Abstract
Purpose:
Our study aims were (a) to examine laryngeal vestibular closure (LVC) temporal measures in healthy adults across tasks used in the Modified Barium Swallow Impairment Profile (MBSImP) protocol to establish normative reference values and (b) to examine influences of age, gender, and swallow task on LVC temporal measures.
Method:
A retrospective analysis of 195 healthy adults (85 men, 110 women; age range: 21–89 years) who participated in a videofluoroscopic swallowing study was completed. Seven swallow tasks of standardized viscosities and volumes, as per the MBSImP protocol, were analyzed to measure time-to-LVC and LVC duration (LVCd). Descriptive statistics were employed for all measures of interest. Regression modeling was used to explore relationships between LVC temporal measures (time-to-LVC, LVCd) with age, gender, and swallow task. The relationship between time-to-LVC and LVCd was also explored.
Results:
Significant findings included an increasing trend in LVCd across age (older individuals had a longer LVCd), with women demonstrating a greater increase. Related to viscosity, LVCd was significantly shorter for pudding compared to thin liquid. Furthermore, when compared to 5-ml tasks, LVCd was significantly longer in cup tasks, while time-to-LVC was significantly shorter. An association was also observed between time-to-LVC and LVCd: As time-to-LVC decreased, LVCd increased.
Conclusions:
LVCd was influenced by age, gender, and swallow task. Longer time-to-LVC was observed in older individuals, particularly older women, and with thin liquids. Study findings contribute to adult normative reference values for LVC temporal measures (time-to-LVC and LVCd) across MBSImP swallowing tasks.
Supplemental Material:
Laryngeal vestibular closure (LVC) is a primary defense for airway protection during swallowing. Various biomechanical events occur to close the laryngeal inlet and prevent bolus entry, including laryngeal elevation, pharyngeal shortening, anterior hyoid movement, and tongue base retraction, all of which contribute to full epiglottic inversion (Pearson et al., 2012, 2013; Vose & Humbert, 2019). If any of these events go awry, the bolus may enter the airway, potentially contributing to undesirable consequences such as aspiration pneumonia (Giraldo-Cadavid et al., 2020; Marik, 2001).
Videofluoroscopic swallowing studies (VFSSs) are the gold standard to assess the various components of oropharyngeal swallowing physiology, and airway invasion is a primary clinical outcome of note. The Modified Barium Swallow Impairment Profile (MBSImP) is an evidence-based protocol that is widely used by clinicians during VFSS assessments to standardize bolus administration (Martin-Harris et al., 2008; Northern Speech Services [NSS], 2020). Targeted management of laryngeal approximation dysfunction relies on identification of the underlying cause of the airway invasion as observed during the VFSS; however, these factors are inconsistently reported by dysphagia clinicians (Vose et al., 2018). Airway invasion due to delayed or incomplete LVC requires a unique and targeted approach to therapy, and merely reporting a binary outcome of normal or abnormal LVC remains insufficient to inform clinical practice. Additional information on kinematic and temporal abnormalities of laryngeal approximation can be determined using tools such as the Analysis of Swallowing Physiology: Events, Kinematics & Timing (ASPEKT) method (Steele et al., 2019).
Evidence supports that, within certain patient populations, such as those with Parkinson's disease and oropharyngeal cancer, LVC values outside a normative range, such as a longer time-to-LVC and a shorter LVC duration (LVCd), increase the risk for bolus airway invasion (Barbon et al., 2020; Curtis et al., 2020; Dumican et al., 2023). As a result, there has been an increasing interest in elucidating the mechanics of LVC as well as how various participant demographics and bolus factors may influence LVC. Normative reference values are essential for clinicians to delineate typical from atypical findings to better inform management plans (e.g., Mancopes et al., 2021; Molfenter et al., 2019; Steele et al., 2019). To date, research on LVC measures have included both healthy and disordered populations using various bolus protocols under videofluoroscopy. Due to these inconsistencies, the current evidence base is not in agreement with regard to how time-to-LVC and LVCd are influenced by participant demographics (i.e., age and gender) and bolus characteristics (i.e., viscosity and volume). For example, a recent study by Mancopes et al. (2021) did not observe any age, gender, or volume effects on time-to-LVC in healthy adults but did observe an age effect on LVCd. Specifically, the authors observed an increase of 4 ms in LVCd for each additional year of age (Mancopes et al., 2021). This age effect, however, was not observed in a study of 44 healthy adults by Molfenter et al. (2019). Differences in study findings may be related to differences in tasks and bolus administration techniques. Mancopes et al. used natural cup sips of thin liquid, while Molfenter et al. employed a controlled amount (5 ml) of nectar-thick liquid. Steele et al. (2019), however, did not find differences in LVCd with various swallow tasks in their healthy adult cohort, including varying thicknesses of liquids employed (International Dysphagia Diet Standardisation Initiative [IDDSI] Levels 0–4). While previous research has explored aspects of swallowing kinematics, none has analyzed a large database to determine normative reference values for LVC temporal measures using MBSImP protocol tasks.
Therefore, the purposes of this study were (a) to examine LVC temporal measures in healthy adults across tasks used in the MBSImP protocol to establish normative reference values with a large sample of healthy adults and (b) to examine the influence of demographic (age, gender) and MBSImP swallow task (viscosity, volume) factors on LVC temporal measures. Consistent with previous research findings on related measures with smaller samples, we hypothesized that (a) time-to-LVC would lengthen with increasing bolus viscosity (Humbert et al., 2018) and shorten with increasing volume (Herzberg et al., 2019). While there is limited reporting on the effect of bolus properties on time-to-LVC, we hypothesize that increased sensory input form larger bolus volumes may shorten reaction times, whereas a longer reaction time is expected with increased bolus viscosities due to the slowed bolus transit time; (b) LVCd would be longer with increased bolus volume (Molfenter & Steele, 2012) but not with increasing viscosity (Steele et al., 2019); (c) with increasing age, time-to-LVC would be shorter but LVCd would be longer (Humbert et al., 2018); and (d) there would be no difference in time-to-LVC and LVCd between genders.
Method
This cross-sectional study received institutional review board approval. Participants provided informed consent before study participation.
Study Participants
A normative VFSS database of 195 healthy adult participants was retrospectively analyzed (Garand et al., 2022). Original inclusion criteria were as follows: (a) ≥ 21 years of age and (b) the ability to safely eat and drink all viscosities of solids and liquids without dietary restrictions or modifications (Garand et al., 2022). Exclusionary criteria included participants with (a) known allergies or dietary restrictions to barium or other materials provided during the swallow study; (b) hiatal hernia > 2 cm; (c) current or a history of pulmonary disease; (d) current or a history of neurological condition or disease, such as stroke; (e) current or a history of head or neck cancer; (f) a history of anterior neck surgery; (g) an inability to self-feed; and (h) current or a history of dysphagia or suspected dysphagia per self-report as well as (i) women who were pregnant or were suspected to be pregnant (Garand et al., 2022). Participants who had a history of dental, tonsil/adenoid, or sinus surgical procedures were included if they met all other criteria (Garand et al., 2022).
