Thus, prevention and reversal of renal dysfunction by targeted regulation of metabolism has become one of research focus points.
Polyamines, including spermidine, spermine, and its precursor putrescine, are ubiquitous and essential biomolecules for human cell metabolism. The presence of polyamine metabolism disorders has been documented in a wide range of acute and chronic human diseases and cancers [8–10], which could serve as a biomarker for the diagnosis of some diseases [11]. Putrescine is considered to be a uremic toxin [12], while several studies have reported that spermine and spermidine exert beneficial effects on a variety of non-neoplastic diseases [9, 13]. Natural spermine and spermidine are organic cations containing multiple amino groups (−NH2) that interact with negatively charged macromolecules, enabling them to function in a multitude of cellular processes, including immune response, chromatin organization, gene regulation, cell proliferation and differentiation, and cell death. Clinical and experimental studies have shown that the level of polyamines and their regulatory enzymes were altered in kidney diseases. Considering the multiple physiological effects of polyamines, supplementing spermine and spermidine or targeting polyamine regulatory proteins has become potential therapeutic strategies for many diseases [13–15]. This review intends to present current evidence on the role of spermine and spermidine in improving renal function in human and animal models. It also focuses on consequent implications for the management of patients with CKD.
Polyamine Metabolism and Transport
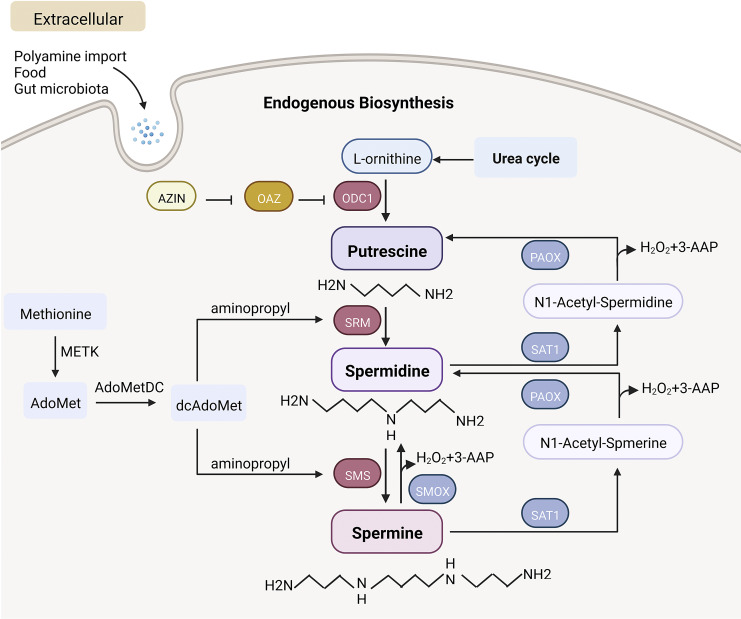
The pioneering studies of Herb and Celia Tabor in collaboration with Sanford Rosenthal in the late 1950s first described the quantification and biosynthetic pathway of polyamine metabolism [16]. Polyamines originate from both exogenous uptake and endogenous biosynthesis. Intracellular ornithine from the urea cycle is first decarboxylated to putrescine by the rate-limiting enzyme ornithine decarboxylase 1 (ODC1). Diamine putrescine is in turn converted to trimine spermidine by spermidine synthase (SRM). Spermidine then catalyzes the aminopropyl transfer reaction through spermine synthase (SMS) to produce spermine. During the process, methionine is converted to S-adenosylmethionine (AdoMet) by S-adenosylmethionine synthase (METK). AdoMet produces the aminopropyl group necessary for SRM and SMS activities [10, 17] by the second rate-limiting enzyme in polyamine biosynthesis, S-adenosylmethionine decarboxylase (AdoMetDC). Alternatively, spermine can be directly catabolized to spermidine by spermine oxidase (SMOX) and produce 3-aminopropanal and H2O2. Spermine and spermidine are acetylated by spermidine/spermine N(1)-acetyltransferase 1 (SAT1) to produce N-acetyl-spermine and N-acetyl-spermidine, which are secreted from cells or back converted into spermidine and putrescine by peroxisomal N(1)-acetyl-spermine/spermidine oxidase (PAOX) [18, 19]. Eventually, the polyamines are excreted in urine and feces.
The dynamic properties of enzymes involved in polyamine metabolism have been revealed in the studies. ODC1, as the most critical enzyme in the biosynthetic pathway, has received the most attention. The half-life of ODC1 in the kidneys of mice is approximately 23 min [20]. ODC1 is degraded by the 26S proteasome without ubiquitination [21], and the regulation of ODC1 is mediated by antienzymes (OAZ) (OAZ1, OAZ2, and OAZ3) and controlled by antienzyme inhibitors (AZIN) such as AZIN1 and AZIN2 [22, 23]. The properties of other enzymes in the biosynthetic pathway are limited and require further exploration. Additionally, the current understanding of the biochemical characterization of the key enzymes in polyamine reverse conversion (SAT1, SMOX, PAOX) includes the expression level, location, and protein stability of these enzymes. For example, SAT1 is mainly expressed in cytoplasm and mitochondria. SAT1 turnover is very rapid, with a half-life of approximately 15 min, and undergoes degradation at the 26S proteasome following polyubiquitination. Of note, SAT1 is a highly inducible enzyme that is elevated in response to tissue injury and inflammation [24, 25]. The unstable and readily inducible SAT1 is considered a rate-limiting enzyme for polyamine reverse conversion. Both PAOX and SMOX are flavin adenine dinucleotide -dependent oxidases [26], but they differ in their preferred oxidation substrates; it has been shown that PAOX is located in the cytoplasm and peroxisomes [27], while SMOX is located in the cytoplasm and nucleus [28, 29]. Based on some factors, SMOX may be the more metabolically important oxidase of these two enzymes. First, PAOX mRNA level in HEK-293 cells was much lower than SAT1 and SMOX mRNA. Second, protein turnover of PAOX was very slow. Finally, and most importantly, when transfected into HEK-293 cells, PAOX had only a slight effect on the intracellular polyamine pool. Furthermore, SMOX was also easily induced by various stimuli, and overexpression of SMOX significantly reduced the pool of available spermine [27, 30]. These findings could help us develop drugs that target the enzymes of polyamine metabolism to correct abnormalities in the polyamine pool caused by the disease.
In addition to endogenous metabolism, exogenous uptake can be supplemented by intake of polyamine-rich foods or secretion from the gut microbiota [9]. As spermine and spermidine cannot be enzymatically degraded in the gastrointestinal tract, oral doses of polyamines could be rapidly absorbed from the gut and distributed throughout the body [31]. There is limited information about the precise mechanism of polyamine transport system in mammals. Extracellular polyamines can be efficiently taken up by the heparan sulfate side chain of circulating glypican-1 on the cell surface and enter the cell by endocytosis [32, 33]. ATP13A2, a member of P5B-ATPases, exists in lysosomes and is responsible for the release of polyamines into the cytosol [34]. Cryo-electron microscopy revealed that spermine uptake was accomplished through electronegative breaks arrayed in transmembrane segments 2, 4, and 6 [35]. This information helps us understand the pathways of polyamine uptake in detail and to design drugs that can interfere with the uptake and excretion of polyamines, thereby stabilizing the polyamine pool. Immunostaining of spermine was mainly observed in the cytoplasm but also in the nucleus [33]. However, the distribution of polyamines in the cytoplasm is still unknown. Is it present in subcellular organelles? And is the distribution of polyamines different in each organelle? Recently, it has been reported that with the strong selective binding between the host-guest complexation of amphiphilic sulfonatocalix [5]arene (SC5A12C) assembly with lucigenin and spermine and the biocompatibility of SC5A12C components, ideal bioimaging of spermine in living cells has been achieved, which can be used to better monitor the activity process of spermine in cells [36]. Figure 1 schematically shows the basic process of mammalian polyamine metabolism.
Fig. 1.
Major process of endogenous biosynthesis and exogenous uptake of polyamines. ODC1, ornithine decarboxylase 1; SRM, spermidine synthase; SMS, spermine synthase; SMOX, spermine oxidase; SAT1, spermidine/spermine N(1)-acetyltransferase 1; PAOX, peroxisomal N(1)-acetyl-spermine/spermidine oxidase; METK, S-adenosylmethionine synthase; AdoMet, S-adenosylmethionine; dcAdoMet, decarboxylated S-adenosylmethionine; AdoMetDC, adenosylmethionine decarboxylase; 3-AAP, 3-acetamidopropanal; AZIN, antienzyme inhibitors; OAZ, ODC antienzymes. Created with BioRender.com.
Polyamines in Physiological Processes
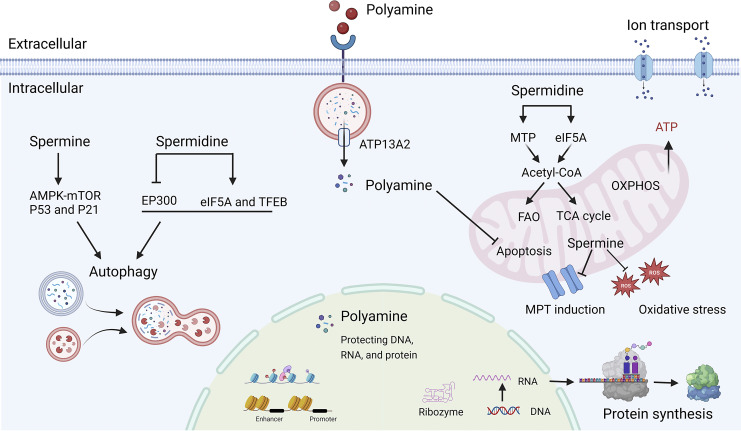
Advances in our understanding of polyamine molecular and cellular functions have led to increased interest in targeting polyamine metabolism for therapeutic benefit. Putrescine is considered to be a uremic toxin, and little information is available. We focused on the biological effects of spermine and spermidine. To date, spermine and spermidine were reported to play important roles in many cellular processes, such as maintaining mitochondrial homeostasis and inhibiting oxidative stress; improving autophagy; protecting RNA, DNA, and proteins, affecting cell transcription and translation, and promoting cell proliferation and differentiation [37]; regulating sodium, potassium, calcium ion channel [38–40]. These important cellular functions of polyamine help maintain general cell homeostasis. Spermine and spermidine have four and three amino groups, respectively, and the more amino groups, the easier it is to establish polar bonds with different anions and thus exert biological functions. Spermine and spermidine have similar biological activities on the surface, but some experiments demonstrated that spermine had greater potency [13]. However, the precise biochemical functions of polyamine remain not fully understood. Figure 2 Scheme of physiological processes influenced by polyamine metabolism in humans.
