Abstract
Background and aims:
Intrahepatic cholestasis of pregnancy (ICP) is a special liver disease during pregnancy, characterized by abnormal bile acid metabolism. However, there is no consensus on how to group women with ICP based on the time of diagnosis worldwide. This study aimed to adopt a new grouping model of women with ICP, and the time from diagnosis to delivery was defined as the monitoring period.
Methods:
This retrospective real-world data study was conducted across multiple centers and included 3172 women with ICP. The study first evaluated the significant difference in medication and nonmedication during different monitoring times. The least absolute shrinkage and selection operator (LASSO) model was then used to screen nine risk factors based on the predictors. The model's discrimination, clinical usefulness, and calibration were assessed using the area under the receiver operating characteristic (ROC) curve, decision curve, and calibration analysis.
Results:
The incidence of preeclampsia risk in ICP patients without drug intervention increased with the extension of the monitoring period. However, the risk of preeclampsia decreased in ICP patients treated with ursodeoxycholic acid. A predictive nomogram and risk score model was developed based on nine risk factors. The area under the ROC curve of the nomogram was 0.765 [95% confidence interval (CI): 0.724–0.807] and 0.812 (95% CI: 0.736–0.889) for the validation cohort.
Conclusions:
This study found that a longer ICP monitoring period could lead to adverse pregnancy outcomes in the absence of drug intervention, especially preeclampsia. A predictive nomogram and risk score model was developed to better manage ICP patients, maintain pregnancy to term delivery, and minimize the risk of severe adverse maternal and fetal outcomes.
Keywords: intrahepatic cholestasis of pregnancy, monitoring times, nomogram, preeclampsia, ursodeoxycholic acid
INTRODUCTION
Intrahepatic cholestasis of pregnancy (ICP) is a common pregnancy-related disease caused by a metabolic disorder of the liver, but the etiology and pathogenesis are not yet fully understood [1]. The clinical symptoms of ICP are characterized by skin itching and elevated serum total bile acid (TBA) concentrations, which are generally considered harmless for pregnant women. Additionally, ICP can increase the risk of adverse outcomes for fetuses, including preterm birth, meconium-stained amniotic fluid, neonatal unit admission, intrauterine fetal distress, and stillbirth [2].
Most studies on maternal and fetal outcomes of ICP patients are grouped based on the levels of TBA [3]. For example, studies have shown that only extremely high concentrations (TBA ≥ 100 μmol/l) are significantly associated with an increased risk of stillbirth, while there are no serious maternal and fetal outcomes in women with TBA levels of 40–99 μmol/l and less than 40 μmol/l [4,5]. However, the majority of women with ICP have a maximum TBA of less than 100 μmol/l, and bile acid metabolism can be affected by various factors such as environment, diet, and heredity [6]. Another perspective in ICP research focuses on the timing of onset and its adverse effects on pregnant women and infants. This has led to two grouping modes: early-onset ICP and late-onset ICP. However, there is no consensus on the definition of ICP grouping time worldwide, resulting in different standards of delineation. For instance, some studies define the grouping time as 28 weeks of pregnancy at diagnosis [7,8], while others use 33 weeks [9]. These differences in interpretation can lead to varying analysis results.
In this study, we aimed to improve the grouping of ICP patients by introducing a new term: the ICP monitoring period. This refers to the time between the diagnosis of ICP and delivery, during which clinical monitoring and management take place. Based on this concept, we divided patients into three groups: short-term monitoring (<1 week), medium and long-term monitoring (≥1 week to <1 month), and long-term monitoring (≥1 month). Through various statistical analyses, we found that the risk of preeclampsia in ICP patients without drug intervention increased with longer monitoring periods. However, ICP patients who received timely drug treatment (mainly ursodeoxycholic acid) during the monitoring period had a reduced risk of preeclampsia and were able to carry their pregnancy to term. Furthermore, clinicians can use the constructed nomogram and risk score model to evaluate the risk of complicated preeclampsia in ICP patients and develop personalized management plans quickly and easily.
METHODS
Ethics approval
The study has been approved by the ethics committee of Chongqing Medical University (ID: 20220627). During the data collection and analysis process, all personally identifiable information of pregnant women was removed to ensure the privacy of the participants.
Study design and population
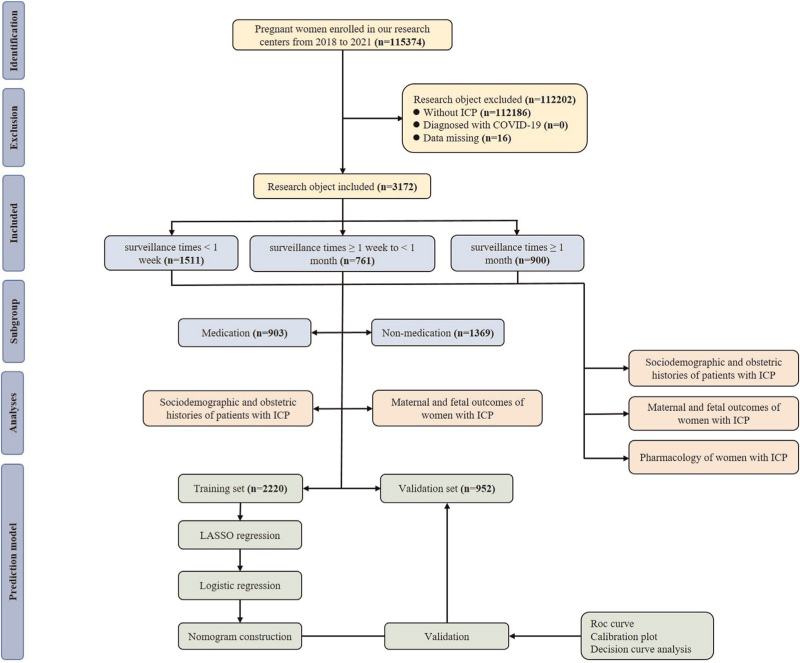
This retrospective real-world data study was conducted across two Grade III and Grade A hospitals in Chongqing, China, namely the First Affiliated Hospital of Chongqing Medical University and the Women and Children's Hospital of Chongqing Medical University from 2018 to 2021. These two hospitals are the largest maternity hospitals in Chongqing, and the total number of newborns exceeded 10 000 and 15 000, respectively. The electronic medical records of both hospitals systematically stored details of all pregnant women, including sociodemographic data, birth history, and various clinical information. The diagnosis of ICP was conducted by a gynecologist in accordance with the guidelines for the diagnosis and treatment of ICP [10]. The criteria for diagnosis included unexplained pruritus in pregnant women, abnormal liver function tests, normal TBA or elevated TBA levels (10 μmol/l), no other identifiable liver disease, and resolution after delivery. Mild ICP was diagnosed when pruritus was present, and the fasting serum bile acid concentration was greater than 10 μmol/l but less than 40 μmol/l, and severe ICP is diagnosed as the fasting serum bile acid concentration is greater than 40 μmol/l. Exclusion criteria were women without ICP, those diagnosed with the COVID-19 virus, or those with missing data. After the screening, a total of 3172 women with ICP were included for further analysis. To construct and validate the prediction model, patients were randomly divided into a training cohort (n = 2220) and a validation cohort (n = 952) at a ratio of 7 : 3 using the random number table method. For a detailed flowchart, please refer to Fig. 1.
