Abstract
PURPOSE
Patritumab deruxtecan, or HER3-DXd, is an antibody-drug conjugate consisting of a fully human monoclonal antibody to human epidermal growth factor receptor 3 (HER3) attached to a topoisomerase I inhibitor payload via a stable tetrapeptide-based cleavable linker. We assessed the efficacy and safety of HER3-DXd in patients with epidermal growth factor receptor (EGFR)–mutated non–small-cell lung cancer (NSCLC).
METHODS
This phase II study (ClinicalTrials.gov identifier: NCT04619004) was designed to evaluate HER3-DXd in patients with advanced EGFR-mutated NSCLC previously treated with EGFR tyrosine kinase inhibitor (TKI) therapy and platinum-based chemotherapy (PBC). Patients received HER3-DXd 5.6 mg/kg intravenously once every 3 weeks or an uptitration regimen (3.2 → 4.8 → 6.4 mg/kg). The primary end point was confirmed objective response rate (ORR; RECIST 1.1) by blinded independent central review (BICR), with a null hypothesis of 26.4% on the basis of historical data.
RESULTS
Enrollment into the uptitration arm closed early on the basis of a prespecified benefit-risk assessment of data from the phase I U31402-A-U102 trial. In total, 225 patients received HER3-DXd 5.6 mg/kg once every 3 weeks. As of May 18, 2023, median study duration was 18.9 (range, 14.9-27.5) months. Confirmed ORR by BICR was 29.8% (95% CI, 23.9 to 36.2); median duration of response, 6.4 months; median progression-free survival, 5.5 months; and median overall survival, 11.9 months. The subgroup of patients with previous osimertinib and PBC had similar outcomes. Efficacy was observed across a broad range of pretreatment tumor HER3 membrane expression levels and across diverse mechanisms of EGFR TKI resistance. In patients with nonirradiated brain metastases at baseline (n = 30), the confirmed CNS ORR by BICR per CNS RECIST was 33.3% (95% CI, 17.3 to 52.8). The safety profile (National Cancer Institute Common Terminology Criteria for Adverse Events v5.0) was manageable and tolerable, consistent with previous observations.
CONCLUSION
After tumor progression with EGFR TKI therapy and PBC in patients with EGFR-mutated NSCLC, HER3-DXd once every 3 weeks demonstrated clinically meaningful efficacy with durable responses, including in CNS metastases. A phase III trial in EGFR-mutated NSCLC after progression on an EGFR TKI is ongoing (HERTHENA-Lung02; ClinicalTrials.gov identifier: NCT05338970).
INTRODUCTION
For patients with epidermal growth factor receptor (EGFR)–mutated advanced non–small-cell lung cancer (NSCLC), initial treatment typically includes one or two EGFR tyrosine kinase inhibitor (TKI) regimens comprising either a third-generation EGFR TKI or a first- or second-generation EGFR TKI followed by a third-generation EGFR TKI when the EGFR T790M mutation is detected.1 After disease progression on EGFR TKI therapy, patients are usually treated with platinum-based chemotherapy (PBC; with or without an immune checkpoint inhibitor and antiangiogenic therapy).2,3 Salvage therapies after disease progression on PBC have limited efficacy; recent retrospective and real-world analyses of salvage therapies in patients with EGFR-mutated NSCLC after failure of EGFR TKI therapy and PBC reported median progression-free survival (PFS) in the range of 2.8-3.3 months (Data Supplement, Table S1 [online only]).4,5
CONTEXT
Key Objective
This primary analysis of the phase II HERTHENA-Lung01 study evaluated the antitumor activity and safety of patritumab deruxtecan (HER3--DXd) in patients with epidermal growth factor receptor (EGFR)–mutated non–small-cell lung cancer (NSCLC) after progression on EGFR tyrosine kinase inhibitor therapy and platinum-based chemotherapy.
Knowledge Generated
HER3-DXd once every 3 weeks demonstrated clinically meaningful efficacy in patients with previously treated EGFR-mutated NSCLC. The safety profile was manageable and tolerable, consistent with previous studies in similar patient populations.
Relevance (T.E. Stinchcombe)
-
The optimal therapy for patients with EGFR-mutant NSCLC after osimertinib and platinum-based therapy is undefined, and patritumab deruxtecan has shown preliminary activity in this patient population. Antibody drug conjugates have a novel mechanism of action and may become standard therapies in the treatment of NSCLC in the future.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
Human epidermal growth factor receptor 3 (HER3; receptor tyrosine-protein kinase erbB-3 [ERBB3]) expression, which has been reported in 83% of NSCLC tumors6 and in 85%-100% of tumors harboring an activating EGFR mutation,7,8 is implicated in resistance to EGFR TKI therapy9,10 and is associated with metastatic progression and shorter relapse-free survival.11 Patritumab deruxtecan (also known as HER3-DXd or U3-1402) is an investigational HER3-directed antibody-drug conjugate composed of a human immunoglobulin G1 monoclonal antibody to HER3 (patritumab) covalently linked to a topoisomerase I inhibitor payload (DXd, an exatecan derivative) via a tetrapeptide-based cleavable linker.12-15 After binding to HER3, HER3-DXd is translocated to the lysosome, where the linker is cleaved by lysosomal enzymes that are upregulated in tumor cells. The cytotoxic payload is then free to enter the nucleus, leading to cell death and, because the payload is membrane permeable, a potential bystander antitumor effect.12,15-17 A phase I dose-escalation/dose-expansion study of HER3-DXd in heavily pretreated patients with EGFR-mutated NSCLC showed that HER3-DXd 5.6 mg/kg administered intravenously once every 3 weeks was associated with a tolerable and manageable safety profile and resulted in a confirmed objective response rate (ORR) of 39% in patients with previous osimertinib and PBC.7 In the phase I study, HER3-DXd once every 3 weeks was effective in patients with diverse mechanisms of resistance to EGFR TKIs, including EGFR-dependent and -independent mechanisms.7
The promising data from the phase I trial led to initiation of the phase II HERTHENA-Lung01 trial of HER3-DXd once every 3 weeks in patients with EGFR-mutated NSCLC who had previously been treated with EGFR TKI therapy and PBC.
METHODS
Study Design and Participants
HERTHENA-Lung01 (ClinicalTrials.gov identifier: NCT04619004) is a multicenter, open-label, randomized, two-arm, phase II study of HER3-DXd once every 3 weeks in previously treated patients with locally advanced or metastatic NSCLC with EGFR-activating mutations (exon 19 deletion or L858R) being conducted in 122 locations in North America, Europe, East Asia, Southeast Asia, and Australia (Data Supplement, Fig S1).
Eligible patients were adults whose disease had progressed on or after their most recent therapy. Previous therapies must have included ≥1 EGFR TKI and ≥1 PBC regimen in any sequence (there was no restriction on the maximum number of previous lines of therapy). After initiation of enrollment, the protocol was amended to require previous treatment with osimertinib. Patients with clinically inactive or treated brain metastases who were asymptomatic (ie, who were without neurologic signs or symptoms and who did not require treatment with corticosteroids or anticonvulsants) were eligible; patients with previous or current evidence of interstitial lung disease (ILD) were excluded. An Eastern Cooperative Oncology Group performance status of 0 or 1 was required at screening.
Patients received one of two dose schedules of HER3-DXd administered intravenously once every 3 weeks. Patients in arm 1 received a fixed-dose regimen of 5.6 mg/kg; those in arm 2 received an uptitration regimen: cycle 1 day 1, 3.2 mg/kg; cycle 2 day 1, 4.8 mg/kg; cycle 3 day 1; and subsequent cycles, 6.4 mg/kg. The uptitration arm was included because preliminary data from the first-in-human trial of HER3-DXd (U31402-A-J101; in metastatic breast cancer) provided tentative support that the uptitration regimen might be associated with a reduced frequency of hematopoietic suppression. The HERTHENA-Lung01 protocol specified that ongoing recruitment into each arm would be reassessed based on a benefit-risk analysis of data from the ongoing phase I U31402-A-U102 study assessing the two dose regimens in a similar patient population.
The study Protocol (online only) was approved by the institutional review board for each institution and was conducted in compliance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use consolidated Guideline for Good Clinical Practice, and applicable regulatory requirements. All patients provided written informed consent before participation in the study.
Biomarker Analyses
All patients consented to provide pretreatment tumor biopsy material, either from a biopsy at study entry or from archival tissue from a biopsy ≤3 months before signing the tissue consent form and after their tumor had progressed on or after the most recent previous therapy.
HER3 immunohistochemistry (IHC) was performed centrally on formalin-fixed, paraffin-embedded tissue using anti-HER3 clone SP438 (investigational use only), a rabbit monoclonal antibody developed by Ventana Medical Systems, Inc. HER3 membrane expression on tumor cells was quantified by H-scores. H-score (range, 0-300) was defined as the sum of the percentage of IHC 1+ (weak staining) plus two times the percentage of IHC 2+ (moderate staining) plus three times the percentage of IHC 3+ (strong staining).
Baseline genomic alterations were analyzed centrally in formalin-fixed, paraffin-embedded tumor tissue using the Oncomine Comprehensive Assay v3 (Thermo Fisher Scientific, Waltham, MA) and in circulating tumor DNA from blood using the GuardantOMNI assay (Guardant Health, Palo Alto, CA; a variant allelic frequency of 0.1% was used as a threshold for mutation detection).