Fig. 2.
Cellular physiological processes mediated by polyamines. MTP, mitochondrial trifunctional protein; eIF5A, translation factor eukaryotic initiation factor 5A; EP300, E1A-associated protein P300; FAO, fatty acid oxidation; TCA, tricarboxylic acid cycle; OXPHOS, oxidative phosphorylation; MPT, mitochondrial permeability transition; ROS, reactive oxygen species. Created with BioRender.com.
Maintenance of Mitochondrial Homeostasis
The kidney requires adequate energy for maintenance of homeostasis, blood filtration, nutrient reabsorption, fluid and electrolyte regulation, acid-base balance, and blood pressure regulation [41]. Thus, mitochondria are abundant in the kidney, especially in the tubules. The dysfunction of mitochondria could result in oxidative stress and different types of cell death (apoptosis, necrosis, pyroptosis, and ferroptosis), contributing to the development of kidney disease [42, 43]. Polyamines maintain mitochondrial homeostasis by regulating multiple pathways, thereby modulating mitochondrial respiration [44] and exerting antioxidant and antiapoptotic effects [45–47]. Polyamines bind to mitochondrial surface phospholipids due to the strong affinity of basic amines for these lipids [47]. Spermine is transported or released through mitochondria to inhibit mitochondrial permeability transition (MPT) [48]. MPT induction is presented as mitochondrial swelling and loss of membrane potential (ΔΨ). Spermine could prevent MPT induction through scavenging reactive oxygen species or electrostatic interaction and restore the mitochondrial bioenergetic function collapsed by MPT induction [46, 49]. In addition, the late lysosomal transporter ATP13A2 pumps spermine into the cytosol, leading to decreased oxidative stress [50]. However, spermine has also been reported to induce MPT and apoptosis due to its oxidation to produce toxic metabolites [48]. Spermidine has been reported to have effects similar to spermine, modulating mitochondrial respiration through translation factor eukaryotic initiation factor 5A (eIF5A) hypusination [44] or directly binding to mitochondrial trifunctional protein to enhance fatty acid oxidation [51]. These results provide a reliable basis for treatment of mitochondrial damage in kidney disease.
Improving Autophagy
Spermine and spermidine are autophagy agonists [52–54]. Cytoplasmic spermine immunostaining was co-localized with the autophagosome marker LC3, suggesting a close correlation between spermine and autophagy [33]. Spermine increases the number of both active autophagosomes and lysosomes. One study reported that spermine increased microtubule acetylation and facilitated the retrograde transport of autophagosomes from the cell periphery to lysosomes located near the nucleus, which assisted the fusion of autophagosomes and lysosomes and enhanced autolysosomal flux to clear prion aggregates [55]. Spermine was also reported to be involved in the induction of autophagy through histone deacetylation and the activation of p53 transcription and p21 promoter [53]. Furthermore, spermine boosted autophagy through AMPK-mTOR signaling pathway to improve ischemic cardiomyopathy and diabetic nephropathy [56, 57]. In addition, spermidine has been reported to enhance autophagy to protect the lung [58] and heart [59] and alleviate age-induced memory disorders [60, 61]. By inhibiting the acetyltransferase E1A-associated protein P300 (EP300), spermidine increased autophagy flux as potent as rapamycin [62, 63]. Spermidine promoted autophagy through eIF5A hypusination and TFEB translation to reverse B cell senescence [64]. The cardiac protective effect of spermidine in mice could be counteracted by deletion of ATG5 [59]. Interestingly, a positive feedback mechanism was also found by which autophagy itself could maintain polyamine metabolism [65]. Based on these findings, the ODC1 inhibitor difluoromethylornithine (DFMO) is proposed as a novel autophagy inhibitor due to the depletion of polyamines [54]. These data suggest that spermine and spermidine may have therapeutic potential as autophagy modulators.
Protecting DNA, RNA, and Protein
The function of polyamines is profound and extensive, and they can stabilize chromatin and ribozyme and protect self-RNA from RNase digestion [66]. In this regard, the protective effect of spermine is better than spermidine and putrescine [66]. Polyamine affects the activities of histone acetyltransferases and deacetylases, a family of ribozymes that modify gene expression by modulating chromatin structure [67]. Spermine and spermidine protect replicating DNA against damage by singlet oxygen [68]. Tubulin is an important cytoskeletal protein that assembles into various forms through interactions with a range of cytokines. Observation of protein structure by solution X-ray scattering and cryo-transmission electron microscope revealed that spermine could promote and stabilize tubulin assembly [69]. Depletion of cellular polyamines by overexpression of SAT1 caused DNA damage and cell cycle G2 arrest, leading to a complete arrest of translation and growth in mammalian cells [37, 70]. Combined with previous knowledge, cell proliferation and differentiation are known to depend on chromatin stability and gene expression [71, 72]. Thus, a more thorough understanding of the pathophysiological roles of polyamines might provide a potential therapeutic opportunity.
Polyamine and Immunoregulation
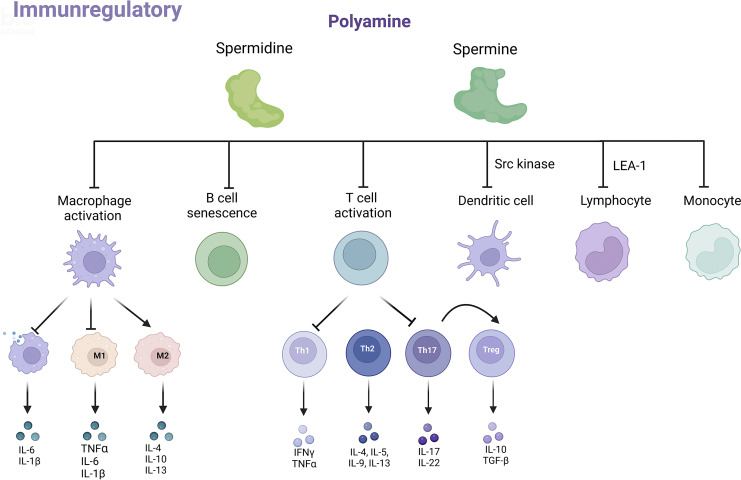
Inflammation is a major feature of both acute kidney injury (AKI) and CKD, and persistent inflammation leads to irreversible renal damage and ultimately organ failure [73]. Current research has highlighted the profound roles of polyamines in the mammalian immune system (shown in Fig. 3).
Fig. 3.
Role of polyamines in the immune system. Spermine and spermidine affect the phenotype and function of immune cells, including macrophages, T and B cells, dendritic cells, and monocytes, to play the role of immunomodulator. IL, interleukin; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; TGF-β,transforming growth factor-β; M1, macrophage 1 type; M2, macrophage 2 type; Th1, T helper 1; Th2, T helper 2; Th17, T helper 17; Treg, regulatory T cells; Src, proto-oncogene tyrosine-protein kinase Src; LEA-1, lymphocyte function-associated antigen 1. Created with BioRender.com.
Macrophages
Polyamines inhibit macrophage secretion of inflammatory factors and regulate their activation and differentiation [44]. A study by William J. Janssen et al. has shown that macrophages engulfing apoptotic cells increased polyamine import through Rac1 and PI3 kinase-dependent mechanisms, promoted polyamine accumulation, and inhibited interleukin-1β (IL-1β) and interleukin-6 release [74]. Compared with other polyamines, spermine was more potent in inhibiting the production of inflammatory cytokines in lipopolysaccharide (LPS)-stimulated mouse macrophages [75]. Spermine also effectively inhibited the high mobility group box protein 1-induced release of inflammatory cytokines in peritoneal macrophages, thereby attenuating sepsis [76]. A study reported that spermine accumulated in the cytoplasm of E. piscicida-infected macrophages and inhibited K+ efflux-dependent NLRP3 inflammasome activation. Moreover, spermine significantly reduced LPS or nigericin-induced lactate dehydrogenase release, IL-1β secretion, and caspase-1 activation [77]. It is well known that macrophages are highly heterogeneous and plastic and can be activated or polarized under the induction of different tissue environments and pathogenic factors, showing different functional phenotypes, mainly divided into proinflammatory M1 type and anti-inflammatory M2 type. Spermine was found to improve thioacetamide-induced acute liver injury, which inhibited liver-resident macrophage polarization toward the M1 type and promoted M2 polarization by enhancing ATG5-mediated autophagy [78]. Similarly, spermidine induced macrophage differentiation into M2 phenotype through increasing mitochondrial reactive oxygen species and autophagy. Adoptive transfer of spermidine-treated macrophages ameliorated inflammatory bowel disease in mice [79].
T Cell
A massive increase in polyamine synthesis occurred during T cell proliferation and activation [80]. Total polyamine levels were reduced in regulatory T cells (Tregs) and nonpathogenic T helper 17 (Th17) cells, compared with pathogenic Th17 cells which secreted inflammatory factors and promoted inflammation. Among them, pathogenic Th17 cells had higher levels of putrescine and acetylputrescine. However, spermidine and acetylspermidine did not differ within pathogenic and nonpathogenic T cells, and spermine was not detected [81]. Regulation of polyamine levels could affect T-cell proliferation and differentiation. Simultaneously blocking polyamine uptake and inhibiting ODC1-depleted intracellular polyamine pool to suppress T-cell proliferation [82]. Similarly, a recent report demonstrated that polyamine metabolism determined CD4+ Th cell differentiation and function by regulating the synthesis of the amino acid hypusine and influencing tricarboxylic acid cycle and histone acetylation [83]. Additionally, spermine significantly inhibited CD4+T-cell activation and subsequent T helper 1 (Th1) and Th17 differentiation through the inhibition of ERK phosphorylation [84]. Spermidine shifted the polarization of Th17 cells toward Foxp3+ Treg cells in an autophagy-related manner [85]. However, supplementation of spermine and spermidine suppressed release of inflammatory cytokines but enhanced CD4+ and CD8+ T-cell activation [86]. These data suggest that polyamines may modulate inflammation by regulating T-cell proliferation and activation. Th1, T helper 2 (Th2), Th17, and Tregs are the major subsets of effector CD4+ Th cells. Th1 and Th17 cells are thought to contribute to fibrosis and inflammation during CKD progression. Th2 cells are considered to be protective via releasing interleukin-4 (IL-4), and Tregs have been shown to prevent inflammation and promote repair [73]. More research is needed to explore the effects of polyamines on different subtypes of T cells and their effects on the kidney.