VFSS Procedure
Participants underwent VFSS in an adult radiology fluoroscopic suite performed by an experienced speech-language pathologist and a radiology assistant. The participants were given 12 swallow tasks per the MBSImP protocol (Martin-Harris et al., 2008; NSS, 2020). For the purposes of this study, only seven tasks derived from the lateral viewing plane were analyzed: (a) a second trial of 5 ml of thin liquid barium (IDDSI Level 0) via teaspoon, (b) a single cup sip of thin liquid barium (IDDSI Level 0), (c) 5-ml nectar-thick liquid barium (IDDSI Level 2) via teaspoon, (d) a single cup sip of nectar-thick liquid barium (IDDSI Level 2), (e) 5 ml of honey-thick liquid barium (IDDSI Level 3) via teaspoon, (f) 5 ml of barium pudding (IDDSI Level 4) via teaspoon, and (g) half of a cookie (IDDSI Level 7) coated with 3-ml barium pudding. The first 5 ml trial of thin liquid was not included since this task is specifically meant to acclimate the individual to the testing environment and barium, which is consistent with the MBSImP protocol (NSS, 2020). Also consistent with the MBSImP protocol, participants were provided instructions to hold the bolus in their mouth until cued by the clinician to swallow for the following tasks: 5-ml trials of thin, nectar-thick, honey-thick, and cup-sip thin and nectar-thick liquid barium trials (NSS, 2020). Continuous fluoroscopy was employed, and images were digitally recorded using the Digital Swallowing Workstation Model 7100 (Kay Elemetrics Corp.) or TIMS DICOM System (TIMS Medical) at a rate of 30 frames per second.
LVC Measures
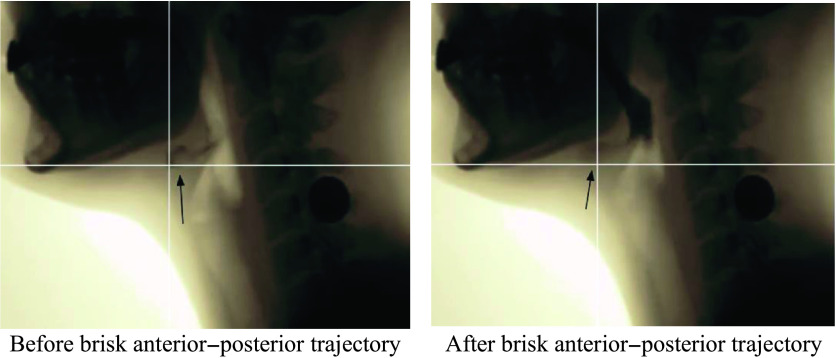
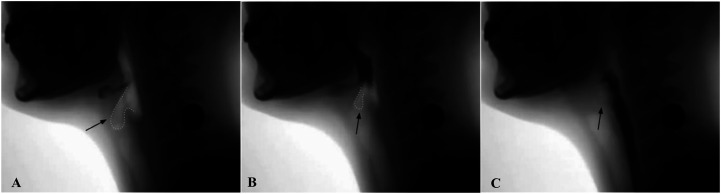
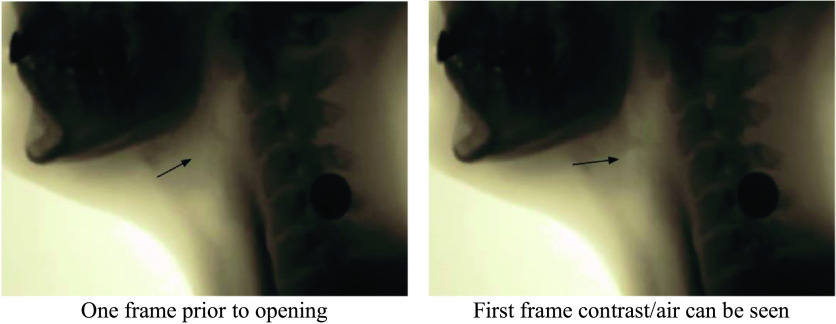
VFSS imaging data were transferred to MP4 files, and individual swallow tasks were spliced for randomized analysis. The individual swallow task video clips were then converted to AVI format (https://cloudconvert.com/; Lunaweb GmbH) and JPEG files via VirtualDub (http://www.virtualdub.org/). JPEG files were then imported to ImageJ (National Institutes of Health; https://imagej.nih.gov), a free image processing software program, to complete LVC temporal measures using the ASPEKT method. As per ASPEKT, LVC is defined as the first frame in which there is maximum approximation of the arytenoids to the epiglottis during a swallow (Steele et al., 2019). Three event frames from each swallow were used to calculate time-to-LVC and LVCd: (a) the first frame of the hyoid burst (i.e., the first frame where the hyoid makes a brisk anterior–superior trajectory; see Figure 1), (b) the first frame of LVC (i.e., the first frame in which the arytenoids are at the furthest point of approximation to the epiglottic petiole; see Figure 2), and (c) the first frame of laryngeal vestibular opening (LVO; i.e., the first frame in which any amount of air or contrast can be detected within the laryngeal vestibule post-closure; see Figure 3).
Figure 1.
Images illustrating before hyoid burst occurs and the first frame of hyoid burst.
Figure 2.
Images illustrating laryngeal vestibule open and partially closed as well as the first frame of laryngeal vestibular closure.
Figure 3.
Images illustrating one frame before and the first frame of laryngeal vestibular opening.
To calculate the time-to-LVC (in milliseconds), the first LVC frame was subtracted from the first hyoid burst frame. Time-to-LVC was then divided by 30 and multiplied by 1,000 (Time-to-LVC = [(First Hyoid Burst Frame − First LVC Frame) / 30] × 1,000) (Steele et al., 2019). To calculate LVCd (in milliseconds), the first frame of LVC was subtracted from the first frame of LVO, divided by 30, and multiplied by 1,000 (LVCd = [(First LVO Frame − First LVC Frame) / 30] × 1,000) (Steele et al., 2019).