FIGURE 1.
The flow chart of the study.
Data collection
All demographic and clinical data were obtained from electronic medical records at two hospitals, including maternal and neonatal information and outcomes. Medical records are extracted simultaneously by two data collectors, and two different descriptions are checked and processed for accurate data extraction.
Definitions
The height and weight before pregnancy were reported in the prenatal survey by all the participants. Body mass index (BMI) was classified into four categories according to Chinese guidelines: normal weight (BMI 18.5–23.9 kg/m2), overweight (BMI 24.0–27.9 kg/m2), and obese (BMI ≥28 kg/m2) [11]. Maternal outcomes included the mode of delivery, gestational diabetes mellitus A1 (GDM A1, treat with dietary therapy), gestational diabetes mellitus A2 (GDM A2, needed pharmacologic treatment), gestational hypertension, preeclampsia, postpartum hemorrhage (defined as >1000 ml), placenta accreta spectrum, placenta residue, polyhydramnios [defined as an Amniotic Fluid Index (AFV) of > 2000 ml], and oligohydramnios (defined as an AFV that is less than 200 ml) [12,13]. According to the criteria of the International Association of the Diabetes and Pregnancy Study Groups Consensus Panel (IADPSG/WHO), GDM was defined as the following: after 8–10 h of overnight fasting all the pregnant women during 24–28 gestational weeks were given a 75 g oral glucose tolerance test (OGTT), the diagnosis was made when any of the following criteria were met, including fasting glucose ≥ 5.1 mmol/l, 1 h glucose ≥ 10.0 mmol/l, or 2 h glucose ≥ 8.5 mmol/l [14]. Gestational hypertension (HDP) is defined as. systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg at least two times apart, occurring after 20 weeks’ gestation without significant proteinuria according to the International Association for the Study of Hypertension in Pregnancy. Preeclampsia was diagnosed by hypertension accompanied by proteinuria ≥300 mg in 24 h, or at least two readings ‘++’ on the dipstick in the test of midstream or catheter urine samples within 24 h [15].
Fetal outcomes include the small for gestational age (defined as birthweight <10th percentile), fetal growth restriction (FGR, fetal weight below the third percentile), macrosomia (birthweight > 4000 g), meconium-stained amniotic fluid, preterm birth (defined as <37 weeks of gestation), fetal distress, umbilical cord around neck or knots, congenital malformation, chromosomal abnormalities, low Apgar score (<7 after 1 min or 5 min), admissions to medium care unit and neonatal intensive care unit, and stillbirth (from 24 weeks of gestation to 7 days after delivery). All outcome measures are based on previous large-scale studies on comparative programs and national guidelines [16,17].
Statistical analysis
The collected data was analyzed using statistical methods to describe both categorical and continuous variables. The mean ± SD was used to manifest the continuous variables with a normal distribution, such as maternal age and gestational weight gain. Categorical variables, including gravidity, parity, body mass index, and other sociodemographic and obstetric histories, were described using frequency and percentages. Under the circumstances that the continuous variables obey a normal distribution, the one-way ANOVA was used if the inter-group variance is homogeneity. If the variance is inhomogeneity, the rank sum test (the Kruskal–Wallis H test) was used. Post hoc analysis was performed using L–S–D correction results for pairwise comparisons between groups with ≥3 independent groups. Results with significant differences between the two groups were shown in tables. Chi-square or Fisher's exact tests were used to compare categorical variables. Univariate logistic regression was used to analyze maternal and fetal outcomes of women with ICP in different monitoring groups. Results were presented as odds ratios (OR) and 95% confidence intervals (CIs). Factors with P-value <0.10 were considered as possible confounding factors and evaluated by backward elimination strategy in multivariate logical regression analysis, such as age, birth plurality, the severity of intrahepatic cholestasis of pregnancy, assisted reproductive technology, use of medication, and skin itch. These data were presented in the form of adjusted odds ratios (AOR) and 95% CI. Subgroup analyses were conducted to evaluate the significant difference in medication and nonmedication during different monitoring times. Missing data in the variables used for statistical analysis were not more than 5%, and multiple imputations were used to account for missing data with baseline efficacy variables, gestational weight gain, body mass index, gravidity, and parity as explanatory variables.
The area under the working characteristic curve (AUC) and C-index were used to evaluate the discrimination. The calibration was evaluated by a calibration diagram and the Hosmer-Lemeshow test. The cutoff value combined with the DCA curve was used to evaluate the clinical usefulness. A two-tailed P-value <0.05 was considered significant. All data refer to the pregnant women with ICP enrolled in our cohort study, and all analyses were performed by Statistical Package for the Social Sciences 26.0 software (SPSS 26.0, IBM Corporation, Chicago, Illinois, USA), and R software (Version v.4.4.1, Vienna, Austria).
RESULTS
Sociodemographic and obstetric histories of pregnant women with different ICP monitoring periods
A total of 3172 pregnant women with ICP who met the study criteria during the study period were included in the First Affiliated Hospital of Chongqing Medical University and the Women and Children's Hospital of Chongqing Medical University (Table S1). The length of time from the diagnosis of ICP to delivery was defined as the ICP monitoring period. Of the 3172 women, 1511 (48%) were classified as short-term monitoring (group 1), 761 (24%) as having medium monitoring (group 2), and 900 (28%) as having long-term monitoring (group 3) (Table S1). The data showed that the proportion of skin itch symptoms in the three groups were 29.5% (group 1), 41.4% (group 2), and 44.6% (group 3), respectively, but the severity of ICP in group 3 was not the most serious. Additionally, the proportion of women receiving drug therapy in group 3 (58.5%) was higher than that in group 1 (32.8%) and group 2 (53.6%) (Table S1). There were no significant differences in the gestational weight gain, gravidity, parity, abortion, Pregestation BMI, cigarette smoking, alcohol consumption, history of hepatobiliary disease, history of ICP, and history of abnormal gestation and birth among the three groups. Moreover, only a few pregnant women with endometriosis, polycystic ovary syndrome, gallbladder stones, pancreatic disease, and Rh negative were found, and there was no significant difference. These finding suggested that the baseline characteristics of the three groups were basically the same. The characteristics of other significant differences in the sociodemographic and obstetric history were identified as potential confounding factors, and the subsequent analysis was conducted using a multiple regression model for postadjustment analysis.
Comparison of maternal and fetal outcomes in women with intrahepatic cholestasis of pregnancy during three different monitoring periods
To determine the clinical significance of the different ICP monitoring periods, the impact of each of the periods on maternal and fetal outcomes was assessed using group 3 as a reference, and the specific analysis results are presented in Table 1. Overall, we observed that different ICP monitoring periods during pregnancy did not have a significant effect on the expectant women, and there was no statistical significance in the related pregnancy complications, including gestational diabetes mellitus, gestational hypertension, placenta accreta, rupture of membranes, polyhydramnios, and oligohydramnios. However, it is worth noting that group 1 (AOR 1.55, 95% CI: 1.05–2.29, P = 0.028) and group 2 (AOR 1.64, 95% CI: 1.06–2.54, P = 0.026) were associated with preeclampsia, and only group 2 (AOR 1.35, 95% CI: 1.10–1.81, P = 0.039) was associated with preterm premature. The difference was statistically significant after adjusting the potential confounding factors by multiple Logistic regression analysis.