To date, the research on polyamines and immune cells mainly focuses on macrophages and T cells, and there is less information known about other immune cells. An interventional study demonstrated that consumption of the polyamine-rich food natto increased the blood levels of spermine, reduced the expression of lymphocyte function-associated antigen 1 (LFA-1) on monocytes, and suppressed the aging-associated proinflammatory state of healthy male volunteers [87]. Besides, LFA-1 is closely associated with the activation of immune function and the progression of inflammation. Polyamines, especially spermine, have a strong inhibitory effect on LFA-1 expression and adhesion ability of human lymphocyte via enhancing the methylation of integrin subunit alpha L (ITGAL, combines with the beta 2 chain to form LFA-1) [88, 89]. In addition, a study published this year found that the administration of spermidine can reverse the inhibition state of c-Myc and glycolytic dysfunction in lymphocyte natural killer cells caused by colon cancer, restore the killing activity of natural killer cells, and play the antitumor function [90]. On the other hand, spermidine can trigger immunosuppressive indoleamine 2,3-dioxygenase 1 signaling by directly activating proto-oncogene tyrosine-protein kinase Src (Src kinase) in dendritic cells [91]. Group 3 innate lymphoid cells (ILC3s) are RORγT + lymphocytes, and exogenous supplementation of putrescine or its biosynthetic substrate ornithine enhanced ILC3 production of interleukin-22 (IL-22). The absence of ODC1 in ILC3s significantly reduced IL-22 production, which alleviated autoimmune colitis but impaired the ability to fight bacterial infections [92, 93]. These findings also suggest that the effects of polyamines on immune cells may play divergent roles in different diseases progression. At present, the role of polyamines in immune response to kidney disease is still a mystery. Understanding the potential role of polyamines in regulation of immune system is attractive to enable the development of appropriately targeted therapeutic agents for kidney disease.
Polyamines in Kidney Diseases
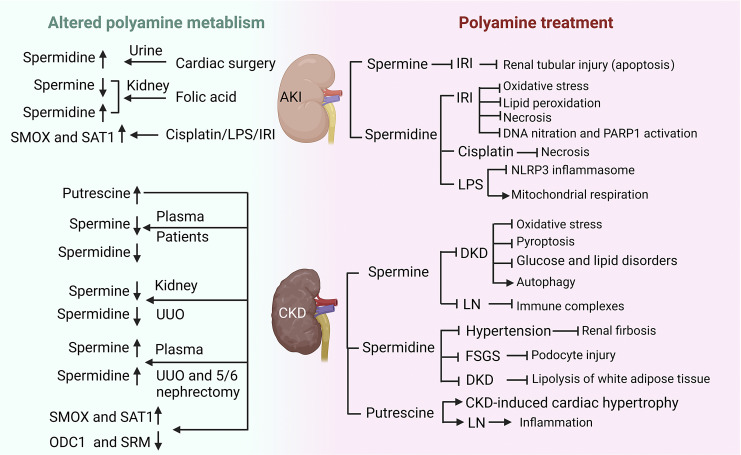
In 1995, in situ hybridization histochemistry was used to show the mRNA of Odc1 and Sat1 in different parts of the kidney of male rats. Odc1 expression was 30-fold higher in the medulla than in the cortex. Odc1 expression was mainly distributed in the cortex-medullary junction, especially in the proximal straight tubular epithelial cells of the outer medulla. Sat1 expression was higher in the cortex than in the medulla, concentrated in the tubular epithelial cells of the vascular pole of the glomeruli and in the distal straight tubules of the outer medulla [94]. Thus, polyamines appear to undergo different metabolic fates in various morphologically and functionally partitioned parts of the nephron in the kidney. The catabolic enzyme Sat1 was upregulated, while anabolic enzymes (Odc1 and Sms) were downregulated in different models of AKI and CKD [95]. Although several in vitro and in vivo studies have investigated the changes of polyamines in the blood or kidney, the effects of polyamine supplementation in kidney diseases are still open areas of research. Studies have found that polyamines and glucocorticoid hormones shared many similarities in their metabolic effects, including anti-inflammatory and stabilizing chromatin structure [96]. Although glucocorticoids are well known for their anti-inflammatory and immunosuppressive properties in treating kidney diseases such as nephrotic syndrome, they also cause many adverse reactions. Whether polyamines could avoid these side effects and become better drugs for the treatment of kidney disease is worth further exploration. Figure 4 is a simple scheme of the involvement of polyamines in different kidney diseases, and details are provided below. Thus, direct renal action of polyamine would be expected.
Fig. 4.
Alterations of polyamine metabolism in kidney diseases and therapeutic effects of polyamine supplementation. The green background showed the changes in polyamine metabolism in AKI and CKD, and the pink background indicated the effect of polyamine supplementation on kidney diseases. AKI, acute kidney injury; CKD, chronic kidney disease; IRI, ischemia-reperfusion injury; FSGS, focal segmental glomerulosclerosis; DKD, diabetic kidney disease; LN, lupus nephritis; UUO, unilateral ureteral obstruction; LPS, lipopolysaccharide; ODC1, ornithine decarboxylase 1; SRM, spermidine synthase; SMOX, spermine oxidase; SAT1, spermidine/spermine N(1)-acetyltransferase 1. Created with BioRender.com.
Acute Kidney Injury
Clinical observational studies or trials on polyamines and AKI are still very few. A case-control observational study reported that patients with high levels of urinary spermidine before cardiac surgery had an increased risk of AKI, suggesting that spermidine may be a novel and promising biomarker to identify those patients at higher predisposition to develop AKI [97]. However, the role of polyamines in the blood and urine of patients with AKI in diagnosis remains to be further studied. Renal ischemia-reperfusion injury (IRI), nephrotoxic drugs (such as cisplatin), and sepsis are common risk factors for AKI. Renal tubular repair may be completed after mild AKI, but incomplete or maladaptive repair usually occurs after severe or recurrent AKI, leading to the development of CKD [98]. Intraperitoneal injection of spermine protected against IRI-induced renal tubular injury and also inhibited the apoptosis of tubular epithelial cells in mice [99], showing potential clinical applications in settings where AKI might be anticipated, including kidney transplantation or cardiothoracic surgery. Furthermore, spermidine supplementation markedly attenuated kidney dysfunction and tubular damage after IRI by reducing oxidative stress, lipid peroxidation, and necrosis [100]. Another study determined the protective mechanism of spermidine during IRI by blocking DNA nitration and PARP1 activation [101]. Exogenous spermidine significantly attenuated cisplatin-induced tubular necrosis and renal dysfunction but did not affect cell apoptosis [102]. A recent study revealed that oral supplementation of spermidine protected against LPS-induced kidney injury and inflammation by regulating macrophage NLRP3 inflammasome activation and mitochondrial respiration in an eIF5A hypusination-related pathway [103]. Dysregulation of polyamine metabolism-related enzymes is common in the kidneys of AKI models. Both ODC1 and SAT1 activities were reported to be significantly altered in the kidneys of folic acid-induced AKI rats. ODC1 peaked at 12 h with a 6-fold increase and then declined rapidly. SAT1 activity also peaked at 12 h but rapidly declined to levels only slightly above control levels within 18 h. Kidney spermidine levels slightly increased while spermine levels decreased in these mice [104]. Therefore, regulation of polyamine biosynthesis and degradation enzymes can affect polyamine pools and their biological effects. Manoocher Soleimani’s team reported elevated expression of SAT1 under various AKI models, including IRI, LPS, and cisplatin-induced AKI [105–107], and suggested that SAT1 may be a universal and critical polyamine-metabolizing enzyme in AKI. Overexpression of SAT1 in kidney cells resulted in the reduction of intracellular spermine and spermidine concentrations and increased putrescine concentrations, which caused the release of toxic metabolites such as H2O2 to affect cell growth and recapitulated different phenotypic features of renal IRI [108]. Conversely, ablation of SAT1 or the use of the polyamine oxidase inhibitor MDL72527 protected against AKI [105–107]. Therefore, manipulation of polyamine metabolism might provide a new therapeutic opportunity for AKI.
Chronic Kidney Disease
CKD is caused by many pathological factors, including inflammation, energy metabolism disorder, oxidative stress, cell senescence, with the development of renal fibrosis. Diabetes and hypertension are the top causes of CKD, and other causes include aging, glomerulonephritis, and polycystic kidney disease [1, 3, 109–111]. Early identification, assessment of prognosis, and selection of the best treatment options are needed to prevent CKD progression and reduce the risk of cardiovascular morbidity and mortality. Previous studies have reported dysregulation of polyamine metabolism in both primary and secondary CKD, and the beneficial effects of spermine and spermidine supplementation have been confirmed in disease models. Therefore, the polyamine metabolic system is an attractive target for pharmacological intervention.