Objectives and End Points
The primary end point was confirmed ORR (complete response [CR] or partial response [PR], confirmed at ≥4 weeks) by blinded independent central review (BICR) according to RECIST version 1.1.18 Imaging (magnetic resonance imaging [MRI] or contrast-enhanced computed tomography [CT]) of the chest, abdomen, pelvis, and brain was conducted at baseline, every 6 weeks to week 24, and every 12 weeks thereafter.
Secondary end points included duration of response by BICR (key secondary end point); confirmed ORR and duration of response by investigator; PFS, disease control rate, time to response, and best percentage change from baseline in the sum of diameters by BICR and the investigator; overall survival (OS); safety; correlation of baseline HER3 expression with efficacy measures; and antidrug antibodies for HER3-DXd.
An additional assessment of confirmed objective response of intracranial tumors by BICR using CNS RECIST was performed (CNS BICR; Data Supplement, Methods). This exploratory analysis was outside of the protocol but was planned before unblinding.
Safety Measures
Adverse events were coded using the Medical Dictionary for Regulatory Activities and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Potential cases of ILD were reviewed by an independent ILD adjudication committee.
Statistical Analysis
The study sample size was based on the null hypothesis that the primary end point of confirmed ORR would be 26.4% (the upper bound of the exact 95% CI of the ORR [23%] observed in the ramucirumab plus docetaxel arm from the REVEL trial in patients with stage IV NSCLC that progressed on PBC).19 A one-sample exact binomial test for single proportions with a nominal two-sided significance of 5% was estimated to have approximately 91% power to detect the difference between the null hypothesis and an alternative hypothesis of an ORR of 37% when the sample size was 210.
The primary analysis occurred when all patients had either a minimum of 9 months of follow-up or had discontinued from the study earlier. Primary efficacy and safety analyses were performed in all patients who received ≥1 dose of the study drug.
The 95% CIs for response end points were calculated using the Clopper-Pearson method. Time-to-event end points, including duration of response, PFS, and OS, were estimated using the Kaplan-Meier method, and two-sided 95% CIs for the median were calculated using the Brookmeyer-Crowley method.
RESULTS
Patient Disposition, Demographics, and Disease Characteristics
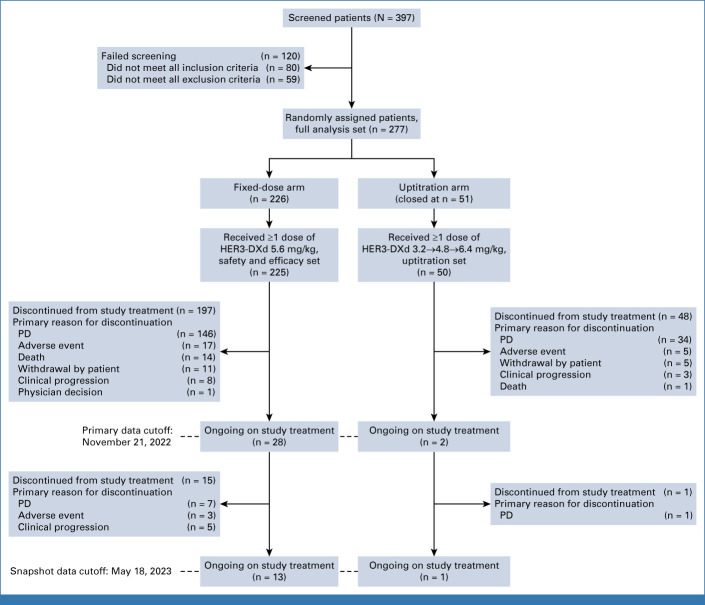
Between February 2, 2021, and February 18, 2022, 277 patients were enrolled (full analysis set; Fig 1). On the basis of the protocol-specified benefit-risk analysis of the data in the ongoing phase I U31402-A-U102 study of the two dose regimens in a similar population, the uptitration arm was closed after 51 patients had been enrolled; 50 received ≥1 dose of HER3-DXd once every 3 weeks (Data Supplement, Tables S2-S4; the benefit-risk of using the uptitration regimen was consistent with the phase I study). Enrollment into the 5.6-mg/kg fixed-dose arm was continued; 226 patients were enrolled, and 225 received ≥1 dose of HER3-DXd once every 3 weeks and made up both the efficacy and safety populations.
FIG 1.
CONSORT diagram (patient disposition). HER3, human epidermal growth factor receptor 3; PD, progressive disease.
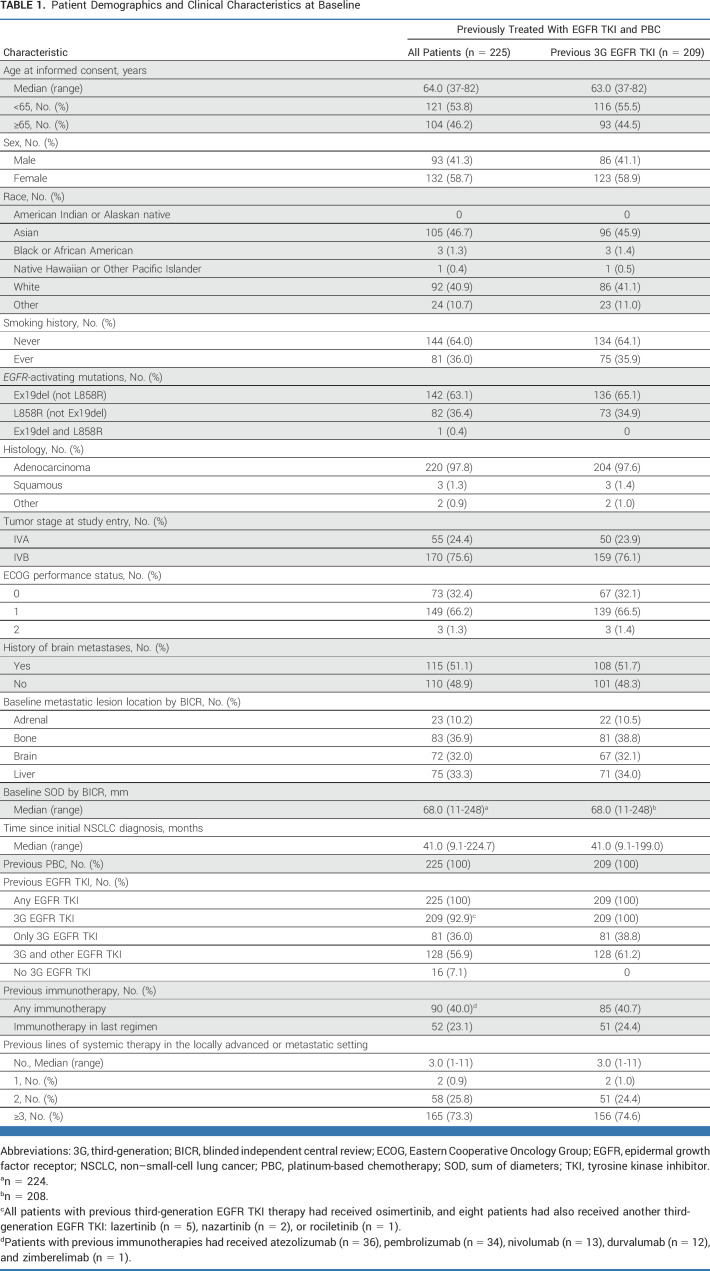
The primary data cutoff was November 21, 2022 (Fig 1; Data Supplement, Table S3). At the subsequent snapshot data cutoff (May 18, 2023; median duration of follow-up, 18.9 [range, 14.9-27.5] months]), 13 patients (n = 225; HER3-DXd 5.6 mg/kg once every 3 weeks) were ongoing on study treatment and 212 had discontinued (Fig 1). Patients had a median age of 64 years, 58.7% were female, and most were Asian (46.7%) or White (40.9%; Table 1). A history of brain metastases was noted in 51.1% of patients, and 32.0% had radiologic evidence of brain metastases at baseline (by BICR). Baseline radiologic evidence of bone and liver metastases was seen in 36.9% and 33.3% of patients, respectively (both by BICR). Median number of previous lines of systemic therapy in the locally advanced/metastatic setting was 3 (range, 1-11; Table 1). All patients had a previous EGFR TKI and previous PBC, and 209 (92.9%) had been previously treated with a third-generation EGFR TKI (all osimertinib); the demographics and disease characteristics of this subgroup were similar to those of the overall population. Forty percent of patients had received immunotherapy, which was the most recent previous regimen for 23.1% of patients (median time since the last immunotherapy dose was 3.4 [range, 1-56] months).
TABLE 1.