Statistical Analysis
Descriptive statistics were calculated for all measures of interest. All data were modeled under parametric assumptions. Pairwise comparisons were performed to determine differences in time-to-LVC and LVCd across three age categories (21–39 years, 40–59 years, and 60+ years) for each swallow task. Age categories were determined based on previous literature available at the time of data collection (e.g., Hiss et al., 2001; Mendell & Logemann, 2007; Rademaker et al., 1998). A Bonferroni correction was applied to adjust for multiple comparisons, which revealed a statistical significance level of p ≤ .0167. Regression models using a general estimating equations framework were employed to determine relationships between LVC temporal measures and the following factors: age, gender, and swallow tasks. Furthermore, interactions among factors were also considered. Comparisons between viscosity and volume were conducted utilizing the output from the associated generalized estimating equation models. Mean differences were calculated using contrast/estimate statements with Wald-type tests chosen to determine statistical significance. To explore the effect of viscosity, only 5-ml tasks were included (i.e., thin, nectar, honey, and pudding). Similarly, to explore the effect of volume, only the 5-ml and cup-sip tasks for thin and nectar-thick liquids were analyzed. Finally, the relationship between time-to-LVC and LVCd was explored across all model variables (age, gender, and swallow task) utilizing a generalized estimating equation regression model similar to the approach described above. Models were fit using the geeglm function from the R library geepack in R Version 4.0.3. Statistical significance was determined using a p value of < .05.
Reliability
Ten percent (n = 137) of the swallows were randomly selected to determine interrater reliability between two raters (C. P. and S. W. A.). Ratings were considered in agreement if they were within a three-frame tolerance (0.1 s). A three-frame tolerance was selected in accordance with previous research assessing swallowing kinematics (Donohue et al., 2021; Steele et al., 2019). Interrater reliability was calculated using a two-way random effects model. Intraclass correlation coefficients and 95% confidence intervals were calculated (Cohen, 1988). Initial interrater reliability (n = 137 of 1,349) was observed to be excellent for all three frame selections (all ps < .001); therefore, C. P. rated approximately 66% of the study sample, and S. W. A. rated approximately 33% of the study sample. Intrarater and interrater reliability were completed again once all swallows were analyzed using a random sample of 10% of the remaining swallows. Disagreements were resolved through consensus meetings between the two raters (C. P. and S. W. A.) and two senior authors (A. N.-M. and K. L. G.). A review, repeat measurement, and discussion technique was used until consensus was met (Steele et al., 2019).
Results
Of the original 1,365 swallows extracted from the database, 1,349 swallows were analyzed. Ten swallows were analyzed for only one temporal measure due to onset of the VFSS recording beginning after the hyoid had made the initial trajectory or due to the recording ending before LVO. Three video files were missing due to the swallow task not having been recorded, and three swallows were eliminated due to lack of clarity or technical issue preventing analysis.
Participant Demographics
This study included a total of 195 participants (85 men, 110 women) between the ages of 21 and 89 years (see Table 1).
Table 1.
Participant demographics across age categories.
| Variable | 21–39 years (n = 70) |
40–59 years (n = 70) |
60–79 years (n = 55) |
Total (N = 195) |
|---|---|---|---|---|
| Age (years), M (SD) | 28.2 (4.6) | 48.9 (6.2) | 68.7 (8.0) | 47 (17.4) |
| Gender | ||||
| Female (n = 110) | 36 (51) | 35 (50) | 39 (71) | 110 |
| Male (n = 85) | 34 (49) | 35 (50) | 16 (29) | 85 |
Note. Data are reported as frequency (percentage) unless otherwise stated.
Reliability
There was excellent intrarater and interrater agreement (see Table 2; Shrout & Fleiss, 1979).
Table 2.
Interrater and intrarater reliability across frame selection for LVC measures.
| Measurement | Intrarater ICC (Rater 1) |
Intrarater ICC (Rater 2) |
95% CI | p value | Interpretation | Interrater ICC | 95% CI | p value | Interpretation |
|---|---|---|---|---|---|---|---|---|---|
| Hyoid burst frame | 1 | 1 | [1, 1] | < .001 | Excellent | .956 | [.939, .969] | < .001 | Excellent |
| LVC frame | 1 | 1 | [1, 1] | < .001 | Excellent | .957 | [.940, .969] | < .001 | Excellent |
| LVC offset frame | 1 | 1 | [1, 1] | < .001 | Excellent | .956 | [.938, .969] | < .001 | Excellent |
Note. LVC = laryngeal vestibular closure; ICC = interclass correlation coefficient; CI = confidence interval.
Descriptive Analyses
Table 3 provides an aggregate summary of time-to-LVC and LVCd. Tables 4–6 provide descriptive analyses for time-to-LVC and LVCd across age categories, between genders, and across swallow tasks, respectively. Further descriptive analyses across age category, gender, and swallow tasks are provided in Supplemental Material S1. Means and standard deviations of timing measures (in milliseconds) have also been converted to frames across Tables 3–6 for reader interpretation of clinically meaningful differences.
Table 3.
Descriptive statistics of time-to-LVC and LVCd.
| LVC measure |
M ± SD (ms) |
Median (ms) |
Minimum, maximum (ms) |
95% CI (ms) |
M ± SD (frames) |
|---|---|---|---|---|---|
| Time-to-LVC | 163.29 ± 127.84 | 166.67 | −1,700, 999 | [156.5, 170.08] | 4.89 ± 3.83 |
| LVCd | 478.35 ± 164.56 | 433.33 | 166.67, 2,366.67 | [469.61, 487.09] | 14.34 ± 4.93 |
Note. LVC = laryngeal vestibular closure; LVCd = laryngeal vestibular closure duration; CI = confidence interval.
Table 4.
Descriptive statistics of time-to-LVC and LVCd across age categories.
| LVC measure | Age category (years) |
M ± SD (ms) |
Median (ms) |
Minimum, maximum (ms) |
95% CI (ms) |
M ± SD (frames) |
|---|---|---|---|---|---|---|
| Time-to-LVC | 21–39 | 167.07 ± 91.99 | 166.67 | −566.67, 999 | [158.91, 175.24] | 5.01 ± 2.74 |
| 40–59 | 162.31 ± 127.31 | 166.67 | −1,700, 999 | [151.01, 173.61] | 4.867 ± 3.82 | |
| 60+ | 159.73 ± 163.11 | 166.67 | −800, 999 | [143.39, 176.08] | 4.79 ± 4.89 | |
| LVCd | 21–39 | 440.19 ± 126.99 | 400.00 | 233.33, 1,300 | [428.92, 451.46] | 13.19 ± 3.81 |
| 40–59 | 477.95 ± 168.06 | 466.67 | 166.67, 2,366.67 | [463.03, 492.86] | 14.33 ± 5.04 | |
| 60+ | 508.56 ± 155.77 | 466.67 | 233.33, 1,466.67 | [508.58, 546.29] | 15.24 ± 4.67 |
Note. LVC = laryngeal vestibular closure; LVCd = laryngeal vestibular closure duration; CI = confidence interval.