Diabetic kidney disease is the main microvascular complication of diabetes mellitus and the most common cause of end-stage renal disease (ESRD). The pathogenesis of diabetic kidney disease is very complex and involves hyperglycemia-mediated intracellular metabolic disturbances, autophagy, oxidative stress, and endoplasmic reticulum stress, resulting in the increased mesangial matrix, basement membrane thickening, and extensive persistent proteinuria. Spermine supplements in type 1 diabetic rats attenuated renal dysfunction and podocyte injury by inhibiting AMPK-mTOR signaling pathway [56]. Spermine can also reverse high glucose or oxidized low-density lipoprotein-induced oxidative stress and pyroptosis in macrophages by activating Nrf2 pathway [112]. In addition, spermine and spermidine could reduce body weight and correct glucose and lipid disorders in diabetic rats [113–115]. The beneficial effect of spermine and spermidine may be attributed to the induction of autophagy [116], alleviation of metabolic endotoxemia, as well as the enhancement of intestinal barrier function [117]. On the other hand, polyamines have been widely reported to promote lipolysis of white adipose tissue and have weight loss effects [52, 118]. It was unexpected that when metabolomics was used to explore the renal protective mechanism of the sodium-glucose cotransporter type 2 inhibitor empagliflozin in male TALLYHO/Jng (obese and insulin resistant) mice, they found that empagliflozin downregulated multiple major polyamine metabolites, spermine, spermidine, putrescine, and N-acetyl-putrescine in the renal cortex, among which the content of spermine was reduced by more than 50% [119]. Unfortunately, the comparison of renal polyamine levels between diabetic mice and healthy control mice was lacking in this study, and more studies are needed to elucidate the underlying mechanisms.
Lupus nephritis (LN), a glomerulonephritis, is one of the most serious organ manifestations of the autoimmune disease systemic lupus erythematosus (SLE). Plasma spermine and spermidine were decreased in 44 patients with SLE, and spermidine levels were correlated with complement C3 and urinary protein/creatinine ratio [120], which may help develop metabolic markers for disease activity or progression to LN in SLE patients. Families of patients with childhood-onset SLE may have a strong genetic susceptibility. A large clinical study identified loss-of-function variants in SAT1 by whole exome sequencing caused X-linked childhood SLE and evaluated the impact of splice site variants and frameshift variants by using CRISPR/Cas9-mediated knock-in mice. The results showed that young hemizygous male and homozygous female Sat1 p. Glu92leufs*6 knock-in mice spontaneously developed splenomegaly, glomerular enlargement with leukocyte infiltration, proteinuria, and increased expression of type I interferon-induced genes [121]. In LN pathogenesis, immune complexes involving DNA binding to anti-DNA autoantibodies are deposited in the kidney and eventually cause kidney damage. Spermine could dose-dependently inhibit the interaction of SLE anti-DNA to calf thymus DNA and promote the dissociation of preformed immune complexes in plasmas of patients and normal human subjects [122]. In 1994, T. J. THOMAS et al. first demonstrated dysregulation of ODC1 in 3 strains of autoimmune mice with the lpr genetic background (MRL-lpr/Ipr, C3H-Ipr/lpr, and C57BL/6J-lpr/lpr, classical animal model of SLE). Compared to control BALB/c kidney, the ODC1 activity, and protein levels were higher in the 3 strains of lpr-gene mice, but the Odc1 mRNA level was lower. The increased ODC1 activity in the kidneys of these mice was associated with the development of LN [123]. The levels of polyamines were increased in the kidneys of SLE mice (MRL-lpr/lpr mice), with putrescine being the most obvious. When DFMO was added to drinking water to inhibit ODC1 activity and reduce putrescine, it significantly attenuated renal injury caused by SLE, including glomerulonephritis, interstitial inflammation, perivascular inflammation, and vasculitis [124]. Sex hormones, estrogen and testosterone, are known to influence disease activity and the therapeutic effect of SLE [125]. Testosterone and 17-estradiol have been shown to enhance ODC1 activity and increase the concentrations of spermidine and spermine in rat renal tubular epithelial cells, providing clues that polyamines may be involved in these effects of the hormones [126]. Collectively, these data suggest that polyamine pathway could be a novel target for LN prevention and therapeutic strategies.
Kidney fibrosis is the final and common pathological feature of CKD progressing to ESRD with different etiologies. Kidney fibrosis includes glomerular sclerosis, tubule interstitial fibrosis, arteriosclerosis, and perivascular fibrosis in the vascular system, which progressively leads to loss of renal function [127, 128]. In patients with CKD, especially with ESRD, putrescine levels are increased, but spermine and spermidine levels are decreased in plasma. More significantly, plasma spermine is negatively correlated with serum creatinine and urea nitrogen [129–131]. This phenomenon may be related to increased SMOX activity in CKD patients, which leads to increased spermine degradation [129]. Another study from Japan has shown that there is no significant difference between spermine and spermidine in the blood of CKD patients before and after hemodialysis, but hemodialysis caused a decrease in spermine/spermidine ratio, and this ratio was positively correlated with skeletal muscle mass index and serum albumin in hemodialysis patients [132]. Besides, elevated levels of spermine in the urine were found in children with cystic fibrosis [133]. Polyamine changes have also been found in animal models of CKD, such as unilateral ureteral obstruction (UUO) and 5/6 nephrectomy models, well-established models of experimental CKD characterized by interstitial fibrosis. The level of spermine and spermidine was downregulated in the obstructed kidney, which could be explained by the reduction of ODC1 protein in UUO kidneys or TGF-β1-induced renal tubular epithelial cells [134]. However, opposite changes have been reported, with increased blood spermine and spermidine in both 5/6 nephrectomy rats at 12-week and UUO rats at 2-week [135, 136]. A recent study comprehensively tested the changes of polyamine in mice at 21 days of unilateral ischemia-reperfusion and showed that in mice of renal injury, the blood levels of putrescine, spermidine, and spermine were increased; the content of putrescine and spermine in kidney tissue were decreased and spermidine was increased; while the urinary polyamine did not significantly change [137]. We speculated that there are several possible reasons why the aforementioned studies have produced inconsistent results in humans and mice. First, the etiology of CKD patients observed in clinical studies has not been classified. Is there any difference in polyamine metabolism among patients with different etiologies of CKD? In terms of animal model of renal fibrosis, UUO and 5/6 nephrectomy mice only partially simulated the pathological status of CKD patients, which may lead to differences. Second, animal studies suggested that the increased level of serum spermine and spermidine in CKD rats was mainly related to gut microbial dysbiosis. The difference in gut microbiota composition between rats and humans or different stage of CKD may explain the opposite result. The different species may not have the same pattern of polyamine metabolism to resist disease. Third, differences in experimental methods can lead to different results. However, more research may be needed to explore the changes of polyamine metabolism during CKD development.
Although there is no evidence of the direct beneficial effect of spermine or spermidine on renal fibrosis, several studies have reported that polyamines have anti-fibrosis and antiaging effects in various organs [138, 139]. Exogenous spermine supplementation could improve diabetic myocardial fibrosis [140, 141]. Spermidine attenuated liver fibrosis [142, 143] and pulmonary fibrosis in animal models [58]. In vitro studies have demonstrated that depletion of cellular polyamines by DFMO increased the expression of TGF-β type I receptor [144] and activated the TGF-β-Smad signaling pathway in intestinal epithelial cells [145]. DFMO also aggravated TGF-β1-induced transformation of kidney epithelial cells into a fibroblast phenotype and the expression of mesenchymal markers, whereas exogenous polyamine supplementation almost completely abolished the combined effects of DFMO and TGF-β1 [146]. The kidney is known to be a prime target organ of hypertensive damage, and Frank Madeo has demonstrated that spermidine supplementation ameliorated high salt-induced hypertension and inhibited renal damage, including glomerulosclerosis, renal interstitial fibrosis, and elevated urinary lipocalin-2 levels in Dahl rats [59]. This team is also running a phase 3 clinical trial to evaluate the effect of spermidine on hypertensive patients, but this study is ongoing, and we look forward to their findings. Notably, in the heart, ODC1 deficiency and oral administration of DFMO significantly counteracted 5/6 nephrectomy-induced polyamine accumulation in cardiomyocytes and attenuated cardiac hypertrophy, while addition of putrescine significantly aggravated CKD-induced cardiac hypertrophy in vivo and in vitro [147]. This difference may be due to the different effects from different polyamine metabolites. Further experiments are needed to evaluate the protective effect and side effects of polyamine metabolism on CKD.
Others
Focal segmental glomerulosclerosis, a type of nephrotic syndrome, is poorly responsive to various treatments. Tillmann Bork et al. have demonstrated impaired autophagy and the reduction of ODC1 and SRM in podocytes of focal segmental glomerulosclerosis and revealed a positive feedback mechanism by which spermidine-activated autophagy and autophagy itself maintained polyamine metabolism [65]. Hence, spermidine supplementation provides a new perspective on treating glomerular disease. Multiple comorbidities associated with immunosuppressive therapy, including infections, osteoporosis, and cardiovascular and reproductive effects, remain a concern. CKD patients are prone to abnormal mineral bone metabolism, which leads to an increased risk of osteoporosis and fragility fractures. Warmth exposure promoted polyamine biosynthesis in the microbiota, leading to higher levels of polyamines in the body and preventing osteoporosis [148]. Exogenous spermine and spermidine supplementation could protect against osteoporosis by preventing bone loss, and its mechanism is related to the disruption of differentiation and maturation of osteoclasts [148, 149]. Vascular calcification is a common vascular lesion in CKD patients, which increases the risk of cardiovascular events and mortality in patients with CKD. Spermidine treatment significantly inhibited mineral deposition and osteogenic differentiation to suppress vascular calcification in both rat and human vascular smooth muscle cells by regulating SIRT1 signaling pathway [150]. Triptolide is widely used in antitumor and immunosuppressive therapy, but the severe testicular toxicity largely limits its clinical application in humans. Exogenous spermine supplementation ameliorated triptolide-induced testicular dysfunction. The protective mechanism of spermine was due to increasing the expression of heat shock protein 70 to improve early and late spermatogenic events [151]. These results suggest that polyamines can be used in combination with other drugs to reduce the toxic side effects.
Potential Limitations
Systemic application of polyamines in patients with kidney disease could potentially be limited by adverse effects. Attention should be paid to the dosage of spermine or spermidine supplementation, as low doses can be protective, but high concentrations of spermine are cytotoxic [20, 89]. Furthermore, screening patients with kidney diseases for spermine or spermidine supplementation should be carefully performed. According to current literature, patients with cancer, certain viral infections, pulmonary hypertension, and asthma should not supplement spermine. There have been many reviews on the close relationship between polyamines and cancer [18, 152, 153]; thus, extra attention needs to be paid to their serious tumor-causing side effect. A diverse group of viruses depend on polyamines for replication [154, 155]. Polyamine supplementation might worsen viral infection-related clinical course and exacerbate kidney dysfunction including SARS-CoV-2 (COVID-19) infection [156, 157]. However, spermine has also been reported to inhibit the replication of vesicular stomatitis virus, a negative single-stranded RNA rhabdovirus, in Jurkat T cells [158]. When we consider the use of exogenous polyamine supplementation, it is important to consider whether the patient has a certain viral infection. Other aspects include that spermine administration promoted pulmonary artery smooth muscle cell proliferation and migration and aggravated vascular remodeling in a rodent model of pulmonary arterial hypertension [159, 160]; spermine promoted the survival and activation of human eosinophils [161]; and knockout of SAT1 or SMOX-induced polyamine accumulation produced asthma-like features in naive mice [162]. Therefore, supplementation of spermidine or spermine in such patients should be carefully reviewed for potential adverse effects.