Patient Demographics and Clinical Characteristics at Baseline
Efficacy
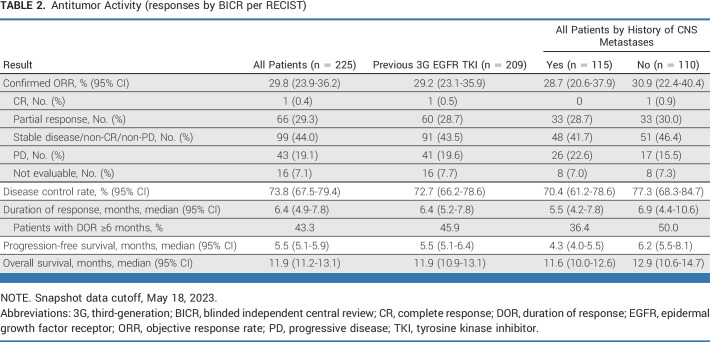
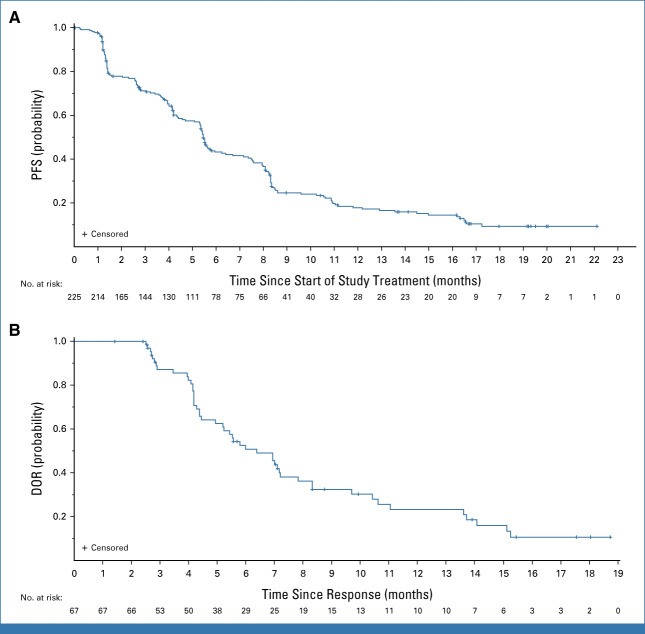
The confirmed ORR (by BICR) was 29.8% (n = 225; 95% CI, 23.9 to 36.2; Table 2). Median duration of response was 6.4 (95% CI, 4.9 to 7.8) months (Table 2; Fig 2B), and 43.3% of patients had a duration of response ≥6 months. Median PFS was 5.5 (95% CI, 5.1 to 5.9) months (Fig 2A). Among patients with baseline and postbaseline target lesion evaluation available (210 of 225), the majority had a reduction in tumor size (Fig 3). Median OS was 11.9 (95% CI, 11.2 to 13.1) months (Table 2). Antitumor activity was similar in patients with and those without a history of CNS metastases (Table 2). Efficacy observed in the subgroup of patients with a previous third-generation EGFR TKI was similar to that in the full efficacy population (Table 2).
TABLE 2.
Antitumor Activity (responses by BICR per RECIST)
FIG 2.
Kaplan-Meier plots of (A) PFS and (B) DOR, both by BICR per RECIST 1.1. Snapshot data cutoff, May 18, 2023. BICR, blinded independent central review; DOR, duration of response; PFS, progression-free survival.
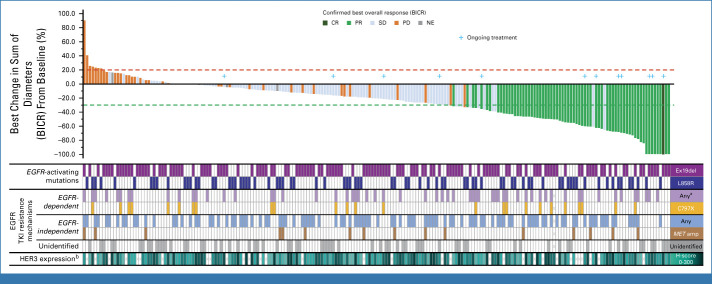
FIG 3.
Waterfall plot of best percentage change in the sum of diameters from baseline (n = 225). Snapshot data cutoff, May 18, 2023. Below the waterfall plot, categories of genomic alterations before treatment with HER3-DXd are flagged for each patient (more details on genomic alterations can be found in the Data Supplement, Fig S4) and pretreatment HER3 IHC membrane H-score is shown as a heatmap. Two hundred and ten patients had evaluable target lesion measurements at both baseline and post baseline and are included. aT790M was not included as an EGFR-dependent mechanism of EGFR TKI resistance. bPretreatment (within 3 months before baseline) tumor HER3 membrane IHC H-score. BICR, blinded independent central review; CR, complete response; EGFR, epidermal growth factor receptor; HER3, human epidermal growth factor receptor 3; IHC, immunohistochemistry; MET, MET proto-oncogene, receptor tyrosine kinase; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease; TKI, tyrosine kinase inhibitor.
In the efficacy population, consistent confirmed ORRs were observed across protocol-defined baseline subgroups on the basis of patient demographics, disease state, and previous treatment (95% CI were overlapping for all comparisons; Data Supplement, Fig S2). In the uptitration arm (n = 50), confirmed ORR was 16.0% (95% CI, 7.2 to 29.1) and PFS was 6.7 (95% CI, 4.2 to 8.8) months.
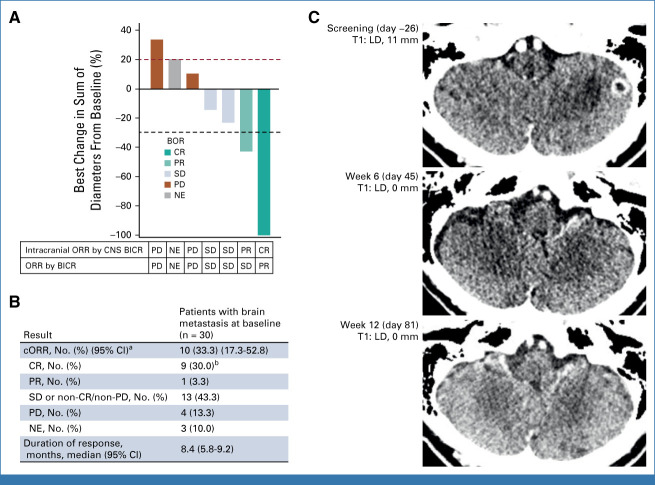
An analysis of intracranial response was performed in 30 patients from the efficacy population who had brain metastases at baseline (by CNS BICR) with no previous radiotherapy of the brain (Fig 4). Target lesions were identified in seven of these patients; the remainder had only nontarget lesions. The CNS ORR (by CNS BICR) was 33.3% (10/30 patients; 95% CI, 17.3 to 52.8). There were nine CNS CRs (eight of which were in patients with only nontarget lesions) and one PR. These responses were based on CT imaging alone (five CRs and one PR), MRI alone (two CRs), or CT imaging with MRI confirmation (two CRs). Best CNS responses of stable disease (including non-CR/non-progressive disease [PD]) and PD were observed in 43.3% (13/30) and 13.3% (4/30) of patients, respectively.
FIG 4.
Outcomes by CNS BICR in patients with brain metastases at baseline with no previous radiotherapy. (A) Best change from baseline in the sum of diameters of measurable target brain lesions. (B) Intracranial antitumor activity in all patients with brain metastasis at baseline (including nontarget lesions). (C) Contrast-enhanced CT series for the patient with a measurable target lesion who had a confirmed complete intracranial response. aCR + PR. bEight patients had only nontarget lesions. BICR, blinded independent central review; BOR, best overall response; cORR, confirmed objective response rate; CR, complete response; CT, computed tomography; LD, longest diameter; NE, not estimable/not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; T, target. Snapshot data cutoff, May 18, 2023.
Association of Biomarkers With Efficacy
Among 193 patients from the efficacy population with evaluable tumor tissue, the median pretreatment HER3 membrane H-score was 198 (range, 0-300; seven patients had a membrane H-score of 0). Responses were observed across the range of pretreatment tumor HER3 membrane expression (Fig 3; Data Supplement, Fig S3; two of seven patients with a membrane H-score of 0 had a confirmed PR).
Genomic alterations known to be associated with EGFR TKI resistance were detected in tumors from 147 of 224 patients in the efficacy population with available baseline data (Data Supplement, Fig S4). Confirmed ORR was 32.4% (11/34) in patients with only EGFR-dependent resistance mechanisms, 27.2% (22/81) in patients with only EGFR-independent resistance mechanisms, and 37.5% (12/32) in patients with both (Data Supplement, Table S5). In patients with no identified EGFR TKI resistance–associated genomic alterations, confirmed ORR was 27.3% (21/77).
Safety
At the primary data cutoff (November 22, 2022), the median duration of treatment in the safety population was 5.5 (range, 0.7-18.2) months, with median dose intensity of 5.5 (range, 3.2-6.0) mg/kg/cycle and median relative dose intensity of 97.7% (range, 57.1%-107.8%; Data Supplement, Table S4). Treatment-emergent adverse events (TEAEs) of grade ≥3 and ≥4 severity occurred in 64.9% and 28.9% of patients, respectively. The most common grade ≥3 TEAEs were hematologic toxicities; those occurring in >15% of patients were thrombocytopenia (20.9%; grouped preferred term) and neutropenia (19.1%; grouped preferred term; Fig 5). Median time to first onset of grade ≥3 events was 8 (range, 7-243) days for thrombocytopenia and 21 (range, 8-299) days for neutropenia; the corresponding median event duration was 13 (95% CI, 10 to 15) days and 7 (95% CI, 6 to 8) days, respectively.
FIG 5.
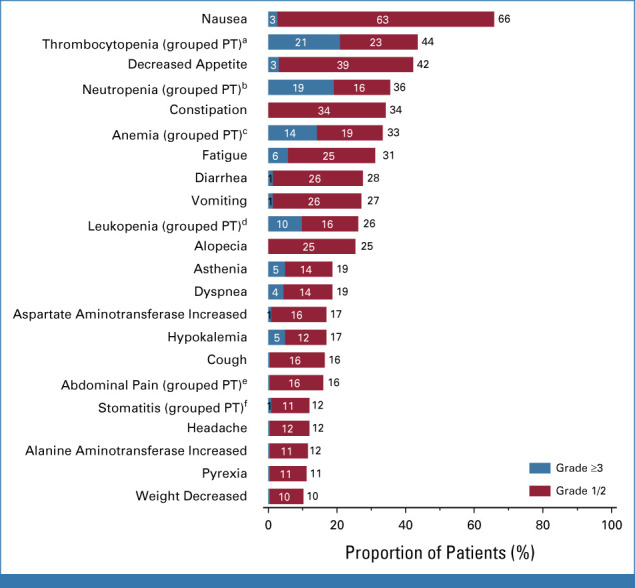
Most common TEAEs occurring in ≥10% of patients (n = 225). Primary data cutoff, November 21, 2022. aPlatelet count decreased, thrombocytopenia. bNeutropenia, neutrophil count decreased. cAnemia, hematocrit decreased, hemoglobin decreased, red blood cell count decreased. dLeukopenia, white blood cell count decreased. eAbdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper. fAphthous ulcer, mouth ulceration, oral mucosa erosion, oral mucosal blistering, stomatitis. PT, preferred term; TEAE, treatment-emergent adverse event.