Table 5.
Descriptive statistics of time-to-LVC and LVCd between genders.
| LVC measure | Gender |
M ± SD (ms) |
Median (ms) |
Minimum, maximum (ms) |
95% CI (ms) |
M ± SD (frames) |
|---|---|---|---|---|---|---|
| Time-to-LVC | Male | 181.79 ± 84.24 | 166.67 | −366.67, 500 | [138.26, 159.73] | 5.45 ± 2.53 |
| Female | 149 ± 151.78 | 133.33 | −1.700, 999 | [138.26, 159.73] | 4.47 ± 4.55 | |
| LVCd | Male | 454.16 ± 135.07 | 433.33 | 166.67, 1,933.33 | [443.29, 465.04] | 13.61 ± 4.05 |
| Female | 497.04 ± 182.03 | 466.67 | 233.33, 2,366.67 | [484.17, 509.92] | 14.90 ± 5.46 |
Note. LVC = laryngeal vestibular closure; LVCd = laryngeal vestibular closure duration; CI = confidence interval.
Table 6.
Descriptive statistics of time-to-LVC and LVCd across swallow tasks.
| LVC measure | Swallow task |
M ± SD (ms) |
Median (ms) |
Minimum, maximum (ms) |
95% CI (ms) |
M ± SD (frames) |
|---|---|---|---|---|---|---|
| Time-to-LVC | 5-ml thin | 168.71 ± 118.56 | 166.67 | −300, 999 | [151.96, 185.45] | 5.06 ± 3.55 |
| Cup thin | 127.52 ± 141.19 | 133.33 | −566.67, 999 | [107.58, 147.46] | 3.82 ± 4.23 | |
| 5-ml nectar | 163.93 ± 98.88 | 166.67 | −333.33, 999 | [149.96, 177.89] | 4.91 ± 2.96 | |
| Cup nectar | 130.08 ± 189.83 | 133.33 | −1,700, 999 | [103.27, 156.89] | 3.90 ± 5.69 | |
| 5-ml honey | 168.88 ± 127.78 | 166.67 | −733.33, 999 | [150.84, 186.93] | 5.06 ± 3.83 | |
| 5-ml pudding | 170.09 ± 79.8 | 166.67 | −500, 400 | [158.821, 181.36] | 5.90 ± 2.39 | |
| Solid | 213.85 ± 85.67 | 200 | 33.33, 500 | [201.75, 225.95] | 6.41 ± 2.57 | |
| LVCd | 5-ml thin | 462.54 ± 143.16 | 433.33 | 200, 999 | [442.32, 482.76] | 13.86 ± 4.29 |
| Cup thin | 555.36 ± 182.68 | 533.33 | 233.33, 1,300 | [529.56, 581.16] | 16.65 ± 5.48 | |
| 5-ml nectar | 469.73 ± 142.54 | 466.67 | 233.33, 1,066.67 | [449.6, 489.86] | 14.08 ± 4.27 | |
| Cup nectar | 532.98 ± 228.27 | 500 | 266.67, 2,366.67 | [500.74, 565.22] | 15.98 ± 6.84 | |
| 5-ml honey | 476.06 ± 161.17 | 433.33 | 233.33, 1,266.67 | [453.29, 498.82] | 14.27 ± 4.83 | |
| 5-ml pudding | 429.06 ± 99.99 | 400 | 233.33, 966.67 | [414.94, 443.18] | 12.86 ± 2.99 | |
| Solid | 422.74 ± 115.23 | 400 | 166.67, 1,066.67 | [406.46, 439.01] | 12.67 ± 3.45 |
Note. LVC = laryngeal vestibular closure; LVCd = laryngeal vestibular closure duration; CI = confidence interval.
Time-to-LVC: Participant Demographics and Influence of Swallow Task Factors
Mean time-to-LVC was longest in adults aged 21–39 years (167 ± 92 ms) and shortest for adults > 60 years of age (160 ± 163 ms; see Table 4); however, there were no significant differences between age categories for any swallow task (all ps > .22; results not shown). Women had a shorter mean time-to-LVC (149 ± 152 ms) compared with men (182 ± 84 ms; see Table 5). There was no statistically significant interaction between age and gender (p = .08; see Table 7).
Table 7.
Model results for time-to-LVC.
| Variable | Estimate | SE | Wald statistic | p value |
|---|---|---|---|---|
| Intercept | 174.60 | 17.10 | 104.22 | < .001 |
| Cup thin | −41.19 | 12.51 | 10.84 | < .001 |
| 5-ml nectar | −4.78 | 9.45 | 0.26 | .61 |
| Cup nectar | −38.63 | 13.83 | 7.80 | .01 |
| 5-ml honey | 0.18 | 10.49 | 0.00 | .99 |
| 5-ml pudding | 1.38 | 8.91 | 0.02 | .88 |
| Solid | 45.14 | 10.09 | 19.99 | < .001 |
| Gender | −11.52 | 23.29 | 0.24 | .62 |
| Age | −0.41 | 0.41 | 0.98 | .32 |
| Gender × Age | 0.96 | 0.54 | 3.12 | .08 |
Note. LVC = laryngeal vestibular closure.
Pairwise comparisons controlling for age and gender revealed significant differences across various swallow tasks (see Table 8). For example, the average time-to-LVC for 5-ml thin liquid (168.71 ± 118.56 ms) was 41 ms longer (p < .001) than a cup sip of thin liquid (127.52 ± 141.19 ms).
Table 8.
Pairwise comparison results for time-to-LVC after controlling for age and gender.
| Comparison | Difference | Wald statistic | p value |
|---|---|---|---|
| 5-ml thin vs. cup thin | 41.19 | 10.84 | < .001 |
| 5-ml thin vs. 5-ml nectar | 4.78 | 0.26 | .61 |
| 5-ml thin vs. 5-ml honey | −0.18 | 0.00 | .99 |
| 5-ml thin vs. 5-ml pudding | −1.38 | 0.02 | .88 |
| Cup thin vs. cup nectar | −2.56 | 0.03 | .86 |
| 5-ml nectar vs. cup nectar | 33.85 | 9.61 | < .001 |
| 5-ml nectar vs. 5-ml honey | −4.96 | 0.24 | .62 |
| 5-ml nectar vs. 5-ml pudding | −6.16 | 0.55 | .46 |
| 5-ml honey vs. 5-ml pudding | −1.20 | 0.02 | .89 |
Note. LVC = laryngeal vestibular closure.
For viscosity, no significant differences in time-to-LVC were observed (all ps > .05), and there was no significant interaction between age and gender (p = .27). See Table 9 for full details of the model.
Table 9.