Conclusions
As a global health problem, CKD has a devastating effect on people’s health and life and puts a huge burden on society. Targeting the universal polyamine system in kidney diseases regardless of the divergent etiology and pathogenesis offers a promising strategy because it can be beneficial to the kidney by modulating multiple pathophysiological processes likely through reducing inflammation, stabilizing impaired mitochondrial homeostasis and inhibiting oxidative stress, improving autophagy, and protecting DNA, RNA, and proteins. However, there are still unsolved risks and limitations in polyamine utility due to adverse effects. The indications and contraindications for the treatment with spermine or spermidine must be carefully defined in upcoming clinical trials. In addition, the kidney is a heterogeneous organ composed of more than 20 types of cells, including renal parenchymal cells, vascular cells, and circulating immune cells involved in kidney pathogenesis. The effects of polyamines on the different cells may be different or even opposite. Thus, specific target-oriented drug delivery methods need to be considered, and the drug structure should be optimized to enhance their efficacy and reduce their toxicity. The suitability and corresponding mechanisms of polyamines and related enzymes for therapeutic intervention need to be further assessed. Overall, the polyamine system has exciting potential as a therapeutic target that could offer multisystem protection to improve outcomes for patients with kidney diseases.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was funded by National Natural Science Foundation of China (82270716 and 82070698), Guangdong Provincial Key Laboratory of Nephrology (2020B1212060028), and National Health Commission Key Laboratory of Clinical Nephrology (Sun Yat-Sen University).
Author Contributions
All authors discussed the article’s content. Dan Luo wrote the manuscript. Xiaohui Lu, Yi Li, and Yiping Xu revised the text. Yi Zhou and Haiping Mao designed and revised the work.
Funding Statement
This study was funded by National Natural Science Foundation of China (82270716 and 82070698), Guangdong Provincial Key Laboratory of Nephrology (2020B1212060028), and National Health Commission Key Laboratory of Clinical Nephrology (Sun Yat-Sen University).
References
- 1. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–304. 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96(5):1048–50. 10.1016/j.kint.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 3. Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 4. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piret SE, Guo Y, Attallah AA, Horne SJ, Zollman A, Owusu D, et al. Kruppel-like factor 6-mediated loss of BCAA catabolism contributes to kidney injury in mice and humans. Proc Natl Acad Sci U S A. 2021;118(23):e2024414118. 10.1073/pnas.2024414118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21(1):37–46. 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao H, Luo J, Zhang Y, Mao X, Wen P, Ding H, et al. Tuberous sclerosis 1 (Tsc1) mediated mTORC1 activation promotes glycolysis in tubular epithelial cells in kidney fibrosis. Kidney Int. 2020;98(3):686–98. 10.1016/j.kint.2020.03.035. [DOI] [PubMed] [Google Scholar]
- 8. Sanchez-Jimenez F, Medina MA, Villalobos-Rueda L, Urdiales JL. Polyamines in mammalian pathophysiology. Cell Mol Life Sci. 2019;76(20):3987–4008. 10.1007/s00018-019-03196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359(6374):eaan2788. 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- 10. Casero RA Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6(5):373–90. 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 11. Amin M, Tang S, Shalamanova L, Taylor RL, Wylie S, Abdullah BM, et al. Polyamine biomarkers as indicators of human disease. Biomarkers. 2021;26(2):77–94. 10.1080/1354750X.2021.1875506. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Sekula P, Wuttke M, Wahrheit J, Hausknecht B, Schultheiss UT, et al. Genome-wide association studies of metabolites in patients with CKD identify multiple loci and illuminate tubular transport mechanisms. J Am Soc Nephrol. 2018;29(5):1513–24. 10.1681/ASN.2017101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soda K. Overview of polyamines as nutrients for human healthy long life and effect of increased polyamine intake on DNA methylation. Cells. 2022;11(1):164. 10.3390/cells11010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zahedi K, Barone S, Soleimani M. Polyamine catabolism in acute kidney injury. Int J Mol Sci. 2019;20(19):4790. 10.3390/ijms20194790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Madeo F, Hofer SJ, Pendl T, Bauer MA, Eisenberg T, Carmona-Gutierrez D, et al. Nutritional aspects of spermidine. Annu Rev Nutr. 2020;40:135–59. 10.1146/annurev-nutr-120419-015419. [DOI] [PubMed] [Google Scholar]
- 16. Pegg AE. Introduction to the thematic minireview series: sixty plus years of polyamine research. J Biol Chem. 2018;293(48):18681–92. 10.1074/jbc.TM118.006291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stanley BA, Pegg AE, Holm I. Site of pyruvate formation and processing of mammalian S-adenosylmethionine decarboxylase proenzyme. J Biol Chem. 1989;264(35):21073–9. 10.1016/s0021-9258(19)30047-x. [DOI] [PubMed] [Google Scholar]
- 18. Holbert CE, Cullen MT, Casero RA Jr, Stewart TM. Polyamines in cancer: integrating organismal metabolism and antitumour immunity. Nat Rev Cancer. 2022;22(8):467–80. 10.1038/s41568-022-00473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Proietti E, Rossini S, Grohmann U, Mondanelli G. Polyamines and kynurenines at the intersection of immune modulation. Trends Immunol. 2020;41(11):1037–50. 10.1016/j.it.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 20. Seely JE, Poso H, Pegg AE. Effect of androgens on turnover of ornithine decarboxylase in mouse kidney. Studies using labeling of the enzyme by reaction with [14C] alpha-difluoromethylornithine. J Biol Chem. 1982;257(13):7549–53. 10.1016/s0021-9258(18)34414-4. [DOI] [PubMed] [Google Scholar]
- 21. Murakami Y, Matsufuji S, Kameji T, Hayashi S, Igarashi K, Tamura T, et al. Ornithine decarboxylase is degraded by the 26S proteasome without ubiquitination. Nature. 1992;360(6404):597–9. 10.1038/360597a0. [DOI] [PubMed] [Google Scholar]
- 22. Kahana C. The antizyme family for regulating polyamines. J Biol Chem. 2018;293(48):18730–5. 10.1074/jbc.TM118.003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2(3):188–94. 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 24. Cervelli M, Amendola R, Polticelli F, Mariottini P. Spermine oxidase: 10 years after. Amino Acids. 2012;42(2–3):441–50. 10.1007/s00726-011-1014-z. [DOI] [PubMed] [Google Scholar]
- 25. Casero RA, Pegg AE. Polyamine catabolism and disease. Biochem J. 2009;421(3):323–38. 10.1042/BJ20090598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seiler N. Polyamine oxidase, properties and functions. Prog Brain Res. 1995;106:333–44. 10.1016/s0079-6123(08)61229-7. [DOI] [PubMed] [Google Scholar]
- 27. Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J. 2003;370(Pt 1):19–28. 10.1042/BJ20021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cervelli M, Bellini A, Bianchi M, Marcocci L, Nocera S, Polticelli F, et al. Mouse spermine oxidase gene splice variants. Nuclear subcellular localization of a novel active isoform. Eur J Biochem. 2004;271(4):760–70. 10.1111/j.1432-1033.2004.03979.x. [DOI] [PubMed] [Google Scholar]
- 29. Murray-Stewart T, Wang Y, Goodwin A, Hacker A, Meeker A, Casero RA Jr. Nuclear localization of human spermine oxidase isoforms - possible implications in drug response and disease etiology. FEBS J. 2008;275(11):2795–806. 10.1111/j.1742-4658.2008.06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J. 2002;367(Pt 3):665–75. 10.1042/BJ20020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bardocz S, Duguid TJ, Brown DS, Grant G, Pusztai A, White A, et al. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73(6):819–28. 10.1079/bjn19950087. [DOI] [PubMed] [Google Scholar]
- 32. Belting M, Mani K, Jonsson M, Cheng F, Sandgren S, Jonsson S, et al. Glypican-1 is a vehicle for polyamine uptake in mammalian cells: a pivital role for nitrosothiol-derived nitric oxide. J Biol Chem. 2003;278(47):47181–9. 10.1074/jbc.M308325200. [DOI] [PubMed] [Google Scholar]
- 33. Cheng F, Fransson LA, Mani K. Common traffic routes for imported spermine and endosomal glypican-1-derived heparan sulfate in fibroblasts. Exp Cell Res. 2018;364(2):133–42. 10.1016/j.yexcr.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 34. van Veen S, Martin S, Van den Haute C, Benoy V, Lyons J, Vanhoutte R, et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature. 2020;578(7795):419–24. 10.1038/s41586-020-1968-7. [DOI] [PubMed] [Google Scholar]
- 35. Li P, Wang K, Salustros N, Gronberg C, Gourdon P. Structure and transport mechanism of P5B-ATPases. Nat Commun. 2021;12(1):3973. 10.1038/s41467-021-24148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tian HW, Chang YX, Hu XY, Shah MR, Li HB, Guo DS. Supramolecular imaging of spermine in cancer cells. Nanoscale. 2021;13(36):15362–8. 10.1039/d1nr04328e. [DOI] [PubMed] [Google Scholar]
- 37. Mandal S, Mandal A, Johansson HE, Orjalo AV, Park MH. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc Natl Acad Sci U S A. 2013;110(6):2169–74. 10.1073/pnas.1219002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266(5187):1068–72. 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- 39. Fleidervish IA, Libman L, Katz E, Gutnick MJ. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 2008;105(48):18994–9. 10.1073/pnas.0803464105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D’Hooge R, Van de Vijver G, Van Bogaert PP, Marescau B, Vanholder R, De Deyn PP. Involvement of voltage- and ligand-gated Ca2+ channels in the neuroexcitatory and synergistic effects of putative uremic neurotoxins. Kidney Int. 2003;63(5):1764–75. 10.1046/j.1523-1755.2003.00912.x. [DOI] [PubMed] [Google Scholar]