Two grade ≥3 bleeding events occurred within ±14 days of a laboratory abnormality of grade ≥3 platelet count decreased (GI hemorrhage and hemothorax, n = 1 each). Two grade ≥3 neutropenic infection events occurred within ±14 days of a laboratory abnormality of grade ≥3 neutrophil count decreased (sepsis and septic shock, n = 1 each). All four of these events were determined by investigators to be unrelated to study drug and the patients recovered (Data Supplement, Results).
TEAEs were associated with dose interruption in 40.4% of patients (91/225), dose reduction in 21.3% (48/225), and treatment discontinuation in 7.1% (16/225; Data Supplement, Table S4). Treatment-related TEAEs were associated with death in 1.8% (4/225) of patients (Data Supplement, Table S4; pneumonitis, pneumonia, GI perforation, and respiratory failure [n = 1 each]).
The potential occurrence of ILD was identified in the safety data for 19 patients from the safety population (N = 225), of whom 12 (5.3%) were adjudicated to have had ILD (Data Supplement, Table S6; all were adjudicated as drug-related [one grade 1, eight grade 2, two grade 3, and one grade 5]). Median time to onset of adjudicated ILD was 53 (range, 9-230) days.
TEAE rates were numerically higher in patients who received previous immunotherapy (90/225) versus those who did not (135/225), although the differences were generally small (Data Supplement, Table S7). The rate of adjudicated ILD (all events were adjudicated as drug-related) was lower in patients with no previous immunotherapy (4%) than in patients with previous immunotherapy (8%; Data Supplement, Table S8).
DISCUSSION
For patients with advanced EGFR-mutated NSCLC that has progressed after EGFR TKI therapy and PBC, current treatment options provide only limited clinical benefit. To our knowledge, the results of HERTHENA-Lung01 presented here provide the largest evaluation to date of the efficacy and safety of HER3-DXd once every 3 weeks monotherapy in this population, and also provide the largest assessment of a targeted therapy after the failure of osimertinib. The confirmed ORR in this study (29.8%; 95% CI, 23.9 to 36.2), with a median duration of response of 6.4 months, was higher than that reported in the ramucirumab plus docetaxel group in the REVEL study (22.9%; 95% CI, 19.7 to 26.4). Although the lower bound of the ORR 95% CI in this study included the null hypothesis of 26.4% that was based on data from the REVEL study, the comparison is limited by several considerations: treatment was in the second line in the REVEL study rather than the setting of third line or later for this study; a low proportion of patients in the REVEL study had NSCLC with a demonstrated EGFR-activating mutation and 36% of patients in the REVEL study had a treatment interval of ≥9 months between the time of last treatment and study entry, suggesting the presence of more indolent disease. A recent real-world analysis (which was performed subsequent to the initiation of this study) of treatment in patients with EGFR-mutated NSCLC after EGFR TKI therapy and PBC showed that the median real-world PFS in this treatment setting was 3.3 (95% CI, 2.8 to 4.4) months and median OS was 8.6 (95% CI, 7.4 to 9.8) months (Data Supplement, Table S1).5 In this study, HER3-DXd once every 3 weeks was associated with a median PFS of 5.5 (95% CI, 5.1 to 5.9) months and a median OS of 11.9 (95% CI, 11.2 to 13.1) months (Table 2).
Both the HERTHENA-Lung01 and the previously reported U1027 study (Data Supplement, Table S1) showed compelling evidence of efficacy for HER3-DXd once every 3 weeks in patients with EGFR-mutated NSCLC after the failure of EGFR TKI and PBC. Both studies showed clinical benefit across subgroups, including those characterized by tumor HER3 expression, mechanisms of resistance to EGFR TKI therapy, and the presence or absence of brain metastasis; however, there were numerical differences in the ORR and PFS observed in the two studies. Potential determinants of efficacy in the two studies that might have accounted for the observed differences included the presence of bone metastases, the proportions of Asian versus non-Asian patients, and the number of previous lines of treatment, although no predominant prognostic determinant was identified. No substantive differences in study conduct accounted for the distinct clinical observations. Both studies show that HER3-DXd once every 3 weeks provides meaningful clinical benefit to patients whose available treatment options provide only transient clinical benefit.
HER3-DXd once every 3 weeks treatment yielded clinically meaningful responses in NSCLC CNS metastases (Fig 4), which have been reported in 70% of patients with advanced EGFR-mutated NSCLC.20 This analysis was added because of the emerging understanding of the potential for intracranial response with large-molecule therapies.21 Although the Response Assessment in Neuro-Oncology Brain Metastases criteria22 are the current standard, required data for corticosteroid use and neurologic symptoms were not routinely collected in this study. A potential limitation of the CNS RECIST data was that small brain lesions can be difficult to assess using CT imaging. The CNS penetration and pharmacodynamic activity of HER3-DXd in patients with CNS metastasis are being evaluated further in the recently initiated PARAMETer window-of-opportunity study (ClinicalTrials.gov identifier: NCT05620914).
HER3-DXd once every 3 weeks elicited tumor responses in a diverse group of EGFR-mutated NSCLC tumors, including clinical efficacy across a broad range of pretreatment tumor HER3 membrane expression, consistent with previous observations.7,23 Additional correlative analyses of the HER3 IHC data are ongoing and are incorporating other parameters, including cytoplasmic expression of the receptor. Studies in preclinical models have shown that the dynamics of receptor internalization and turnover in addition to receptor expression level have the potential to affect the efficacy of HER3-DXd.24 In HERTHENA-Lung01, antitumor activity was also observed across subgroups of EGFR TKI resistance detected at baseline, including tumors harboring EGFR-dependent, EGFR-independent, or unidentified mechanisms of resistance. Durable responses to HER3-DXd once every 3 weeks were also seen in patients with tumors with EGFR C797X or MET amplification, which comprise the most common mechanisms of resistance to third-generation EGFR TKIs.25 Thus, HER3-DXd once every 3 weeks is associated with durable efficacy across a broad spectrum of EGFR-mutated NSCLC subtypes.
HER3-DXd once every 3 weeks had a manageable safety profile with a low rate of treatment-related discontinuation due to AEs. Thrombocytopenia was the most frequent grade ≥3 TEAE, and it typically occurred early in treatment and was transient; bleeding events in the setting of thrombocytopenia were rare. Grade ≥3 neutropenia was also transient and was rarely associated with fever and/or infection.
The incidence of adjudicated drug-related ILD (5.3% [grade 5, 0.4%]) was consistent with previous reports of HER3-DXd once every 3 weeks in patients with NSCLC,7 and the majority of instances were grade 1 or 2 in severity. Vigilant observation for signs and symptoms associated with ILD, prompt interruption of HER3-DXd, and intervention with high-dose corticosteroid treatment may mitigate the development of severe ILD.
In summary, HER3-DXd once every 3 weeks demonstrated clinically meaningful efficacy, including durable intracranial responses, with a manageable safety profile in patients with previously treated EGFR-mutated NSCLC. Clinical evaluation of HER3-DXd once every 3 weeks is ongoing in a phase III trial versus PBC (HERTHENA-Lung02; ClinicalTrials.gov identifier: NCT05338970) in patients with EGFR-mutated NSCLC after progression on EGFR TKI treatment.
ACKNOWLEDGMENT
The authors thank the patients, their families, and their caregivers for their participation. The authors also thank Frédérique Cantero and Mike Vigliotti for their contributions. Medical editorial assistance was provided by Amos Race, PhD, CMPP (ArticulateScience, LLC), and funded by Daiichi Sankyo, Inc.