Model results for viscosity and time-to-LVC.
| Variable | Estimate | SE | Wald statistic | p value |
|---|---|---|---|---|
| Intercept | 164.71 | 18.56 | 78.78 | < .001 |
| Nectar | −4.78 | 9.45 | 0.26 | .61 |
| Honey | 0.18 | 10.49 | 0.00 | .99 |
| Pudding | 1.38 | 8.91 | 0.02 | .88 |
| Gender | 0.73 | 25.69 | 0.00 | .98 |
| Age | −0.19 | 0.48 | 0.16 | .69 |
| Gender × Age | 0.66 | 0.60 | 1.23 | .27 |
Note. LVC = laryngeal vestibular closure.
For volume, cup sip tasks were significant. Compared to 5-ml tasks (168.71 ± 118.56 ms), cup sip tasks (127.52 ± 141.19 ms), on average, demonstrated a 38-ms reduction in time-to-LVC (p < .001), and there was a significant interaction between age and gender (p = .03). See Table 10 for full details of the model.
Table 10.
Model results for volume and time-to-LVC.
| Variable | Estimate | SE | Wald statistic | p value |
|---|---|---|---|---|
| Intercept | 183.20 | 23.82 | 59.16 | < .001 |
| Cup sip | −37.52 | 8.68 | 18.68 | < .001 |
| Nectar | −1.11 | 9.33 | 0.01 | .91 |
| Gender | −31.19 | 29.39 | 1.13 | .29 |
| Age | −0.66 | 0.55 | 1.47 | .23 |
| Gender × Age | 1.48 | 0.69 | 4.61 | .03 |
Note. LVC = laryngeal vestibular closure.
LVCd: Participant Demographics and Influence of Swallow Task Factors
Mean LVCd was shortest in adults aged 21–39 years (440 ± 127 ms) and longest in adults > 60 years of age (509 ± 156 ms; see Table 4); significant differences were observed between age groups. When comparing the youngest to the oldest age category, significant differences were observed for the following tasks: 5-ml thin (p = .004), 5-ml nectar (p = .008), cup sip nectar (p < .001), 5-ml honey (p < .001), 5-ml pudding (p = .002), and solid (p = .009). When comparing the middle age category to the oldest age category, significant differences were observed for the 5-ml honey task (p < .001). When comparing the middle age category to the youngest age category, significant differences were observed for the 5-ml thin task (p = .005) and 5-ml pudding task (p = .014). All other comparisons were nonsignificant (ps > .0167). For all significant differences observed, the older individuals demonstrated longer LVCd times relative to their younger counterparts.
With respect to gender, female participants had a longer mean LVCd (497 ± 182 ms) compared with male participants (454 ± 135 ms; see Table 5). There was also a statistically significant interaction between age and gender (p < .001; see Table 11).
Table 11.
Model results for laryngeal vestibular closure duration.
| Variable | Estimate | SE | Wald statistic | p value |
|---|---|---|---|---|
| Intercept | 342.35 | 26.55 | 166.32 | < .001 |
| Cup thin | 92.83 | 12.66 | 53.74 | < .001 |
| 5-ml nectar | 7.19 | 8.81 | 0.67 | .41 |
| Cup nectar | 70.44 | 14.69 | 23.00 | < .001 |
| 5-ml honey | 13.52 | 11.43 | 1.40 | .24 |
| 5-ml pudding | −33.48 | 9.98 | 11.25 | < .001 |
| Solid | −39.80 | 10.93 | 13.27 | < .001 |
| Gender | 78.11 | 35.73 | 4.78 | .03 |
| Age | 2.82 | 0.57 | 24.08 | < .001 |
| Gender × Age | −2.41 | 0.79 | 9.31 | < .001 |
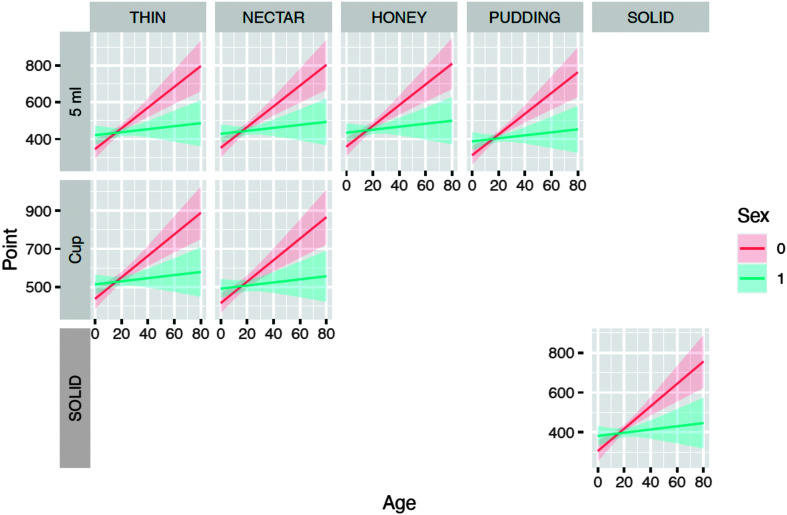
Figure 4 illustrates the increasing trend in LVCd for both genders across age; women (solid line) demonstrated a greater increase than men (dotted line). This increase in LVCd by age is noted across all bolus volumes and viscosities.
Figure 4.
Expected laryngeal vestibular closure duration times across age by task and gender.
Pairwise comparisons controlling for age and gender revealed significant differences across various swallow tasks (see Table 12). For example, the average LVCd for 5-ml thin liquid was approximately 93 ms shorter when compared to a cup sip (p < .001). In contrast, average LVCd for 5-ml thin liquid was approximately 33 ms longer compared to pudding (p < .001).
Table 12.
Pairwise comparison results for laryngeal vestibular closure duration after controlling for age and gender.
| Comparison | Difference | Wald statistic | p value |
|---|---|---|---|
| 5-ml thin vs. cup thin | −92.83 | 53.74 | < .001 |
| 5-ml thin vs. 5-ml nectar | −7.19 | 0.67 | .41 |
| 5-ml thin vs. 5-ml honey | −13.52 | 1.40 | .24 |
| 5-ml thin vs. 5-ml pudding | 33.48 | 11.25 | < .001 |
| Cup thin vs. cup nectar | 22.38 | 2.00 | .16 |
| 5-ml nectar vs. cup nectar | −63.25 | 21.29 | < .001 |
| 5-ml nectar vs. 5-ml honey | −6.33 | 0.33 | .56 |
| 5-ml nectar vs. 5-ml pudding | 40.67 | 16.21 | < .001 |
| 5-ml honey vs. 5-ml pudding | 47.00 | 22.62 | < .001 |
For viscosity, model results revealed that only pudding was significantly different compared to thin liquid, with a 34-ms reduction on average for LVCd for the pudding task (p < .001; results not shown). No further significant differences were observed comparing viscosities (all ps > .05). There was a significant interaction between age and gender (p < .001). Full details of the model can be found in Table 13.