TABLE 1.
Maternal and fetal outcomes of women with ICP during different monitoring times
| Group 1 (<1 week) | Group 2 (≥1 week to <1 month) | |||||||
| Outcomes | Crude OR (95% CI)† | P § | Adjusted OR (95% CI)§ | P § | Crude OR (95% CI)† | P † | Adjusted OR (95% CI)§ | P § |
| Maternal outcomes | ||||||||
| Cesarean delivery | 1.01 (0.84,1.20) | 0.969 | 1.05 (0.87,1.27) | 0.592 | 1.59 (1.28,1.98) | <0.001∗∗∗ | 1.50 (1.20,1.89) | <0.001∗∗∗ |
| Forceps assisted | 1.23 (0.65,2.34) | 0.526 | 1.45 (0.75,2.81) | 0.273 | 1.19 (0.57,2.52) | 0.642 | 1.28 (0.60,2.71) | 0.526 |
| Gestational diabetes mellitus A1 | 1.14 (0.91,1.43) | 0.269 | 1.07 (0.84,1.36) | 0.574 | 1.30 (1.01,1.69) | 0.048∗ | 1.18 (0.91,1.54) | 0.220 |
| Gestational diabetes mellitus A2 | 1.12 (0.62,2.04) | 0.700 | 0.98 (0.53,1.82) | 0.959 | 1.33 (0.69,2.58) | 0.398 | 1.14 (0.58,1.54) | 0.704 |
| Gestational hypertension | 0.80 (0.50,1.27) | 0.333 | 0.69 (0.43,1.12) | 0.132 | 1.23 (0.75,2.02) | 0.414 | 1.01 (0.60,1.68) | 0.985 |
| Preeclampsia | 1.21 (0.81,1.81) | 0.364 | 1.55 (1.05,2.29) | 0.028∗ | 1.33 (0.85,2.09) | 0.209 | 1.64 (1.06,2.54) | 0.026∗ |
| Placenta accreta spectrum | 1.13 (0.81,1.58) | 0.477 | 1.01 (0.71,1.42) | 0.899 | 1.22 (0.84,1.79) | 0.300 | 1.08 (0.73,1.60) | 0.696 |
| Preterm premature rupture of membranes | 1.23 (0.99,1.54) | 0.067 | 1.09 (0.86,1.37) | 0.482 | 1.35 (1.02,1.80) | 0.038∗ | 1.35 (1.10,1.81) | 0.039∗ |
| Polyhydramnios | 1.62 (1.06,2.49) | 0.027 | 1.63 (1.05,2.54) | 0.030∗ | 1.27 (0.77,2.12) | 0.351 | 1.21 (0.72,2.03) | 0.476 |
| Oligohydramnios | 0.81 (0.63,1.05) | 0.116 | 0.80 (0.61,1.05) | 0.101 | 0.78 (0.57,1.06) | 0.111 | 0.82 (0.60,1.12) | 0.215 |
| Fetal outcomes | ||||||||
| Low birth weight | 0.90 (0.73,1.11) | 0.330 | 0.88 (0.71,1.10) | 0.261 | 1.45 (1.15,1.82) | 0.002∗∗ | 1.46 (1.15,1.84) | 0.002∗∗ |
| Fetal growth restriction | 1.24 (0.65,2.36) | 0.515 | 1.22 (0.62,2.39) | 0.574 | 1.36 (0.66,2.80) | 0.406 | 1.48 (0.70,3.11) | 0.304 |
| Macrosomia | 1.25 (0.76,2.07) | 0.384 | 1.48 (0.87,2.54) | 0.150 | 0.77 (0.40,1.48) | 0.429 | 0.76 (0.38,1.54) | 0.445 |
| Preterm delivery | 0.95 (0.76,1.18) | 0.622 | 0.93 (0.74,1.18) | 0.549 | 1.59 (1.24,2.02) | <0.001∗∗∗ | 1.58 (1.24,2.02) | <0.001∗∗∗ |
| Stillbirth | 2.09 (0.43,10.08) | 0.359 | 2.47 (0.49,12.59) | 0.275 | 0.59 (0.05,6.53) | 0.668 | 0.51 (0.05,5.67) | 0.584 |
| Fetal distress | 1.11 (0.88,1.38) | 0.379 | 1.08 (0.85,1.36) | 0.539 | 1.06 (0.82,1.38) | 0.660 | 1.03 (0.81,1.38) | 0.661 |
| Meconium-stained amniotic fluid | 1.28 (1.06,1.54) | 0.009∗∗ | 1.22 (1.03,1.48) | 0.042∗ | 1.31 (1.01,1.69) | 0.042∗ | 1.30 (1.02,1.67) | 0.042∗ |
| Umbilical cord around the neck | 1.19 (0.58,2.47) | 0.633 | 1.26 (0.58,2.73) | 0.558 | 1.19 (0.51,2.75) | 0.692 | 1.32 (0.55,3.14) | 0.531 |
| Fetus with congenital malformation | 1.23 (0.65,2.34) | 0.526 | 1.45 (0.75,2.81) | 0.273 | 1.19 (0.57,2.25) | 0.642 | 1.28 (0.60,2.71) | 0.526 |
| 1 min Apgar score <7 | 0.76 (0.28,2.06) | 0.595 | 1.02 (0.36,2.84) | 0.974 | 1.18 (0.41,3.39) | 0.753 | 1.14 (0.39,3.30) | 0.813 |
| 5 min Apgar <7 | 0.40 (0.07,2.38) | 0.311 | 0.76 (0.13,4.60) | 0.765 | 0.39 (0.04,3.79) | 0.420 | 0.38 (0.04,3.77) | 0.405 |
| Intensive care unit admission | 0.96 (0.74,1.26) | 0.778 | 0.95 (0.72,1.25) | 0.708 | 1.59 (1.20,2.12) | 0.001∗∗ | 1.57 (1.17,2.09) | 0.002∗∗ |
∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Group 3 (≥1 month) was used as the reference group.
Single variable logistic regression analysis.
Adjusted for age, birth plurality, the severity of intrahepatic cholestasis of pregnancy, assisted reproductive technology, using of medication, skin itch.
Further analysis of fetal outcomes showed that only group 2 had preterm delivery (AOR 1.35, 95% CI: 1.10–1.81, P = 0.039), low birth weight (AOR 1.46, 95% CI: 1.15–1.84, P = 0.002), and intensive care unit admission (AOR 1.57, 95% CI: 1.17–2.09, P = 0.002). These outcomes were clinically related to each other. Additionally, the statistical results of group 1 were consistent with those of group 2 among the three fetal outcomes, as pregnant women in group 1 were diagnosed with ICP, and the proportion of gestational weeks ≥ 37 was the highest (71.6%, Table S1). Nevertheless, short-term, medium-term, and long-term ICP monitoring did not lead to serious adverse outcomes such as fetal growth restriction, stillbirth, and fetal distress. In general, there were no serious maternal and infant outcomes and concurrent preeclampsia in long-term monitoring, considering the highest proportion of using of medication in group 3 (58.5%, Table S1), we speculated that the drug may have played a protective role to a certain extent.