Helena A. Yu
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo, Blueprint Medicines, Janssen, C4 Therapeutics, Cullinan Oncology, Black Diamond Therapeutics, Taiho Oncology, AbbVie
Research Funding: AstraZeneca (Inst), Astellas Pharma (Inst), Lilly (Inst), Novartis (Inst), Pfizer (Inst), Daiichi Sankyo (Inst), Cullinan Oncology (Inst), Janssen Oncology (Inst), Erasca, Inc (Inst), Blueprint Medicines (Inst), Black Diamond Therapeutics (Inst)
Other Relationship: Astellas Pharma
Yasushi Goto
Honoraria: AstraZeneca, Boehringer Ingelheim, Lilly Japan, Chugai Pharma, Taiho Pharmaceutical, Ono Pharmaceutical, MSD, Pfizer, Bristol Myers Squibb Japan, Novartis, Thermo Fisher Scientific, Merck, Guardant Health AMEA, Takeda, Daiichi Sankyo/Astra Zeneca, Daiichi Sankyo/UCB Japan, Amgen, Janssen, Sandoz, Nichiiko
Consulting or Advisory Role: Lilly, Boehringer Ingelheim, Taiho Pharmaceutical, Pfizer, Novartis, AstraZeneca, Chugai Pharma, Guardant Health AMEA, Daiichi Sankyo/UCB Japan, Janssen, Ono Pharmaceutical
Research Funding: AbbVie (Inst), Lilly Japan (Inst), Pfizer (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Bristol Myers Squibb Japan (Inst), Chugai Pharma (Inst), Taiho Pharmaceutical (Inst), MSD (Inst), AstraZeneca (Inst), Novartis (Inst), Merck Serono (Inst), Genomic Health (Inst), CMIC (Inst), Takeda (Inst), EPS Holdings (Inst), IQvia (Inst), Daiichi Sankyo/UCB Japan (Inst), Janssen (Inst), Amgen (Inst), EP Croit Co (Inst), Astellas Amgen BioPharama (Inst), Bayer (Inst), Preferred Network (Inst)
Hidetoshi Hayashi
Honoraria: Ono Pharmaceutical, Bristol Myers Squibb Japan, Lilly, Boehringer Ingelheim, AstraZeneca Japan, Chugai Pharma, Pfizer, MSD, Novartis, Merck Serono, Amgen, Daiichi Sankyo/UCB Japan, Guardant Health, Takeda
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Janssen
Research Funding: Ono Pharmaceutical, Boehringer Ingelheim, AstraZeneca, AbbVie (Inst), AC Medical (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Lilly Japan (Inst), EPS Associates Co., Ltd (Inst), GlaxoSmithKline (Inst), Japan Clinical Research Operations (Inst), Kyowa Hakko Kirin (Inst), Merck Serono (Inst), Novartis (Inst), Otsuka (Inst), PAREXEL (Inst), Pfizer (Inst), PPD-SNBL (Inst), Quintiles Inc (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Sysmex (Inst)
Patents, Royalties, Other Intellectual Property: Sysmex
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Turning Point Therapeutics, Daiichi Sankyo
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, Peervoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: GRIFOLS
Uncompensated Relationships: Member of the Scientific Advisory Committee—Hospital Universitari Parc Taulí, SEOM (Sociedad Española de Oncología Médica), President from 2021-2023, “ETOP IBCSG Partners” Member of the Scientific Committee
James Chih-Hsin Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, Takeda, Lilly, Pfizer
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Clovis Oncology, Lilly, MSD Oncology, Merck Serono, Celgene, Bayer, Pfizer, Ono Pharmaceutical, Bristol Myers Squibb, Boehringer Ingelheim (Inst), Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Takeda, Amgen, Incyte, Merck Serono (Inst), Janssen (Inst), GlaxoSmithKline (Inst), Amgen (Inst), Takeda (Inst), Daiichi Sankyo (Inst), AstraZeneca (Inst), Novartis (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Kiyotaka Yoh
Honoraria: Chugai Pharma, AstraZeneca, Lilly Japan, Kirin Pharmaceuticals, Bristol Myers Squibb Japan, Taiho Pharmaceutical, Janssen, Daiichi Sankyo/UCB Japan, Boehringer Ingelheim, Novartis
Consulting or Advisory Role: Boehringer Ingelheim
Research Funding: Lilly Japan (Inst), AstraZeneca (Inst), Pfizer (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), MSD (Inst), Takeda (Inst), Daiichi Sankyo (Inst), AbbVie (Inst)
Se-Hoon Lee
Honoraria: AstraZeneca/MedImmune, Roche, Merck, Lilly, Amgen
Consulting or Advisory Role: AstraZeneca, Roche, Merck, Pfizer, Lilly, BMS/Ono, Takeda, Janssen, IMBdx
Research Funding: Merck, AstraZeneca, Lunit
Travel, Accommodations, Expenses: Novartis
Luis Paz-Ares
Leadership: Altum Sequencing, Stab Therapeutics
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck, Roche
Benjamin Besse
Research Funding: AstraZeneca (Inst), Inivata (Inst), AbbVie (Inst), Amgen (Inst), Sanofi (Inst), Daiichi Sankyo (Inst), Janssen Oncology (Inst), Roche/Genentech (Inst), Aptitude Health (Inst), Chugai Pharma (Inst), Genzyme (Inst), Ipsen (Inst), Turning Point Therapeutics (Inst), Eisai (Inst), Ellipses Pharma (Inst), Genmab (Inst), Hedera Dx (Inst), MSD Oncology (Inst), PharmaMar (Inst), Taiho Pharmaceutical (Inst), SOCAR (Inst)
Paolo Bironzo
Honoraria: AstraZeneca, Bristol Myers Squibb, MSD Oncology, Roche, Takeda, Novartis, Sanofi
Consulting or Advisory Role: Roche, Janssen Oncology, Pierre Fabre, Amgen, Seagen, Regeneron
Research Funding: Roche (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Amgen, Daiichi Sankyo/Arqule
Dong-Wan Kim
Research Funding: Alpha Biopharma (Inst), AstraZeneca/MedImmune (Inst), Hanmi (Inst), Janssen (Inst), Merus (Inst), Mirati Therapeutics (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Roche/Genentech (Inst), Takeda (Inst), TP Therapeutics (Inst), Xcovery (Inst), Yuhan (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Chong Kun Dang Pharmaceutical (Inst), BridgeBio Pharma (Inst), GlaxoSmithKline (Inst), Merck (Inst), inno.N (Inst)
Melissa L. Johnson
Consulting or Advisory Role: Genentech/Roche (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Merck (Inst), Sanofi (Inst), Mirati Therapeutics (Inst), Ribon Therapeutics (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Gritstone Bio (Inst), Janssen Oncology (Inst), Lilly (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Axelia Oncology (Inst), Black Diamond Therapeutics (Inst), CytomX Therapeutics (Inst), EcoR1 Capital (Inst), Editas Medicine (Inst), Genmab (Inst), IDEAYA Biosciences (Inst), ITeos Therapeutics (Inst), Oncorus (Inst), Regeneron (Inst), Turning Point Therapeutics (Inst), Astellas Pharma (Inst), Checkpoint Therapeutics (Inst), Genocea Biosciences (Inst), Molecular Axiom (Inst), Novartis (Inst), Revolution Medicines (Inst), Takeda (Inst), VBL Therapeutics (Inst), ArriVent Biopharma (Inst), Pyramid Biosciences (Inst), SeaGen (Inst)
Research Funding: EMD Serono (Inst), Kadmon (Inst), Janssen (Inst), Mirati Therapeutics (Inst), Genmab (Inst), Pfizer (Inst), AstraZeneca (Inst), Stem CentRx (Inst), Novartis (Inst), Array BioPharma (Inst), Regeneron (Inst), Merck (Inst), Hengrui Pharmaceutical (Inst), Lycera (Inst), BeiGene (Inst), Tarveda Therapeutics (Inst), Loxo (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), Sanofi (Inst), CytomX Therapeutics (Inst), Dynavax Technologies (Inst), Corvus Pharmaceuticals (Inst), Incyte (Inst), Genocea Biosciences (Inst), Gritstone Bio (Inst), Amgen (Inst), Genentech/Roche (Inst), Adaptimmune (Inst), Syndax (Inst), Neovia Oncology (Inst), Acerta Pharma (Inst), Takeda (Inst), Shattuck Labs (Inst), GlaxoSmithKline (Inst), Apexigen (Inst), Atreca (Inst), OncoMed (Inst), Lilly (Inst), Immunocore (Inst), University of Michigan (Inst), TCR2 Therapeutics (Inst), Arcus Biosciences (Inst), Ribon Therapeutics (Inst), BerGenBio (Inst), Calithera Biosciences (Inst), Tmunity Therapeutics, Inc (Inst), Seven and Eight Biopharmaceuticals (Inst), Rubius Therapeutics (Inst), Curis (Inst), Silicon Therapeutics (Inst), Dracen (Inst), PMV Pharma (Inst), Artios (Inst), BioAtla (Inst), Elicio Therapeutics (Inst), Erasca, Inc (Inst), Harpoon (Inst), Helsinn Healthcare (Inst), Hutchison MediPharma (Inst), IDEAYA Biosciences (Inst), IGM Biosciences (Inst), Memorial Sloan Kettering Cancer Center (Inst), NeoImmuneTech (Inst), Numab (Inst), Relay Therapeutics (Inst), Revolution Medicines (Inst), Tempest Therapeutics (Inst), Tizona Therapeutics, Inc (Inst), Turning Point Therapeutics (Inst), Vyriad (Inst), Y-mAbs Therapeutics (Inst), Exelixis (Inst), Fate Therapeutics (Inst), Merus (Inst), Black Diamond Therapeutics (Inst), Kartos Therapeutics (Inst), Carisma Therapeutics (Inst), Rain Therapeutics (Inst), Nuvalent, Inc (Inst), Palleon Pharmaceuticals (Inst), EQRx (Inst), Immunitas (Inst)
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Genentech, Incyte, Merck, Pfizer, Sanofi
Yi-Long Wu
Honoraria: AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical, BeiGene Beijing
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), BMS (Inst)
Thomas John