- 41. Jiang M, Bai M, Lei J, Xie Y, Xu S, Jia Z, et al. Mitochondrial dysfunction and the AKI-to-CKD transition. Am J Physiol Ren Physiol. 2020;319(6):F1105–16. 10.1152/ajprenal.00285.2020. [DOI] [PubMed] [Google Scholar]
- 42. Doke T, Susztak K. The multifaceted role of kidney tubule mitochondrial dysfunction in kidney disease development. Trends Cell Biol. 2022;32(10):841–53. 10.1016/j.tcb.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21(2):85–100. 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 44. Puleston DJ, Buck MD, Klein Geltink RI, Kyle RL, Caputa G, O’Sullivan D, et al. Polyamines and eIF5A hypusination modulate mitochondrial respiration and macrophage activation. Cell Metab. 2019;30(2):352–63.e8. 10.1016/j.cmet.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci U S A. 1998;95(19):11140–5. 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sava IG, Battaglia V, Rossi CA, Salvi M, Toninello A. Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic Biol Med. 2006;41(8):1272–81. 10.1016/j.freeradbiomed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 47. Mandal S, Mandal A, Park MH. Depletion of the polyamines spermidine and spermine by overexpression of spermidine/spermine N¹-acetyltransferase 1 (SAT1) leads to mitochondria-mediated apoptosis in mammalian cells. Biochem J. 2015;468(3):435–47. 10.1042/BJ20150168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grancara S, Dalla Via L, Garcia-Argaez AN, Ohkubo S, Pacella E, Manente S, et al. Spermine cycling in mitochondria is mediated by adenine nucleotide translocase activity: mechanism and pathophysiological implications. Amino Acids. 2016;48(10):2327–37. 10.1007/s00726-016-2264-6. [DOI] [PubMed] [Google Scholar]
- 49. Grancara S, Martinis P, Manente S, Garcia-Argaez AN, Tempera G, Bragadin M, et al. Bidirectional fluxes of spermine across the mitochondrial membrane. Amino Acids. 2014;46(3):671–9. 10.1007/s00726-013-1591-0. [DOI] [PubMed] [Google Scholar]
- 50. Vrijsen S, Besora-Casals L, van Veen S, Zielich J, Van den Haute C, Hamouda NN, et al. ATP13A2-mediated endo-lysosomal polyamine export counters mitochondrial oxidative stress. Proc Natl Acad Sci U S A. 2020;117(49):31198–207. 10.1073/pnas.1922342117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Al-Habsi M, Chamoto K, Matsumoto K, Nomura N, Zhang B, Sugiura Y, et al. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science. 2022;378(6618):eabj3510. 10.1126/science.abj3510. [DOI] [PubMed] [Google Scholar]
- 52. Liao CY, Kummert OMP, Bair AM, Alavi N, Alavi J, Miller DM, et al. The autophagy inducer spermidine protects against metabolic dysfunction during overnutrition. J Gerontol A Biol Sci Med Sci. 2021;76(10):1714–25. 10.1093/gerona/glab145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chae YB, Kim MM. Activation of p53 by spermine mediates induction of autophagy in HT1080 cells. Int J Biol Macromol. 2014;63:56–63. 10.1016/j.ijbiomac.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 54. Vanrell MC, Cueto JA, Barclay JJ, Carrillo C, Colombo MI, Gottlieb RA, et al. Polyamine depletion inhibits the autophagic response modulating Trypanosoma cruzi infectivity. Autophagy. 2013;9(7):1080–93. 10.4161/auto.24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Phadwal K, Kurian D, Salamat MKF, MacRae VE, Diack AB, Manson JC. Spermine increases acetylation of tubulins and facilitates autophagic degradation of prion aggregates. Sci Rep. 2018;8(1):10004. 10.1038/s41598-018-28296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang X, Zhang L, Chen Z, Li S, Che B, Wang N, et al. Exogenous spermine attenuates diabetic kidney injury in rats by inhibiting AMPK/mTOR signaling pathway. Int J Mol Med. 2021;47(3):27. 10.3892/ijmm.2021.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duan Q, Yang W, Jiang D, Tao K, Dong A, Cheng H. Spermine ameliorates ischemia/reperfusion injury in cardiomyocytes via regulation of autophagy. Am J Transl Res. 2016;8(9):3976–85. [PMC free article] [PubMed] [Google Scholar]
- 58. Baek AR, Hong J, Song KS, Jang AS, Kim DJ, Chin SS, et al. Spermidine attenuates bleomycin-induced lung fibrosis by inducing autophagy and inhibiting endoplasmic reticulum stress (ERS)-induced cell death in mice. Exp Mol Med. 2020;52(12):2034–45. 10.1038/s12276-020-00545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22(12):1428–38. 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sigrist SJ, Carmona-Gutierrez D, Gupta VK, Bhukel A, Mertel S, Eisenberg T, et al. Spermidine-triggered autophagy ameliorates memory during aging. Autophagy. 2014;10(1):178–9. 10.4161/auto.26918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. 2013;16(10):1453–60. 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- 62. Pietrocola F, Lachkar S, Enot DP, Niso-Santano M, Bravo-San Pedro JM, Sica V, et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015;22(3):509–16. 10.1038/cdd.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. du Toit A, De Wet S, Hofmeyr JHS, Muller-Nedebock KK, Loos B. The precision control of autophagic flux and vesicle dynamics-A micropattern approach. Cells. 2018;7(8):94. 10.3390/cells7080094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang H, Alsaleh G, Feltham J, Sun Y, Napolitano G, Riffelmacher T, et al. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol Cell. 2019;76(1):110–25.e9. 10.1016/j.molcel.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liang W, Yamahara K, Hernando-Erhard C, Lagies S, Wanner N, Liang H, et al. A reciprocal regulation of spermidine and autophagy in podocytes maintains the filtration barrier. Kidney Int. 2020;98(6):1434–48. 10.1016/j.kint.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 66. Lou F, Sun Y, Xu Z, Niu L, Wang Z, Deng S, et al. Excessive polyamine generation in keratinocytes promotes self-RNA sensing by dendritic cells in psoriasis. Immunity. 2020;53(1):204–16.e10. 10.1016/j.immuni.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 67. Sakamoto A, Terui Y, Uemura T, Igarashi K, Kashiwagi K. Polyamines regulate gene expression by stimulating translation of histone acetyltransferase mRNAs. J Biol Chem. 2020;295(26):8736–45. 10.1074/jbc.RA120.013833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khan AU, Mei YH, Wilson T. A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc Natl Acad Sci U S A. 1992;89(23):11426–7. 10.1073/pnas.89.23.11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dharan R, Shemesh A, Millgram A, Zalk R, Frank GA, Levi-Kalisman Y, et al. Hierarchical assembly pathways of spermine-induced tubulin conical-spiral architectures. Acs Nano. 2021;15(5):8836–47. 10.1021/acsnano.1c01374. [DOI] [PubMed] [Google Scholar]
- 70. Zahedi K, Bissler JJ, Wang Z, Josyula A, Lu L, Diegelman P, et al. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am J Physiol Cell Physiol. 2007;292(3):C1204–15. 10.1152/ajpcell.00451.2006. [DOI] [PubMed] [Google Scholar]
- 71. Li Y, Hartemink AJ, MacAlpine DM. Cell-cycle-dependent chromatin dynamics at replication origins. Genes. 2021;12(12):1998. 10.3390/genes12121998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. de la Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet. 2006;7(6):461–73. 10.1038/nrg1882. [DOI] [PubMed] [Google Scholar]
- 73. Fu Y, Xiang Y, Li H, Chen A, Dong Z. Inflammation in kidney repair: mechanism and therapeutic potential. Pharmacol Ther. 2022;237:108240. 10.1016/j.pharmthera.2022.108240. [DOI] [PubMed] [Google Scholar]
- 74. McCubbrey AL, McManus SA, McClendon JD, Thomas SM, Chatwin HB, Reisz JA, et al. Polyamine import and accumulation causes immunomodulation in macrophages engulfing apoptotic cells. Cell Rep. 2022;38(2):110222. 10.1016/j.celrep.2021.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Paul S, Kang SC. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm Res. 2013;62(7):681–8. 10.1007/s00011-013-0620-5. [DOI] [PubMed] [Google Scholar]
- 76. Zhu S, Ashok M, Li J, Li W, Yang H, Wang P, et al. Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol Med. 2009;15(7–8):275–82. 10.2119/molmed.2009.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jiang J, Wang W, Sun F, Zhang Y, Liu Q, Yang D. Bacterial infection reinforces host metabolic flux from arginine to spermine for NLRP3 inflammasome evasion. Cell Rep. 2021;34(10):108832. 10.1016/j.celrep.2021.108832. [DOI] [PubMed] [Google Scholar]
- 78. Zhou S, Gu J, Liu R, Wei S, Wang Q, Shen H, et al. Spermine alleviates acute liver injury by inhibiting liver-resident macrophage pro-inflammatory response through ATG5-dependent autophagy. Front Immunol. 2018;9:948. 10.3389/fimmu.2018.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu R, Li X, Ma H, Yang Q, Shang Q, Song L, et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic Biol Med. 2020;161:339–50. 10.1016/j.freeradbiomed.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 80. Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–82. 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wagner A, Wang C, Fessler J, DeTomaso D, Avila-Pacheco J, Kaminski J, et al. Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell. 2021;184(16):4168–85.e21. 10.1016/j.cell.2021.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wu R, Chen X, Kang S, Wang T, Gnanaprakasam JR, Yao Y, et al. De novo synthesis and salvage pathway coordinately regulate polyamine homeostasis and determine T cell proliferation and function. Sci Adv. 2020;6(51):eabc4275. 10.1126/sciadv.abc4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Puleston DJ, Baixauli F, Sanin DE, Edwards-Hicks J, Villa M, Kabat AM, et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell. 2021;184(16):4186–202.e20. 10.1016/j.cell.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zheng R, Kong M, Wang S, He B, Xie X. Spermine alleviates experimental autoimmune encephalomyelitis via regulating T cell activation and differentiation. Int Immunopharmacol. 2022;107:108702. 10.1016/j.intimp.2022.108702. [DOI] [PubMed] [Google Scholar]