Maternal and fetal outcomes of intrahepatic cholestasis of pregnancy women with and without medication during three different monitoring periods
To investigate whether drugs could improve the pregnancy outcome of women with ICP, variable control was carried out based on the grouping of monitoring periods. Sociodemographic and obstetric histories of women with ICP without or with medication during three monitoring periods are shown in Tables S2 and S3. The comparison revealed that the risk rate of preeclampsia was lower in group 1 (AOR 0.47, 95% CI: 0.26–0.86, P = 0.014) and group 2 (AOR 0.67, 95% CI: 0.51–0.88, P = 0.004) than in group 3 without medication, and there were no other serious maternal and fetal outcomes (Table 2). Further analysis showed that ICP women with medication had the opposite result, where the risk rate of preeclampsia in group 3 was significantly lower than that in group 1 (AOR 1.55, 95% CI: 1.09–2.21, P = 0.014) and group 2 (AOR 1.51, 95% CI: 1.04–2.18, P = 0.030, Table 3). These results suggest that longer monitoring times increase the likelihood of preeclampsia in women with ICP without drug intervention. However, using corresponding drugs in clinical management may reduce the incidence of ICP complicated with preeclampsia. Additionally, while ICP did not cause severe maternal and fetal outcomes, group 2 had a statistically significant incidence of preterm delivery, low birth weight, and intensive care unit admission, which were considered to be cascade effects caused by the highest proportion of twin pregnancies (18.6%, Table S3), and this was consistent with the overall analysis (Table S1 and Table 1).
TABLE 2.
Maternal and fetal outcomes of ICP women without medication during different monitoring times.
| Group 1 (<1 week) | Group 2 (≥1 week to <1 month) | |||||||
| Outcomes | Crude OR (95% CI)† | P § | Adjusted OR (95% CI)§ | P § | Crude OR (95% CI)† | P † | Adjusted OR (95% CI)§ | P § |
| Maternal outcomes | ||||||||
| Cesarean delivery | 1.30 (1.01,1.66) | 0.040∗ | 1.33 (1.03,1.71) | 0.031∗ | 2.10 (1.52,2.91) | <0.001∗∗∗ | 1.88 (1.34,2.63) | <0.001∗∗∗ |
| Forceps assisted | 1.37 (0.45,4.16) | 0.576 | 1.40 (0.46,4.28) | 0.555 | 0.79 (0.18,3.56) | 0.760 | 0.74 (0.16,3.39) | 0.698 |
| Gestational diabetes mellitus A1 | 0.82 (0.60,1.13) | 0.223 | 0.76 (0.55,1.04) | 0.089 | 1.06 (0.73,1.54) | 0.745 | 0.90 (0.61,1.31) | 0.569 |
| Gestational diabetes mellitus A2 | 0.76 (0.36,1.63) | 0.485 | 0.67 (0.31,1.46) | 0.316 | 1.05 (0.43,2.56) | 0.910 | 0.87 (0.35,2.16) | 0.767 |
| Gestational hypertension | 1.42 (0.85,2.36) | 0.177 | 1.27 (0.76,2.13) | 0.365 | 1.51 (0.84,2.74) | 0.172 | 1.24 (0.68,2.28) | 0.480 |
| Preeclampsia | 0.50 (0.28,0.89) | 0.020∗ | 0.47 (0.26,0.86) | 0.014∗ | 0.70 (0.54,0.91) | 0.009∗∗ | 0.67 (0.51,0.88) | 0.004∗∗ |
| Placenta accreta spectrum | 0.97 (0.62,1.52) | 0.897 | 0.94 (0.59,1.48) | 0.774 | 1.35 (0.80,2.26) | 0.264 | 1.17 (0.69,2.00) | 0.558 |
| Preterm premature rupture of membranes | 1.21 (0.89,1.64) | 0.216 | 1.17 (0.86,1.60) | 0.313 | 0.59 (0.39,0.89) | 0.012∗ | 0.65 (0.42,0.99) | 0.046∗ |
| Polyhydramnios | 1.38 (0.75,2.51) | 0.300 | 1.32 (0.72,2.43) | 0.369 | 0.98 (0.45,2.10) | 0.949 | 0.85 (0.39,1.89) | 0.697 |
| Oligohydramnios | 0.85 (0.59,1.24) | 0.399 | 0.791 (0.54,1.15) | 0.217 | 0.87 (0.55,1.38) | 0.585 | 0.84 (0.53,1.33) | 0.451 |
| Fetal outcomes | ||||||||
| Low birth weight | 0.87 (0.64,1.17) | 0.351 | 0.85 (0.63,1.15) | 0.294 | 1.35 (0.95,1.91) | 0.091 | 1.30 (0.91,86) | 0.143 |
| Fetal growth restriction | 1.26 (0.54,2.95) | 0.597 | 1.24 (0.52,2.93) | 0.629 | 0.90 (0.30,2.70) | 0.850 | 0.83 (0.27,2.52) | 0.738 |
| Macrosomia | 1.02 (0.49,2.13) | 0.951 | 1.05 (0.50,2.19) | 0.905 | 0.41 (0.13,1.33) | 0.139 | 0.44 (0.13,1.41) | 0.166 |
| Preterm delivery | 0.90 (0.66,1.23) | 0.511 | 0.87 (0.63,1.20) | 0.391 | 1.31 (0.91,1.90) | 0.147 | 1.25 (0.86,1.81) | 0.248 |
| Fetal distress | 1.08 (0.70,1.66) | 0.734 | 1.02 (0.66,1.58) | 0.921 | 0.83 (0.48,1.45) | 0.570 | 0.84 (0.48,1.47) | 0.538 |
| Meconium-stained amniotic fluid | 1.01 (0.74,1.39) | 0.941 | 0.98 (0.71,1.35) | 0.893 | 1.01 (0.68,1.47) | 0.992 | 1.01 (0.68,1.49) | 0.978 |
| Umbilical cord around the neck | 0.78 (0.39,1.55) | 0.475 | 0.67 (0.33,1.36) | 0.269 | 0.98 (0.70,1.37) | 0.908 | 1.07 (0.70,1.50) | 0.706 |
| 1 min Apgar score <7 | 1.83 (0.21,15.71) | 0.528 | 1.77 (0.20,15.36) | 0.607 | 1.05 (0.07,16.87) | 0.972 | 1.22 (0.08,19.96) | 0.888 |
| Intensive care unit admission | 0.91 (0.63,1.33) | 0.636 | 0.92 (0.62,1.35) | 0.659 | 1.57 (1.03,2.41) | 0.037∗ | 1.53 (0.99,2.36) | 0.055 |
The sample size of the outcomes Stillbirth, Fetus with congenital malformation and 5 min Apgar score <7 is too small to present.
∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Group 3 (≥1 month) was used as the reference group.
Single variable logistic regression analysis.
Adjusted for age, birth plurality, the severity of intrahepatic cholestasis of pregnancy, assisted reproductive technology, and skin itch.
TABLE 3.
Maternal and fetal outcomes of ICP women with medication during different monitoring times.
| Group 1 (<1 week) | Group 2 (≥ 1 week to <1 month) | |||||||
| Outcomes | Crude OR (95% CI)† | P § | Adjusted OR (95% CI)§ | P § | Crude OR (95% CI)† | P † | Adjusted OR (95% CI)§ | P § |
| Maternal outcomes | ||||||||
| Cesarean delivery | 0.85 (0.65,1.11) | 0.229 | 0.85 (0.64,1.11) | 0.222 | 1.28 (0.95,1.73) | 0.102 | 1.28 (0.95,1.73) | 0.108 |
| Forceps assisted | 1.50 (0.66,3.41) | 0.333 | 1.55 (0.68,3.54) | 0.299 | 1.45 (0.61,3.45) | 0.402 | 1.55 (0.65,3.69) | 0.326 |
| Gestational diabetes mellitus A1 | 1.14 (0.58,2.23) | 0.710 | 1.07 (0.54,2.10) | 0.852 | 1.72 (0.90,3.28) | 0.101 | 1.70 (0.89,3.25) | 0.109 |
| Gestational diabetes mellitus A2 | 1.70 (0.65,4.41) | 0.279 | 1.64 (0.63,4.28) | 0.310 | 1.68 (0.62,4.56) | 0.306 | 1.66 (0.61,4.50) | 0.320 |
| Gestational hypertension | 1.35 (0.62,2.91) | 0.449 | 1.31 (0.60,2.83) | 0.497 | 1.99 (0.95,4.18) | 0.069 | 1.83 (0.86,3.88) | 0.116 |
| Preeclampsia | 1.57 (1.11,2.23) | 0.011∗ | 1.55 (1.09,2.21) | 0.014∗ | 1.53 (1.06,2.21) | 0.023∗ | 1.51 (1.04,2.18) | 0.030∗ |
| Placenta accreta spectrum | 1.19 (0.70,2.01) | 0.520 | 1.16 (0.68,1.97) | 0.581 | 1.03 (0.58,1.83) | 0.924 | 1.02 (0.58,1.82) | 0.942 |
| Preterm premature rupture of membranes | 0.90 (0.62,1.30) | 0.577 | 0.90 (0.62,1.30) | 0.566 | 0.89 (0.61,1.32) | 0.570 | 0.88 (0.60,1.31) | 0.538 |
| Polyhydramnios | 1.92 (1.03,3.60) | 0.041∗ | 1.97 (1.05,3.70) | 0.034∗ | 1.57 (0.80,3.09) | 0.195 | 1.58 (0.80,3.12) | 0.186 |
| Oligohydramnios | 0.75 (0.51,1.12) | 0.159 | 0.75 (0.50,1.11) | 0.151 | 0.70 (0.46,1.07) | 0.100 | 0.71 (0.47,1.09) | 0.120 |
| Fetal outcomes | ||||||||
| Low birth weight | 0.93 (0.68,1.27) | 0.652 | 0.94 (0.69,1.29) | 0.705 | 1.53 (1.12,2.08) | 0.007∗∗ | 1.54 (1.13,2.10) | 0.006∗∗ |
| Fetal growth restriction | 0.76 (0.24,2.41) | 0.643 | 0.78 (0.24,2.47) | 0.666 | 1.87 (0.71,4.97) | 0.207 | 1.93 (0.79,5.14) | 0.187 |
| Macrosomia | 1.67 (0.82,3.39) | 0.156 | 1.61 (0.79,3.28) | 0.189 | 1.10 (0.49,2.48) | 0.819 | 0.98 (0.43,2.27) | 0.970 |
| Preterm delivery | 0.95 (0.67,1.33) | 0.742 | 0.96 (0.69,1.36) | 0.833 | 1.84 (1.33,2.54) | <0.001∗∗∗ | 1.86 (1.34,2.58) | <0.001∗∗∗ |
| Stillbirth | 1.07 (0.15,7.62) | 0.947 | 1.03 (0.14,7.40) | 0.977 | 0.65 (0.06,7.17) | 0.723 | 0.60 (0.05,6.66) | 0.678 |
| Fetal distress | 1.35 (0.84,2.16) | 0.211 | 1.32 (0.83,2.12) | 0.387 | 1.11 (0.67,1.86) | 0.680 | 1.1 (0.66,1.84) | 0.712 |
| Meconium-stained amniotic fluid | 1.15 (0.82,1.60) | 0.421 | 1.16 (0.83,1.62) | 0.246 | 1.59 (1.14,2.22) | 0.007 | 1.58 (1.13,2.20) | 0.008∗∗ |
| Umbilical cord around the neck | 0.98 (0.74,1.29) | 0.863 | 0.99 (0.74,1.31) | 0.916 | 1.03 (0.76,1.38) | 0.865 | 1.06 (0.79,1.43) | 0.711 |
| 1 min Apgar score <7 | 0.71 (0.20,2.53) | 0.598 | 0.67 (0.19,2.40) | 0.540 | 1.30 (0.42,4.06) | 0.651 | 1.24 (0.39,3.87) | 0.717 |
| 5 min Apgar <7 | 0.71 (0.12,4.28) | 0.710 | 0.70 (0.12,4.21) | 0.694 | 0.43 (0.05,4.16) | 0.467 | 0.44 (0.05,4.22) | 0.473 |
| Intensive care unit admission | 1.01 (0.68,1.51) | 0.950 | 1.05 (0.71,1.57) | 0.804 | 1.60 (1.09,2.35) | 0.016∗ | 1.61 (1.10,2.38) | 0.016∗ |
∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Group 3 (≥1 month) was used as the reference group.
Single variable logistic regression analysis.
Adjusted for age and severity of intrahepatic cholestasis of pregnancy.
Pharmacology of women with intrahepatic cholestasis of pregnancy during three different monitoring periods
In the cohort of the medication group, one or more drugs are typically used in clinical management based on the actual symptoms of pregnant women with ICP. Table S4 shows the details of drug usage. The results indicate that the three monitoring groups primarily used a single drug, with proportions of 62.0% (group 1), 61.0% (group 2), and 66.4% (group 3), respectively. Ursodeoxycholic acid accounted for the highest proportion of the four commonly used drugs for treating ICP, representing 80.4, 84.8, and 84.7% in the three monitoring groups, respectively. The second most commonly used drug was reduced glutathione tablets, accounting for 32.9, 30.1, and 28.9%, respectively. As for postmenstrual age (PMA) treatment, there was no significant difference was found in using heparin and progesterone treatment among the three monitoring groups.