Honoraria: AstraZeneca/MedImmune, Roche/Genentech, Bristol Myers Squibb, MSD Oncology
Consulting or Advisory Role: AstraZeneca, Pfizer, AstraZeneca/MedImmune, Roche/Genentech, Ignyta, Boehringer Ingelheim, Novartis, MSD Oncology, Merck KGaA, Bristol Myers Squibb, Amgen (Inst), PharmaMar (Inst), Specialised Therapeutics, Gilead Sciences, Seagen (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Roche, AstraZeneca, Bristol Myers Squibb, Roche, Merck Sharp & Dohme
Steven Kao
Honoraria: Pfizer (Inst), AstraZeneca (Inst), Roche (Inst), Bristol Myers Squibb (Inst), MSD Oncology (Inst), Takeda (Inst), BeiGene
Consulting or Advisory Role: AstraZeneca, Pfizer (Inst), MSD Oncology, BMSi, Roche, Amgen, BeiGene
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Toshiyuki Kozuki
Honoraria: Chugai Pharma, AstraZeneca, Lilly Japan, Ono Pharmaceutical, Bristol Myers Squibb Japan, Nippon Boehringer Ingelheim, Nippon Kayaku, Taiho Pharmaceutical, MSD, Novartis, Pfizer, Merck, Daiichi Sankyo, Takeda, Bayer, AbbVie, Sawai Pharmaceutical Co, Amgen
Consulting or Advisory Role: AstraZeneca, Chugai Pharma, Daiichi Sankyo/UCB Japan, Ono Pharmaceutical, AbbVie, Pfizer, Bayer Yakuhin
Research Funding: Chugai Pharma (Inst), AstraZeneca (Inst), Merck (Inst), MSD (Inst), Taiho Pharmaceutical (Inst), Kyowa-Hakko Kirin (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Bristol Myers Squibb Japan (Inst), Eisai (Inst), Amgen (Inst), AbbVie (Inst), Sanofi (Inst), Dizal Pharma (Inst), Gilead Sciences (Inst), Pfizer (Inst)
Erminia Massarelli
Honoraria: AstraZeneca
Consulting or Advisory Role: Lilly, Janssen Scientific Affairs, Sanofi, Bristol Myers Squibb Foundation, Daiichi Sankyo Co, AbbVie, Mirati Therapeutics, Fusion Pharmaceuticals, Iovance Biotherapeutics, Gilead Sciences
Speakers' Bureau: Merck, AstraZeneca, Takeda, Lilly, Mirati Therapeutics
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck, Genentech/Roche, Pfizer, AstraZeneca
Jyoti Patel
Consulting or Advisory Role: AbbVie, AstraZeneca, Takeda Science Foundation, Genentech, Anheart Therapeutics
Travel, Accommodations, Expenses: Tempus
Egbert Smit
Honoraria: AstraZeneca, Daiichi Sankyo/Astra Zeneca, Merck KGaA, Boehringer Ingelheim
Consulting or Advisory Role: Lilly (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Merck KGaA (Inst), MSD Oncology (Inst), Takeda (Inst), Bayer (Inst), Merck KGaA (Inst), Novartis (Inst), Daiichi Sankyo (Inst), Seagen (Inst)
Research Funding: Boehringer Ingelheim (Inst), Bayer (Inst), Roche/Genentech (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst)
Karen L. Reckamp
Consulting or Advisory Role: Amgen, Takeda, AstraZeneca, Seagen, Genentech, Blueprint Medicines, Daiichi Sankyo/Lilly, EMD Serono, Janssen Oncology, Lilly, Merck KGaA, GlaxoSmithKline, Mirati Therapeutics
Research Funding: Genentech/Roche (Inst), Janssen Oncology (Inst), Calithera Biosciences (Inst), Elevation Oncology (Inst), Daiichi Sankyo/Astra Zeneca (Inst), Blueprint Medicines
Qian Dong
Employment: Daiichi Sankyo
Stock and Other Ownership Interests: Daiichi Sankyo
Pomy Shrestha
Employment: Daiichi Sankyo
Stock and Other Ownership Interests: Daiichi Sankyo
Travel, Accommodations, Expenses: Daiichi Sankyo
Pang-Dian Fan
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Consulting or Advisory Role: Guidry & East
Parul Patel
Employment: Merck KGaA
Stock and Other Ownership Interests: Merck, Daiichi Sankyo/Lilly
Travel, Accommodations, Expenses: Daiichi Sankyo/Lilly
Andrea Sporchia
Employment: Daiichi Sankyo Europe GmbH, MorphoSys
David W. Sternberg
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Dalila Sellami
Employment: Daiichi-Sankyo Inc, Radius Pharmaceutical
Stock and Other Ownership Interests: Daiichi Sankyo, Janssen-Ortho
Pasi A. Jänne
Stock and Other Ownership Interests: Gatekeeper Pharmaceuticals, Loxo
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, AstraZeneca, Merrimack, Chugai Pharma, Roche/Genentech, Loxo, Mirati Therapeutics, Araxes Pharma, Ignyta, Lilly, Takeda, Novartis, Biocartis, Voronoi Health Analytics, SFJ Pharmaceuticals Group, Sanofi, Daiichi Sankyo, Silicon Therapeutics, Nuvalent, Inc, Eisai, Bayer, Syndax, AbbVie, Allorion Therapeutics, Accutar Biotech, Transcenta, Monte Rosa Therapeutics, Scorpion Therapeutics, Merus, Frontier Medicines, Hongyun Biotech, Duality Biologics
Research Funding: AstraZeneca (Inst), Astellas Pharma (Inst), Daiichi Sankyo (Inst), Lilly (Inst), Boehringer Ingelheim (Inst), Puma Biotechnology (Inst), Takeda (Inst), Revolution Medicines (Inst)
Patents, Royalties, Other Intellectual Property: I am a co-inventor on a DFCI owned patent on EGFR mutations licensed to Lab Corp. I receive post-marketing royalties from this invention
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 5351
Listen to the podcast by Dr Westin at jcopodcast.libsyn.com
PRIOR PRESENTATION
Presented in part at the 2023 International Association for the Study of Lung Cancer World Conference, Singapore, September 9-12, 2023.
SUPPORT
Supported by Daiichi Sankyo, Inc.
CLINICAL TRIAL INFORMATION
H.A.Y. and Y.G. contributed equally to this work.
DATA SHARING STATEMENT
Anonymized individual participant data on completed studies and applicable supporting clinical study documents may be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of the company and our clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo/.
AUTHOR CONTRIBUTIONS
Conception and design: Helena A. Yu, Yasushi Goto, James Chih-Hsin Yang, Yi-Long Wu, Thomas John, Jyoti Patel, Pang-Dian Fan, Parul Patel, David W. Sternberg, Dalila Sellami, Pasi A. Jänne
Provision of study materials or patients: Hidetoshi Hayashi, Enriqueta Felip, James Chih-Hsin Yang, Martin Reck, Luis Paz-Ares, Dong-Wan Kim, Melissa L. Johnson, Thomas John, Steven Kao, Toshiyuki Kozuki, Erminia Massarelli, Jyoti Patel, Egbert Smit, Karen L. Reckamp, Pasi A. Jänne
Collection and assembly of data: Helena A. Yu, Hidetoshi Hayashi, Luis Paz-Ares, Benjamin Besse, Melissa L. Johnson, Yi-Long Wu, Steven Kao, Toshiyuki Kozuki, Erminia Massarelli, Jyoti Patel, Karen L. Reckamp, Pomy Shrestha, Parul Patel, Andrea Sporchia, David W. Sternberg, Pasi A. Jänne
Data analysis and interpretation: Helena A. Yu, Yasushi Goto, Enriqueta Felip, James Chih-Hsin Yang, Martin Reck, Kiyotaka Yoh, Se-Hoon Lee, Paolo Bironzo, Dong-Wan Kim, Melissa L. Johnson, Yi-Long Wu, Thomas John, Steven Kao, Egbert Smit, Karen L. Reckamp, Qian Dong, Pomy Shrestha, Pang-Dian Fan, Parul Patel, Andrea Sporchia, David W. Sternberg, Dalila Sellami
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
HERTHENA-Lung01, a Phase II Trial of Patritumab Deruxtecan (HER3-DXd) in Epidermal Growth Factor Receptor–Mutated Non–Small-Cell Lung Cancer After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Helena A. Yu
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo, Blueprint Medicines, Janssen, C4 Therapeutics, Cullinan Oncology, Black Diamond Therapeutics, Taiho Oncology, AbbVie
Research Funding: AstraZeneca (Inst), Astellas Pharma (Inst), Lilly (Inst), Novartis (Inst), Pfizer (Inst), Daiichi Sankyo (Inst), Cullinan Oncology (Inst), Janssen Oncology (Inst), Erasca, Inc (Inst), Blueprint Medicines (Inst), Black Diamond Therapeutics (Inst)
Other Relationship: Astellas Pharma
Yasushi Goto
Honoraria: AstraZeneca, Boehringer Ingelheim, Lilly Japan, Chugai Pharma, Taiho Pharmaceutical, Ono Pharmaceutical, MSD, Pfizer, Bristol Myers Squibb Japan, Novartis, Thermo Fisher Scientific, Merck, Guardant Health AMEA, Takeda, Daiichi Sankyo/Astra Zeneca, Daiichi Sankyo/UCB Japan, Amgen, Janssen, Sandoz, Nichiiko
Consulting or Advisory Role: Lilly, Boehringer Ingelheim, Taiho Pharmaceutical, Pfizer, Novartis, AstraZeneca, Chugai Pharma, Guardant Health AMEA, Daiichi Sankyo/UCB Japan, Janssen, Ono Pharmaceutical
Research Funding: AbbVie (Inst), Lilly Japan (Inst), Pfizer (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Bristol Myers Squibb Japan (Inst), Chugai Pharma (Inst), Taiho Pharmaceutical (Inst), MSD (Inst), AstraZeneca (Inst), Novartis (Inst), Merck Serono (Inst), Genomic Health (Inst), CMIC (Inst), Takeda (Inst), EPS Holdings (Inst), IQvia (Inst), Daiichi Sankyo/UCB Japan (Inst), Janssen (Inst), Amgen (Inst), EP Croit Co (Inst), Astellas Amgen BioPharama (Inst), Bayer (Inst), Preferred Network (Inst)
Hidetoshi Hayashi
Honoraria: Ono Pharmaceutical, Bristol Myers Squibb Japan, Lilly, Boehringer Ingelheim, AstraZeneca Japan, Chugai Pharma, Pfizer, MSD, Novartis, Merck Serono, Amgen, Daiichi Sankyo/UCB Japan, Guardant Health, Takeda