- 85. Carriche GM, Almeida L, Stuve P, Velasquez L, Dhillon-LaBrooy A, Roy U, et al. Regulating T-cell differentiation through the polyamine spermidine. J Allergy Clin Immunol. 2021;147(1):335–48.e11. 10.1016/j.jaci.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 86. Fischer M, Ruhnau J, Schulze J, Obst D, Floel A, Vogelgesang A. Spermine and spermidine modulate T-cell function in older adults with and without cognitive decline ex vivo. Aging. 2020;12(13):13716–39. 10.18632/aging.103527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Soda K, Uemura T, Sanayama H, Igarashi K, Fukui T. Polyamine-rich diet elevates blood spermine levels and inhibits pro-inflammatory status: an interventional study. Med Sci. 2021;9(2):22. 10.3390/medsci9020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Soda K, Kano Y, Nakamura T, Kasono K, Kawakami M, Konishi F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J Immunol. 2005;175(1):237–45. 10.4049/jimmunol.175.1.237. [DOI] [PubMed] [Google Scholar]
- 89. Kano Y, Soda K, Konishi F. Suppression of LFA-1 expression by spermine is associated with enhanced methylation of ITGAL, the LFA-1 promoter area. PLoS One. 2013;8(2):e56056. 10.1371/journal.pone.0056056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. He H, Song Z, Lin S, Wang Y, Wang G. Exploring the effect of polyamines on NK cell function in colorectal cancer process based on glycolysis. Int Immunopharmacol. 2023;117:109944. 10.1016/j.intimp.2023.109944. [DOI] [PubMed] [Google Scholar]
- 91. Mondanelli G, Bianchi R, Pallotta MT, Orabona C, Albini E, Iacono A, et al. A relay pathway between arginine and tryptophan metabolism confers immunosuppressive properties on dendritic cells. Immunity. 2017;46(2):233–44. 10.1016/j.immuni.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sah P, Zenewicz LA. The polyamine putrescine is a positive regulator of group 3 innate lymphocyte activation. Immunohorizons. 2023;7(1):41–8. 10.4049/immunohorizons.2200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Peng V, Cao S, Trsan T, Bando JK, Avila-Pacheco J, Cleveland JL, et al. Ornithine decarboxylase supports ILC3 responses in infectious and autoimmune colitis through positive regulation of IL-22 transcription. Proc Natl Acad Sci U S A. 2022;119(45):e2214900119. 10.1073/pnas.2214900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bettuzzi S, Marinelli M, Strocchi P, Davalli P, Cevolani D, Corti A. Different localization of spermidine/spermine N1-acetyltransferase and ornithine decarboxylase transcripts in the rat kidney. FEBS Lett. 1995;377(3):321–4. 10.1016/0014-5793(95)01359-8. [DOI] [PubMed] [Google Scholar]
- 95. Sieckmann T, Ögel N, Kelterborn S, Boivin FJ, Schley G, Fähling M, et al. Different murine models of kidney injury reveal a common pattern of dysregulation within the polyamine system in favour of its catabolic pathways. FASEB J. 2022;36:S1. 10.1096/fasebj.2022.36.s1.r3345. [DOI] [Google Scholar]
- 96. Bjelaković G, Stojanović I, Jevtović Stoimenov T, Pavlović D, Kocić G, Rossi S, et al. Metabolic correlations of glucocorticoids and polyamines in inflammation and apoptosis. Amino Acids. 2010;39(1):29–43. 10.1007/s00726-010-0489-3. [DOI] [PubMed] [Google Scholar]
- 97. Martin-Lorenzo M, Ramos-Barron A, Gutierrez-Garcia P, Martin-Blazquez A, Santiago-Hernandez A, Rodrigo Calabia E, et al. Urinary spermidine predicts and associates with in-hospital acute kidney injury after cardiac surgery. Antioxidants. 2021;10(6):896. 10.3390/antiox10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264–76. 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yan B, Min SJ, Xu B, Zhang C, Pei J, Zhang W, et al. The protective effects of exogenous spermine on renal ischemia-reperfusion injury in rats. Transl Androl Urol. 2021;10(5):2051–66. 10.21037/tau-21-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim J. Spermidine rescues proximal tubular cells from oxidative stress and necrosis after ischemic acute kidney injury. Arch Pharm Res. 2017;40(10):1197–208. 10.1007/s12272-017-0957-3. [DOI] [PubMed] [Google Scholar]
- 101. Kim J. Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation. Anat Cell Biol. 2017;50(3):200–6. 10.5115/acb.2017.50.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yoon SP, Kim J. Exogenous spermidine ameliorates tubular necrosis during cisplatin nephrotoxicity. Anat Cell Biol. 2018;51(3):189–99. 10.5115/acb.2018.51.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li X, Zhou X, Liu X, Li X, Jiang X, Shi B, et al. Spermidine protects against acute kidney injury by modulating macrophage NLRP3 inflammasome activation and mitochondrial respiration in an eIF5A hypusination-related pathway. Mol Med. 2022;28(1):103. 10.1186/s10020-022-00533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Matsui I, Pegg AE. Induction of spermidine N1-acetyltransferase in rat kidney by treatment with folic acid. FEBS Lett. 1982;139(2):205–8. 10.1016/0014-5793(82)80852-1. [DOI] [PubMed] [Google Scholar]
- 105. Zahedi K, Barone S, Destefano-Shields C, Brooks M, Murray-Stewart T, Dunworth M, et al. Activation of endoplasmic reticulum stress response by enhanced polyamine catabolism is important in the mediation of cisplatin-induced acute kidney injury. PLoS One. 2017;12(9):e0184570. 10.1371/journal.pone.0184570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zahedi K, Barone S, Wang Y, Murray-Stewart T, Roy-Chaudhury P, Smith RD, et al. Proximal tubule epithelial cell specific ablation of the spermidine/spermine N1-acetyltransferase gene reduces the severity of renal ischemia/reperfusion injury. PLoS One. 2014;9(11):e110161. 10.1371/journal.pone.0110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zahedi K, Barone S, Kramer DL, Amlal H, Alhonen L, Janne J, et al. The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury. Am J Physiol Cell Physiol. 2010;299(1):C164–74. 10.1152/ajpcell.00512.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang Z, Zahedi K, Barone S, Tehrani K, Rabb H, Matlin K, et al. Overexpression of SSAT in kidney cells recapitulates various phenotypic aspects of kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2004;15(7):1844–52. 10.1097/01.asn.0000131525.77636.d5. [DOI] [PubMed] [Google Scholar]
- 109. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–52. 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 110. Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov. 2016;15(8):568–88. 10.1038/nrd.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16(5):269–88. 10.1038/s41581-019-0248-y. [DOI] [PubMed] [Google Scholar]
- 112. Qiu Y, Li L, Guo X, Liu J, Xu L, Li Y. Exogenous spermine inhibits high glucose/oxidized LDL-induced oxidative stress and macrophage pyroptosis by activating the Nrf2 pathway. Exp Ther Med. 2022;23(4):310. 10.3892/etm.2022.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sadasivan SK, Vasamsetti B, Singh J, Marikunte VV, Oommen AM, Jagannath MR, et al. Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur J Pharmacol. 2014;729:94–9. 10.1016/j.ejphar.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 114. Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ. Effect of spermine on lipid profile and HDL functionality in the streptozotocin-induced diabetic rat model. Life Sci. 2008;82(5–6):301–7. 10.1016/j.lfs.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 115. Mendez JD, Balderas FL. Inhibition by L-arginine and spermidine of hemoglobin glycation and lipid peroxidation in rats with induced diabetes. Biomed Pharmacother. 2006;60(1):26–31. 10.1016/j.biopha.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 116. Fernandez AF, Barcena C, Martinez-Garcia GG, Tamargo-Gomez I, Suarez MF, Pietrocola F, et al. Autophagy couteracts weight gain, lipotoxicity and pancreatic beta-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis. 2017;8(8):e2970. 10.1038/cddis.2017.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ma L, Ni Y, Wang Z, Tu W, Ni L, Zhuge F, et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes. 2020;12(1):1–19. 10.1080/19490976.2020.1832857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Monelli E, Villacampa P, Zabala-Letona A, Martinez-Romero A, Llena J, Beiroa D, et al. Angiocrine polyamine production regulates adiposity. Nat Metab. 2022;4(3):327–43. 10.1038/s42255-022-00544-6. [DOI] [PubMed] [Google Scholar]
- 119. Bangarbale S, Shepard BD, Bansal S, Jayatilake MM, Kurtz R, Levi M, et al. Renal metabolome in obese mice treated with Empagliflozin suggests a reduction in cellular respiration. Biomolecules. 2022;12(9):1176. 10.3390/biom12091176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kim HA, Lee HS, Shin TH, Jung JY, Baek WY, Park HJ, et al. Polyamine patterns in plasma of patients with systemic lupus erythematosus and fever. Lupus. 2018;27(6):930–8. 10.1177/0961203317751860. [DOI] [PubMed] [Google Scholar]
- 121. Xu L, Zhao J, Sun Q, Xu X, Wang L, Liu T, et al. Loss-of-function variants in SAT1 cause X-linked childhood-onset systemic lupus erythematosus. Ann Rheum Dis. 2022;81(12):1712–21. 10.1136/ard-2022-222795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wang X, Stearns NA, Li X, Pisetsky DS. The effect of polyamines on the binding of anti-DNA antibodies from patients with SLE and normal human subjects. Clin Immunol. 2014;153(1):94–103. 10.1016/j.clim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hsu HC, Seibold JR, Thomas TJ. Regulation of ornithine decarboxylase in the kidney of autoimmune mice with the lpr gene. Autoimmunity. 1994;19(4):253–64. 10.3109/08916939409071351. [DOI] [PubMed] [Google Scholar]