Least absolute shrinkage and selection operator regression analysis in training cohort
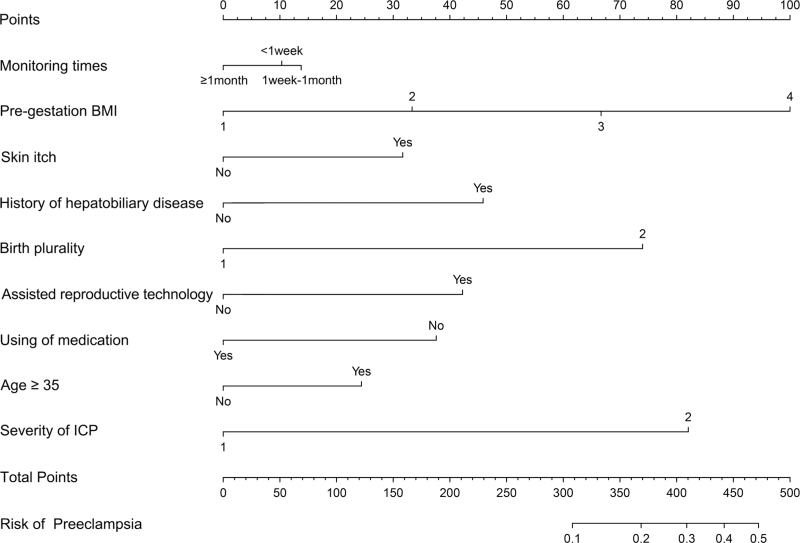
Finally, this study screened the potential risk variables and constructed prediction models for women with ICP during different ICP monitoring periods. Least absolute shrinkage and selection operator (LASSO) regression analysis was used to select independent risk factors for a linear regression model by reducing the regression coefficients of variables to zero. Regression coefficients were assumed to be zero and the corresponding variables were excluded from the model. The variables with nonzero regression coefficients were considered independent risk factors and were more closely related to the outcome event. Seventeen potential risk factors were introduced, and LASSO regression was performed using 10-fold cross-validation (Figure S1). Finally, nine variables with nonzero regression coefficients were selected, including monitoring time, pregestation BMI, skin itch, history of hepatobiliary disease, birth plurality, assisted reproductive technology, use of medication, age ≥35, and severity of ICP. These nine potential risk factors were combined into a prediction model and displayed using a nomogram (Fig. 2).
FIGURE 2.
Nomogram for predicting preeclampsia in women with ICP during different monitoring times.
Validation of nomogram for women with intrahepatic cholestasis of pregnancy
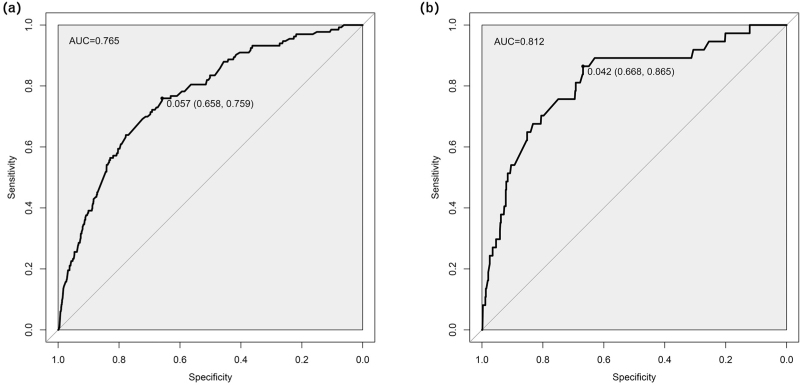
The C-index was used to estimate the performance of the predictive model. In the derived cohort, the area under the ROC curve was 0.765 (95% CI: 0.724–0.807), and the sensitivity and specificity were 65.8 and 75.9%, respectively. In the validation cohort, C-index was 0.812 (95% CI: 0.736–0.889) and the sensitivity and specificity were 66.8% and 86.5% respectively (Fig. 3). Moreover, bootstrap resampled validation results (number = 1000) confirmed the stable performance of the nomograms in the validation set. The C-indexes of the nomogram prediction model in the training cohort and validation cohort were >0.70, showing good discrimination of the constructed model.
FIGURE 3.
Comparison of the ROC curves of the nomogram model. (a) The ROC curve of the training set. (b) The ROC curve of the validation set. The x-axis represents 1-specificity, and the y-axis represents sensitivity. The part below the black line is the area under the ROC curve of the model.
The calibration curves of the nomogram prediction model in the training cohort and the validation cohort showed that the predicted probabilities were in good agreement with the actual probabilities (Figure S2). The Hosmer-Lemeshow test results of the nomogram prediction model in the training cohort and validation cohort were χ2 = 7.567 (P = 0.477) and χ2 = 4.299 (P = 0.745). All P-values of the Hosmer-Lemeshow test nomogram prediction model were greater than 0.05 in the training group and the validation group and the difference was not statistically significant. This means the model is highly calibrated.
DCA curves of the training cohort and validation cohort were drawn to estimate clinical usefulness (Figure S3). The net benefit to the patient was greater than the other two curves when the marginal probabilities were between 2 and 27%. The horizontal line indicates that none of the patients developed preeclampsia and was not treated, and no intervention benefit was obtained. The diagonal line suggests that all patients developed preeclampsia and the benefit after treatment. Thus, clinical DCA shows that the line chart prediction model has good clinical practicability within these ranges.
Clinical application of the nomogram
Take a patient with ICP as an example (Figure S4), and present the detailed clinical information as follows: monitoring times (1 week to 1 month), pregestation BMI (24–27.9), skin itch, with a history of the hepatobiliary disease, birth plurality is twins, assisted reproductive technology, no using of medication, age < 35, the severity of ICP is severe. According to the nomogram, the total score was approximately 397, and the predicted risk of preeclampsia was approximately 27%. Therefore, some interventions should be taken to reduce the risk of patients developing preeclampsia resulting in adverse pregnancy outcomes.
DISCUSSION
Cholestasis is a common concomitant disease in pregnant women that is a serious health burden for both pregnant women and fetuses. Due to the lack of comprehensive experimental research, little is known about the pathogenesis and clinical treatment of ICP. Therefore, ICP is still a difficult disease to manage during pregnancy. The hallmark symptom of ICP is skin itch, which mainly occurs in the palms and soles, ranging from mild to severe, typically worse at night [18]. Obvious clinical symptoms usually occur in the second and third trimesters of pregnancy, with up to 80% of women appearing after 30 weeks [17], which is a critical period for the rapid development of fetal organs. It seriously affects the physical and mental health and sleep quality of pregnant women, affecting the healthy development of the fetus. Serum total bile acid combined with transaminase have been the most commonly used biomarkers to diagnose ICP in clinical practice [19]. At present, the bile acid level of 10–15 μmol/l is used as the cutoff value for evaluating ICP [5,20]. Since many laboratory cutoffs are determined by the general population, including men and nonpregnant women, the pregnancy-specific cutoff remains to be verified. Although many studies had reported that premature rate elevated with the increase of bile acid concentration, an extremely high bile acid level (≥100 μmol/l) was associated with the risk of stillbirth [4,16,21]. To our knowledge, there is no study on the threshold of abnormal bile acid as a drug intervention.