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Janssen
Research Funding: Ono Pharmaceutical, Boehringer Ingelheim, AstraZeneca, AbbVie (Inst), AC Medical (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Lilly Japan (Inst), EPS Associates Co., Ltd (Inst), GlaxoSmithKline (Inst), Japan Clinical Research Operations (Inst), Kyowa Hakko Kirin (Inst), Merck Serono (Inst), Novartis (Inst), Otsuka (Inst), PAREXEL (Inst), Pfizer (Inst), PPD-SNBL (Inst), Quintiles Inc (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Sysmex (Inst)
Patents, Royalties, Other Intellectual Property: Sysmex
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Turning Point Therapeutics, Daiichi Sankyo
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, Peervoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: GRIFOLS
Uncompensated Relationships: Member of the Scientific Advisory Committee—Hospital Universitari Parc Taulí, SEOM (Sociedad Española de Oncología Médica), President from 2021-2023, “ETOP IBCSG Partners” Member of the Scientific Committee
James Chih-Hsin Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, Takeda, Lilly, Pfizer
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Clovis Oncology, Lilly, MSD Oncology, Merck Serono, Celgene, Bayer, Pfizer, Ono Pharmaceutical, Bristol Myers Squibb, Boehringer Ingelheim (Inst), Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Takeda, Amgen, Incyte, Merck Serono (Inst), Janssen (Inst), GlaxoSmithKline (Inst), Amgen (Inst), Takeda (Inst), Daiichi Sankyo (Inst), AstraZeneca (Inst), Novartis (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis, Sanofi/Regeneron, Daiichi Sankyo Europe GmbH
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen, Mirati Therapeutics, Sanofi/Aventis
Kiyotaka Yoh
Honoraria: Chugai Pharma, AstraZeneca, Lilly Japan, Kirin Pharmaceuticals, Bristol Myers Squibb Japan, Taiho Pharmaceutical, Janssen, Daiichi Sankyo/UCB Japan, Boehringer Ingelheim, Novartis
Consulting or Advisory Role: Boehringer Ingelheim
Research Funding: Lilly Japan (Inst), AstraZeneca (Inst), Pfizer (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), MSD (Inst), Takeda (Inst), Daiichi Sankyo (Inst), AbbVie (Inst)
Se-Hoon Lee
Honoraria: AstraZeneca/MedImmune, Roche, Merck, Lilly, Amgen
Consulting or Advisory Role: AstraZeneca, Roche, Merck, Pfizer, Lilly, BMS/Ono, Takeda, Janssen, IMBdx
Research Funding: Merck, AstraZeneca, Lunit
Travel, Accommodations, Expenses: Novartis
Luis Paz-Ares
Leadership: Altum Sequencing, Stab Therapeutics
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck, Roche
Benjamin Besse
Research Funding: AstraZeneca (Inst), Inivata (Inst), AbbVie (Inst), Amgen (Inst), Sanofi (Inst), Daiichi Sankyo (Inst), Janssen Oncology (Inst), Roche/Genentech (Inst), Aptitude Health (Inst), Chugai Pharma (Inst), Genzyme (Inst), Ipsen (Inst), Turning Point Therapeutics (Inst), Eisai (Inst), Ellipses Pharma (Inst), Genmab (Inst), Hedera Dx (Inst), MSD Oncology (Inst), PharmaMar (Inst), Taiho Pharmaceutical (Inst), SOCAR (Inst)
Paolo Bironzo
Honoraria: AstraZeneca, Bristol Myers Squibb, MSD Oncology, Roche, Takeda, Novartis, Sanofi
Consulting or Advisory Role: Roche, Janssen Oncology, Pierre Fabre, Amgen, Seagen, Regeneron
Research Funding: Roche (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Amgen, Daiichi Sankyo/Arqule
Dong-Wan Kim
Research Funding: Alpha Biopharma (Inst), AstraZeneca/MedImmune (Inst), Hanmi (Inst), Janssen (Inst), Merus (Inst), Mirati Therapeutics (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Roche/Genentech (Inst), Takeda (Inst), TP Therapeutics (Inst), Xcovery (Inst), Yuhan (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Chong Kun Dang Pharmaceutical (Inst), BridgeBio Pharma (Inst), GlaxoSmithKline (Inst), Merck (Inst), inno.N (Inst)
Melissa L. Johnson
Consulting or Advisory Role: Genentech/Roche (Inst), AstraZeneca (Inst), Calithera Biosciences (Inst), Merck (Inst), Sanofi (Inst), Mirati Therapeutics (Inst), Ribon Therapeutics (Inst), AbbVie (Inst), GlaxoSmithKline (Inst), Gritstone Bio (Inst), Janssen Oncology (Inst), Lilly (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Axelia Oncology (Inst), Black Diamond Therapeutics (Inst), CytomX Therapeutics (Inst), EcoR1 Capital (Inst), Editas Medicine (Inst), Genmab (Inst), IDEAYA Biosciences (Inst), ITeos Therapeutics (Inst), Oncorus (Inst), Regeneron (Inst), Turning Point Therapeutics (Inst), Astellas Pharma (Inst), Checkpoint Therapeutics (Inst), Genocea Biosciences (Inst), Molecular Axiom (Inst), Novartis (Inst), Revolution Medicines (Inst), Takeda (Inst), VBL Therapeutics (Inst), ArriVent Biopharma (Inst), Pyramid Biosciences (Inst), SeaGen (Inst)
Research Funding: EMD Serono (Inst), Kadmon (Inst), Janssen (Inst), Mirati Therapeutics (Inst), Genmab (Inst), Pfizer (Inst), AstraZeneca (Inst), Stem CentRx (Inst), Novartis (Inst), Array BioPharma (Inst), Regeneron (Inst), Merck (Inst), Hengrui Pharmaceutical (Inst), Lycera (Inst), BeiGene (Inst), Tarveda Therapeutics (Inst), Loxo (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), Sanofi (Inst), CytomX Therapeutics (Inst), Dynavax Technologies (Inst), Corvus Pharmaceuticals (Inst), Incyte (Inst), Genocea Biosciences (Inst), Gritstone Bio (Inst), Amgen (Inst), Genentech/Roche (Inst), Adaptimmune (Inst), Syndax (Inst), Neovia Oncology (Inst), Acerta Pharma (Inst), Takeda (Inst), Shattuck Labs (Inst), GlaxoSmithKline (Inst), Apexigen (Inst), Atreca (Inst), OncoMed (Inst), Lilly (Inst), Immunocore (Inst), University of Michigan (Inst), TCR2 Therapeutics (Inst), Arcus Biosciences (Inst), Ribon Therapeutics (Inst), BerGenBio (Inst), Calithera Biosciences (Inst), Tmunity Therapeutics, Inc (Inst), Seven and Eight Biopharmaceuticals (Inst), Rubius Therapeutics (Inst), Curis (Inst), Silicon Therapeutics (Inst), Dracen (Inst), PMV Pharma (Inst), Artios (Inst), BioAtla (Inst), Elicio Therapeutics (Inst), Erasca, Inc (Inst), Harpoon (Inst), Helsinn Healthcare (Inst), Hutchison MediPharma (Inst), IDEAYA Biosciences (Inst), IGM Biosciences (Inst), Memorial Sloan Kettering Cancer Center (Inst), NeoImmuneTech (Inst), Numab (Inst), Relay Therapeutics (Inst), Revolution Medicines (Inst), Tempest Therapeutics (Inst), Tizona Therapeutics, Inc (Inst), Turning Point Therapeutics (Inst), Vyriad (Inst), Y-mAbs Therapeutics (Inst), Exelixis (Inst), Fate Therapeutics (Inst), Merus (Inst), Black Diamond Therapeutics (Inst), Kartos Therapeutics (Inst), Carisma Therapeutics (Inst), Rain Therapeutics (Inst), Nuvalent, Inc (Inst), Palleon Pharmaceuticals (Inst), EQRx (Inst), Immunitas (Inst)
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Genentech, Incyte, Merck, Pfizer, Sanofi
Yi-Long Wu
Honoraria: AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical, BeiGene Beijing
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), BMS (Inst)
Thomas John
Honoraria: AstraZeneca/MedImmune, Roche/Genentech, Bristol Myers Squibb, MSD Oncology
Consulting or Advisory Role: AstraZeneca, Pfizer, AstraZeneca/MedImmune, Roche/Genentech, Ignyta, Boehringer Ingelheim, Novartis, MSD Oncology, Merck KGaA, Bristol Myers Squibb, Amgen (Inst), PharmaMar (Inst), Specialised Therapeutics, Gilead Sciences, Seagen (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Roche, AstraZeneca, Bristol Myers Squibb, Roche, Merck Sharp & Dohme
Steven Kao
Honoraria: Pfizer (Inst), AstraZeneca (Inst), Roche (Inst), Bristol Myers Squibb (Inst), MSD Oncology (Inst), Takeda (Inst), BeiGene
Consulting or Advisory Role: AstraZeneca, Pfizer (Inst), MSD Oncology, BMSi, Roche, Amgen, BeiGene
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Toshiyuki Kozuki
Honoraria: Chugai Pharma, AstraZeneca, Lilly Japan, Ono Pharmaceutical, Bristol Myers Squibb Japan, Nippon Boehringer Ingelheim, Nippon Kayaku, Taiho Pharmaceutical, MSD, Novartis, Pfizer, Merck, Daiichi Sankyo, Takeda, Bayer, AbbVie, Sawai Pharmaceutical Co, Amgen
Consulting or Advisory Role: AstraZeneca, Chugai Pharma, Daiichi Sankyo/UCB Japan, Ono Pharmaceutical, AbbVie, Pfizer, Bayer Yakuhin
Research Funding: Chugai Pharma (Inst), AstraZeneca (Inst), Merck (Inst), MSD (Inst), Taiho Pharmaceutical (Inst), Kyowa-Hakko Kirin (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Bristol Myers Squibb Japan (Inst), Eisai (Inst), Amgen (Inst), AbbVie (Inst), Sanofi (Inst), Dizal Pharma (Inst), Gilead Sciences (Inst), Pfizer (Inst)
Erminia Massarelli
Honoraria: AstraZeneca
Consulting or Advisory Role: Lilly, Janssen Scientific Affairs, Sanofi, Bristol Myers Squibb Foundation, Daiichi Sankyo Co, AbbVie, Mirati Therapeutics, Fusion Pharmaceuticals, Iovance Biotherapeutics, Gilead Sciences