Table 13.
Model results for viscosity and laryngeal vestibular closure duration times.
| Variable | Estimate | SE | Wald statistic | p value |
|---|---|---|---|---|
| Intercept | 342.27 | 26.28 | 172.59 | < .001 |
| Nectar | 7.19 | 8.81 | 0.67 | .41 |
| Honey | 13.52 | 11.43 | 1.40 | .24 |
| Pudding | −33.48 | 9.98 | 11.25 | < .001 |
| Gender | 87.02 | 38.15 | 5.20 | .02 |
| Age | 2.73 | 0.58 | 22.40 | < .001 |
| Gender × Age | −2.55 | 0.80 | 10.02 | < .001 |
For volume, cup sip tasks were significant. Compared to 5-ml tasks, cup sip tasks, on average, demonstrate a 78-ms increase in LVCd (p < .001). There was a significant interaction between age and gender (p < .001). Full details of the model can be found in Table 14.
Table 14.
Model results for volume and laryngeal vestibular closure duration.
| Variable | Estimate | SE | Wald statistic | p value |
|---|---|---|---|---|
| Intercept | 332.30 | 33.13 | 100.62 | < .001 |
| Cup sip | 78.04 | 9.71 | 64.53 | < .001 |
| Nectar | −7.60 | 9.18 | 0.69 | .41 |
| Gender | 97.19 | 43.45 | 5.00 | .03 |
| Age | 3.20 | 0.71 | 20.38 | < .001 |
| Gender × Age | −2.88 | 0.97 | 8.88 | < .001 |
Relationship Between Time-to-LVC and LVCd
A significant association was observed between time-to-LVC and LVCd, which revealed that as time-to-LVC shortened, LVCd lengthened (p < .001). This relationship remained after controlling for swallow task, age, and gender.
Discussion
This study evaluated time-to-LVC and LVCd in healthy adults to determine normative reference values across MBSImP swallow tasks, while also examining the influence of age, gender, and swallow task factors on these measures. These findings indicate that both participant demographics and swallow task factors influence LVC measures. Overall, a significant negative relationship was found between LVCd and time-to-LVC, as shorter reaction times were associated with longer durations. Details of all findings are discussed below.
Time-to-LVC
Participant Demographic Trends
The main effects of this study indicate that while older adults (> 60 years of age) had a shorter mean time-to-LVC, there was no significant difference between age groups. Furthermore, the main effects of gender showed that women had a shorter time-to-LVC compared with male peers. The results of this study found no significant interaction between age and gender for time-to-LVC. This is contrary to some research, for example, in a study by Humbert et al. (2018), older adults were observed to have a longer time-to-LVC compared to their younger counterparts. Muscle function is anticipated to decline during typical aging, with evidence supporting deteriorations in swallowing function in older adults (e.g., Molfenter et al., 2019; Robbins et al., 2006), which may account for the longer time-to-LVC seen in some study samples. However, given the relatively large sample and the more complex data analyses employed, the results of this study are likely to be more generalizable to the larger population. Older adults may be compensating for previously reported age-related changes. Alternatively, laryngeal muscle fibers may not be affected by sarcopenia to the same degree as pharyngeal or lingual muscles. Laryngeal muscles control respiration, phonation, and airway protection, all of which are endurance activities (Hoh, 2005). The laryngeal muscles also have the capability for sudden high-intensity movements, such as when sneezing and coughing (Hoh, 2005). Given our limited knowledge of how sarcopenia affects the larynx, further research is required.
Available evidence is contradictory regarding the impact of gender. For example, Humbert et al. (2018) observed a longer time-to-LVC in men, while findings from Molfenter et al. (2019) and Steele et al. (2019) did not find a gender effect. Methodological differences may explain contradictory findings (e.g., swallow tasks employed, sample size). An additional consideration is that men often have a larger volume in the laryngeal and hypopharyngeal regions (Inamoto et al., 2011), which may create a longer distance for the arytenoids to travel to abut the epiglottic petiole, therefore contributing to a longer time-to-LVC. The authors of this study are not aware of available evidence that has examined how differences in anatomical size influence time-to-LVC, but this area of research is worthy of exploration in the future.
Swallow Task Factors (Viscosity, Volume)
Viscosity did not significantly influence time-to-LVC within this study, which contradicts previous reports (Humbert et al., 2018; Steele et al., 2019). For example, in a healthy cohort, Steele et al. (2019) observed a longer time-to-LVC in thin liquids compared with other consistencies. The current study cohort, however, had a larger sample size and employed controlled volumes. Uncontrolled volumes may have influenced findings in the Steele et al. study since participants generally consumed a larger volume of thin liquid compared to the thicker liquid consistencies trialed. While Humbert et al. (2018) did find differences in time-to-LVC across viscosities, this was likely due to their use of additional stimuli not administered within our study (i.e., frozen boluses and mixed consistencies). When comparing consistencies included in this study (i.e., thin and pudding), our findings are congruent, and there is no significant difference in reaction time.
LVCd
Participant Demographic Trends
The main effects of this study indicate that there was a significant difference in mean LVCd between age groups, with reaction time increasing with age. With regard to gender, women had a longer mean LVCd compared with male peers. There was a significant interaction between age and gender for LVCd. Specifically, there was an increasing trend for both genders across age, with women demonstrating a greater increase in LVCd. The age effect is consistent with study findings from Mancopes et al. (2021), with the authors proposing that a longer LVCd may be due to compensation as age-related changes alter swallowing mechanics. Similar findings were also observed in a study by Humbert et al. (2018), in which they also noted more variability in older adults, consistent with the hypothesis of age-related alterations in swallowing function and inherent complexity in the underlying mechanics of LVC. Molfenter et al. (2019) did not find an influence of age on LVCd; however, their sample of 44 healthy individuals were aged 65 years and older, with an age range of 68–86 years, and this limited age range may have prevented an age effect from being observed.
Swallow Task Factors (Viscosity, Volume)
Cup sips of thin liquid had the longest LVCd, while solid and pudding thick boluses had the shortest LVCd. The difference in LVCd was found to be significant when pudding was compared to thin liquids, which is consistent with findings by Humbert et al. (2018). Their study employed different swallow tasks to capture viscosity differences, so there is a lack of direct comparison across all bolus trials. Steele et al. (2019) did not observe an effect of viscosity, although this discrepancy may be due to sample size difference (195 participants in this study compared to 38) and current study comparisons across tightly controlled volumes of 5 ml (compared to unrestricted sips in the study by Steele et al., 2019).