In recent years, both scholars and clinicians have been focusing more on ICP and have discovered that it not only affects liver metabolism but also leads to maternal co-morbidity, such as preeclampsia and gestational diabetes mellitus [22]. Previous study has shown that the incidence of preeclampsia increased among patients with ICP, both in singleton and twin pregnancies. Interestingly, the incidence is not related to the duration of exposure to excess bile acid or the time of onset of ICP [23]. Another study found that maternal TBA levels were normal or decreased to the normal range, but preeclampsia still occurred [24]. This could be because the initial elevated TBA levels would trigger a cascade of onsets that later lead to preeclampsia. Additionally, a study showed that the incidence of gestational diabetes mellitus was higher in women predisposed to developing ICP [25]. Moreover, the proportion of women with combined ICP and gestational diabetes mellitus who developed preeclampsia during pregnancy increased significantly [26]. Our study did not find a direct relationship between ICP and the incidence of gestational diabetes mellitus, but it supported the view that ICP is more likely to develop into preeclampsia, and the longer the monitoring period, the higher the incidence of preeclampsia (Table 2).
Ursodeoxycholic acid, a naturally occurring bile acid, is less synthesized in the human body. It can increase bile acid excretion by upregulating hepatic metabolizing enzymes and bile acid transporters, thus improving cholestasis [27]. Ursodeoxycholic acid is widely recommended in the guidelines for the management of ICP in many countries and has become the first-line drug for clinical treatment of ICP [28–30], although studies have shown that it does not reduce the adverse perinatal outcomes in ICP women [31,32]. A meta-analysis showed that ursodeoxycholic acid effectively improved pruritus in women with ICP and had a good safety profile for use during pregnancy [33]. The relief of pruritus in ICP women is not only an effective treatment for the disease but also has far-reaching significance for the mental health of pregnant women and fetal gestation. Moreover, our study found that the incidence of preeclampsia was higher in ICP patients who were not treated with drugs and had longer monitoring periods (Table 2); while the incidence of preeclampsia decreased significantly in long-term monitored ICP patients (≥1 month) after receiving drug treatment (Table 3). To our knowledge, there are no reports about ursodeoxycholic acid preventing the occurrence of preeclampsia in patients with ICP. One mechanism suggested that ursodeoxycholic acid may inhibit organic anion-transporting polypeptide 4A1-mediated bile acid transfer and taurocholate-induced placental vasoconstriction [34]. Therefore, we speculated that ursodeoxycholic acid may improve preeclampsia induced by elevated blood pressure through this pathway, further research are needed to confirm it.
Based on the LASSO regression analysis data from the training cohort study, we developed a nomogram and validated the model to predict the incidence of preeclampsia in individuals with ICP. The calibration curve demonstrated that the accurate cumulative hazard predictions of the nomogram were highly fitted with the actual situation, and the decision curve analysis show excellent prediction. Clinicians could evaluate the possibility of combined with preeclampsia according to the risk factors of newly admitted women with ICP and assist them in carrying out individual clinical management of ICP patients and identifying the best time to use drugs. The nomogram uses routine clinical variables readily available to clinicians, making it valuable in clinical practice.
In conclusion, this study found that the longer ICP monitoring periods without drug intervention were likely to lead to adverse pregnancy outcomes, especially preeclampsia. Ursodeoxycholic acid treatment for ICP may prevent the risk of preeclampsia to some extent. The results of this study are expected to provide a new strategy for better clinical management of ICP, selecting the appropriate timing of drug use during different monitoring periods and providing a basis for further study of the pharmacological mechanism of ursodeoxycholic acid in the treatment of ICP.
Limitations
Although this is the first large-scale cohort study about the monitoring time on pregnancy outcomes in pregnant women with ICP and what role ursodeoxycholic acid plays, there are still some limitations. Firstly, this is a retrospective cohort study, so it is difficult to obtain and quantify the diet, exercise, and mental state of pregnant women with ICP, limiting us from exploring the interference of these factors. Furthermore, although we used standard statistical analysis including subgroup analysis and other methods to imitate the actual situation, there is still a gap with RCT research. Thus, more evidence is needed to evaluate the conclusion of this study.
ACKNOWLEDGEMENTS
The authors also thank all involved laboratory technicians for their help with data collection and analysis.
Author contributions: Conceptualization, Q-Y.C., Z.-H.L., L.W., and Y.-X.W.; methodology, Q-Y.C., Z.-H.L., B.-N.D., and T.-H.L.; software, Q-Y.C., Z.-H.L., X.Luo, and L.-F.L.; validation, B.-N.D., Y.C., and C.-Y.L.; investigation, Q-Y.C., B.-N.D., L.-F.L., C.-Y.L., and Y.C.; resources, X.Luo., X.Lan., T.-H.L., and L.W.; data curation, Q-Y.C., Z.-H.L., B.-N.D., and T.-H.L.; writing—original draft preparation, Q-Y.C., Z.-H.L., and T.-H.L.; writing—review and editing, X.Luo, Y.-X.W., and L.W.; visualization, Q-Y.C., Z.-H.L., and, C.-Y.L.; supervision, X.Luo, Y.-X.W., and L.W.; project administration, L. W. and Y.-X.W.; funding acquisition, T.-H.L. and Y.-X.W. All authors have read and agreed to the published version of the manuscript.
Consent for publication: Not applicable.
Ethics approval and consent to participate: This study has been approved by the ethics committee of Chongqing Medical University, and the content of the approval includes the oral informed consent acquisition scheme and privacy protection method (ID: 20220627). The whole study was carried out in strict accordance with the ethical standards of the institutional research committee and the 1964 Helsinki declaration and its later amendments.
Funding: This study was supported by the National Natural Science Foundation of China (Grant number 82271707) and the Program for Youth Innovation in Future Medicine, Chongqing Medical University (Grant number W0068).
Availability of data and materials: The data underlying this article will be provided by the corresponding author on reasonable request.
Conflicts of interest
The authors declared that they have no conflict of interest.
Supplementary Material
Q.-Y.C. and Z.-H.L. contributed equally to this work.
Abbreviations: AFV, Amniotic Fluid Index; AOR, adjusted odds ratios; BMI, body mass index; CI, confidence interval; DCA, decision curve analysis; FGR, fetal growth restriction; GDM A1, gestational diabetes mellitus A1; GDM A2, gestational diabetes mellitus A2; HDP, gestational hypertension; ICP, intrahepatic cholestasis of pregnancy; OGTT, oral glucose tolerance test; OR, odds ratios; PMA, postmenstrual age; ROC, receiver operating characteristic; TBA, total bile acids
Supplemental digital content is available for this article.