Speakers' Bureau: Merck, AstraZeneca, Takeda, Lilly, Mirati Therapeutics
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck, Genentech/Roche, Pfizer, AstraZeneca
Jyoti Patel
Consulting or Advisory Role: AbbVie, AstraZeneca, Takeda Science Foundation, Genentech, Anheart Therapeutics
Travel, Accommodations, Expenses: Tempus
Egbert Smit
Honoraria: AstraZeneca, Daiichi Sankyo/Astra Zeneca, Merck KGaA, Boehringer Ingelheim
Consulting or Advisory Role: Lilly (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Merck KGaA (Inst), MSD Oncology (Inst), Takeda (Inst), Bayer (Inst), Merck KGaA (Inst), Novartis (Inst), Daiichi Sankyo (Inst), Seagen (Inst)
Research Funding: Boehringer Ingelheim (Inst), Bayer (Inst), Roche/Genentech (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst)
Karen L. Reckamp
Consulting or Advisory Role: Amgen, Takeda, AstraZeneca, Seagen, Genentech, Blueprint Medicines, Daiichi Sankyo/Lilly, EMD Serono, Janssen Oncology, Lilly, Merck KGaA, GlaxoSmithKline, Mirati Therapeutics
Research Funding: Genentech/Roche (Inst), Janssen Oncology (Inst), Calithera Biosciences (Inst), Elevation Oncology (Inst), Daiichi Sankyo/Astra Zeneca (Inst), Blueprint Medicines
Qian Dong
Employment: Daiichi Sankyo
Stock and Other Ownership Interests: Daiichi Sankyo
Pomy Shrestha
Employment: Daiichi Sankyo
Stock and Other Ownership Interests: Daiichi Sankyo
Travel, Accommodations, Expenses: Daiichi Sankyo
Pang-Dian Fan
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Consulting or Advisory Role: Guidry & East
Parul Patel
Employment: Merck KGaA
Stock and Other Ownership Interests: Merck, Daiichi Sankyo/Lilly
Travel, Accommodations, Expenses: Daiichi Sankyo/Lilly
Andrea Sporchia
Employment: Daiichi Sankyo Europe GmbH, MorphoSys
David W. Sternberg
Employment: Daiichi Sankyo, Inc
Stock and Other Ownership Interests: Daiichi Sankyo, Inc
Dalila Sellami
Employment: Daiichi-Sankyo Inc, Radius Pharmaceutical
Stock and Other Ownership Interests: Daiichi Sankyo, Janssen-Ortho
Pasi A. Jänne
Stock and Other Ownership Interests: Gatekeeper Pharmaceuticals, Loxo
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, AstraZeneca, Merrimack, Chugai Pharma, Roche/Genentech, Loxo, Mirati Therapeutics, Araxes Pharma, Ignyta, Lilly, Takeda, Novartis, Biocartis, Voronoi Health Analytics, SFJ Pharmaceuticals Group, Sanofi, Daiichi Sankyo, Silicon Therapeutics, Nuvalent, Inc, Eisai, Bayer, Syndax, AbbVie, Allorion Therapeutics, Accutar Biotech, Transcenta, Monte Rosa Therapeutics, Scorpion Therapeutics, Merus, Frontier Medicines, Hongyun Biotech, Duality Biologics
Research Funding: AstraZeneca (Inst), Astellas Pharma (Inst), Daiichi Sankyo (Inst), Lilly (Inst), Boehringer Ingelheim (Inst), Puma Biotechnology (Inst), Takeda (Inst), Revolution Medicines (Inst)
Patents, Royalties, Other Intellectual Property: I am a co-inventor on a DFCI owned patent on EGFR mutations licensed to Lab Corp. I receive post-marketing royalties from this invention
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hendriks LE, Kerr K, Menis J, et al. : Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34:339-357, 2023 [DOI] [PubMed] [Google Scholar]
- 2.Han B, Yang L, Wang X, et al. : Efficacy of pemetrexed-based regimens in advanced non-small cell lung cancer patients with activating epidermal growth factor receptor mutations after tyrosine kinase inhibitor failure: A systematic review. Onco Targets Ther 11:2121-2129, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi H, Sugawara S, Fukuda Y, et al. : A randomized phase II study comparing nivolumab with carboplatin-pemetrexed for EGFR-mutated NSCLC with resistance to EGFR tyrosine kinase inhibitors (WJOG8515L). Clin Cancer Res 28:893-902, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang CJ, Hung JY, Tsai MJ, et al. : The salvage therapy in lung adenocarcinoma initially harbored susceptible EGFR mutation and acquired resistance occurred to the first-line gefitinib and second-line cytotoxic chemotherapy. BMC Pharmacol Toxicol 18:21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel JD, Meng J, Phani S, et al. : AACR 2023 abstract 6754: Clinical characteristics, real-world treatment patterns, and clinical outcomes among patients with previously treated metastatic or unresectable EGFR-mutated non-small cell lung cancer in the United States. Cancer Res 83:6754, 2023 [Google Scholar]
- 6.Scharpenseel H, Hanssen A, Loges S, et al. : EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci Rep 9:7406, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jänne PA, Baik C, Su WC, et al. : Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov 12:74-89, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonesaka K, Tanizaki J, Maenishi O, et al. : HER3 augmentation via blockade of EGFR/AKT signaling enhances anticancer activity of HER3-targeting patritumab deruxtecan in EGFR-mutated non-small cell lung cancer. Clin Cancer Res 28:390-403, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Mishra R, Patel H, Alanazi S, et al. : HER3 signaling and targeted therapy in cancer. Oncol Rev 12:355, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA, Zejnullahu K, Mitsudomi T, et al. : MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316:1039-1043, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Zhang R, Yan H, et al. : Prognostic significance of HER3 in patients with malignant solid tumors. Oncotarget 8:67140-67151, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto Y, Koyama K, Kamai Y, et al. : A novel HER3-targeting antibody-drug conjugate, U3-1402, exhibits potent therapeutic efficacy through the delivery of cytotoxic payload by efficient internalization. Clin Cancer Res 25:7151-7161, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Koganemaru S, Kuboki Y, Koga Y, et al. : U3-1402, a novel HER3-targeting antibody-drug conjugate, for the treatment of colorectal cancer. Mol Cancer Ther 18:2043-2050, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Nakada T, Sugihara K, Jikoh T, et al. : The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo) 67:173-185, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Ogitani Y, Aida T, Hagihara K, et al. : DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 22:5097-5108, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Ueno S, Hirotani K, Abraham R, et al. : U3-1402a, a novel HER3-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a potent tumor efficacy. Cancer Res 77:3092, 2017. 28377455 [Google Scholar]
- 17.Yonesaka K, Takegawa N, Watanabe S, et al. : An HER3-targeting antibody-drug conjugate incorporating a DNA topoisomerase I inhibitor U3-1402 conquers EGFR tyrosine kinase inhibitor-resistant NSCLC. Oncogene 38:1398-1409, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Garon EB, Ciuleanu T-E, Arrieta O, et al. : Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 384:665-673, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Ge M, Zhuang Y, Zhou X, et al. : High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J Neurooncol 135:413-418, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Jerusalem G, Park YH, Yamashita T, et al. : Trastuzumab deruxtecan in HER2-positive metastatic breast cancer patients with brain metastases: A DESTINY-Breast01 subgroup analysis. Cancer Discov 12:2754-2762, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin NU, Lee EQ, Aoyama H, et al. : Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol 16:e270-e278, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Hayashi H, Yu HA, Baik C, et al. : First report of cohort 3 and extended follow-up data from the U31402-A-U102 study of HER3-DXd in EGFR-mutated NSCLC. Presented at the Japanese Society of Medical Oncology Annual Meeting, Fukuoka, Japan, March 16-18, 2023 (abst e70378)
- 24.Komatsu N, Sato S, Muramatsu S, et al. : Abstract 3996: The impact of HER3 dynamics on the efficacy of HER3-DXd, a novel HER3 directed antibody-drug conjugate. Cancer Res 83:3996, 2023 [Google Scholar]
- 25.Ramalingam SS, Cheng Y, Zhou C, et al. : Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol 29:viii740, 2018 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized individual participant data on completed studies and applicable supporting clinical study documents may be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of the company and our clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo/.