- 124. Gunnia UB, Amenta PS, Seibold JR, Thomas TJ. Successful treatment of lupus nephritis in MRL-lpr/lpr mice by inhibiting ornithine decarboxylase. Kidney Int. 1991;39(5):882–90. 10.1038/ki.1991.111. [DOI] [PubMed] [Google Scholar]
- 125. Kim JW, Kim HA, Suh CH, Jung JY. Sex hormones affect the pathogenesis and clinical characteristics of systemic lupus erythematosus. Front Med. 2022;9:906475. 10.3389/fmed.2022.906475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jotova I, Wang C, Tabib A, Dimitrov O, Bachrach U. Effects of testosterone and 17, beta-estradiol on the polyamine metabolism in cultivated normal rat kidney epithelial cells. Amino Acids. 2000;18(4):353–61. 10.1007/pl00010322. [DOI] [PubMed] [Google Scholar]
- 127. Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6(11):643–56. 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- 128. Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16–36. 10.1016/j.mam.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 129. Igarashi K, Ueda S, Yoshida K, Kashiwagi K. Polyamines in renal failure. Amino Acids. 2006;31(4):477–83. 10.1007/s00726-006-0264-7. [DOI] [PubMed] [Google Scholar]
- 130. Sakata K, Kashiwagi K, Sharmin S, Ueda S, Irie Y, Murotani N, et al. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem Biophys Res Commun. 2003;305(1):143–9. 10.1016/s0006-291x(03)00716-2. [DOI] [PubMed] [Google Scholar]
- 131. Sakata K, Kashiwagi K, Sharmin S, Ueda S, Igarashi K. Acrolein produced from polyamines as one of the uraemic toxins. Biochem Soc Trans. 2003;31(2):371–4. 10.1042/bst0310371. [DOI] [PubMed] [Google Scholar]
- 132. Sanayama H, Ito K, Ookawara S, Uemura T, Imai S, Kiryu S, et al. Positive correlation between relative concentration of spermine to spermidine in whole blood and skeletal muscle mass index: a possible indicator of sarcopenia and prognosis of hemodialysis patients. Biomedicines. 2023;11(3):746. 10.3390/biomedicines11030746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Russell DH, Rosenblum MG, Beckerman RC, Durie BG, Taussig LM, Barnett DR. Altered polyamine metabolism in cystic fibrosis. Pediatr Res. 1979;13(10):1137–40. 10.1203/00006450-197910000-00011. [DOI] [PubMed] [Google Scholar]
- 134. Li R, Chung AC, Dong Y, Yang W, Zhong X, Lan HY. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int. 2013;84(6):1129–44. 10.1038/ki.2013.272. [DOI] [PubMed] [Google Scholar]
- 135. Feng YL, Cao G, Chen DQ, Vaziri ND, Chen L, Zhang J, et al. Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol Life Sci. 2019;76(24):4961–78. 10.1007/s00018-019-03155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chen L, Chen DQ, Liu JR, Zhang J, Vaziri ND, Zhuang S, et al. Unilateral ureteral obstruction causes gut microbial dysbiosis and metabolome disorders contributing to tubulointerstitial fibrosis. Exp Mol Med. 2019;51(3):1–18. 10.1038/s12276-019-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sieckmann T, Schley G, Ogel N, Kelterborn S, Boivin FJ, Fahling M, et al. Strikingly conserved gene expression changes of polyamine regulating enzymes among various forms of acute and chronic kidney injury. Kidney Int. 2023;104(1):90–107. 10.1016/j.kint.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 138. Xu TT, Li H, Dai Z, Lau GK, Li BY, Zhu WL, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging. 2020;12(7):6401–14. 10.18632/aging.103035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Madeo F, Bauer MA, Carmona-Gutierrez D, Kroemer G. Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy. 2019;15(1):165–8. 10.1080/15548627.2018.1530929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hu J, Lu X, Zhang X, Shao X, Wang Y, Chen J, et al. Exogenous spermine attenuates myocardial fibrosis in diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress and the canonical Wnt signaling pathway. Cell Biol Int. 2020;44(8):1660–70. 10.1002/cbin.11360. [DOI] [PubMed] [Google Scholar]
- 141. Wang Y, Chen J, Li S, Zhang X, Guo Z, Hu J, et al. Exogenous spermine attenuates rat diabetic cardiomyopathy via suppressing ROS-p53 mediated downregulation of calcium-sensitive receptor. Redox Biol. 2020;32:101514. 10.1016/j.redox.2020.101514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yue F, Li W, Zou J, Jiang X, Xu G, Huang H, et al. Spermidine prolongs lifespan and prevents liver fibrosis and hepatocellular carcinoma by activating MAP1S-mediated autophagy. Cancer Res. 2017;77(11):2938–51. 10.1158/0008-5472.CAN-16-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Liu P, de la Vega MR, Dodson M, Yue F, Shi B, Fang D, et al. Spermidine confers liver protection by enhancing NRF2 signaling through a MAP1S-mediated noncanonical mechanism. Hepatology. 2019;70(1):372–88. 10.1002/hep.30616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rao JN, Li L, Bass BL, Wang JY. Expression of the TGF-beta receptor gene and sensitivity to growth inhibition following polyamine depletion. Am J Physiol Cell Physiol. 2000;279(4):C1034–44. 10.1152/ajpcell.2000.279.4.C1034. [DOI] [PubMed] [Google Scholar]
- 145. Liu L, Santora R, Rao JN, Guo X, Zou T, Zhang HM, et al. Activation of TGF-beta-Smad signaling pathway following polyamine depletion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G1056–67. 10.1152/ajpgi.00151.2003. [DOI] [PubMed] [Google Scholar]
- 146. Compagnone A, Bandino A, Meli F, Bravoco V, Cravanzola C, Parola M, et al. Polyamines modulate epithelial-to-mesenchymal transition. Amino Acids. 2012;42(2–3):783–9. 10.1007/s00726-011-0995-y. [DOI] [PubMed] [Google Scholar]
- 147. Han W, Du C, Zhu Y, Ran L, Wang Y, Xiong J, et al. Targeting myocardial mitochondria-STING-polyamine Axis prevents cardiac hypertrophy in chronic kidney disease. JACC Basic Transl Sci. 2022;7(8):820–40. 10.1016/j.jacbts.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chevalier C, Kieser S, Çolakoğlu M, Hadadi N, Brun J, Rigo D, et al. Warmth prevents bone loss through the gut microbiota. Cell Metab. 2020;32(4):575–90.e7. 10.1016/j.cmet.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yamamoto T, Hinoi E, Fujita H, Iezaki T, Takahata Y, Takamori M, et al. The natural polyamines spermidine and spermine prevent bone loss through preferential disruption of osteoclastic activation in ovariectomized mice. Br J Pharmacol. 2012;166(3):1084–96. 10.1111/j.1476-5381.2012.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Liu X, Chen A, Liang Q, Yang X, Dong Q, Fu M, et al. Spermidine inhibits vascular calcification in chronic kidney disease through modulation of SIRT1 signaling pathway. Aging Cell. 2021;20(6):e13377. 10.1111/acel.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhao Q, Huang JF, Cheng Y, Dai MY, Zhu WF, Yang XW, et al. Polyamine metabolism links gut microbiota and testicular dysfunction. Microbiome. 2021;9(1):224. 10.1186/s40168-021-01157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tse RT, Wong CY, Chiu PK, Ng CF. The potential role of spermine and its acetylated derivative in human malignancies. Int J Mol Sci. 2022;23(3):1258. 10.3390/ijms23031258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Novita Sari I, Setiawan T, Seock Kim K, Toni Wijaya Y, Won Cho K, Young Kwon H. Metabolism and function of polyamines in cancer progression. Cancer Lett. 2021;519:91–104. 10.1016/j.canlet.2021.06.020. [DOI] [PubMed] [Google Scholar]
- 154. Huang M, Zhang W, Chen H, Zeng J. Targeting polyamine metabolism for control of human viral diseases. Infect Drug Resist. 2020;13:4335–46. 10.2147/IDR.S262024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Firpo MR, Mounce BC. Diverse functions of polyamines in virus infection. Biomolecules. 2020;10(4):628. 10.3390/biom10040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Andrade Silva M, da Silva A, do Amaral MA, Fragas MG, Camara NOS. Metabolic alterations in SARS-CoV-2 infection and its implication in kidney dysfunction. Front Physiol. 2021;12:624698. 10.3389/fphys.2021.624698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Migliaccio MG, Di Mauro M, Ricciolino R, Spiniello G, Carfora V, Verde N, et al. Renal involvement in COVID-19: a review of the literature. Infect Drug Resist. 2021;14:895–903. 10.2147/IDR.S288869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Grandvaux N, Gaboriau F, Harris J, tenOever BR, Lin R, Hiscott J. Regulation of arginase II by interferon regulatory factor 3 and the involvement of polyamines in the antiviral response. FEBS J. 2005;272(12):3120–31. 10.1111/j.1742-4658.2005.04726.x. [DOI] [PubMed] [Google Scholar]
- 159. He YY, Yan Y, Jiang X, Zhao JH, Wang Z, Wu T, et al. Spermine promotes pulmonary vascular remodelling and its synthase is a therapeutic target for pulmonary arterial hypertension. Eur Respir J. 2020;56(5):2000522. 10.1183/13993003.00522-2020. [DOI] [PubMed] [Google Scholar]
- 160. Zhu L, Xiao R, Zhang X, Lang Y, Liu F, Yu Z, et al. Spermine on endothelial extracellular vesicles mediates smoking-induced pulmonary hypertension partially through calcium-sensing receptor. Arterioscler Thromb Vasc Biol. 2019;39(3):482–95. 10.1161/ATVBAHA.118.312280. [DOI] [PubMed] [Google Scholar]
- 161. Ilmarinen P, Moilanen E, Erjefalt JS, Kankaanranta H. The polyamine spermine promotes survival and activation of human eosinophils. J Allergy Clin Immunol. 2015;136(2):482–4.e11. 10.1016/j.jaci.2014.12.1922. [DOI] [PubMed] [Google Scholar]
- 162. Jain V, Raina S, Gheware AP, Singh R, Rehman R, Negi V, et al. Reduction in polyamine catabolism leads to spermine-mediated airway epithelial injury and induces asthma features. Allergy. 2018;73(10):2033–45. 10.1111/all.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]