Study Limitations
There were limitations to this retrospective analysis. There was an imbalance between genders for participants above the age of 60 years, with fewer men in the oldest age category. Therefore, there was an imbalance in sample size across age categories, with the oldest age category having the fewest participants (n = 55). Furthermore, although strict eligibility criteria were employed in the collection of the normative database, the cross-sectional design prohibited a longitudinal assessment that would document any changes in medical diagnoses where subclinical alterations may have already been present at the time of study data collection. Other limitations include self-report of dysphagia and inclusion of previous/current smokers, with the latter potentially altering aerodigestive mucosa and sensitivity that can negatively impact swallowing and other protective functions (Kim et al., 2020). In addition, volume was not controlled during the self-administered cup sips. Previous evidence suggests the average natural sip is between 11 and 14 ml in adults (Steele et al., 2019) and does not appear to be influenced by age (Mancopes et al., 2021). Therefore, we are confident that our results reflect a true comparison between smaller (5-ml) and larger (cup sip) volumes. Furthermore, it is possible that other factors may have contributed to LVC timing. This study did not consider the location of the bolus at swallow onset. Future work may consider including this component to elucidate the influencing factors of LVC. Finally, the liquid swallow tasks (i.e., thin, nectar, and honey) employed in this study required the patient to hold the bolus until the clinician cued them to swallow, as per the MBSImP protocol. Previous research by Daniels et al. (2007) notes that temporal measures can vary during cued tasks compared to noncued tasks, although LVC measures were not specifically collected for that study. Despite these limitations, our cohort of a relatively large sample size and employment of MBSImP protocol swallow tasks help to fill gaps in the current literature by providing normative ranges and exploration of influential factors on LVC temporal measures.
Conclusions
The findings of this study contribute to normative reference values of two LVC temporal measures—time-to-LVC and LVCd—as well as the influence of demographic and bolus factors in the context of the widely used MBSImP protocol. Normative reference values allow a direct comparison to patient performance and provide objective measures to document change in patient performance over time for clinicians who employ the MBSImP protocol of bolus administration and the ASPEKT method of analyses. Understanding how participant demographics and bolus task characteristics alter LVC measures supports clinicians in enhanced clinical practices to optimize patient outcomes.
Ethics Approval
Institutional review board approval was initially obtained from the Medical University of South Carolina (protocol number: 00011566).
Author Contributions
Sophia Werden Abrams: Data curation, Validation, Writing – original draft, Writing – review & editing. Courtney Petersen: Data curation, Validation, Writing – review & editing. Jonathan Beall: Formal analysis, Visualization, Writing – review & editing. Ashwini Namasivayam-MacDonald: Validation, Conceptualization, Writing – review & editing. Dahye Choi: Formal analysis, Writing – review & editing. Kendrea L. (Focht) Garand: Conceptualization, Project administration, Data curation, Funding acquisition, Writing – original draft.
Data Availability Statement
The data that support the findings of this study are available on request from Kendrea L. (Focht) Garand.
Supplementary Material
Acknowledgments
This work was partially supported by the Veterans Affairs CDA-1 (RR&D1IK1RX001628-01A1 to Kendrea Garand); the National Institute on Deafness and Other Communication Disorders (K24DC12801 to Bonnie-Martin Harris); the South Carolina Clinical & Translational Research Institute, with an academic home at the Medical University of South Carolina, National Center for Advancing Translational Sciences (TL1 TR000061 to Kathleen Brady, project principal investigator: Kendrea Garand); the Evelyn Trammell Trust to Bonnie Martin-Harris; and the American Speech-Language-Hearing Foundation to Kendrea Garand. Related to the historical normative data set, the authors wish to acknowledge Cephus Simmons and clinicians from the Evelyn Trammell Institute for Voice and Swallowing at the Medical University of South Carolina, including R. Jordan Hazelwood, Kate Davidson, Julie Blair, and Brittni Carnes, for their assistance with original videofluoroscopic data collection. They would also like to acknowledge Madeline Faur for her assistance in the final edits of the tables and supplemental materials.
Funding Statement
This work was partially supported by the Veterans Affairs CDA-1 (RR&D1IK1RX001628-01A1 to Kendrea Garand); the National Institute on Deafness and Other Communication Disorders (K24DC12801 to Bonnie-Martin Harris); the South Carolina Clinical & Translational Research Institute, with an academic home at the Medical University of South Carolina, National Center for Advancing Translational Sciences (TL1 TR000061 to Kathleen Brady, project principal investigator: Kendrea Garand); the Evelyn Trammell Trust to Bonnie Martin-Harris; and the American Speech-Language-Hearing Foundation to Kendrea Garand. Related to the historical normative data set, the authors wish to acknowledge Cephus Simmons and clinicians from the Evelyn Trammell Institute for Voice and Swallowing at the Medical University of South Carolina, including R. Jordan Hazelwood, Kate Davidson, Julie Blair, and Brittni Carnes, for their assistance with original videofluoroscopic data collection.