REFERENCES
- 1.Xiao J, Li Z, Song Y, Sun Y, Shi H, Chen D, et al. Molecular pathogenesis of intrahepatic cholestasis of pregnancy. Can J Gastroenterol Hepatol 2021; 2021:6679322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DD, Rood KM. Intrahepatic cholestasis of pregnancy. Clin Obstet Gynecol 2020; 63:134–151. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen JH, Lykke JA. Differentiated timing of induction for women with intrahepatic cholestasis of pregnancy – a historical cohort study. Acta Obstet Gynecol Scand 2021; 100:279–285. [DOI] [PubMed] [Google Scholar]
- 4.Ovadia C, Seed PT, Sklavounos A, Geenes V, Di Ilio C, Chambers J, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet 2019; 393:899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huri M, Seravalli V, Lippi C, Tofani L, Galli A, Petraglia F, et al. Intrahepatic cholestasis of pregnancy – time to redefine the reference range of total serum bile acids: a cross-sectional study. BJOG 2022; 129:1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diken Z, Usta IM, Nassar AH. A clinical approach to intrahepatic cholestasis of pregnancy. Am J Perinatol 2014; 31:1–8. [DOI] [PubMed] [Google Scholar]
- 7.Jin J, Pan SL, Huang LP, Yu YH, Zhong M, Zhang GW. Risk factors for adverse fetal outcomes among women with early- versus late-onset intrahepatic cholestasis of pregnancy. Int J Gynaecol Obstet 2015; 128:236–240. [DOI] [PubMed] [Google Scholar]
- 8.Ma Z, Liu Y, Chai L, Jin G, Sun Y, Zhou S, et al. Metabolic changes in bile acids with pregnancy progression and their correlation with perinatal complications in intrahepatic cholestasis of pregnant patients. Sci Rep 2023; 13:1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labbe C, Delesalle C, Creveuil C, Dreyfus M. Early and later intrahepatic cholestasis of pregnancy (ICP): study of adverse pregnancy outcomes. Gynecol Obstet Fertil Senol 2018; 46:388–394. [DOI] [PubMed] [Google Scholar]
- 10.Obstetrics Subgroup CSoO, Gynecology CMA. Guidelines for diagnosis and treatment of intrahepatic cholestasis of pregnancy (2015). Zhonghua Fu Chan Ke Za Zhi 2015; 50:481–485. [PubMed] [Google Scholar]
- 11.Chen C, Lu FC. Department of Disease Control Ministry of Health PRC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci 2004; 17: (Suppl): 1–36. [PubMed] [Google Scholar]
- 12.Magann EF, Morton ML, Nolan TE, Martin JN, Jr, Whitworth NS, Morrison JC. Comparative efficacy of two sonographic measurements for the detection of aberrations in the amniotic fluid volume and the effect of amniotic fluid volume on pregnancy outcome. Obstet Gynecol 1994; 83:959–962. [DOI] [PubMed] [Google Scholar]
- 13.Dubil EA, Magann EF. Amniotic fluid as a vital sign for fetal wellbeing. Australas J Ultrasound Med 2013; 16:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Association of D, Pregnancy Study Groups Consensus P, Metzger BE, Gabbe SG, Persson B, Buchanan TA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33:676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 2001; 20:IX–XIV. [DOI] [PubMed] [Google Scholar]
- 16.Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology 2004; 40:467–474. [DOI] [PubMed] [Google Scholar]
- 17.Geenes V, Chappell LC, Seed PT, Steer PJ, Knight M, Williamson C. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population-based case-control study. Hepatology 2014; 59:1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ovadia C, Williamson C. Intrahepatic cholestasis of pregnancy: recent advances. Clin Dermatol 2016; 34:327–334. [DOI] [PubMed] [Google Scholar]
- 19.Manzotti C, Casazza G, Stimac T, Nikolova D, Gluud C. Total serum bile acids or serum bile acid profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy. Cochrane Database Syst Rev 2019; 7:CD012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakita T, Parikh LI, Ramsey PS, Huang CC, Zeymo A, Fernandez M, et al. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol 2015; 213:570.e571–e578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brouwers L, Koster MP, Page-Christiaens GC, Kemperman H, Boon J, Evers IM, et al. Intrahepatic cholestasis of pregnancy: maternal and fetal outcomes associated with elevated bile acid levels. Am J Obstet Gynecol 2015; 212:100.e101-e107. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Gao J, Liu J, Wang X, He J, Sun J, et al. Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes and preeclampsia. Ann Transl Med 2020; 8:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raz Y, Lavie A, Vered Y, Goldiner I, Skornick-Rapaport A, Landsberg Asher Y, et al. Severe intrahepatic cholestasis of pregnancy is a risk factor for preeclampsia in singleton and twin pregnancies. Am J Obstet Gynecol 2015; 213:395.e1–395.e8. [DOI] [PubMed] [Google Scholar]
- 24.Deng W, Zhang L, Du Q, Li Y, Chen J, Du L, et al. The association of serum total bile acid with new-onset hypertension during pregnancy. BMC Pregnancy Childbirth 2022; 22:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martineau M, Raker C, Powrie R, Williamson C. Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes. Eur J Obstet Gynecol Reprod Biol 2014; 176:80–85. [DOI] [PubMed] [Google Scholar]
- 26.Axelsen SM, Kampmann U, Koefoed AS, McIntyre D, Ovesen PG, Fuglsang J. Intrahepatic cholestasis of pregnancy: Association with glycaemic control in gestational diabetes. Diabet Med 2021; 38:e14574. [DOI] [PubMed] [Google Scholar]
- 27.Roma MG, Toledo FD, Boaglio AC, Basiglio CL, Crocenzi FA, Sanchez Pozzi EJ. Ursodeoxycholic acid in cholestasis: linking action mechanisms to therapeutic applications. Clin Sci (Lond) 2011; 121:523–544. [DOI] [PubMed] [Google Scholar]
- 28.Bicocca MJ, Sperling JD, Chauhan SP. Intrahepatic cholestasis of pregnancy: review of six national and regional guidelines. Eur J Obstet Gynecol Reprod Biol 2018; 231:180–187. [DOI] [PubMed] [Google Scholar]
- 29.Bacq Y, le Besco M, Lecuyer AI, Gendrot C, Potin J, Andres CR, et al. Ursodeoxycholic acid therapy in intrahepatic cholestasis of pregnancy: results in real-world conditions and factors predictive of response to treatment. Dig Liver Dis 2017; 49:63–69. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Liu XH, Qi HB, Li Z, Fu XD, Chen L, et al. Ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy: a multicentered randomized controlled trial. Eur Rev Med Pharmacol Sci 2015; 19:3770–3776. [PubMed] [Google Scholar]
- 31.Chappell LC, Bell JL, Smith A, Linsell L, Juszczak E, Dixon PH, et al. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial. Lancet 2019; 394:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yule CS, Holcomb DS, Kraus AC, Brown CEL, McIntire DD, Nelson DB. Cholestasis: a prospective study of perinatal outcomes and time to symptom improvement. Am J Perinatol 2021; 38:414–420. [DOI] [PubMed] [Google Scholar]
- 33.Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta-analysis. Gastroenterology 2012; 143:1492–1501. [DOI] [PubMed] [Google Scholar]
- 34.Lofthouse EM, Torrens C, Manousopoulou A, Nahar M, Cleal JK, O’Kelly IM, et al. Ursodeoxycholic acid inhibits uptake and vasoconstrictor effects of taurocholate in human placenta. FASEB J 2019; 33:8211–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]