References
- Barbon, C. E., Chepeha, D. B., Hope, A. J., Peladeau-Pigeon, M., Waito, A. A., & Steele, C. M. (2020). Mechanisms of impaired swallowing on thin liquids following radiation treatment for oropharyngeal cancer. Journal of Speech, Language, and Hearing Research, 63(9), 2870–2879. 10.1044/2020_JSLHR-19-00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (1988). Set correlation and contingency tables. Applied Psychological Measurement, 12(4), 425–434. 10.1177/014662168801200410 [DOI] [Google Scholar]
- Curtis, J. A., Molfenter, S., & Troche, M. S. (2020). Predictors of residue and airway invasion in Parkinson's disease. Dysphagia, 35(2), 220–230. 10.1007/s00455-019-10014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, S. K., Schroeder, M. F., DeGeorge, P. C., Corey, D. M., & Rosenbek, J. C. (2007). Effects of verbal cue on bolus flow during swallowing. American Journal of Speech-Language Pathology, 16(2), 140–147. 10.1044/1058-0360(2007/018) [DOI] [PubMed] [Google Scholar]
- Donohue, C., Khalifa, Y., Perera, S., Sejdić, E., & Coyle, J. L. (2021). How closely do machine ratings of duration of UES opening during videofluoroscopy approximate clinician ratings using temporal kinematic analyses and the MBSImP? Dysphagia, 36(4), 707–718. 10.1007/s00455-020-10191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumican, M., Watts, C., Drulia, T., & Zhang, Y. (2023). Dysphagia presentation, airway invasion, and gender differences in a clinically based sample of people with Parkinson's disease. Dysphagia, 38(1), 353–366. 10.1007/s00455-022-10472-y [DOI] [PubMed] [Google Scholar]
- Garand, K. L., Beall, J., Hill, E. G., Davidson, K., Blair, J., Pearson, W., Jr., & Martin-Harris, B. (2022). Effects of presbyphagia on oropharyngeal swallowing observed during modified barium swallow studies. Journal of Nutrition, Health & Aging, 26(11), 973–980. 10.1007/s12603-022-1854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Cadavid, L. F., Pantoja, J. A., Forero, Y. J., Gutiérrez, H. M., & Bastidas, A. R. (2020). Aspiration in the fiberoptic endoscopic evaluation of swallowing associated with an increased risk of mortality in a cohort of patients suspected of oropharyngeal dysphagia. Dysphagia, 35(2), 369–377. 10.1007/s00455-019-10036-7 [DOI] [PubMed] [Google Scholar]
- Herzberg, E. G., Brates, D., & Molfenter, S. M. (2019). Physiological compensation for advanced bolus location at swallow onset: A retrospective analysis in healthy seniors. Journal of Speech, Language, and Hearing Research, 62(12), 4351–4355. 10.1044/2019_jslhr-19-00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiss, S. G., Treole, K., & Stuart, A. (2001). Effect of age, gender, and repeated measures on intraoral air pressure in normal adults. Journal of Voice, 15(2), 159–164. 10.1016/S0892-1997(01)00017-0 [DOI] [PubMed] [Google Scholar]
- Hoh, J. F. Y. (2005). Laryngeal muscle fibre types. Acta Physiologica Scandinavica, 183(2), 133–149. 10.1111/j.1365-201X.2004.01402.x [DOI] [PubMed] [Google Scholar]
- Humbert, I. A., Sunday, K. L., Karagiorgos, E., Vose, A. K., Gould, F., Greene, L., Azola, A., Tolar, A., & Rivet, A. (2018). Swallowing kinematic differences across frozen, mixed, and ultrathin liquid boluses in healthy adults: Age, sex, and normal variability. Journal of Speech, Language, and Hearing Research, 61(7), 1544–1559. 10.1044/2018_jslhr-s-17-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamoto, Y., Fujii, N., Saitoh, E., Baba, M., Okada, S., Katada, K., Ozeki, Y., Kanamori, D., & Palmer, J. B. (2011). Evaluation of swallowing using 320-detector-row multislice CT. Part II: Kinematic analysis of laryngeal closure during normal swallowing. Dysphagia, 26(3), 209–217. 10.1007/s00455-010-9276-2 [DOI] [PubMed] [Google Scholar]
- Kim, J. W., Choi, H., Jung, J., & Kim, H. J. (2020). Risk factors for aspiration pneumonia in patients with dysphagia undergoing videofluoroscopic swallowing studies: A retrospective cohort study. Medicine, 99(46), Article e23177. 10.1097/MD.0000000000023177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancopes, R., Gandhi, P., Smaoui, S., & Steele, C. M. (2021). Which physiological swallowing parameters change with healthy aging? OBM Geriatrics, 5(1), Article 153. 10.21926/obm.geriatr.2101153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik, P. E. (2001). Aspiration pneumonitis and aspiration pneumonia. The New England Journal of Medicine, 344(9), 665–671. 10.1056/NEJM200103013440908 [DOI] [PubMed] [Google Scholar]
- Martin-Harris, B., Brodsky, M. B., Michel, Y., Castell, D. O., Schleicher, M., Sandidge, J., Maxwell, R., & Blair, J. (2008). MBS measurement tool for swallow impairment–MBSImp: Establishing a standard. Dysphagia, 23(4), 392–405. 10.1007/s00455-008-9185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell, D. A., & Logemann, J. A. (2007). Temporal sequence of swallow events during the oropharyngeal swallow. Journal of Speech, Language, and Hearing Research, 50(5), 1256–1271. 10.1044/1092-4388(2007/088) [DOI] [PubMed] [Google Scholar]
- Molfenter, S. M., Lenell, C., & Lazarus, C. L. (2019). Volumetric changes to the pharynx in healthy aging: Consequence for pharyngeal swallow mechanics and function. Dysphagia, 34(1), 129–137. 10.1007/s00455-018-9924-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfenter, S. M., & Steele, C. M. (2012). Temporal variability in the deglutition literature. Dysphagia, 27(2), 162–177. 10.1007/s00455-012-9397-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northern Speech Services. (2020). Modified Barium Swallow Impairment Profile. https://www.mbsimp.com
- Pearson, W. G., Jr., Hindson, D. F., Langmore, S. E., & Zumwalt, A. C. (2013). Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. International Journal of Radiation Oncology, Biology, Physics, 85(3), 735–740. 10.1016/j.ijrobp.2012.07.2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, W. G., Jr., Langmore, S. E., Yu, L. B., & Zumwalt, A. C. (2012). Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia, 27(4), 445–451. 10.1007/s00455-011-9392-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker, A. W., Pauloski, B. R., Colangelo, L. A., & Logemann, J. A. (1998). Age and volume effects on liquid swallowing function in normal women. Journal of Speech, Language, and Hearing Research, 41(2), 275–284. 10.1044/jslhr.4102.275 [DOI] [PubMed] [Google Scholar]
- Robbins, J., Bridges, A. D., & Taylor, A. (2006). Oral, pharyngeal and esophageal motor function in aging. GI Motility Online. Advance online publication. https://www.nature.com/gimo/contents/pt1/full/gimo39.html [Google Scholar]
- Shrout, P. E., & Fleiss, J. L. (1979). Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin, 86(2), 420–428. 10.1037/0033-2909.86.2.420 [DOI] [PubMed] [Google Scholar]
- Steele, C. M., Peladeau-Pigeon, M., Barbon, C. A. E., Guida, B. T., Namasivayam-MacDonald, A. M., Nascimento, W. V., Smaoui, S., Tapson, M. S., Valenzano, T. J., Waito, A. A., & Wolkin, T. S. (2019). Reference values for healthy swallowing across the range from thin to extremely thick liquids. Journal of Speech, Language, and Hearing Research, 62(5), 1338–1363. 10.1044/2019_JSLHR-S-18-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose, A., & Humbert, I. (2019). “Hidden in plain sight”: A descriptive review of laryngeal vestibule closure. Dysphagia, 34(3), 281–289. 10.1007/s00455-018-9928-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose, A. K., Kesneck, S., Sunday, K., Plowman, E., & Humbert, I. (2018). A survey of clinician decision making when identifying swallowing impairments and determining treatment. Journal of Speech, Language, and Hearing Research, 61(11), 2735–2756. 10.1044/2018_JSLHR-S-17-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from Kendrea L. (Focht) Garand.